Plasma Glutathione Concentrations Are Associated with Leukocyte and Neutrophils’ Counts in Clozapine-Treated Patients
Abstract
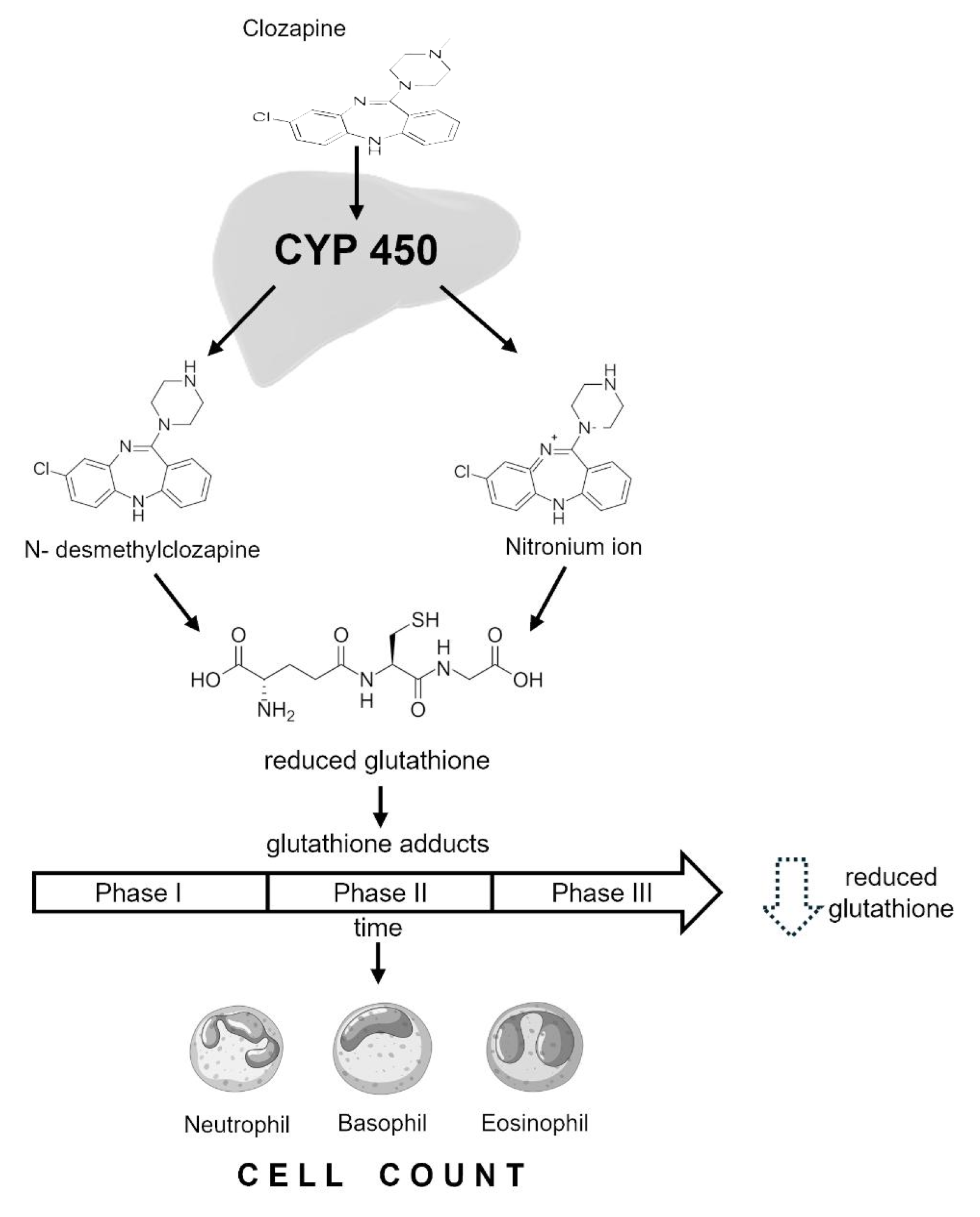
1. Introduction
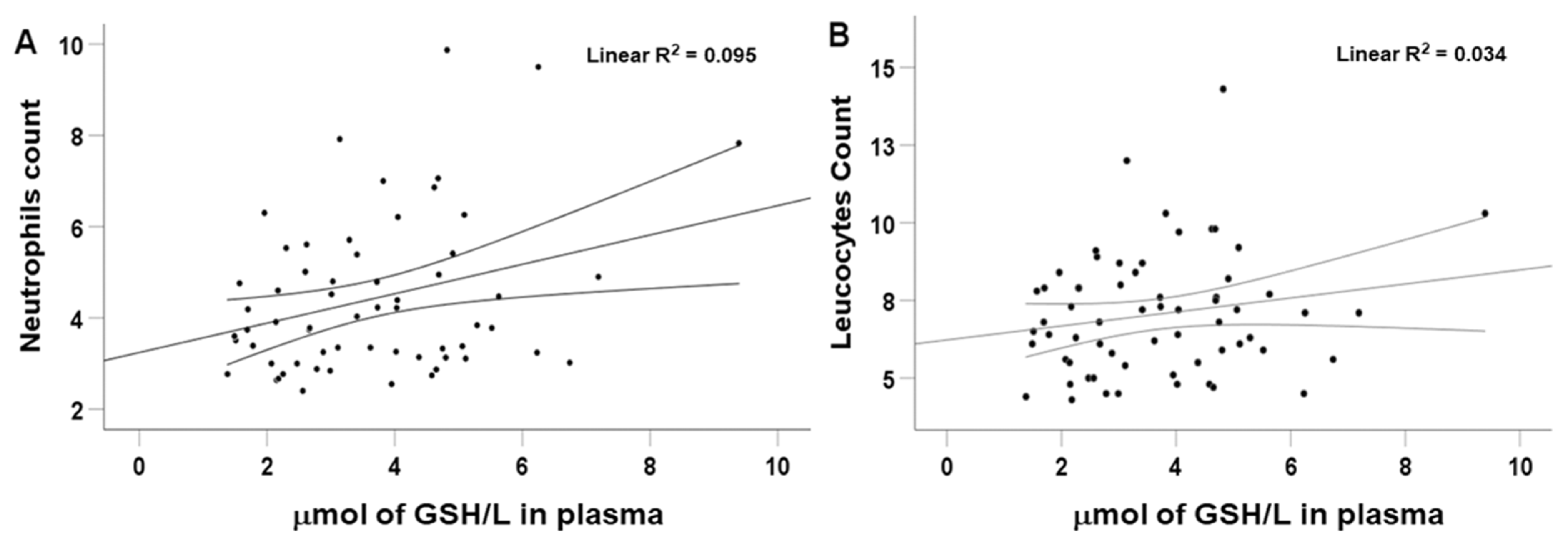
