Efficacy of SGLT2 Inhibitors, GLP-1 Receptor Agonists, DPP-4 Inhibitors, and Sulfonylureas on Moderate-to-Severe COPD Exacerbations Among Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy, Selection Criteria, and Data Extraction
2.2. Endpoint Definition
2.3. Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
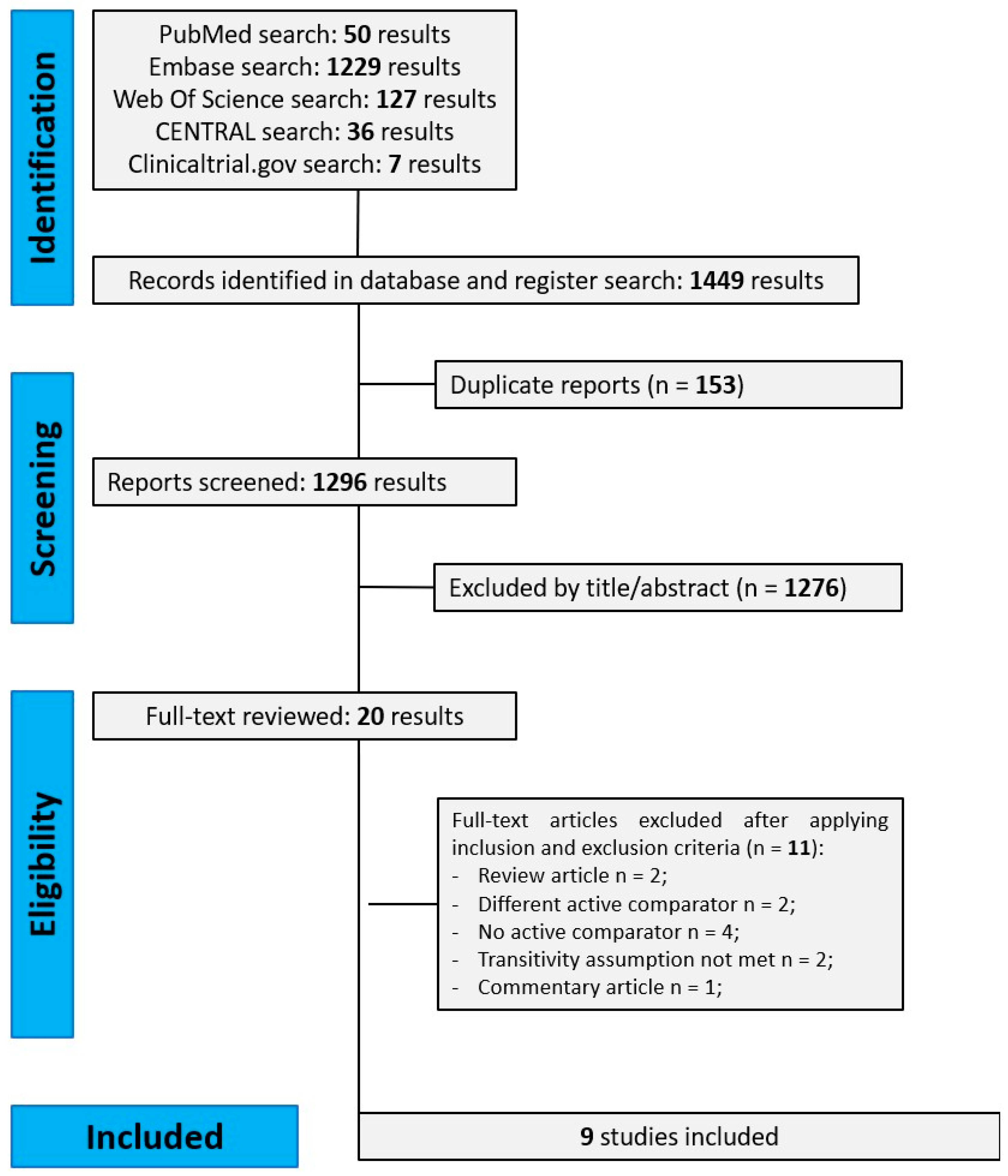
3.1. Literature Search and Study Characteristics
3.2. Structure of NMA
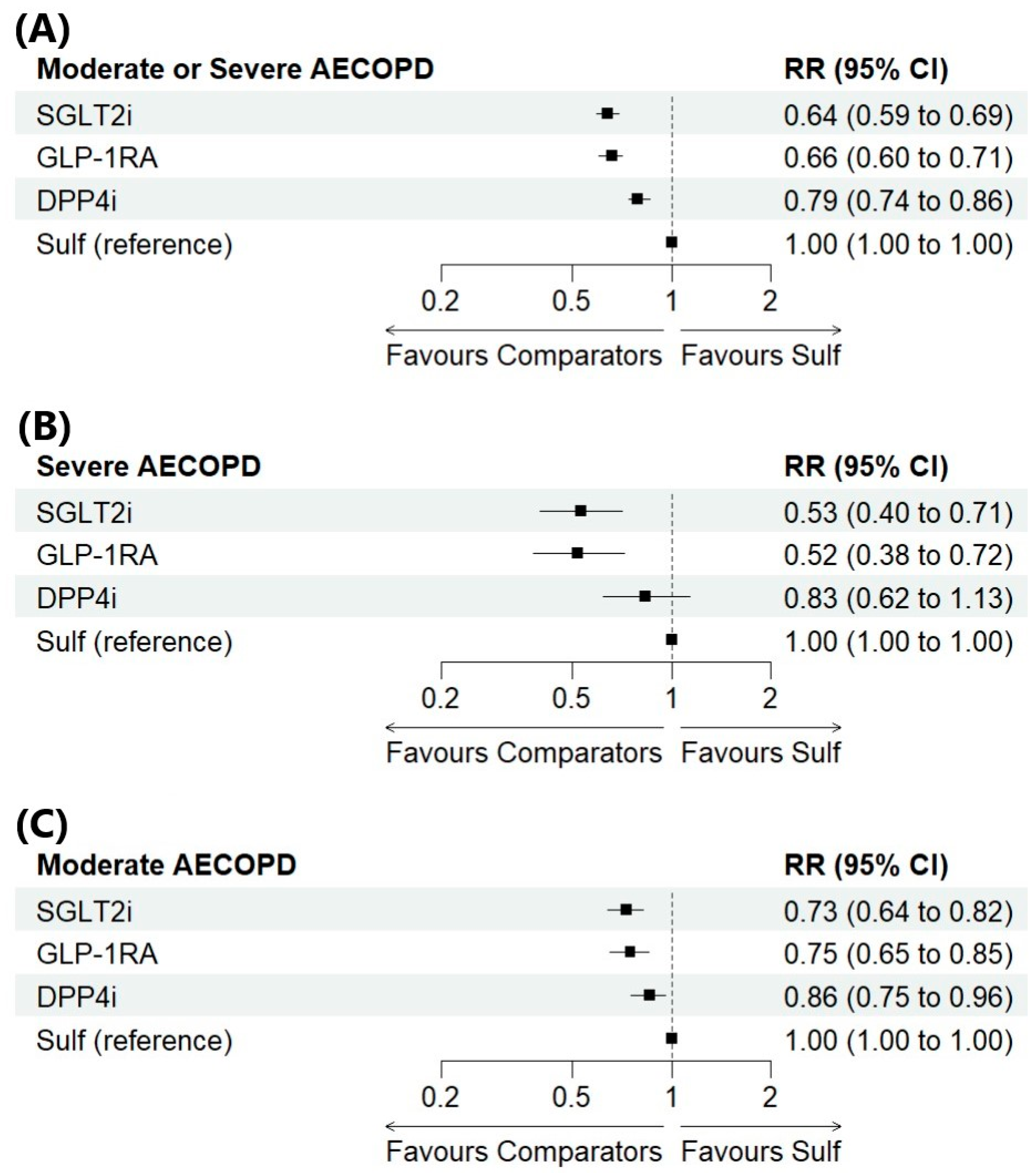
3.3. NMA Results for the Primary and Secondary Outcomes
3.4. Ranking of Treatment Strategies
3.5. Results of Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COPD | Chronic Obstructive Pulmonary Disease |
| CVD | Cardiovascular Disease |
| DPP4is | Dipeptidyl Peptidase-4 Inhibitor |
| FEV1 | Linear Dichroism |
| FVC | Forced Vital Capacity |
| GLP-1RAs | Glucagon-Like Peptide-1 Receptor Agonist |
| MOOSE | Meta-Analyses Of Observational Studies In Epidemiology |
| NMA | Network Meta-Analysis |
| PROSPERO | International Prospective Register Of Systematic Reviews |
| RCT | Randomised Controlled Trial |
| ROBINS-I | Risk Of Bias In Non-Randomised Studies Of Interventions |
| RR | Risk Ratio |
| SGLT2is | Sodium-Glucose Cotransporter-2 Inhibitors |
| SUCRA | Surface Under The Cumulative Ranking Curve |
| T2DM | Type 2 Diabetes Mellitus |
| 95% CI | 95% Credible Interval |
References
- 2025 GOLD Report. Available online: https://goldcopd.org/2025-gold-report/ (accessed on 13 April 2025).
