Two Sides of the Same Coin for Health: Adaptogenic Botanicals as Nutraceuticals for Nutrition and Pharmaceuticals in Medicine
Abstract
1. Introduction
| Definition: Adaptogens are natural compounds or plant extracts that enhance the adaptability and survival of living organisms in response to stress. Therapeutic category and action: adaptogens, tonic, immunomodulatory [4,15,16,17,18,19,20,21,22,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49], Chemical class: predominantly tetracyclic triterpene, phenethyl- and phenylpropanoid glycosides, lignans, etc. Pharmacological activity: adaptogenic, tonic, stress-protective, stimulating Mechanism of action: multitarget effects on the neuroendocrine-immune system; adaptogens are adaptive stress response modifiers of cellular and organismal defense systems, activating intracellular and extracellular adaptive signaling pathways, expression of stress-activated proteins, neuropeptides, antioxidant enzymes, and anti-apoptotic proteins of an organism, resulting in nonspecific resistance to various stressors and increased survival [1,2,3,14,23,33,34,35,36]. |
- How to distinguish adaptogens from other herbs that are claimed as putative adaptogens, e.g., Eurycoma longifolia [57]?
2. Progress, Trends, Pitfalls, and Challenges in the Adaptogens Research
| • Adaptogens are medicinal substances causing the “state of nonspecifically increased resistance” of the organism. • Only those preparations that meet the following requirements may be included in the group of adaptogens: (a) An adaptogen should be innocuous and cause minimal disorders in the physiological functions of an organism; (b) The action of an adaptogen should be nonspecific, i.e., it should increase resistance to adverse influences of a wide range of factors of physical, chemical, and biological nature, (c) An adaptogen may possess normalizing action irrespective of the direction of the foregoing pathologic changes. • The adaptogens are nontoxic compounds with polyvalent mechanisms of action and pharmacological effects related to adaptability and survival. • Adaptogens are substances that elicit in an organism a state of nonspecifically raised resistance, allowing them to counteract stressor signals and to adapt to exceptional strain. • Adaptogens are metabolic regulators that increase the ability of an organism to adapt to environmental factors and to avoid damage from such factors. • Plant adaptogens are agents that reduce the damaging effects of various stressors due to the reduction in the reactivity of the host defense system. They adapt organisms to stress and have a curative impact on stress-induced disorders • Adaptogenic substances have the capacity to normalize body functions and strengthen systems compromised by stress. They have a protective effect on health against a wide variety of environmental assaults and emotional conditions. • Adaptogens comprise a pharmacotherapeutic group of herbal preparations used to: increase attention and endurance in fatigue and prevent/mitigate/reduce stress-induced impairments and disorders related to neuro-endocrine and immune systems. • Botanical adaptogens are plant extracts, or specific constituents of plant extracts, which function to increase survival in animals and humans by stimulating their adaptability to stress by inducing adaptive responses. • Adaptogens are stress-response modifiers that increase an organism’s nonspecific resistance to stress by increasing its ability to adapt and survive. • Botanical adaptogens are metabolic regulators that increase survival by increasing adaptability in stress. • Adaptogens are natural compounds or plant extracts that increase the adaptability and survival of living organisms to stress. • Adaptogen—any of various natural substances used in herbal medicine to normalize and regulate the systems of the body. https://www.dictionary.com/browse/adaptogen (assessed on 3 September 2025). • Adaptogens are currently defined as a therapeutic category/ pharmacological group of herbal medicines or/and nutritional products, increasing adaptability, survival, and resilience in stress and aging by triggering intracellular and extracellular adaptive signaling pathways of cellular and organismal defense systems (stress system, e.g., neuroendocrine-immune complex • Adaptogens act as chronic eustress, activating adaptive stress response, resilience, and overall survival. Adaptogens trigger the defense adaptive stress response of the organism to stressors, leading to the extension of the limits of resilience to overload, brain fatigue, and mental and aging disorders [3] • The term “adaptogen” refers to a time-dependent physiological adaptation process of the organism in response to the repeated administration of a plant substance that activates intracellular and extracellular adaptive signaling pathways and triggers an adaptive stress response [23]. |
- (i)
- Steroids-like tetracyclic and pentacyclic triterpenes, such as ginsenosides, withanolides, cucurbitacines, and andrographolides, which structurally resemble the catabolic hormones corticosteroids that inactivate the stress system to protect against overreaction to stressors; and anabolic-androgenic steroids structurally related to testosterone and estrogen androgenic steroid hormones which regulate lipids and sugar metabolism via hypothalamus–pituitary–adrenal hypothalamus–pituitary–adrenal (HPA)-axis = hypothalamus–pituitary–adrenal axis [1,2,3,15,67,68].
- (ii)
- Catecholamine-like phenolic compounds such as phenylpropanoids, phenylethane derivatives, and lignans, having similar to neurotransmitter hormones pharmacophores and suggesting an effect on the sympathetic nervous system, possibly implying an impact in the early stages of the stress response [1,2,3],
- (iii)
- Oxylipins [2,15,69] (unsaturated C-18 trihydroxy and epoxy polyhydroxylated unsaturated fatty acids), homologous to anti-inflammatory resolvins and pro-inflammatory leukotrienes [70], which are involved in the pathogenesis of Alzheimer’s disease. Rhodiola rosea, Withania somnifera, and Eleutherococcus senticosus downregulate the expression of key genes (ALOX5AP, DPEP2, LTC4S) involved in the biosynthesis of leukotrienes A, B, C, D, and E, resulting in inhibition of the leukotriene signaling pathway, suggesting their potential benefits in Alzheimer’s disease [3,71].
- The stress factors inducing the activation of the c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK)-mediated adaptive signaling pathway are heat shock, irradiation, reactive oxygen species, cytotoxic drugs, inflammatory cytokines, hormones, growth factors, and other stresses. Activating the JNK/MAPK10 signaling pathway promotes cell death and apoptosis by upregulating proapoptotic genes.
- The activation of the extracellular-signal-regulated kinase (ERK) pathway is initiated by hormones and stresses to trigger endothelial cell proliferation during angiogenesis, T cell activation, long-term potentiation in hippocampal neurons, phosphorylation of the transcription factor p53, activation of phospholipase A2 in mast cells, followed by activation of biosynthesis of leukotrienes and inflammation/allergy, etc.
- The third major stress-activated p38 signaling pathway contributes to the control of inflammation, the release of cytokines by macrophages and neutrophils, apoptosis, cell differentiation, and cell cycle regulation [3].
2.1. Stress-Protective, Stimulating, and Tonic Activity of Adaptogens
2.2. What Is Necessary and Sufficient to Be Classified as an Adaptogenic Plant?
- An appropriate in vivo model in animals
- An appropriate in vitro model
- Mechanism of action by a proper biochemical assay
- Mechanism of action by an appropriate molecular biology technique in combination with network pharmacology analysis
- Clinical trials in stress-induced and aging-related disorders
2.3. Progress and Trends in Adaptogens Research
2.4. Pitfalls in Adaptogen Research: Inadequate Assignment of Some Plants to Adaptogens or Insufficient Scientific Data
- Active compounds of Eurycoma are 4300 dalton glycopeptides consisting of 36 amino acids.
“Eurycoma contains a group of small peptides referred to as “eurypeptides” with 4300 dalton glycopeptides 36 amino acids that are known to have effects in improving energy status and sex drive in studies of rodents. Animal studies have shown that many of the effects of the extract are mediated by its glycoprotein components. Typical dosage recommendations, based on traditional use and on the available scientific evidence in humans, including dieters and athletes, call for 50–200 mg/day of a water-extracted tongkat ali root standardized to 22% eurypeptides.”
- According to the authors Talbott et al., 2013 [111], the mechanism of action is related to “the bioactive complex 4300 dalton glycopeptides (“eurypeptides” with 36 amino acids) has been shown to activate the CYP17 enzyme (17 alphahydroxylase and 17,20 lyase) to enhance the metabolism of pregnenolone and progesterone to yield more DHEA (dehydroepiandrosterone) and androstenedione, respectively [114]. This glycoprotein water-soluble extract of Eurycoma longifolia has been shown to deliver anti-aging and antistress benefits subsequent to its testosterone balancing effects”.
- Insufficient description of the study medication (see above),
- Randomization (a method used to generate the random allocation sequence, including details of restriction)
- Implementation (who generated the allocation sequence, enrolled participants, assigned participants to their groups, etc.)
- Blinding (preparation had the same appearance, test, and odor as placebo; how care providers, those assessing outcomes, were blinded; how the success of blinding was evaluated)
- Allocation concealment (the mechanism used to implement the random allocation sequence, such as sequentially numbered containers, describing any steps taken to conceal the sequence until interventions were assigned; it is not clear whether the sequence was concealed until interventions were assigned),
- Procedure for treatment compliance (how measurements of compliance of individual patients with the treatment regimen under study were documented).
- Monitoring,
- Settings and locations where the data were collected,
- Quality assurance and quality control,
- Deviations from the protocol
- Selective reporting
- The trial was conducted per ICH guidelines for GCP.
- Voucher specimen (i.e., retention sample was retained and, if so, where it is kept or deposited).
- The role of the study sponsor/funder,
- Inappropriate statistical tools and statistical analysis (e.g., lack of between-groups comparison of changes from the baseline by two-way ANOVA, etc.).
2.5. Dual-Use Dilemma and Inconsistencies in Botanical Risk Assessments in the Case of Withania somnifera
- In India, Withania somnifera is officially included in the Indian Herbal Pharmacopeia [154] where the monograph outlines: (i)—the plant names, (ii)—geographical distribution, (iii)—macroscopic and microscopic description of the roots, (iv)—chemical constituents (steroidal lactones including withanone, withaferin A, withanolides I, II, III, A, D, E, F, G, H, I, J, K, L, M, WS-L, P, and S, withasomnidienone, withanolide C, and alkaloids viz., cuscohygrine, anahygrine, tropine, pseudotropine, anaferine, isopellatierine, 3-tropyltigloate), (v)—Assays/analytical methods including HPLC conditions fingerprints, identifying withaferin A in extracts and withanolide J in vitro culture, (v)—quantitative standards (including total alkaloids (in total about0,2%), (vi)—adulteration, (vii)—pharmacology section, (viii)—reported activities including antistress, immunomodulatory, anticancer, antioxidant, an-ticonvulsive, anthelminthic, antiarthritic, chemopreventive, antibacterial, cardiopro-tective, antidepressant, antitoxic, hypoglycemic, diuretic, hypercholesterolemic, im-munosuppressive, antiradical, and adaptogenic activities, and (ix)—therapeutic category: adaptogen.
- In Europe, EMEA conducted a comprehensive literature search and reviewed available data, including information on the market in the European Union obtained from HMPC members. This review concluded that the requirements for establishing a Community herbal monograph on traditional herbal medicinal products containing Withania somnifera (L.) are met. Dunal, radix are not fulfilled since:
- ○
- the requirement of the definition of the ‘herbal preparation’ as the HMPC has not been able to find adequate evidence allowing a description of the herbal preparations (insufficient extract specification according to pharmaceutical requirements,
- ○
- lack of adequate evidence allowing a demonstration of at least 30 years of medicinal use, including at least 15 years in the European Union.
- Ashwagandha is allowed as a food supplement in the UK. The Medicines and Healthcare Products Regulatory Agency (MHRA) has approved clinical trials involving Ashwagandha, indicating its acceptance within specific regulatory frameworks [161].
- Ashwagandha is available as a dietary supplement in Germany. However, the Federal Institute for Risk Assessment (BfR) has recommended its inclusion in the EU’s list of substances for which safety has not been conclusively established, suggesting caution in its use [162].
- Ashwagandha is included in the Australian Register of Therapeutic Goods (ARTG), with over 320 listed medicines containing it, reflecting its acceptance in therapeutic products [163]
- Within the Association of Southeast Asian Nations (ASEAN), Ashwagandha is not uniformly included in national pharmacopeias. However, efforts are underway to harmonize traditional medicine regulations across member states. The ASEAN Common Technical Document (ACTD) framework is being utilized to standardize quality, safety, and efficacy requirements for herbal products, such as Ashwagandha [55].
