Alpha Particle Emitter Radiolabeled Antibodies in Cancer Therapy: Current Status, Challenges, and Future Prospects
Abstract
1. Introduction
2. α-Emitters for RIT
2.1. Actinium-225 (225Ac)
2.2. Bismuth-213 (213Bi)

2.3. Thorium-227 (227Th)
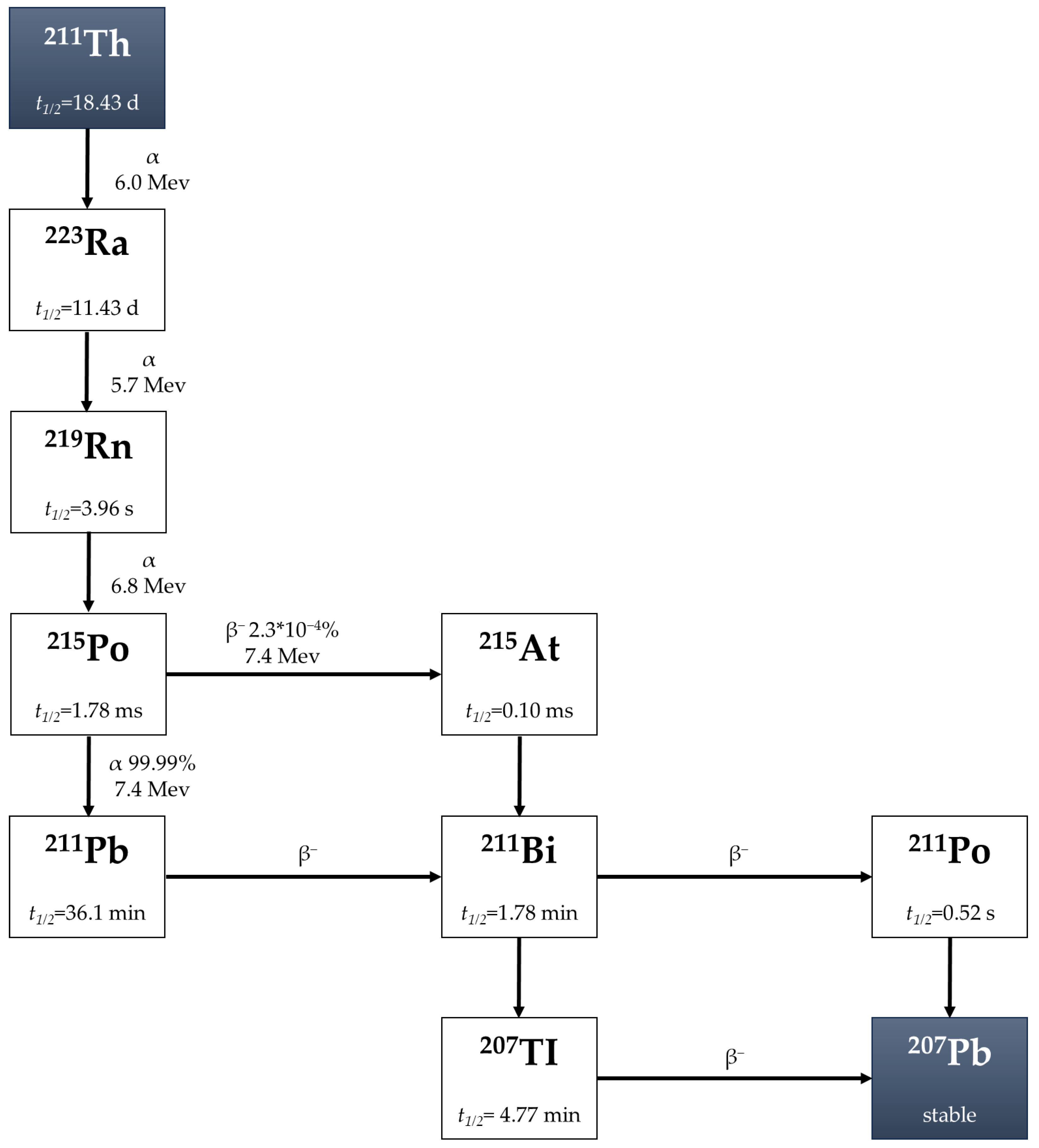
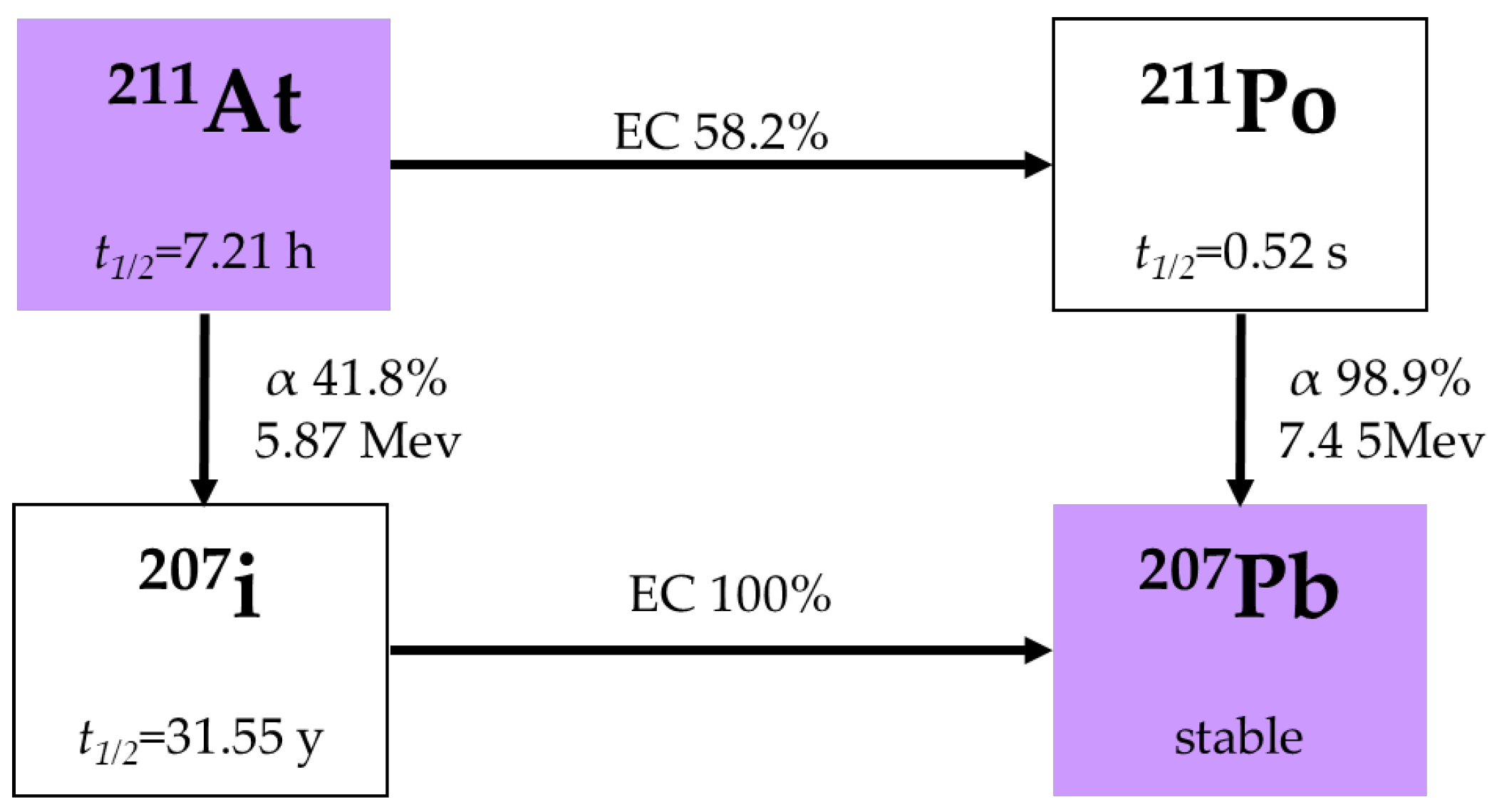
2.4. Astatine-211 (211At)
3. An In Vivo Generator of 212Bi for RIT
Lead-212 (212Pb)
4. Availability and Production Cost Analysis
5. Preclinical and Clinical Study
6. Antibody Labeling
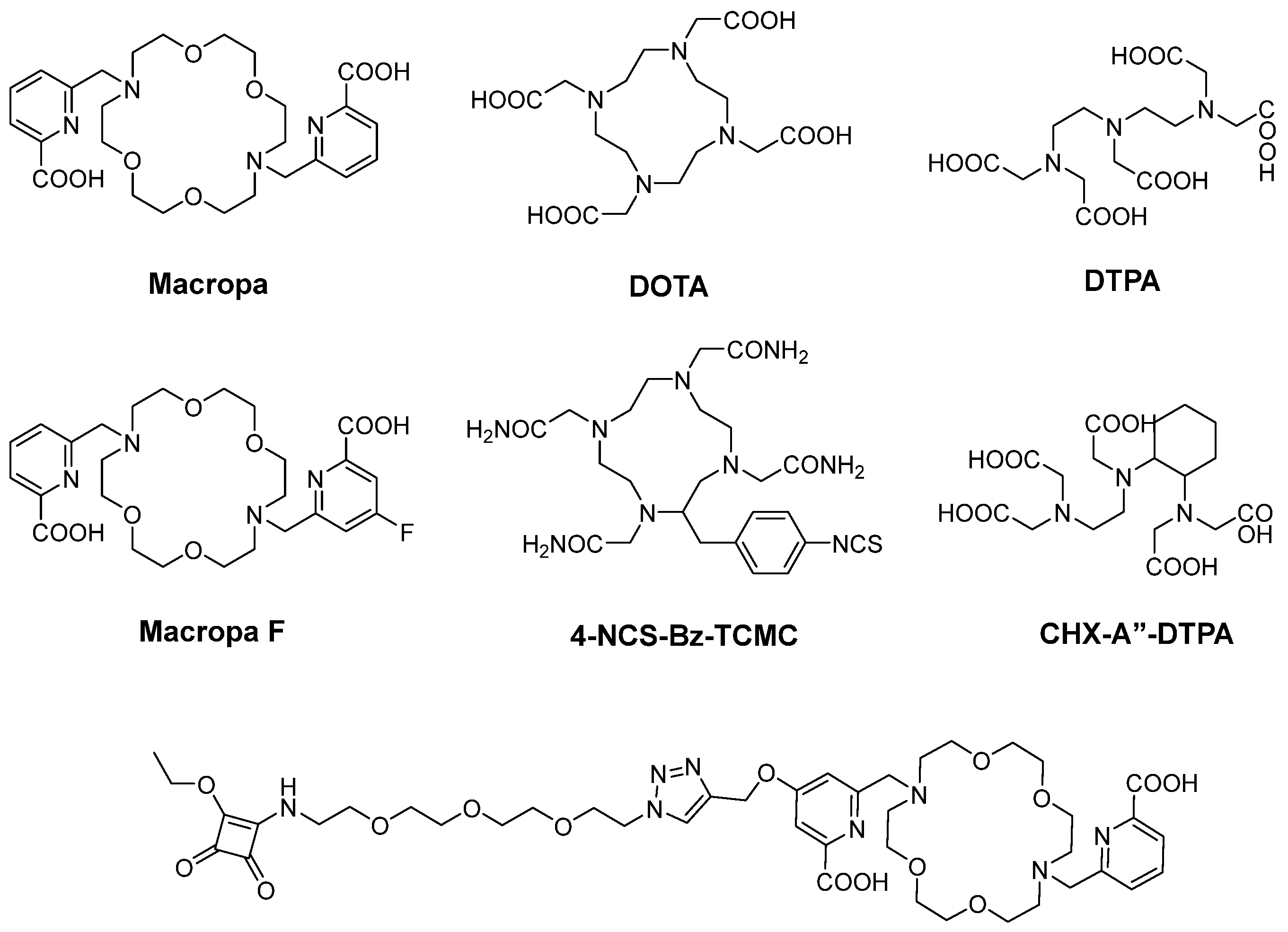
6.1. Radiometal Labeling
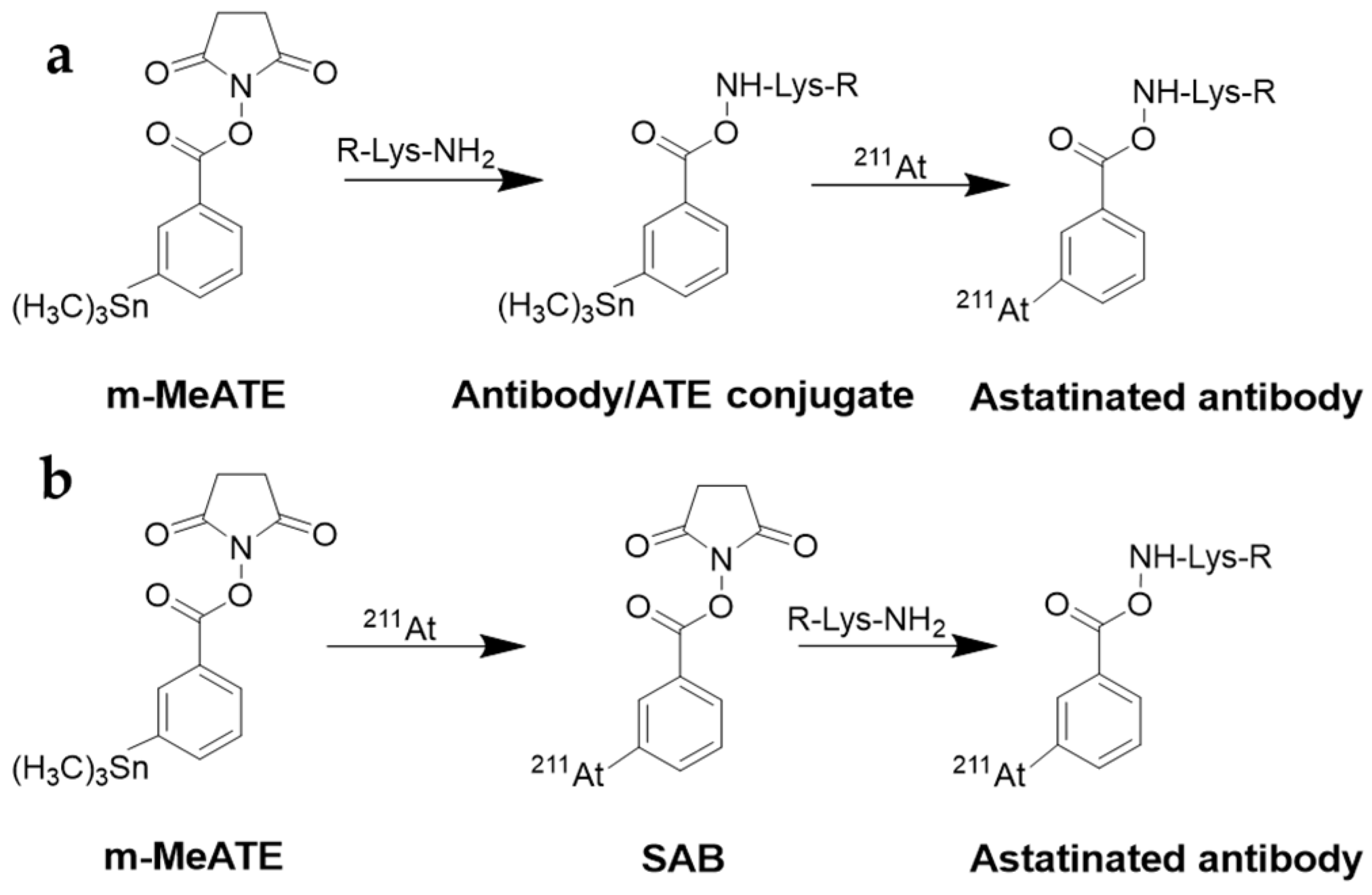
6.2. Labeling with Radiohologens
7. Strategies to Improve Therapeutic Effect and Reduce Toxicity
7.1. Antibody Fragments
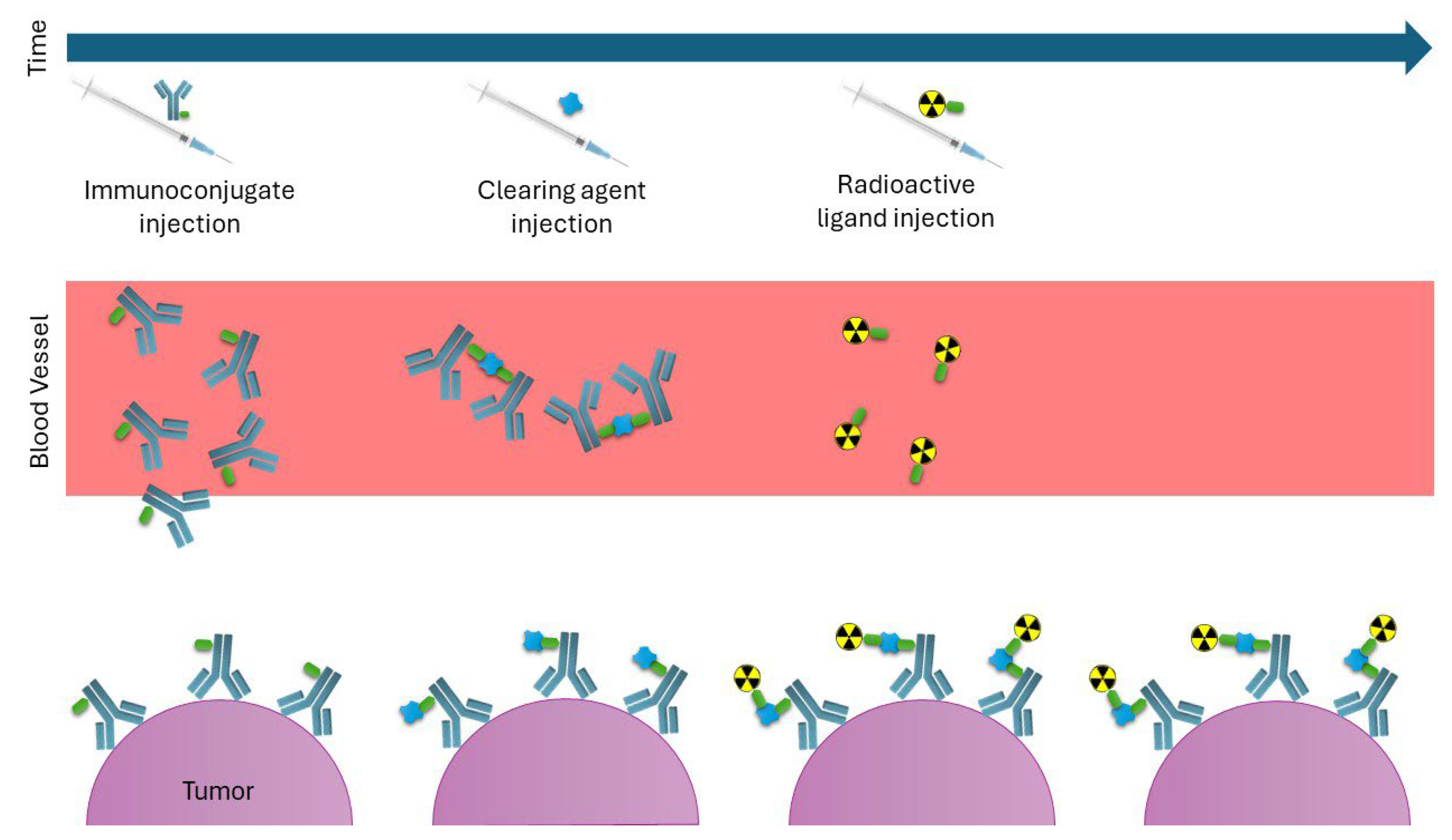
7.2. Pretargeting Strategy
7.3. Local Injection/Infusion
7.4. Combination of Pretargeted Therapy with Local Injection
7.5. Theranostics
8. Future Prospects
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosch, F.; Rosich, L. The Contributions of Paul Ehrlich to Pharmacology: A Tribute on the Occasion of the Centenary of His Nobel Prize. Pharmacology 2008, 82, 171–179. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Gansow, O.A.; Brechbiel, M.W.; Mirzadeh, S.; Colcher, D.; Roselli, M. Chelates and Antibodies: Current Methods and New Directions. Cancer Treat. Res. 1990, 51, 153–171. [Google Scholar] [CrossRef]
- Witzig, T.E.; Flinn, I.W.; Gordon, L.I.; Emmanouilides, C.; Czuczman, M.S.; Saleh, M.N.; Cripe, L.; Wiseman, G.; Olejnik, T.; Multani, P.S.; et al. Treatment with Ibritumomab Tiuxetan Radioimmunotherapy in Patients with Rituximab-Refractory Follicular Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2002, 20, 3262–3269. [Google Scholar] [CrossRef]
