1. Introduction
Type 2 diabetes mellitus (T2DM) is a prevalent endocrine disease characterized primarily by hyperglycemia, impaired glucose tolerance, insulin resistance, and disrupted glucose and lipid homeostasis [
1]. Global diabetes prevalence in 2021 was estimated to be approximately 10.5% of the population (536.6 million) and is projected to rise to 12.2% (783.2 million) by 2045, according to recent IDF statistics [
2]. T2DM accounts for nearly 90% of all diabetes mellitus cases globally [
3] and can lead to dysfunction of multiple organs and systems, including cardiovascular disease and nephropathy, among others. Meanwhile, an increasing severity of metabolic syndrome symptoms, such as hyperglycemia and hyperlipidemia, has become exceedingly prevalent worldwide owing to cases of overweight and obesity, with changes in dietary habits and lifestyles [
4]. However, the mechanisms underlying glucose and lipid metabolism dysregulation in T2DM are complex. Previous research has indicated significant associations with systemic inflammation responses, oxidative damage, and impaired insulin sensitivity [
5,
6]. In addition, IRS-1 (insulin receptor substrate-1), peroxisome proliferator-activated receptors (PPARs), AMP-activated protein kinase, and other metabolic products, including lipids, amino acids, and carbohydrate metabolites, are strongly implicated in glucose and lipid metabolism disorders of diabetes mellitus [
7,
8]. Therefore, the prevention and management of T2DM are critically significant. During the past few decades, dietary management and medication for blood glucose and lipid reduction have been the main therapies for T2DM, including biguanides, sulfonylureas, thiazolidinediones, insulin, statins, and others. Nevertheless, most of them have failed to delay diabetes progression and exhibit adverse reactions or toxicity despite their temporary efficacy [
9]. Consequently, the exploration of safe and effective glycolipid-regulating drugs with multiple target actions in the treatment of T2DM is an essential objective.
Traditional Chinese medicine (TCM) has a long history in promoting human health and treating chronic diseases based on a holistic view. It is commonly used in the form of herbal formulas, which leverage the synergistic effects among various herbs, and TCM is also known for its lower rates of drug resistance and side effects [
10]. Some TCM herbs have proven to be remarkably efficient in managing T2DM and related cardiovascular illnesses, based on the pathogenesis of Qi blockage, phlegm dampness, and blood stasis accumulation in Chinese medicine [
11]. Accordingly, TCM herbs can relieve symptoms and slow the progression of diabetes mellitus through therapeutic strategies such as replenishing Qi and strengthening the spleen, resolving phlegm and dampness, and dispelling stasis and eliminating turbidity. Qihua Tongtiao Formula (QHTTF) is a further modification based on Huangqi Baizhu Decoction from
the Yellow Emperor’s Inner Canon and Zhizhu Decoction from
the Synopsis of the Golden Chamber, while incorporating clinical treatment experience. The formula is composed of Huangqi (
Astragalus membranaceus), Baizhu (
Atractylodes macrocephala koidz), Zhishi (
Aurantii fructus immaturus), Chaihu (
Radix bupleuri), Chuanxiong (
Ligusticum chuanxiong hort), Danggui (
Angelicae sinensis radix), Laifuzi (
Raphanus sativus), and Zhuling
(Polyporus umbellatus). It was prepared as a decoction for experimental research. In our preliminary study, QHTTF showed a significant therapeutic effect on glycemic control and lipid regulation in Sprague Dawley diabetic rats and has been proven effective in lowering fasting blood glucose and lipid levels of those with type 2 diabetes. Thus, we further conducted the experimental study presented in this paper to clarify the underlying mechanisms of QHTTF in treating glucose and lipid metabolic disorders of T2DM.
With the advent of large-scale data and artificial intelligence, the integration of informatics technologies—such as network pharmacology, molecular biology techniques, multi-omics technologies, and biological experiment methods—offers a promising approach for the comprehensive illustration of TCM formula principles [
12]. In recent years, numerous studies have been conducted by combining network pharmacology methods and metabolomics, and these studies have contributed to clarifying the pathophysiology of glycolipid metabolic disorders in T2DM [
13]. Notably, metabolomics examines the human body as an integrated system characterized by its holistic, dynamic, and non-invasive nature. This approach is closely aligned with the principles of holistic and dynamic concepts in TCM. Furthermore, metabolomics is generally used to describe abnormal metabolism during the progression of glycolipid metabolic disorders. Meanwhile, network pharmacology constructs interaction networks in the context of biological systems and explores the connections between multiple drug targets via the “disease-targets-drug” network [
14,
15]. Thus, traditional Chinese medicine and modern approaches like network pharmacology and metabolomics, with their integrated concepts, can provide insights into disease mechanisms and explore disease-related targets for T2DM treatment.
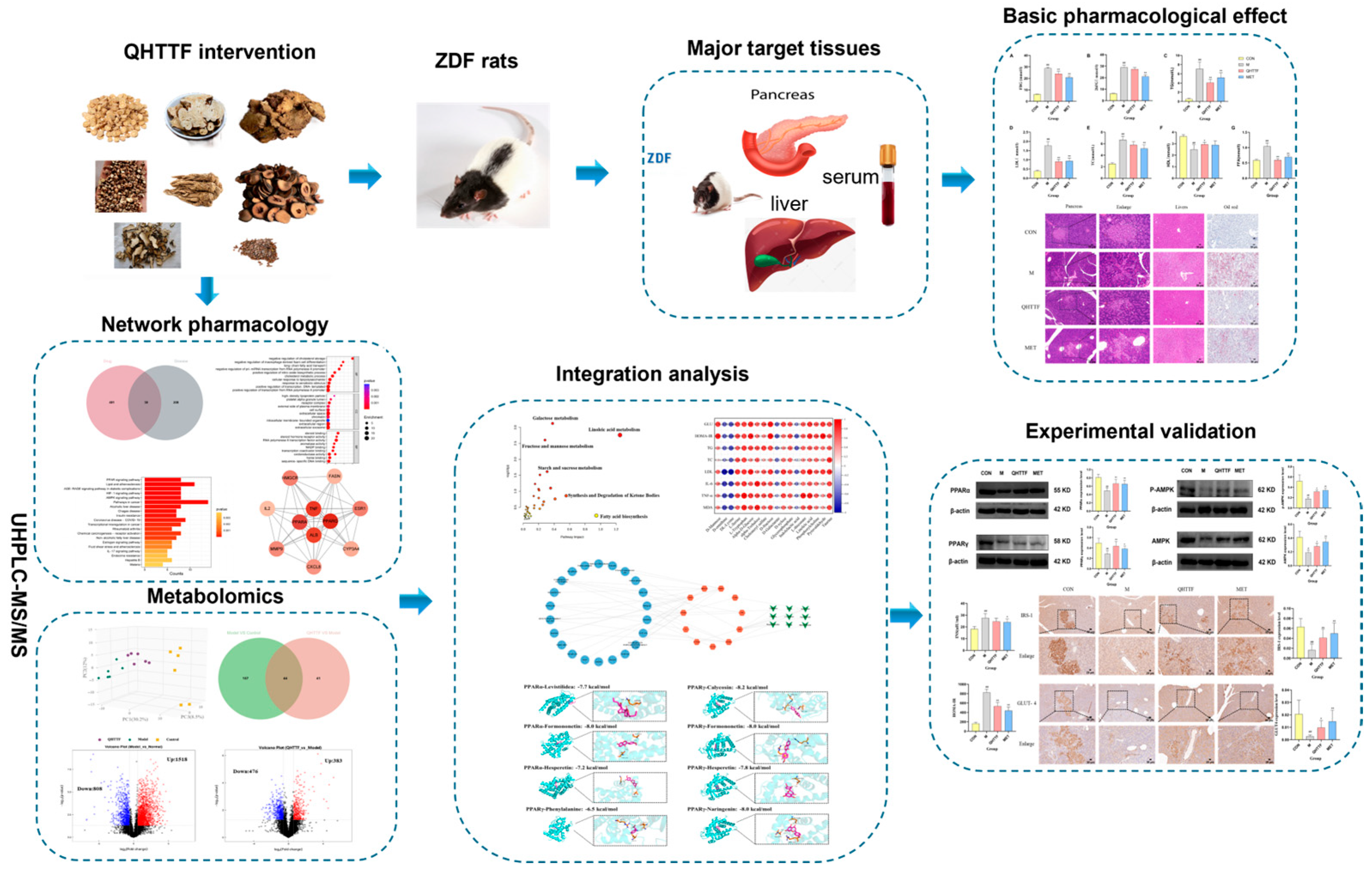
In the present study, the components of QHTTF were determined by means of ultra-performance liquid chromatography–mass spectrometry (UPLC-MS/MS), which is a high-throughput technique. Subsequently, a “compound-target-metabolite” network was established using network pharmacology combined with metabolomics to identify the primary targets and therapeutic metabolic pathways. Furthermore, an experimental assay was developed in ZDF rats induced by high-fat diets to illustrate the related mechanisms of improving glycolipid metabolic disorders. The workflow of this study is illustrated in
Figure 1.