2.5.1. Food and Drug Authorities
- In April 2023, Denmark banned Withania, stating that it is impossible to find a safe dose given the current data. The ban was based on a finding in 2020 by the Danish Technical University (DTU) that ashwagandha may induce abortions and has a possibly harmful effect on thyroid and sex hormones [52]. This decision was based on only one study in rodents, which found that ashwagandha reduced sperm quality and quantity [172], and one trial in humans suggested that ashwagandha might increase thyroid hormone levels [173], which could potentially cause delirium, heart failure, and dehydration [173].
- In Report of the Expert Committee constituted by the Ministry of Ayush, Govt. of India. The Central Council for Research in Ayurvedic Sciences, the experts state that “Numerous safety studies consistently demonstrate that standardized Ashwagandha (Withania somnifera) root extract is safe for human consumption. The scientific data reveal that Ashwagandha root is well-tolerated across a wide range of doses, with no adverse outcomes reported in diverse demographic and clinical cohorts. Thus, the Expert Committee holds a strong view that the current evidence supporting the safety and efficacy of Ashwagandha root is robust, allowing it to be safely recommended and integrated into global health practices. This reinforces its standing as a safe herb in both traditional and contemporary health contexts; nevertheless, using correctly identified species is crucial, and stringent quality control should be carried out suitably in accordance with applicable regulatory norms. In view of its scientifically proven safety, the globally available and popular Indian herb Ashwagandha (Withania Somnifera) can be judiciously prescribed by the Ayurvedic physicians for its health benefits in humans [54].The report by the DTU Food Institute (published in May 2020) needs a reconsideration due to two main reasons:
- ○
- The report is not taking into consideration the different properties concerning function and safety between different plant parts of Ashwagandha
- ○
- There is evolving evidence on the safety and efficacy of Ashwagandha published since 2020 (more than 400 papers), DTU report [52], there are glaring instances of a lack of rigorous scientific scrutiny in the DTU risk assessment report, including conclusions drawn from publications of predatory journals” [54].
- The National Institute for Public Health and the Environment (RIVM) studied whether herbal preparations containing Withania somnifera are harmful to health, concluding that the herb can induce harmful effects in individuals who are sensitive to it. It is unknown which individuals are sensitive to Withania somnifera. As a precaution, RIVM advises consumers not to use herbal preparations containing Withania somnifera, especially during pregnancy [165].
- In contrast, the EFSA assessment report and scientific opinion support the safety of ashwagandha [166]. As quoted, “In addition to its historical uses and pre-clinical studies, in the multiple published human clinical studies, ashwagandha and its preparations have been investigated in healthy adults, children, the elderly, and diseased populations. Although the primary objective of the majority of these trials was to investigate efficacy for health benefits, these studies provide insight into the potential safety and ‘tolerability’ of ashwagandha and its preparations in a diverse population. In healthy adults and children, Ashwagandha root has been used at doses of up to 5000 mg/day without adverse effects. Of the over 60 human clinical studies identified, more than 30 were placebo-controlled, double-blind clinical trials. As double-blind placebo-controlled trials are least likely to result in bias, these trials provide an opportunity to assess safety. Adverse effects of ashwagandha root or its preparations are rarely reported in humans. In some clinical trials, adverse effects were reported; however, the incidence was similar to that of the placebo group. A majority of the adverse events were transient and minor, and the frequency of these events was not related to the duration of intake. The findings from all of these studies support the safety-in-use of Ashwagandha root preparations [166].
2.5.2. Dietary Supplement Manufacturers
- All Ashwagandha are not the same. All studies are product-specific, meaning they are conducted exclusively on the extract contained in KSM66. In all studies, the dose of 2 × 300 mg has been used, i.e., one capsule in the morning and one in the evening. Experience, combined with safety studies, shows that in cases of increased need, the dose can be doubled [168].
- In response to the DTU report, an expert report of the World Ashwagandha Council and Ixoreal Biomed Inc., Los Angeles, California, USA, and Heyderabad, India, presented data from 79 human clinical studies, 29 Toxicity studies, and 130 preclinical studies. The report also discusses observations by DTU Food Institute and presents data-driven responses to the comments. The results suggest that Ashwagandha root extract) It is clinically beneficial for reducing stress and anxiety by balancing cortisol levels, enhancing memory and cognition, increasing sex drive, endurance, and strength, as well as promoting muscle growth and recovery, and improving sleep quality and overall quality of life. The data assure its safety, as no serious adverse events have been reported in any of the clinical trials. Animal studies include acute and long-term toxicity assessment, including reproductive and developmental toxicity. The authors claim that KSM-66 Ashwagandha extract is safe and conclude that the report by the DTU Food Institute needs a reconsideration due to two main reasons:
- ○
- The report does not take into consideration the different properties concerning function and safety between different plant parts of Ashwagandha
- ○
- There is evolving evidence on the safety and efficacy of Ashwagandha published since 2020 [167].
2.5.3. Independent Reviewers
- ○
- Dose-dependent reversal effect of Withania demonstrated in gene-expression study on cultivated brain cell culture, suggesting inhibition of corticotropin-releasing hormone CRH signaling pathways and inflammation in the concentration of 1.5 mg/L, and opposite effect—activation of CRH signaling pathway leading to the pro-inflammatory impacts in higher concentration of 5 mg/L [3,35]. A similar dose-dependent reversal effect of Withania was observed on the Electrophysiological Activity in Hippocampal Long-Term Potentiation, a Synaptic Model of Memory in the hippocampus slice model in vitro. In concentrations of 0.5–1.0 mg/L, Withania extract significantly increases pyramidal cell activity; however, at a higher dose of 2.5–10 mg/L, the effect decreases (Supplement S1, Figure S12).
- ○
- Inconsistencies result from studies dealing with diverse Withania extracts of diverse chemical composition, depending on numerous factors during harvesting, cultivation, methods of extraction of herbal substance, processing of herbal product, etc., ensuring sustainable quality and reproducible effects in pharmacological and clinical studies can lead to contradictory results.
- ○
- Surprisingly, the quality of the studies of Withania, which raised concerns about the safety, was not scrupulously assessed in the DPU report. For example, the reporting of the clinical trial (registered at ClinicalTrials.gov, Identifier: NCT00761761) of Ashwagandha on thyroid and sex hormones [173,174,175] does not meet the CONSORT and ICH GCP requirements and cannot be taken into account for warning and banning Ashwagandha in Denmark and the Netherlands. The placebo/verum blinding and assignment procedures were not sufficiently described. Assessment criteria must be consistent across all studies.
2.6. Key Issues Identified
- Blurring of Pharmacological and Nutritional Frameworks
- 2.
- Inappropriate Aggregation of Data from Different Plant Parts
- 3.
- Selective and Outdated Use of Scientific Literature
- 4.
- Absence of Peer Review and Transparency
- 5.
- Regulatory Disparity and Industry Impact
2.7. Critical Assessment of Common Technical Documentation Submitted by Drug Manufacturers to Drug Authorities
- The published clinical trials exhibit considerable deficiencies in their quality and show methodological problems.
- ○
- insufficiently characterized herbal preparations,
- ○
- open (label) studies,
- ○
- small sample size,
- ○
- missing ITT analysis, regardless of a detailed description of dropouts and reasons for exclusion in the analysis of an outcome measure,
- ○
- healthy subjects,
- ○
- The efficacy score has not been validated
- ○
- The results from trials on clinical pharmacology are contradictory.
- ○
- There is a lack of independent replications of the single studies.
2.8. Other Challenges in Adaptogens Research
3. Pharmaceuticals vs. Nutraceuticals and Dietary Supplements
3.1. Regulatory Classification, Purpose of Use, Dose and Potency, Labeling and Claims
- Complex multi-component botanicals, particularly of fixed combinations of herbal preparations with reproducible and sustainable quantitative and qualitative chemical composition. That is a challenge in analytical chemistry and standardization, ensuring the production of acceptable quality products. Supplement S4. The more components a product has, the more resources and time are spent on quality control, and the higher the product’s cost.
- Differences in requirements for manufacturers of medicines and food products in different countries.
- Some tasks and goals of manufacturers and regulatory authorities are different, which can sometimes lead to ambiguous results or conflicts of interest.
3.2. Proposed Solutions
- Establish Internationally Harmonized Guidelines for evaluating botanicals based on their intended use (e.g., pharmaceutical vs. dietary Supplement), incorporating traditional use data alongside modern scientific methods.
- Encourage Peer Review and Transparency in national assessments by mandating public disclosure of methodologies, data sources, and expert affiliations.
- Create a Tiered Evidence Framework that allows differentiated standards of proof for traditional botanical supplements versus pharmaceutical candidates.
- Promote International Scientific Dialogs among regulators, researchers, and industry to develop consensus positions and avoid unilateral bans that may lack scientific rigor.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panossian, A. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.A.; et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress- and aging-related diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Lemerond, T.; Efferth, T. Adaptogens in Long-Lasting Brain Fatigue: An Insight from Systems Biology and Network Pharmacology. Pharmaceuticals 2025, 18, 261. [Google Scholar] [CrossRef]
- Lazarev, N.V. General and specific in action of pharmacological agents. Farmacol. Toxicol. 1958, 21, 81–86. [Google Scholar]
- Lazarev, N.V.; Ljublina, E.I.; Ljublina, M.A. State of nonspecific resistance. Patol. Fiziol. Exp. Terapia 1959, 3, 16–21. [Google Scholar]
- Canguilhem, G. Essai sur quelques problèmes concernant le normal et le pathologique. In The Normal and the Pathological; Fawcett, C.R.; Cohen, R.S., Translators; Zone Books: New York, NY, USA, 1991; Available online: https://www.undergroundbooks.net/pages/books/11553/georges-canguilhem-michel-foucault-intro/the-normal-and-the-pathological (accessed on 10 February 2025).
- Anonymous. What is health? The ability to adapt. Editorial. Lancet 2009, 373, 781. [Google Scholar] [CrossRef]
- Cannon, W.B. Stresses and strains of homeostasis. Am. J. Med. Sci. 1935, 189, 13–14. [Google Scholar] [CrossRef]
- Selye, H. Confusion and controversy in the stress field. J. Hum. Stress 1975, 1, 37–44. [Google Scholar] [CrossRef]
- Selye, H. Stress without distress. Brux. Med. 1976, 56, 205–210. [Google Scholar]
- Brekhman, I.I.; Dardymov, I.V. New substances of plant origin which increase nonspecific resistance. Annu. Rev. Pharmacol. 1969, 9, 419–430. [Google Scholar] [CrossRef]
- Wagner, H.; Nörr, H.; Winterhoff, H. Plant adaptogens. Phytomedicine 1994, 1, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.H.; Elwin-Lewis, M.P.F. (Eds.) Panaceas, Adaptogens, and Tonics. In Medical Botany: Plants Affecting Human Health, 2nd ed.; JohnWiley & Sons, Inc.: Hoboken, NJ, USA, 2003; Part II, Chapter 18; pp. 608–628. [Google Scholar]
- Panossian, A.; Efferth, T. Network Pharmacology of Adaptogens in the Assessment of Their Pleiotropic Therapeutic Activity. Pharmaceuticals 2022, 15, 1051. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G. Adaptogens: Tonic herbs for fatigue and stress. Altern. Compliment. Ther. 2003, 9, 327–332. [Google Scholar] [CrossRef]
- Eleutherococcus. In British Herbal Pharmacopoeia 1996; British Herbal Medicine Association: Exeter, UK, 1996; pp. 74–75.
- Ginseng. In British Herbal Pharmacopoeia 1996; British Herbal Medicine Association: Exeter, UK, 1996; pp. 89–90.
- Eleutherococcus. In British Herbal Compendium; A Handbook of Scientific Information on Widely Used Plant Drugs; Bredley, P.R., Ed.; British Herbal Medicine Association: Exeter, UK, 1996; Volume 1, pp. 89–91. [Google Scholar]
- Ginseng. In British Herbal Compendium; A Handbook of Scientific Information on Widely Used Plant Drugs; Bredley, P.R., Ed.; British Herbal Medicine Association: Exeter, UK, 1996; Volume 1, pp. 115–118. [Google Scholar]
- Farnsworth, N.R.; Kinghorn, A.D.; Soejarto, D.D.; Waller, D.P. Siberian Ginseng (Eleutherococcus senticosus): Current Status as an Adaptogen. In Economic and Medicinal Plant Research; Wagner, H., Hikino, H., Farnsworth, N.R., Eds.; Academic Press: London, UK, 1985; Volume 1, pp. 155–215. [Google Scholar]
- Farnsworth, N.R.; Waller, D.; Strelkova, L.M. Use of Eleutherococcus senticosus in United States: Problems, prospects and literature update. In Proceedings of the Second International Symposium on Eleutherococcus; Far Eastern Scientific Center, USSR Academy of Sciences: Vladivostok, Russia, 1986; pp. 47–51. [Google Scholar]
- EMEA/HMPC/102655/2007; Reflection Paper on the Adaptogenic Concept. European Medicines Agency: London, UK, 2008; pp. 1–6. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-adaptogenic-concept_en.pdf (accessed on 3 September 2025).
