Moringa (Moringa oleifera) Leaf Attenuates the High-Cholesterol Diet-Induced Adverse Events in Zebrafish: A 12-Week Dietary Intervention Resulted in an Anti-Obese Effect and Blood Lipid-Lowering Properties
Abstract
1. Introduction
2. Results
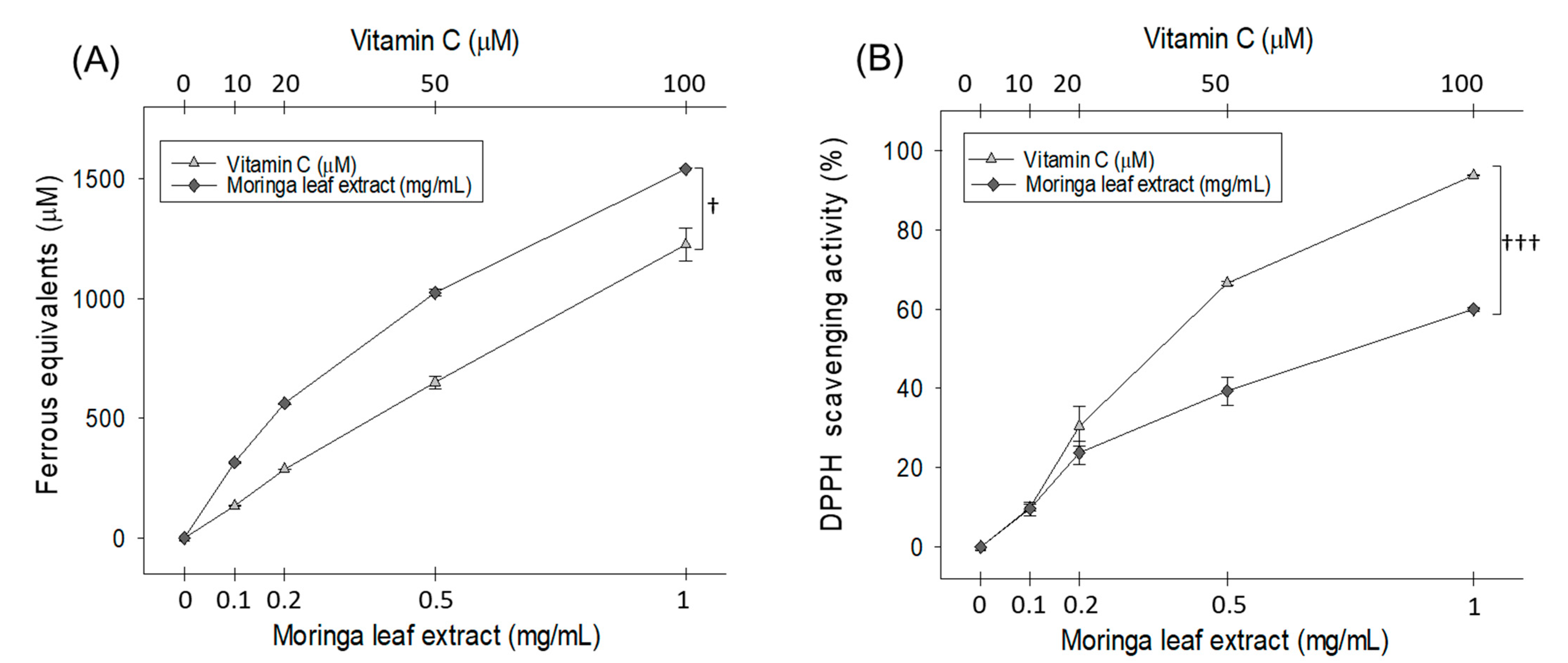
2.1. In Vitro Antioxidant Activity
2.2. Moringa Rescues Zebrafish Embryos from the CML-Induced Oxidative Stress
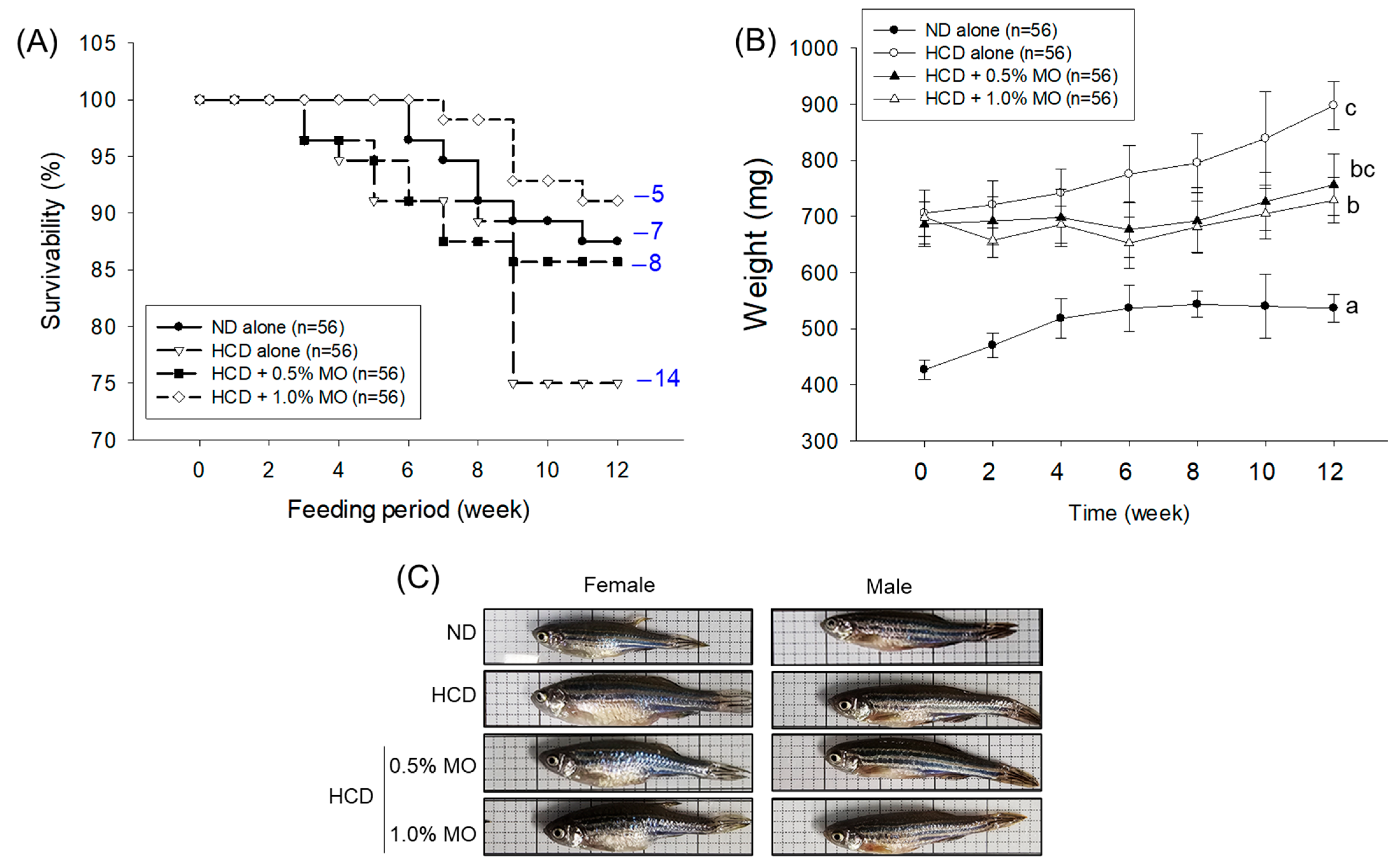
2.3. Effect of Moringa on Hyperlipidemic Zebrafish Survivability and Body Weight
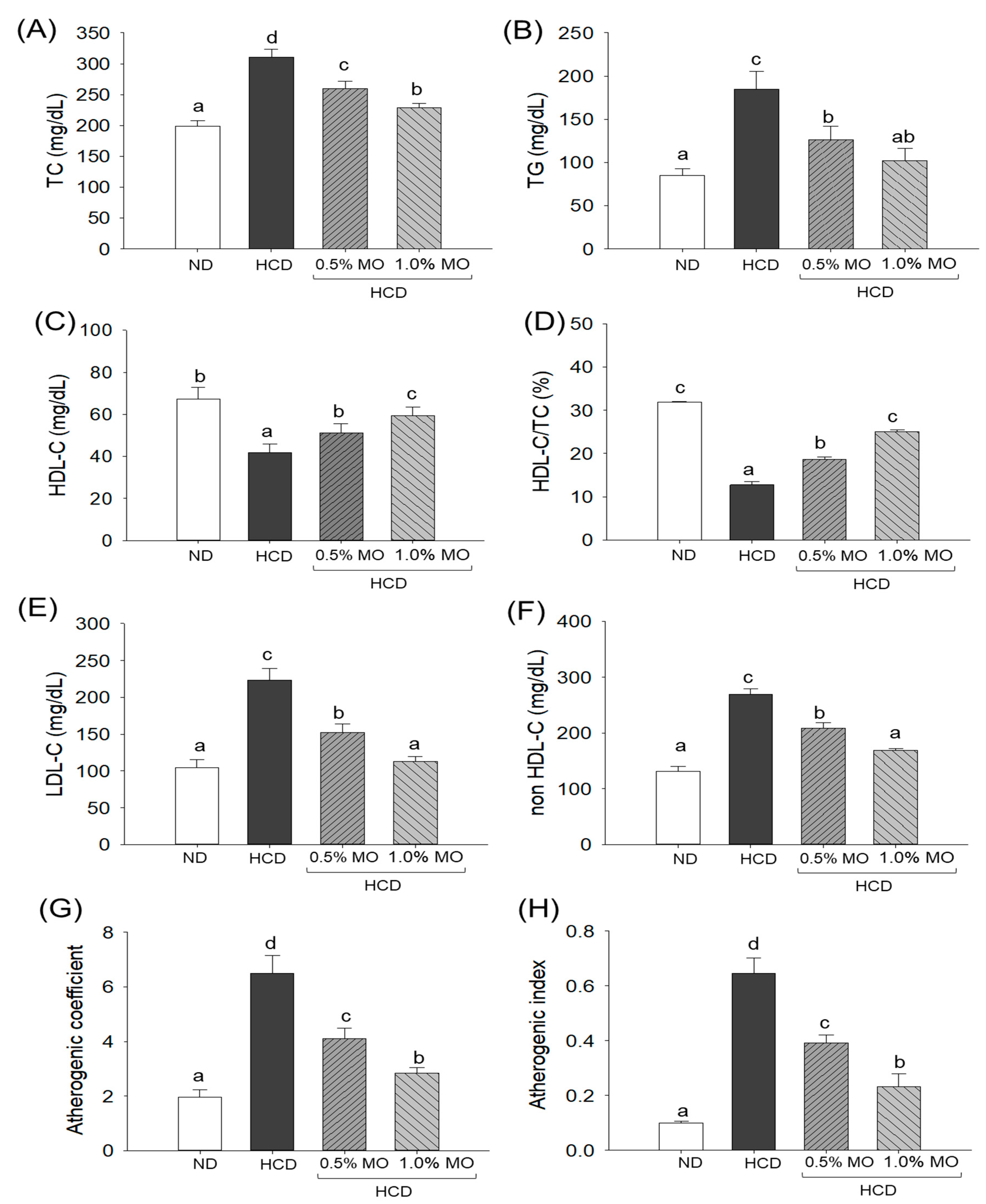
2.4. Moringa Positively Modulates the Plasma Lipid Profile of Hyperlipidemic Zebrafish
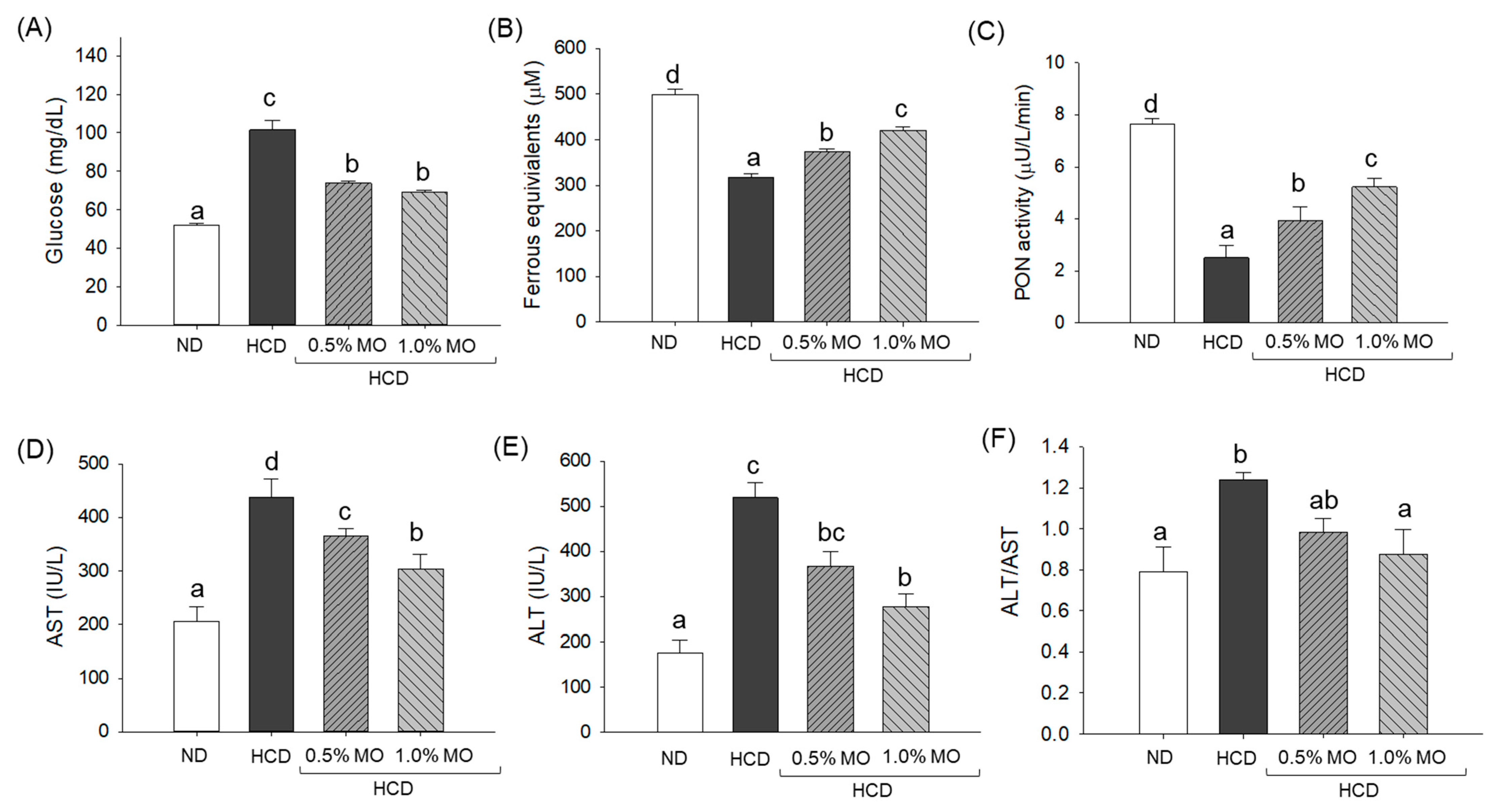
2.5. Quantification of Blood Glucose, Antioxidants, and Hepatic Function Biomarkers
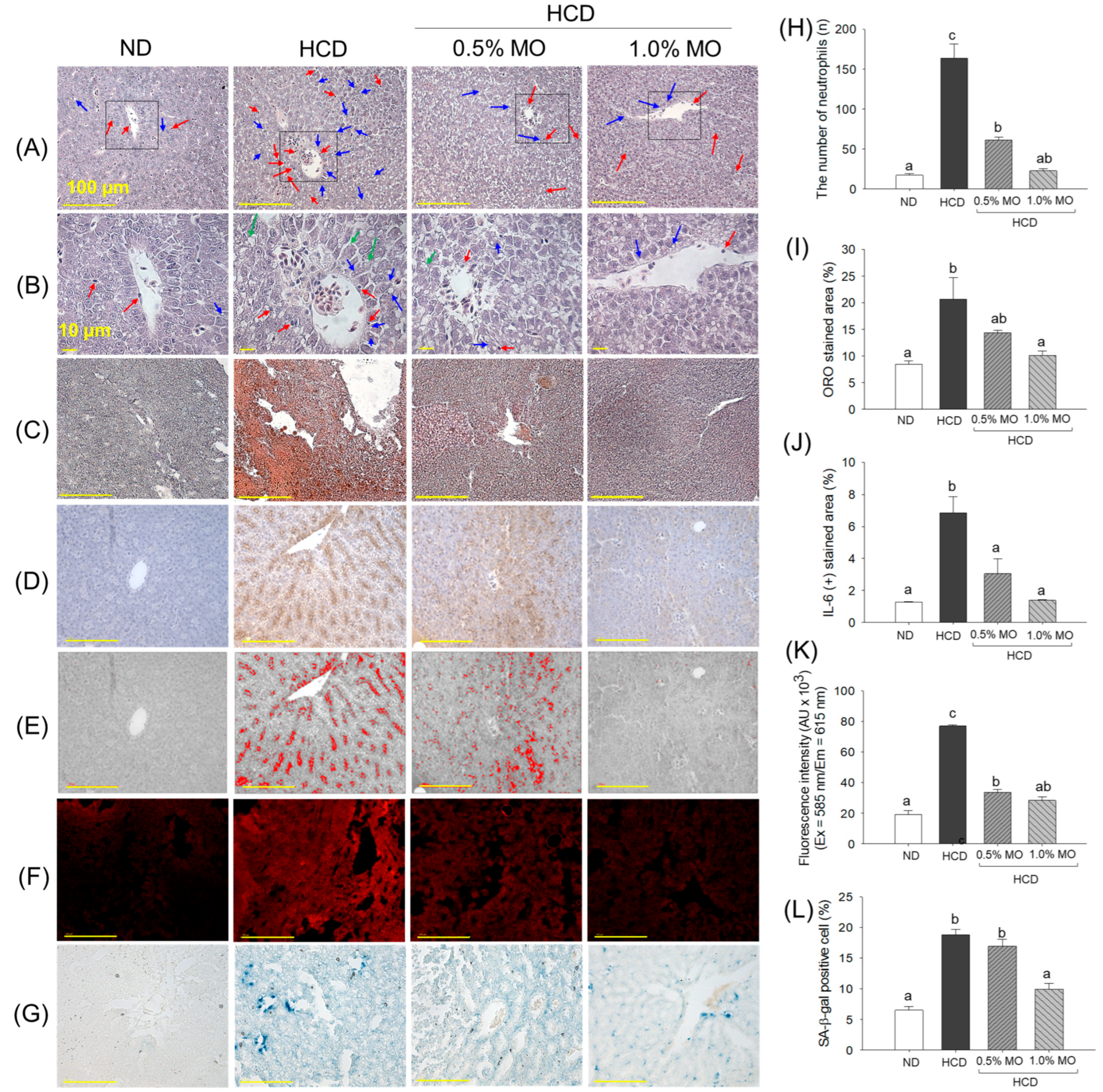
2.6. Moringa Protects Against the High-Cholesterol Diet-Triggered Liver Damage of Zebrafish
2.7. Moringa Prevents High-Cholesterol Diet-Induced Kidney Damage, Ros Generation, and Cellular Senescence
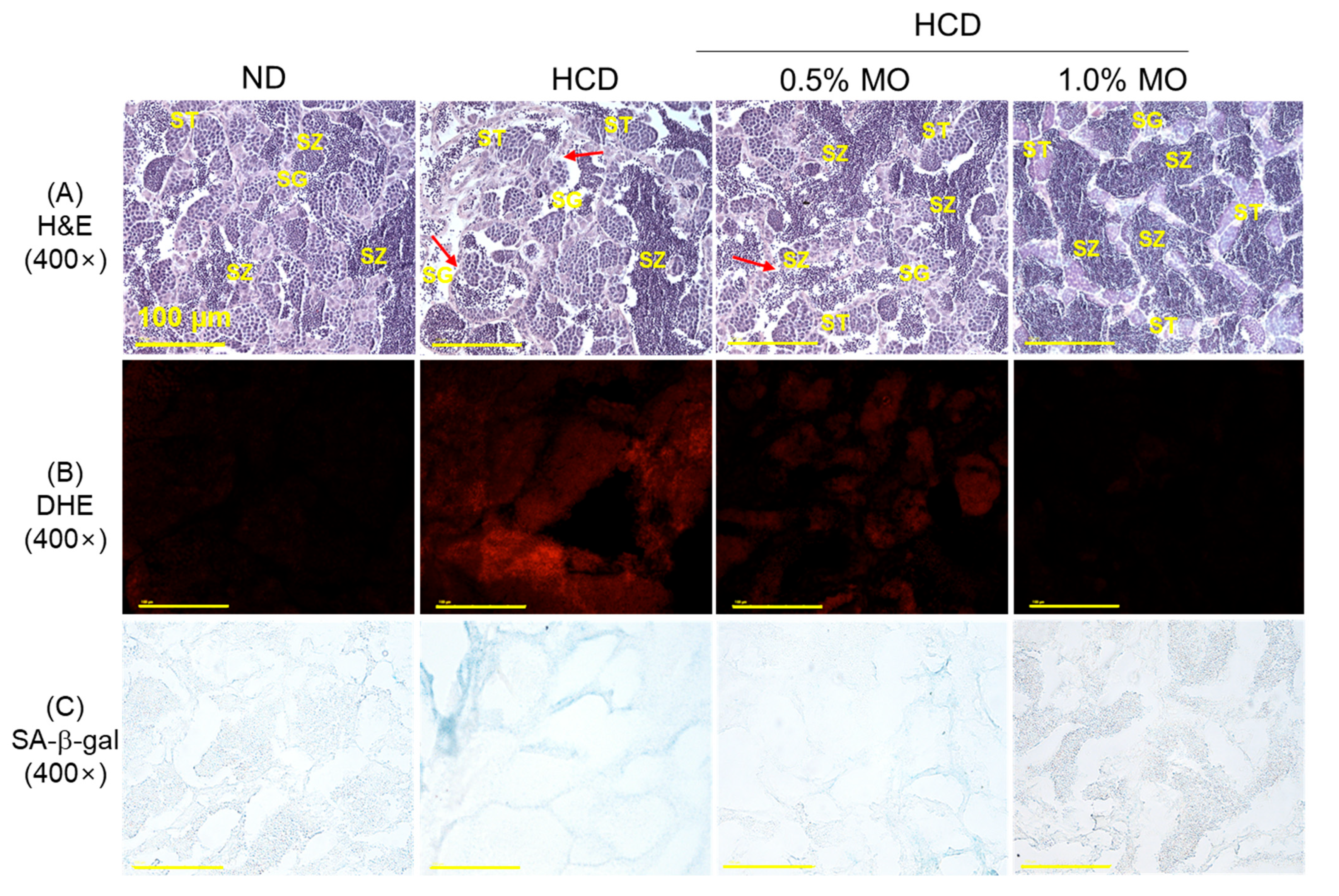
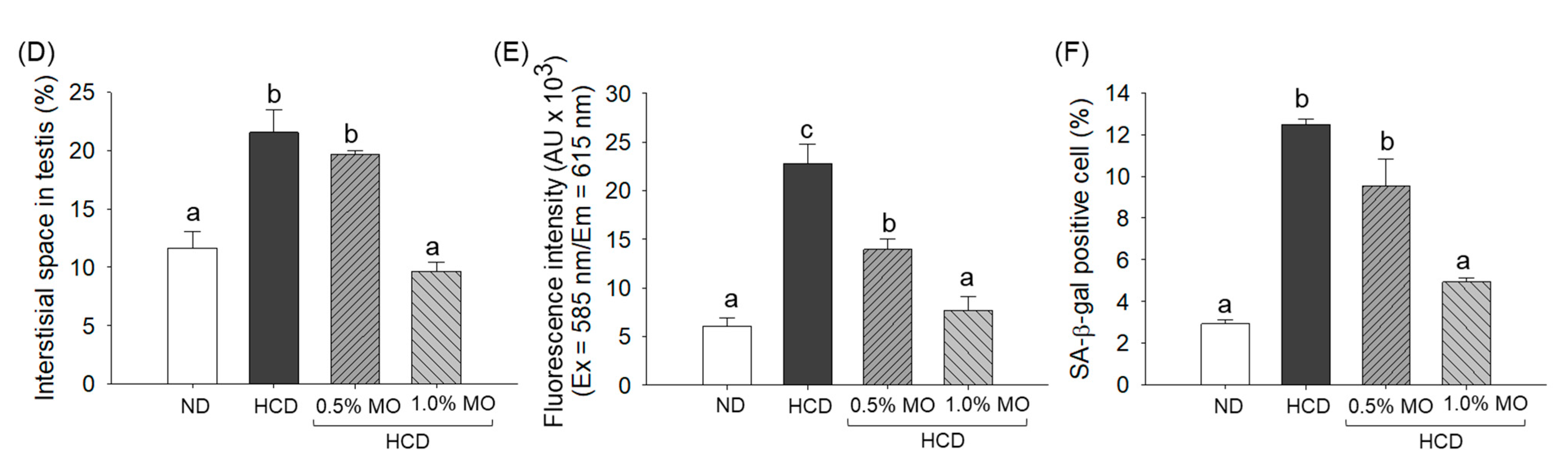
2.8. Moringa Prevents High-Cholesterol Diet-Induced Testes Damage
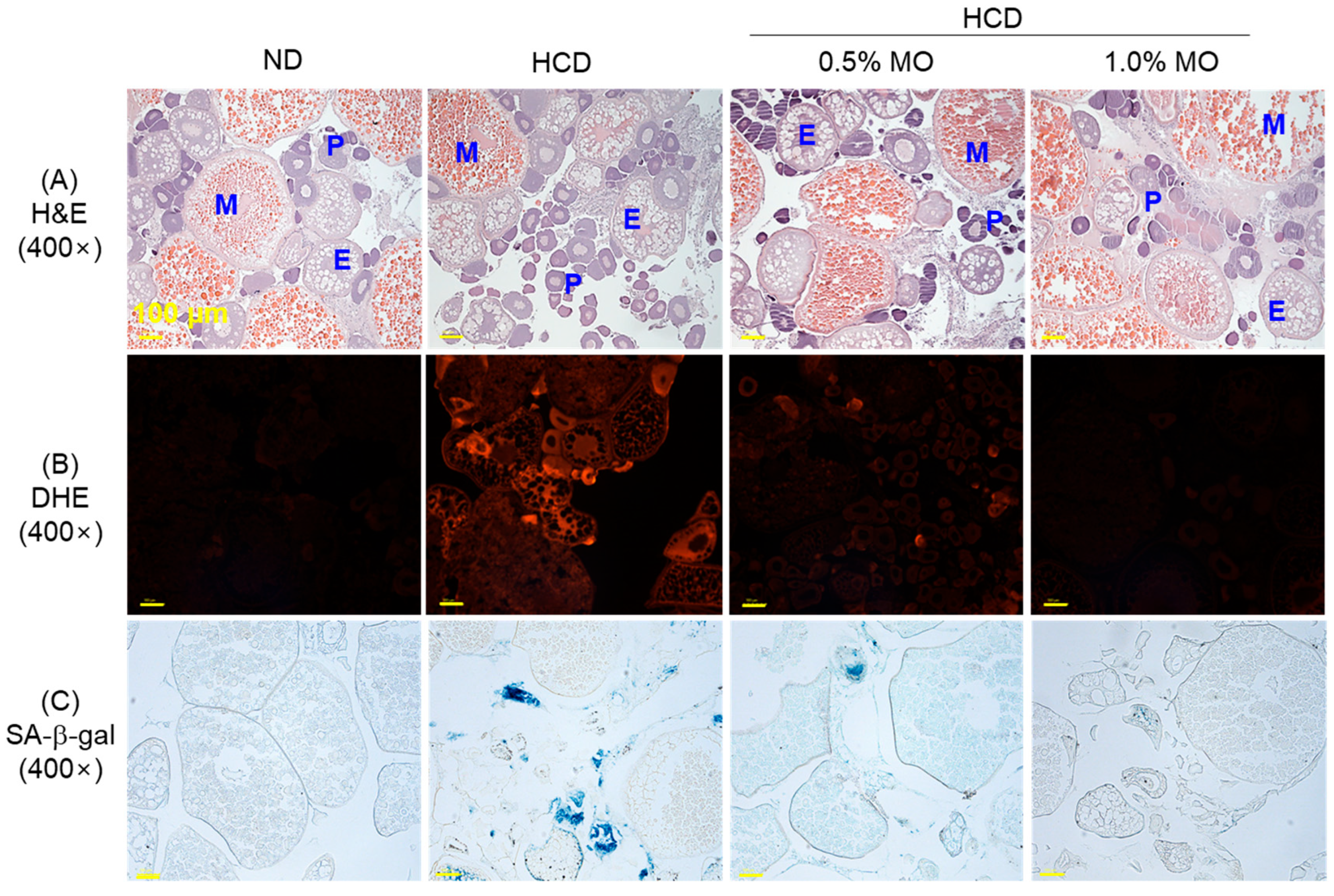
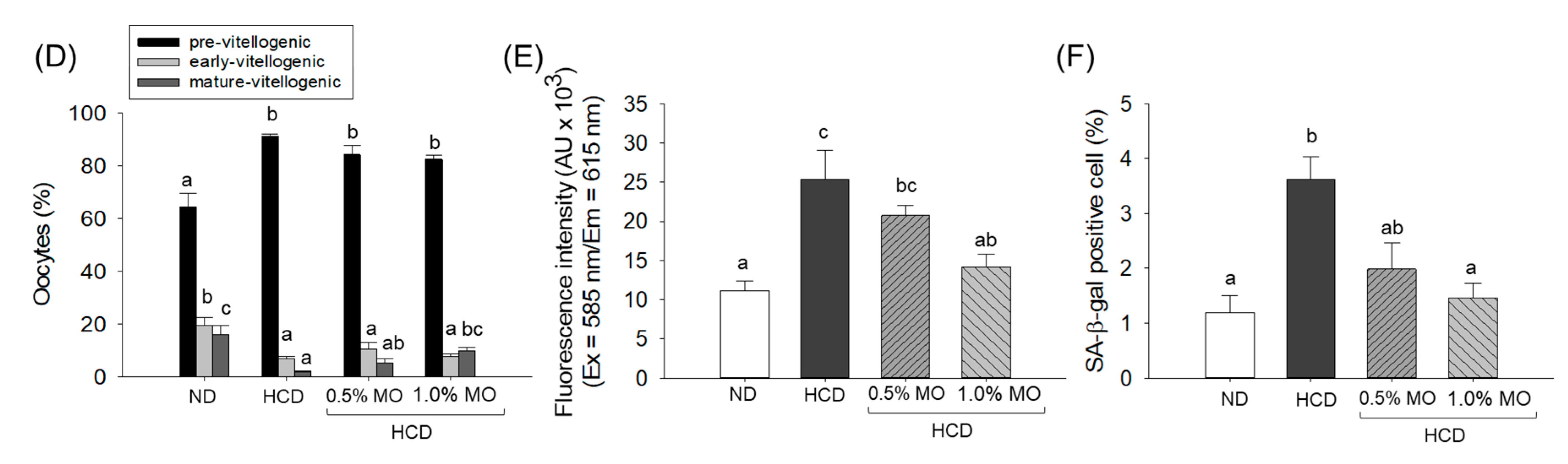
2.9. Moringa Prevents Ovary Damage of a High-Cholesterol Diet-Consuming Zebrafish
2.10. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Plant Material and Extraction Method
4.3. In Vitro Antioxidant Ability
4.4. Culturing of Zebrafish and Embryo Production
4.5. Microinjection to Zebrafish Embryos
4.6. Dihydroethidium (DHE) and Acridine Orange (AO) Staining
4.7. Preparation of Different Diets, Zebrafish Feeding, and Food Consumption Efficacy
4.8. Blood and Organ Collection
4.9. Lipid Profile and Biochemical Analysis of Blood
4.10. Histological Analysis, Immunohistochemical (IHC), Dihydroethidium (DHE), and Senescent Staining
4.11. In Silico Evaluation
4.11.1. Preparation of Ligands and Receptor
4.11.2. Molecular Docking
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tak, Y.J.; Lee, S.Y. Anti-Obesity Drugs: Long-Term Efficacy and Safety: An Updated Review. World J. Men’s Health 2021, 39, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Masood, B.; Moorthy, M. Causes of obesity: A review. Clin. Med. 2023, 23, 284–291. [Google Scholar] [CrossRef]
