Modulation of Macrophage Polarization by Traditional Chinese Medicine in HFpEF: A Review of Mechanisms and Therapeutic Potentials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Method
2.2. Inclusion and Exclusion Criteria
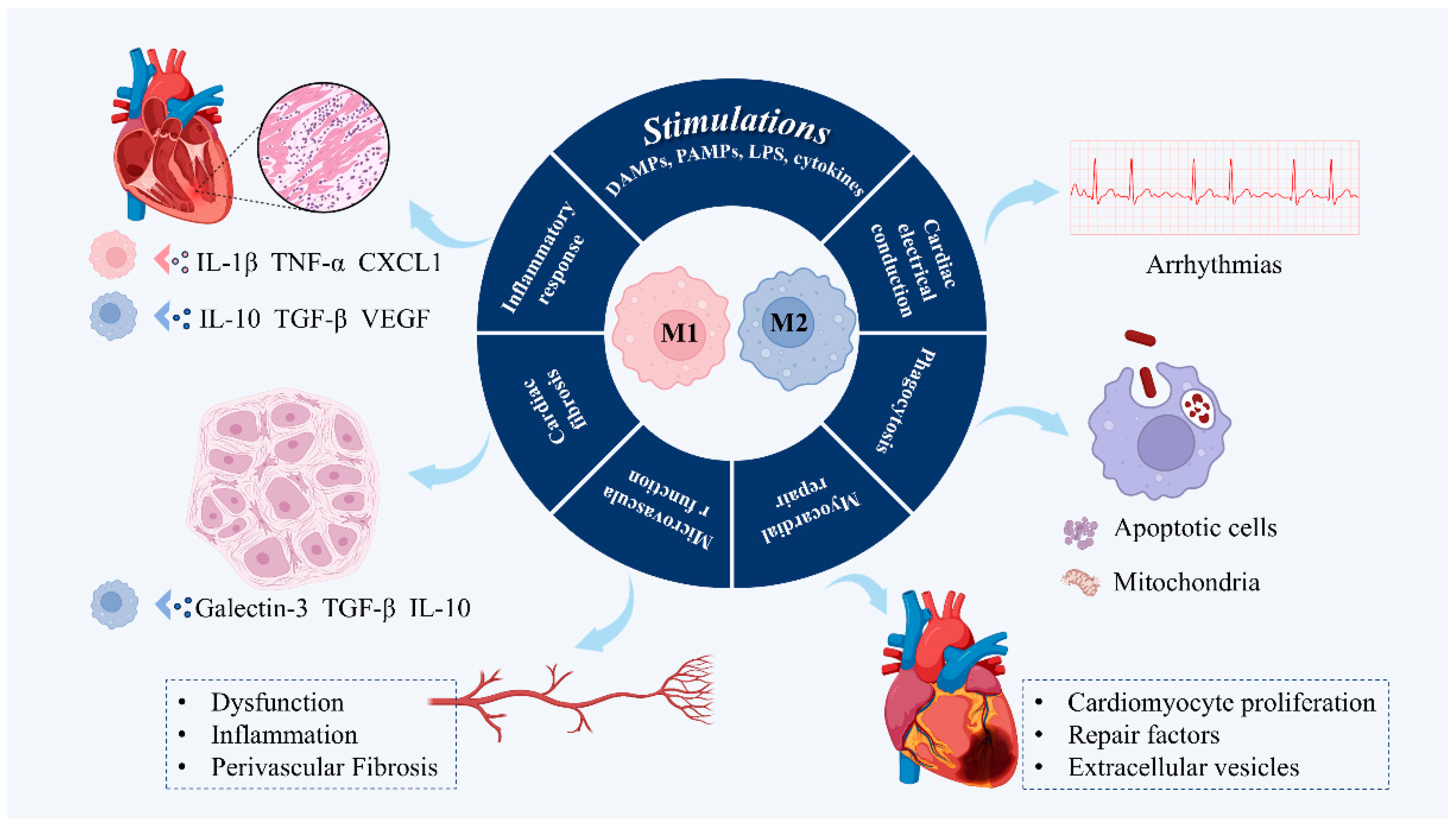
3. Dual Role of Macrophage Phenotypic Plasticity in the Pathogenesis of HFpEF
3.1. Regulating Inflammatory Response
3.2. Regulation of Cardiac Fibrosis
3.3. Regulation of Microvascular Function
3.4. Promotes Myocardial Repair
3.5. Phagocytosis
3.6. Facilitating Cardiac Electrical Conduction
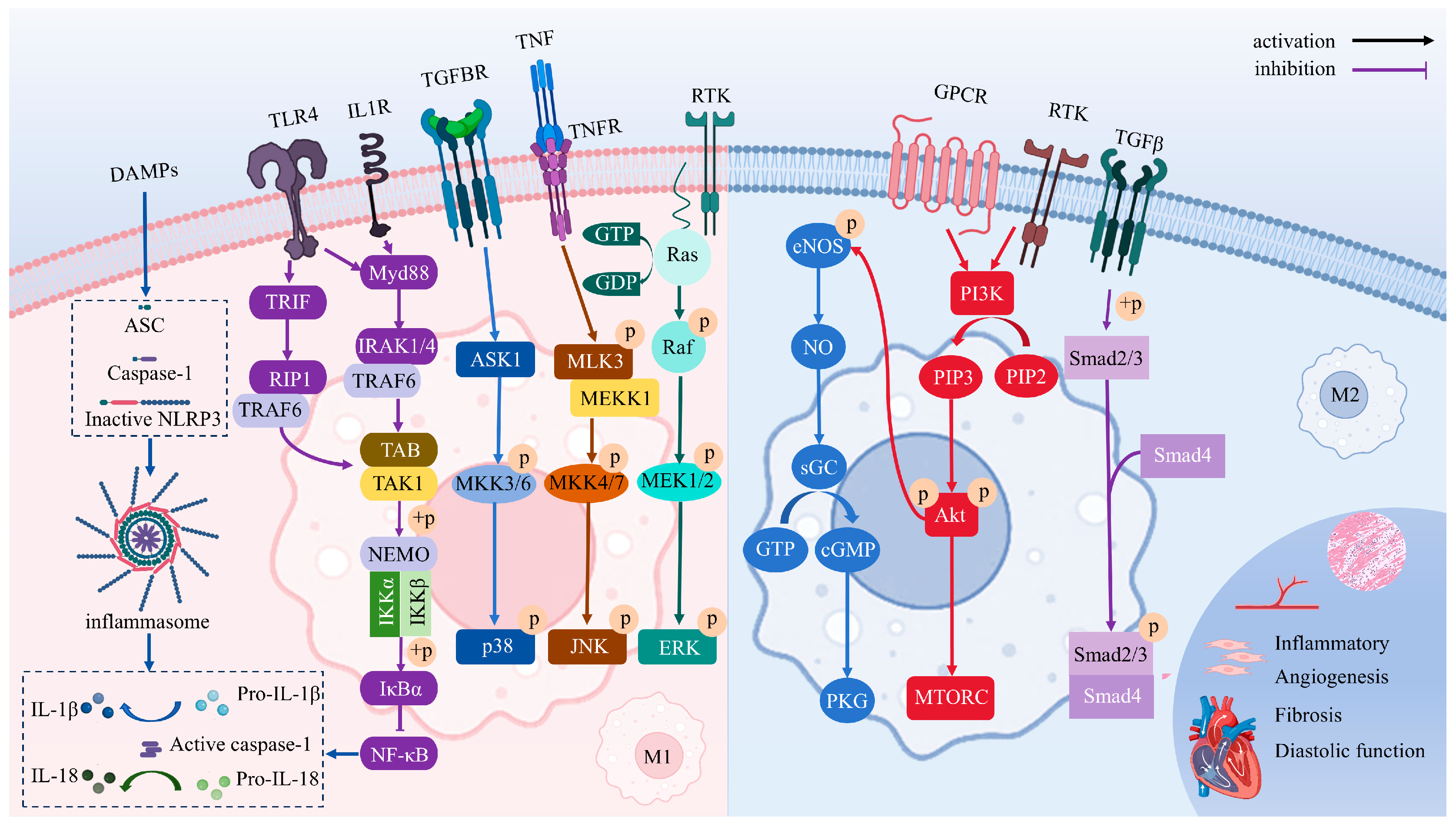
4. Signaling Pathways Driving Macrophage Polarization
4.1. NO/cGMP/PKG
4.2. TGF-β/Smads
4.3. TLRs/NF-κB
4.4. NLRP3 Inflammasome
4.5. PI3K/Akt
4.6. MAPK
5. TCM Treats HFpEF by Modulating Macrophages
5.1. Active Metabolites of TCM
5.1.1. Anti-Inflammatory Activity
5.1.2. Anti-Oxidative Stress
5.1.3. Anti-Myocardial Fibrosis
5.1.4. Promote Lymphangiogenesis
5.2. TCM Formulas
6. Conclusions
7. Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalogeropoulos, A.P.; Butler, J. Worsening Cardiovascular Disease Epidemiology in the United States. J. Am. Coll. Cardiol. 2022, 80, 579–583. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global Burden of Heart Failure: A Comprehensive and Updated Review of Epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. J. Heart Fail. 2024, 26, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Rutten, F.H.; Lee, M.M.; Hawkins, N.M.; Petrie, M.C. Heart Failure with Preserved Ejection Fraction: Everything the Clinician Needs to Know. Lancet 2024, 403, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Shahid, I.; Bennis, A.; Rakisheva, A.; Metra, M.; Butler, J. Global Epidemiology of Heart Failure. Nat. Rev. Cardiol. 2024, 21, 717–734. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the Impact of Heart Failure in the United States: A Policy Statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary Microvascular Rarefaction and Myocardial Fibrosis in Heart Failure with Preserved Ejection Fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef]
- Shah, S.J.; Borlaug, B.A.; Kitzman, D.W.; McCulloch, A.D.; Blaxall, B.C.; Agarwal, R.; Chirinos, J.A.; Collins, S.; Deo, R.C.; Gladwin, M.T.; et al. Research Priorities for Heart Failure with Preserved Ejection Fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation 2020, 141, 1001–1026. [Google Scholar] [CrossRef]
- Mishra, S.; Kass, D.A. Cellular and Molecular Pathobiology of Heart Failure with Preserved Ejection Fraction. Nat. Rev. Cardiol. 2021, 18, 400–423. [Google Scholar] [CrossRef]
- Alcaide, P.; Kallikourdis, M.; Emig, R.; Prabhu, S.D. Myocardial Inflammation in Heart Failure with Reduced and Preserved Ejection Fraction. Circ. Res. 2024, 134, 1752–1766. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Otero-Santiago, M.; Anido-Varela, L.; Moraña-Fernández, S.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Gualillo, O.; González-Juanatey, J.R.; et al. Role of Sodium-Glucose Co-Transporter 2 Inhibitors in the Regulation of Inflammatory Processes in Animal Models. Int. J. Mol. Sci. 2022, 23, 5634. [Google Scholar] [CrossRef] [PubMed]
- Rykova, E.Y.; Klimontov, V.V.; Shmakova, E.; Korbut, A.I.; Merkulova, T.I.; Kzhyshkowska, J. Anti-Inflammatory Effects of SGLT2 Inhibitors: Focus on Macrophages. Int. J. Mol. Sci. 2025, 26, 1670. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-Specific Treatment of Heart Failure with Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, X.; Sun, Y.; Jiao, B.; Yao, J.; Hu, Y.; Deng, Q.; Dong, J.; Wang, W.; Wang, Y.; et al. Transcriptional Regulation of Macrophages in Heart Failure. Front. Cardiovasc. Med. 2023, 10, 1148041. [Google Scholar] [CrossRef]
- Moskalik, A.; Niderla-Bielińska, J.; Ratajska, A. Multiple Roles of Cardiac Macrophages in Heart Homeostasis and Failure. Heart Fail. Rev. 2022, 27, 1413–1430. [Google Scholar] [CrossRef]
- Qin, D.; Zhang, Y.; Liu, F.; Xu, X.; Jiang, H.; Su, Z.; Xia, L. Spatiotemporal Development and the Regulatory Mechanisms of Cardiac Resident Macrophages: Contribution in Cardiac Development and Steady State. Acta Physiol. 2024, 240, e14088. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, H.; Tang, B.; Luo, Y.; Yang, Y.; Zhong, X.; Chen, S.; Xu, X.; Huang, S.; Liu, C. Macrophages in Cardiovascular Diseases: Molecular Mechanisms and Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 130. [Google Scholar] [CrossRef]
- Rizzoni, D.; De Ciuceis, C.; Szczepaniak, P.; Paradis, P.; Schiffrin, E.L.; Guzik, T.J. Immune System and Microvascular Remodeling in Humans. Hypertension 2022, 79, 691–705. [Google Scholar] [CrossRef]
- Yang, P.; Chen, Z.; Huang, W.; Zhang, J.; Zou, L.; Wang, H. Communications between Macrophages and Cardiomyocytes. Cell Commun. Signal 2023, 21, 206. [Google Scholar] [CrossRef]
- Lanzer, J.D.; Wienecke, L.M.; Ramirez Flores, R.O.; Zylla, M.M.; Kley, C.; Hartmann, N.; Sicklinger, F.; Schultz, J.-H.; Frey, N.; Saez-Rodriguez, J.; et al. Single-Cell Transcriptomics Reveal Distinctive Patterns of Fibroblast Activation in Heart Failure with Preserved Ejection Fraction. Basic. Res. Cardiol. 2024, 119, 1001–1028. [Google Scholar] [CrossRef]
- Kuppe, C.; Ramirez Flores, R.O.; Li, Z.; Hayat, S.; Levinson, R.T.; Liao, X.; Hannani, M.T.; Tanevski, J.; Wünnemann, F.; Nagai, J.S.; et al. Spatial Multi-Omic Map of Human Myocardial Infarction. Nature 2022, 608, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.-Y.; Hou, C.; Jia, F.; Xue, C.; Li, X.-L.; Yang, L.; Jiang, W.-Y.; Zhang, X. Transcutaneous Auricular Vagus Nerve Stimulation Ameliorates Heart Failure with Preserved Ejection Fraction Through Regulating Macrophage Polarization Mediated by Alpha7nAChR. Cardiovasc. Drugs Ther. 2025. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yao, J.; Li, C.; Cui, L.; Liu, Y.; Liu, X.; Wang, G.; Dong, J.; Deng, Q.; Hu, Y.; et al. Shexiang Tongxin Dropping Pills Promote Macrophage Polarization-Induced Angiogenesis Against Coronary Microvascular Dysfunction via PI3K/Akt/mTORC1 Pathway. Front. Pharmacol. 2022, 13, 840521. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Jia, X.; Li, C.; Ma, X.; Tong, H.; Liu, M.; Li, L. Baicalein Alleviates Cardiomyocyte Death in EAM Mice by Inhibiting the JAK-STAT1/4 Signalling Pathway. Phytomedicine 2024, 128, 155558. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, J.; Li, R.; Zhang, Y.; Tao, Y.; Hou, Y.; Yang, L.; Zhang, Y.; Wu, J.; Meng, X. Traditional Chinese Medicine in Regulating Macrophage Polarization in Immune Response of Inflammatory Diseases. J. Ethnopharmacol. 2024, 325, 117838. [Google Scholar] [CrossRef]
- Jian, X.; Liu, Y.; Zhao, Z.; Zhao, L.; Wang, D.; Liu, Q. The Role of Traditional Chinese Medicine in the Treatment of Atherosclerosis through the Regulation of Macrophage Activity. Biomed. Pharmacother. 2019, 118, 109375. [Google Scholar] [CrossRef]
- Gianopoulos, I.; Daskalopoulou, S.S. Macrophage Profiling in Atherosclerosis: Understanding the Unstable Plaque. Basic. Res. Cardiol. 2024, 119, 35–56. [Google Scholar] [CrossRef]