- Panossian, A.; Efferth, T. Entschlüsselung des pleiotropen, therapeutischen Potenzials von Adaptogenen. Z. Phytother. J. Phytother. 2025, 46, 121–133. [Google Scholar] [CrossRef]
- Samuelsson, G.; Bohlin, L. Drugs of Natural Origin: A Treatise of Pharmacognosy, 6th ed.; Swedish Academy of Pharmaceutical Sciences: Stockholm, Sweden, 2009; p. 776. Available online: https://www.routledge.com/Drugs-of-Natural-Origin-A-Treatiseof-Pharmacognosy-Seventh-Edition/Samuelsson-Bohlin/p/book/9789198094251 (accessed on 10 February 2025).
- Winston, D. Adaptogens: Herbs for Strength, Stamina, and Stress Relief; Simon and Schuster: New York, NY, USA, 2019; 432p, Available online: https://books.google.se/books/about/Adaptogens.html?id=ZdOPDwAAQBAJ&redir_esc=y (accessed on 10 February 2025).
- Winston, D.; Maimes, S. Adaptogens. Herbs for Strength, Stamina, and Stress Relief; Simon and Schuster: New York, NY, USA, 2007; 336p, Available online: https://www.amazon.com/Adaptogens-Strength-Stamina-Stress-Relief/dp/1594771588 (accessed on 10 February 2025).
- Yance, D.R. Adaptogens in Herbal Herbalism, Adaptogens in Medical Herbalism: Elite Herbs and Natural Compounds for Mastering Stress, Aging, and Chronic Disease; Healing Arts Press: Rochester, NY, USA, 2013; 672p, ISBN 13 978-1-62055-100-4. Available online: https://adaptogensbook.com/ (accessed on 10 February 2025).
- Brown, R.P.; Gerbarg, P.L.; Graham, B. The Rhodiola Revolution: Transform Your Health with the Herbal Breakthrough of the 21st Century; Harmony/Rodale/Convergent: New York, NY, USA, 2004; 260p, Available online: https://www.goodreads.com/book/show/1256256.The_Rhodiola_Revolution (accessed on 10 February 2025).
- Kuhn, M.A.; Winston, D. Herbal Therapy and Supplements; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; Medical—592p; 2000—Medical—430p; Available online: https://books.google.se/books/about/Herbal_Therapy_and_Supplements.html?id=puxU3qzD5_YC&redir_esc=y (accessed on 10 February 2025).
- Der Marderosian, A.; Beutler, J.A. The Review of Natural Products, 6th ed.; Wolters Kluwer Health Kluwer: Waltham, MA, USA; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; 1672p, Available online: https://www.amazon.com/Natural-%20Prod-ucts-DerMarderosian-Lawrence-Paperback/dp/B011DC8AO6/ref=monarch_sidesheet_title (accessed on 10 February 2025).
- Heinrich, M.; Barnes, J.; Prieto-Garcia, J.; Gibbons, S.; Williamson, E.M. Fundamentals of Pharmacognosy and Phytotherapy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; 4th Edition; Elsevier: Amsterdam, The Netherlands, 2023; p. 340. ISBN 9780323834346. [Google Scholar]
- Bone, K.; Mills, S. Principles and Practice of Phytotherapy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; 1056p, ISBN 9780702052972. Available online: https://www.sciencedirect.com/book/9780443069925/principles-and-practice-ofphytotherapy (accessed on 10 February 2025).
- Panossian, A.; Seo, E.J.; Efferth, T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 2018, 50, 257–284. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Hamm, R.; Kadioglu, O.; Wikman, G.; Efferth, T. Synergy and Antagonism of Active Constituents of ADAPT-232 on Transcriptional Level of Metabolic Regulation of Isolated Neuroglial. Cells Front. Neurosci. 2013, 7, 16. [Google Scholar] [CrossRef]
- Panossian, A.; Lemerond, T.; Efferth, T. State-of-the-Art Review on Botanical Hybrid Preparations in Phytomedicine and Phytotherapy Research: Background and Perspectives. Pharmaceuticals 2024, 17, 483. [Google Scholar] [CrossRef]
- Panossian, A.; Abdelfatah, S.; Efferth, T. Network Pharmacology of Ginseng (Part II): The Differential Effects of Red Ginseng and Ginsenoside Rg5 in Cancer and Heart Diseases as Determined by Transcriptomics. Pharmaceuticals 2021, 14, 1010. [Google Scholar] [CrossRef] [PubMed]
- EMA/HMPC/232100/2011. Assessment Report on Rhodiola rosea L., Rhizoma et Radix. Based on Article 16d (1), Article 16f and Article 16h of Directive 2001/83/EC as Amended Traditional Use. Final. 27 March 2012. Available online: https://magistralbr.caldic.com/storage/product-files/680546566.pdf (accessed on 10 February 2025).
- EMA/HMPC/24177/2023. European Union Herbal Monograph on Rhodiola rosea L., Rhizoma et Radix. Draft—Revision 1. Committee on Herbal Medicinal Products (HMPC). 19 July 2023. pp. 1–6. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-rhodiola-rosea-l-rhizoma-et-radix-revision-1_en.pdf (accessed on 3 September 2025).
- EMA/HMPC/321232/2012. Assessment Report on Panax ginseng C.A. Meyer, Radix. Based on Article 16d (1), Article 16f and Article 16h of Directive 2001/83/EC as Amended (Traditional Use). Final. 25 March 2014. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-panaxginseng-camey-radix-revision-1_en.pdf (accessed on 3 September 2025).
- EMA/HMPC/680615/2013. Assessment Report on Eleutherococcus senticosus (Rupr. et Maxim.) Maxim., Radix. Based on Article 16d (1), Article 16f and Article 16h of Directive 2001/83/EC as Amended (Traditional Use). Final. 25 March 2014. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-eleutherococcus-senticosus-rupr-et-maxim-maxim-radix_en.pdf (accessed on 3 September 2025).
- Panossian, A.G. Adaptogens in mental and behavioral disorders. Psychiatr. Clin. N. Am. 2013, 36, 49–64. [Google Scholar] [CrossRef]
- Panossian, A.; Gerbarg, P. Potential Use of Plant Adaptogens in Age-related Disorders. In Complementary, Alternative, and Integrative Interventions in Mental Health and Aging; Lavretsky, H., Sajatovic, M., Reynolds, C.F., III, Eds.; Oxford University Press: New York, NY, USA, 2016; pp. 197–211. [Google Scholar]
- Panossian, A.; Amsterdam, J. Adaptogens in Psychiatric Practice. In Complementary and Integrative Treatments; Gerbarg, P.L., Muskin, P.R., Brown, R.P., Eds.; American Psychiatric Association Publishing: Arlington, TX, USA, 2018; 425p, Chapter 8; pp. 155–181. Available online: https://www.appi.org/Complementary_and_Integrative_Treatments_in_Psychiatric_Practice (accessed on 3 September 2025).
- Amsterdam, J.D.; Panossian, A.G. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine 2016, 23, 770–783. [Google Scholar] [CrossRef]
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharmacol. 2011, 21, 841–860. [Google Scholar] [CrossRef]
- Darbinyan, V.; Aslanyan, G.; Amroyan, E.; Gabrielyan, E.; Malmström, C.; Panossian, A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord. J. Psychiatry 2007, 61, 2343–2348. [Google Scholar] [CrossRef]
- Soldati, F. Ginseng, Panax. In Encyclopedia of Dietary Supplements; Marcel Dekker: New York, NY, USA, 2005; pp. 265–277. [Google Scholar]
- Shikov, A.N.; Tsitsilin, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Heinrich, M. Traditional and Current Food Use of Wild Plants Listed in the Russian Pharmacopoeia. Front. Pharmacol. 2017, 8, 841. [Google Scholar] [CrossRef]
- Panossian, A.; Brendler, T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals 2020, 13, 236. [Google Scholar] [CrossRef]
- Brekhman, I.I. Valeology—The Science of Health; Physical Education and Sport: Moscow, Russia, 1990; 208p. [Google Scholar]
- Brekhman, I.I. Pharmacosanation Medicaments. In Man and Biologically Active Substances: The Effect of Drugs, Diet and Pollution on Health, 1st ed.; Pergamon Press: New York, NY, USA, 1980; Chapter 3; pp. 49–59. Available online: https://openlibrary.org/books/OL4425643M/Man_and_biologically_active_substances (accessed on 3 September 2025).
- Danish Technical University (DTU). Food Institute’s Risk Assessments of Withania somnifera (Ashwagandha) Root; DTU DOCX No. 19/1030299; Danish Technical University (DTU): Kongens Lyngby, Denmark, 2020. [Google Scholar]
- Danish Technical University (DTU). Food Institute’s Risk Assessments of Withania somnifera (Ashwagandha) Root; DTU DOCX 25/100205; Danish Technical University (DTU): Kongens Lyngby, Denmark, 2025. [Google Scholar]
- Ministry of Ayush, Government of India, New Delhi. Safety of Ashwagandha. (Withania somnifera); Report of the Expert Committee Constituted by Ministry of Ayush Govt. of India; Central Council for Research in Ayuredic Sciences, Ministry of Ayush, Government of India: New Delhi, India, 2020; pp. 1–70. Available online: https://ayush.gov.in/images/domains/quality_standards/safetyReportAshwagandha.pdf (accessed on 3 September 2025).
- Ramadoss, M.S.K.; Koumaravelou, K. Regulatory Requirement Sufficiency for Registration of Traditional Herbal Medicine-Based Products in Association of Southeast Asian Nations Regions: A Model Study with Ashwagandha Tablets. Indian J. Pharm. Sci. 2022, 84, 501–512. [Google Scholar] [CrossRef]
- Patwardhan, B.; Chaturvedi, S.; Tillu, G.; Deshpande, S.; Hegde, B.M. Danish ban on Ashwagandha: Truth, evidence, ethics, and regulations. J. Ayurveda Integr. Med. 2024, 15, 101028. [Google Scholar] [CrossRef]
- Sukardiman; Mutiah, R.; Handayani, R. Potential and mechanisms of indigenous Indonesian medicinal plants in treating sexual dysfunction: A systematic review and pharmacological network overview. Heliyon 2025, 11, e42501. [Google Scholar] [CrossRef] [PubMed]
- Definition of Health. Available online: https://www.publichealth.com.ng/world-health-organizationwho-definition-of-health/ (accessed on 3 September 2025).
- Panossian, A.; Lemerond, T. The Milestones of Adaptogens Research and Use in Medicine; Vide Leaf: Hyderabad, India, 2022; pp. 1–78. Available online: https://videleaf.com/product/the-milestones-of-adaptogens-research-and-use-in-medicine/ (accessed on 3 September 2025).
- Panossian, A.; Lemerond, T. ADAPTOGENS: Proven Plant Medicines Scientifically Researched to Regain and Restore Your Health; TTN PublishingL: Kingwood, TX, USA, 2022; ISBN 978-1-952507-29-8. Available online: https://www.amazon.com/Adaptogens-Proven-Plant-Medicines-Scientifically/dp/1952507294 (accessed on 3 September 2025).