- de Oliveira, L.L.H.; de Assis, A.C.R.; Giraldez, V.Z.R.; Scudeler, T.L.; Soares, P.R. Dyslipidemia: A Narrative Review on Pharmacotherapy. Pharmaceuticals 2024, 17, 289. [Google Scholar] [CrossRef]
- Crichton, G.E.; Alkerwi, A. Physical activity, sedentary behavior time and lipid levels in the Observation of Cardiovascular Risk Factors in Luxembourg study. Lipids Health Dis. 2015, 14, 87. [Google Scholar] [CrossRef]
- Chang, Y.H.; Hung, H.Y. Recent advances in natural anti-obesity compounds and derivatives based on in vivo evidence: A mini-review. Eur. J. Med. Chem. 2022, 237, 114405. [Google Scholar] [CrossRef]
- Elizalde-Romero, C.A.; Leyva-López, N.; Contreras-Angulo, L.A.; Cabanillas Ponce de-León, R.; Rodriguez-Anaya, L.Z.; León-Félix, J.; Heredia, J.B.; Beltrán-Ontiveros, S.A.; Gutiérrez-Grijalva, E.P. Current Evidence of Natural Products against Overweight and Obesity: Molecular Targets and Mechanisms of Action. Receptors 2024, 3, 362–379. [Google Scholar] [CrossRef]
- Bais, S.; Singh, G.S.; Sharma, R. Anti-obesity and hypolipidemic activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Adv. Biol. 2014, 2014, 162914. [Google Scholar] [CrossRef]
- Redha, A.A.; Perna, S.; Riva, A.; Petrangolini, G.; Peroni, G.; Nichetti, M.; Iannello, G.; Naso, M.; Faliva, M.A.; Rondanelli, M. Novel insights on anti-obesity potential of the miracle tree, Moringa oleifera: A systematic review. J. Funct. Foods 2021, 84, 104600. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Jiao, R.; Ma, K.Y. Cholesterol-lowering nutraceuticals and functional foods. J. Agric. Food Chem. 2008, 56, 8761–8773. [Google Scholar] [CrossRef]
- Gul, P.; Khan, J.; Li, Q.; Liu, K. Moringa oleifera in a modern time: A comprehensive review of its nutritional and bioactive composition as a natural solution for managing diabetes mellitus by reducing oxidative stress and inflammation. Food Res. Int. 2025, 201, 115671. [Google Scholar] [CrossRef]
- Villegas-Vazquez, E.Y.; Gómez-Cansino, R.; Marcelino-Pérez, G.; Jiménez-López, D.; Quintas-Granados, L.I. Unveiling the Miracle Tree: Therapeutic Potential of Moringa oleifera in Chronic Disease Management and Beyond. Biomedicines 2025, 13, 634. [Google Scholar] [CrossRef]