- Wang, Y.; Han, R.; Ding, X.; Feng, W.; Gao, R.; Ma, A. Chronic Obstructive Pulmonary Disease across Three Decades: Trends, Inequalities, and Projections from the Global Burden of Disease Study 2021. Front. Med. 2025, 12, 1564878. [Google Scholar] [CrossRef]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, Regional, and National Age-Sex Specific All-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Lopez, A.D.; Shibuya, K.; Rao, C.; Mathers, C.D.; Hansell, A.L.; Held, L.S.; Schmid, V.; Buist, S. Chronic Obstructive Pulmonary Disease: Current Burden and Future Projections. Eur. Respir. J. 2006, 27, 397–412. [Google Scholar] [CrossRef]
- World Health Organization. Projections of Global Deaths from 2016 to 2060. Available online: https://colinmathers.com/2022/05/10/projections-of-global-deaths-from-2016-to-2060/ (accessed on 25 August 2025).
- Kerr, E.A.; Heisler, M.; Krein, S.L.; Kabeto, M.; Langa, K.M.; Weir, D.; Piette, J.D. Beyond Comorbidity Counts: How Do Comorbidity Type and Severity Influence Diabetes Patients’ Treatment Priorities and Self-Management? J. Gen. Intern. Med. 2007, 22, 1635–1640. [Google Scholar] [CrossRef]
- Chen, W.; Thomas, J.; Sadatsafavi, M.; FitzGerald, J.M. Risk of Cardiovascular Comorbidity in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Lancet Respir. Med. 2015, 3, 631–639. [Google Scholar] [CrossRef]
- Walter, R.E.; Beiser, A.; Givelber, R.J.; O’Connor, G.T.; Gottlieb, D.J. Association between Glycemic State and Lung Function: The Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2003, 167, 911–916. [Google Scholar] [CrossRef]
- McKeever, T.M.; Weston, P.J.; Hubbard, R.; Fogarty, A. Lung Function and Glucose Metabolism: An Analysis of Data from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2005, 161, 546–556. [Google Scholar] [CrossRef]
- Raslan, A.S.; Quint, J.K.; Cook, S. All-Cause, Cardiovascular and Respiratory Mortality in People with Type 2 Diabetes and Chronic Obstructive Pulmonary Disease (COPD) in England: A Cohort Study Using the Clinical Practice Research Datalink (CPRD). Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 1207–1218. [Google Scholar] [CrossRef]
- Di Martino, G.; Di Giovanni, P.; Cedrone, F.; Michela, D.; Meo, F.; Scampoli, P.; Romano, F.; Staniscia, T. The Impact of COPD on Hospitalized Patients with Diabetes: A Propensity Score Matched Analysis on Discharge Records. Healthcare 2022, 10, 885. [Google Scholar] [CrossRef]
- Kanie, T.; Mizuno, A.; Takaoka, Y.; Suzuki, T.; Yoneoka, D.; Nishikawa, Y.; Tam, W.W.S.; Morze, J.; Rynkiewicz, A.; Xin, Y.; et al. Dipeptidyl Peptidase-4 Inhibitors, Glucagon-like Peptide 1 Receptor Agonists and Sodium-Glucose Co-Transporter-2 Inhibitors for People with Cardiovascular Disease: A Network Meta-Analysis. Cochrane Database Syst. Rev. 2021, 10, CD013650. [Google Scholar] [CrossRef]