- Witzig, T.E.; Gordon, L.I.; Cabanillas, F.; Czuczman, M.S.; Emmanouilides, C.; Joyce, R.; Pohlman, B.L.; Bartlett, N.L.; Wiseman, G.A.; Padre, N.; et al. Randomized Controlled Trial of Yttrium-90–Labeled Ibritumomab Tiuxetan Radioimmunotherapy Versus Rituximab Immunotherapy for Patients with Relapsed or Refractory Low-Grade, Follicular, or Transformed B-Cell Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2002, 20, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.S.; Tuck, M.; Estes, J.; Kolstad, A.; Ross, C.W.; Zasadny, K.; Regan, D.; Kison, P.; Fisher, S.; Kroll, S.; et al. 131I-Tositumomab Therapy as Initial Treatment for Follicular Lymphoma. N. Engl. J. Med. 2005, 352, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic Value of Tumor Oxygenation in 397 Head and Neck Tumors after Primary Radiation Therapy. An International Multi-Center Study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.; Bezak, E.; Allen, B. Biomedical Physics in Radiotherapy for Cancer; Springer Science: Berlin/Heidelberg, Germany, 2012; ISBN 978-0-85729-732-7. [Google Scholar]
- Khazaei Monfared, Y.; Heidari, P.; Klempner, S.J.; Mahmood, U.; Parikh, A.R.; Hong, T.S.; Strickland, M.R.; Esfahani, S.A. DNA Damage by Radiopharmaceuticals and Mechanisms of Cellular Repair. Pharmaceutics 2023, 15, 2761. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, G.; Han, W.; Xu, S.; Wu, L. Radiation Target: Moving from Theory to Practice. Nucl. Anal. 2022, 1, 100024. [Google Scholar] [CrossRef]
- Hatcher-Lamarre, J.L.; Sanders, V.A.; Rahman, M.; Cutler, C.S.; Francesconi, L.C. Alpha Emitting Nuclides for Targeted Therapy. Nucl. Med. Biol. 2021, 92, 228–240. [Google Scholar] [CrossRef]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. α-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies—Part 1. J. Nucl. Med. 2018, 59, 878–884. [Google Scholar] [CrossRef]
- Chan, H.S.; de Blois, E.; Morgenstern, A.; Bruchertseifer, F.; de Jong, M.; Breeman, W.; Konijnenberg, M. In Vitro Comparison of 213Bi- and 177Lu-Radiation for Peptide Receptor Radionuclide Therapy. PLoS ONE 2017, 12, e0181473. [Google Scholar] [CrossRef] [PubMed]