- Santoriello, C.; Zon, L.I. Hooked! modeling human disease in zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Liu, C.; Miller, Y.I. Zebrafish models of dyslipidemia: Relevance to atherosclerosis and angiogenesis. Transl. Res. 2014, 163, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Stemple, D.L.; Barroso, I. The emerging use of zebrafish to model metabolic disease. Dis. Models Mech. 2013, 6, 1080–1088. [Google Scholar] [CrossRef]
- Trigo, C.; Castelló, M.L.; Ortolá, M.D.; García-Mares, F.J.; Desamparados Soriano, M. Moringa oleifera: An unknown crop in developed countries with great potential for industry and adapted to climate change. Foods 2021, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-O.; Park, C.-I.; Jin, S.-J.; Park, M.-R.; Choi, I.-Y.; Park, C.-H.; Adnan, M. Comparison in content of total polyphenol, flavonoid, and antioxidant capacity from different organs and extruded condition of Moringa oleifera Lam. Processes 2022, 10, 819. [Google Scholar] [CrossRef]
- Lee, B.H.; Hsu, W.H.; Hsu, Y.W.; Pan, T.M. Suppression of dimerumic acid on hepatic fibrosis caused from carboxymethyl-lysine (CML) by attenuating oxidative stress depends on Nrf2 activation in hepatic stellate cells (HSCs). Food Chem. Toxicol. 2013, 62, 413–419. [Google Scholar] [CrossRef]
- Wang, Z.; Bao, Z.; Ding, Y.; Xu, S.; Du, R.; Yan, J.; Li, L.; Sun, Z.; Shao, C.; Gu, W. Nε-carboxymethyl-lysine-induced PI3K/Akt signaling inhibition promotes foam cell apoptosis and atherosclerosis progression. Biomed. Pharmacother. 2019, 115, 108880. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Bahuguna, A.; Kang, D.-J. Long-term supplementation of ozonated sunflower oil improves dyslipidemia and hepatic inflammation in hyperlipidemic zebrafish: Suppression of oxidative stress and inflammation against carboxymethyllysine toxicity. Antioxidants 2023, 12, 1240. [Google Scholar] [CrossRef]
- Gaens, K.H.; Goossens, G.H.; Niessen, P.M.; van Greevenbroek, M.M.; van der Kallen, C.J.; Niessen, H.W.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.; Blaak, E.E.; et al. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1199–1208. [Google Scholar] [CrossRef]