- López-Cano, C.; Ciudin, A.; Sánchez, E.; Tinahones, F.J.; Barbé, F.; Dalmases, M.; García-Ramírez, M.; Soto, A.; Gaeta, A.M.; Pellitero, S.; et al. Liraglutide Improves Forced Vital Capacity in Individuals with Type 2 Diabetes: Data from the Randomized Crossover LIRALUNG Study. Diabetes 2022, 71, 315–320. [Google Scholar] [CrossRef]
- Toki, S.; Goleniewska, K.; Reiss, S.; Zhang, J.; Bloodworth, M.H.; Stier, M.T.; Zhou, W.; Newcomb, D.C.; Ware, L.B.; Stanwood, G.D.; et al. Glucagon-like Peptide 1 Signaling Inhibits Allergen-Induced Lung IL-33 Release and Reduces Group 2 Innate Lymphoid Cell Cytokine Production In Vivo. J. Allergy Clin. Immunol. 2018, 142, 1515–1528.e8. [Google Scholar] [CrossRef]
- Au, P.C.M.; Tan, K.C.B.; Cheung, B.M.Y.; Wong, I.C.K.; Wong, Y.; Cheung, C.-L. Association Between SGLT2 Inhibitors vs DPP-4 Inhibitors and Risk of Pneumonia Among Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, e1719–e1726. [Google Scholar] [CrossRef]
- Kotnala, S.; Kim, Y.; Rajput, C.; Reddyvari, H.; Bolla, S.; Marchetti, N.T.; Kosmider, B.; Bahmed, K.; Sajjan, U.S. Contribution of Dipeptidyl Peptidase 4 to Non-Typeable Haemophilus Influenzae-Induced Lung Inflammation in COPD. Clin. Sci. 2021, 135, 2067–2083. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P.A. Graphical Methods and Numerical Summaries for Presenting Results from Multiple-Treatment Meta-Analysis: An Overview and Tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.J.; Caldwell, D.M.; Ades, A.E. Checking Consistency in Mixed Treatment Comparison Meta-Analysis. Stat. Med. 2010, 29, 932–944. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Welton, N.J.; Ades, A.E. Evidence Synthesis for Decision Making 6: Embedding Evidence Synthesis in Probabilistic Cost-Effectiveness Analysis. Med. Decis. Mak. 2013, 33, 671–678. [Google Scholar] [CrossRef]
- Turner, R.M.; Davey, J.; Clarke, M.J.; Thompson, S.G.; Higgins, J.P. Predicting the Extent of Heterogeneity in Meta-Analysis, Using Empirical Data from the Cochrane Database of Systematic Reviews. Int. J. Epidemiol. 2012, 41, 818–827. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook/current (accessed on 14 September 2024).
- Albogami, Y.; Cusi, K.; Daniels, M.J.; Wei, Y.-J.J.; Winterstein, A.G. Glucagon-Like Peptide 1 Receptor Agonists and Chronic Lower Respiratory Disease Exacerbations Among Patients with Type 2 Diabetes. Diabetes Care 2021, 44, 1344–1352. [Google Scholar] [CrossRef]
- Pradhan, R.; Lu, S.; Yin, H.; Yu, O.H.Y.; Ernst, P.; Suissa, S.; Azoulay, L. Novel Antihyperglycaemic Drugs and Prevention of Chronic Obstructive Pulmonary Disease Exacerbations among Patients with Type 2 Diabetes: Population Based Cohort Study. BMJ 2022, 379, e071380. [Google Scholar] [CrossRef]
- Foer, D.; Strasser, Z.H.; Cui, J.; Cahill, K.N.; Boyce, J.A.; Murphy, S.N.; Karlson, E.W. Association of GLP-1 Receptor Agonists with Chronic Obstructive Pulmonary Disease Exacerbations among Patients with Type 2 Diabetes. Am. J. Respir. Crit. Care Med. 2023, 208, 1088–1100. [Google Scholar] [CrossRef]