- Graf, F.; Fahrer, J.; Maus, S.; Morgenstern, A.; Bruchertseifer, F.; Venkatachalam, S.; Fottner, C.; Weber, M.M.; Huelsenbeck, J.; Schreckenberger, M.; et al. DNA Double Strand Breaks as Predictor of Efficacy of the Alpha-Particle Emitter Ac-225 and the Electron Emitter Lu-177 for Somatostatin Receptor Targeted Radiotherapy. PLoS ONE 2014, 9, e88239. [Google Scholar] [CrossRef]
- Ballisat, L.; De Sio, C.; Beck, L.; Guatelli, S.; Sakata, D.; Shi, Y.; Duan, J.; Velthuis, J.; Rosenfeld, A. Dose and DNA Damage Modelling of Diffusing Alpha-Emitters Radiation Therapy Using Geant4. Phy Med. 2024, 121, 103367. [Google Scholar] [CrossRef]
- Cruz-Nova, P.; Trujillo-Nolasco, M.; Aranda-Lara, L.; Ferro-Flores, G.; Ocampo-García, B. Radiobiological Effect of Alpha Particles. The Scientific Basis of Targeted Alpha-Particle Therapy. Nucl. Med. Biol. 2025, 146–147, 109044. [Google Scholar] [CrossRef]
- Wenker, S.T.M.; van Lith, S.A.M.; Tamborino, G.; Konijnenberg, M.W.; Bussink, J.; Heskamp, S. The Potential of Targeted Radionuclide Therapy to Treat Hypoxic Tumor Cells. Nucl. Med. Biol. 2025, 140–141, 108971. [Google Scholar] [CrossRef]
- Pouget, J.-P.; Constanzo, J. Revisiting the Radiobiology of Targeted Alpha Therapy. Front. Med. 2021, 8, 692436. [Google Scholar] [CrossRef] [PubMed]
- Minnix, M.; Adhikarla, V.; Caserta, E.; Poku, E.; Rockne, R.; Shively, J.E.; Pichiorri, F. Comparison of CD38-Targeted α-Versus β-Radionuclide Therapy of Disseminated Multiple Myeloma in an Animal Model. J. Nucl. Med. 2021, 62, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.B.; Allen, K.J.H.; Malo, M.E.; Frank, C.; Dadachova, E. Comparison of Radiobiological Effects Induced by Radiolabeled Antibodies in Human Cancer Cells and Fungal Cells. Int. J. Radiat. Biol. 2025, 101, 521–530. [Google Scholar] [CrossRef]
- Hassan, M.; Bokhari, T.H.; Lodhi, N.A.; Khosa, M.K.; Usman, M. A Review of Recent Advancements in Actinium-225 Labeled Compounds and Biomolecules for Therapeutic Purposes. Chem. Biol. Drug Des. 2023, 102, 1276–1292. [Google Scholar] [CrossRef]