- Panico, C.; Felicetta, A.; Kunderfranco, P.; Cremonesi, M.; Salvarani, N.; Carullo, P.; Colombo, F.; Idini, A.; Passaretti, M.; Doro, R.; et al. Single-Cell RNA Sequencing Reveals Metabolic Stress-Dependent Activation of Cardiac Macrophages in a Model of Dyslipidemia-Induced Diastolic Dysfunction. Circulation 2023, 150, 1517–1532. [Google Scholar] [CrossRef]
- Venkatesan, T.; Toumpourleka, M.; Niewiadomska, M.; Farhat, K.; Morris, L.; Elkholey, K.; Maryam, B.; Cordova, A.; Darby, I.G.; Whyte, S.; et al. Vagal Stimulation Rescues HFpEF by Altering Cardiac Resident Macrophage Function. Circ. Res. 2025, 137, 5. [Google Scholar] [CrossRef]
- Hulsmans, M.; Schloss, M.J.; Lee, I.-H.; Bapat, A.; Iwamoto, Y.; Vinegoni, C.; Paccalet, A.; Yamazoe, M.; Grune, J.; Pabel, S.; et al. Recruited Macrophages Elicit Atrial Fibrillation. Science 2023, 381, 231–239. [Google Scholar] [CrossRef]
- DeBerge, M.; Shah, S.J.; Wilsbacher, L.; Thorp, E.B. Macrophages in Heart Failure with Reduced versus Preserved Ejection Fraction. Trends Mol. Med. 2019, 25, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Rodolico, D.; Hill, J.A. Metabolic Inflammation in Heart Failure with Preserved Ejection Fraction. Cardiovasc. Res. 2021, 117, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Zhou, X.; Shan, W.; Chen, C.; Ge, W.; Cui, J.; Yi, W.; Sun, Y. Crosstalk between Macrophages and Cardiac Cells after Myocardial Infarction. Cell Commun. Signal 2023, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, S.; Liu, F.; Xia, L.; Wang, C.; Sandoghchian Shotorbani, S.; Xu, H.; Chakrabarti, S.; Peng, T.; Su, Z. Renewal of Embryonic and Neonatal-Derived Cardiac-Resident Macrophages in Response to Environmental Cues Abrogated Their Potential to Promote Cardiomyocyte Proliferation via Jagged-1–Notch1. Acta Pharm. Sin. B 2023, 13, 128–141. [Google Scholar] [CrossRef]
- Zuo, W.; Sun, R.; Ji, Z.; Ma, G. Macrophage-Driven Cardiac Inflammation and Healing: Insights from Homeostasis and Myocardial Infarction. Cell Mol. Biol. Lett. 2023, 28, 81. [Google Scholar] [CrossRef]
- Bajpai, G.; Bredemeyer, A.; Li, W.; Zaitsev, K.; Koenig, A.L.; Lokshina, I.; Mohan, J.; Ivey, B.; Hsiao, H.-M.; Weinheimer, C.; et al. Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury. Circ. Res. 2019, 124, 263–278. [Google Scholar] [CrossRef]
- Chakarov, S.; Lim, H.Y.; Tan, L.; Lim, S.Y.; See, P.; Lum, J.; Zhang, X.-M.; Foo, S.; Nakamizo, S.; Duan, K.; et al. Two Distinct Interstitial Macrophage Populations Coexist across Tissues in Specific Subtissular Niches. Science 2019, 363, eaau0964. [Google Scholar] [CrossRef]
- Doddapattar, P.; Dev, R.; Ghatge, M.; Patel, R.B.; Jain, M.; Dhanesha, N.; Lentz, S.R.; Chauhan, A.K. Myeloid Cell PKM2 Deletion Enhances Efferocytosis and Reduces Atherosclerosis. Circ. Res. 2022, 130, 1289–1305. [Google Scholar] [CrossRef]
- Peet, C.; Ivetic, A.; Bromage, D.I.; Shah, A.M. Cardiac Monocytes and Macrophages after Myocardial Infarction. Cardiovasc. Res. 2020, 116, 1101–1112. [Google Scholar] [CrossRef]
- Ma, Y.; Mouton, A.J.; Lindsey, M.L. Cardiac Macrophage Biology in the Steady-State Heart, the Aging Heart, and Following Myocardial Infarction. Transl. Res. 2018, 191, 15–28. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Ren, W.; Sun, Z.; Zhang, Y.; Wu, N.; Diao, H. Cardiomyocyte Proliferation: Advances and Insights in Macrophage-Targeted Therapy for Myocardial Injury. Genes. Dis. 2024, 12, 101332. [Google Scholar] [CrossRef]
- Niskala, A.; Heijman, J.; Dobrev, D.; Jespersen, T.; Saljic, A. Targeting the NLRP3 Inflammasome Signalling for the Management of Atrial Fibrillation. Br. J. Pharmacol. 2024, 181, 4939–4957. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, D.; Heijman, J.; Hiram, R.; Li, N.; Nattel, S. Inflammatory Signalling in Atrial Cardiomyocytes: A Novel Unifying Principle in Atrial Fibrillation Pathophysiology. Nat. Rev. Cardiol. 2023, 20, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zhu, Y.; Kuai, Y.; Chen, B.; Zhao, Q.; Yu, W. Macrophage MKL1 Contributes to Cardiac Fibrosis in a Mouse Model of Myocardial Infarction. Life Sci. 2024, 356, 123036. [Google Scholar] [CrossRef] [PubMed]
- Merino-Merino, A.; Gonzalez-Bernal, J.; Fernandez-Zoppino, D.; Saez-Maleta, R.; Perez-Rivera, J.-A. The Role of Galectin-3 and ST2 in Cardiology: A Short Review. Biomolecules 2021, 11, 1167. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, M.; Wang, X.; Liu, Q.; Su, H.; Sun, B.; Guo, Z.; Tian, B.; Gan, H.; Gong, C.; et al. Single-Cell RNA Sequencing Reveals S100a9hi Macrophages Promote the Transition from Acute Inflammation to Fibrotic Remodeling after Myocardial Ischemia-reperfusion. Theranostics 2024, 14, 1241–1259. [Google Scholar] [CrossRef]
- Shen, J.-L.; Xie, X.-J. Insight into the Pro-Inflammatory and Profibrotic Role of Macrophage in Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Pharmacol. 2020, 76, 276–285. [Google Scholar] [CrossRef]
- Shen, S.-C.; Xu, J.; Cheng, C.; Xiang, X.-J.; Hong, B.-Y.; Zhang, M.; Gong, C.; Ma, L.-K. Macrophages Promote the Transition from Myocardial Ischemia Reperfusion Injury to Cardiac Fibrosis in Mice through GMCSF/CCL2/CCR2 and Phenotype Switching. Acta Pharmacol. Sin. 2024, 45, 959–974. [Google Scholar] [CrossRef]
- Amrute, J.M.; Luo, X.; Penna, V.; Yang, S.; Yamawaki, T.; Hayat, S.; Bredemeyer, A.; Jung, I.-H.; Kadyrov, F.F.; Heo, G.S.; et al. Targeting Immune-Fibroblast Cell Communication in Heart Failure. Nature 2024, 635, 423–433. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling through Coronary Microvascular Endothelial Inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Matsiukevich, D.; Kovacs, A.; Li, T.; Kokkonen-Simon, K.; Matkovich, S.J.; Oladipupo, S.S.; Ornitz, D.M. Characterization of a Robust Mouse Model of Heart Failure with Preserved Ejection Fraction. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H203–H231. [Google Scholar] [CrossRef] [PubMed]
- Meagher, P.; Adam, M.; Civitarese, R.; Bugyei-Twum, A.; Connelly, K.A. Heart Failure with Preserved Ejection Fraction in Diabetes: Mechanisms and Management. Can. J. Cardiol. 2018, 34, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Manabe, I. Cellular Interplay between Cardiomyocytes and Nonmyocytes in Cardiac Remodeling. Int. J. Inflam. 2011, 2011, 535241. [Google Scholar] [CrossRef]
- Tromp, J.; Lim, S.L.; Tay, W.T.; Teng, T.-H.K.; Chandramouli, C.; Ouwerkerk, W.; Wander, G.S.; Sawhney, J.P.S.; Yap, J.; MacDonald, M.R.; et al. Microvascular Disease in Patients with Diabetes with Heart Failure and Reduced Ejection Versus Preserved Ejection Fraction. Diabetes Care 2019, 42, 1792–1799. [Google Scholar] [CrossRef]
- Simmonds, S.J.; Cuijpers, I.; Heymans, S.; Jones, E.A.V. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020, 9, 242. [Google Scholar] [CrossRef]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac Inflammation Contributes to Changes in the Extracellular Matrix in Patients with Heart Failure and Normal Ejection Fraction. Circ. Heart Fail 2011, 4, 44–52. [Google Scholar] [CrossRef]
- Hulsmans, M.; Sager, H.B.; Roh, J.D.; Valero-Muñoz, M.; Houstis, N.E.; Iwamoto, Y.; Sun, Y.; Wilson, R.M.; Wojtkiewicz, G.; Tricot, B.; et al. Cardiac Macrophages Promote Diastolic Dysfunction. J. Exp. Med. 2018, 215, 423–440. [Google Scholar] [CrossRef]