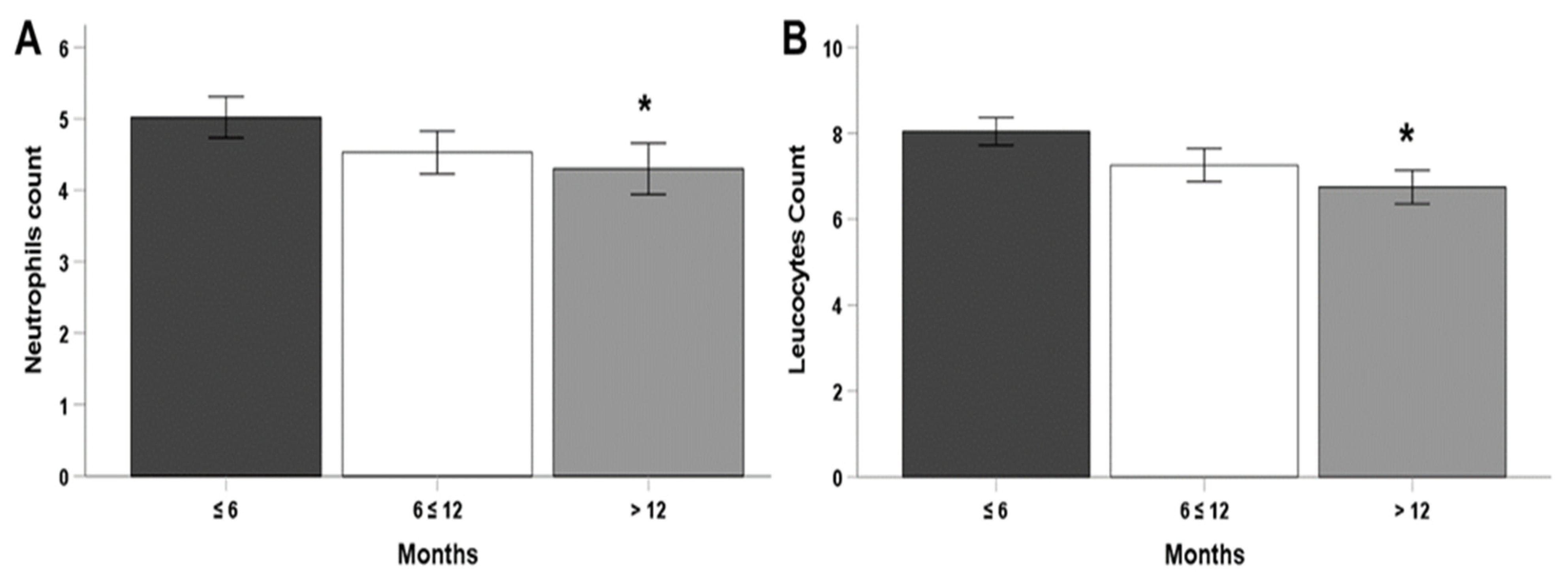
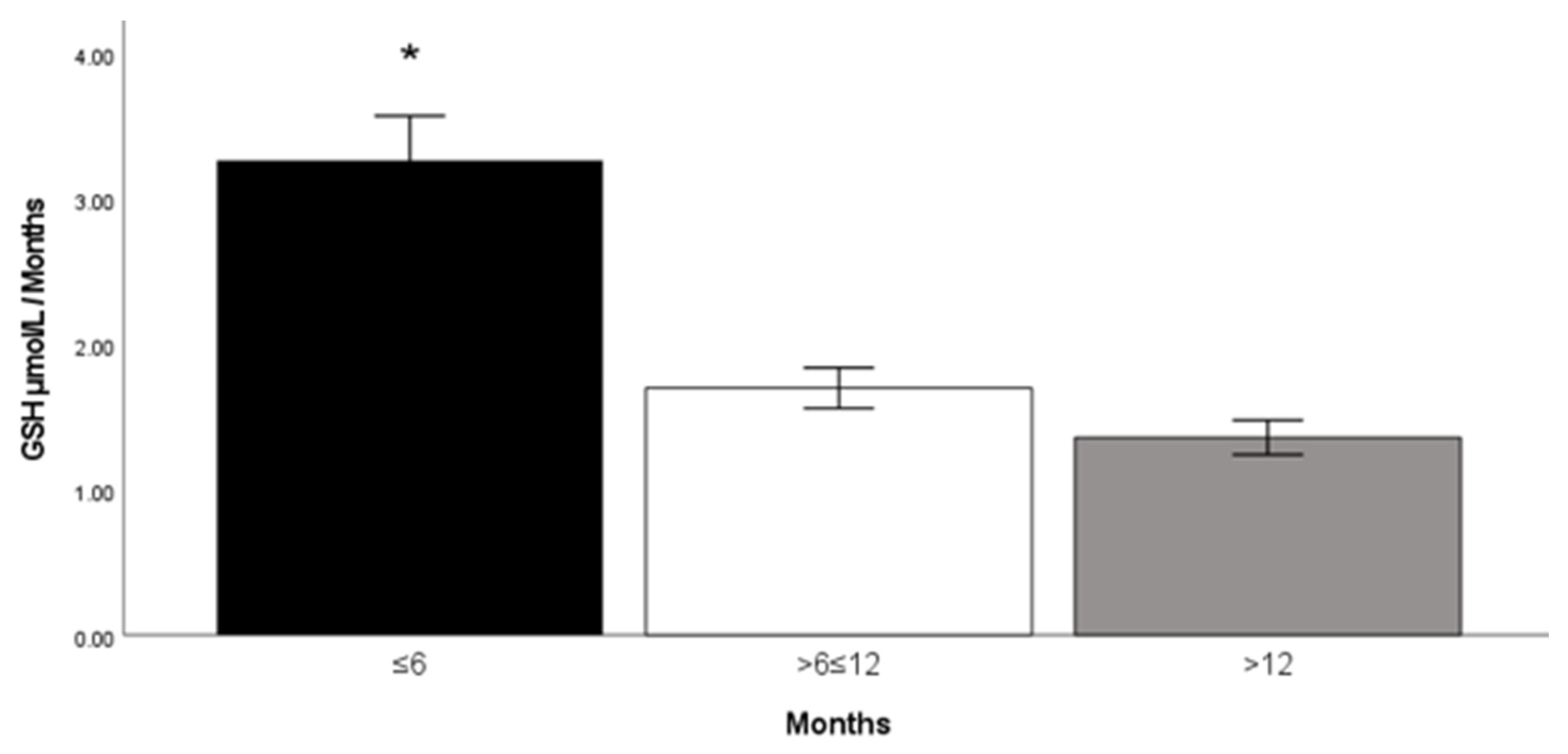
2. Results
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Study Design and Population
4.2. Determination of Plasma Clozapine and N-Desmethylclozapine
4.3. Determination of Reduced Glutathione (GSH)
4.4. Hematological Analysis
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Mental Health—Our World in Data. Mental Health. 2020. Available online: https://ourworldindata.org/mental-health (accessed on 6 January 2020).