- Boll, R.A.; Malkemus, D.; Mirzadeh, S. Production of Actinium-225 for Alpha Particle Mediated Radioimmunotherapy. Appl. Radiat. Isot. 2005, 62, 667–679. [Google Scholar] [CrossRef]
- Nagatsu, K.; Suzuki, H.; Fukada, M.; Ito, T.; Ichinose, J.; Honda, Y.; Minegishi, K.; Higashi, T.; Zhang, M.-R. Cyclotron Production of 225Ac from an Electroplated 226Ra Target. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 279–289. [Google Scholar] [CrossRef]
- Apostolidis, C.; Molinet, R.; McGinley, J.; Abbas, K.; Möllenbeck, J.; Morgenstern, A. Cyclotron Production of Ac-225 for Targeted Alpha. Appl. Radiat. Isot. 2005, 62, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Bicak, M.; Lückerath, K.; Kalidindi, T.; Phelps, M.E.; Strand, S.-E.; Morris, M.J.; Radu, C.G.; Damoiseaux, R.; Peltola, M.T.; Peekhaus, N.; et al. Genetic Signature of Prostate Cancer Mouse Models Resistant to Optimized HK2 Targeted α-Particle Therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 15172–15181. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Xu, M.; Cai, J.; Chen, J.; Liu, Y.; Su, Q.; Li, Z.; Liu, Z. 225 Ac-Labeled Antibody for Fibroblast Activation Protein-Targeted Alpha Therapy. Chem. Biomed. Imaging 2023, 1, 628–636. [Google Scholar] [CrossRef]
- Garg, R.; Allen, K.J.H.; Dawicki, W.; Geoghegan, E.M.; Ludwig, D.L.; Dadachova, E. 225Ac-labeled CD33-targeting Antibody Reverses Resistance to Bcl-2 Inhibitor Venetoclax in Acute Myeloid Leukemia Models. Cancer Med. 2021, 10, 1128–1140. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Thomas, C.; Sartor, A.O.; Sun, M.; Stangl-Kremser, J.; Bissassar, M.; Vallabhajosula, S.; Castellanos, S.H.; Nauseef, J.T.; Sternberg, C.N.; et al. Prostate-Specific Membrane Antigen–Targeting Alpha Emitter via Antibody Delivery for Metastatic Castration-Resistant Prostate Cancer: A Phase I Dose-Escalation Study of 225 Ac-J591. J. Clin. Oncol. 2024, 42, 842–851. [Google Scholar] [CrossRef]