- Glezeva, N.; Voon, V.; Watson, C.; Horgan, S.; McDonald, K.; Ledwidge, M.; Baugh, J. Exaggerated Inflammation and Monocytosis Associate with Diastolic Dysfunction in Heart Failure with Preserved Ejection Fraction: Evidence of M2 Macrophage Activation in Disease Pathogenesis. J. Card. Fail. 2015, 21, 167–177. [Google Scholar] [CrossRef]
- O’Rourke, S.A.; Dunne, A.; Monaghan, M.G. The Role of Macrophages in the Infarcted Myocardium: Orchestrators of ECM Remodeling. Front. Cardiovasc. Med. 2019, 6, 101. [Google Scholar] [CrossRef]
- Yuan, X.; Braun, T. Multimodal Regulation of Cardiac Myocyte Proliferation. Circ. Res. 2017, 121, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Mongirdienė, A.; Liobikas, J. Phenotypic and Functional Heterogeneity of Monocyte Subsets in Chronic Heart Failure Patients. Biology 2022, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Pan, Y.; Ning, X.; Shi, X.; Zhong, J.; Fan, X.; Li, W.; Teng, Y.; Liu, X.; Yu, B.; et al. Targeted Heart Repair by Tβ4-Loaded Cardiac-Resident Macrophage-Derived Extracellular Vesicles Modified with Monocyte Membranes. Acta Biomater. 2023, 169, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, M.; Lang, Y.; Zhang, J.; Wang, Y.; Zheng, H.; Zheng, Y.; Zhou, B. Subepicardial Adipose Genes Contribute to the Deterioration of Heart Failure Preserved Ejection Fraction. Front. Cardiovasc. Med. 2025, 12, 1501397. [Google Scholar] [CrossRef]
- Grune, J.; Lewis, A.J.M.; Yamazoe, M.; Hulsmans, M.; Rohde, D.; Xiao, L.; Zhang, S.; Ott, C.; Calcagno, D.M.; Zhou, Y.; et al. Neutrophils Incite and Macrophages Avert Electrical Storm after Myocardial Infarction. Nat. Cardiovasc. Res. 2022, 1, 649–664. [Google Scholar] [CrossRef]
- Jia, D.; Chen, S.; Bai, P.; Luo, C.; Liu, J.; Sun, A.; Ge, J. Cardiac Resident Macrophage-Derived Legumain Improves Cardiac Repair by Promoting Clearance and Degradation of Apoptotic Cardiomyocytes After Myocardial Infarction. Circulation 2022, 145, 1542–1556. [Google Scholar] [CrossRef]
- Tan, H.; Li, W.; Pang, Z.; Weng, X.; Gao, J.; Chen, J.; Wang, Q.; Li, Q.; Yang, H.; Dong, Z.; et al. Genetically Engineered Macrophages Co-Loaded with CD47 Inhibitors Synergistically Reconstruct Efferocytosis and Improve Cardiac Remodeling Post Myocardial Ischemia Reperfusion Injury. Adv. Health Mater. 2024, 13, e2303267. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245. [Google Scholar] [CrossRef]
- Chen, J.; Fu, C.-Y.; Shen, G.; Wang, J.; Xu, L.; Li, H.; Cao, X.; Zheng, M.-Z.; Shen, Y.-L.; Zhong, J.; et al. Macrophages Induce Cardiomyocyte Ferroptosis via Mitochondrial Transfer. Free Radic. Biol. Med. 2022, 190, 1–14. [Google Scholar] [CrossRef]
- Nicolás-Ávila, J.A.; Pena-Couso, L.; Muñoz-Cánoves, P.; Hidalgo, A. Macrophages, Metabolism and Heterophagy in the Heart. Circ. Res. 2022, 130, 418–431. [Google Scholar] [CrossRef]
- Nicolás-Ávila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martínez, L.; Sánchez-Díaz, M.; Díaz-García, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109.e23. [Google Scholar] [CrossRef]
- Gao, J.; Huang, C.; Kong, L.; Zhou, W.; Sun, M.; Wei, T.; Shen, W. SIRT3 Regulates Clearance of Apoptotic Cardiomyocytes by Deacetylating Frataxin. Circ. Res. 2023, 133, 631–647. [Google Scholar] [CrossRef]
- Cai, S.; Zhao, M.; Zhou, B.; Yoshii, A.; Bugg, D.; Villet, O.; Sahu, A.; Olson, G.S.; Davis, J.; Tian, R. Mitochondrial Dysfunction in Macrophages Promotes Inflammation and Suppresses Repair after Myocardial Infarction. J. Clin. Investig. 2023, 133, e159498. [Google Scholar] [CrossRef] [PubMed]
- Sugita, J.; Fujiu, K.; Nakayama, Y.; Matsubara, T.; Matsuda, J.; Oshima, T.; Liu, Y.; Maru, Y.; Hasumi, E.; Kojima, T.; et al. Cardiac Macrophages Prevent Sudden Death during Heart Stress. Nat. Commun. 2021, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522.e20. [Google Scholar] [CrossRef] [PubMed]
- Simon-Chica, A.; Fernández, M.C.; Wülfers, E.M.; Lother, A.; Hilgendorf, I.; Seemann, G.; Ravens, U.; Kohl, P.; Schneider-Warme, F. Novel Insights into the Electrophysiology of Murine Cardiac Macrophages: Relevance of Voltage-Gated Potassium Channels. Cardiovasc. Res. 2022, 118, 798–813. [Google Scholar] [CrossRef]
- Kotecha, D.; Lam, C.S.P.; Van Veldhuisen, D.J.; Van Gelder, I.C.; Voors, A.A.; Rienstra, M. Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J. Am. Coll. Cardiol. 2016, 68, 2217–2228. [Google Scholar] [CrossRef]
- Oraii, A.; McIntyre, W.F.; Parkash, R.; Kowalik, K.; Razeghi, G.; Benz, A.P.; Belley-Côté, E.P.; Conen, D.; Connolly, S.J.; Tang, A.S.L.; et al. Atrial Fibrillation Ablation in Heart Failure with Reduced vs Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. JAMA Cardiol. 2024, 9, 545–555. [Google Scholar] [CrossRef]
- Ninni, S.; Dombrowicz, D.; de Winther, M.; Staels, B.; Montaigne, D.; Nattel, S. Genetic Factors Altering Immune Responses in Atrial Fibrillation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2024, 83, 1163–1176. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, A.; Lin, H.; Wang, X.; Yang, K.; Zhang, H.; Fan, G.; Li, L. Role of Macrophages in Cardiac Arrhythmias: Pathogenesis and Therapeutic Perspectives. Int. Immunopharmacol. 2025, 149, 114206. [Google Scholar] [CrossRef]
- Lin, H.; Liu, H.; Xi, H.; Li, D.; Jiang, P.; Wang, Y.; Cheng, S.; Jiang, H.; Deng, H.; Zhou, X.; et al. Oxygen-Independent Photodynamic Therapy-Mediated Selective Consumption of M1 Macrophage Against Ventricular Arrhythmias via Sympathetic Neuromodulation. Small 2025, 21, 2409244. [Google Scholar] [CrossRef]
- Wilck, N.; Markó, L.; Balogh, A.; Kräker, K.; Herse, F.; Bartolomaeus, H.; Szijártó, I.A.; Gollasch, M.; Reichhart, N.; Strauss, O.; et al. Nitric Oxide-Sensitive Guanylyl Cyclase Stimulation Improves Experimental Heart Failure with Preserved Ejection Fraction. JCI Insight 2018, 3, e96006. [Google Scholar] [CrossRef]
- Mollace, R.; Scarano, F.; Bava, I.; Carresi, C.; Maiuolo, J.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Muscoli, S.; Palma, E.; et al. Modulation of the Nitric Oxide/cGMP Pathway in Cardiac Contraction and Relaxation: Potential Role in Heart Failure Treatment. Pharmacol. Res. 2023, 196, 106931. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Redfield, M.M.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction: A Review. JAMA 2023, 329, 827. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-Induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Zhang, N.; Feng, B.; Ma, X.; Sun, K.; Xu, G.; Zhou, Y. Dapagliflozin Improves Left Ventricular Remodeling and Aorta Sympathetic Tone in a Pig Model of Heart Failure with Preserved Ejection Fraction. Cardiovasc. Diabetol. 2019, 18, 107. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ding, Y.; Chen, W.; Shi, J.; Zhang, C.; Zhao, X.; Li, J.; Han, Z.; Chen, X. VASP Knockdown Ameliorates Lipopolysaccharide-Induced Acute Lung Injury with Inhibition of M1 Macrophage Polarization Through the cGMP-PKG Signaling Pathway. Inflammation 2025. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; Deng, Y.; Yang, Y.; Chen, X.; Chen, Q.; Liu, Y.; Fu, X.; Kwan, H.Y.; You, Y.; et al. Zhenwu Decoction Ameliorates Cardiac Hypertrophy through Activating sGC (Soluble Guanylate Cyclase)—cGMP (Cyclic Guanosine Monophosphate)—PKG (Protein Kinase G) Pathway. J. Ethnopharmacol. 2023, 300, 115705. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, K.; Wang, X.; Guo, K.; Li, X.; Chen, F.; Du, R.; Li, S.; Li, L.; Yang, Z.; et al. Multi-Omics Approach for Identification of Molecular Alterations of QiShenYiQi Dripping Pills in Heart Failure with Preserved Ejection Fraction. J. Ethnopharmacol. 2023, 315, 116673. [Google Scholar] [CrossRef]