3. Discussion
Glycolipid metabolic disorders (GLMDs) represent metabolic dysfunctions caused by impaired glycolipid homeostasis. Diabetes independently contributes to ASCVD risk, and dyslipidemia plays a crucial role in the occurrence of ASCVD in diabetic populations. Diabetic individuals, particularly T2DM patients, exhibit markedly higher dyslipidemia rates than non-diabetic controls, constituting a major risk in ASCVD and diabetes progression [
16]. Diabetic dyslipidemia is caused by multiple complex pathophysiological mechanisms such as insulin resistance, chronic inflammation, oxidative stress, and energy metabolism disorders [
7,
17]. At present, an individual intervention is insufficient to manage multiple metabolic disorders effectively, leading to inadequate lipid management and blood sugar regulation. Based on extensive clinical experience, TCM has developed numerous effective prescription drugs under the guidance of a holistic view and dialectical treatment principles. These formulas inherit ancient formulations, are applied in modern diseases, preserve their essence, and further innovate. From the perspective of TCM theory, T2DM patients with dyslipidemia commonly have Qi deficiency in the spleen and stagnation in the liver. Deficiency and obstruction of Qi often lead to blood stagnation, damp accumulation, and toxic obstruction. Therefore, a herbal compound—QHTTF was developed based on the pathogenesis of the disease and in conjunction with classical Chinese herbal formulas, which exerts effects of strengthening the spleen and resolving dampness, smoothing the liver and regulating Qi, and eliminating turbidity in GLMDs.
The Zucker Diabetic Fatty rats model, induced by high-fat feeding, is commonly used for research on diabetes and obesity. This model is characterized by a genetic mutation in the leptin receptor, leading to hyperglycemia, obesity, hyperinsulinemia, hyperlipidemia, and impaired glucose tolerance [
18,
19]. It is widely employed in research on metabolic syndrome, including diabetes and obesity-related diseases. In our research, we selected Qihua Tongtiao Decoction and Metformin for intervention in ZDF rats to evaluate the effects of Chinese herbal compounds on glucose and lipid metabolism disorders, as well as the underlying mechanisms. Meanwhile, network pharmacology further identified the active ingredients, major targets, and signaling pathways of QHTTF related to the disease, while exploring mechanisms of disease progression and drug action. Broadly targeted metabolomics was also used to assess metabolite differences under various physiological and disease states. Accordingly, by combining the two methods and integrating biological experiments, we further revealed the mechanisms of QHTTF.
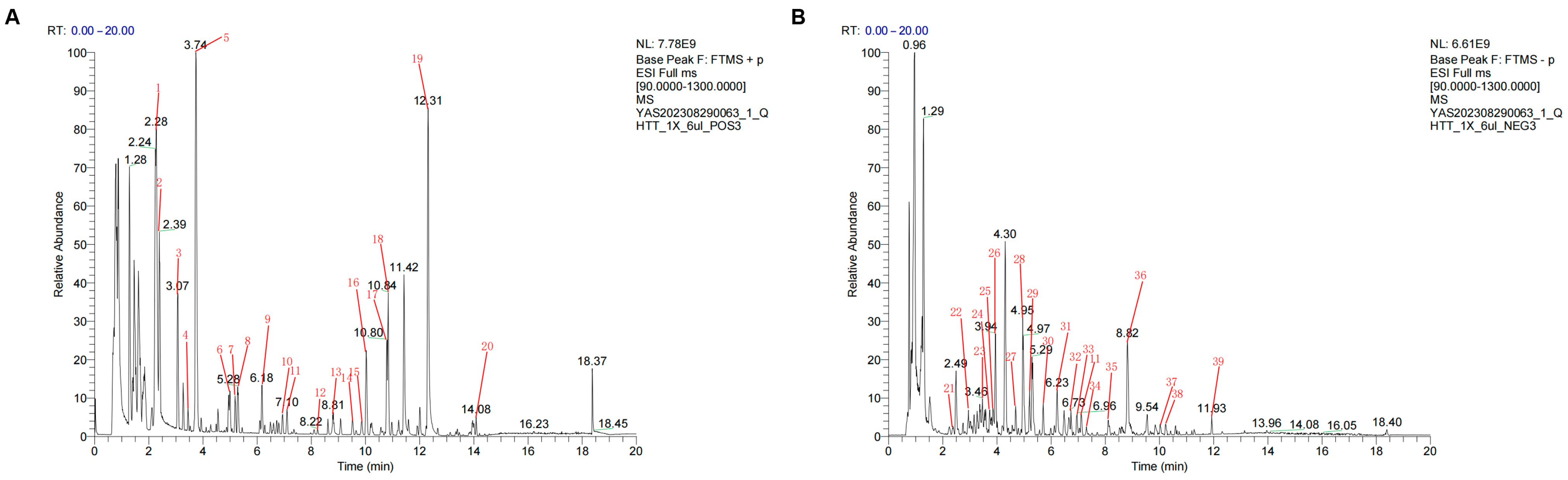
In the present study, we identified 39 active components of QHTTF using UHPLC-Q-MS and further screened 22 major active compounds based on pharmaceutical principles, including Lipinski’s Rule of Five and GI absorption. In this way, calycosin, phenylalanine, hesperetin, naringenin, levistilide A, ferulic acid, sinapine cation, and coumaric acid were identified as the primary active ingredients of QHTTF. These components are derived from Chinese herbs such as
Astragalus,
Atractylodes,
Citrus aurantium,
Ligusticum chuanxiong,
Angelica sinensis,
Raphanus sativus,
Bupleurum, and others based on the 2020 edition of the
China Pharmacopoeia. In particular, most of these components have been reported to influence glucose and lipid levels and ameliorate issue and organ damage in rats with T2DM or dyslipidemia. For instance, modern pharmacological research shows that calycosin and phenylalanine could regulate glucose and lipid metabolism by influencing insulin resistance, inflammation, oxidative stress, and lipid accumulation [
20,
21,
22]. Hesperidin and naringenin exhibit anti-inflammatory and anti-oxidation effects, regulate carbohydrate and lipid homeostasis, and contribute to the amelioration of lipid metabolism disorders and insulin resistance [
23]. In addition, levistilide A primarily exerts anti-inflammatory and antioxidant effects and modulates AMPK/mTOR phosphorylation, as well as altering glucose metabolism levels [
24]. Ferulic acid can also improve glucose and lipid metabolism, as well as restore pancreatic function [
25]. Furthermore, the mechanism of action of coumaric acid mainly involves regulating hepatic lipid metabolism, anti-glycation, anti-oxidant effects, and modulating the expression of liver-related proteins PPAR and AMPK [
26,
27]. Thus, based on modern pharmacological research, QHTTF might improve glucose and lipid metabolism, relieve inflammation and oxidative stress, and alleviate insulin resistance, making it useful in the treatment of diabetes and dyslipidemia.