- Segwatibe, M.K.; Cosa, S.; Bassey, K. Antioxidant and Antimicrobial Evaluations of Moringa oleifera Lam Leaves Extract and Isolated Compounds. Molecules 2023, 28, 899. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Fernando, I.P.S.; Jayasinghe, A.M.K.; Han, E.-J.; Dias, M.K.H.M.; Kang, K.-P.; Moon, S.-I.; Shin, T.-S.; Ma, A.; Ahn, G. Moringa oleifera hot water extract protects Vero cells from hydrogen peroxide-induced oxidative stress by regulating mitochondria-mediated apoptotic pathway and Nrf2/HO-1 Signaling. Foods 2022, 11, 420. [Google Scholar] [CrossRef]
- Kim, H.-L.; Ahn, Y.M.; Lee, S.M.; Seo, C.-S.; Park, S.-H.; Bang, O.-S.; Jung, J. Anti-obesity effects of aqueous extracts of Sunbanghwalmyung-Eum in high-fat- and high-cholesterol-diet-induced obese C57BL/6J Mice. Nutrients 2022, 14, 2929. [Google Scholar] [CrossRef] [PubMed]
- Tréguier, M.; Briand, F.; Boubacar, A.; André, A.; Magot, T.; Nguyen, P.; Krempf, M.; Sulpice, T.; Ouguerram, K. Diet-induced dyslipidemia impairs reverse cholesterol transport in hamsters. Eur. J. Clin. Investig. 2011, 41, 921–928. [Google Scholar] [CrossRef]
- Jahdkaran, M.; Sistanizad, M. From Lipids to Glucose: Investigating the role of dyslipidemia in the risk of insulin resistance. J. Steroid Biochem. Mol. Biol. 2025, 250, 106744. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chanathong, B. α-glucosidase inhibitory activity and lipid-lowering mechanisms of Moringa oleifera Leaf extract. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 803–808. [Google Scholar]
- Zhang, H.L.; Wu, Q.X.; Wei, X.; Qin, X.M. Pancreatic lipase and cholesterol esterase inhibitory effect of Camellia nitidissima Chi flower extracts in vitro and in vivo. Food Biosci. 2020, 37, 100682. [Google Scholar] [CrossRef]
- Heidrich, J.E.; Contos, L.M.; Hunsaker, L.A.; Deck, L.M.; Vander Jagt, D.L. Inhibition of pancreatic cholesterol esterase reduces cholesterol absorption in the Hamster. BMC Pharmacol. 2004, 4, 5. [Google Scholar] [CrossRef]
- Reddy, P.V.; Urooj, A.; Sairam, S.; Ahmed, F.; Prasad, N.N. Hypocholesterolemic effect of Moringa oleifera polyphenols in rats fed high fat-cholesterol diet. Mal. J. Nutr. 2017, 23, 473–478. [Google Scholar]
- Reddy, V.P.; Ahmed, F.; Urooj, A. Inhibition of 3-hydroxy-3-methylglutaryl coenzyme a (HMG Co-A) reductase in liver microsomes by Moringa oleifera L. polyphenols. Int. J. Pharm. Sci. Res. 2012, 3, 2510–2516. [Google Scholar]