- Murray, C.J.; Lopez, A.D. Evidence-based health policy-lessons from the Global Burden of Disease Study. Science 1996, 274, 740–743. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5); American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Mayoral-van Son, J.; de la Foz, V.O.; Martinez-Garcia, O.; Moreno, T.; Parrilla-Escobar, M.; Valdizan, E.M.; Crespo-Facorro, B. Clinical outcome after antipsychotic treatment discontinuation in functionally recovered first-episode nonaffective psychosis individuals: A 3-year naturalistic follow-up study. J. Clin. Psychiatry 2016, 77, 492–500. [Google Scholar] [CrossRef]
- Bronstein, A.C.; Spyker, D.A.; Cantilena, L.R., Jr.; Green, J.L.; Rumack, B.H.; Giffin, S.L. 2008 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 26th Annual Report. Clin. Toxicol. 2009, 47, 911–1084. [Google Scholar] [CrossRef]
- Mowry, J.B.; Spyker, D.A.; Cantilena, L.R., Jr.; Bailey, J.E.; Ford, M. 2012 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin. Toxicol. 2013, 51, 949–1229. [Google Scholar] [CrossRef]
- Diniz, E.; Fonseca, L.; Rocha, D.; Trevizol, A.; Cerqueira, R.; Ortiz, B.; Brunoni, A.R.; Bressan, R.; Correll, C.U.; Gadelha, A. Treatment resistance in schizophrenia: A meta-analysis of prevalence and correlates. Braz. J. Psychiatry 2023, 45, 448–458. [Google Scholar] [CrossRef]
- Beck, K.; McCutcheon, R.; Stephenson, L.; Schilderman, M.; Patel, N.; Ramsay, R.; Howes, O.D. Prevalence of treatment-resistant psychoses in the community: A naturalistic study. J. Psychopharmacol. 2019, 33, 1248–1253. [Google Scholar] [CrossRef]
- Correll, C.U.; Martin, A.; Patel, C.; Benson, C.; Goulding, R.; Kern-Sliwa, J.; Joshi, K.; Schiller, E.; Kim, E. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. Schizophrenia 2022, 8, 5. [Google Scholar] [CrossRef]
- Agid, O.; Crespo-Facorro, B.; de Bartolomeis, A.; Fagiolini, A.; Howes, O.D.; Seppälä, N.; Correll, C.U. Overcoming the barriers to identifying and managing treatment-resistant schizophrenia and to improving access to clozapine: A narrative review and recommendation for clinical practice. Eur. Neuropsychopharmacol. 2024, 84, 35–47. [Google Scholar] [CrossRef] [PubMed]
- de Leon, J.; Schoretsanitis, G.; Smith, R.L.; Molden, E.; Solismaa, A.; Seppälä, N.; Kopeček, M.; Švancer, P.; Olmos, I.; Ricciardi, C. An International Adult Guideline for Making Clozapine Titration Safer by Using Six Ancestry-Based Personalized Dosing Titrations, CRP, and Clozapine Levels. Pharmacopsychiatry 2022, 55, 73–86. [Google Scholar] [CrossRef]
- Mijovic, A.; MacCabe, J.H. Clozapine-induced agranulocytosis. Ann. Hematol. 2020, 99, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Siskind, D.; Sidhu, A.; Cross, J.; Chua, Y.T.; Myles, N.; Cohen, D.; Kisely, S. Systematic review and meta-analysis of rates of clozapine-associated myocarditis and cardiomyopathy. Aust. N. Z. J. Psychiatry 2020, 54, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.J.; Hill, R.; Krum, H.; Liew, D.; Tonkin, A.; Demos, L.; Stephan, K.; McNeil, J. Clozapine-associated myocarditis: A review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003. Drug Saf. 2007, 30, 47–57. [Google Scholar] [CrossRef]
- Bai, Y.M.; Lin, C.C.; Chen, J.Y.; Lin, C.Y.; Su, T.P.; Chou, P. Association of initial antipsychotic response to clozapine and long-term weight gain. Am. J. Psychiatry 2006, 163, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Meyer, J.M. Risk factors for ileus in patients with schizophrenia. Schizophr. Bull. 2012, 38, 592–598. [Google Scholar] [CrossRef]