- Au, P.C.M.; Tan, K.C.B.; Lam, D.C.L.; Cheung, B.M.Y.; Wong, I.C.K.; Kwok, W.C.; Sing, C.-W.; Cheung, C.-L. Association of Sodium-Glucose Cotransporter 2 Inhibitor vs Dipeptidyl Peptidase-4 Inhibitor Use with Risk of Incident Obstructive Airway Disease and Exacerbation Events Among Patients with Type 2 Diabetes in Hong Kong. JAMA Netw. Open 2023, 6, e2251177. [Google Scholar] [CrossRef]
- See, X.Y.; Xanthavanij, N.; Lee, Y.-C.; Ong, T.E.; Wang, T.H.; Ahmed, O.; Chang, Y.-C.; Peng, C.-Y.; Chi, K.-Y.; Chang, Y.; et al. Pulmonary Outcomes of Incretin-Based Therapies in COPD Patients Receiving Single-Inhaler Triple Therapy. ERJ Open Res. 2025, 11, 00803–02024. [Google Scholar] [CrossRef]
- Yen, F.-S.; Hsu, C.-C.; Wei, J.C.-C.; Tsai, F.-J.; Huang, Y.; Yu, T.-S.; Hwu, C.-M. Glucagon-like Peptide-1 Receptor Agonists May Benefit Cardiopulmonary Outcomes in Patients with COPD. Thorax 2024, 79, 1017–1023. [Google Scholar] [CrossRef]
- Chang, T.-C.; Liang, Y.-C.; Lai, C.-C.; Ho, C.-H.; Chen, Y.-C.; Liao, K.-M.; Liang, F.-W. Comparison of SGLT2 and DPP4 Inhibitors on Clinical Outcomes in COPD Patients with Diabetes: A Nationwide Cohort Study. Diabetes Res. Clin. Pract. 2025, 223, 112122. [Google Scholar] [CrossRef]
- Ray, A.; Paik, J.M.; Wexler, D.J.; Sreedhara, S.K.; Bykov, K.; Feldman, W.B.; Patorno, E. Glucose-Lowering Medications and Risk of Chronic Obstructive Pulmonary Disease Exacerbations in Patients with Type 2 Diabetes. JAMA Intern. Med. 2025, e247811. [Google Scholar] [CrossRef]
- Yen, F.-S.; Wei, J.C.-C.; Huang, Y.-H.; Hsu, T.-J.; Wang, S.-T.; Hwu, C.-M.; Hsu, C.-C. SGLT-2 Inhibitors and the Risk of Chronic Obstructive Pulmonary Disease Exacerbations and Mortality in Chronic Obstructive Pulmonary Disease Patients. Ann. Am. Thorac. Soc. 2025, 22, 846–854. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Foer, D.; Beeler, P.E.; Cui, J.; Snyder, W.E.; Mashayekhi, M.; Nian, H.; Luther, J.M.; Karlson, E.W.; Boyce, J.A.; Cahill, K.N. Glucagon-like Peptide-1 Receptor Agonist Use Is Associated with Lower Serum Periostin. Clin. Exp. Allergy 2023, 53, 469–473. [Google Scholar] [CrossRef]
- Toki, S.; Newcomb, D.C.; Printz, R.L.; Cahill, K.N.; Boyd, K.L.; Niswender, K.D.; Peebles, R.S. Glucagon-like Peptide-1 Receptor Agonist Inhibits Aeroallergen-Induced Activation of ILC2 and Neutrophilic Airway Inflammation in Obese Mice. Allergy 2021, 76, 3433–3445. [Google Scholar] [CrossRef]
- Joo, H.; Park, S.J.; Min, K.H.; Rhee, C.K. Association between Plasma Interleukin-33 Level and Acute Exacerbation of Chronic Obstructive Pulmonary Disease. BMC Pulm. Med. 2021, 21, 86. [Google Scholar] [CrossRef]
- Rogliani, P.; Calzetta, L.; Capuani, B.; Facciolo, F.; Cazzola, M.; Lauro, D.; Matera, M.G. Glucagon-Like Peptide 1 Receptor: A Novel Pharmacological Target for Treating Human Bronchial Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2016, 55, 804–814. [Google Scholar] [CrossRef]
