Modified Liuwei Dihuang Decoction Ameliorates Oligoasthenozoospermia in Mice via Modulation of the PI3K/AKT/Nrf2 Signaling Pathway
Abstract
1. Introduction
2. Result
2.1. Bioactive Components and Therapeutic Targets of MLWDH Identified by UPLC-HRMS and Network Pharmacology
2.1.1. Characterization of Principal Constituents in MLWDH via UPLC-HRMS Analysis
2.1.2. Identification and Prioritization of Putative Targets of MLWDH Against OA
2.1.3. Network Construction of Drug-Compound–Target–Disease Associations
2.1.4. KEGG Pathway Enrichment Analysis of Putative Targets
2.1.5. Molecular Docking Analysis of MLWDH Bioactive Compounds with PI3K/AKT Signaling
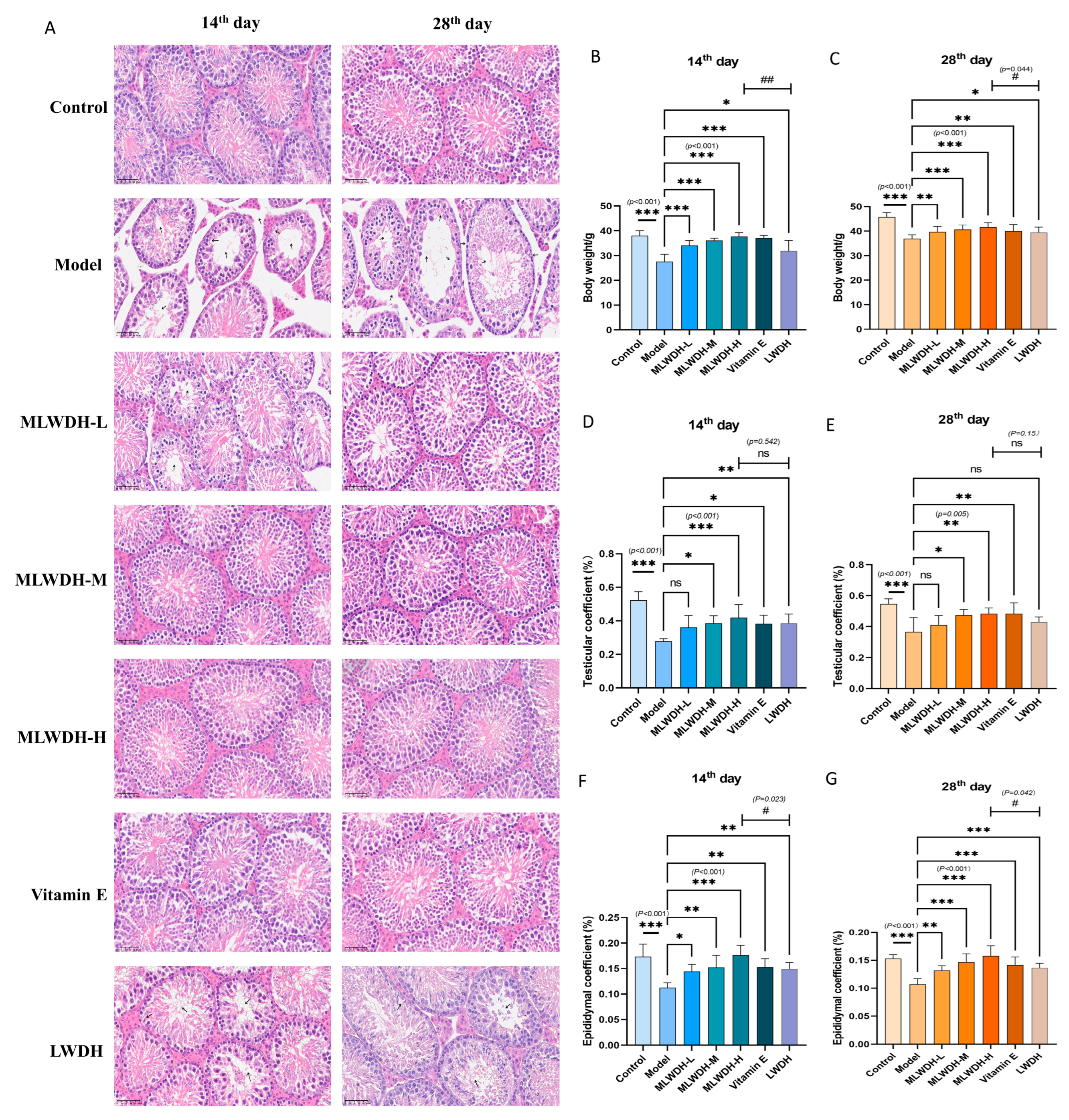
2.2. MLWDH Alleviates CP-Induced Reproductive Organ Injury and Testicular Histopathology
2.3. MLWDH Increases Reproductive Hormone Levels in OA Mice
2.4. MLWDH Improves Sperm Quality and Reduces Morphological Abnormalities in OA Mice
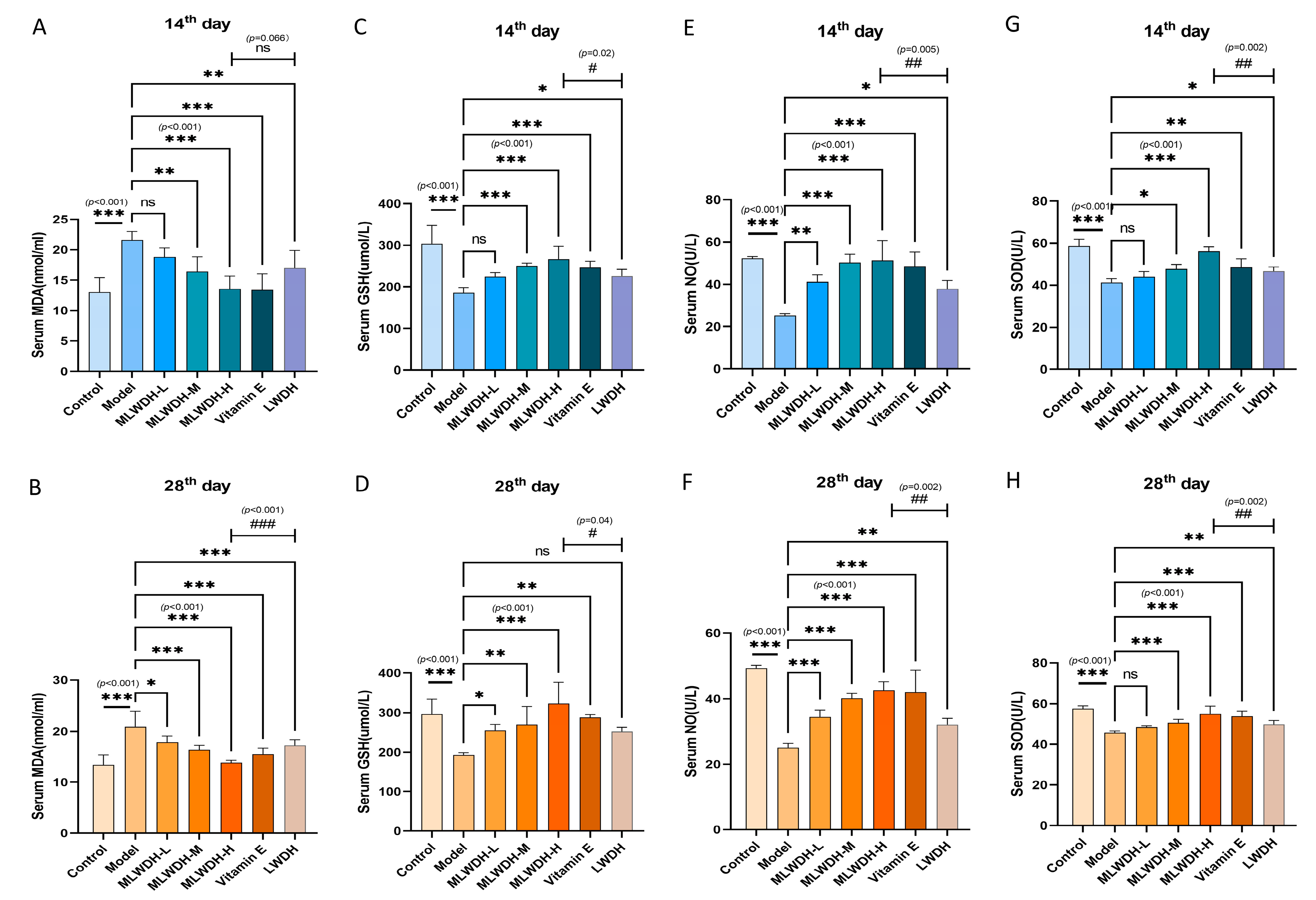
2.5. MLWDH Attenuates Oxidative Stress in OA Mice
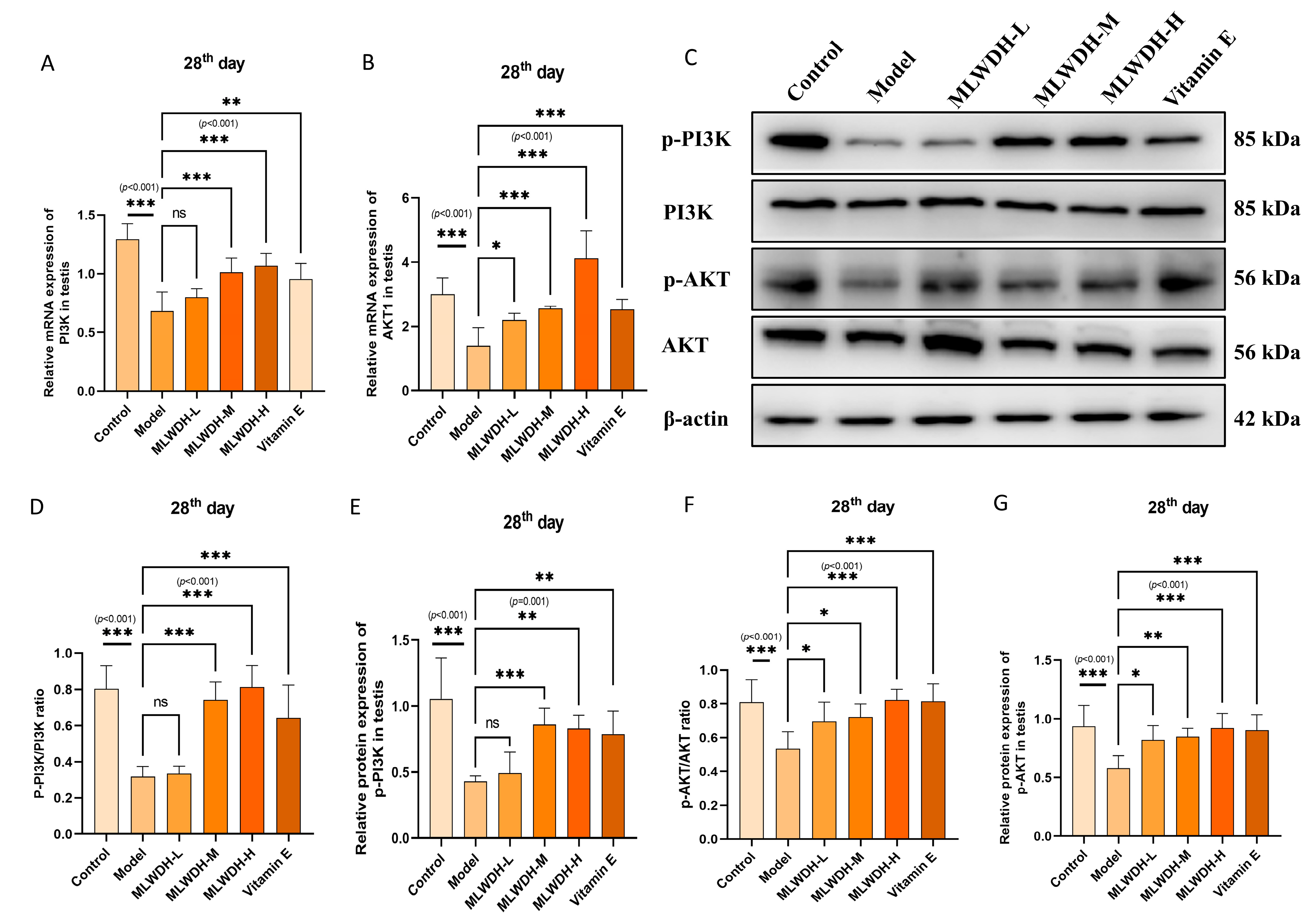
2.6. MLWDH Activates the PI3K/AKT Signaling Pathway in OA Mice
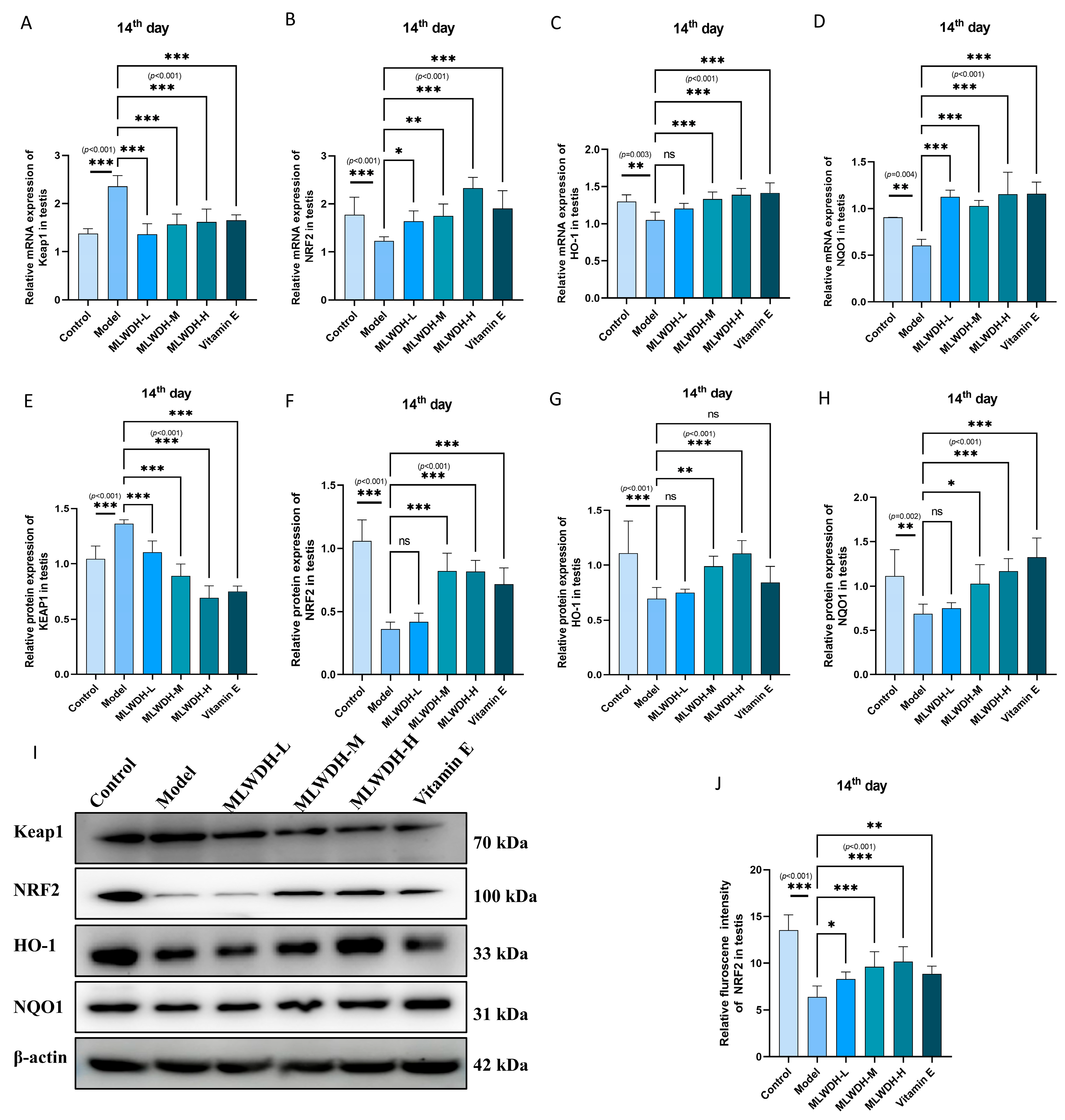
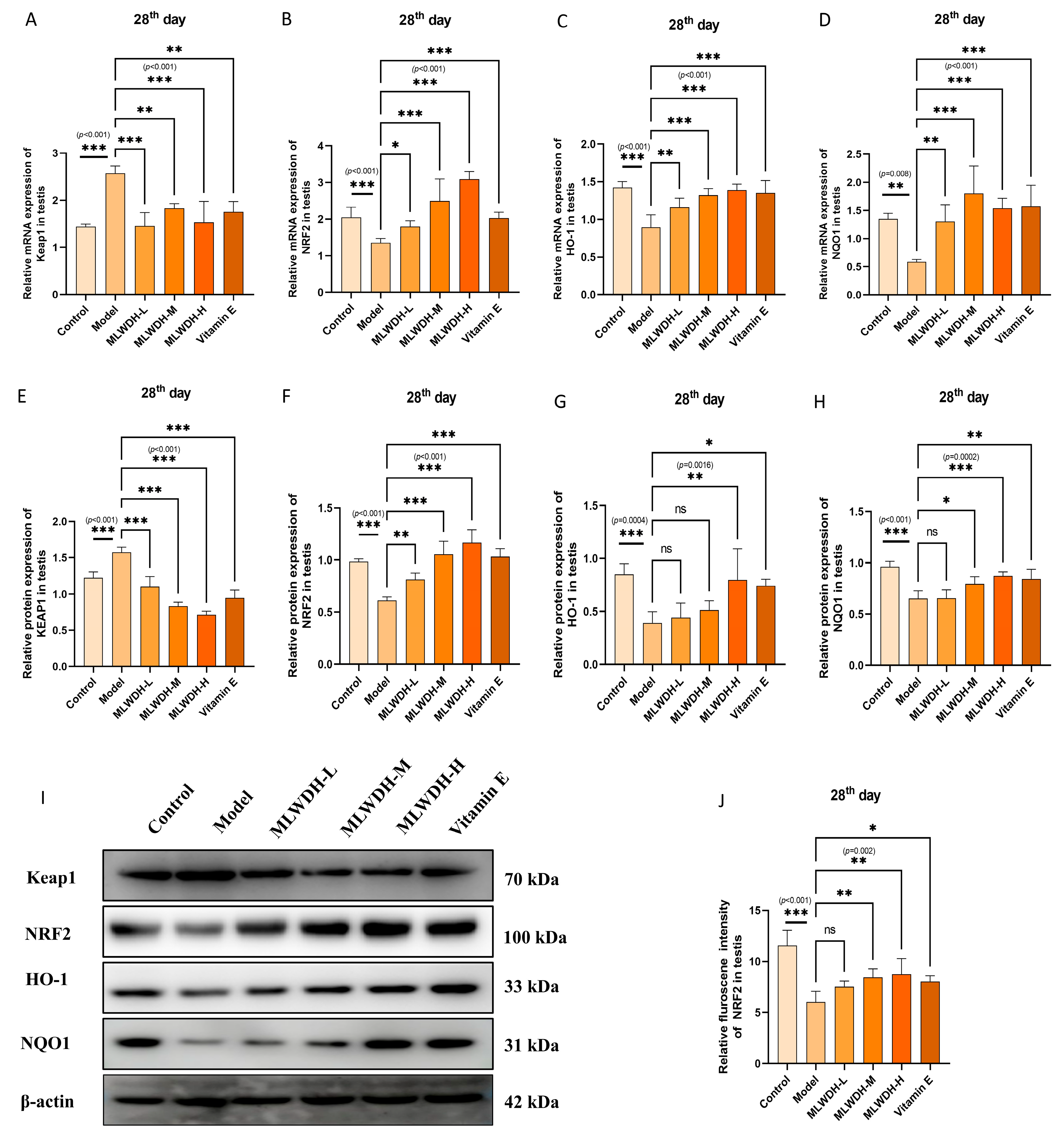
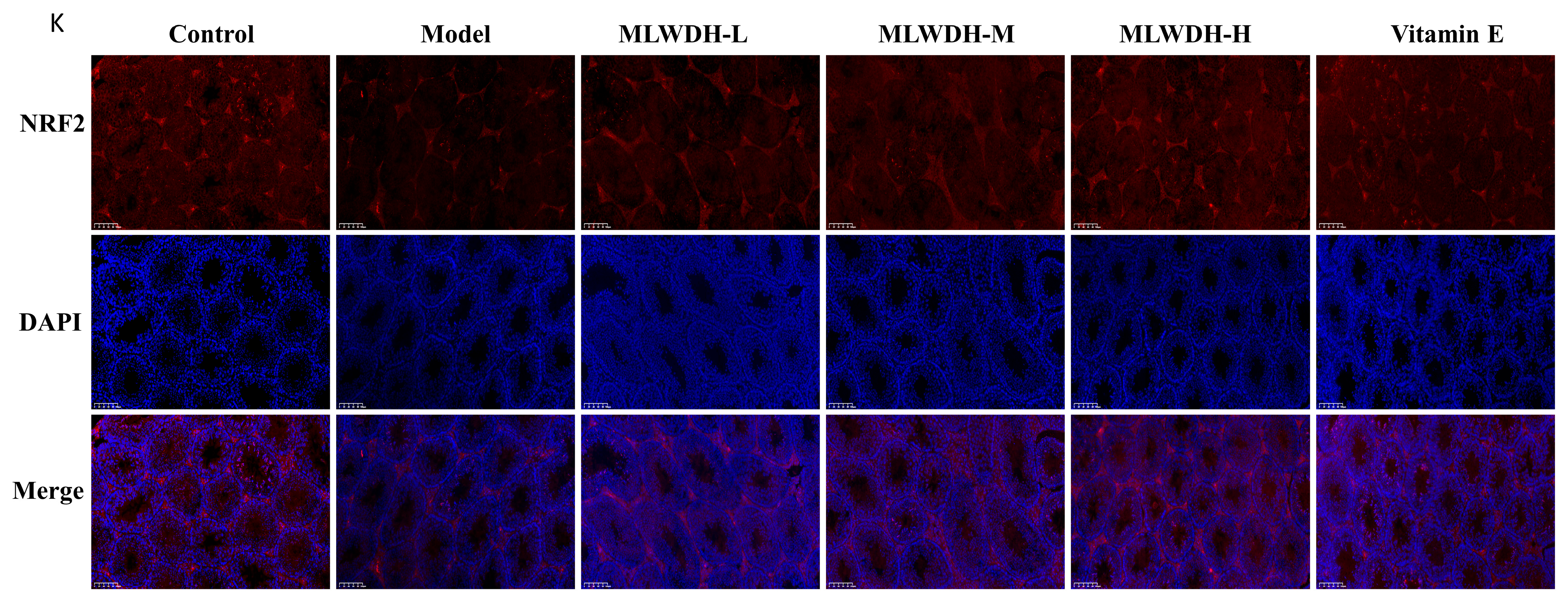
2.7. MLWDH Enhances Antioxidant Defense via the Nrf2/ARE Signaling
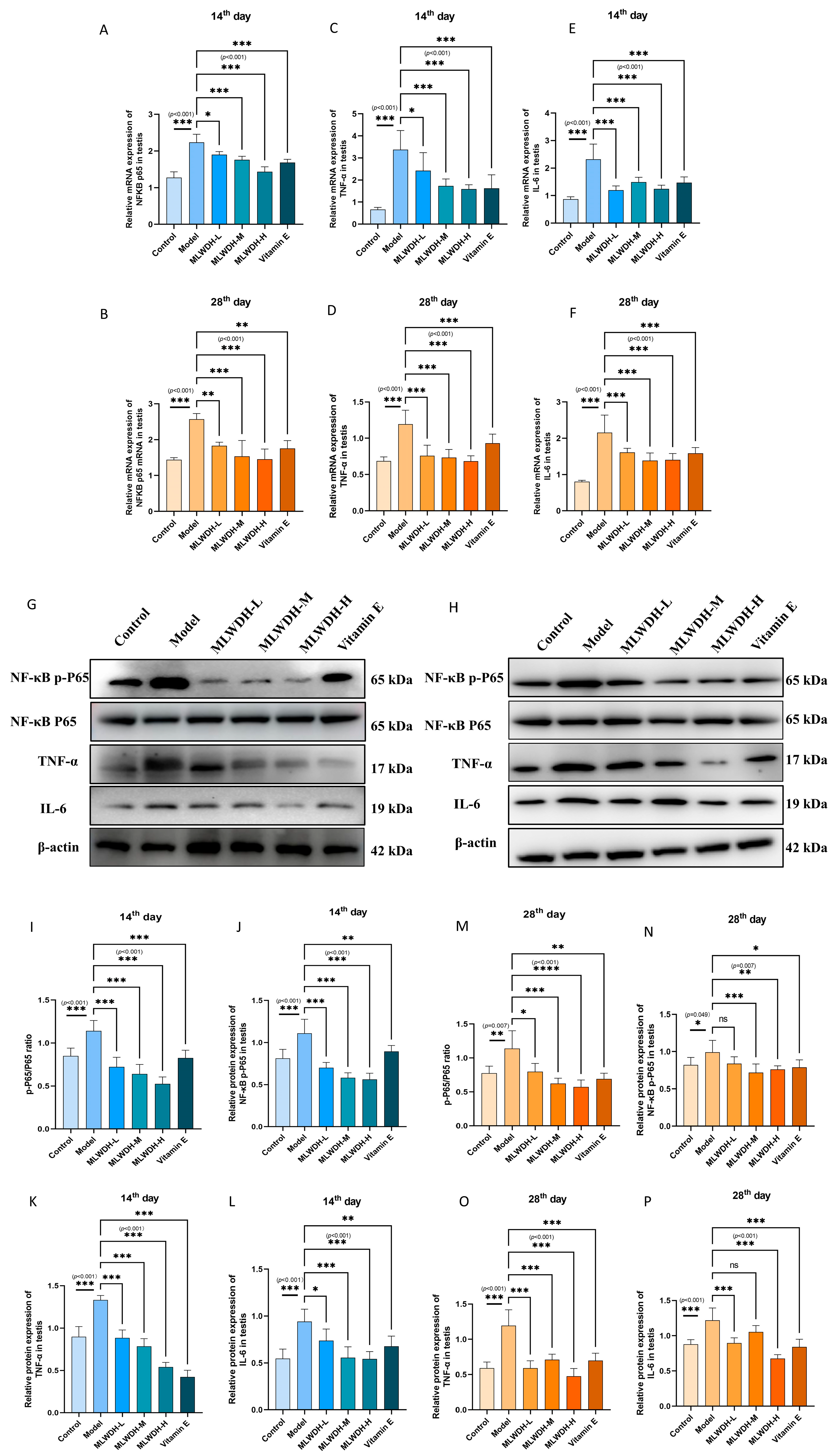
2.8. MLWDH Mitigates Testicular Inflammation via Modulation of NF-κB Signaling
3. Discussion
| Formula | Herbs | Major Active Compounds | Reported Molecular Targets | Pharmacological Actions |
|---|---|---|---|---|
| LWDH | Shudihuang | 5-Hydroxymethyl-2-furaldehyde, Oleic acid, Succinic acid | PI3K/AKT [95,96], Nrf2, HO-1,NQO1 [97], TNF-α, IL-6 [98] | Anti-inflammatory [95,98], antioxidant [97] |
| Shanyurou | Isoleucine, Stearamide, Oleic acid | TNF-α, IL-6 [99] | Anti-inflammatory [99] | |
| Shanyao | Isoleucine, Tyrosine | PI3K/AKT [96], NF-κB [100] | Anti-inflammatory [96,100] | |
| Mudanpi | Benzoic acid, Oleic acid | TNF-α, IL-6 [101] | Anti-inflammatory [101] | |
| Zexie | Stearic acid | PI3K/AKT [102], NF-κB, IL-6 [103] | Anti-inflammatory [102,103] | |
| Fuling | Oleic acid | TNF-α, NF-κB [104], Nrf2, HO-1 [105] | Immune modulation [104], Anti-inflammatory, Antioxidant [105] | |
| Additional herbs in MLWDH | Baishao | Salicylic acid | PI3K/AKT [106,107,108],Nrf2, NF-κB [109,110] | Anti-inflammatory [106,107], Antioxidant [109,110], Inhibits testicular cell apoptosis, Regulates sex hormone balance [53] |
| Chaihu | Oleic acid, 7-hydroxy-6-methoxy-2H-chromen-2-one, Vanillin, Xylitol, Esculetin | PI3K/AKT [111] IL-6,MDA,SOD,GSH [112] | Anti-apoptosis [111], Antioxidant [112,113,114], Erectile dysfunction [56] | |
| Jineijin | Isoleucine | Nrf2, NQO1, HO-1, MDA, SOD [57] | Antioxidant [57], | |
| Wugong | Isoleucine, Tyrosine | TNF-α [55] | Anti-inflammatory [55], Erectile dysfunction [54], | |
| Gouqi | Ferulic acid, Oleic acid, Citric acid, 7-hydroxy-6-methoxy-2H-chromen-2-one | PI3K/AKT [115], Nrf2, Keap1, MDA, SOD, GSH [58], FSH, T, LH, E2 [60] | Promotes cell proliferation [115], Antioxidant [58], Anti-inflammatory [116], Inhibits spermatogenic cell apoptosis [60], Regulates sex hormone balance, Regulates Sertoli cell function, Facilitates spermatogenesis [59] | |
| Rougui | Oleic acid, Caryophyllene oxide, Methyl cinnamate | Testis index, Testosterone, Estradiol [31], PI3K/AKT, NO, NQO1, Nrf2, MDA [117], IL-6, TNF-α, NO, NF-KB [118] | Ameliorates testicular injury [61], Anti-inflammatory, Antioxidant [117,118], Regulates sex hormone balance [31] | |
| Foshou | Kojic acid, Oleic acid, 7-hydroxy-6-methoxy-2H-chromen-2-one, Limonin, Isoleucine | IL-6, TNF-α [63], NF-κB [62] | Anti-inflammatory, Antioxidant [63], Immune modulation [62] | |
| Danggui | Ferulic acid, Azelaic acid, 7-hydroxy-6-methoxy-2H-chromen-2-one, Vanillin, Succinic acid | PI3K/AKT [64], Nrf2 [119], NQO1, TNF-α [120], Keap1 [121], MDA, SOD, NO [30] | Antioxidant [64,119], Improves sperm viability [30], Anti-inflammatory [120] |
4. Materials and Methods
4.1. Preparation of MLWDH
4.2. Identification of the Components of MLWDH by UPLC-HRMS
4.3. Network Pharmacology Analysis
4.3.1. Collection of MLWDH and OA-Related Targets
4.3.2. Creating a PPI Network and Identifying Core Genes
4.3.3. Constructing Drug-Component-Target Networks
4.3.4. Enrichment Analysis
4.3.5. Molecular Docking
4.4. Reagents
4.5. Animals and Treatment
4.6. Pathologic Sampling of Mice
4.7. Determination of Organ Index
4.8. Evaluation of Sperm Characteristics
4.9. Histopathology Analysis
4.10. Serum Biomarkers Analysis
4.11. RT-qPCR Analysis
4.12. Western Blot Analysis
4.13. Immunofluorescence Stain
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARE | Antioxidant Response Elements |
| CP | Cyclophosphamide |
| FSH | Follicle-stimulating Hormone |
| GSH | Glutathione |
| HO-1 | Heme Oxygenase-1 |
| UPLC-HRMS | Ultra performance liquid chromatography-high resolution mass spectrometry |
| Keap1 | Kelch-like ECH-associated Protein 1 |
| KEGG | Kyoto encyclopedia of gene and genomes |
| LH | Luteinizing Hormone |
| LWDH | Liuwei Dihuang Decoction |
| MDA | Malondialdehyde |
| MLWDH | Modified Liuwei Dihuang Decotion |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| NS | Normal Saline |
| OA | Oligoasthenozoospermia |
| PI3K | Phosphatidylinositol 3-kinase |