- Shan, X.; Ji, Z.; Wang, B.; Zhang, Y.; Dong, H.; Jing, W.; Zhou, Y.; Hu, P.; Cui, Y.; Li, Z.; et al. Riboflavin Kinase Binds and Activates Inducible Nitric Oxide Synthase to Reprogram Macrophage Polarization. Redox Biol. 2024, 78, 103413. [Google Scholar] [CrossRef]
- Wu, K.K.-L.; Xu, X.; Wu, M.; Li, X.; Hoque, M.; Li, G.H.Y.; Lian, Q.; Long, K.; Zhou, T.; Piao, H.; et al. MDM2 Induces Pro-Inflammatory and Glycolytic Responses in M1 Macrophages by Integrating iNOS-Nitric Oxide and HIF-1α Pathways in Mice. Nat. Commun. 2024, 15, 8624. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wen, J.; He, A.; Qu, C.; Peng, Y.; Luo, S.; Wang, X. iNOS Contributes to Heart Failure with Preserved Ejection Fraction through Mitochondrial Dysfunction and Akt S-Nitrosylation. J. Adv. Res. 2023, 43, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Gojova, A.; Marchiol-Fournigault, C.; Esposito, B.; Kamaté, C.; Merval, R.; Fradelizi, D.; Tedgui, A. Inhibition of Transforming Growth Factor-β Signaling Accelerates Atherosclerosis and Induces an Unstable Plaque Phenotype in Mice. Circ. Res. 2001, 89, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yuan, H.-Q.; Hao, Y.-M.; Ren, Z.; Qu, S.-L.; Liu, L.-S.; Wei, D.-H.; Tang, Z.-H.; Zhang, J.-F.; Jiang, Z.-S. Macrophage Polarization in Atherosclerosis. Clin. Chim. Acta 2020, 501, 142–146. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Sakowicz, A.; Misztal, M.; von Haehling, S.; Ahmed, A.; Pietrucha, T.; Rysz, J.; Banach, M. Differences in Biochemical and Genetic Biomarkers in Patients with Heart Failure of Various Etiologies. Int. J. Cardiol. 2016, 221, 1073–1080. [Google Scholar] [CrossRef]
- Boichenko, V.; Noakes, V.M.; Reilly-O’Donnell, B.; Luciani, G.B.; Emanueli, C.; Martelli, F.; Gorelik, J. Circulating Non-Coding RNAs as Indicators of Fibrosis and Heart Failure Severity. Cells 2025, 14, 553. [Google Scholar] [CrossRef]
- Tuleta, I.; Hanna, A.; Humeres, C.; Aguilan, J.T.; Sidoli, S.; Zhu, F.; Frangogiannis, N.G. Fibroblast-Specific TGF-β Signaling Mediates Cardiac Dysfunction, Fibrosis, and Hypertrophy in Obese Diabetic Mice. Cardiovasc. Res. 2024, 120, 2047–2063. [Google Scholar] [CrossRef]
- Chen, G.; Xu, H.; Xu, T.; Ding, W.; Zhang, G.; Hua, Y.; Wu, Y.; Han, X.; Xie, L.; Liu, B.; et al. Calycosin Reduces Myocardial Fibrosis and Improves Cardiac Function in Post-Myocardial Infarction Mice by Suppressing TGFBR1 Signaling Pathways. Phytomedicine 2022, 104, 154277. [Google Scholar] [CrossRef]
- Yao, Y.; Hu, C.; Song, Q.; Li, Y.; Da, X.; Yu, Y.; Li, H.; Clark, I.M.; Chen, Q.; Wang, Q.K. ADAMTS16 Activates Latent TGF-β, Accentuating Fibrosis and Dysfunction of the Pressure-Overloaded Heart. Cardiovasc. Res. 2019, 116, 956–969. [Google Scholar] [CrossRef]
- Chowkwale, M.; Lindsey, M.L.; Saucerman, J.J. Intercellular Model Predicts Mechanisms of Inflammation-Fibrosis Coupling after Myocardial Infarction. J. Physiol. 2023, 601, 2635–2654. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Wang, X.; Wang, P.; Essandoh, K.; Cui, S.; Huang, W.; Mu, X.; Liu, Z.; Wang, Y.; et al. GDF3 Protects Mice against Sepsis-Induced Cardiac Dysfunction and Mortality by Suppression of Macrophage Pro-Inflammatory Phenotype. Cells 2020, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Transforming Growth Factor-β in Myocardial Disease. Nat. Rev. Cardiol. 2022, 19, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.-J.; et al. Fibroblast-Specific TGF-β-Smad2/3 Signaling Underlies Cardiac Fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Xiong, S.; Yang, L.; Yang, C.; Qiao, W.; Liu, Y.; Liu, S.; Liu, J.; Dong, G. Ling-Gui-Qi-Hua Formula Alleviates Left Ventricular Myocardial Fibrosis in Rats with Heart Failure with Preserved Ejection Fraction by Blocking the Transforming Growth Factor-Β1 /Smads Signaling Pathway. J. Ethnopharmacol. 2023, 317, 116849. [Google Scholar] [CrossRef]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Cao, X. Self-Regulation and Cross-Regulation of Pattern-Recognition Receptor Signalling in Health and Disease. Nat. Rev. Immunol. 2016, 16, 35–50. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, S.; Chang, S.; Ren, D.; Shali, S.; Li, C.; Yang, H.; Huang, Z.; Ge, J. M2 Macrophage-Derived Exosomes Carry microRNA-148a to Alleviate Myocardial Ischemia/Reperfusion Injury via Inhibiting TXNIP and the TLR4/NF-κB/NLRP3 Inflammasome Signaling Pathway. J. Mol. Cell. Cardiol. 2020, 142, 65–79. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, J.; Jiang, S.; Ma, Z.; Wang, D.; Hu, W.; Deng, C.; Fan, C.; Di, S.; Sun, Y.; et al. The Emerging Role of Toll-like Receptor 4 in Myocardial Inflammation. Cell Death Dis. 2016, 7, e2234. [Google Scholar] [CrossRef]
- Yang, D.; Yang, L.; Cai, J.; Hu, X.; Li, H.; Zhang, X.; Zhang, X.; Chen, X.; Dong, H.; Nie, H.; et al. A Sweet Spot for Macrophages: Focusing on Polarization. Pharmacol. Res. 2021, 167, 105576. [Google Scholar] [CrossRef]
- Deng, T.; Hu, B.; Wang, X.; Ding, S.; Lin, L.; Yan, Y.; Peng, X.; Zheng, X.; Liao, M.; Jin, Y.; et al. TRAF6 Autophagic Degradation by Avibirnavirus VP3 Inhibits Antiviral Innate Immunity via Blocking NFKB/NF-κB Activation. Autophagy 2022, 18, 2781–2798. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, B.; Wang, M.; Zha, Y.; Lu, S.; Zhang, F.; Peng, Y.; Duan, Y.; Zhong, D.; Zhang, S. Daucosterol Alleviates Heart Failure with Preserved Ejection Fraction through Activating PPARα Pathway. Heliyon 2024, 10, e38379. [Google Scholar] [CrossRef]
- Hao, J.-M.; Sun, P.; Zeng, Y.; Zhang, H.; Zhang, Z.-D.; Chang, L.-P.; Hou, Y.-L. Qiliqiangxin Capsule Improves Cardiac Remodeling in Rats with DOCA-Salt-Induced Diastolic Dysfunction. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7264–7275. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 Inflammasome in Inflammatory Diseases. Nat. Rev. Drug Discov. 2018, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and Regulation of the Inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Toldo, S.; Mezzaroma, E.; Buckley, L.F.; Potere, N.; Di Nisio, M.; Biondi-Zoccai, G.; Van Tassell, B.W.; Abbate, A. Targeting the NLRP3 Inflammasome in Cardiovascular Diseases. Pharmacol. Ther. 2022, 236, 108053. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, W.; Zhang, Y.; Li, J.; Zhao, Y.; Pan, L.; Shen, Z.; Chen, W.; Hui, J. Aminooxyacetic Acid Attenuates Post-Infarct Cardiac Dysfunction by Balancing Macrophage Polarization through Modulating Macrophage Metabolism in Mice. J. Cell Mol. Med. 2020, 24, 2593–2609. [Google Scholar] [CrossRef]