- Panossian, A.; Wikman, G.; Wagner, H. Plant adaptogens. III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine 1999, 6, 287–300. [Google Scholar] [CrossRef]
- Panossian, A.G.; Oganessian, A.S.; Ambartsumian, M.; Gabrielian, E.S.; Wagner, H.; Wikman, G. Effects of heavy physical exercise and adaptogens on nitric oxide content in human saliva. Phytomedicine 1999, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wagner, H. Stimulating effect of adaptogens: An overview with particular reference to their efficacy following single dose administration. Phytother. Res. 2005, 19, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Effects of Adaptogens on the Central Nervous System and the Molecular Mechanisms Associated with Their Stress-Protective Activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr. Clin. Pharmacol. 2009, 4, 198–219. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Gabrielian, E.; Wagner, H. Plant adaptogens. II. Bryonia as an adaptogen. Phytomedicine 1997, 4, 85–99. [Google Scholar] [CrossRef]
- Panossian, A.; Gabrielian, E.; Wagner, H. On the mechanism of action of plant adaptogens with particular reference to Cucurbitacin R diglucoside. Phytomedicine 1999, 6, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, A.N.; Panossian, A.G. Trihydroxyoctadecadienoic acids exhibit antiatherosclerotic and antiatherogenic activity. Phytomedicine 1994, 1, 123–126. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.J.; Efferth, T. Effects of anti-inflammatory and adaptogenic herbal extracts on gene expression of eicosanoid signaling pathways in isolated brain cells. Phytomedicine 2019, 60, 152881. [Google Scholar] [CrossRef]
- Noreen, E.E.; Buckley, J.G.; Lewis, S.L.; Brandauer, J.; Stuempfle, K.J. The effects of an acute dose of Rhodiola rosea on endurance exercise performance. J. Strength Cond. Res. 2013, 27, 839–847. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Kaur, P.; Asea, A. Adaptogens exert a stress-protective effect by modulation of expression of molecular chaperones. Phytomedicine 2009, 16, 617–622. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G.; Kaur, P.; Asea, A. Adaptogens stimulate neuropeptide Y and hsp72 expression and release in neuroglia cells. Front. Neurosci. 2012, 6, 6. [Google Scholar] [CrossRef]
- Panossian, A.G.; Dadayan, M.A.; Gevorkian, G.A. The effect of stress and adaptogen cucurbitacin R diglucoside on arachidonic acid metabolism. Probl. Endocrinol. 1989, 35, 58–61. [Google Scholar]
- Wiegant, F.A.C.; Limandjaja, G.C.; Poot, S.A.H.; Bayda, L.A.; Vorontsova, O.N.; Zenina, T.A.; Langelaar-Makkinje, M.; Post, J.A.; Wikman, G. Plant adaptogens activate cellular adaptive mechanisms by causing mild damage. In Adaptation Biology and Medicine: Health Potentials; Lukyanova, L., Takeda, N., Singal, P.K., Eds.; Narosa Publishers: New Delhi, India, 2008; Volume 5, pp. 319–332. Available online: https://www.uu.nl/staff/FACWiegant/Publications (accessed on 3 September 2025).
- Wiegant, F.A.C.; Surinova, S.; Ytsma, E.; Langelaar-Makkinje, M.; Wikman, G.; Post, J.A. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology 2009, 10, 27–42. [Google Scholar] [CrossRef]
- Hovhannisyan, A.; Nylander, M.; Wikman, G.; Panossian, A. Efficacy of Adaptogenic Supplements on Adapting to Stress: A Randomized, Controlled Trial. J. Athl. Enhanc. 2015, 4, 4. [Google Scholar] [CrossRef]
- Anderson, E.M.; Gomez, D.; Caccamise, A.; McPhail, D.; Hearing, M. Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiol. Stress 2019, 10, 100152. [Google Scholar] [CrossRef]
- Panossian, A. Trends and Pitfalls in the Progress of Network Pharmacology Research on Natural Products. Pharmaceuticals 2025, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Abdelfatah, S.; Efferth, T. Network Pharmacology of Red Ginseng (Part I): Effects of Ginsenoside Rg5 at Physiological and Sub-Physiological Concentrations. Pharmaceuticals 2021, 14, 999. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Abdelfatah, S.; Efferth, T. Network Pharmacology of Ginseng (Part III): Antitumor Potential of a Fixed Combination of Red Ginseng and Red Sage as Determined by Transcriptomics. Pharmaceuticals 2022, 15, 1345. [Google Scholar] [CrossRef]
- Chen, K.X.; Jiang, H.L.; Luo, X.M.; Shen, J.H. Drug discovery in postgenome era: Trend and practice. Chin. J. Nat. Med. 2004, 2, 257–260. [Google Scholar]
- Wu, J.; Huang, H.; Chen, G.; Mao, J.; Tian, X.; Zhan, S.; Peng, Z.; Zhu, Y.; Wang, W. Integration of network pharmacology, bioinformatics and experimental verification strategy to discover the pharmacological mechanisms of mogroside acts against pharyngitis. J. Ethnopharmacol. 2025, 344, 119499. [Google Scholar] [CrossRef] [PubMed]
- Noor, F.; Tahir Ul Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multitarget therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Panossian, A.; Hamm, R.; Wikman, G.; Efferth, T. Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: An interactive pathway analysis of the downstream effects using RNA microarray data. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 1325–1348. [Google Scholar] [CrossRef]
- Gao, X.F.; Zhang, J.J.; Gong, X.J.; Li, K.K.; Zhang, L.X.; Li, W. Ginsenoside Rg5: A Review of Anticancer and Neuroprotection with Network Pharmacology Approach. Am. J. Chin. Med. 2022, 50, 2033–2056. [Google Scholar] [CrossRef]
- He, S.; Shi, J.; Chai, H.; Ma, L.; Pei, H.; Zhang, P.; Shi, D.; Li, H. Mechanisms with network pharmacology approach of Ginsenosides in Alzheimer’s disease. Heliyon 2024, 10, e26642. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Du, L.; Zhang, S.; Wang, H.; Kong, L.; Du, G. Network pharmacology and experimental study of phenolic acids in salvia miltiorrhiza bung in preventing ischemic stroke. Front. Pharmacol. 2023, 14, 1108518. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Seo, E.J.; Wikman, G.; Efferth, T. Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome-wide microarray profiling. Phytomedicine 2015, 22, 981–992. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.J.; Efferth, T. Synergy assessments of plant extracts used in the treatment of stress and aging related disorders. Synerg. Res. 2018, 7, 39–49. [Google Scholar] [CrossRef]
- Li, S.; Xiao, W. General expert consensus on the application of network pharmacology in the research and development of new traditional Chinese medicine drugs. Chin. J. Nat. Med. 2025, 23, 129–142. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lv, A.P.; Li, J.; Jiang, M. Application review of network pharmacology in the study of properties theory of traditional Chinese medicine. J. Basic Chin. Med. 2019, 25, 127–130. [Google Scholar]
- Zhou, Z.; Chen, B.; Chen, S.; Lin, M.; Chen, Y.; Jin, S.; Chen, W.; Zhang, Y. Applications of Network Pharmacology in Traditional Chinese Medicine Research. Evid. Based Complement. Altern. Med. 2020, 2020, 1646905. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, D.; Zhou, W.; Wang, L.; Wang, B.; Zhang, T.; Li, S. Network pharmacology: Towards the artificial intelligence-based precision traditional Chinese medicine. Brief. Bioinform. 2023, 25, bbad518. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef]
- Spanakis, M.; Tzamali, E.; Tzedakis, G.; Koumpouzi, C.; Pediaditis, M.; Tsatsakis, A.; Sakkalis, V. Artificial Intelligence Models and Tools for the Assessment of Drug–Herb Interactions. Pharmaceuticals 2025, 18, 282. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.I.; Iyengar, R. Role of systems pharmacology in understanding drug adverse events. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 129–135. [Google Scholar] [CrossRef]
- Young, M.; Hoheisel, J.D.; Efferth, T. Toxicogenomics for the prediction of toxicity related to herbs from traditional Chinese medicine. Planta Med. 2010, 76, 2019–2025. [Google Scholar] [CrossRef]
- Börner, F.U.; Schütz, H.; Wiedemann, P. The fragility of omics risk and benefit perceptions. Toxicol. Lett. 2011, 201, 249–257. [Google Scholar] [CrossRef]
- Aardema, M.J.; MacGregor, J.T. Toxicology and genetic toxicology in the new era of “toxicogenomics”: Impact of “-omics” technologies. Mutat. Res. 2002, 499, 13–25. [Google Scholar] [CrossRef]
- Ouedraogo, M.; Baudoux, T.; Stévigny, C.; Nortier, J.; Colet, J.M.; Efferth, T.; Qu, F.; Zhou, J.; Chan, K.; Shaw, D.; et al. Review of current and “omics” methods for assessing the toxicity (genotoxicity, teratogenicity and nephrotoxicity) of herbal medicines and mushrooms. J. Ethnopharmacol. 2012, 140, 492–512. [Google Scholar] [CrossRef]
- Panossian, A. Challenges in phytotherapy research. Front. Pharmacol. 2023, 14, 1199516. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, Y.; Xu, Y.; Zhu, B.; Piao, J.; Zhu, L.; Yao, L.; Liu, K.; Wang, S.; Zhang, Q.; et al. The effects of different extraction methods on physicochemical, functional and physiological properties of soluble and insoluble dietary fiber from Rubus chingiiHu. Fruits. J. Funct. Foods 2022, 93, 105081. [Google Scholar] [CrossRef]
- Tambi, M.I. Eurycoma longifolia jack: A potent adaptogen in the form of water-soluble extract with the effects of maintaining men’s health. Asian J. Androl. 2006, 8, 49–50. [Google Scholar]
- George, A.; Liske, E.; Chen, C.K.; Ismail, S.B. The Eurycoma longifolia Freeze-Dried Water Extract-Physta Does not Change Normal Ratios of Testosterone to Epitestosterone in Healthy Males. J. Sports Med. Doping Stud. 2013, 3, 2. [Google Scholar]
- George, A.; Henkel, R. Phytoandrogenic properties of Eurycoma longifolia as natural alternative to testosterone replacement therapy. Andrologia 2014, 46, 708–721. [Google Scholar] [CrossRef]
- Talbott, S.M.; Talbott, J.A.; George, A.; Pugh, M. Effect of Tongkat Ali on stress hormones and psychological mood state in moderately stressed subjects. J. Int. Soc. Sports Nutr. 2013, 10, 28. [Google Scholar] [CrossRef]
- Thu, H.E.; Mohamed, I.N.; Hussain, Z.; Jayusman, P.A.; Shuid, A.N. Eurycoma longifolia as a potential adoptogen of male sexual health: A systematic review on clinical studies. Chin. J. Nat. Med. 2017, 15, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Leitão, A.E.; Vieira, M.C.S.; Pelegrini, A.; da Silva, E.L.; Guimarães, A.C.A. A 6-month, double-blind, placebo-controlled, randomized trial to evaluate the effect of Eurycoma longifolia (Tongkat Ali) and concurrent training on erectile function and testosterone levels in androgen deficiency of aging males (ADAM). Maturitas 2021, 145, 78–85. [Google Scholar] [CrossRef]
- Chinnappan, S.M.; George, A.; Pandey, P.; Narke, G.; Choudhary, Y.K. Effect of Eurycoma longifolia standardised aqueous root extract–Physta® on testosterone levels and quality of life in ageing male subjects: A randomised, double-blind, placebo-controlled multicentre study. Food Nutr. Res. 2021, 65, 5647. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Karim, A.A. Tongkat Ali (Eurycoma longifolia Jack): A review on its ethnobotany and pharmacological importance. Fitoterapia 2010, 81, 669–679. [Google Scholar] [CrossRef]
- Rehman, S.U.; Choe, K.; Yoo, H.H. Review on a Traditional Herbal Medicine, Eurycoma longifolia Jack (Tongkat Ali): Its Traditional Uses, Chemistry, Evidence-Based Pharmacology and Toxicology. Molecules 2016, 21, 331. [Google Scholar] [CrossRef]
- Low, B.S.; Das, P.K.; Chan, K.L. Standardized quassinoid-rich Eurycoma longifolia extract improved spermatogenesis and fertility in male rats via the hypothalamic-pituitary-gonadal axis. J. Ethnopharmacol. 2013, 145, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, K.; Finelli, R.; Sikka, S.C.; Panner Selvam, M.K. Eurycoma longifolia (Jack) Improves Serum Total Testosterone in Men: A Systematic Review and Meta-Analysis of Clinical Trials. Medicina 2022, 58, 1047. [Google Scholar] [CrossRef]
- Henkel, R.R.; Wang, R.; Bassett, S.H.; Chen, T.; Liu, N.; Zhu, Y.; Tambi, M.I. Tongkat Ali as a potential herbal supplement for physically active male and female seniors—A pilot study. Phytother. Res. 2014, 28, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Khanijo, T.; Jiraungkoorskul, W. Review Ergogenic Effect of Long Jack, Eurycoma Longifolia. Pharmacogn. Rev. 2016, 10, 139–142. [Google Scholar] [CrossRef]
- George, A.; Udani, J.; Abidin, N.Z.; Yusof, A. Efficacy and safety of Eurycoma longifolia (Physta®) water extract plus 690 multivitamins on quality of life, mood and stress: A randomized placebo-controlled and parallel study. Food Nutr. Res. 2018, 62, 1374. [Google Scholar] [CrossRef]
- Farag, M.A.; Ajayi, A.O.; Taleb, M.; Wang, K.; Ayoub, I.M. A Multifaceted Review of Eurycoma longifolia Nutraceutical Bioactives: Production, Extraction, and Analysis in Drugs and Biofluids. ACS Omega 2023, 8, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.L.; Barnes, J. Tongkat Ali/Long Jack. J. Prim. Health Care 2022, 14, 380–382. [Google Scholar] [CrossRef]
- Muniandy, S.; Yahya, H.M.; Shahar, S.; Kamisan Atan, I.; Mahdy, Z.A.; Rajab, N.F.; George, A.; Chinnappan, S.M. Effects of Eurycoma longifolia Jack standardised water extract (Physta) on well-being of perimenopausal and postmenopausal women: Protocol for a randomised, double-blinded, placebo-controlled, parallel group study. BMJ Open 2023, 13, e073323. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Boon, H.; Rochon, P.; Moher, D.; Barnes, J.; Bombardier, C.; CONSORT Group. Recommendations for reporting randomized controlled trials of herbal interventions: Explanation and elaboration. J. Clin. Epidemiol. 2006, 59, 1134–1149. [Google Scholar] [CrossRef]
- ICH E6 (R3) Guideline for Good Clinical Practice (GCP)—Step 5, EMA/CHMP/ICH/135/1995 Legal Effective Date: 23 July 2025. Available online: https://www.ema.europa.eu/en/ich-e6-good-clinical-practice-scientific-guideline (accessed on 3 September 2025).