| P-PI3K | Phosphorylated PI3K |
| PPI | protein–protein interaction |
| ROS | Reactive Oxygen Species |
| SCs | Sertoli Cells |
| SOD | Superoxide Dismutase |
| TCM | Traditional Chinese Medicine |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology Database |
References
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Murshidi, M.M.; Choy, J.T.; Eisenberg, M.L. Male Infertility and Somatic Health. Urol. Clin. N. Am. 2020, 47, 211–217. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, Y.; Zhu, L.; Li, T.; Guan, Y.; Zhao, S.; Wang, Q.; Wang, J.; Li, L. Hua-Tan-Sheng-Jing Decoction Treats Obesity With Oligoasthenozoospermia by Up-Regulating the PI3K-AKT and Down-Regulating the JNK MAPK Signaling Pathways: At the Crossroad of Obesity and Oligoasthenozoospermia. Front. Pharmacol. 2022, 13, 896434. [Google Scholar] [CrossRef]
- Wu, D.; Wang, T.; Liu, H.; Xu, F.; Xie, S.; Tong, X.; Li, L.; Peng, D.; Kong, L. Wuzi-Yanzong-Wan prevents oligoasthenospermia due to TAp73 suppression by affecting cellular junction remodeling in testicular tissue in mice. J. Ethnopharmacol. 2023, 302, 115867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, L.; An, Q.; Hu, W.; Liu, J.; Tang, X. Effects of Qilin pills on spermatogenesis, reproductive hormones, oxidative stress, and the TSSK2 gene in a rat model of oligoasthenospermia. BMC Complement. Med. Ther. 2020, 20, 42. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Habibi-Rezaei, M.; Arese, M.; Saso, L.; Moosavi-Movahedi, A.A. Nrf2 Modulation in Breast Cancer. Biomedicines 2022, 10, 2668. [Google Scholar] [CrossRef]
- Nakamura, B.N.; Lawson, G.; Chan, J.Y.; Banuelos, J.; Cortes, M.M.; Hoang, Y.D.; Ortiz, L.; Rau, B.A.; Luderer, U. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic. Biol. Med. 2010, 49, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Ding, X.; Ge, B.; Wang, M.; Zhou, H.; Sang, R.; Yu, Y.; Xu, L.; Zhang, X. Inonotus obliquus polysaccharide ameliorates impaired reproductive function caused by Toxoplasma gondii infection in male mice via regulating Nrf2-PI3K/AKT pathway. Int. J. Biol. Macromol. 2020, 151, 449–458. [Google Scholar] [CrossRef]
- Liu, T.; Xia, Q.; Lv, Y.; Wang, Z.; Zhu, S.; Qin, W. ErZhiFormula prevents UV-induced skin photoaging by Nrf2/HO-1/NQO1 signaling: An in vitro and in vivo studies. J. Ethnopharmacol. 2023, 309, 115935. [Google Scholar] [CrossRef]
- Papaiahgari, S.; Zhang, Q.; Kleeberger, S.R.; Cho, H.Y.; Reddy, S.P. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid. Redox Signal 2006, 8, 43–52. [Google Scholar] [CrossRef]
- Chen, K.Q.; Wei, B.H.; Hao, S.L.; Yang, W.X. The PI3K/AKT signaling pathway: How does it regulate development of Sertoli cells and spermatogenic cells? Histol. Histopathol. 2022, 37, 621–636. [Google Scholar] [CrossRef]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, J.; Li, X.; Chen, Q.; Hong, Z.; Zheng, L.; Huang, S.; Mo, P.; Li, C.; Wang, R.; et al. Protective effect of Huangqi-Guizhi-Wuwutang against cyclophosphamide-induced spermatogenesis dysfunction in mice by promoting steroid hormone biosynthesis. J. Ethnopharmacol. 2024, 319, 117260. [Google Scholar] [CrossRef]
- Cui, F.; Zhang, Y.; Fan, Y. Adjuvant treatment with Wu-Zi-Yan-Zong formula for abnormal sperm parameters associated with male infertility: A meta-analysis of randomized controlled trials. Front. Pharmacol. 2025, 16, 1580705. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xue, X.; Zhou, J.; Qiu, Z.; Wang, B.; Yin, Z.; Ou, G.; Zhou, Q. L-carnitine combined with traditional Chinese medicine for male infertility: A systematic review and meta-analysis. Heliyon 2024, 10, e36680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cheng, X.; Zhang, Y. Effect of Liuwei Dihuang decoction, a traditional Chinese medicinal prescription, on the neuroendocrine immunomodulation network. Pharmacol. Ther. 2016, 162, 170–178. [Google Scholar] [CrossRef]
- Lu, Z.; Huang, M.; Lin, H.; Wang, G.; Li, H. Network pharmacology and molecular docking approach to elucidate the mechanisms of Liuwei Dihuang pill in diabetic osteoporosis. J. Orthop. Surg. Res. 2022, 17, 314. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, X.; Lin, B.; Fang, J.; Jin, J.; He, Q. Liuwei Dihuang Pills Inhibit Podocyte Injury and Alleviate IgA Nephropathy by Directly Altering Mesangial Cell-Derived Exosome Function and Secretion. Front. Pharmacol. 2022, 13, 889008. [Google Scholar] [CrossRef]
- Qiu, Z.; Dong, J.; Xue, C.; Li, X.; Liu, K.; Liu, B.; Cheng, J.; Huang, F. Liuwei Dihuang Pills alleviate the polycystic ovary syndrome with improved insulin sensitivity through PI3K/Akt signaling pathway. J. Ethnopharmacol. 2020, 250, 111965. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, B.; Geng, L.; Zhang, J.; Qin, G. Xiaokang Liuwei Dihuang decoction ameliorates the immune infertility of male rats induced by lipopolysaccharide through regulating the levels of sex hormones, reactive oxygen species, pro-apoptotic and immune factors. Biomed. Pharmacother. 2021, 139, 111514. [Google Scholar] [CrossRef]
- Zhang, K.; Weng, H.; Yang, J. Protective effect of Liuwei Dihuang Pill on cisplatin-induced reproductive toxicity and genotoxicity in male mice. J. Ethnopharmacol. 2020, 247, 112269. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Yang, L.; Zou, Q.; Zhao, S.; Hu, N. Liuweidihuang Pill Alleviates Inflammation of the Testis via AMPK/SIRT1/NF-kappaB Pathway in Aging Rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 2792738. [Google Scholar] [CrossRef]