- Robertson, A.K.H.; Ramogida, C.F.; Schaffer, P.; Radchenko, V. Development of 225Ac Radiopharmaceuticals: TRIUMF Perspectives and Experiences. Curr. Radiopharm. 2018, 11, 156–172. [Google Scholar] [CrossRef]
- Franchi, S.; Di Marco, V.; Tosato, M. Bismuth Chelation for Targeted Alpha Therapy: Current State of the Art. Nucl. Med. Biol. 2022, 114–115, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson-Lutz, A.; Bäck, T.; Aneheim, E.; Hultborn, R.; Palm, S.; Jacobsson, L.; Morgenstern, A.; Bruchertseifer, F.; Albertsson, P.; Lindegren, S. Therapeutic Efficacy of α-Radioimmunotherapy with Different Activity Levels of the 213Bi-Labeled Monoclonal Antibody MX35 in an Ovarian Cancer Model. EJNMMI Res. 2017, 7, 38. [Google Scholar] [CrossRef]
- Rosenblat, T.L.; McDevitt, M.R.; Mulford, D.A.; Pandit-Taskar, N.; Divgi, C.R.; Panageas, K.S.; Heaney, M.L.; Chanel, S.; Morgenstern, A.; Sgouros, G.; et al. Sequential Cytarabine and α-Particle Immunotherapy with Bismuth-213–Lintuzumab (HuM195) for Acute Myeloid Leukemia. Clin. Cancer Res. 2010, 16, 5303–5311. [Google Scholar] [CrossRef]
- Sgouros, G.; Ballangrud, Ã.; Jurcic, J.G.; Mcdevitt, M.R.; Humm, J.L.; Erdi, Y.E.; Mehta, B.M.; Finn, R.D.; Larson, S.M.; Scheinberg, D.A. Pharmacokinetics and Dosimetry of an A-Particle Emitter Labeled Antibody: 213Bi-HuM195 (Anti-CD33) in Patients with Leukemia. J. Nucl. Med. 1999, 40, 1935–1946. [Google Scholar] [PubMed]
- Gustafsson, A.M.E.; Bäck, T.; Elgqvist, J.; Jacobsson, L.; Hultborn, R.; Albertsson, P.; Morgenstern, A.; Bruchertseifer, F.; Jensen, H.; Lindegren, S. Comparison of Therapeutic Efficacy and Biodistribution of 213Bi- and 211At-Labeled Monoclonal Antibody MX35 in an Ovarian Cancer Model. Nucl. Med. Biol. 2012, 39, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Jurcic, J.G.; Larson, S.M.; Sgouros, G.; McDevitt, M.R.; Finn, R.D.; Divgi, C.R.; Ballangrud, Å.M.; Hamacher, K.A.; Ma, D.; Humm, J.L.; et al. Targeted α Particle Immunotherapy for Myeloid Leukemia. Blood 2002, 100, 1233–1239. [Google Scholar] [CrossRef]