- Michail, N. ‘Incorrect and Misleading’: Industry Defends Ashwagandha Root Extract Safety Amid Danish Assessment. 25 May 2023. Available online: https://www.vitafoodsinsights.com/botanicals-herbs/-incorrect-and-misleading-industry-defends-ashwagandha-root-extract-safety-amid-danish-assessment (accessed on 3 September 2025).
- Indian Express. Denmark’s Ban on Ashwagandha Lacks Scientific Rigor. 2024. Available online: https://indianexpress.com/article/cities/pune/denmarks-ban-on-ashwagandha-ban-based-on-2020-report-that-lacks-scientific-rigor-says-ayush-ministry-9435664/ (accessed on 3 September 2025).
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Banerjee, S.; Biswas, S.; Das, B.; Kar, A.; Katiyar, C.K. Withania somnifera (L.) Dunal—Modern perspectives of an ancient Rasayana from Ayurveda. J. Ethnopharmacol. 2021, 264, 113157. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Szeląg, M.; Szklanny, D.; Judasz, E.; Kaczmarek, G.; et al. Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics 2023, 15, 1057. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.U.; Sultan, M.T.; Baig, I.; Abbas, A.; Noman, A.M.; Zinedine, A.; Bartkiene, E.; Rocha, J.M. A comprehensive review on the bioactivity and pharmacological attributes of Withania somnifera. Nat. Prod. Res. 2025, 1–25, Advance online publication. [Google Scholar] [CrossRef]
- Anupama, N.; Rathi, P.; Sharma, A. Clinical safety profile of Ashwagandha root in human trials. J. Ayurveda Integr. Med. 2023, 14, 100420. [Google Scholar]
- Kalaivani, P.; Siva, R.; Gayathri, V.; Langade, D. Ninety-day repeated dose toxicity of Ashwagandha (Withania somnifera) root extract in Wistar rats. Toxicol. Rep. 2023, 11, 189–198. [Google Scholar] [CrossRef]
- Tandon, N.; Yadav, S.S. Safety and clinical effectiveness of Withania Somnifera (Linn.) Dunal root in human ailments. J. Ethnopharmacol. 2020, 255, 112768. [Google Scholar] [CrossRef]
- Verma, N.; Gupta, S.K.; Tiwari, S.; Mishra, A.K. Safety of Ashwagandha Root Extract: A Randomized, Placebo-Controlled, Study in Healthy Volunteers. Complement. Ther. Med. 2021, 57, 102642. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.G.; Gothwad, A.; Ganu, G.; Girme, A.; Modi, S.J.; Hingorani, L. Clinical safety and tolerability evaluation of Withania somnifera (L.) Dunal (Ashwagandha) root extract in healthy human volunteers. J. Ayurveda Integr. Med. 2024, 15, 100859. [Google Scholar] [CrossRef]
- Mandlik Ingawale, D.S.; Namdeo, A.G. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J. Diet. Suppl. 2021, 18, 183–226. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, V.; Vijayakumar, V.; Balakrishnan, A.; Bhandari, R.; Boopalan, D.; Ponnurangam, R.; Sankaralingam Thirupathy, V.; Kuppusamy, M. Effects of Ashwagandha (Withania somnifera) on stress and anxiety: A systematic review and meta-analysis. Explore 2024, 20, 103062. [Google Scholar] [CrossRef]
- Pratte, M.A.; Nanavati, K.B.; Young, V.; Morley, C.P. An alternative treatment for anxiety: A systematic review of human trial results reported for the Ayurvedic herb ashwagandha (Withania somnifera). J. Altern. Complement. Med. 2014, 20, 901–908. [Google Scholar] [CrossRef]
- Durg, S.; Shivaram, S.B.; Bavage, S. Withania somnifera (Indian ginseng) in male infertility: An evidence-based systematic review and meta-analysis. Phytomedicine 2018, 50, 247–256. [Google Scholar] [CrossRef]
- Pérez-Gómez, J.; Villafaina, S.; Adsuar, J.C.; Merellano-Navarro, E.; Collado-Mateo, D. Effects of Ashwagandha (Withania somnifera) on VO2max: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1119. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-blind, Randomized, Placebo-controlled Study. Cureus 2019, 11, e5797. [Google Scholar] [CrossRef]
- Yerram, C.; Jillella, A.; Reddy, V. Effects of Withania somnifera root extract serum application on hair health in healthy adults: A prospective, double-blind, randomized, parallel, placebo-controlled study. J. Ayurveda Integr. Med. 2023, 14, 100817. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body Weight Management in Adults Under Chronic Stress Through Treatment with Ashwagandha Root Extract: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Evid.-Based Complement. Altern. Med. 2016, 22, 96–106. [Google Scholar] [CrossRef]
- Salve, J.; Pate, S.; Khokan, D.; Langade, D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-blind, Randomized, Placebo-controlled Clinical Study. Cureus 2019, 11, e6466. [Google Scholar]
- Priyanka, G.; Anil Kumar, B.; Lakshman, M.; Manvitha, V.; Kala Kumar, B. Adaptogenic and Immunomodulatory Activity of Ashwagandha Root Extract: An Experimental Study in an Equine Model. Front. Vet. Sci. 2020, 7, 541112. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Seshadri, S.; Golla, K.H.; Sampara, P. Efficacy and safety of standardized Ashwagandha (Withania somnifera) root extract on reducing stress and anxiety in domestic dogs: A randomized controlled trial. J. Vet. Behav. 2022, 51, 8–15. [Google Scholar] [CrossRef]
- Devarasetti, A.K.; Bharani, K.K.; Khurana, A.; Anand, S.; Kollipaka, R.; Saranu, V.D.T.; Hanuman, D.D.V.; Addanki, V.K.; Chetlah, V.S.; Banothu, A.K. Adaptogenic Ashwagandha root extract modulates inflammatory markers in feline stress management: A double-blind placebo-controlled clinical trial. J. Appl. Anim. Res. 2024, 52, 2335921. [Google Scholar] [CrossRef]
- Długołęcka, B.; Jowko, E.; Kotowska, J.; Gierczuk, D. Effects of Ashwagandha (Withania somnifera) Supplementation on Body Composition and Blood Health Indices in Professional Wrestlers. Pol. J. Sport Tour. 2023, 30, 26–32. [Google Scholar] [CrossRef]
- Langade, D.; Thakare, V.; Kanchi, S.; Kelgane, S. Clinical evaluation of the pharmacological impact of ashwagandha root extract on sleep in healthy volunteers and insomnia patients: A double-blind, randomized, parallel-group, placebo-controlled stud. J. Ethnopharmacol. 2020, 264, 113276. [Google Scholar] [CrossRef]
- Kelgane, S.B.; Jaysing Salve, J.; Sampara, P.; Debnath, K. Efficacy and Tolerability of Ashwagandha Root Extract in the Elderly for Improvement of General Well-Being and Sleep: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. Cureus 2020, 12, e7083. [Google Scholar] [CrossRef]
- The Indian Herbal Pharmacopeia; Revised New Edition 2002; Indian Drug Manufacturers’ Association: Mumbai, India. 2002; pp. 467–478. Available online: https://www.scribd.com/document/59798407/Indian-Herbal-Pharmacopoeia-Vol-i (accessed on 3 September 2025).
- Withaniae somniferae Radix—Herbal Medicinal Product|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/herbal/withaniae-somniferae-radix (accessed on 3 September 2025).
- Public Statement on Withania somnifera (L.) Dunal, Radix. EMA/HMPC/681519/2012. Committee on Herbal Medicinal Products (HMPC). 9 July 2013. Available online: https://www.ema.europa.eu/en/documents/public-statement/final-public-statement-withania-somnifera-l-dunal-radix-first-version_en.pdf (accessed on 3 September 2025).
- Herbal Medicinal Products and Natural Medicinal Products. Available online: https://www.lakemedelsverket.se/en/permission-approval-and-control/marketing-authorisation/herbal-medicinal-products-and-natural-medicinal-products (accessed on 3 September 2025).
- Food Supplements. Available online: https://www.livsmedelsverket.se/en/business-legislation-and-control/legislation-food-business/food-supplements (accessed on 3 September 2025).
- United States Pharmacopeia. Ashwagandha Root. Dietary Supplement Monographs, Ashwagandha Root; USP-NF: Rockville, MD, USA, Pharmacopeia ID: GUID-8F4D7EDA-40ED-44A2-A3AD-E13C8E7638BF_2_en-US. [CrossRef]
- HPLC Analysis of Ashwagandha Supplements Using Alliance™ iS HPLC System. Available online: https://www.waters.com/nextgen/us/en/library/application-notes/2024/hplc-analysis-of-ashwagandha-supplements-using-alliance-is-hplc-system.html (accessed on 3 September 2025).
- Is Ashwagandha Legal in the UK: Regulations and Availability. Available online: https://oxford-origins.com/blogs/learn/is-ashwagandha-legal-in-the-uk-regulations-and-availability (accessed on 3 September 2025).
- Ashwagandha: Food Supplements with Potential Health Risks. 10 September 2024. Available online: https://www.bfr.bund.de/cm/349/ashwagandha-food-supplements-with-potential-health-risks.pdf (accessed on 3 September 2025).
- Australian Register of Therapeutic Goods (ARTG). Available online: https://www.tga.gov.au/products/australian-register-therapeutic-goods-artg (accessed on 3 September 2025).
- McKeown, M. Why Did Denmark Ban Ashwagandha? 2 June 2023. Available online: https://www.mcgill.ca/oss/article/critical-thinking-health-and-nutrition/why-did-denmark-ban-ashwagandha (accessed on 3 September 2025).
- Ashwagandha (Withania somnifera) Root. Summary of Safety Data. Food Supple ments Europe. EFSA. March 2023. pp. 1–26. Available online: https://www.npninfo.nl/wp-content/uploads/2025/03/FSE-Ashwaganda-Mar-2023-F.pdf (accessed on 3 September 2025).
- de Heer, J.A. Risk Assessment of Herbal Preparations Containing Withania somnifera (Ashwagandha). RIVM Letter Report 2024-0029. Available online: https://www.rivm.nl/bibliotheek/rapporten/2024-0029.pdf (accessed on 3 September 2025).