- LIU, Y.; GE, P. Current Status of Traditional Chinese Medicine in the Treatment of Oligozoospermia and Asthenozoospermia. J. Guizhou Univ. Tradit. Chin. Med. 2019, 41, 79–83. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, M.; YAN, F.; ZHANG, T.; WANG, X. Mechanism of Asperuloside on Antioxidative Stress and Anti-apoptosis in Rats with Oligoasthenospermia. World Chin. Med. 2024, 19, 2584–2589+2595. (In Chinese) [Google Scholar]
- Zeng, L.F.; Cao, Y.; Wang, L.; Dai, Y.K.; Hu, L.; Wang, Q.; Zhu, L.T.; Bao, W.H.; Zou, Y.P.; Chen, Y.B.; et al. Role of Medicinal Plants for Liver-Qi Regulation Adjuvant Therapy in Post-stroke Depression: A Systematic Review of Literature. Phytother. Res. 2017, 31, 40–52. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Zhou, A. Research progress on bioactive components and health benefits of finger citron and prospects for microbial transformation. China Natl. Knowl. Infrastruct. 2025, 1–24. (In Chinese) [Google Scholar]
- Sun, M.; Hu, K.; Yang, X.; Liu, Y.; Chen, Q.; Zhou, T. HU Kai-wen’s clinical experience in treating malignant tumors with Gecko and Centipede. China J. Tradit. Chin. Med. Pharm. 2018, 33, 3929–3931. [Google Scholar]
- Feng, J.; Li, H.; Wang, J.; Dai, H.; Bao, B.; Deng, S.; Long, Z.; Xu, H.; Wang, B. Mechanism of effects of Shuizhi-Wugong medicinal pair on improving diabetic erectile dyfunction in rats based on PI3K/AKT/mTOR signaling pathway. J. Beijing Univ. Tradit. Chin. Med. 2021, 44, 1118–1125. (In Chinese) [Google Scholar]
- Zhang, K.; Zhang, L.; Chai, J. Protective effect of Angelica sinensis polysaccharide (asp) on cryopreservation-induced damage to semen of chongming white goats. Cryo Lett. 2025, 46, 186–196. (In Chinese) [Google Scholar] [CrossRef]
- Fang, Q. Research progress of Rougui. Tradit. Chin. Drug Res. Clin. Pharmacol. 2007, 4, 249–252. [Google Scholar] [CrossRef]
- Bezerra-Filho, C.S.M.; Barboza, J.N.; Souza, M.T.S.; Sabry, P.; Ismail, N.S.M.; de Sousa, D.P. Therapeutic Potential of Vanillin and its Main Metabolites to Regulate the Inflammatory Response and Oxidative Stress. Mini Rev. Med. Chem. 2019, 19, 1681–1693. [Google Scholar] [CrossRef]
- Chen, J.; Jia, X.; Hu, Y.; Zhao, X.; Cheng, Y.; Lu, L.; Zhong, S.; You, J.; Zou, T. Benzoic acid as a dietary supplement mitigates inflammation and intestinal injury in acute enterotoxigenic Escherichia coli-infected mice without adverse effects in healthy mice. Food Funct. 2025, 16, 3195–3210. [Google Scholar] [CrossRef]
- Gart, E.; van Duyvenvoorde, W.; Caspers, M.P.M.; van Trigt, N.; Snabel, J.; Menke, A.; Keijer, J.; Salic, K.; Morrison, M.C.; Kleemann, R. Intervention with isoleucine or valine corrects hyperinsulinemia and reduces intrahepatic diacylglycerols, liver steatosis, and inflammation in Ldlr-/-.Leiden mice with manifest obesity-associated NASH. FASEB J. 2022, 36, e22435. [Google Scholar] [CrossRef]
- Huang, S.C.; Yen, G.C.; Chang, L.W.; Yen, W.J.; Duh, P.D. Identification of an antioxidant, ethyl protocatechuate, in peanut seed testa. J. Agric. Food Chem. 2003, 51, 2380–2383. [Google Scholar] [CrossRef]
- Kilari, G.; Balakrishnan, S. In Vitro Antiproliferative Activity and Phytochemicals Screening of Extracts of the Freshwater Microalgae, Chlorochromonas danica. Appl. Biochem. Biotechnol. 2023, 195, 534–555. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Lee, B.H.; Wei, K. 5-Hydroxymethylfurfural Mitigates Lipopolysaccharide-Stimulated Inflammation via Suppression of MAPK, NF-kappaB and mTOR Activation in RAW 264.7 Cells. Molecules 2019, 24, 275. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, G.; He, Z.; Huang, H.; Yang, J.; Nie, M. Kojic acid alleviates osteoarthritis by attenuating inflammation and restoring impaired autophagy through regulating NF-kappaB and PI3K/AKT/mTOR signaling pathways: An in vivo and in vitro study. Int. Immunopharmacol. 2025, 147, 114022. [Google Scholar] [CrossRef]
- Molnar, G.A.; Kun, S.; Selley, E.; Kertesz, M.; Szelig, L.; Csontos, C.; Boddi, K.; Bogar, L.; Miseta, A.; Wittmann, I. Role of Tyrosine Isomers in Acute and Chronic Diseases Leading to Oxidative Stress—A Review. Curr. Med. Chem. 2016, 23, 667–685. [Google Scholar] [CrossRef]
- Msomi, N.Z.; Erukainure, O.L.; Salau, V.F.; Olofinsan, K.A.; Islam, M.S. Xylitol improves antioxidant, purinergic and cholinergic dysfunction, and lipid metabolic homeostasis in hepatic injury in type 2 diabetic rats. J. Food Biochem. 2022, 46, e14040. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M. Succinic acid treatment enhances energy metabolism and antioxidant biosynthesis in radish sprouts. J. Biotechnol. 2025, 404, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Uddin, G.; Choudhary, M.I. Short Communication: Discovery and molecular docking simulation of 7-hydroxy-6-methoxy-2H-chromen-2-one as a LOX Inhibitor. Pak. J. Pharm. Sci. 2019, 32, 217–220. [Google Scholar]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- Santa-Maria, C.; Lopez-Enriquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J. Dermatolog. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.; Zhu, Y.; Han, J.; Li, Y.; Yang, X.; Yang, G.; Song, G.; Li, S.; Li, Y.; Cheng, C.; et al. Caryophyllene Oxide Induces Ferritinophagy by Regulating the NCOA4/FTH1/LC3 Pathway in Hepatocellular Carcinoma. Front. Pharmacol. 2022, 13, 930958. [Google Scholar] [CrossRef]