- Rogliani, P.; Matera, M.G.; Calzetta, L.; Hanania, N.A.; Page, C.; Rossi, I.; Andreadi, A.; Galli, A.; Coppola, A.; Cazzola, M.; et al. Long-Term Observational Study on the Impact of GLP-1R Agonists on Lung Function in Diabetic Patients. Respir. Med. 2019, 154, 86–92. [Google Scholar] [CrossRef]
- Altintas Dogan, A.D.; Hilberg, O.; Hess, S.; Jensen, T.T.; Bladbjerg, E.-M.; Juhl, C.B. Respiratory Effects of Treatment with a Glucagon-Like Peptide-1 Receptor Agonist in Patients Suffering from Obesity and Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obs. Pulmon Dis. 2022, 17, 405–414. [Google Scholar] [CrossRef]
- Briere, D.A.; Bueno, A.B.; Gunn, E.J.; Michael, M.D.; Sloop, K.W. Mechanisms to Elevate Endogenous GLP-1 Beyond Injectable GLP-1 Analogs and Metabolic Surgery. Diabetes 2018, 67, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Brikman, S.; Dori, G. Sodium Glucose Cotransporter2 Inhibitor-Possible Treatment for Patients with Diabetes, Pulmonary Disease and CO2 Retention. Med. Hypotheses 2020, 139, 109631. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.G.N.; Noguera, A.; Sauleda, J.; Sala, E.; Pons, J.; Busquets, X. Systemic Effects of Chronic Obstructive Pulmonary Disease. Eur. Respir. J. 2003, 21, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Vanfleteren, L.E.G.W. Does COPD Stand for “COmorbidity with Pulmonary Disease”? Eur. Respir. J. 2015, 45, 14–17. [Google Scholar] [CrossRef]
- Pirera, E.; Di Raimondo, D.; D’Anna, L.; Tuttolomondo, A. Risk Trajectory of Cardiovascular Events after an Exacerbation of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Eur. J. Intern. Med. 2025, 135, 74–82. [Google Scholar] [CrossRef]
- Burke, H.; Wilkinson, T.M.A. Unravelling the Mechanisms Driving Multimorbidity in COPD to Develop Holistic Approaches to Patient-Centred Care. Eur. Respir. Rev. 2021, 30, 210041. [Google Scholar] [CrossRef]
- Ma, H.; Lin, Y.-H.; Dai, L.-Z.; Lin, C.-S.; Huang, Y.; Liu, S.-Y. Efficacy and Safety of GLP-1 Receptor Agonists versus SGLT-2 Inhibitors in Overweight/Obese Patients with or without Diabetes Mellitus: A Systematic Review and Network Meta-Analysis. BMJ Open 2023, 13, e061807. [Google Scholar] [CrossRef]
- Liu, Y.; Ruan, B.; Jiang, H.; Le, S.; Liu, Y.; Ao, X.; Huang, Y.; Shi, X.; Xue, R.; Fu, X.; et al. The Weight-Loss Effect of GLP-1RAs Glucagon-Like Peptide-1 Receptor Agonists in Non-Diabetic Individuals with Overweight or Obesity: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2023, 118, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.J.; Sim, B.; Teo, Y.H.; Teo, Y.N.; Chan, M.Y.; Yeo, L.L.L.; Eng, P.C.; Tan, B.Y.Q.; Sattar, N.; Dalakoti, M.; et al. Efficacy of GLP-1 Receptor Agonists on Weight Loss, BMI, and Waist Circumference for Patients With Obesity or Overweight: A Systematic Review, Meta-Analysis, and Meta-Regression of 47 Randomized Controlled Trials. Diabetes Care 2025, 48, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Chan, K.Y.; Lo, K. Sodium-Glucose Co-Transporter 2 Inhibitors on Weight Change and Cardiometabolic Profiles in Individuals with Overweight or Obesity and without Diabetes: A Meta-Analysis. Obes. Rev. 2021, 22, e13336. [Google Scholar] [CrossRef]