- Golino, M.; Moroni, F.; Carbone, S.; Corna, G.; Trankle, C.; Billingsley, H.E.; Del Buono, M.G.; Talasaz, A.H.; Thomas, G.K.; De Ponti, R.; et al. Differential Response to Interleukin-1 Blockade with Anakinra on Cardiorespiratory Fitness in Patients with Heart Failure with Preserved Ejection Fraction Stratified According to Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2023, 12, e031251. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Arena, R.; Biondi-Zoccai, G.; Canada, J.M.; Oddi, C.; Abouzaki, N.A.; Jahangiri, A.; Falcao, R.A.; Kontos, M.C.; Shah, K.B.; et al. Effects of Interleukin-1 Blockade with Anakinra on Aerobic Exercise Capacity in Patients with Heart Failure and Preserved Ejection Fraction (from the D-HART Pilot Study). Am. J. Cardiol. 2014, 113, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Trankle, C.R.; Canada, J.M.; Carbone, S.; Buckley, L.; Kadariya, D.; Del Buono, M.G.; Billingsley, H.; Wohlford, G.; Viscusi, M.; et al. IL-1 Blockade in Patients with Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2018, 11, e005036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-S.; He, Q.-Z.; Qin, C.H.; Little, P.J.; Weng, J.-P.; Xu, S.-W. Therapeutic Potential of Colchicine in Cardiovascular Medicine: A Pharmacological Review. Acta Pharmacol. Sin. 2022, 43, 2173–2190. [Google Scholar] [CrossRef]
- Shen, S.; Duan, J.; Hu, J.; Qi, Y.; Kang, L.; Wang, K.; Chen, J.; Wu, X.; Xu, B.; Gu, R. Colchicine Alleviates Inflammation and Improves Diastolic Dysfunction in Heart Failure Rats with Preserved Ejection Fraction. Eur. J. Pharmacol. 2022, 929, 175126. [Google Scholar] [CrossRef]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Aksoylar, H.I.; Horng, T. Control of Macrophage Metabolism and Activation by mTOR and Akt Signaling. Semin. Immunol. 2015, 27, 286–296. [Google Scholar] [CrossRef]
- Ieronymaki, E.; Daskalaki, M.G.; Lyroni, K.; Tsatsanis, C. Insulin Signaling and Insulin Resistance Facilitate Trained Immunity in Macrophages Through Metabolic and Epigenetic Changes. Front. Immunol. 2019, 10, 1330. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox Regulation of the Immune Response. Cell Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- You, Z.; Yang, Z.; Cao, S.; Deng, S.; Chen, Y. The Novel KLF4/BIG1 Regulates LPS-Mediated Neuro-Inflammation and Migration in BV2 Cells via PI3K/Akt/NF-kB Signaling Pathway. Neuroscience 2022, 488, 102–111. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- McClain, K.L.; Bigenwald, C.; Collin, M.; Haroche, J.; Marsh, R.A.; Merad, M.; Picarsic, J.; Ribeiro, K.B.; Allen, C.E. Histiocytic Disorders. Nat. Rev. Dis. Primers 2021, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Huang, Z.; Lin, L.; Fang, Z.; Li, W.; Luo, W.; Wu, G.; Huang, Z.; Liang, G. Tussilagone Attenuates Atherosclerosis through Inhibiting MAPKs-Mediated Inflammation in Macrophages. Int. Immunopharmacol. 2023, 119, 110066. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Zhong, J.; Liu, H.; Li, W.; Chen, M.; Xu, L.; Zhang, W.; Zhang, Z.; Wei, Z.; et al. LRG1 Promotes Atherosclerosis by Inducing Macrophage M1-like Polarization. Proc. Natl. Acad. Sci. USA 2024, 121, e2405845121. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Long, Q.; Yang, H.; Yang, H.; Tang, Y.; Liu, B.; Zhou, Z.; Yuan, J. Sacubitril/Valsartan Inhibits M1 Type Macrophages Polarization in Acute Myocarditis by Targeting C-Type Natriuretic Peptide. Biomed. Pharmacother. 2024, 174, 116535. [Google Scholar] [CrossRef]
- Yuan, L.; Bu, S.; Du, M.; Wang, Y.; Ju, C.; Huang, D.; Xu, W.; Tan, X.; Liang, M.; Deng, S.; et al. RNF207 Exacerbates Pathological Cardiac Hypertrophy via Post-Translational Modification of TAB1. Cardiovasc. Res. 2023, 119, 183–194. [Google Scholar] [CrossRef]
- Hu, B.; Xu, L.; Li, Y.; Bai, X.; Xing, M.; Cao, Q.; Liang, H.; Song, S.; Ji, A. A peptide inhibitor of macrophage migration in atherosclerosis purified from the leech Whitmania pigra. J. Ethnopharmacol. 2020, 254, 112723. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Huang, X.-R.; Zhang, J.; Wu, W.; Chen, J.; Wan, S.; Yu, X.-Y.; Lan, H.-Y. Neuropeptide Y Attenuates Cardiac Remodeling and Deterioration of Function Following Myocardial Infarction. Mol. Ther. 2022, 30, 881–897. [Google Scholar] [CrossRef]
- Tian, J.; Li, W.; Zeng, L.; Li, Y.; Du, J.; Li, Y.; Li, B.; Su, G. HBI-8000 Improves Heart Failure with Preserved Ejection Fraction via the TGF-Β1/MAPK Signalling Pathway. J. Cell Mol. Med. 2024, 28, e18238. [Google Scholar] [CrossRef]
- Li, S.; Shi, Y.; Yuan, S.; Ruan, J.; Pan, H.; Ma, M.; Huang, G.; Ji, Q.; Zhong, Y.; Jiang, T. Inhibiting the MAPK Pathway Improves Heart Failure with Preserved Ejection Fraction Induced by Salt-Sensitive Hypertension. Biomed. Pharmacother. 2024, 170, 115987. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Meng, W.; Zheng, Y.; Guan, X.; Shao, C.; Li, X.; Hu, D.; Wang, M.; Guo, H. Dihydrotanshinone I Improves Cardiac Function by Promoting Lymphangiogenesis after Myocardial Ischemia-Reperfusion Injury. Eur. J. Pharmacol. 2025, 989, 177245. [Google Scholar] [CrossRef]
- Xu, S.; Hu, C.; Han, J.; Luo, W.; Huang, L.; Jiang, Y.; Samorodov, A.V.; Wang, Y.; Huang, J. Schisandrin B Alleviates Angiotensin II-Induced Cardiac Inflammatory Remodeling by Inhibiting the Recruitment of MyD88 to TLRs in Mouse Cardiomyocytes. Int. Immunopharmacol. 2024, 139, 112660. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wu, M.; Xu, J.; Xu, Q.; Wan, F.; Zhong, H.; Zhang, J.; Zhou, G.; Qin, H.; Li, H.; et al. Mechanism of Vanillic Acid against Cardiac Fibrosis Induced by Isoproterenol in Mice Based on Drp1/HK1/NLRP3 and Mitochondrial Apoptosis Signaling Pathways. Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China J. Chin. Mater. Medica 2025, 50, 2193–2208. [Google Scholar]
- Yu, T.; Xu, J.; Wang, Q.; Han, X.; Tu, Y.; Wang, Y.; Luo, W.; Wang, M.; Liang, G. 20(S)-Ginsenoside Rh2 Inhibits Angiotensin-2 Mediated Cardiac Remodeling and Inflammation Associated with Suppression of the JNK/AP-1 Pathway. Biomed. Pharmacother. 2023, 169, 115880. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wu, G.; Luo, W.; Li, W.; Li, X.; Dai, C.; Huang, W.; Liang, G. Leonurine Attenuates Angiotensin II-Induced Cardiac Injury and Dysfunction via Inhibiting MAPK and NF-κB Pathway. Phytomedicine 2023, 108, 154519. [Google Scholar] [CrossRef]
- Wang, M.; Luo, W.; Yu, T.; Liang, S.; Sun, J.; Zhang, Y.; Han, X.; Long, X.; Liang, G.; Li, G. Corynoline Protects Ang II-Induced Hypertensive Heart Failure by Increasing PPARα and Inhibiting NF-κB Pathway. Biomed. Pharmacother. 2022, 150, 113075. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y.; Pan, Z.; Hu, X.; Xu, Y.; Huang, Z.; Sun, Z.; Yuan, M.; Shi, J.; Huang, P.; et al. Gentiopicroside Alleviates Cardiac Inflammation and Fibrosis in T2DM Rats through Targeting Smad3 Phosphorylation. Phytomedicine 2022, 106, 154389. [Google Scholar] [CrossRef]