- Yang, R.; Song, C.; Chen, J.; Zhou, L.; Jiang, X.; Cao, X.; Sun, Y.; Zhang, Q. Limonin ameliorates acetaminophen-induced hepatotoxicity by activating Nrf2 antioxidative pathway and inhibiting NF-kappaB inflammatory response via upregulating Sirt1. Phytomedicine 2020, 69, 153211. [Google Scholar] [CrossRef]
- Yang, Z.; Xue, B.; Song, G.; Shi, S. Effects of citric acid on antioxidant system and carbon-nitrogen metabolism of Elymus dahuricus under Cd stress. Ecotoxicol. Environ. Saf. 2022, 233, 113321. [Google Scholar] [CrossRef]
- Zhang, D.; Jing, B.; Chen, Z.N.; Li, X.; Shi, H.M.; Zheng, Y.C.; Chang, S.Q.; Gao, L.; Zhao, G.P. Ferulic acid alleviates sciatica by inhibiting neuroinflammation and promoting nerve repair via the TLR4/NF-kappaB pathway. CNS Neurosci. Ther. 2023, 29, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, Q.; Li, X. Esculetin: A review of its pharmacology and pharmacokinetics. Phytother. Res. 2022, 36, 279–298. [Google Scholar] [CrossRef]
- Zou, D.; Meng, X.; Wang, B.; Dai, Y.; Yang, R.; Suo, Y.; Wu, Y.; Yang, W.; Lin, R. Analysis of pharmacological mechanisms and targets mining of Wuzi-Yanzong-Wan for treating non-obstructive oligoasthenospermia. Biomed. Pharmacother. 2019, 115, 108898. [Google Scholar] [CrossRef]
- Lian, F.; Wu, H.C.; Sun, Z.G. Effects of Liuwei Dihuang Granule ([symbols; see text]) on the outcomes of in vitro fertilization pre-embryo transfer in infertility women with Kidney-yin deficiency syndrome and the proteome expressions in the follicular fluid. Chin. J. Integr. Med. 2014, 20, 503–509. [Google Scholar] [CrossRef]
- Song, L.; Wang, J.; Gong, M.; Zhang, Y.; Zhang, Z.; Wang, Y.; Wu, X.; Guo, X. Study on the detoxifying mechanism of processing on Tripterygium wilfordii-induced testicular reproductive toxicity. J. Li-Shizhen Tradit. Chin. Med. 2021, 32, 2664–2667. (In Chinese) [Google Scholar]
- Lin, J.; Zhang, B.; Zhao, L.; Kang, Z.; Li, Q.; Tong, X. The Experience of Tong Xiaolin in Utilizing “Toxic” Traditional Chinese Medicine for Treating Metabolic Diseases. J. Tradit. Chin. Med. 2024, 65, 1213–1218. (In Chinese) [Google Scholar] [CrossRef]
- Liu, D.Y.; Zhao, H.M.; Cheng, S.M.; Rao, Y.; Huang, X.Y.; Zuo, Z.Q.; Lei, M.; Guan, Y.M.; Liu, H.N.; Lu, A.P. Scorpio and Scolopendra attenuate inflammation and articular damage in rats with collagen-induced arthritis. J. Ethnopharmacol. 2012, 141, 603–607. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Zhu, Z.; Yang, R.; Wen, Q.; Guo, F.; Zhang, F.; Zhang, C.; Zhao, C. The current status and rules of clinical application of traditional Chinese medicine for treating erectile dysfunction via data mining. Chin. J. Hum. Sex. 2023, 32, 5–10. [Google Scholar]
- Ding, Y.; Zhou, C.; Wang, Y. Effects of polysaccharides from Endothelium Corneum Gigeriae Galli on high glucose-induced islet β cell activity and Nrf2 signaling pathway. Shanghai J. Tradit. Chin. Med. 2024, 58, 90–96. (In Chinese) [Google Scholar] [CrossRef]
- Lin, Z.; Yang, M.; Liu, J.; Yang, C.; Liang, L.; Pan, J.; Yang, Q.; Pan, L. Study on the Anti-Fatigue Effects of Polysaccharides from Erjing Formula. Curr. Pharm. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Liu, D.; Bai, X.; Tang, Y.; Liu, G.; Fan, X.; Chen, Y.; Ma, R.; Chang, Z. Research progress on the mechanism of action of Fructus Lycii treating male infertility. Glob. Tradit. Chin. Med. 2021, 14, 1166–1171. (In Chinese) [Google Scholar]
- Nan, Y.; Li, Q.; Zhao, F. Effect of lycium barbarum polysaccharide on erectile function and sex hormones of rat with kidney yang-deficiency syndrome. Shanxi J. Tradit. Chin. Med. 2019, 35, 52–55. (In Chinese) [Google Scholar]
- Zhang, Z.; Zhao, W.; Sun, X.; Yan, T.; Zhang, Y. Effects of Rougui (Cinnamomi Cortex) on Expression of Related mRNA and Histopathological Changes of Hypothalamus—Pituitary—Target Gland Axis in Rats with Kidney Yang Deficiency. Chin. Arch. Tradit. Chin. Med. 2023, 41, 59–63+264. (In Chinese) [Google Scholar] [CrossRef]
- Cao, R.; Hao, E.; Du, Z.; Li, Z.; Chen, F.; Wu, D.; Hou, X.; Deng, J. Ingredients, Pharmacological Activities and Predictive Analysis of Q-markers of Guangfoshou (Citri Sarcodactylis Fructus). Chin. Arch. Tradit. Chin. Med. 2022, 40, 135–145+277. (In Chinese) [Google Scholar] [CrossRef]
- Lombardo, G.E.; Cirmi, S.; Musumeci, L.; Pergolizzi, S.; Maugeri, A.; Russo, C.; Mannucci, C.; Calapai, G.; Navarra, M. Mechanisms Underlying the Anti-Inflammatory Activity of Bergamot Essential Oil and Its Antinociceptive Effects. Plants 2020, 9, 704. [Google Scholar] [CrossRef]
- Lu, J.; Wang, C. Ferulic acid from Angelica sinensis (Oliv.) Diels ameliorates lipid metabolism in alcoholic liver disease via AMPK/ACC and PI3K/AKT pathways. J. Ethnopharmacol. 2025, 338, 119118. [Google Scholar] [CrossRef]
- Abel, M.H.; Baker, P.J.; Charlton, H.M.; Monteiro, A.; Verhoeven, G.; De Gendt, K.; Guillou, F.; O’Shaughnessy, P.J. Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology 2008, 149, 3279–3285. [Google Scholar] [CrossRef] [PubMed]