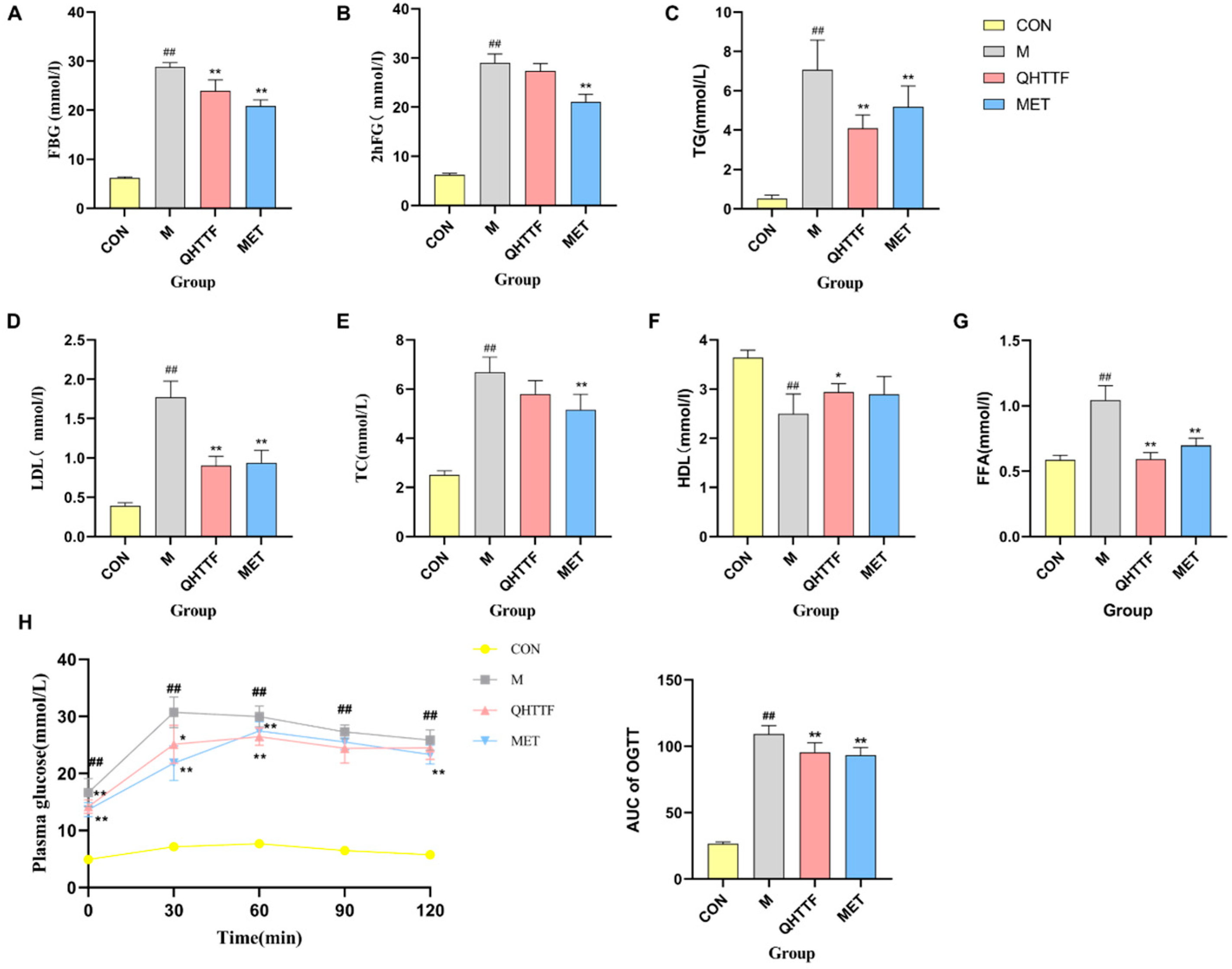
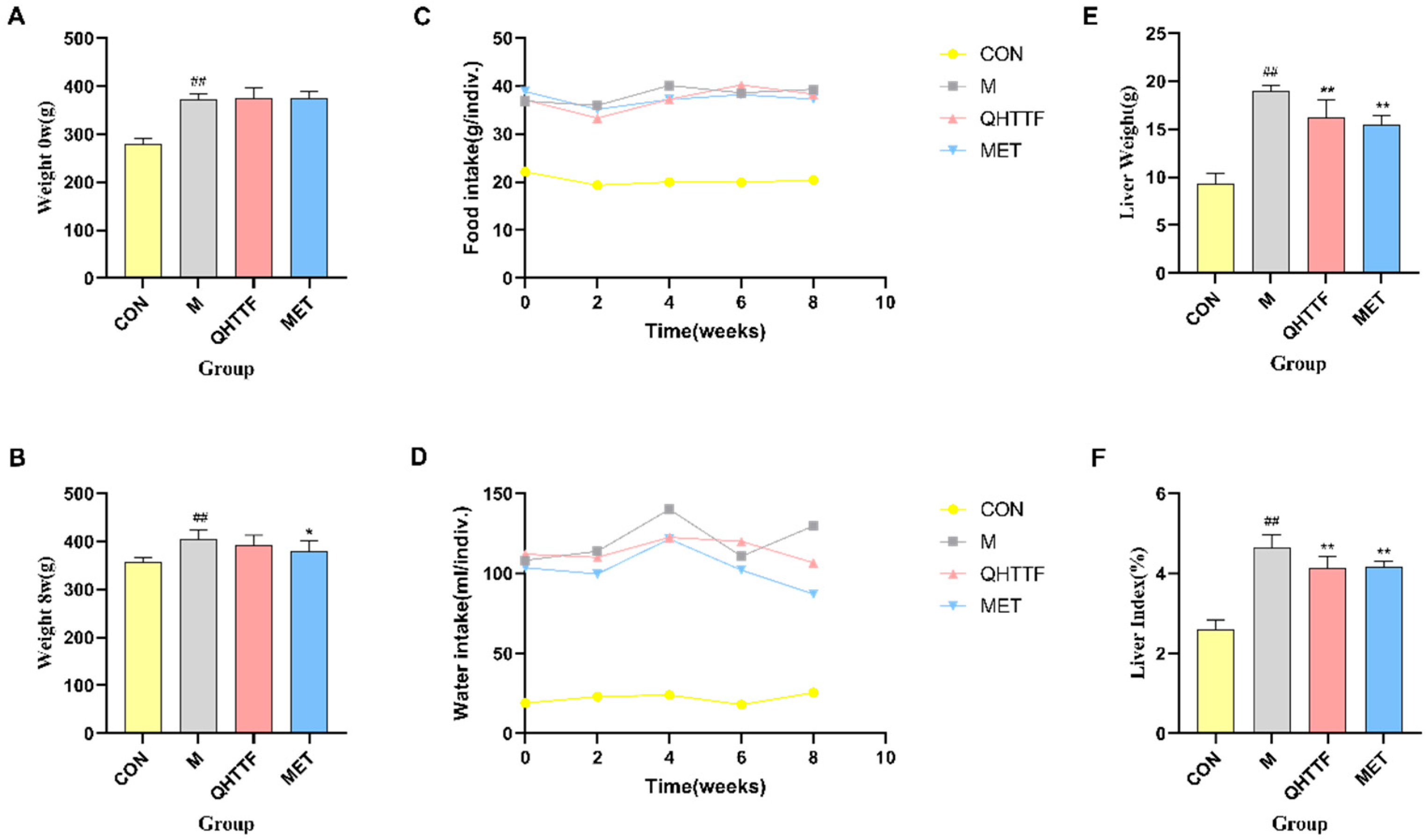
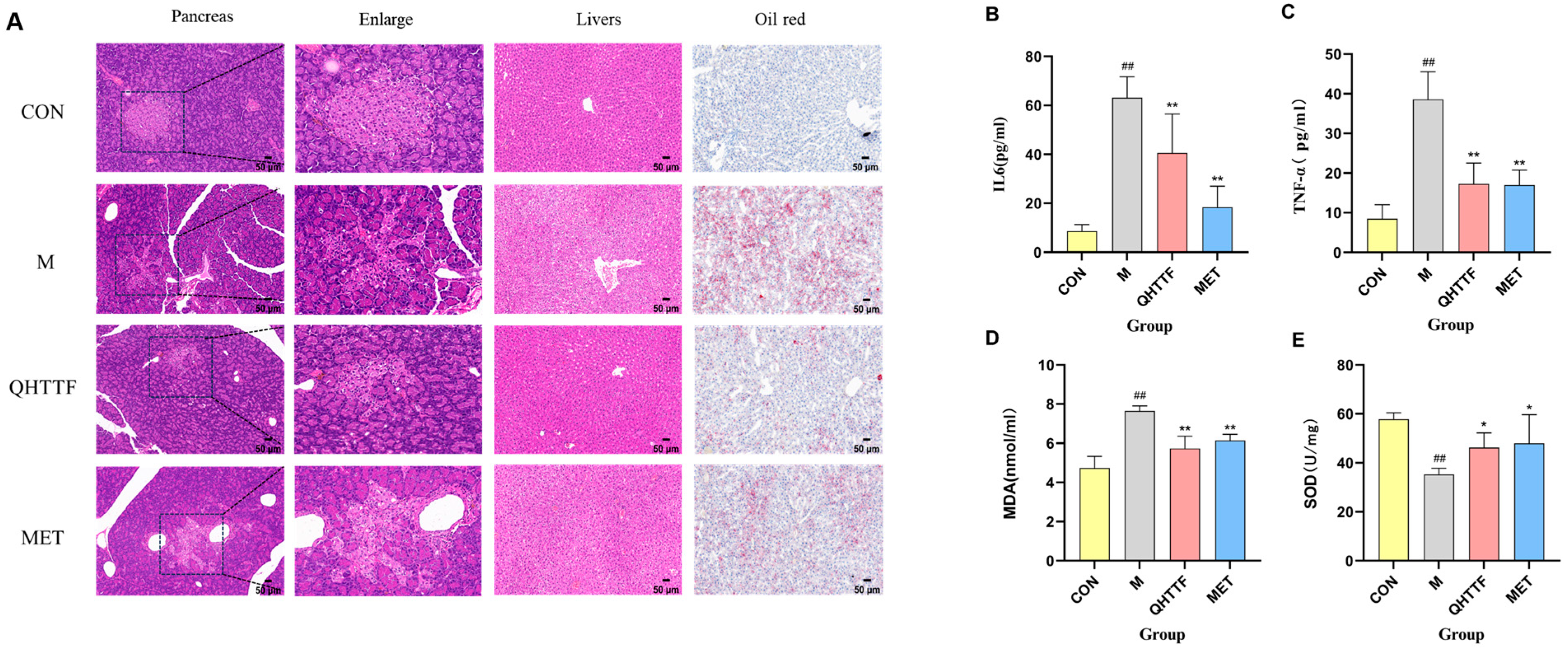
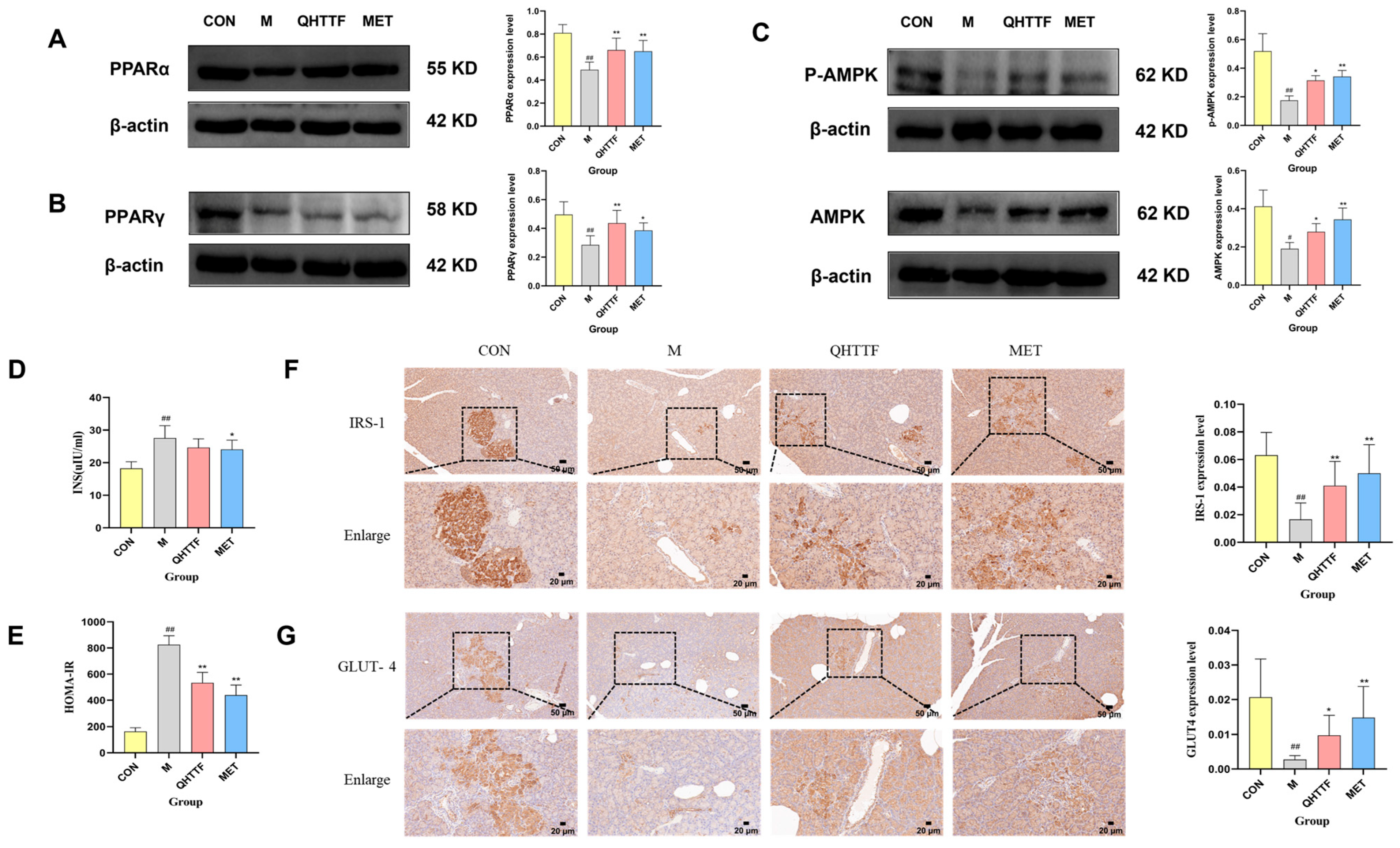
The animal experiment also demonstrated that QHTTF decreased the serum levels of GLU, TG, LDL-C, and FFA and significantly reduced the AUC of OGTT blood glucose in rats. This suggests that this herbal medicine could lower fasting blood glucose and abnormal lipid levels in ZDF rats. Patients with glucolipid metabolic disorders may experience increased oxidative stress and chronic inflammation due to excessive calorie consumption and insufficient physical activity. Meanwhile, as key metabolic regulators, the liver and pancreas are centrally involved in GLMDs [
28]. Histopathological staining showed that model group rats exhibited aggravated pathological damage, inflammatory infiltration, and increased cell necrosis in the liver and pancreas, as well as severe lipid accumulation in hepatocytes. After QHTTF intervention, the rats showed notable improvements in islet morphology, a significant reduction in cell vacuolation and necrosis, and a decrease in hepatic lipid accumulation. These improvements in fatty degeneration were superior to those in the metformin group. Additionally, the Chinese medicine formula could reduce the liver weight and liver index in ZDF rats to some extent. Relative to the model group, QHTTF treatment exhibited effects similar to those of metformin therapy. However, body weight loss showed a notable trend in the Met group, while that of the QHTTF group was slightly reduced but not statistically significant (
p > 0.05), indicating that the herbal medicine has limited efficacy in reducing body weight and alleviating obesity in ZDF rats. Another discovery of our present study was that QHTTF significantly reduced the serum levels of inflammatory factors IL-6, TNF-α, and the lipid peroxidation marker MDA, while increasing the levels of SOD, potentially exerting further effects on the glucose and lipid metabolism of rats. These basic pharmacological effects—including reduced GLU, TG, and LDL accumulation, alleviated FFA levels, and inhibited inflammation and oxidative stress—were consistent with previous pharmacological research on the major components of QHTTF.