- Tosato, M.; Favaretto, C.; Kleynhans, J.; Burgoyne, A.R.; Gestin, J.-F.; van der Meulen, N.P.; Jalilian, A.; Köster, U.; Asti, M.; Radchenko, V. Alpha Atlas: Mapping Global Production of α-Emitting Radionuclides for Targeted Alpha Therapy. Nucl. Med. Biol. 2025, 142–143, 108990. [Google Scholar] [CrossRef] [PubMed]
- Kondev, F.; McCutchan, E.; Singh, B.; Tuli, J. Nuclear Data Sheets for A = 227. Nucl. Data Sheets 2016, 132, 257–354. [Google Scholar] [CrossRef]
- Murray, I.; Rojas, B.; Gear, J.; Callister, R.; Cleton, A.; Flux, G.D. Quantitative Dual-Isotope Planar Imaging of Thorium-227 and Radium-223 Using Defined Energy Windows. Cancer Biother. Radiopharm. 2020, 35, 530–539. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J. Clin. Oncol. 2014, 32, 3059–3067. [Google Scholar] [CrossRef]
- Lindén, O.; Bates, A.T.; Cunningham, D.; Hindorf, C.; Larsson, E.; Cleton, A.; Pinkert, J.; Huang, F.; Bladt, F.; Hennekes, H.; et al. 227Th-Labeled Anti-CD22 Antibody (BAY 1862864) in Relapsed/Refractory CD22-Positive Non-Hodgkin Lymphoma: A First-in-Human, Phase I Study. Cancer Biother. Radiopharm. 2021, 36, 672–681. [Google Scholar] [CrossRef]
- Hagemann, U.B.; Wickstroem, K.; Hammer, S.; Bjerke, R.M.; Zitzmann-Kolbe, S.; Ryan, O.B.; Karlsson, J.; Scholz, A.; Hennekes, H.; Mumberg, D.; et al. Advances in Precision Oncology: Targeted Thorium-227 Conjugates As a New Modality in Targeted Alpha Therapy. Cancer Biother. Radiopharm. 2020, 35, 497–510. [Google Scholar] [CrossRef]
- Bayer First-in-Human Study of BAY2287411 Injection, a Thorium-227 Labeled Antibody-Chelator Conjugate, in Patients with Tumors Known to Express Mesothelin. Available online: https://clinicaltrials.gov/study/NCT03507452 (accessed on 5 June 2025).