- Li, Y.; Feng, L.; Li, G.; An, J.; Zhang, S.; Li, J.; Liu, J.; Ren, J.; Yang, L.; Qi, Z. Resveratrol Prevents ISO-Induced Myocardial Remodeling Associated with Regulating Polarization of Macrophages through VEGF-B/AMPK/NF-kB Pathway. Int. Immunopharmacol. 2020, 84, 106508. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Yan, L.; He, Q.; Xie, H.; Chen, M. Resveratrol Ameliorates Cardiac Remodeling in a Murine Model of Heart Failure with Preserved Ejection Fraction. Front. Pharmacol. 2021, 12, 646240. [Google Scholar] [CrossRef]
- Qi, W.; Boliang, W.; Xiaoxi, T.; Guoqiang, F.; Jianbo, X.; Gang, W. Cardamonin Protects against Doxorubicin-Induced Cardiotoxicity in Mice by Restraining Oxidative Stress and Inflammation Associated with Nrf2 signalingCardamonin. Biomed. Pharmacother. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Zhang, N.; Shou, B.; Chen, L.; Lai, X.; Luo, Y.; Meng, X.; Liu, R. Cardioprotective Effects of Latifolin Against Doxorubicin-Induced Cardiotoxicity by Macrophage Polarization in Mice. J. Cardiovasc. Pharmacol. 2020, 75, 564–572. [Google Scholar] [CrossRef]
- Ni, S.-H.; Sun, S.-N.; Zhou, Z.; Li, Y.; Huang, Y.-S.; Li, H.; Wang, J.-J.; Xiao, W.; Xian, S.-X.; Yang, Z.-Q.; et al. Arctigenin Alleviates Myocardial Infarction Injury through Inhibition of the NFAT5-Related Inflammatory Phenotype of Cardiac Macrophages/Monocytes in Mice. Lab. Investig. 2020, 100, 527–541. [Google Scholar] [CrossRef]
- Li, R.; Lu, K.; Wang, Y.; Chen, M.; Zhang, F.; Shen, H.; Yao, D.; Gong, K.; Zhang, Z. Triptolide Attenuates Pressure Overload-Induced Myocardial Remodeling in Mice via the Inhibition of NLRP3 Inflammasome Expression. Biochem. Biophys. Res. Commun. 2017, 485, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Luo, W.; Han, J.; Zhang, Q.; Ji, L.; Samorodov, A.V.; Pavlov, V.N.; Zhuang, Z.; Yang, D.; Yin, L.; et al. Schisandrin B Protects against LPS-Induced Inflammatory Lung Injury by Targeting MyD88. Phytomedicine 2023, 108, 154489. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Cui, J.; Li, X.; Chen, S.; Gao, K.; Mei, X. Modified ZIF-8 Nanoparticles for Targeted Metabolic Treatment of Acute Spinal Cord Injury. ACS Appl. Mater. Interfaces 2024, 16, 14503–14509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Luo, S.; Zhao, T.; Wu, M.; Huang, L.; Zhang, L.; Huang, Y.; Gao, H.; Sun, X.; Gong, T.; et al. Scavenger Receptor A-Mediated Nanoparticles Target M1 Macrophages for Acute Liver Injury. Asian J. Pharm. Sci. 2023, 18, 100813. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Y.; Wang, J.; Wang, X.; Chen, X.; Yin, K.; Zhu, X. Leonurine Improves Atherosclerosis by Activating Foam Cell Autophagy and Metabolic Remodeling via METTL3-Mediated AKT1S1 mRNA Stability Modulation. Phytomedicine 2024, 134, 155939. [Google Scholar] [CrossRef]
- Hyam, S.R.; Lee, I.-A.; Gu, W.; Kim, K.-A.; Jeong, J.-J.; Jang, S.-E.; Han, M.J.; Kim, D.-H. Arctigenin Ameliorates Inflammation in Vitro and in Vivo by Inhibiting the PI3K/AKT Pathway and Polarizing M1 Macrophages to M2-like Macrophages. Eur. J. Pharmacol. 2013, 708, 21–29. [Google Scholar] [CrossRef]
- Lin, R.; Duan, J.; Mu, F.; Bian, H.; Zhao, M.; Zhou, M.; Li, Y.; Wen, A.; Yang, Y.; Xi, M. Cardioprotective Effects and Underlying Mechanism of Radix Salvia Miltiorrhiza and Lignum Dalbergia Odorifera in a Pig Chronic Myocardial Ischemia Model. Int. J. Mol. Med. 2018, 42, 2628–2640. [Google Scholar] [CrossRef]
- Mu, F.; Duan, J.; Bian, H.; Yin, Y.; Zhu, Y.; Wei, G.; Guan, Y.; Wang, Y.; Guo, C.; Wen, A.; et al. Cardioprotective Effects and Mechanism of Radix Salviae Miltiorrhizae and Lignum Dalbergiae Odoriferae on Rat Myocardial Ischemia/Reperfusion Injury. Mol. Med. Rep. 2017, 16, 1759–1770. [Google Scholar] [CrossRef]
- Qin, L.; Tan, J.; Lv, X.; Zhang, J. Vanillic Acid Alleviates Liver Fibrosis through Inhibiting Autophagy in Hepatic Stellate Cells via the MIF/CD74 Signaling Pathway. Biomed. Pharmacother. 2023, 168, 115673. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Tang, Z.; Li, B.; Yu, J.; Li, W.; Liu, Z.; Tian, C. Resveratrol against Cardiac Fibrosis: Research Progress in Experimental Animal Models. Molecules 2021, 26, 6860. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sun, K.; Liu, Y.; Yin, X.; Zhu, H.; Yu, F.; Zhao, W. Resveratrol-Loaded Macrophage Exosomes Alleviate Multiple Sclerosis through Targeting Microglia. J. Control Release 2023, 353, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Chen, Y.; Lyu, W.; He, X.; Ye, Z.; Huang, C.-Y.; He, X.-L.; Chen, X.; Chen, X.; Zhang, B.; et al. Ginsenoside Rh2 Augmented Anti-PD-L1 Immunotherapy by Reinvigorating CD8+ T Cells via Increasing Intratumoral CXCL10. Pharmacol. Res. 2023, 198, 106988. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, S.; Li, Y.; Shao, P.; Jiang, J. Inhibition of Macrophage Polarization and Pyroptosis in Collagen-Induced Arthritis through MSC-Exo and Ginsenoside Rh2. Arthritis Res. Ther. 2025, 27, 6. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Zhu, G.; Xi, X.; Li, K.; Wu, C.; Huang, L. Corynoline Attenuates LPS-Induced Acute Lung Injury in Mice by Activating Nrf2. Int. Immunopharmacol. 2017, 48, 96–101. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Liu, Y.; Tan, X.; Liu, L.; Zhang, B.; Li, Z.; Kang, L.; Hu, L. Corynoline Protects Chronic Pancreatitis via Binding to PSMA2 and Alleviating Pancreatic Fibrosis. J. Gastroenterol. 2024, 59, 1037–1051. [Google Scholar] [CrossRef]
- Yong, Q.; Huang, C.; Chen, B.; An, J.; Zheng, Y.; Zhao, L.; Peng, C.; Liu, F. Gentiopicroside Improves NASH and Liver Fibrosis by Suppressing TLR4 and NLRP3 Signaling Pathways. Biomed. Pharmacother. 2024, 177, 116952. [Google Scholar] [CrossRef]
- Song, J.; He, G.-N.; Dai, L. A Comprehensive Review on Celastrol, Triptolide and Triptonide: Insights on Their Pharmacological Activity, Toxicity, Combination Therapy, New Dosage Form and Novel Drug Delivery Routes. Biomed. Pharmacother. 2023, 162, 114705. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, K.; Zhu, Z.; Shao, F.; Qian, R.; Wang, C.; Dong, H.; Li, Y.; Gao, Z.; Zhao, J. Triptolide with Hepatotoxicity and Nephrotoxicity Used in Local Delivery Treatment of Myocardial Infarction by Thermosensitive Hydrogel. J. Nanobiotechnology 2023, 21, 227. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Li, W.; Zhang, Q.; Jiang, Y.; Guo, D.; Sun, X.; Lu, W.; Li, C.; Wang, Y. TFEB-NF-κB Inflammatory Signaling Axis: A Novel Therapeutic Pathway of Dihydrotanshinone I in Doxorubicin-Induced Cardiotoxicity. J. Exp. Clin. Cancer Res. CR 2020, 39, 93. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Zhou, R.; Ju, D.; Li, M.; Wang, H.; Pan, L.; Wang, X.; Han, M.; Yu, Y. Zhen-Wu-Tang Protects against Myocardial Fibrosis by Inhibiting M1 Macrophage Polarization via the TLR4/NF-κB Pathway. Phytomedicine 2024, 130, 155719. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tan, Y.-Q.; Zhang, J.-Y.; Zhang, Y.; Su, L.-Q.; Hu, Z.-X.; Hu, S.-Y. Shenfu Injection Regulates Macrophage Polarization via TLR4/NF-κB Pathway to Reduce Inflammation in Chronic Heart Failure. Zhongguo Zhong Yao Za Zhi 2024, 49, 3574–3582. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; He, X.; Ouyang, X.; Zhang, X.; Li, J.; Chen, X.; Liu, D.; Sun, S.; Li, Z.; Li, S.; et al. Effects and Mechanisms of Xinyang Tablets Improving Uremic Cardiomyopathy Mice by Inhibiting Osteopontin-Mediated Cardiac Fibrosis Signaling. China J. Tradit. Chin. Med. Pharm. 2024, 39, 2273–2280. [Google Scholar]