- A Safety Report on Ashwagandha Root Extract an Evaluation of the Risk Assessment Made by DTU Food Institute 2020. World Ashwagandha Council and Ixoreal Biomed Inc, USA. March 2023. pp. 1–74. Available online: https://drinktr.getonnet.dev/wp-content/uploads/2023/12/3.-Ashwagandha-Safety-Report.pdf (accessed on 3 September 2025).
- Studies. All Ashwagandha Are Not The same. Available online: https://en.ksm66.se/studier (accessed on 3 September 2025).
- Gupta, D.P.; Langade, D. Safety Opinion of Standardized Ashwagandha Root Extract. March 2024. Available online: https://cdn.prod.website-files.com/5e68cf7fabaae796200b13dc/65f2d081c085e3ecd906f836_Appendix%202_Safety%20Opinion%20of%20Standardized%20Ashwagandha%20Root%20Extract.pdf (accessed on 3 September 2025).
- Opinion: Safety Evaluation on the Root Part vs. The Entire Plant/Aerial Parts. Available online: https://cdn.prod.website-files.com/5e68cf7fabaae796200b13dc/65f2d0817311671eb9fe81e0_Appendix%201_SAFETY%20EVALUATION_ROOT%20PART%20VS.%20AERIAL%20PARTS.pdf (accessed on 3 September 2025).
- KSM66—A Safety Assessment. Available online: https://en.medicinegarden.se/press/genomgang-av-sakerhet-och-kliniska-studier-avseende-ksm66 (accessed on 3 September 2025).
- Singh, A.; Singh, K.D.; Shekhawat, P.S. Spermicidal activity and antifertility activity of ethanolic extract of Withania somnifera in male albino rats. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 227–232. [Google Scholar]
- Gannon, J.M.; Forrest, P.E.; Roy Chengappa, K.N. Subtle changes in thyroid indices during a placebo-controlled study of an extract of Withania somnifera in persons with bipolar disorder. J. Ayurveda Integr. Med. 2014, 5, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Sensoril(Ashwaganhda)for Bipolar Disorder. Available online: https://ichgcp.net/clinical-trials-registry/NCT00761761 (accessed on 3 September 2025).
- Chengappa, K.N.R.; Bowie, C.R.; Schlicht, P.J.; Fleet, D.; Brar, J.S.; Jindal, R. Randomized placebo-controlled adjunctive study of an extract of Withania somnifera for cognitive dysfunction in bipolar disorder. J. Clin. Psychiatry 2013, 74, 1076–1083. [Google Scholar] [CrossRef]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on Stress and the Stress-Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Curr Neuropharmacol. 2021, 19, 1468–1495. [Google Scholar] [CrossRef]
- Punja, S.; Shamseer, L.; Olson, K.; Vohra, S. Rhodiola rosea for mental and physical fatigue in nursing students: A randomized controlled trial. PLoS ONE 2014, 9, e108416. [Google Scholar] [CrossRef]
- Schröter, H.-B.; Neumann, D.; Katritzky, A.R.; Swinbourne, F.J. Withasomnine. A pyrazole alkaloid from Withania somnifera Dun. Tetrahedron 1966, 22, 2895–2897. [Google Scholar] [CrossRef]
- Rajalakshmy, M.R.; Geetha, G. Isolation and identification of withasomnine, withanolides and butein from industrial herbal marc of Withania somnifera (L.) Dunal. Indian J. Nat. Prod. Resour. 2016, 7, 116–124. [Google Scholar]
- Akgul, Y.; Ferreira, D.; Abourashed, E.A.; Khan, I.A. Lotaustralin from Rhodiola rosea roots. Fitoterapia 2004, 75, 612–614. [Google Scholar] [CrossRef]
- Langeder, J.; Grienke, U.; Döring, K.; Jafari, M.; Ehrhardt, C.; Schmidtke, M.; Rollinger, J.M. High-performance Countercurrent Chromatography to Access Rhodiola rosea Influenza Virus Inhibiting Constituents. Planta Med. 2021, 87, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Dimpfel, W.; Schombert, L.; Panossian, A.G. Assessing the Quality and Potential Efficacy of Commercial Extracts of Rhodiola rosea L. by Analyzing the Salidroside and Rosavin Content and the Electrophysiological Activity in Hippocampal Long-Term Potentiation, a Synaptic Model of Memory. Front. Pharmacol. 2018, 9, 425. [Google Scholar] [CrossRef]
- Legal and Regulatory Framework for Herbal Medicines; Association Européenne des Spécialités Pharmaceutiques Grand Public Association of the European Self-Medication Industry Europäischer Verband der Arzneimittel-Hersteller (AESGP): Brussels Belgium, 2010; pp. 1–245.
- European Pharmacopoeia (Ph. Eur.). Available online: https://www.edqm.eu/en/european-pharmacopoeia (accessed on 3 September 2025).
- European Union Monographs and List Entries. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/herbal-medicinal-products/european-union-monographs-list-entries (accessed on 3 September 2025).
- Final Guideline on Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products—Revision 3. EMA/HMPC/CHMP/CVMP/201116/20051 Rev. 318. Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products. January 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-herbal-medicinal-products-traditional-herbal-medicinal-products-revision-2_en.pdf (accessed on 3 September 2025).
- Guideline on declaration of herbal substances and herbal preparations in herbal medicinal products/traditional herbal medicinal products in the SPC. EMEA/HMPC/CHMP/CVMP/287539/2005. 11 March 2010. Declaration of herbal substances and herbal preparations in herbal medicinal products/traditional herbal medicinal products—Scientific guideline. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/final-guideline-quality-herbal-medicinal-productstraditional-herbal-medicinal-products-revision-3_en.pdf. (accessed on 3 September 2025).
- Guideline on Good Agricultural and Collection Practice (GACP) for Starting Materials of Herbal Origin—Revision 1. EMA/HMPC/246816/2005 Rev. 1. Good Agricultural and Collection Practice for Starting Materials of Herbal Origin. 9 July 2025. Available online: https://www.ema.europa.eu/en/good-agricultural-collection-practice-starting-materials-herbal-origin-scientific-guideline. (accessed on 3 September 2025).
- Guideline on Quality of Combination Herbal Medicinal Products/Traditional Herbal Medicinal Products. EMEA/HMPC/CHMP/CVMP/214869/2006. Quality of Combination Herbal Medicinal Products/Traditional Herbal Medicinal Products. 23 June 2008. Available online: https://www.ema.europa.eu/en/quality-combination-herbal-medicinal-products-traditional-herbal-medicinal-products-scientific-guideline (accessed on 3 September 2025).
- Guideline on Specifications: Test Procedures and Acceptance Criteria for Herbal Substances, Herbal Preparations and Herbal Medicinal Products/Traditional Herbal Medicinal Products—Revision 3. EMA/HMPC/CHMP/CVMP/162241/2005. Specifications: Test Procedures and Acceptance Criteria for Herbal Substances, Herbal Preparations and Herbal Medicinal Products/Traditional Herbal Medicinal Products—Scientific Guideline. 18 January 2022. Available online: https://www.ema.europa.eu/en/specifications-test-procedures-acceptance-criteria-herbal-substances-herbal-preparations-herbal-medicinal-products-traditional-herbal-medicinal-products-scientific-guideline (accessed on 3 September 2025).
- Herbal Medicinal Products: Scientific Guidelines. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/scientific-guidelines/multidisciplinary-guidelines/herbal-medicinal-products-scientific-guidelines (accessed on 3 September 2025).
- Herbal: European Union Herbal Monographs. Available online: https://efsa.onlinelibrary.wiley.com/authored-by/ContribRaw/EFSA+Scientific+Committee (accessed on 3 September 2025).
- Kroes, B.H. The legal framework governing the quality of (traditional) herbal medicinal products in the European Union. J. Ethnopharmacol. 2014, 158 Pt B, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Peschel, W. The Traditional Herbal Medicine Directive within the European regulatory framework for Herbal Products. Bol. Latinoam. Caribe Plant. Med. Aromat. 2007, 6, 102–111. [Google Scholar]
- Qu, L.; Zou, W.; Wang, Y.; Wang, M. European regulation model for herbal medicine: The assessment of the EU monograph and the safety and efficacy evaluation in marketing authorization or registration in Member States. Phytomed. Int. J. Phytother. Phytopharm. 2018, 42, 219–225. [Google Scholar] [CrossRef]
- Guidance for Industry. General/Specific Intended Use. 1998. Available online: https://www.fda.gov/media/71966/download (accessed on 3 September 2025).
- Current Good Manufacturing Practice—Guidance for Human Drug Compounding Outsourcing Facilities Under Section 503B of the FD&C Act Guidance for Industry. U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Pharmaceutical Quality/Manufacturing Standards (GMP), Revision 2. January 2020. Available online: https://www.fda.gov/media/88905/download (accessed on 3 September 2025).
- Federal Registers of 23 September 1997 (62 FR 49826), 5 June 1998 (63 FR 30615), and 15 January 1997 (62 FR 2218). Available online: https://www.federalregister.gov/ (accessed on 3 September 2025).
- ICH: Q 7: Good Manufacturing Practice for Active Pharmaceutical Ingredients—Step 5, CPMP/ICH/4106/00. 2000. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-7-good-manufacturing-practice-active-pharmaceutical-ingredients-step-5_en.pdf (accessed on 3 September 2025).
- State Pharmacopoeia of the USSR. State Pharmacopoeia of the USSR, 14th ed.; Medicina: Moscow, Russia, 2018; Part 2. Available online: https://pharmacopeia.ru/ (accessed on 3 September 2025).
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China, English ed.; National Pharmacopoeia Committee: Beijing, China, 2010. Available online: https://books.google.se/books/about/Pharmacopoeia_of_the_People_s_Republic_o.html?id=Z49IXwAACAAJ&redir_esc=y (accessed on 3 September 2025).
- WHO Good Practices for Pharmaceutical Quality Control Laboratories. Annex 1. World Health Organization WHO Technical Report Series, No. 957. 2010. Available online: https://www.who.int/docs/default-source/medicines/norms-and-standards/guidelines/quality-control/trs957-annex1-goodpractices-harmaceuticalqualitycontrol-laboratories.pdf (accessed on 3 September 2025).
- Jain, S.D.; Shrivastava, S.K.; Agrawal, A.; Gupta, A.K. WHO guidelines for quality control of herbal medicines: From cultivation to consumption. Int. J. Pharm. Chem. Anal. 2024, 11, 212–225. [Google Scholar] [CrossRef]
- Swedish Medical Products Agency. Herbal Medicines. Available online: https://www.lakemedelsverket.se/en/search?q=herbal%20medicines (accessed on 3 September 2025).
- Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress. Available online: https://ods.od.nih.gov/About/DSHEA_Wording.aspx (accessed on 3 September 2025).
- Andrews, K.W.; Gusev, P.A.; McNeal, M.; Savarala, S.; Dang, P.T.V.; Oh, L.; Atkinson, R.; Pehrsson, P.R.; Dwyer, J.T.; Saldanha, L.G.; et al. Dietary Supplement Ingredient Database (DSID) and the application of analytically based estimates of ingredient amount to intake calculations. J. Nutr. 2018, 148, 1413S–1421S. [Google Scholar] [CrossRef]
- Botanical Drug Development Guidance for Industry. U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Pharmaceutical Quality/CMC Revision 1. December 2016. pp. 1–30. Available online: https://www.fda.gov/files/drugs/published/Botanical-Drug-Development--Guidance-for-Industry.pdf (accessed on 3 September 2025).
- Coates, P.M.; Bailey, R.L.; Blumberg, J.B.; El-Sohemy, A.; Floyd, E.; Goldenberg, J.Z.; Gould Shunney, A.; Holscher, H.D.; Nkrumah-Elie, Y.; Rai, D.; et al. The Evolution of Science and Regulation of Dietary Supplements: Past, Present, and Future. J. Nutr. 2024, 154, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Dietary Supplement Health and Education Act of 1994. Public Law 103-417 [Internet], 1994. 1994. Available online: https://www.congress.gov/103/statute/STATUTE-108/STATUTE-108-Pg4325.pdf (accessed on 12 March 2025).
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Food Labeling; Nutrient Content Claims: Definition for “High Potency” Claim for Dietary Supplements and Definition of “Antioxidant” for Use in Nutrient Content Claims for Dietary Supplements and Conventional Foods. Department of Health and Human Services Food and Drug Administration. 21 CFR Part 101. [Docket No. 95N–0347]. Federal Register/Vol. 60, No. 249/Thursday, December 28, 1995/Proposed Rules. Available online: https://www.govinfo.gov/content/pkg/FR-1995-12-28/pdf/95-31193.pdf (accessed on 3 September 2025).