- Colpi, G.M.; Francavilla, S.; Haidl, G.; Link, K.; Behre, H.M.; Goulis, D.G.; Krausz, C.; Giwercman, A. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology 2018, 6, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, W.; Wang, Z.; Huang, R.; Xia, H.; Li, Z.; Deng, J.; Ye, T.; Huang, Y.; Yang, Y. Hormone Regulation in Testicular Development and Function. Int. J. Mol. Sci. 2024, 25, 5805. [Google Scholar] [CrossRef] [PubMed]
- Bruno, C.; Basile, U.; Vergani, E.; Napodano, C.; Oliva, A.; Gulli, F.; Meucci, E.; Silvestrini, A.; Orlando, P.; Silvestri, S.; et al. Inflammation and Oxidative Stress in Seminal Plasma: Search for Biomarkers in Diagnostic Approach to Male Infertility. J. Pers. Med. 2022, 12, 857. [Google Scholar] [CrossRef]
- Wang, C.N.; Sang, M.M.; Gong, S.N.; Yang, J.F.; Cheng, C.Y.; Sun, F. Two resveratrol analogs, pinosylvin and 4,4′-dihydroxystilbene, improve oligoasthenospermia in a mouse model by attenuating oxidative stress via the Nrf2-ARE pathway. Bioorg Chem. 2020, 104, 104295. [Google Scholar] [CrossRef]
- Zareba, P.; Colaci, D.S.; Afeiche, M.; Gaskins, A.J.; Jorgensen, N.; Mendiola, J.; Swan, S.H.; Chavarro, J.E. Semen quality in relation to antioxidant intake in a healthy male population. Fertil. Steril. 2013, 100, 1572–1579. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Agarwal, A.; Prabakaran, S.; Allamaneni, S. What an andrologist/urologist should know about free radicals and why. Urology 2006, 67, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Chaiswing, L.; Zhong, W.; Oberley, T.D. Distinct redox profiles of selected human prostate carcinoma cell lines: Implications for rational design of redox therapy. Cancers 2011, 3, 3557–3584. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Wang, X.; Zhou, T.; Cheng, J.; Wang, C.; Sun, F.; Zhao, X. Inhibition of ferroptosis attenuates oligospermia in male Nrf2 knockout mice. Free Radic. Biol. Med. 2022, 193, 421–429. [Google Scholar] [CrossRef]
- Li, S.; Song, Z.; Liu, T.; Liang, J.; Yuan, J.; Xu, Z.; Sun, Z.; Lai, X.; Xiong, Q.; Zhang, D. Polysaccharide from Ostrea rivularis attenuates reproductive oxidative stress damage via activating Keap1-Nrf2/ARE pathway. Carbohydr. Polym. 2018, 186, 321–331. [Google Scholar] [CrossRef]
- Turk, E.; Ozan Tekeli, I.; Ozkan, H.; Uyar, A.; Cellat, M.; Kuzu, M.; Yavas, I.; Alizadeh Yegani, A.; Yaman, T.; Guvenc, M. The protective effect of esculetin against aluminium chloride-induced reproductive toxicity in rats. Andrologia 2021, 53, e13930. [Google Scholar] [CrossRef]
- Guvvala, P.R.; Ravindra, J.P.; Selvaraju, S.; Arangasamy, A.; Venkata, K.M. Ellagic and ferulic acids protect arsenic-induced male reproductive toxicity via regulating Nfe2l2, Ppargc1a and StAR expressions in testis. Toxicology 2019, 413, 1–12. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, M.; Wang, Y.; Li, M.; Wu, Y.; Xu, R.; Zhang, Q.; Jia, J.; Huang, Y.; Zheng, X.; et al. Oleic acid alleviates LPS-induced acute kidney injury by restraining inflammation and oxidative stress via the Ras/MAPKs/PPAR-gamma signaling pathway. Phytomedicine 2022, 94, 153818. [Google Scholar] [CrossRef]
- Meroni, S.B.; Riera, M.F.; Pellizzari, E.H.; Galardo, M.N.; Cigorraga, S.B. FSH activates phosphatidylinositol 3-kinase/protein kinase B signaling pathway in 20-day-old Sertoli cells independently of IGF-I. J. Endocrinol. 2004, 180, 257–265. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Zhang, X.; Liu, Y.; Su, Z.; Dawar, F.U.; Dan, H.; He, Y.; Gui, J.F.; Mei, J. Leucine mediates autophagosome-lysosome fusion and improves sperm motility by activating the PI3K/Akt pathway. Oncotarget 2017, 8, 111807–111818. [Google Scholar] [CrossRef]
- Bereczki, D., Jr.; Balla, J.; Bereczki, D. Heme Oxygenase-1: Clinical Relevance in Ischemic Stroke. Curr. Pharm. Des. 2018, 24, 2229–2235. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress. Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yao, M.; Qi, J.; Song, R.; Wang, L.; Li, J.; Zhou, X.; Chang, D.; Huang, Q.; Li, L.; et al. Puerarin inhibited oxidative stress and alleviated cerebral ischemia-reperfusion injury through PI3K/Akt/Nrf2 signaling pathway. Front. Pharmacol. 2023, 14, 1134380. [Google Scholar] [CrossRef]
- Fu, H.; Sen, L.; Zhang, F.; Liu, S.; Wang, M.; Mi, H.; Liu, M.; Li, B.; Peng, S.; Hu, Z.; et al. Mesenchymal stem cells-derived extracellular vesicles protect against oxidative stress-induced xenogeneic biological root injury via adaptive regulation of the PI3K/Akt/NRF2 pathway. J. Nanobiotechnol. 2023, 21, 466. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, T.; Zheng, X.; Kang, L.; Wang, J.; Wei, Y.; Wu, Y.; Shen, L.; Long, C.; Wei, G.; et al. Protective effects of melatonin on DEHP-induced apoptosis and oxidative stress in prepubertal testes via the PI3K/AKT pathway. Environ. Toxicol. 2024, 39, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zhang, Y.; Huang, M.; Liu, J.; Wang, C.; Zhang, C.; Cao, J.; Zhang, Q.; Jiang, L. Circ-CREBBP inhibits sperm apoptosis via the PI3K-Akt signaling pathway by sponging miR-10384 and miR-143-3p. Commun. Biol. 2022, 5, 1339. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Billah, M.M.; Khatiwada, S.; Lecomte, V.; Morris, M.J.; Maloney, C.A. Ameliorating high-fat diet-induced sperm and testicular oxidative damage by micronutrient-based antioxidant intervention in rats. Eur. J. Nutr. 2022, 61, 3741–3753. [Google Scholar] [CrossRef]