- Shi, L.; Deng, J.; Yin, E.; Chen, X.; Du, X. Effect of Fangji Fulingtang on Macrophage Polarization and Oxidative Stress in Mouse Model of Myocardial Fibrosis. Chin. J. Exp. Tradit. Med. Formulae 2023, 29, 11–18. [Google Scholar] [CrossRef]
- Anwaier, G.; Xie, T.-T.; Pan, C.-S.; Li, A.-Q.; Yan, L.; Wang, D.; Chen, F.-K.; Weng, D.-Z.; Sun, K.; Chang, X.; et al. QiShenYiQi Pill Ameliorates Cardiac Fibrosis After Pressure Overload-Induced Cardiac Hypertrophy by Regulating FHL2 and the Macrophage RP S19/TGF-Β1 Signaling Pathway. Front. Pharmacol. 2022, 13, 918335. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, K.; Liu, M.; Su, J.; Qin, X.; Wang, X.; Zhang, J.; Li, S.; Fan, G. An Herbal Preparation Ameliorates Heart Failure with Preserved Ejection Fraction by Alleviating Microvascular Endothelial Inflammation and Activating NO-cGMP-PKG Pathway. Phytomedicine 2021, 91, 153633. [Google Scholar] [CrossRef]
- Liu, B.; He, Y.; Lu, R.; Zhou, J.; Bai, L.; Zhang, P.; Ye, S.; Wu, J.; Liang, C.; Zhou, Y.; et al. Zhen-Wu-Tang Protects against Podocyte Injury in Rats with IgA Nephropathy via PPARγ/NF-κB Pathway. Biomed. Pharmacother. 2018, 101, 635–647. [Google Scholar] [CrossRef]
- Han, Y.; Huang, L.; Zhong, G.; Chang, X.; Zhu, Q.; Xu, M.; Mingtai, C.; Men, L.; Wang, L. Evaluation of the Safety and Efficacy of Zhenwu Decoction as Adjuvant Therapy for the Treatment of Heart Failure with Reduced Ejection Fraction: A Protocol for Systematic Review and Meta-Analysis. Medicine 2022, 101, e28672. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, Y.; Li, K. Zhenwu Decoction for Chronic Heart Failure: Protocol for a Systematic Review and Meta-Analysis. Medicine 2018, 97, e11559. [Google Scholar] [CrossRef]
- Liu, B.; Cao, Y.; Wang, D.; Zhou, Y.; Zhang, P.; Wu, J.; Chen, J.; Qiu, J.; Zhou, J. Zhen-Wu-Tang Induced Mitophagy to Protect Mitochondrial Function in Chronic Glomerulonephritis via PI3K/AKT/mTOR and AMPK Pathways. Front. Pharmacol. 2021, 12, 777670. [Google Scholar] [CrossRef]
- Mok, H.L.; Cheng, K.W.; Xu, Y.; Huang, C.; Lyu, C.; Xu, J.; Hu, D.; Zhu, L.; Lin, C.; Tan, H.-Y.; et al. Modified Zhenwu Decoction Suppresses Chronic Colitis via Targeting Macrophage CCR2/Fyn/P38 MAPK Signaling Axis. Phytomedicine 2024, 129, 155694. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Wan, W.; Guo, Y.; Cui, Y.; Liu, W.; Guo, F. Effects of Shenfu Injection on Myocardial Adenosine Receptors in Rats with Myocardial Ischemia-Reperfusion Postconditioning. Hum. Exp. Toxicol. 2021, 40, S300–S309. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Qin, C.; Wang, Z.; Gao, L.; Zhang, S.; Feng, Y.; Liu, J.; Tao, L. Effect of Shenfu Injection in Patients with Septic Shock: A Systemic Review and Meta-Analysis for Randomized Clinical Trials. J. Ethnopharmacol. 2024, 320, 117431. [Google Scholar] [CrossRef]
- Tao, L.; Mo, Z.; Li, Z.; Li, S.; Luo, Z.; Li, D.; Wang, D.; Zhu, W.; Ding, B. Efficacy and Safety of Shenfu Injection on Acute Heart Failure: A Systematic Review and Meta-Analysis. Phytomedicine 2023, 110, 154641. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Li, Z.; Mo, Z.; Luo, Z.; Li, D.; Wang, D.; Zhu, W.; Ding, B. Efficacy and Safety of Shenfu Injection for the Treatment of Post-Acute Myocardial Infarction Heart Failure: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 1027131. [Google Scholar] [CrossRef]
- Xu, F.-F.; Xie, X.-F.; Hu, H.-Y.; Tong, R.-S.; Peng, C. Shenfu Injection: A Review of Pharmacological Effects on Cardiovascular Diseases. Front. Pharmacol. 2024, 15, 1279584. [Google Scholar] [CrossRef]
- Wu, H.; Wu, W.; Li, R.; Chen, Y.; Wang, S. Effects of Xinyang Tablet on Brain Natriuretic Peptide, Ultrasensitive C-Reactive Protein and Cardiac Function in Patients with Acute Decompensated Heart Failure. Tradit. Chin. Drug Res. Clin. Pharmacol. 2011, 22, 220–223. [Google Scholar] [CrossRef]
- Salloum, F.N.; Chau, V.Q. Osteopontin in HFpEF: More Than Just a Remodeling-Specific Biomarker. J. Am. Coll. Cardiol. 2019, 73, 2719–2721. [Google Scholar] [CrossRef]
- Wu, G.; Yao, Z.; Zhang, X.; Li, G. Effect of Fangji Fuling Tang Adjuvant Therapy on Orphan Nuclear Receptors and Ventricular Remodeling in Peripheral Blood Mononuclear Cells from Patients with Refractory Heart Failure. Clin. J. Chin. Med. 2022, 14, 43–45. [Google Scholar]
- Wang, M.; Shan, Y.; Wu, C.; Cao, P.; Sun, W.; Han, J.; Shen, L.; Chen, J.; Yu, P.; Chen, X. Efficacy and Safety of Qishen Yiqi Dripping Pill for Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 626375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, S.; Fan, Z.; Zhang, Z.; Zhang, X.; Zhao, Z.; Wang, X.; Mao, J. Effect of Qishen Yiqi Dripping Pills on the Classification of Ejection Fraction in Patients with Ischaemic Heart Failure: A Prospective Cohort Study. Phytomedicine 2025, 139, 156530. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.M.; Panjrath, G.S.; Amancherla, K.; Davis, L.L.; Deswal, A.; Dixon, D.L.; Januzzi, J.L.; Yancy, C.W. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure with Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2023, 81, 1835–1878. [Google Scholar] [CrossRef]
- Usman, M.S.; Bhatt, D.L.; Hameed, I.; Anker, S.D.; Cheng, A.Y.Y.; Hernandez, A.F.; Jones, W.S.; Khan, M.S.; Petrie, M.C.; Udell, J.A.; et al. Effect of SGLT2 Inhibitors on Heart Failure Outcomes and Cardiovascular Death across the Cardiometabolic Disease Spectrum: A Systematic Review and Meta-Analysis. Lancet Diabetes Endocrinol. 2024, 12, 447–461. [Google Scholar] [CrossRef]
- Chen, P.; Ning, X.; Li, W.; Pan, Y.; Wang, L.; Li, H.; Fan, X.; Zhang, J.; Luo, T.; Wu, Y.; et al. Fabrication of Tβ4-Exosome-Releasing Artificial Stem Cells for Myocardial Infarction Therapy by Improving Coronary Collateralization. Bioact. Mater. 2022, 14, 416–429. [Google Scholar] [CrossRef]
- Rai, A.; Claridge, B.; Lozano, J.; Greening, D.W. The Discovery of Extracellular Vesicles and Their Emergence as a Next-Generation Therapy. Circ. Res. 2024, 135, 1. [Google Scholar] [CrossRef]
- Izzo, A.A.; Borrelli, F.; Capasso, R. Herbal Medicine: The Dangers of Drug Interaction. Trends Pharmacol. Sci. 2002, 23, 358–359. [Google Scholar] [CrossRef] [PubMed]

| Name | Structure | Source | In Vivo | Action Time | In Vitro | Targets or Related Signal Pathways | Changes in Macrophages | Ref. |
|---|---|---|---|---|---|---|---|---|
| Dihydrotanshinone I | 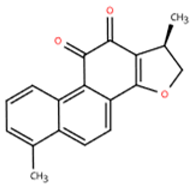 | Salvia miltiorrhiza | Ligation of the left anterior descending coronary artery in SD rats | 2 weeks | 350 μM H2O2 or 6 h OGD followed by 24 h reoxygenation-induced human lymphatic endothelial cells | LYVE-1, PROX1, VEGF-C, VEGFR-3, VE-cadherin, IGF-1, IGF-1R↑ | M1↓ | [141] |
| Schisandrin B | 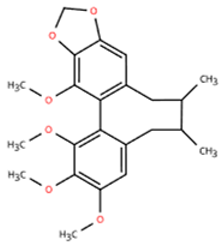 | Schisandra chinensis | AngII (1.4 mg/kg) s.c. in C57BL/6J wild-type mice | 2 weeks | 1 μM AngⅡ-induced H9c2 and primary rat cardiomyocytes | (1) Myd88/TLR signaling pathway (2)β-Mhc, Tgfb, Anp, α-Ska, Il6, Tnf, Col1a1, α-SkA↓ | F4/80 macrophages↓ | [142] |