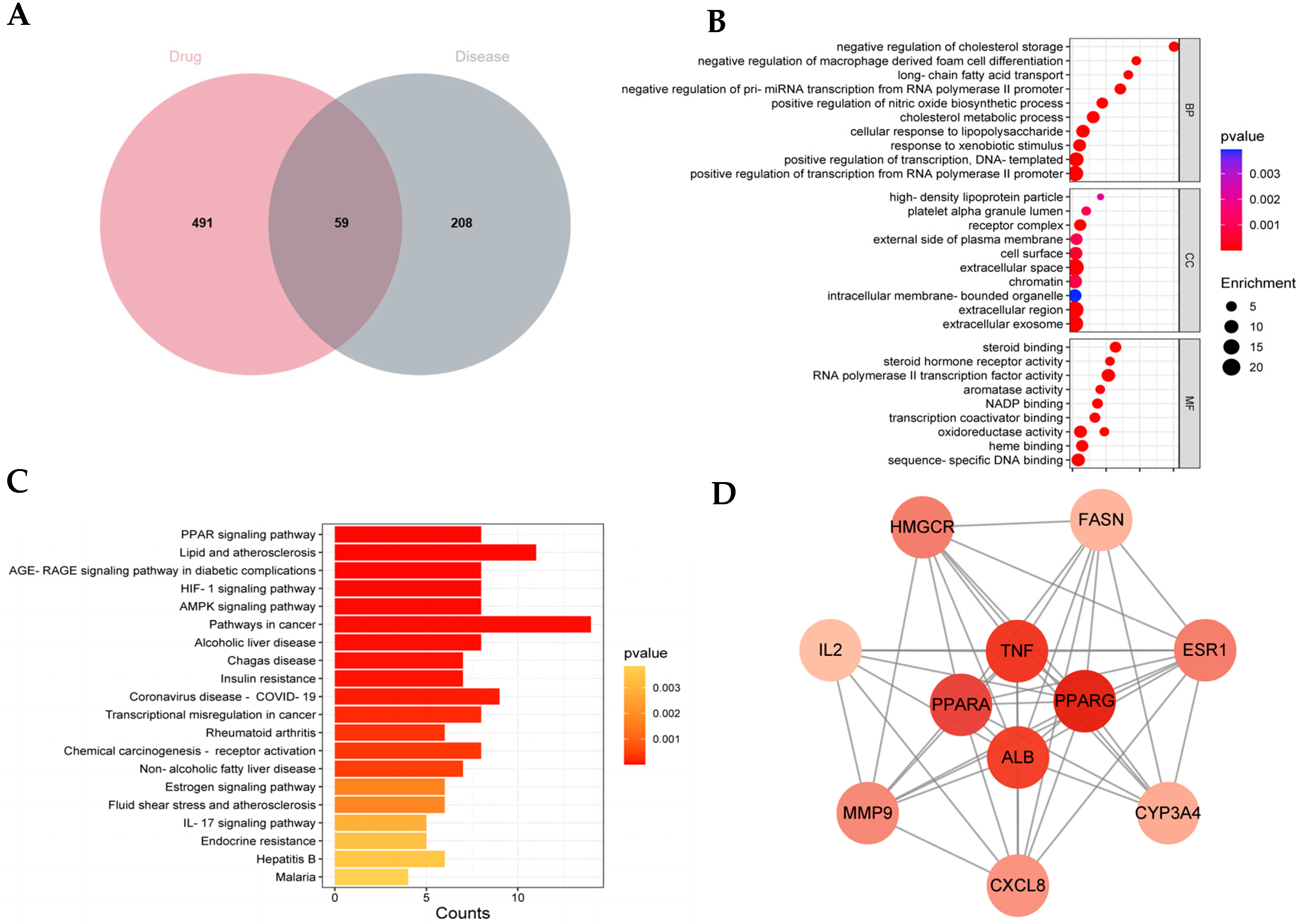
In the current research, we employed network pharmacology and metabolomic analysis to investigate the potential mechanism of QHTTF against glucolipid metabolic disease. Network pharmacology is a comprehensive approach to predict the major active ingredients, targets, and related signaling pathways involving GLMDs and drugs. We identified 59 common drug-disease intersection targets and performed enrichment analysis on them. The GO enrichment analysis revealed that the relevant BP included cholesterol metabolic process, cellular response to lipopolysaccharide, response to xenobiotic stimulus, and oxidoreductase activity, among others. This is consistent with previous studies linking cholesterol metabolism and lipopolysaccharide response to insulin resistance and T2DM [
29,
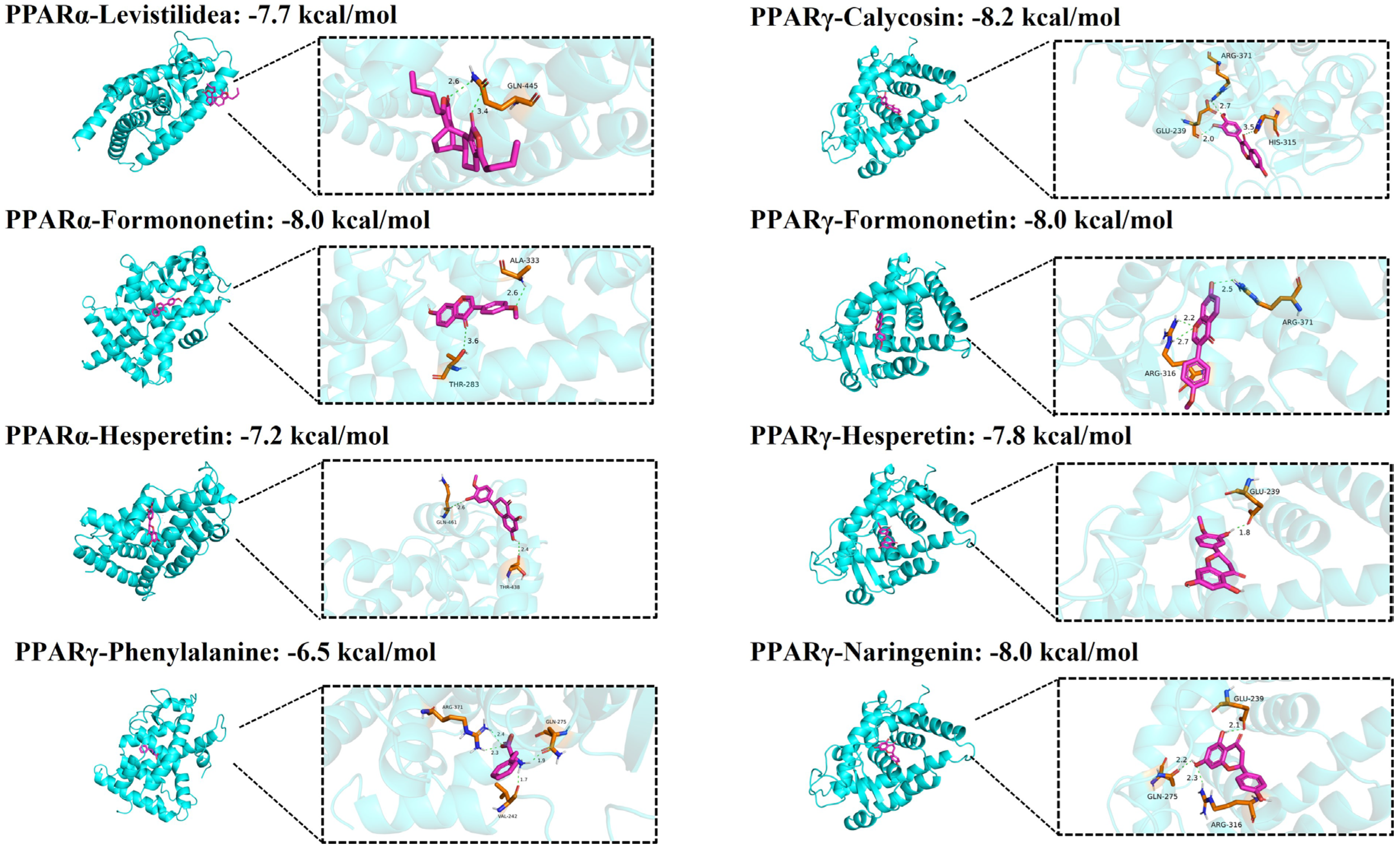
30]. At the same time, KEGG pathway analysis provided evidence that the signaling pathways mainly involved the PPAR, AMPK pathways, insulin resistance, lipid and atherosclerosis, and alcoholic liver disease, among others. Furthermore, 11 potential core targets were acquired according to the PPI network, which mainly contained PPARα, PPARγ, TNF, ALB, ESR1, etc. Thus, our analysis suggested that the PPAR and AMPK signaling pathway and insulin resistance may play crucial roles in the therapeutic effect of QHTTF. In addition, molecular docking analysis showed that the compounds of calycosin, formononetin, phenylalanine, naringenin, hesperetin, and levistilide A have great binding affinity to PPARα and PPARγ. This indicated that the main active components of QHTTF might regulate glucolipid metabolism disorders in ZDF rats by modulating PPARα and PPARγ targets.