- Corson, D.R.; MacKenzie, K.R.; Segrè, E. Artificially Radioactive Element 85. Phys. Rev. 1940, 58, 672–678. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Zalutsky, M. Astatine Radiopharmaceuticals: Prospects and Problems. Curr. Radiopharm. 2008, 1, 177–196. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Pruszynski, M. Astatine-211: Production and Availability. Curr. Radiopharm. 2011, 4, 177–185. [Google Scholar] [CrossRef]
- Sevenois, M.B.C.; Miller, B.W.M.; Jensen, H.J.; D’Huyvetter, M.; Covens, P. Optimized Cyclotron Production of 211At: The Challenge of 210Po-Characterization. Radiat. Phys. Chem. 2023, 212, 111155. [Google Scholar] [CrossRef]
- Laszlo, G.S.; Orozco, J.J.; Kehret, A.R.; Lunn, M.C.; Huo, J.; Hamlin, D.K.; Scott Wilbur, D.; Dexter, S.L.; Comstock, M.L.; O’Steen, S.; et al. Development of [211At]Astatine-Based Anti-CD123 Radioimmunotherapy for Acute Leukemias and Other CD123+ Malignancies. Leukemia 2022, 36, 1485–1491. [Google Scholar] [CrossRef]
- O’Steen, S.; Comstock, M.L.; Orozco, J.J.; Hamlin, D.K.; Wilbur, D.S.; Jones, J.C.; Kenoyer, A.; Nartea, M.E.; Lin, Y.; Miller, B.W.; et al. The α-Emitter Astatine-211 Targeted to CD38 Can Eradicate Multiple Myeloma in a Disseminated Disease Model. Blood 2019, 134, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Fred Hutchinson Cancer Center 211At-OKT10-B10 and Fludarabine Alone or in Combination with Cyclophosphamide and Low-Dose TBI Before Donor Stem Cell Transplant for the Treatment of Newly Diagnosed, Recurrent, or Refractory High-Risk Multiple Myeloma. Available online: https://clinicaltrials.gov/study/NCT04579523?intr=OKT10-B10&rank=2 (accessed on 5 June 2025).
- Nakaya, A.; Qiu, H.; Santos, E.B.; Hamlin, D.K.; Wilbur, D.S.; Storb, R.; Sandmaier, B.M. Addition of Astatine-211-Labeled Anti-CD45 Antibody to TBI as Conditioning for DLA-Identical Marrow Transplantation: A Novel Strategy to Overcome Graft Rejection in a Canine Presensitization Model: “Radioimmunotherapy to Overcome Transfusion-Induced Sensitization”. Transplant. Cell. Ther. 2021, 27, 476.e1–476.e7. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Garg, P.K.; Friedman, H.S.; Bigner, D.D. Labeling Monoclonal Antibodies and F(Ab′)2 Fragments with the Alpha-Particle-Emitting Nuclide Astatine-211: Preservation of Immunoreactivity and in Vivo Localizing Capacity. Proc. Natl. Acad. Sci. USA 1989, 86, 7149–7153. [Google Scholar] [CrossRef]
- Stenberg, V.Y.; Juzeniene, A.; Bruland, Ø.S.; Larsen, R.H. In Situ Generated 212Pb-PSMA Ligand in a 224Ra-Solution for Dual Targeting of Prostate Cancer Sclerotic Stroma and PSMA-Positive Cells. Curr. Radiopharm. 2020, 13, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sagastume, E.A.; Lee, D.; McAlister, D.; DeGraffenreid, A.J.; Olewine, K.R.; Graves, S.; Copping, R.; Mirzadeh, S.; Zimmerman, B.E.; et al. 203/212Pb Theranostic Radiopharmaceuticals for Image-Guided Radionuclide Therapy for Cancer. Curr. Med. Chem. 2020, 27, 7003–7031. [Google Scholar] [CrossRef]
- Edem, P.E.; Fonslet, J.; Kjær, A.; Herth, M.; Severin, G. In Vivo Radionuclide Generators for Diagnostics and Therapy. Bioinorg. Chem. Appl. 2016, 2016, 6148357. [Google Scholar] [CrossRef]
- Kokov, K.V.; Egorova, B.V.; German, M.N.; Klabukov, I.D.; Krasheninnikov, M.E.; Larkin-Kondrov, A.A.; Makoveeva, K.A.; Ovchinnikov, M.V.; Sidorova, M.V.; Chuvilin, D.Y. 212Pb: Production Approaches and Targeted Therapy Applications. Pharmaceutics 2022, 14, 189. [Google Scholar] [CrossRef]
- McNeil, B.L.; Robertson, A.K.H.; Fu, W.; Yang, H.; Hoehr, C.; Ramogida, C.F.; Schaffer, P. Production, Purification, and Radiolabeling of the 203Pb/212Pb Theranostic Pair. EJNMMI Radiopharm. Chem. 2021, 6, 6. [Google Scholar] [CrossRef]
- Durand-Panteix, S.; Monteil, J.; Sage, M.; Garot, A.; Clavel, M.; Saidi, A.; Torgue, J.; Cogne, M.; Quelven, I. Preclinical Study of 212Pb Alpha-Radioimmunotherapy Targeting CD20 in Non-Hodgkin Lymphoma. Br. J. Cancer 2021, 125, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Quelven, I.; Monteil, J.; Sage, M.; Saidi, A.; Mounier, J.; Bayout, A.; Garrier, J.; Cogne, M.; Durand-Panteix, S. 212Pb α-Radioimmunotherapy Targeting CD38 in Multiple Myeloma: A Preclinical Study. J. Nucl. Med. 2020, 61, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, T.; Hua, J.; Wang, Q.; Su, Y.; Chen, P.; Bidlingmaier, S.; Li, A.; Xie, Z.; Bidkar, A.P.; et al. CD46 Targeted 212Pb Alpha Particle Radioimmunotherapy for Prostate Cancer Treatment. J. Exp. Clin. Cancer Res. 2023, 42, 61. [Google Scholar] [CrossRef] [PubMed]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M. Dose Escalation and Dosimetry of First-in-Human α Radioimmunotherapy with 212Pb-TCMC-Trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef]