| Vanillic acid | 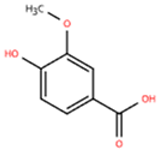 | Salvia miltiorrhiza, Mint, Pueraria lobata, etc. | ISO (10 mg/kg) s.c. in C57BL/6J mice | 2 weeks | - | (1) Drp1/HK1/NLRP3 signaling pathway (2) ROS, LDH, IL-1β, IL-6, IL-18, TNF-α, MDA, MPO, iNOS, Coll I, Coll III, α-SMA↓ (3) IL-4, IL-10, CAT, GSH, SOD, T-AOC, Arg-1 ↑ | M1↓, M2↑ | [143] |
| 20(S)-ginsenoside Rh2 | 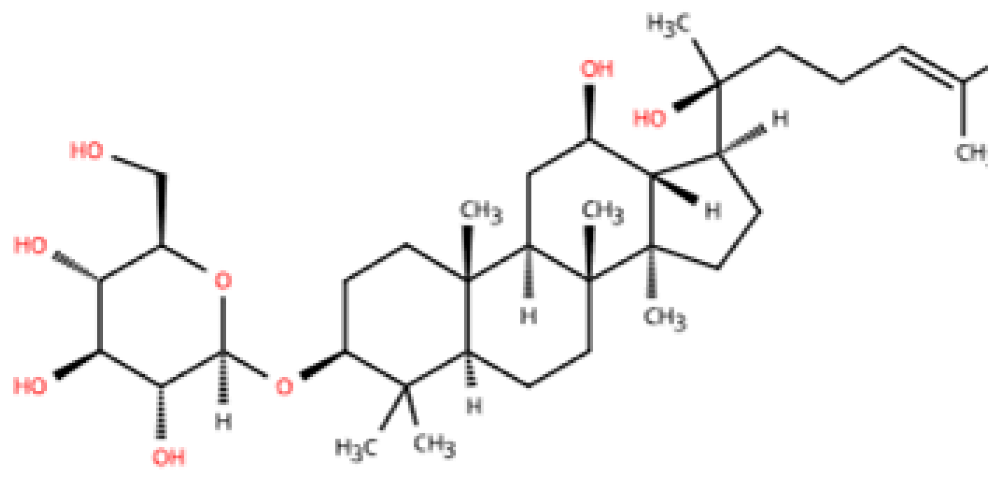 | Ginseng | Ang-II infusion (1 ng/kg/min) via micro-osmotic pump implanted in C57BL/6 mice | 2 weeks | Ang-II-induced NRVMs | (1) JNK/AP-1 signaling pathway (2) Il1b, Il6, Tnfa, TGF-β1, β-MyHC, collagen I↓ | F4/80 mRNA↓ | [144] |
| Leonurine | 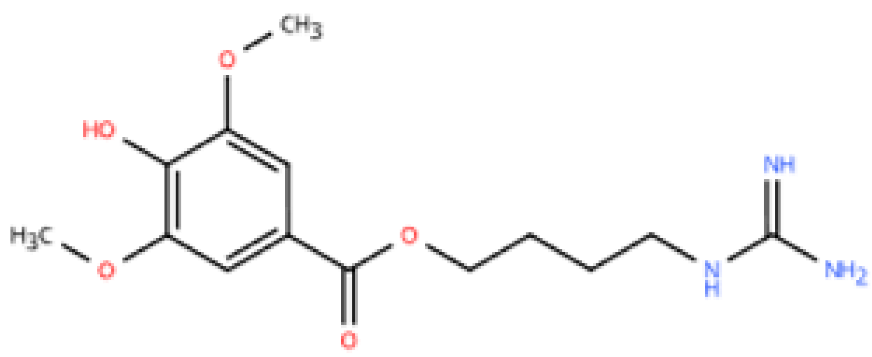 | Leonurus japonicus Houtt | Ang-II infusion (1000 ng/kg/min) via micro-osmotic pump implanted in C57BL/6 mice | 2 weeks | Ang II (1 μM)-induced H9c2 cells and NRVMs | (1) MAPK signaling pathway (2) NF-κB signaling pathway (3) Il1b, Il6, Tnfa, β-MyHC, collagen I, TGF-β1↓ | macrophage marker F4/80↓ | [145] |
| Corynoline | 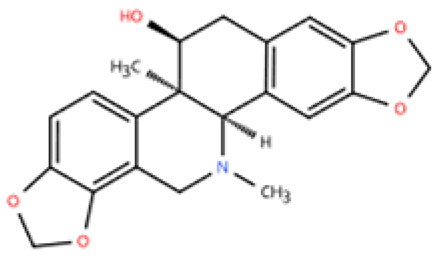 | Corydalis bungeana Herba | Ang-II infusion (1000 ng/kg/min) via micro-osmotic pump implanted in C57BL/6 mice | 2 weeks | Ang II (1 μM)-induced H9c2 cells | (1) NF-κB signaling pathway↓ (2) TGF-β1, β-MyHC, collagen I, Il1b, Il6, Tnfa↓ (3) PPARα↑ | M1↓ | [146] |
| Gentiopicroside | 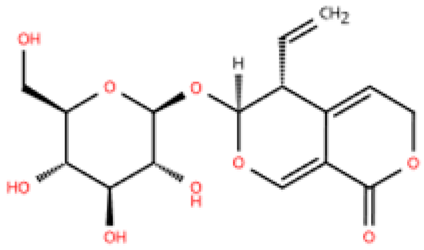 | Gentiana manshurica Kitagawa | High-fat diet and streptozotocin (50 mg/kg) i.p. in Sprague Dawley rats | 8 weeks | 30 mM high glucose-induced cardiac fibroblasts | (1) Smad3, collagen I and III, IL-1β, IL-6, TNF-α, MDA, NOX2, NOX4↓ (2) SOD↑ | M1↓ | [147] |
| Resveratrol | 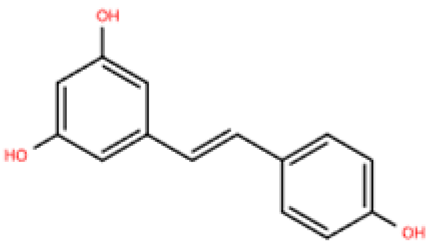 | Grapes, berries, Polygonum cuspidatum, etc. | ISO (50 mg/kg) s.c. in BALB/c mice | 1 week | 50 μmol/L ISO-induced RAW264.7 | (1) VEGFB/AMPK/NF-кB signaling pathway (2) Nppa, Il6, Tnf, Ccl2, Ptgs2, Icam1, Vcam1↓ (3) Il10, Il1rn↑ | M1↓, M2↑ | [148] |
| Resveratrol | 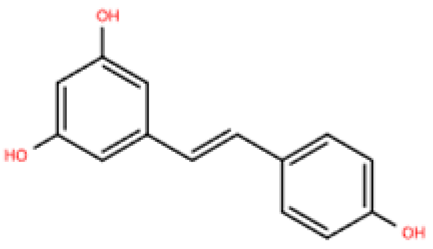 | Same as above | Uninephrectomy surgery in C57BL/6 mice | 4 weeks | 10 ng/mL TGF-β-induced cardiac fibroblasts | (1) TGF-β/Smad3 signaling pathway (2) IL-1β, IL-6, TNF-α, Col1a1, Col3a1, Nos2, GSH, CAT, SOD↓ (3) Sirt1, eNOS↑ | M1↓, M2↑ | [149] |
| Cardamonin | 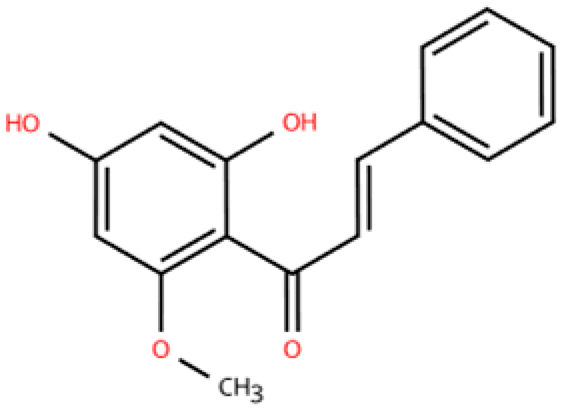 | Alpinia plant | DOX (5 mg/kg) i.p. in C57BL/6J mice | 4 weeks | 5 μM DOX-induced HL-1 | (1) Nrf2 signaling pathway↑ (2) Caspase-3, Keap1, NF-κB, MDA, ROS, TNF-α, IL-1β, IL-6, IL-18↓ (3) SOD, GSH, CAT↑ | macrophage marker F4/80↓ | [150] |
| Latifolin | 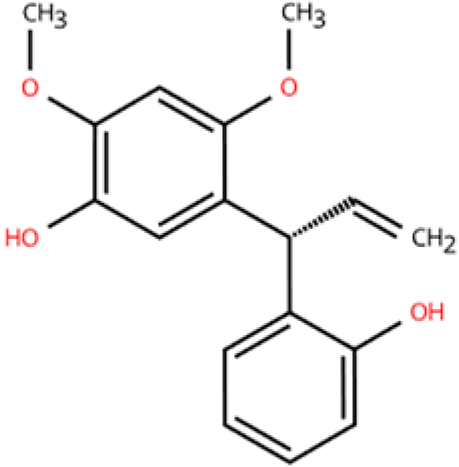 | Lignum dalbergiae odoriferae | DOX (20 mg/kg) i.p. in C57BL/6 mice | 12 days | 100 ng/mL LPS and 30 ng/mL IFN-γ-induced peritoneal macrophages | (1) Nos2, Il6, Il1b, Tnf, LDH↓ (2) Il10, Il4ra↑ | M1↓, M2↑ | [151] |
| Arctigenin | 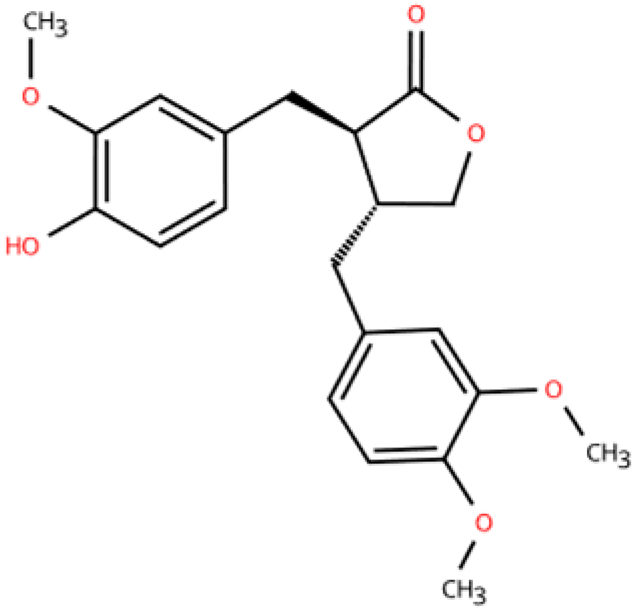 | Arctium lappa and Forsythia suspensa | Coronary artery ligation in C57BL/6 mice | 18 weeks | 0.2 mg/mL LPS-induced RAW264.7 | (1) JAK/STAT signaling pathway (2) NF-κB signaling pathway (3) NFAT5, Il6, Tnfa↓ | M1,M2c↓ M2a, M2b, M2d↑ | [152] |
| Triptolide | 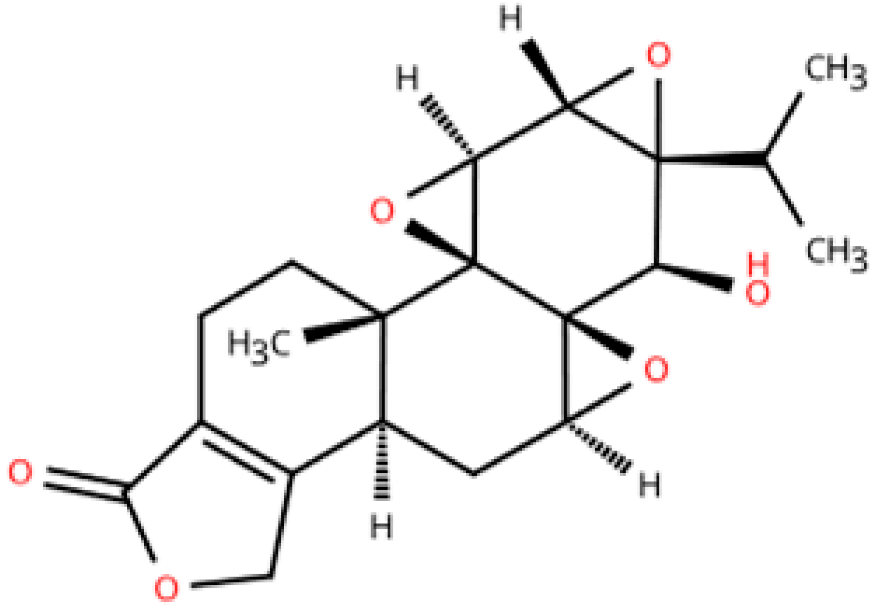 | Tripterygium wilfordii Hook F | Transverse aortic constriction operation in C57/BL6 mice | 6 weeks | - | (1) NLRP3 inflammasome↓ (2) Il1b, Il18, Ccl2, Vcam1, Collagen I and III, ASC↓ (3) TGFβ1, p-Smad3↓ | macrophage marker F4/80↓ | [153] |
| Name | Main Herbal Ingredients | In Vivo | Action Time | In Vitro | Mechanisms | Changes in Macrophages | Ref. |
|---|---|---|---|---|---|---|---|
| Zhen Wu Tang | Aconitum carmichaelii, Poria cocos, Atractylodes macrocephala, Zingiber officinale, Paeonia lactiflora | ISO (2.5 mg/kg) i.p. in Kunming mice | 30 days | 1 μg/mL LPS-induced RAW 264.7 cells | (1) TLR4/NF-κB signaling pathway (2) α-SMA, collagen I, and collagen III, iNOS↓ | M1↓ | [172] |
| Shenfu Injection | Panax ginseng, Aconitum carmichaelii | ISO (7.5 mg/kg) i.p. in C57BL/6J mice | 15 days | - | (1) TLR4/NF-κB signaling pathway (2) IL-6, TNFα↓ (3) IL-10, Arg-1↑ | M1↓, M2↑ | [173] |
| Xinyang Tablet | Panax ginseng, Epimedium brevicornum, Astragalus membranaceus, Leonurus japonicus, Isatis tinctoria, Lepidium apetalum, Plantago asiatica | Uninephrectomy surgery in C57BL/6 mice | 8 weeks | - | OPN, α-SMA, acta2, col1a1, col2a1, mmp3, mmp9↓ | macrophage marker F4/80↓ | [174] |
| Fangji Fuling tang | Stephania tetrandra, Poria cocos, Cinnamomum verum, Astragalus mongholicus, Glycyrrhiza uralensis | ISO (5 mg/kg) s.c. in C57BL/6J mice | 2 weeks | - | (1) TGF-β1, TNF-α, IL-1β, IL-6, SOD, GSH↓ (2) IL-10, MDA↑ | M1↓, M2↑ | [175] |
| QiShenYiQi Pill | Astragalus mongholicus, Panax notoginseng, Salvia miltiorrhiza, Dalbergia odorifera | Ascending aortic stenosis surgery in Sprague–Dawley rats | 6 weeks | 1 μM Ang II-induced H9c2, 10 ng/mL TGF-β1-induced RDF, 100 ng/ml | (1) TGF-β1/Smads signaling pathway (2) RPS19, MCP-1, MDA, LDH, cleaved caspase-9, cleaved caspase-3, MMP2, MMP9, TIMP1, collagen I, collagen III↓ (3) FHL2, TIMP2, ATP↑ | M1↓, M2↓ | [176] |
| QiShenYiQi Pill | Same as above | L-NAME and high-fat diet for C57BL/6N male mice | 4 weeks | - | (1) NO/cGMP/PKG signaling pathway (2) NF-κB, NLRP3, TNF-α, MCP-1, ROS, Icam1, Vcam1, Sele↓ | No significant changes | [177] |
| Category | Representative Agents | Action Time | Main Pathways Targeted | Macrophage Regulation | Mechanistic Features |
|---|---|---|---|---|---|
| Metabolites | Resveratrol, vanillic acid, gentiopicroside, leonurine, arctigenin | Mostly 14 days | NF-κB, MAPK, JNK/AP-1, NLRP3, TGFβ/Smads, AMPK, JAK/STAT, Nrf2 | Mostly M1↓, some M2↑ | Single pathway focused Mainly inhibit inflammation, fibrosis, and oxidative stress |
| Formulas | Zhen Wu Tang, Shenfu Injection, Xinyang Tablet, QiShenYiQi Pill, Fangji Fuling Tang | Over 14 days | TLR4/NF-κB, TGFβ/Smads, OPN, NO/cGMP/PKG, PI3K/Akt/mTOR | M1↓, M2↑ or bidirectional | Several pathways, systemic regulation across immune-metabolic-fibrotic axes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Yuan, J.; Cheng, P.; Yang, T.; Liu, Q.; Li, T.; Li, C.; Qu, H.; Zhou, H. Modulation of Macrophage Polarization by Traditional Chinese Medicine in HFpEF: A Review of Mechanisms and Therapeutic Potentials. Pharmaceuticals 2025, 18, 1317. https://doi.org/10.3390/ph18091317
Liu C, Yuan J, Cheng P, Yang T, Liu Q, Li T, Li C, Qu H, Zhou H. Modulation of Macrophage Polarization by Traditional Chinese Medicine in HFpEF: A Review of Mechanisms and Therapeutic Potentials. Pharmaceuticals. 2025; 18(9):1317. https://doi.org/10.3390/ph18091317
Chicago/Turabian StyleLiu, Chunqiu, Jinfeng Yuan, Peipei Cheng, Tao Yang, Qian Liu, Tianshu Li, Chuyi Li, Huiyan Qu, and Hua Zhou. 2025. "Modulation of Macrophage Polarization by Traditional Chinese Medicine in HFpEF: A Review of Mechanisms and Therapeutic Potentials" Pharmaceuticals 18, no. 9: 1317. https://doi.org/10.3390/ph18091317
APA StyleLiu, C., Yuan, J., Cheng, P., Yang, T., Liu, Q., Li, T., Li, C., Qu, H., & Zhou, H. (2025). Modulation of Macrophage Polarization by Traditional Chinese Medicine in HFpEF: A Review of Mechanisms and Therapeutic Potentials. Pharmaceuticals, 18(9), 1317. https://doi.org/10.3390/ph18091317