- van Valkenhoef, G.; Lu, G.; de Brock, B.; Hillege, H.; Ades, A.E.; Welton, N.J. Automating Network Meta-Analysis. Res. Synth. Methods 2012, 3, 285–299. [Google Scholar] [CrossRef] [PubMed]

| Author and Year | Intervention (n) | Comparator (n) | Age (Mean ± SD) | Male (I:C) a | Body Mass Index b (I:C) a | Follow-up (Median and IQR) | COPD Severity (FEV1% Predicted) d | COPD Exacerbation History | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Comparator | ||||||||
| Albogami, 2021 [25] | GLP1-RA (4091) | DPP4i (12,445) | 53.6 ± 7.8 | 53 ± 8 | 41.1:42.5 | N/A | 1 year | N/A | Severe AECOPD DPP4i: 2.3; GLP-1RA: 2.3; |
| Pradhan, 2022 [26] | GLP1-RA (1252) | Sulfonylurea (14,259) | 61.4 ± 8.9 | 61.1 ± 9.2 | 50:50 | <30: 6:6.6 | 1 year (0.4–2.3) | GLP-1RA <30: 2.6; 30–80: 55.8; >80: 12.5; Missing: 29.2; DPP4i <30: 2.9; 30–80: 58; >80:14.1; Missing: 25; SGLT2i <30: 1.8; 30–80: 57.9; >80: 14.7; Missing: 25.6; Sulfonylurea <30: 3.5; 30–80: 57.4; >80: 11.8; Missing: 27.3; | Severe AECOPD GLP-1RA: 25.8; DPP4i: 26.9; SGLT2i: 21; |
| ≥30: 91.6:90.0 | |||||||||
| DPP4i (8731) | Sulfonylurea (18,204) | 69.3 ± 10.7 | 68.8 ± 10.7 | 55.4:55.2 | <30: 35.1:34.3 | 1 year (0.4–2.2) | |||
| ≥30: 63.9:64.7 | |||||||||
| SGLT2i (2956) | Sulfonylurea (10,841) | 62.9 ± 9.0 | 62.7 ± 9.1 | 58.5:58.0 | <30: 20.8:21.7 | 0.9 year (0.3–2.0) | |||
| ≥30: 78.3:77.4 | |||||||||
| Foer, 2023 [27] | DPP4i (260) | GLP1-RA (328) | 72.2 ± 11.8 c | 67.2 ± 9.6 c | 61.1:57.6 | 29.7:34.9 e | 6 months | DPP4i GOLD 1: 12.3; GOLD 2: 53.7; GOLD 3: 28.7; GOLD 4: 5.3; GLP-1RA GOLD 1: 13.7; GOLD 2: 52.6; GOLD 3: 30; GOLD 4: 3.8; SGLT2i GOLD 1: 12.9; GOLD 2: 52.6; GOLD 3: 31.2; GOLD 4: 3.3; Sulfonylurea GOLD 1: 11.8; GOLD 2: 49.9; GOLD 3: 29.8; GOLD 4: 8.5; | Number of exacerbations ≤12 months. n (SD) DPP4i: 1.1 (2.1); GLP-1RA: 1.13 (2.04); SGLT2i: 1.36 (2.32); Sulfonylurea: 1.24 (2.21); |
| SGLT2i (353) | GLP1-RA (328) | 71.5 ± 10.0 c | 67.2 ± 9.6 c | 67.1:57.6 | 30.8:34.9 e | ||||
| Sulfonylurea (701) | GLP1-RA (328) | 70.1 ± 9.5 c | 67.2 ± 9.6 c | 59.3:57.6 | 30.7:34.9 e | ||||
| Au, 2023 [28] | DPP4i (1524) | SGLT2i (381) | 62.3 ± 10.8 | 62.2 ± 10.7 | 51.0:52.0 | N/A | DPP4i: 2.3 (1.0–3.5) SGLT2i: 1.5 (0.5–3.0) | N/A | N/A |
| See, 2024 [29] | GLP1-RA (1751) | DPP4i (1751) | 68.2 ± 8.7 | 68.3 ± 9.7 | 52.7:52.4 | Overweight: 20.9:20.9 Class I: 26.8:25.6 Class II: 19.6:18.6 Class III–IV: 15.2:15.4 | 1 year | Mean (SD) GLP-1RA: 61.9 (17.2); DPP4i: 61.7 (19.7); | N/A |
| Yen, 2024 [30] | GLP1-RA (7506) | SGLT2i (7506) | 58.6 ± 9.8 | 58.7 ± 9.4 | 42.5:42.5 | ≥ 30: 9.18:9.29 | GLP1-RA: 2.51 years SGLT2i: N/A | N/A | ≥2 Moderate AECOPD SGLT2i: 30.4; GLP-1RA: 31.07; ≥2 Severe AECOPD SGLT2i: 1.95; GLP-1RA: 2.03; |
| Chang, 2025 [31] | SGLT2i (188) | DPP4i (181) | <70 year: 33.5% 70–79 years: 42.5% ≥80 years: 23.9% | <70 year: 34.8% 70–79 years: 41.9% ≥80 years: 23.2% | 97.9:96.1 | N/A | 1 year | N/A | ≥2 Moderate AECOPD DPP4i: 6.63; SGLT2i: 1.60; ≥2 severe AECOPD DPP4i: 17.68; SGLT2i: 8.51; |