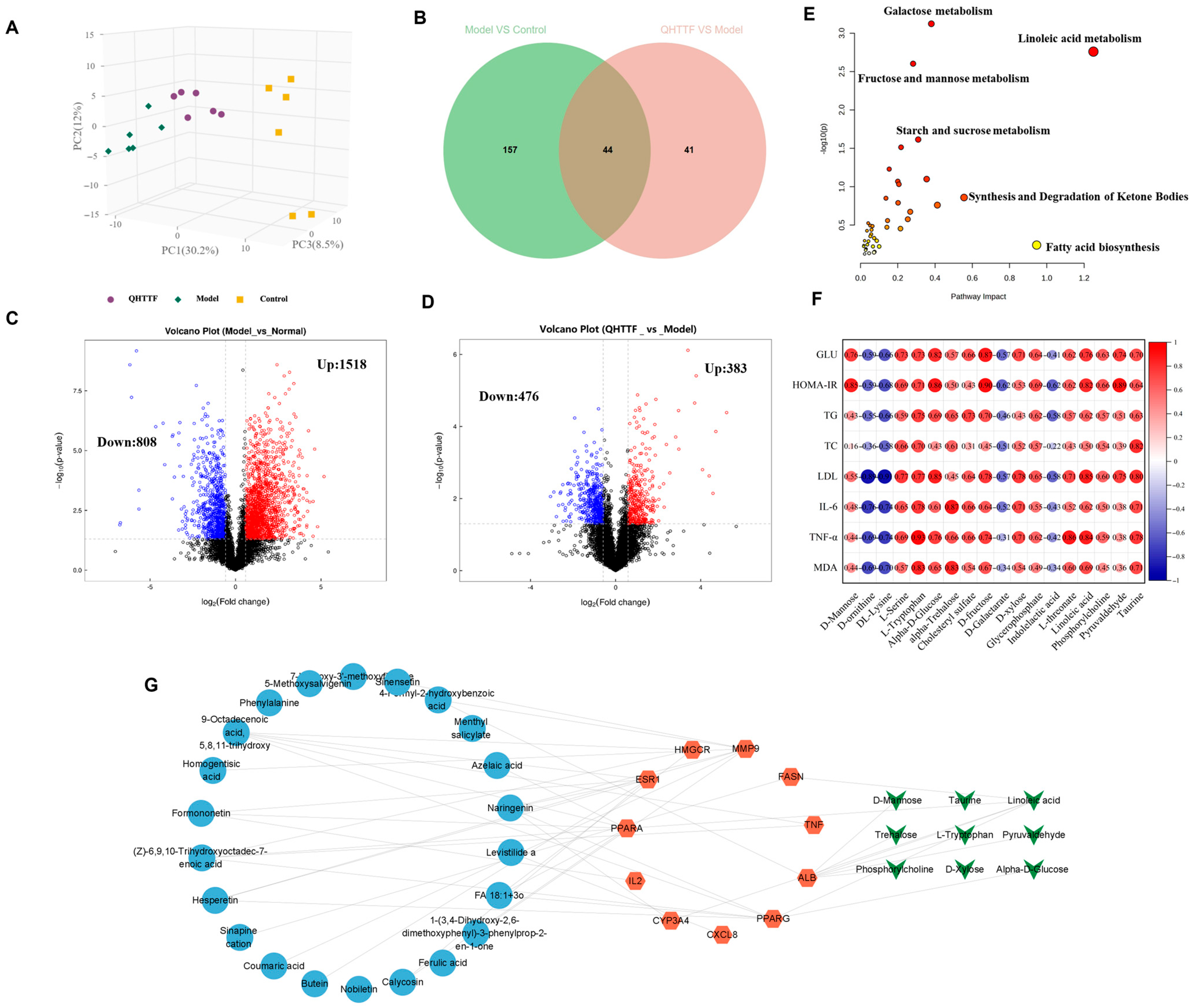
On the other hand, in the metabolomics results, we observed an imbalance of amino acids, fatty acids, and some gluconic acid in ZDF rats. QHTTF significantly decreased the levels of alpha-D-glucose, D-fructose, linoleic acid, D-xylose, and Pyruvaldehyde and increased the levels of D-Galactarate, Indolelactic acid, and DL-Lysine, among others. Subsequently, integrated analysis of the intersecting targets and differential metabolites revealed that they were primarily enriched in pathways including linoleic acid, fructose and mannose, galactose, fatty acid biosynthesis, and synthesis and degradation of ketone bodies. Recent studies also show that the dysregulation of linoleic acid metabolism, D-fructose, and D-glucose metabolism was associated with type 2 diabetes risk, and their excessive accumulation could lead to metabolic disturbances in T2DM [
31,
32]. Meanwhile, galactose and glycerolipid metabolisms were the primary disrupted pathways in diabetic conditions based on clinical trials [
33]. Additionally, the heatmap revealed that most of the metabolites exhibited a positive correlation with the levels of blood glucose, lipids, inflammatory factors, and oxidative markers. Hence, by integrating network pharmacology, metabolomics, and previous pharmacological research, we hypothesized that the main ingredients of QHTTF may regulate PPAR-associated targets, AMPK, and insulin signaling proteins. This regulation could influence α-D-glucose metabolism, phosphorylcholine, linoleic acid metabolism, and D-galactarate metabolism, thereby mitigating inflammation reactions, oxidative stress, and insulin resistance to ultimately influence glucose and lipid metabolism in ZDF rats.
Consequently, to investigate the underlying mechanism of QHTTF, we performed biological experiments to confirm the results of the network analysis above. Western blot analysis suggested that QHTTF might upregulate the expression of PPARγ and PPARα, and increase the phosphorylation (p-AMPK) and total levels of AMPK. Peroxisome proliferator-activated receptors (PPARs) are important ligand-induced transcription factors that play crucial roles in glucose-lipid metabolism [
34]. PPAR receptors are involved in regulating lipid and carbohydrate metabolism, homeostasis, and cellular processes such as proliferation, differentiation, and inflammation [
35,
36]. Within the PPAR family, three isoforms have been identified: PPARα, PPARγ, and PPARβ/δ. The role of PPARβ/δ has received less attention in research due to its limited clinical association with metabolic diseases. PPARα is mainly expressed in hepatic and skeletal muscle tissues, making a significant impact on regulating lipid catabolism [
37]. Activation of PPARα promotes fatty acid oxidation and reduces blood lipid levels. Meanwhile, PPARγ is one of the primary targets for regulating glucose and lipid in the liver, and it serves as a key modulator of systemic lipid metabolism and insulin sensitivity [
38]. In addition, PPARγ agonists can effectively treat diabetes and lipid metabolism disorders as insulin sensitizers. Moreover, PPARγ activation can regulate the PI3K/Akt insulin signaling pathway, which enhances insulin sensitivity, inhibits lipogenesis, and suppresses glucagon secretion, thereby improving glycolipid metabolism [
39,
40]. Notably, modulating the AMPK/Sirt1/PPARγ pathway has been shown to alleviate glucolipid metabolism disorders in fatty liver mice triggered by high-fat and high-fructose diets, thereby exerting hepatoprotective, anti-hyperlipidemic, and anti-inflammatory effects [
41]. AMP-activated protein kinase is a central regulator of cellular energy balance and a master sensor of lipid and glucose metabolism [
42]. AMPK is activated by phosphorylation at Thr172 (p-AMPK). Once activated, it can regulate multiple metabolic pathways, including promoting fatty acid oxidation and glycolysis, inhibiting fatty acid synthesis, reducing inflammation, and responding to nutrient stress to enhance insulin sensitivity [
43]. Furthermore, in vivo studies confirm that sustained AMPK activation could mediate the metabolic effects of PPARβ/δ and play a crucial role in glucose and lipid metabolism, as well as improve hepatic function [
44].
Another finding of our study was that QHTTF and metformin significantly increased the expression of IRS-1 and GLUT4 proteins in the pancreas. Both of them are essential proteins in the insulin signaling pathway. Insulin receptor substrate-1 (IRS-1) serves as a substrate for the insulin receptor tyrosine kinase and plays a critical role in insulin signaling. When insulin binds to its receptor, it triggers IRS-1 phosphorylation, which subsequently activates the PI3K and AKT targets, thereby promoting glucose transport into cells, regulating glucose metabolism, and maintaining normal blood glucose levels [
45]. Studies have indicated that IRS-1 can coordinate skeletal muscle metabolism via the AKT and AMPK pathways. In T2DM models, the expression of p-AMPK/AMPK, IRS-1, and GLUT4 proteins also showed a similar trend, contributing to improved insulin sensitivity and glucolipid metabolism [
46,
47]. Additionally, in hepatic tissues, insulin can suppress gluconeogenesis and lipolysis through IRS-1, while simultaneously promoting fatty acid synthesis and the secretion of low-density lipoprotein (LDL), thereby maintaining a healthy balance of blood lipids [
48]. GLUT4 is an insulin-regulated glucose transporter, primarily responsible for the uptake of glucose into adipose and muscle cells, playing an essential role in glucose homeostasis. When insulin binds to receptors on skeletal muscle and adipocyte surfaces, it activates the PI3K-AKT pathway, facilitating the translocation and fusion of GLUT4 with the plasma membrane, thereby enabling efficient glucose uptake [
49]. The dysfunction or deficiency of GLUT4 can reduce cellular sensitivity to insulin, resulting in insulin resistance and subsequent disturbances in glucose metabolism [
50,
51]. Therefore, the inhibition of IRS-1 and GLUT4 due to various factors can result in insulin resistance and disruptions in glucose and lipid homeostasis. This study suggested that QHTTF treatment may regulate insulin resistance by upregulating IRS-1 and GLUT4 expression in pancreatic tissues, potentially indicating modulation of the insulin signaling pathway.
Nevertheless, there are also some limitations to our research that need further improvement. First, the lack of blinding during drug administration and outcome assessment could introduce potential bias. Secondly, the active ingredients of QHTTF can be identified by analyzing drug-containing serum in animal experiments, which might better reflect the biologically active components. This study was only a preliminary exploration of pharmacodynamics, and the pharmacokinetic characteristics should be explored in subsequent research. Next, we employed an untargeted approach but lacked targeted validation for key metabolites. Future studies should employ targeted mass spectrometry to confirm the absolute concentrations and changes of these metabolites, which is essential for strengthening our conclusions and enhancing the translational relevance of our findings. Moreover, other results of network and metabolic enrichment analyses could be further examined to elucidate the mechanism of QHTTF in treating glucolipid metabolism disorders. Finally, and equally important, we did not set up dosage groups for the TCM formula (QHTTF) in this study. This decision was based on the high cost and the results of preliminary experiments, which showed no clear dose-dependent effect, with the moderate dose yielding the best therapeutic outcome. However, the findings of our study are limited to the selected dosage. Further validation of the dose–response relationship via multi-dose gradient experiments is needed in future studies. At the same time, we are considering conducting the cellular experiments on QHTTF-related signaling pathways to elucidate the underlying mechanisms. This remains a considerable potential area for future investigation.