- Liu, G.H.; Qu, J.; Shen, X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jia, X.Y.; Zhang, S.H.; Wu, Y.Z.; Xu, L.S.; Han, J.G.; Yu, W.; Zhou, Q.H. Metformin activates the PI3K/AKT/BDNF axis to attenuate postoperative cognitive dysfunction. Neuropharmacology 2025, 265, 110262. [Google Scholar] [CrossRef]
- Gao, H.L.; Cui, Q.; Wang, J.Q.; Ashby, C.R., Jr.; Chen, Y.; Shen, Z.X.; Chen, Z.S. The AKT inhibitor, MK-2206, attenuates ABCG2-mediated drug resistance in lung and colon cancer cells. Front. Pharmacol. 2023, 14, 1235285. [Google Scholar] [CrossRef]
- Lin, Q.; Ge, X.; Gao, L.; Chen, Y.; Su, T.; Ma, M.; Wang, H.; Chen, C.; Han, B.; Liu, D. Betaine alleviates spermatogenic cells apoptosis of oligoasthenozoospermia rat model by up-regulating methyltransferases and affecting DNA methylation. Phytomedicine 2024, 129, 155713. [Google Scholar] [CrossRef]
- Hu, S.J.; Chen, G.C.; Wang, F.Y.; Fang, Y.Q.; Wang, S.Q.; Song, Z.L.; Zhao, Z.H.; Zhang, Q.L.; Meng, X.Y.; Zhang, Q.Y.; et al. Network pharmacology analysis uncovers the mechanism of Shudihuang-Shanzhuyu herb pair in prevention and treatment of diabetic osteoporosis via PI3K/AKT pathway. J. Ethnopharmacol. 2025, 345, 119581. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Z.; Lin, Y.; Zheng, Y.; Liu, Z.; Li, Y.; Huang, L.; Chen, Z.; Zhu, L. Shanyao regulates the PI3K/AKT/P21 pathway to promote oogonial stem cell proliferation and stemness restoration to alleviate premature ovarian insufficiency. J. Ethnopharmacol. 2025, 340, 119168. [Google Scholar] [CrossRef]
- Chen, X.; Zuo, J.; Hu, T.; Shi, X.; Zhu, Y.; Wu, H.; Xia, Y.; Shi, W.; Wei, W. Exploration of the Effect and Mechanism of Fructus Lycii, Rehmanniae Radix Praeparata, and Paeonia lactiflora in the Treatment of AMD Based on Network Pharmacology and in vitro Experimental Verification. Drug Des. Devel Ther. 2021, 15, 2831–2842. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Feng, W.; Zhang, Z.; Li, H. Structural characterization and immunomodulatory activities of two polysaccharides from Rehmanniae Radix Praeparata. Int. J. Biol. Macromol. 2021, 186, 385–395. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, D.; Zhou, R.; Yang, C.; Zhang, Y.; Lian, F.; Tong, X. Mechanism of Cornus Officinalis in Treating Diabetic Kidney Disease Based on Network Pharmacology. Evid. Based Complement. Alternat. Med. 2022, 2022, 1799106. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Zou, Y.; Liao, S.; Gao, Q.; Li, Q. Molecular Targets and Mechanisms of 6,7-Dihydroxy-2,4-dimethoxyphenanthrene from Chinese Yam Modulating NF-kappaB/COX-2 Signaling Pathway: The Application of Molecular Docking and Gene Silencing. Nutrients 2023, 15, 883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ma, Y.; Li, Y.; Wang, Y.; Zhang, L.; Zheng, M.; Tian, Y.; Zhang, R.; Yang, K.; Li, J.; et al. Paeonol prevents sepsis-associated encephalopathy via regulating the HIF1A pathway in microglia. Int. Immunopharmacol. 2024, 143, 113287. [Google Scholar] [CrossRef]
- Shi, M.F.; Liu, X.B.; Ma, X.N.; Feng, W.; Zhang, Y.F.; Lin, C.S.; Liu, Q.P.; Xu, Q. Study on the effect and mechanism of ZeXie decoction in treating MSU-induced acute gouty arthritis model through PI3K-AKT-mTOR signaling pathway. Int. Immunopharmacol. 2025, 150, 114214. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, B.; Song, D.; Xi, J.; Hao, W.; Yuan, J.; Gao, C.; Cui, Z.; Cheng, Z. Alisol A Alleviates Arterial Plaque by Activating AMPK/SIRT1 Signaling Pathway in apoE-Deficient Mice. Front. Pharmacol. 2020, 11, 580073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, J.; Zheng, D.; Zhao, P.; Wang, Y.; Zhao, J.; Li, P. Immunomodulatory Activity and Its Mechanisms of Two Polysaccharides from Poria cocos. Molecules 2023, 29, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Niu, X.; Yu, J.; Xiao, X.; Li, W.; Zang, L.; Hu, Z.; Siu-Po Ip, P.; Li, W. Poria cocos polysaccharides attenuated ox-LDL-induced inflammation and oxidative stress via ERK activated Nrf2/HO-1 signaling pathway and inhibited foam cell formation in VSMCs. Int. Immunopharmacol. 2020, 80, 106173. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, Q.; Ao, Q.; Luo, C.; Wang, B.; Bai, C.; Ge, X.; Wang, Y.; Wang, J.; et al. On the Core Prescriptions and Their Mechanisms of Traditional Chinese Medicine in Hepatitis B, Liver Cirrhosis, and Liver Cancer Treatment. J. Oncol. 2022, 2022, 5300523. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, Q.; Liu, M. A Network Pharmacology Approach to Explore the Potential Mechanisms of Huangqin-Baishao Herb Pair in Treatment of Cancer. Med. Sci. Monit. 2020, 26, e923199. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, G.; Yang, J.; Liu, X.; Chen, Z.; Liu, D.; Huang, Y.; Yang, F.; Luo, W. Integrated network pharmacology, transcriptomics and metabolomics to explore the material basis and mechanism of Danggui-Baishao herb pair for treating hepatic fibrosis. J. Ethnopharmacol. 2025, 337, 118834. [Google Scholar] [CrossRef]
- Chen, I.C.; Lin, T.H.; Hsieh, Y.H.; Chao, C.Y.; Wu, Y.R.; Chang, K.H.; Lee, M.C.; Lee-Chen, G.J.; Chen, C.M. Formulated Chinese Medicine Shaoyao Gancao Tang Reduces Tau Aggregation and Exerts Neuroprotection through Anti-Oxidation and Anti-Inflammation. Oxid. Med. Cell Longev. 2018, 2018, 9595741. [Google Scholar] [CrossRef]