- Tabbon, P.; Sripanidkulchai, B.; Sripanidkulchai, K. Hypocholesterolemic mechanism of phenolics-enriched extract from Moringa oleifera leaves in HepG2 cell lines. Songklanakarin J. Sci. Technol. 2016, 38, 155–161. [Google Scholar]
- Ray, S. Role of statins in the management of dyslipidaemia. Indian. Heart J. 2024, 76 (Suppl. S1), S33–S37. [Google Scholar] [CrossRef]
- Cho, K.-H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Srivastava, S.; Kumar, R.; Maurya, A.K.; Chanda, D. Experimental assessment of Moringa oleifera Leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evid.-Based Complement. Alternat. Med. 2012, 2012, 519084. [Google Scholar] [CrossRef] [PubMed]
- Almatrafi, M.M.; Vergara-Jimenez, M.; Murillo, A.G.; Norris, G.H.; Blesso, C.N.; Fernandez, M.L. Moringa Leaves Prevent Hepatic Lipid Accumulation and Inflammation in Guinea Pigs by Reducing the Expression of Genes Involved in Lipid Metabolism. Int. J. Mol. Sci. 2017, 18, 1330. [Google Scholar] [CrossRef]
- Oladipupo, S.O.; Ezenabor, E.H.; Ojo, A.B.; Ogunlakin, A.D.; Ojo, O.A. Interplay of the pathophysiological mechanisms of non-alcoholic fatty liver disease, diabetes mellitus, and inflammation: A growing threat to public health. Obes. Med. 2025, 55, 100613. [Google Scholar] [CrossRef]
- Joung, H.; Kim, B.; Park, H.; Lee, K.; Kim, H.-H.; Sim, H.-C.; Do, H.-J.; Hyun, C.-K.; Do, M.-S. Fermented Moringa oleifera decreases hepatic adiposity and ameliorates glucose intolerance in high-fat diet-induced obese mice. J. Med. Food 2017, 20, 439–447. [Google Scholar] [CrossRef]
- Reyes-Gordillo, K.; Shah, R.; Muriel, P. Oxidative stress and inflammation in hepatic diseases: Current and future therapy. Oxid. Med. Cell. Longev. 2017, 2017, 3140673. [Google Scholar] [CrossRef]
- Chiș, A.; Noubissi, P.A.; Pop, O.-L.; Mureșan, C.I.; Fokam Tagne, M.A.; Kamgang, R.; Fodor, A.; Sitar-Tăut, A.-V.; Cozma, A.; Orășan, O.H. Bioactive compounds in Moringa oleifera: Mechanisms of action, focus on their anti-inflammatory properties. Plants 2023, 13, 20. [Google Scholar] [CrossRef]