4. Materials and Methods
4.1. QHTTF Preparation
Huangqi (2304305), Baizhu (2210008), Chaihu (2304019), Zhishi (2303087), Danggui (2305003), Chuanxiong (2303011), Zhuling (2207014), and Laifuzi (2302021) were all purchased from Beijing Yanjing Pharmaceutical Co., Ltd. (Beijing, China) and used at a proportion of 5:4:4:3:3:3:2:2. All herbs were further prepared as decoctions, which were immersed in purified water for one hour, and boiled twice over medium and low heat. The herbal decoction was strained with gauze, and the filtrate was collected, then concentrated to 1 g/mL of raw drugs and refrigerated at 4 °C. The daily dosage of QHTTF administered to rats is based on the clinical daily prescription amount for adult humans: (78 g raw herbs/60 kg body weight) × 6.3 (The multiplication factor of 6.3 is a standard constant used to adjust the dosage from humans to rats). In addition, according to our preliminary experiments, the medium dose of QHTTF administered via gavage to diabetic rats was determined to be the optimal dose. Therefore, additional dose groups were not included in this study.
4.2. Instrumentation and Reagents
An automatic biochemical analyzer from Japan (Toshiba, TBA40FR, Kanagawa, Japan), a blood glucose meter (ACCU-CHEK Performa, Roche Diagnostics GmbH, Mannheim, Germany), Gamma radioimmunoassay counter (XH-6080, Xi’an, China), fully automatic microplate reader (MULTISKAN MK3, Thermo, Waltham, MA, USA), Benchtop High-Speed Refrigerated Centrifuge (Allegra X-30R, Beckman Coulter, Inc., Brea, CA, USA), Electric Incubator (Tianjin Taisite, DH4000A, Tianjin, China), Electrophoresis Apparatus (Servicebio BV-2, Servicebio BT-2, Wuhan, Hubei, China), Chemiluminescent Imaging and Analysis System (JUNYI MINICHEMI, Beijing, China), 3DHISTECH (Budapest, Hungary) Pannoramic (Desk/Midi/250/1000), AB Triple SCIEX TOF 6600 mass spectrometer system (SCIEX. Ltd., Shanghai, China), and Ultra-High Performance Liquid Chromatography (UHPLC) System (Thermo Scientific, Waltham, MA, USA) were used in this study.
The FBG (no. B2007), TG (no. B2001), T-CHO (no. B2002), LDL-C (no. B2004), HDL(no. B2003), and FFA (no. B1101) reagents were all acquired from Beijing Beijian New Creative Source Biotechnology Co., Ltd. (Beijing, China). Insulin (no. F01PZA) was purchased from the Beijing Northern Biotechnology Research Institute (Beijing, China). The IL6 ELISA kit (no. EMC003.96) and TNF-a ELISA (no. EMC102a.96) were obtained from NeoBioscience Technology Co., Ltd. (Beijing, China). The SOD kit (no. A001-3-2) and the MDA (no. A003-1) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Furthermore, HE and Oil Red O staining solutions (G1016, Servicebio, Wuhan, China) were also utilized in this research.
4.3. The Chemical Constituent Analysis of QHTTF
The QHTTF decoction was prepared, and the sample (600 μL) was placed into a 1.5 mL Eppendorf tube. Next, we added 400 μL of pure methanol and vortexed the mixture for 10 s. Then, we took 200 μL of the aforementioned solution and added 200 μL of a 40% methanol aqueous solution and mixed for an additional 10 s. Finally, it was centrifuged for 15 min at 16,000× g at 4 °C, and we took the supernatant, which is the desired solution. For chromatographic analysis, a Vanquish UHPLC system with an ACQUITY HSS T3 analytical column (2.1 × 100 mm, 1.8 μm) was applied to separate the components at 35 °C. The flow rate was kept at 0.3 mL/min, and the mobile phase consisted of A (0.1% formic acid in water) with B (formic acid acetonitrile) to conduct further gradient elution. In addition, mass spectrometry data, including primary and secondary mass spectra, were analyzed by using a Q-Exactive HFX mass spectrometer integrated with the UHPLC platform. The positive and negative ionization modes were 3800 V (ESI+)/3500 V (ESI−), sheath gas flow 40 L/min, ion transport tube temperature 320 °C, and atomization temperature 350 °C, respectively.
Data analysis was conducted through XCMS software 4.6.0 for peak matching, RT adjustment, and peak area extraction. Compounds were identified by searching the local TCM high-resolution mass spectrometry database of the Institute of Biophysics, Chinese Academy of Sciences, with a primary mass error of less than 25 ppm and a secondary fragment spectrum match score greater than 0.7. Currently, it is widely accepted that a score above 0.7 ensures reliable identification.
4.4. QHTTF Network Pharmacology Analysis
The Herb Pharmacology database (HERB) (
http://herb.ac.cn/, accessed on 6 March 2024), SwissTarget Prediction (
http://www.swisstargetprediction.ch/, accessed on 6 March 2024), uniport data (
https://www.uniprot.org/, accessed on 9 March 2024), and Swiss ADME (
http://www.swissadme.ch/, accessed on 9 March 2024) were used to identify the targets of QHTTF, which are based on the main chemical components obtained from mass spectrometry and the Rule of Five principles (Lipinski, Ghose, Veber, Egan, and Muegge). At the same time, the selected compounds fulfilled two or more of the five criteria that were evaluated as “yes” and exhibited a high GI absorption rating. Subsequently, the potential targets of T2DM combined with hyperlipidemia or lipid metabolism disorders were searched from the Drug Bank (
http://go.Drugbank.com/, accessed on 13 March 2024), OMIM (
https://omim.org/, accessed on 13 March 2024), and Gene Cards (
https://www.genecards.org/, accessed on 13 March 2024 ) databases. Then, all duplicates were removed, and the intersection targets along with genes of QHTTF and T2DM with hyperlipidemia were identified and visualized by a Venn diagram from the Bioinformatics web platform (
https://www.bioinformatics.com.cn, accessed on 16 March 2024). Moreover, the screened overlapping targets and genes were submitted to the String database (
https://string-db.org/, accessed on 16 March 2024) to build the PPI network and then visualized through Cytoscape 3.9.1 software. Finally, to verify the functions of the intersected genes, Gene Ontology and Kyoto Encyclopedia of Genes and Genomes (GO and KEGG) pathway enrichment analyses were conducted using the DAVID database (
https://david.ncifcrf.gov, accessed on 18 March 2024), and the 20 most enriched pathways were represented through the Bioinformatics analysis platform.
4.5. Molecular Docking Analysis
The active components of QHTTF were identified through LC-MS analysis and prioritized via Lipinski’s Rule of Five and docking scores. Molecular docking against core therapeutic targets, including PPARα, PPARγ, and TNF-α, was performed using AutoDock Vina 1.5.6, with protein structures modeled via SWISS-MODEL (PDB format) and ligands prepared from public databases (SDF format). The most frequent low-energy conformations were selected and visualized using PyMOL 3.1 and Discovery Studio 2019, with the best binding and conformational robustness effect for output.
4.6. Animals and Drug Administration
Male and SPF-grade diabetic obese rats (Zucker Diabetic Fatty rats, ZDF) of 8 weeks of age were utilized in our study, which were purchased from Beijing Vital River Biotech Co., Ltd. (Beijing, China) and induced by high-fat diets (K5008) for 6 weeks as the research subjects. The high-fat diet K5008 (protein 26.84%, fat 16.71%, and carbohydrates 56.44%) was obtained from Beijing Keao Xieli feed Co., Ltd. (Beijing, China). The rats were housed at the experimental animal center of the Institute of Basic Theory of Traditional Chinese Medicine of the Chinese Academy of Chinese Medical Sciences under conditions of 20–26 °C, 40–70% relative humidity, with a 12 h light/dark cycle and free access to drinking water. In total, 32 ZDF rats were randomly assigned to four groups by an independent third party using a random number table generated through computer—CON (healthy control), M (diabetic model), QHTTF (traditional Chinese medicine), and MET (metformin) groups. Rats in the QHTTF group were given 10 mL/kg of Chinese herb remedy and 10 mL/kg metformin solution in the western medicine group, according to the clinical dose conversion for humans, while rats were offered the same volume of saline in the CON and Model groups. After 8 weeks of Chinese medicine treatment, the rats were sacrificed with pentobarbital. Then, the rats’ serum was collected and preserved at −80 °C, and the liver and pancreas were extracted, packaged, and rapidly fixed in 4% paraformaldehyde. Subsequently, various biochemical indicators and molecular biology tests were conducted. This experimental protocol was approved by the Ethics Committee of the Chinese Academy of Chinese Medical Sciences, approval number: IBTCMCACMS21-2303-01.