| Ray, 2025 [32] | SGLT2i (27,991) | DPP4i (27,991) | 70.5 ± 8.6 | 70.7 ± 8.8 | 50.8:50.5 | ≥30: 39.7:39.7 | Composite SGLT2i: 145 days (61–335) DPP4i: 147 days (62–336) Severe SGLT2i: 150 days (67–354) DPP4i: 147 days (69–356) | N/A | Moderate/severe AECOPD SGLT2i: 4.4; GLP-1RA: 4.1; DPP4i: 4.4; Severe AECOPD SGLT2i: 2.5; GLP-1RA: 2.4; DPP4i: 2.5; Moderate AECOPD SGLT2i: 8.5; GLP-1RA: 8.0; DPP4i: 8.3; |
| GLP1-RA (32,107) | DPP4i (32,107) | 70.4 ± 8.5 | 70.4 ± 8.2 | 45.1:45.1 | ≥30: 45.2:45.1 | Composite GLP1-RA: 142 days (63–339) DPP4i: 147 days (62–336) Severe GLP1-RA: 147 days (69–356) DPP4i: 164 days (73–377) | |||
| SGLT2i (36,218) | GLP1-RA (36,218) | 69.7 ± 8.7 | 69.7 ± 8.7 | 48.1:47.9 | ≥30: 46.1:46.1 | Composite SGLT2i: 141 days (61–316) GLP1-RA: 139 days (65–314) Severe SGLT2i: 147 days (67–329) GLP1-RA: 144 days (70–331) | |||
| Yen, 2025 [33] | DPP4i (452) | SGLT2i (452) | 60.4 ± 9.7 | 60.1 ± 10.1 | 57.1:57.7 | ≥30: 4.9:7.1 | DPP4i: N/A SGLT2i: 2.61 years | N/A | Moderate AECOPD SGLT2i: 31.2; DPP4i: 30.3; Sulfonylurea: 27.5; Severe AECOPD SGLT2i:37.6; DPP4i: 36.7; Sulfonylurea: 32.7; |
| Sulfonylurea (312) | SGLT2i (312) | 61.2 ± 10.4 | 60.7 ± 10.2 | 50.6:50.6 | ≥30: 8.3:8.6 | Sulfonylurea: N/A SGLT2i: 2.61 years | |||
| Values | SGLT2i | GLP-1RA | DPP4i | Sulf |
|---|---|---|---|---|
| Moderate or severe AECOPD | 0.938 | 0.729 | 0.333 | 0.000 |
| Severe AECOPD | 0.818 | 0.847 | 0.299 | 0.035 |
| Moderate AECOPD | 0.892 | 0.768 | 0.337 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirera, E.; Di Raimondo, D.; D’Anna, L.; Tuttolomondo, A. Efficacy of SGLT2 Inhibitors, GLP-1 Receptor Agonists, DPP-4 Inhibitors, and Sulfonylureas on Moderate-to-Severe COPD Exacerbations Among Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Pharmaceuticals 2025, 18, 1337. https://doi.org/10.3390/ph18091337
Pirera E, Di Raimondo D, D’Anna L, Tuttolomondo A. Efficacy of SGLT2 Inhibitors, GLP-1 Receptor Agonists, DPP-4 Inhibitors, and Sulfonylureas on Moderate-to-Severe COPD Exacerbations Among Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Pharmaceuticals. 2025; 18(9):1337. https://doi.org/10.3390/ph18091337
Chicago/Turabian StylePirera, Edoardo, Domenico Di Raimondo, Lucio D’Anna, and Antonino Tuttolomondo. 2025. "Efficacy of SGLT2 Inhibitors, GLP-1 Receptor Agonists, DPP-4 Inhibitors, and Sulfonylureas on Moderate-to-Severe COPD Exacerbations Among Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis" Pharmaceuticals 18, no. 9: 1337. https://doi.org/10.3390/ph18091337
APA StylePirera, E., Di Raimondo, D., D’Anna, L., & Tuttolomondo, A. (2025). Efficacy of SGLT2 Inhibitors, GLP-1 Receptor Agonists, DPP-4 Inhibitors, and Sulfonylureas on Moderate-to-Severe COPD Exacerbations Among Patients with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis. Pharmaceuticals, 18(9), 1337. https://doi.org/10.3390/ph18091337