- Food Labeling: Requirements for Nutrient Content Claims, Health Claims, and Statements of Nutritional Support for Dietary Supplements. Department of Health AND Human Services Food and Drug Administration. 21 CFR Part 101. Docket No. 95N–0282. Federal Register/Vol. 60, No. 249/Thursday, December 28, 1995/Proposed Rules. Available online: https://www.govinfo.gov/content/pkg/FR-1995-12-28/pdf/95-31193.pdf (accessed on 3 September 2025).
- Johnston, D. Natural standard herb and supplement reference: Evidence based clinical reviews. Ulst. Med. J. 2006, 75, 166. [Google Scholar]
- Label Claims for Conventional Foods and Dietary Supplements. Available online: https://www.fda.gov/food/nutrition-food-labeling-and-critical-foods/label-claims-conventional-foods-and-dietary-supplements (accessed on 3 September 2025).
- Ulbricht, C.E.; Basch, E.M. Natural Standard Herb and Supplement Reference: Evidence-Based Clinical Reviews; Mosby: Amsterdam, The Netherlands, 2005; 1040p, ISBN 0-323-02994-9. [Google Scholar]
- NIH Office of Dietary Supplements: Mission, Origin, and Mandate. Available online: https://ods.od.nih.gov/About/MissionOriginMandate.aspx (accessed on 12 March 2025).
- NIST Measurements and Standards for Botanical Dietary Supplements. Available online: https://www.nist.gov/programs-projects/measurements-and-standards-botani (accessed on 12 March 2025).
- ODS Analytical Methods and Reference Materials Program. Dietary Supplement Analytical Methods and Reference Materials Program (AMRM). Available online: https://ods.od.nih.gov/Research/AMRMProgramWebsite.aspx (accessed on 3 September 2025).
- Pasiakos, S.M.; Kuszak, A.J. Strengthening Dietary Supplement Research: An Expanded Priority for the NIH Office of Dietary Supplements. Am. J. Clin. Nutr. 2025, 121, 1217–1219. [Google Scholar] [CrossRef]
- Understanding Dietary Supplement Claims. Available online: https://www.chpa.org/news/2021/11/understanding-dietary-supplement-claims (accessed on 3 September 2025).
- Botanicals. Available online: https://www.efsa.europa.eu/en/topics/topic/botanicals (accessed on 3 September 2025).
- Compendium of Botanicals. Available online: https://www.efsa.europa.eu/en/data-report/compendium-botanicals (accessed on 3 September 2025).
- EU Register of Health Claims. Available online: https://food.ec.europa.eu/food-safety/labelling-and-nutrition/nutrition-and-health-claims/eu-register-health-claims_en (accessed on 3 September 2025).
- EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2006/1925/oj/eng (accessed on 3 September 2025).
- “General Function” Claims Under Article 13.1 of the EC Regulation on Nutrition and Health Claims Structure/Function Claim. Available online: https://www.fda.gov/food/nutrition-food-labeling-and-critical-foods/structurefunction-claims (accessed on 3 September 2025).
- Guidance on Safety Assessment of Botanicals and Botanical Preparations Intended for Use as Ingredients in Food Supplements. EFSA J. 2009, 7, 1249. [CrossRef]
- Health Claims. Available online: https://ec.europa.eu/food/food-feed-portal/screen/health-claims/eu-register (accessed on 3 September 2025).
- Health Claims. Available online: https://www.efsa.europa.eu/en/topics/topic/health-claims (accessed on 3 September 2025).
- Nutrition and Health Claims. Available online: https://food.ec.europa.eu/food-safety/labelling-and-nutrition/nutrition-and-health-claims_en (accessed on 3 September 2025).
- Nutrition Claims. Available online: https://food.ec.europa.eu/food-safety/labelling-and-nutrition/nutrition-and-health-claims/nutrition-claims_en (accessed on 3 September 2025).
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: http://data.europa.eu/eli/reg/2006/1924/oj (accessed on 3 September 2025).
- EFSA—The European Food Safety Authority. Available online: https://www.efsa.europa.eu/en/about/about-efsa (accessed on 3 September 2025).
- Ginseng Radix. In WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 1999; Volume 1, pp. 168–182.
- Radix Eleutherococci. In WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2002; Volume 2, pp. 83–96.
- Fructus Schisandrae. In WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2007; Volume 3, pp. 296–313.
- Radix Withaniae. In WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2009; Volume 4, pp. 373–391.
- Upton, R. (Ed.) Ashwagandha Root. Withania somnifera. Analytical, Quality Control, and Therapeutic Monograph; American Herbal Phamacopoia and Therapeutic Compendium: Scotts Valley, CA, USA, 2000; pp. 1–26. [Google Scholar]
- Upton, R. (Ed.) Schisandra Berry. Schisandra chinensis. Analytical, Quality Control, and Therapeutic Monograph; American Herbal Phamacopoia and Therapeutic Compendium: Scotts Valley, CA, USA, 1999; pp. 1–28. [Google Scholar]
- Radix Ginseng. In WHO Monographs on Medicinal Plants Commonly Used in the Newly Independent States (NIS); World Health Organization: Geneva, Switzerland, 2010; Volume 5, pp. 141–160.
- European Union Herbal Monograph on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba. EMA/HMPC/39453/2015. Committee on Herbal Medicinal Products (HMPC). 2 February 2016. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-sideritis-scardica-griseb-sideritis-clandestina-bory-chaub-hayek-sideritis-raeseri-boiss-heldr-sideritis-syriaca-l-herba_en.pdf (accessed on 3 September 2025).
- Panossian, A. Herba Sideritis: A putative adaptogen for reducing the risk of age-related cognitive decline and neurodegenerative disorders. Phytomed. Plus 2024, 4, 100519. [Google Scholar] [CrossRef]
- Panossian, A.; Lemerond, T. Herba Sideritis: Insights from a Herbal Monograph with Particular Reference to Adaptogenic Potential in Neurodegenerative Disorders and Aging-Related Cognitive Decline, 1st ed.; BP International: London, UK, 2024; pp. 1–58. ISBN 978-93-48119-26-1/978-93-48119-56-8. [Google Scholar] [CrossRef]
- Kligman, E.W. Preventive geriatrics: Basic principles for primary care physicians. Geriatrics 1992, 47, 39–50. [Google Scholar]
- Berezovskiĭ, V.A. Valeology and biophysical medicine. Fiziol. Zh. (1994) 2010, 56, 3–18. [Google Scholar] [CrossRef]
- Ilnitski, A.N.; Prashchayeu, K.I.; Trofimova, S.V.; Birjukova, I.V. Preventive geriatrics vs. anti-aging medicine. Adv Gerontol. 2015, 28, 589–593. [Google Scholar] [PubMed]
- Ruzieva, G.A.; Jabborova, E.J. The science of valeology and its significance in modern medicine. Mod. Educ. Dev. 2025, 26, 127–129. [Google Scholar]

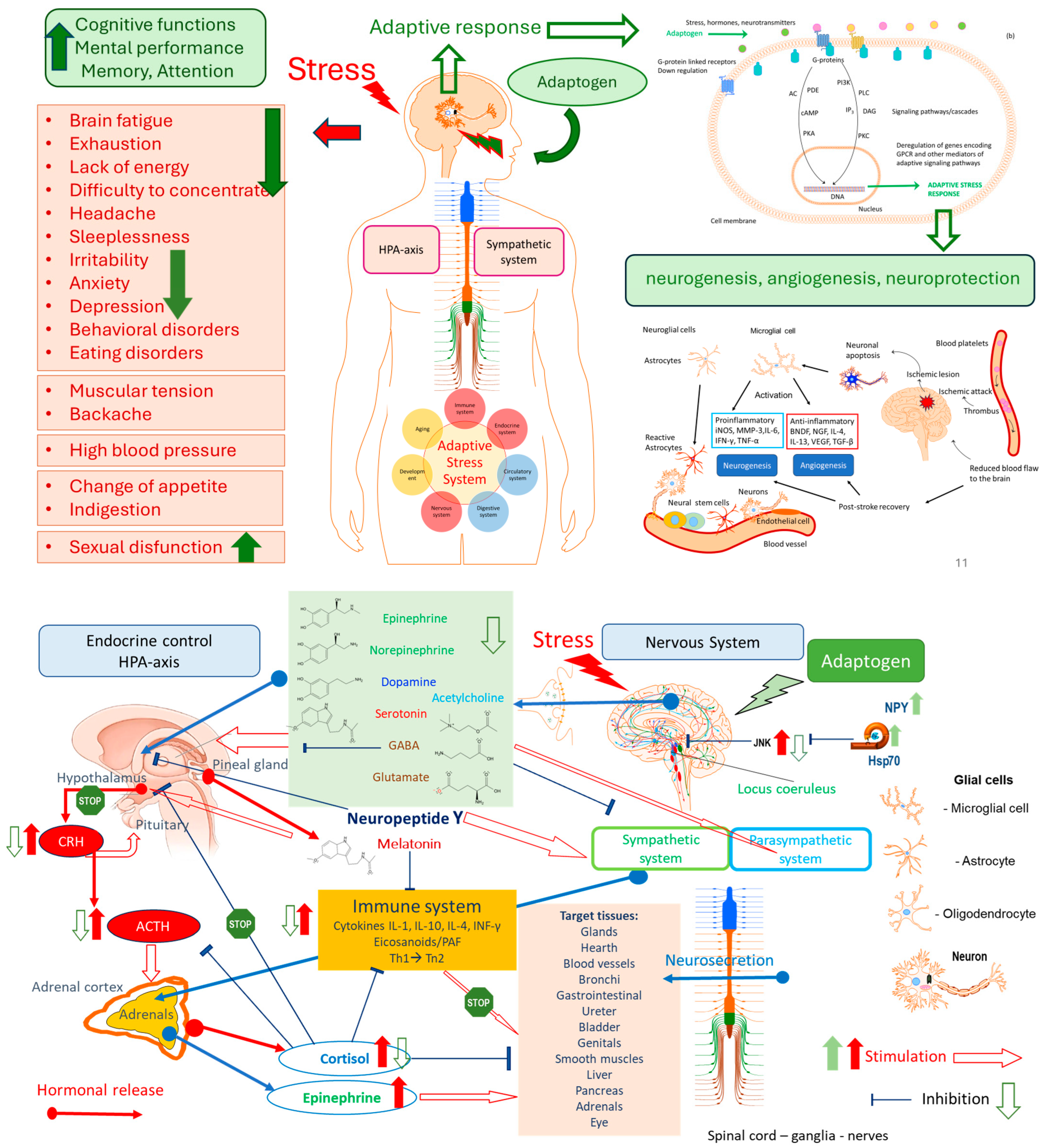

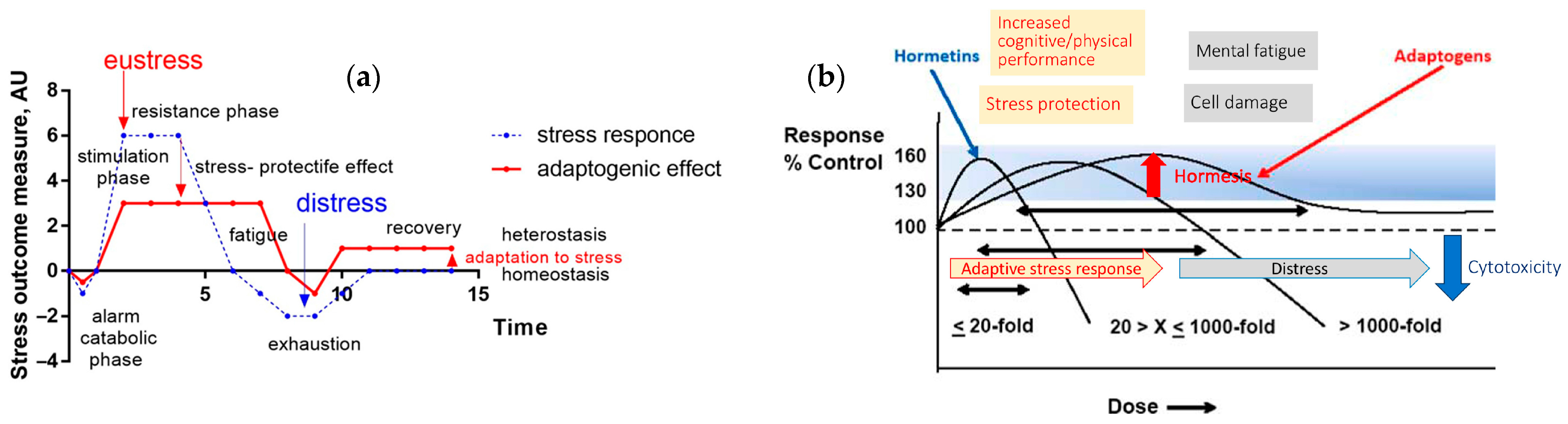
| Feature | Traditional Pharmacology | Network Pharmacology |
|---|---|---|
| Philosophy | Reductionist—targets one gene/protein | Systems-oriented—considers multitarget interactions |
| Target Focus | Single molecule | Multiple targets, often in networks |
| Drug Design Goal | High specificity | Modulation of networks/pathways |
| View of Disease | Caused by the dysfunction of a single entity | Disease as a network perturbation |
| Data Used | Experimental pharmacokinetics/dynamics | Multi-omics, PPI networks, computational modeling |
| Mechanism Identification | Binding affinity and downstream effects | Topological influence on biological networks |
| Predictive Capacity | Limited to known targets | Broader scope; includes off-targets, repurposing, synergy predictions |
| Herbal/TCM Suitability | Not applicable | Especially suitable due to the multi-component nature |
| Validation | Strong experimental support | Requires computational and experimental integration |
| Limitations | Ignores complexity and off-target effects | Data noise, oversimplified networks, context-ignorant models |
| Category | Pharmaceuticals [16,17,18,19,154,157,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205] | Dietary Supplements [158,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233] |
|---|---|---|
| Regulatory Oversight | Very strict | Lenient |
| Regulated by | Strictly regulated by drug authorities (e.g., FDA *, EMA) ** | Governed by food supplement laws (e.g., DSHEA *** in the U.S., EFSA **** in the EU). |
| Pre-market approval | Required (clinical trials, IND, NDA, etc.) | Not required; must follow labeling and safety guidelines. |
| Evidence of Safety and Efficacy | Required clinical + preclinical | Not required; General safety only |
| Efficacy | Must be proven through rigorous clinical trials | No requirement to prove efficacy before marketing |
| Safety | Extensive safety data required (nonclinical + clinical) | Only required to ensure general safety; no clinical trials mandated |
| Quality Standards | High | Moderate |
| Identity and purity | Must meet strict pharmacopoeial standards (e.g., USP †, EP ††) | Less stringent, basic identity and purity testing is often enough, focus on identity, purity, and composition |
| Standardization | Active ingredients must be quantified and consistent | Often contains a range of components; standardization is not always required |