- D’Arrigo, T. Updated Clozapine REMS Program Requires Certification With Few Exceptions. Psychiatr. News 2019, 54. [Google Scholar] [CrossRef]
- Alvir, J.M.; Lieberman, J.A.; Safferman, A.Z.; Schwimmer, J.L.; Schaaf, J.A. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N. Engl. J. Med. 1993, 329, 162–167. [Google Scholar] [CrossRef]
- Lambertenghi-Deliliers, G. Blood dyscrasias in clozapine-treated patients in Italy. Haematologica 2000, 85, 233–237. [Google Scholar]
- Raffa, M.; Mechri, A.; Othman, L.B.; Fendri, C.; Gaha, L.; Kerkeni, A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1178–1183. [Google Scholar] [CrossRef]
- Myles, N.; Myles, H.; Xia, S.; Large, M.; Kisely, S.; Galletly, C.; Bird, R.; Siskind, D. Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatr. Scand. 2018, 138, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Remington, G.; Lee, J.; Agid, O.; Takeuchi, H.; Foussias, G.; Hahn, M.; Fervaha, G.; Burton, L.; Powell, V. Clozapine’s critical role in treatment resistant schizophrenia: Ensuring both safety and use. Expert. Opin. Drug Saf. 2016, 15, 1193–1203. [Google Scholar] [CrossRef]
- Gerson, S.L.; Arce, C.; Meltzer, H.Y. N-desmethylclozapine: A clozapine metabolite that suppresses haemopoiesis. Br. J. Haematol. 1994, 86, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Uetrecht, J.P. Metabolism of Clozapine by Neutrophils: Possible Implications for Clozapine-Induced Agranulocytosis. Drug Saf. 1992, 7, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.P.; Pirmohamed, M.; Naisbitt, D.J.; Uetrecht, J.P.; Park, B.K. Induction of metabolismdependent and -independent neutrophil apoptosis by clozapine. Mol. Pharmacol. 2000, 58, 207–216. [Google Scholar] [CrossRef]
- Chen, A.T.; Chibnall, J.T.; Nasrallah, H.A. Placebo-controlled augmentation trials of the antioxidant NAC in schizophrenia: A review. Ann. Clin. Psychiatry 2016, 28, 190–196. [Google Scholar]
- Matsui, K.; Ishibashi, M.; Kawano, M.; Oshibuchi, H.; Ishigooka, J.; Nishimura, K.; Inada, K. Clozapine-induced agranulocytosis in Japan: Changes in leukocyte/neutrophil counts before and after discontinuation of clozapine. Hum. Psychopharmacol. 2020, 35, e2739. [Google Scholar] [CrossRef]
- Tang, Y.L.; Mao, P.X.; Jiang, F.; Chen, Q.; Wang, C.Y.; Cai, Z.J.; Mitchell, P.B. Clozapine in China. Pharmacopsychiatry 2008, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P. Risk of clozapine-associated agranulocytosis and mandatory white blood cell monitoring. Ann. Pharmacother. 2006, 40, 683–688. [Google Scholar] [CrossRef]
- Ballesteros, A.; Jiang, P.; Summerfelt, A.; Du, X.; Chiappelli, J.; O’Donnell, P.; Kochunov, P.; Hong, L.E. No evidence of exogenous origin for the abnormal glutathione redox state in schizophrenia. Schizophr. Res. 2013, 146, 184–189. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tan, Y.L.; Cao, L.Y.; Wu, G.Y.; Xu, Q.; Shen, Y.; Zhou, D.F. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr. Res. 2006, 81, 291–300. [Google Scholar] [CrossRef]
- Hendouei, N.; Farnia, S.; Mohseni, F.; Salehi, A.; Bagheri, M.; Shadfar, F.; Barzegar, F.; Hoseini, S.D.; Charati, J.Y.; Shaki, F. Alterations in oxidative stress markers and its correlation with clinical findings in schizophrenic patients consuming perphenazine, clozapine and risperidone. Biomed. Pharmacother. 2018, 103, 965972. [Google Scholar] [CrossRef]
- Wiciński, M.; Węclewicz, M.M. Clozapine-induced granulocytosis/ granulocytopenia: Mechanisms and monitoring. Curr. Opin. Helmitol. 2018, 25, 22. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef] [PubMed]