- Parker, S.; May, B.; Zhang, C.; Zhang, A.L.; Lu, C.; Xue, C.C. A Pharmacological Review of Bioactive Constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch. Phytother. Res. 2016, 30, 1445–1473. [Google Scholar] [CrossRef]
- Duan, Z.W.; Liu, Y.; Zhang, P.P.; Hu, J.Y.; Mo, Z.X.; Liu, W.Q.; Ma, X.; Zhou, X.H.; Wang, X.H.; Hu, X.H.; et al. Da-Chai-Hu-Tang Formula inhibits the progression and metastasis in HepG2 cells through modulation of the PI3K/AKT/STAT3-induced cell cycle arrest and apoptosis. J. Ethnopharmacol. 2024, 331, 118293. [Google Scholar] [CrossRef]
- Xirui, Y.; Hui, Z.; Muhammad, S.; Feixue, D.; Dandan, Z.; Jixue, W.; Xingxing, Y. Efficacy of bioactive compounds of Chaihu () on glaucomatous optic atrophy through interleukin-6/hypoxia inducible factor-1alpha signal pathway. J. Tradit. Chin. Med. 2023, 43, 1219–1226. [Google Scholar] [CrossRef]
- Qiang, X.; Xia, T.; Geng, B.; Zhao, M.; Li, X.; Zheng, Y.; Wang, M. Bioactive Components of Lycium barbarum and Deep-Processing Fermentation Products. Molecules 2023, 28, 8044. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.J.; Yue, S.Q.; Zhang, L.Y.; Dou, K.F. Antioxidant activity and hepatoprotective effect of a polysaccharide from Bei Chaihu (Bupleurum chinense DC). Carbohydr. Polym. 2012, 89, 448–452. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Z.; Zhou, X.; Liao, Y.; Peng, Z.; Meng, Z.; Nussler, A.K.; Ma, L.; Xia, H.; Liu, L.; et al. Gouqi-derived Nanovesicles (GqDNVs) promoted MC3T3-E1 cells proliferation and improve fracture healing. Phytomedicine 2025, 142, 156755. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef]
- Sampath, C.; Chukkapalli, S.S.; Raju, A.V.; Alluri, L.S.C.; Srisai, D.; Gangula, P.R. Cinnamaldehyde Protects against P. gingivalis Induced Intestinal Epithelial Barrier Dysfunction in IEC-6 Cells via the PI3K/Akt-Mediated NO/Nrf2 Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 4734. [Google Scholar] [CrossRef]
- Zhang, Y.; Ou, N.; Liu, M.; Wang, K.; Jia, H.; Yu, W.; Li, Y. In vitro Anti-inflammatory Activity of Cinnamon Essential Oil and Cinnamaldehyde and Protective Effect against LPS-induced Inflammatory Injury in RAW 264.7 cells. Acta Vet. Zootech. Sin. 2025, 1, 1–13. (In Chinese) [Google Scholar]
- Lu, X.; Li, J.; Ma, Y.; Khan, I.; Yang, Y.; Li, Y.; Wang, Y.; Liu, G.; Zhang, Z.; Yang, P.; et al. Fermented Angelica sinensis activates Nrf2 signaling and modulates the gut microbiota composition and metabolism to attenuate D-gal induced liver aging. Food Funct. 2023, 14, 215–230. [Google Scholar] [CrossRef]
- Saw, C.L.; Wu, Q.; Su, Z.Y.; Wang, H.; Yang, Y.; Xu, X.; Huang, Y.; Khor, T.O.; Kong, A.N. Effects of natural phytochemicals in Angelica sinensis (Danggui) on Nrf2-mediated gene expression of phase II drug metabolizing enzymes and anti-inflammation. Biopharm. Drug Dispos. 2013, 34, 303–311. [Google Scholar] [CrossRef]
- Dietz, B.M.; Liu, D.; Hagos, G.K.; Yao, P.; Schinkovitz, A.; Pro, S.M.; Deng, S.; Farnsworth, N.R.; Pauli, G.F.; van Breemen, R.B.; et al. Angelica sinensis and its alkylphthalides induce the detoxification enzyme NAD(P)H: Quinone oxidoreductase 1 by alkylating Keap1. Chem. Res. Toxicol. 2008, 21, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Chen, X.; Luo, M.; Yin, M.; Xie, X.; Ai, Y.; Zhang, X.; He, J. Therapeutic potential of Lingjiao Gouteng decoction in acute alcohol intoxication and alcohol-induced brain injury involving the RhoA/ROCK2/NF-kappaB signaling pathway. J. Ethnopharmacol. 2024, 328, 118114. [Google Scholar] [CrossRef]
- Xie, L.; Huang, W.; Li, J.; Chen, G.; Xiao, Q.; Zhang, Y.; He, H.; Wang, Q.; He, J. The protective effects and mechanisms of modified Lvdou Gancao decoction on acute alcohol intoxication in mice. J. Ethnopharmacol. 2022, 282, 114593. [Google Scholar] [CrossRef]
- Ping, W.; Tinglan, Z.; Guohua, Y.; Mengjie, L.; Jin, S.; Jiaqi, Z.; Ke, L.; Yan, F.; Haiyu, X.; Hongjun, Y. Poly-pharmacokinetic strategy-delineated metabolic fate of bioactive compounds in a traditional Chinese medicine formula, Yuanhu Zhitong tablets, using parallel reaction monitoring mode. Phytomedicine 2019, 53, 53–61. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Dong, L.; Liu, L.; Guo, J.; Zhao, L.; Zhang, J.; Bu, D.; Liu, X.; Huo, P.; Cao, W.; et al. HERB: A High-Throughput Experiment- and Reference-Guided Database of Traditional Chinese Medicine. 2025. Available online: http://herb.ac.cn/ (accessed on 2 May 2025).
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.R.; Hem, V.; Katz, K.S.; Ovetsky, M.; Wallin, C.; Ermolaeva, O.; Tolstoy, I.; Tatusova, T.; Pruitt, K.D.; Maglott, D.R.; et al. Gene: A Gene-Centered Information Resource at NCBI. 2025. Available online: https://www.genecards.org/ (accessed on 5 May 2025).
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful New Tools for Exploring 3D Structures of Biological Macromolecules for Basic and Applied Research and Education in Fundamental Biology, Biomedicine, Biotechnology, Bioengineering and Energy Sciences. 2025. Available online: https://www.rcsb.org/ (accessed on 10 May 2025).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New Data Content and Improved Web Interfaces. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 8 May 2025).
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef]