- Kooltheat, N.; Sranujit, R.P.; Chumark, P.; Potup, P.; Laytragoon-Lewin, N.; Usuwanthim, K. An ethyl acetate fraction of Moringa oleifera Lam. inhibits human macrophage cytokine production induced by cigarette smoke. Nutrients 2014, 6, 697–710. [Google Scholar] [CrossRef]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Flensted-Jensen, M.; Oró, D.; Rørbeck, E.A.; Zhang, C.; Madsen, M.R.; Madsen, A.N.; Norlin, J.; Feigh, M.; Larsen, S.; Hansen, H.H. Dietary intervention reverses molecular markers of hepatocellular senescence in the GAN diet-induced obese and biopsy-confirmed mouse model of NASH. BMC Gastroenterol. 2024, 24, 59. [Google Scholar] [CrossRef]
- Xuan, Y.; Wu, D.; Zhang, Q.; Yu, Z.; Yu, J.; Zhou, D. Elevated ALT/AST ratio as a marker for NAFLD risk and severity: Insights from a cross-sectional analysis in the United States. Front. Endocrinol. 2024, 15, 1457598. [Google Scholar] [CrossRef]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Alkhamees, O.A.; Aleisa, A.M.; Alroujayee, A.S. Gender difference following high cholesterol diet induced renal injury and the protective role of rutin and ascorbic acid combination in Wistar albino rats. Lipids Health Dis. 2012, 11, 41. [Google Scholar] [CrossRef]
- Moradi, H.; Pahl, M.V.; Elahimehr, R.; Vaziri, N.D. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl. Res. 2009, 153, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Alkafafy, M.E.; Sayed, S.M.; El-Shehawi, A.M.; El-Shazly, S.; Farouk, S.; Alotaibi, S.S.; Madkour, D.A.; Orabi, S.H.; Elbaz, H.T.; Ahmed, M.M. Moringa oleifera ethanolic extract ameliorates the testicular dysfunction resulted from HFD-induced obesity rat model. Andrologia 2021, 53, e14126. [Google Scholar] [CrossRef]
- Soliman, S.S.; Suliman, A.A.; Fathy, K.; Sedik, A.A. Ovario-protective effect of Moringa oleifera leaf extract against cyclophosphamide-induced oxidative ovarian damage and reproductive dysfunction in female rats. Sci. Rep. 2025, 15, 1054. [Google Scholar] [CrossRef] [PubMed]
- Ka, J.; Jin, S.-W. Zebrafish as an emerging model for dyslipidemia and associated diseases. J. Lipid Atheroscler. 2021, 10, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Maddison, L.A.; Chen, W. Zebrafish as a model for obesity and diabetes. Front. Cell Dev. Biol. 2018, 6, 91. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Song, Y.; Xie, H.; Dong, M. An update on brown adipose tissue and obesity intervention: Function, regulation and therapeutic implications. Front. Endocrinol. 