- Frahnert, C.; Rao, M.L.; Grasmäder, K. Analysis of eighteen antidepressants, four atypical antipsychotics and active metabolites in serum by liquid chromatography: A simple tool for therapeutic drug monitoring. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2003, 794, 35–47. [Google Scholar] [CrossRef]
- Llerena, A.; Berecz, R.; Norberto, M.J.; de la Rubia, A. Determination of clozapine and its Ndesmethyl metabolite by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr B. Biomed. Sci. Appl. 2001, 755, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Titier, K.; Bouchet, S.; Péhourcq, F.; Moore, N.; Molimard, M. High-performance liquid chromatographic method with diode array detection to identify and quantify atypical antipsychotics and haloperidol in plasma after overdose. J. Chromatogr B. Analyt. Technol. Biomed. Life Sci. 2003, 788, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Vaquero-Baez, M.; Díaz-Ruíz, A.; Tristán-López, L.; Aviña-Cervantes, C.; Torner, C.; Ramírez-Bermúdez, J.; Montes, S.; Ríos, C. Clozapine and desmethylclozapine: Correlation with neutrophils and leucocytes counting in Mexican patients with schizophrenia. BMC Psychiatry 2019, 19, 295. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]

| Variable | Women | Men | p |
|---|---|---|---|
| Weight (kg) | 65.47 ± 7.59 | 83.91 ± 15.03 | 0.00 |
| Age (years) | 37.9 ± 10 | 35.17 ± 9.68 | NS |
| Clozapine dosage (mg/day) | 255.88 ± 32.33 | 276.54 ± 28.15 | NS |
| Neutrophils (×103/mL) | 4.143 ± 1.566 | 4.735 ± 1.730 | NS |
| Leukocytes (×103/mL) | 6.708 ± 2.144 | 7.470 ± 1.918 | NS |
| Clozapine concentration (ng/mL) | 537.32 ± 82.31 | 447.144 ± 43.07 | NS |
| N-desmethylclozapine concentration (ng/mL) | 152.507 ± 32.1 | 173.96 ± 28.96 | NS |
| GSH concentration (μmol/L) | 4.13 ± 1.37 | 3.40 ± 1.66 | NS |
| Years of clozapine treatment | 6.47 ± 1.37 | 3.65 ± 0.61 | 0.04 |
| (β) | t | p | |
|---|---|---|---|
| Neutrophils | |||
| Clozapine plasma levels | 0.224 | 1.897 | 0.063 |
| GSH concentration | 0.327 | 2.691 | 0.009 |
| Phase | −0.291 | −2.443 | 0.018 |
| Leucocytes | |||
| GSH concentration | 0.265 | 2.205 | 0.031 |
| Phase | −0.384 | −3.199 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rodríguez, E.J.; Barón-Flores, V.; Ramirez-Bermudez, J.; Aviña-Cervantes, C.; González-Esquivel, D.; Diaz-Ruiz, A.; Ríos, C. Plasma Glutathione Concentrations Are Associated with Leukocyte and Neutrophils’ Counts in Clozapine-Treated Patients. Pharmaceuticals 2025, 18, 1345. https://doi.org/10.3390/ph18091345
Martínez-Rodríguez EJ, Barón-Flores V, Ramirez-Bermudez J, Aviña-Cervantes C, González-Esquivel D, Diaz-Ruiz A, Ríos C. Plasma Glutathione Concentrations Are Associated with Leukocyte and Neutrophils’ Counts in Clozapine-Treated Patients. Pharmaceuticals. 2025; 18(9):1345. https://doi.org/10.3390/ph18091345
Chicago/Turabian StyleMartínez-Rodríguez, Erick José, Verónica Barón-Flores, Jesús Ramirez-Bermudez, Carlos Aviña-Cervantes, Dinora González-Esquivel, Araceli Diaz-Ruiz, and Camilo Ríos. 2025. "Plasma Glutathione Concentrations Are Associated with Leukocyte and Neutrophils’ Counts in Clozapine-Treated Patients" Pharmaceuticals 18, no. 9: 1345. https://doi.org/10.3390/ph18091345
APA StyleMartínez-Rodríguez, E. J., Barón-Flores, V., Ramirez-Bermudez, J., Aviña-Cervantes, C., González-Esquivel, D., Diaz-Ruiz, A., & Ríos, C. (2025). Plasma Glutathione Concentrations Are Associated with Leukocyte and Neutrophils’ Counts in Clozapine-Treated Patients. Pharmaceuticals, 18(9), 1345. https://doi.org/10.3390/ph18091345