4.7. Metabolomics Research and Metabolite Analysis
The APT-BioCloud platform (Shanghai, China) was used for untargeted metabolomics profiling. Briefly, the frozen plasma samples were thawed at 4 °C, and then an appropriate amount of sample was mixed with a cold solution of methanol, acetonitrile, and water (in a 2:2:1 ratio), vortexed well, sonicated at low temperature for 30 min, spun in a centrifuge at 14,000× g at 4 °C for 15 min, and the supernatant was collected for injection and analyzing metabolites.
The serum samples in the CON, M, and QHTTF groups were chromatographically separated via an Agilent 1290 UHPLC system equipped with a HILIC column (25 °C, 0.5 mL/min flow rate, 2 μL injection volume). The mobile phase consisted of (A) 25 mM ammonium acetate/ammonia water and (B) acetonitrile, with the following gradient profile: 0–0.5 min (95% B), 0.5–7 min (65% B), 7–8 min (40% B), 8–9 min (isocratic 40% B), 9–9.1 min (95% B), and 9.1–12 min (isocratic 95% B). Meanwhile, all samples were maintained at 4 °C in the autosampler, and QC samples were inserted into the sample queue to monitor and evaluate the stability of the system and the reliability of the experimental data. An AB Triple 6600 mass spectrometer was applied for the acquisition of the first- and second-level mass spectra of the samples, after separation with Agilent 1290 UHPLC. At the same time, Electrospray Ionization (ESI) was used in both positive and negative ion modes. Moreover, the ESI source parameters were set as follows: the ion source temperature was 600 °C, and the spray voltage (ISVF) was ±5500 V for both positive and negative modes.
The data obtained from UPLC-Q-TOF-MS underwent peak detection, RT adjustment, and peak extraction via XCMS software. Then, the quality of the experimental data was evaluated and analyzed after metabolite structural identification and data preprocessing. Following peak extraction, the resulting data matrix underwent preprocessing to ensure quality and reliability. Missing values, largely resulting from ion intensities below the detection limit, were imputed using the k-nearest neighbors (KNN) algorithm. To reduce systematic variations between samples, the data were normalized by the probabilistic quotient normalization (PQN) method to balance the influence of high and low-abundance metabolites in multivariate statistical modeling. The VIP scores from OPLS-DA (threshold > 1) combined with p-values (<0.05) were selected to identify the differential metabolites in this research.
4.8. Animal Experiments
4.8.1. General Biochemical Analysis
The rats’ blood samples in all groups were collected via the abdominal aorta at the 8th week and followed by centrifugation at 4 °C (3000 r, 15 min). Next, the serum levels of fasting blood glucose (FBG) and 2 h postprandial blood glucose (2 h FG), total cholesterol (TC), triglycerides (TG), free fatty acid (FFA), low-density lipoprotein cholesterol (LDL-C), and HDL-C levels were measured via an automated biochemical analyzer. The insulin bioactivity (INS) was calculated through radioimmunoassay following the manufacturer’s guidelines, while insulin sensitivity was assessed using the HOMA-IR index: [Fasting insulin (μIU/mL) × Fasting glucose]/22.5.
4.8.2. Body Weight and Liver Index
The body mass and daily consumption (food and water) of the different groups of the ZDF rats were determined and recorded every two weeks, and the body weight, liver weight were analyzed at the 0th and 8th weeks. After 8 weeks’ medicine therapy, the body weight was again measured and followed by anesthesia based on their weight. Blood was collected from the abdominal aorta, the liver tissue was isolated, cleaned, and dried, and then weighed. Liver weight index = liver tissue (g)/weight (g) × 100%. Subsequently, the liver was placed in paraformaldehyde for fixation to perform pathology analysis.
4.8.3. Oral Glucose Tolerance Test (OGTT)
The different groups of rats were subjected to an OGTT after a 15 h fasting period and administered a 50% glucose solution by gavage (2 g/kg body weight). The rats’ serum was taken from the tail vein later at 0, 30, 60, 90, and 120 min by using an ACCU-CHEK Performa glucose meter to monitor the glucose levels.
4.8.4. Histopathological Observation and Analysis
After blood collection via abdominal aorta puncture, pancreas and liver tissue were isolated from different groups of rats and fixed in 4% paraformaldehyde. Then washed via saline solution and immersed in 75% ethanol overnight, and afterward, tissue trimming, dehydration, embedding with paraffin, sectioning, and H&E staining were carried out, followed by mounting for microscopic observation. In addition, frozen sections of the rat liver and pancreas tissues were performed using an optical coherence tomography (OCT) compound to embed, with a section thickness of 8 µm, then incubated with Oil Red O staining solution and counterstained with hematoxylin to evaluate for any pathological alterations.
4.8.5. Inflammatory Cytokine and Antioxidant Capability Detection
The blood samples that were collected from different ZDF rat groups were centrifuged and separated at 3000 rpm for 15 min. The obtained sera were used to assess the levels of inflammatory factors and oxidative stress. The concentrations of TNF-α and IL-6 factors were determined using ELISA kits from NeoBioscience Technology Co., Ltd (Shenzhen, China). The SOD activity and MDA contents in serum were quantified with detection kits from Nanjing Jiancheng Institute to evaluate the antioxidant capability of drugs. All the experiments were executed following the manufacturer’s guidelines.
4.8.6. Immunohistochemical Detection of Insulin Pathway-Related Proteins
The pancreas tissue isolated from different rat groups was preserved in 4% paraformaldehyde, followed by embedding in paraffin, and sectioned with 5 μm for an immunohistochemical (IHC) staining experiment. For each rat, three non-consecutive sections from the pancreas were analyzed, and four random, non-overlapping fields of view (20× magnification) from each section were captured and quantified. The sections were incubated with the IRS-1 (Servicebio, GB111506, Wuhan, Hubei, China) and GLUT4 (Immunoway, YT5523, Beijing, China) antibodies at 4 °C overnight after dewaxing, antigen retrieval, endogenous enzyme inhibition, and serum blocking procedure. Subsequently, the sections were exposed to horseradish peroxidase (HRP)-linked secondary antibodies for 1 h at 37 °C, then stained with a DAB kit and counterstained with hematoxylin, dehydrated, and mounted. Ultimately, the slices were viewed and scanned under Pannoramic MIDI microscope and analyzed with Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville Pike, MA, USA).
4.8.7. Western Blot Analysis
The homogenates of hepatic tissues (50 mg) from different ZDF rat groups were dissolved on ice in RIPA lysate (1:10) and protease inhibitors to extract relevant proteins. Then, the total protein extract was centrifuged for 10 min (12,000 rpm, 4 °C) to obtain the supernatant, and the protein concentration was quantified with a BCA assay kit (Solarbio, Beijing, China). The samples (within total protein-30 μg) were separated by SDS-PAGE and electroblotted onto PVDF membranes (Millipore, Bedford, MA, USA) for 40 min, which were incubated later in TBST containing skim milk (Servicebio Technology, Wuhan, China) for 1 h at 37 °C. Subsequently, the PPARα (Immunoway, YT3835, Beijing, China), PPARγ (Proteintech, 16643-1-AP, Wuhan, China), AMPK (Immunoway, YT0216, Beijing, China), and p-AMPK (Immunoway, YP0575) antibodies were added (diluted 1:1000) and reacted at 4 °C all night. The β-actin (ABclonal, AC026, Wuhan, China) was diluted 1:100,000 and incubated overnight at 4 °C. Following that, the protein band intensities were quantified using Image J software (
https://imagej.net/ij/) and normalized to the β-actin loading control. The next day, the secondary antibody was diluted in TBST (1:10,000) and incorporated into the protein bands, incubated for 60 min at room temperature, and then ECL luminescent liquid was added to expose and image through Chemiluminescent Gel Imaging and Analysis System (JY-Clear ECL, Beijing Junyi Oriental Co., Ltd., Beijing, China).
4.9. Statistical Analysis
The results were expressed as the mean ± standard deviation () and analyzed using SPSS 26.0 software. One-way analysis of variance (ANOVA) was performed to determine statistical significance in comparison with the respective control for each experiment. GraphPad Prism 9.5.1 software was utilized to visualize the statistical data, and p-value < 0.05 was regarded as statistically significant.