2023, 13, 1065263. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Bahuguna, A.; Kim, J.-E. Long-term supplementation of Royal Jelly (Raydel®) improves zebrafish growth, embryo production and survivability, blood lipid profile and functionality of vital organs: A 72-weeks’ consumption study. Pharmaceuticals 2024, 17, 324. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Lee, M.-S.; Bahuguna, A. Oral supplementation of ozonated sunflower oil augments plasma antioxidant and anti-inflammatory abilities with enhancement of high-density lipoproteins functionality in rats. Antioxidants 2024, 13, 529. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Al-Ghamdi, T.H.; Atta, I.S. Efficacy of interleukin-6 in the induction of liver cell proliferation after hemi-hepatectomy: Histopathologic and immunohistochemical study. Int. J. Clin. Exp. Pathol. 2020, 13, 1540–1549. [Google Scholar] [PubMed]
- Cho, K.-H.; Bahuguna, A.; Kang, D.-J.; Kim, J.-E. Prolonged supplementation of ozonated sunflower oil bestows an antiaging effect, improves blood lipid profile and spinal deformities, and protects vital organs of zebrafish (Danio rerio) against age-related degeneration: Two-years consumption study. Antioxidants 2024, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Divya, S.; Pandey, V.K.; Dixit, R.; Rustagi, S.; Suthar, T.; Atuahene, D.; Nagy, V.; Ungai, D.; Ahmed, A.E.M.; Kovács, B. Exploring the phytochemical, pharmacological and nutritional properties of Moringa oleifera: A comprehensive review. Nutrients 2024, 16, 3423. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, Nutritional, and Therapeutic Significance of Moringa oleifera Lam. Phytother. Res. PTR 2019, 33, 2870–2903. [Google Scholar] [CrossRef]
- Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa oleifera: An updated comprehensive review of its pharmacological activities, ethnomedicinal, phytopharmaceutical formulation, clinical, phytochemical, and toxicological aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]


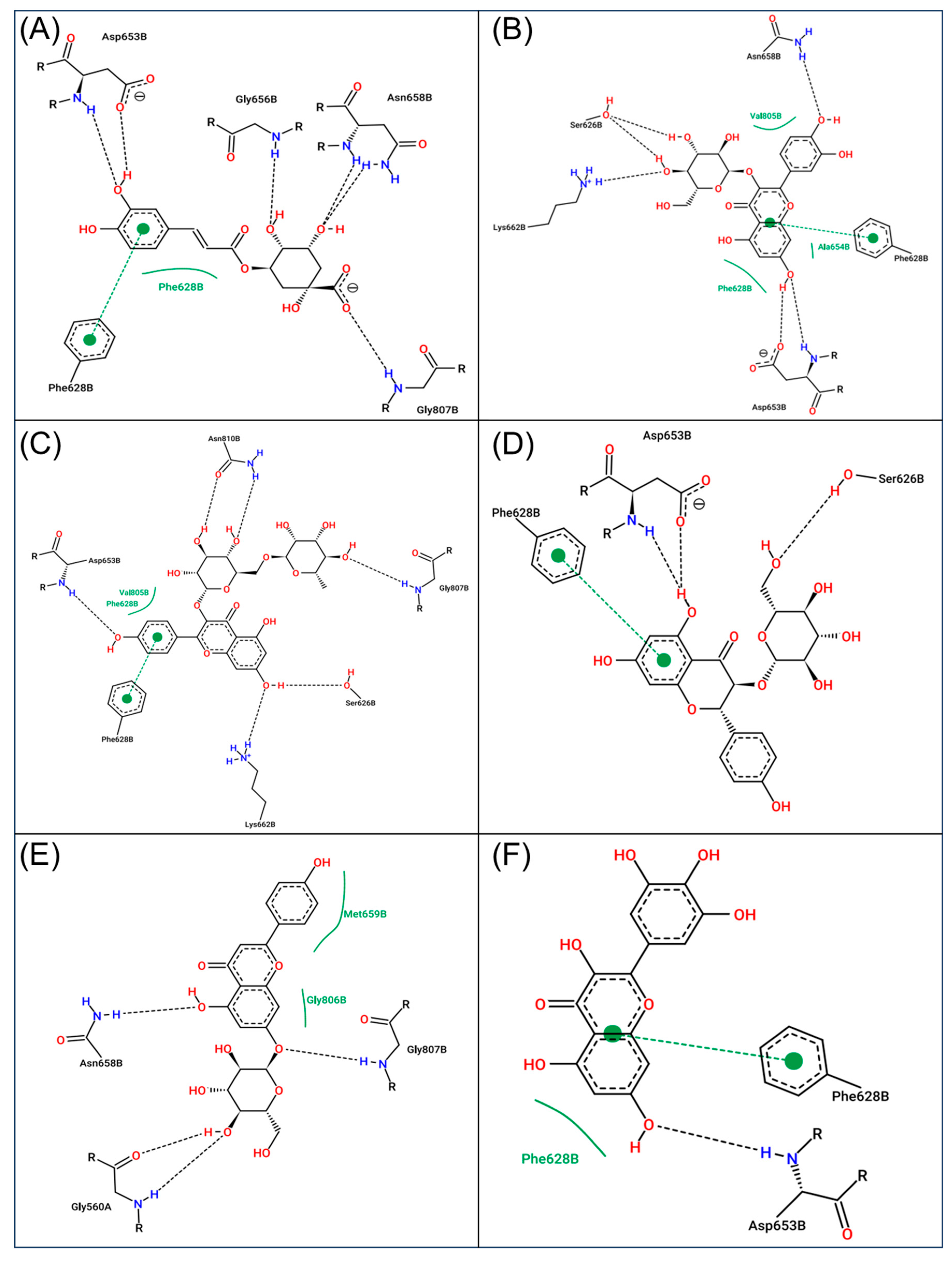
| Phytoconstituent of Moringa oleifera Leaf | Docking Score (kcal/mol) | Residues with H-Bonds | Residues with Hydrophobic Interaction |