- Ye, Y.; Fang, C.; Li, L.; Liu, D.; Wang, Y.; Huang, F. Protective Effect of l-Theanine on Cyclophosphamide-Induced Testicular Toxicity in Mice. J. Agric. Food Chem. 2023, 71, 8050–8060. [Google Scholar] [CrossRef]
- Ganaie, J.A.; Gautam, V.; Shrivastava, V.K. Effects of Kamdhenu Ark and Active Immunization by Gonadotropin Releasing Hormone Conjugate (GnRH-BSA) on Gonadosomatic Indices (GSI) and Sperm Parameters in Male Mus musculus. J. Reprod. Infertil. 2011, 12, 3–7. [Google Scholar] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
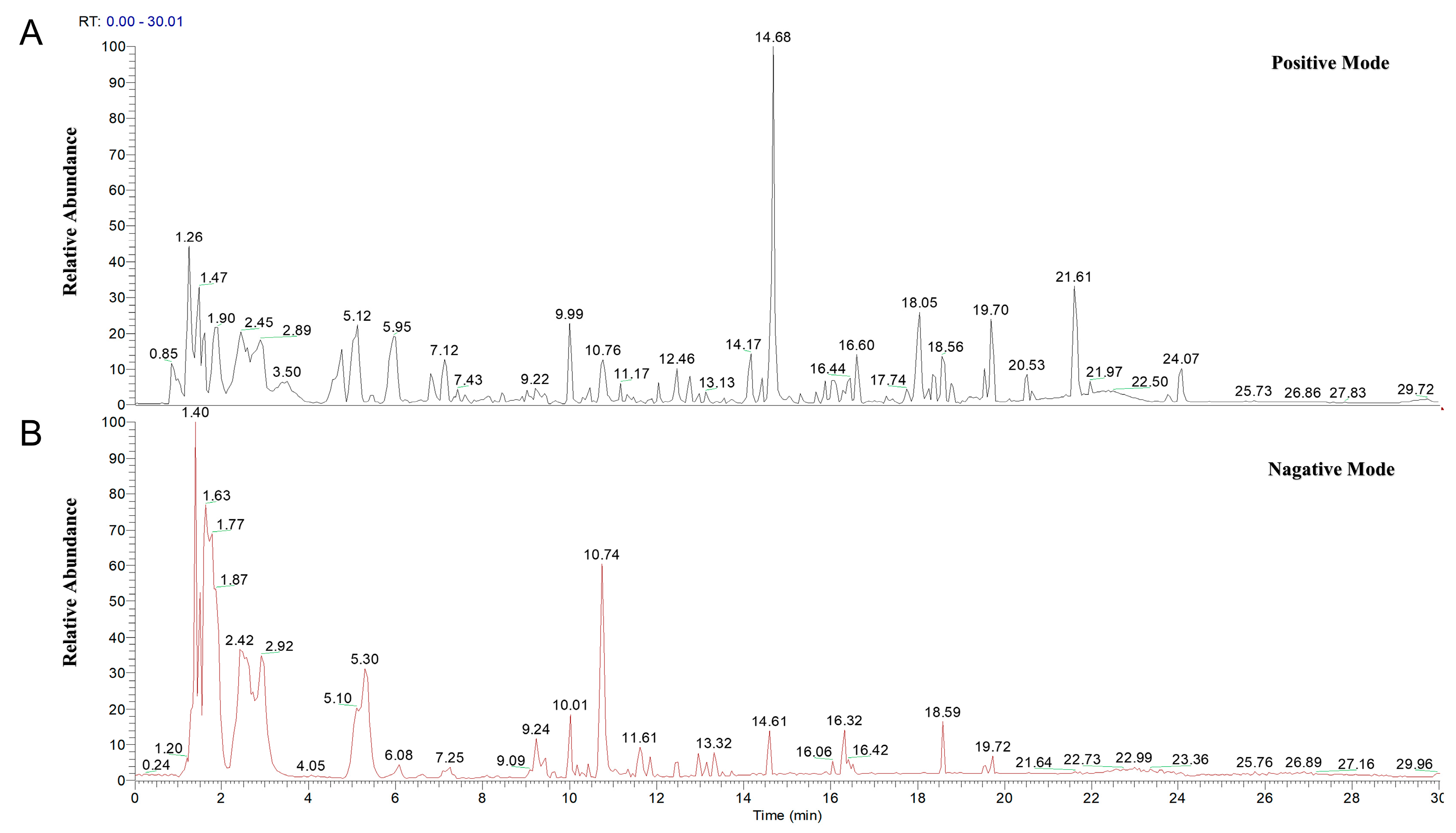
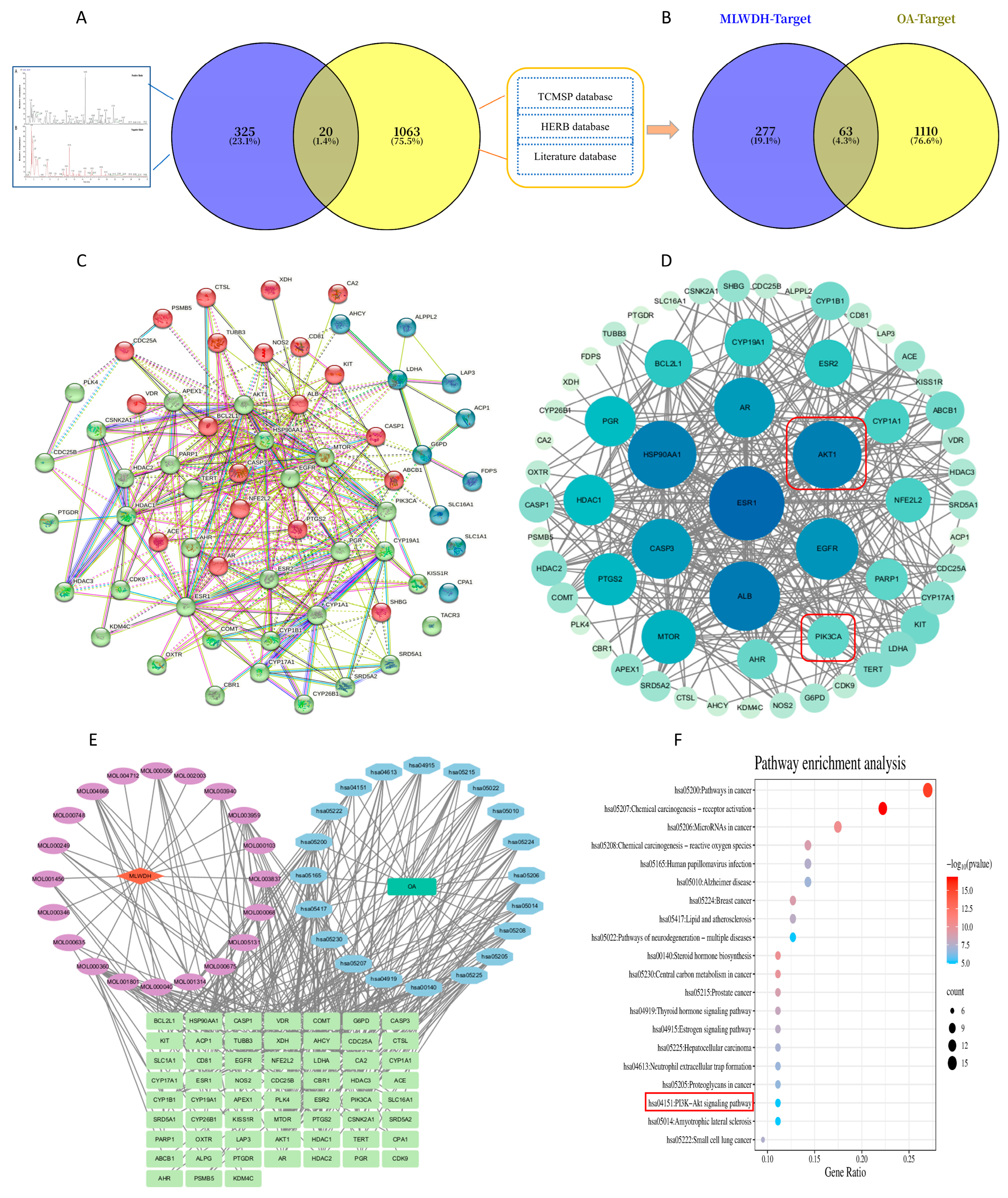






| NO. | Molecular ID | Molecular Name | Formula | Ion | RT [min] |
|---|---|---|---|---|---|
| 1 | MOL000068 | Isoleucine | C6H13NO2 | [M+H]+1 | 2.45 |
| 2 | MOL000360 | Ferulic acid | C10H10O4 | [M+H-H2O]+1 | 11.82 |
| 3 | MOL000103 | Benzoic acid | C7H6O2 | [M-H]−1 | 9.65 |
| 4 | MOL000056 | Tyrosine | C9H11NO3 | [M+H]+1 | 3.00 |
| 5 | MOL000748 | 5-Hydroxymethyl-2-furaldehyde | C6H6O3 | [M+H]+1 | 5.96 |
| 6 | MOL005131 | Kojic acid | C6H6O4 | [M+H]+1 | 7.30 |
| 7 | MOL003940 | Stearamide | C18 H37NO | [M+H]+1 | 22.62 |
| 8 | MOL001314 | Azelaic acid | C9H16O4 | [M-H]−1 | 13.33 |
| 9 | MOL000675 | Oleic acid | C18H34O2 | [M-H]−1 | 22.72 |
| 10 | MOL001801 | Salicylic acid | C7H6O3 | [M-H]−1 | 12.87 |
| 11 | MOL001456 | Citric acid | C6H8O7 | [M-H]−1 | 2.46 |
| 12 | MOL000040 | 7-hydroxy-6-methoxy-2H-chromen-2-one | C10H8O4 | [M+H]+1 | 11.47 |
| 13 | MOL002003 | Caryophyllene oxide | C15H24O | [M+H]+1 | 15.68 |
| 14 | MOL000635 | Vanillin | C8H8O3 | [M+H]+1 | 10.51 |
| 15 | MOL004712 | Xylitol | C5H12O5 | [M+H-H2O]+1 | 1.36 |
| 16 | MOL000249 | Methyl cinnamate | C10H10O2 | [M+H+MeOH]+1 | 10.26 |
| 17 | MOL004666 | Ethyl protocatechuate | C9H10O4 | [M+H]+1 | 11.86 |
| 18 | MOL003959 | Limonin | C26H30O8 | [M+H]+1 | 14.22 |
| 19 | MOL003837 | Esculetin | C9H6O4 | [M-H]−1 | 9.90 |
| 20 | MOL000346 | Succinic acid | C4 H6O4 | [M-H]−1 | 3.15 |
| Ferulic Acid | 7-Hydroxy-6-methoxy-2H-chromen-2-one | Limonin | Esculetin | Oleic Acid | |
|---|---|---|---|---|---|
| PIK3CA | −6.7 kcal/mol | −6.5 kcal/mol | −10.4 kcal/mol | −7 kcal/mol | −6.3 kcal/mol |
| AKT1 | −6.4 kcal/mol | −6.9 kcal/mol | −9.2 kcal/mol | −6.9 kcal/mol | −5.8 kcal/mol |
| Chinese Name | Accepted Name | Weight (g) | Medicinal Part |
|---|---|---|---|
| Shudihuang | Rehmannia glutinosa (Gaertn.) DC. | 30 | Prepared root |
| Shanyurou | Cornus officinalis Siebold & Zucc. | 10 | Flesh of the fruit (dried) |
| Shanyao | Dioscorea polystachya Turcz | 30 | Tuber (dried) |
| Mudanpi | Paeonia suffruticosa Andrews | 10 | Root bark (dried) |
| Zexie | Alisma orientale (Sam.) Juzep | 10 | Tuber (dried) |
| Fuling | Poria cocos (Schw.) Wolf | 15 | Sclerotium (fungal body, dried) |
| Baishao | Paeonia lactiflora Pall | 30 | Root |
| Chaihu | Bupleurum chinense DC | 10 | Root |
| Jineijin | Gallus gallus domesticus Brisson | 20 | Inner lining of the gizzard (dried) |
| Wugong | Scolopendra subspinipes mutilans L. Koch | 3 | Entire dried body |
| Gouqi | Lycium barbarum L | 10 | Fruit (dried) |
| Rougui | Cinnamomum cassia (L.) J. Presl | 5 | Bark |
| Foshou | Citrus medica L. var. sarcodactylis (Noot.) Swingle | 10 | Fruit (dried or fresh) |
| Danggui | Angelica sinensis (Oliv.) Diels | 10 | Root (dried) |
| Gene | Forward | Reverse |
|---|---|---|
| PI3K | 5′-GAAGAAGCTGAACGAGTCGC-3′ | 5′-CCCGACATTCCACGTCTTCT-3′ |
| AKT1 | 5′-CTGCTCCTAGTCCACCACCT-3′ | 5′-AGAGACCTCCATTATCGCTACC-3′ |
| Nrf2 | 5′-AGACATTCCCATTTGTAGATGACC-3′ | 5′-CTCCAGAcGAGCTATTGAGGGACT-3′ |
| KEAP1 | 5′-TCGAAGGCATCCACCCTAAG-3′ | 5′-CTCGAACCACGCTGTCAATCT-3′ |
| HO-1 | 5′-ACAGAGGAACACAAAGACCAGAGT-3′ | 5′-GTGTCTGGGATGAGCTAGTGC-3′ |
| NQO1 | 5′-TGGCCGAACACAAGAAGCTG-3′ | 5′-GCTACGAGCACTCTCTCAAACC-3′ |
| NF-κB p65 | 5′-GTTCACAGACCTGGCATCTG-3′ | 5′-CCTGTCACCAGGCGAGTTAT-3′ |
| TNF-α | 5′-TGTCTCAGCCTCTTCTCATTCC-3′ | 5′-GGTCTGGGCCATAGAACTGAT-3′ |
| IL-6 | 5′-CCAAGAGGTGAGTGCTTCCC-3′ | 5′-CTGTTGTTCAGACTCTCTCCCT-3′ |
| GAPDH | 5′-GTGGACCTCATGGCCTACAT-3′ | 5′-TGTGAGGGAGATGCTCAGTG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Chen, L.; Xu, H.; Li, J.; Liu, Y.; Chen, X.; Luo, M.; Xie, X.; Yin, M.; He, J. Modified Liuwei Dihuang Decoction Ameliorates Oligoasthenozoospermia in Mice via Modulation of the PI3K/AKT/Nrf2 Signaling Pathway. Pharmaceuticals 2025, 18, 1363. https://doi.org/10.3390/ph18091363
Li M, Chen L, Xu H, Li J, Liu Y, Chen X, Luo M, Xie X, Yin M, He J. Modified Liuwei Dihuang Decoction Ameliorates Oligoasthenozoospermia in Mice via Modulation of the PI3K/AKT/Nrf2 Signaling Pathway. Pharmaceuticals. 2025; 18(9):1363. https://doi.org/10.3390/ph18091363
Chicago/Turabian StyleLi, Mingzhu, Linhuang Chen, Haotian Xu, Junlin Li, Yatian Liu, Xiuyun Chen, Minyi Luo, Xinyuan Xie, Mingyu Yin, and Jinyang He. 2025. "Modified Liuwei Dihuang Decoction Ameliorates Oligoasthenozoospermia in Mice via Modulation of the PI3K/AKT/Nrf2 Signaling Pathway" Pharmaceuticals 18, no. 9: 1363. https://doi.org/10.3390/ph18091363
APA StyleLi, M., Chen, L., Xu, H., Li, J., Liu, Y., Chen, X., Luo, M., Xie, X., Yin, M., & He, J. (2025). Modified Liuwei Dihuang Decoction Ameliorates Oligoasthenozoospermia in Mice via Modulation of the PI3K/AKT/Nrf2 Signaling Pathway. Pharmaceuticals, 18(9), 1363. https://doi.org/10.3390/ph18091363