| Contaminants (e.g., heavy metals, microbes) | Tightly controlled with established limits | Limits exist, but are less strictly enforced |
| Batch-to-batch consistency | Mandatory and validated | Expected but not strictly enforced |
| Manufacturing Requirements | Pharmaceutical grade, GMP | Food GMP |
| GMP Standards | Must follow pharmaceutical GMP (e.g., ICH Q7 †††, EU GMP, Part 211) | Must follow food-grade GMP (e.g., FDA Part 111), which are less stringent |
| Process validation | Mandatory for all critical manufacturing steps | Not required for all processes |
| Change control and documentation. | Detailed documentation and validation are required. | Documentation is required, but it is generally simpler. |
| Consistency of Botanical Source Specifics | Standardized | Variable |
| Botanical identity | Must be rigorously confirmed and controlled | Often confirmed, but methods may vary in rigor |
| Extraction process | Fully validated and standardized | May vary; often not standardized |
| Complex mixtures | Defined active constituents or fractions used | Often, a whole plant/extract with variable composition |
| Labeling Claims and Doses | Indications for use in diseases | Health supporting claims |
| Health claims | Treat/cure diseases; medicine list “Dosage” based on age, weight, or condition. Can make therapeutic claims (e.g., “treats depression”) | Support health functions; Supplements list “Suggested use” or “Serving size”. Cannot make disease claims; only “structure/function” claims (e.g., “supports mood”) |
| Labeling accuracy | Must match approved documentation | Must be truthful and not misleading, but with less scrutiny |
| Regulatory Classification |
|
|
| Purpose of Use | Medicines are designed for therapeutic effect and are often used for shorter-term or targeted purposes. | Supplements aim to provide nutritional support and are typically used on a long-term basis. |
| Product Type | Herbal Medicinal Products | Botanical Dietary Supplements |
|---|---|---|
| Regulator | Swedish Medical Products Agency | Swedish Food Agency, |
| Key Legislation: | under EU Directive 2001/83/EC and national provisions |
|
| Classification: |
|
|
| Definitions: |
| |
| Approval Needed | Yes | No (registration only) |
| Approval Requirements |
|
|
| Claims Allowed | Treat/cure diseases | Support health functions (not a cure) |
| Aspect | FDA (U.S.) [196,197,198,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221] | EMA (EU) [157,158,183,184,185,186,187,188,189,190,191,192,193,194,195,222,223,224,225,226,227,228,229,230,231,232,233] |
|---|---|---|
| Governing Bodies | - Pharmaceuticals: Center for Drug Evaluation and Research (CDER) - Supplements: Center for Food Safety and Applied Nutrition (CFSAN) | - Pharmaceuticals: European Medicines Agency (EMA) - Supplements: Regulated at member state level (e.g., Germany: BfArM, France: ANSM) |
| Applicable Legal Frameworks | - Drugs: FD&C Act, 21 CFR - Supplements: Dietary Supplement Health and Education Act (DSHEA, 1994) | - Drugs: Directive 2001/83/EC - Supplements: Food Supplements Directive (2002/46/EC), national laws |
| Botanical Drugs | Defined as botanical drug products, subject to full NDA or IND path (e.g., Veregen®, Mytesi®) | Herbal medicinal products (HMPs), classified into:—Well-established use (WEU)—Traditional use (THMP)—Full marketing authorization |
| Supplements (Botanical) | Treated as foods, not drugs. No pre-market approval. No efficacy proof required. | Also treated as foods, but the EU is more restrictive on claims. Heavily influenced by EFSA assessments. |
| Quality Standards for Botanicals | Encourages use of USP monographs and FDA Botanical Drug Guidance (2004). Must define active constituents or marker compounds. | Uses European Pharmacopeia (Ph. Eur.) monographs. Strict on identity, purity, and standardization. The Herbal Medicinal Products Committee (HMPC) oversees scientific guidelines. |
| Clinical Evidence (Botanical Drugs) | IND → NDA process: requires full clinical trials unless eligible for accelerated approval. | WEU: requires published literature and some clinical data. THMP: based on 30 years of traditional use (15 in the EU), with nonclinical safety evidence only |
| Labeling (Supplements) | Structure/function claims allowed: “supports immune health.” Must carry a disclaimer: “This product is not intended to diagnose, treat, cure, or prevent any disease.” | Health claims reviewed and authorized by EFSA; therapeutic claims prohibited on supplements. Stricter than the FDA. |
| GMP | - Drugs: 21 CFR Part 210/211- Supplements: 21 CFR Part 111 | - Drugs: EU GMP (Annexes)- Supplements: Food GMP (varies by country); less |
| Unique Points | Allows botanical drug development via standard drug approval paths. Dietary supplements are widely available with relatively light regulation, provided safety is ensured. Botanical Drug Development Guidance has been available since 2004. | Provides a specific regulatory framework for traditional herbal medicinal products (THMPs) via simplified registration. More centralized regulation of herbal drugs via EMA’s HMPC. Supplements are subject to tighter control over labeling and claims, often stricter than in the U.S. |
| Example | ||
| Echinacea supplement | Dietary Supplement, no pre-market approval | Food supplement; cannot claim therapeutic effects |
| Echinacea extract as a medicine | Must go through the full IND/NDA process | Can qualify as THMP or WEU based on evidence and monograph |
 —Dietary supplement use.
—Dietary supplement use.| Country | Pharmacopeia Monographs | Rhodiola | Ginseng | Withania | Eleuthero-Coccus | Schisandra | Eurycoma | Sideritis |
|---|---|---|---|---|---|---|---|---|
| Russia | State Pharmacopeia [189,190] | ✅ | ✅ | ✅ | ✅ | |||
| China * | Pharmacopeia of PRC -2010 (Eng) [191] | ✅ | ✅ | ✅ | ||||
| European Union | EP8 European EMA/HMPC Union herbal monograph | ✅ | ✅ | ✅ | ✅ | ✅ | ✅ | |
| United States | USP, USP Herbal Compendium monograph, AHP | ✅ | ✅ |  |  |  | ||
| Germany | Commission E | ✅ | ✅ |  | ✅ | ✅ | ||
| UK | British Pharmacopoea | ✅ |  | ✅ | ||||
| Mongolia | Mongolian Pharmacopeia | ✅ | ✅ | ✅ | ||||
| India * | Indian Herbal Pharmacopeia | ✅ | ✅ | |||||
| Pakistan | Unani/Ayurvedic Pharmacopeia | ✅ | ||||||
| Bangladesh | Unani Pharmacopeia | ✅ | ||||||
| Sri Lanka | Ayurvedic Pharmacopeia | ✅ | ||||||
| South Korea | Korean Herbal Pharmacopeia | ✅ | ✅ | ✅ | ||||
| Japan | Japanese Pharmacopeia | ✅ | ✅ | ✅  | ✅ | |||
| Vietnam | Vietnamese Pharmacopeia | ✅ | ✅ | |||||
| Lanka | ||||||||
| Australia | Australian Register of Therapeutic Goods (ARTG) | ✅ | ||||||
| Malaysia | Malaysian Pharmacopeia | ✅ | ||||||
| Indonesia | Indonesia Pharmacopeia | ✅ | ||||||
| South Africa | CAM regulatory framework |  | ||||||
| WHO | WHO Monographs | ✅ | ✅ | ✅ |
| Botanical | Dietary Supplement Dose | Pharmaceutical Dose |
|---|---|---|
| Ginseng | 100–400 mg/day extract (variable, not always standardized) | 200–400 mg/day standardized extract (1–2 g/day root equivalent) |
| Rhodiola | 100–300 mg/day extract (variable) | 200–600 mg/day standardized extract (3% rosavins, ~1% salidroside) |
| Eleutherococcus | 300–1200 mg/day crude root or extract (variable) | 300–400 mg/day standardized extract (2–4 g/day root equivalent) |
| Schisandra | 200–500 mg/day extract (variable) | 500–1500 mg/day standardized extract OR 1.5–6 g/day dried fruit |
| Ashwagandha | 300–600 mg/day extract (often not standardized) | 500–1000 mg/day standardized extract (3–6 g/day root powder in Ayurveda) |
| Rhodiola | 100–300 mg/day extract (variable) | 200–600 mg/day standardized extract (3% rosavins, ~1% salidroside) |
| St. John’s Wort | 100–300 mg/day extract (variable) | 900 mg/day standardized extract (hypericin/hyperforin defined) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panossian, A.; Lemerond, T. Two Sides of the Same Coin for Health: Adaptogenic Botanicals as Nutraceuticals for Nutrition and Pharmaceuticals in Medicine. Pharmaceuticals 2025, 18, 1346. https://doi.org/10.3390/ph18091346
Panossian A, Lemerond T. Two Sides of the Same Coin for Health: Adaptogenic Botanicals as Nutraceuticals for Nutrition and Pharmaceuticals in Medicine. Pharmaceuticals. 2025; 18(9):1346. https://doi.org/10.3390/ph18091346
Chicago/Turabian StylePanossian, Alexander, and Terrence Lemerond. 2025. "Two Sides of the Same Coin for Health: Adaptogenic Botanicals as Nutraceuticals for Nutrition and Pharmaceuticals in Medicine" Pharmaceuticals 18, no. 9: 1346. https://doi.org/10.3390/ph18091346
APA StylePanossian, A., & Lemerond, T. (2025). Two Sides of the Same Coin for Health: Adaptogenic Botanicals as Nutraceuticals for Nutrition and Pharmaceuticals in Medicine. Pharmaceuticals, 18(9), 1346. https://doi.org/10.3390/ph18091346