|---|---|---|---|
| 3-Caffeoylquinic acid (chlorogenic acid) | −10.33 | Asp653 (2), Gly656, Asn658 (2), Gly807 | Phe628, Ala654, Met655, Met657, Met659, Ala826, Cys827, Pro931, |
| Quercetin-3-O-glucoside (isoquercetin) | −9.45 | Ser626 (2), Asn658, Lys662, Asp653 (2) | Phe628, Met659, Ala654, Val805, Ala826, Cys827, Pro831 |
| Kaempferol 3-O-rutinoside | −8.51 | Asp653, Lys662, Ser626, Gly807, Asn810 (2) | Ala525, Phe628, Ala654, Met659, Ala826, Val805, Cys827, Pro831 |
| Astragalin | −7.76 | Ser626, Asp653 (2) | Phe628, Ala654, Met659, Val805, Ala826, Cys827, Pro831 |
| Apigetrin | −7.49 | Gly560 (2), Asn658, Gly807 | Ala525, Ala654, Phe628, Met655, Met657, Met659, Val805 |
| Myricetin | −7.30 | Asp553 | Phe628, Ala654, Met655, Met659, Val805, Ala826, Cys827, Pro831 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Bahuguna, A.; Lee, Y.; Kim, J.-E.; Lee, S.H.; Djayanti, K. Moringa (Moringa oleifera) Leaf Attenuates the High-Cholesterol Diet-Induced Adverse Events in Zebrafish: A 12-Week Dietary Intervention Resulted in an Anti-Obese Effect and Blood Lipid-Lowering Properties. Pharmaceuticals 2025, 18, 1336. https://doi.org/10.3390/ph18091336
Cho K-H, Bahuguna A, Lee Y, Kim J-E, Lee SH, Djayanti K. Moringa (Moringa oleifera) Leaf Attenuates the High-Cholesterol Diet-Induced Adverse Events in Zebrafish: A 12-Week Dietary Intervention Resulted in an Anti-Obese Effect and Blood Lipid-Lowering Properties. Pharmaceuticals. 2025; 18(9):1336. https://doi.org/10.3390/ph18091336
Chicago/Turabian StyleCho, Kyung-Hyun, Ashutosh Bahuguna, Yunki Lee, Ji-Eun Kim, Sang Hyuk Lee, and Krismala Djayanti. 2025. "Moringa (Moringa oleifera) Leaf Attenuates the High-Cholesterol Diet-Induced Adverse Events in Zebrafish: A 12-Week Dietary Intervention Resulted in an Anti-Obese Effect and Blood Lipid-Lowering Properties" Pharmaceuticals 18, no. 9: 1336. https://doi.org/10.3390/ph18091336
APA StyleCho, K.-H., Bahuguna, A., Lee, Y., Kim, J.-E., Lee, S. H., & Djayanti, K. (2025). Moringa (Moringa oleifera) Leaf Attenuates the High-Cholesterol Diet-Induced Adverse Events in Zebrafish: A 12-Week Dietary Intervention Resulted in an Anti-Obese Effect and Blood Lipid-Lowering Properties. Pharmaceuticals, 18(9), 1336. https://doi.org/10.3390/ph18091336