- Meredith, R.F.; Torgue, J.J.; Rozgaja, T.A.; Banaga, E.P.; Bunch, P.W.; Alvarez, R.D.; Straughn, J.M.; Dobelbower, M.C.; Lowy, A.M. Safety and Outcome Measures of First-in-Human Intraperitoneal α Radioimmunotherapy with 212Pb-TCMC-Trastuzumab. Am. J. Clin. Oncol. 2018, 41, 716–721. [Google Scholar] [CrossRef]
- Jadvar, H.; Quinn, D.I. Targeted α-Particle Therapy of Bone Metastases in Prostate Cancer. Clin. Nucl. Med. 2013, 38, 966–971. [Google Scholar] [CrossRef]
- Filosofov, D.; Baimukhanova, A.; Khushvaktov, J.; Kurakina, E.; Radchenko, V. Potent Candidates for Targeted Alpha Therapy (TAT). Nucl. Med. Biol. 2025, 146–147, 109027. [Google Scholar] [CrossRef]
- Miller, C.; Rousseau, J.; Ramogida, C.F.; Celler, A.; Rahmim, A.; Uribe, C.F. Implications of Physics, Chemistry and Biology for Dosimetry Calculations Using Theranostic Pairs. Theranostics 2022, 12, 232–259. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Goel, S.; Valdovinos, H.F.; Hernandez, R.; Hong, H.; Nickles, R.J.; Cai, W. Matching the Decay Half-Life with the Biological Half-Life: ImmunoPET Imaging with 44Sc-Labeled Cetuximab Fab Fragment. Bioconjug. Chem. 2014, 25, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Ree, S.M.; Greenwood, H.; Young, J.D.; Roberts, R.; Livens, F.R.; Heath, S.L.; Sosabowski, J.K. Selection of Radionuclide(s) for Targeted Alpha Therapy Based on Their Nuclear Decay Properties. Nucl. Med. Commun. 2024, 45, 465–473. [Google Scholar] [CrossRef]
- Ludwig, D.L.; Bryan, R.A.; Dawicki, W.; Geoghegan, E.M.; Liang, Q.; Gokhale, M.; Reddy, V.; Garg, R.; Allen, K.J.H.; Berger, M.S.; et al. Preclinical Development of an Actinium-225-Labeled Antibody Radio-Conjugate Directed Against CD45 for Targeted Conditioning and Radioimmunotherapy. Bio. Blood Marrow Transplant. 2020, 26, S160–S161. [Google Scholar] [CrossRef]
- Rodak, M.; Dekempeneer, Y.; Wojewódzka, M.; Caveliers, V.; Covens, P.; Miller, B.W.; Sevenois, M.B.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T.; et al. Preclinical Evaluation of 225Ac-Labeled Single-Domain Antibody for the Treatment of HER2pos Cancer. Mol. Cancer Ther. 2022, 21, 1835–1845. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Thorek, D.L.J.; Hashimoto, T.; Gondo, T.; Veach, D.R.; Sharma, S.K.; Kalidindi, T.M.; Abou, D.S.; Watson, P.A.; Beattie, B.J.; et al. Feed-Forward Alpha Particle Radiotherapy Ablates Androgen Receptor-Addicted Prostate Cancer. Nat. Commun. 2018, 9, 1629. [Google Scholar] [CrossRef]
- Minnix, M.; Li, L.; Yazaki, P.J.; Miller, A.D.; Chea, J.; Poku, E.; Liu, A.; Wong, J.Y.C.; Rockne, R.C.; Colcher, D.; et al. TAG-72–Targeted α-Radionuclide Therapy of Ovarian Cancer Using 225Ac-Labeled DOTAylated-HuCC49 Antibody. J. Nucl. Med. 2021, 62, 55–61. [Google Scholar] [CrossRef]
- Chung, S.K.; Vargas, D.B.; Chandler, C.S.; Katugampola, S.; Veach, D.R.; McDevitt, M.R.; Seo, S.H.; Vaughn, B.A.; Rinne, S.S.; Punzalan, B.; et al. Efficacy of HER2-Targeted Intraperitoneal 225Ac α-Pretargeted Radioimmunotherapy for Small-Volume Ovarian Peritoneal Carcinomatosis. J. Nucl. Med. 2023, 64, 1439–1445. [Google Scholar] [CrossRef]
- Sudo, H.; Tsuji, A.B.; Sugyo, A.; Harada, Y.; Nagayama, S.; Katagiri, T.; Nakamura, Y.; Higashi, T. FZD10-targeted A-radioimmunotherapy with 225Ac-labeled OTSA101 Achieves Complete Remission in a Synovial Sarcoma Model. Cancer Sci. 2022, 113, 721–732. [Google Scholar] [CrossRef]
- Veach, D.R.; Storey, C.M.; Lückerath, K.; Braun, K.; von Bodman, C.; Lamminmäki, U.; Kalidindi, T.; Strand, S.-E.; Strand, J.; Altai, M.; et al. PSA-Targeted Alpha-, Beta-, and Positron-Emitting Immunotheranostics in Murine Prostate Cancer Models and Nonhuman Primates. Clin. Cancer Res. 2021, 27, 2050–2060. [Google Scholar] [CrossRef]
- Morgan, K.A.; Wichmann, C.W.; Osellame, L.D.; Cao, Z.; Guo, N.; Scott, A.M.; Donnelly, P.S. Tumor Targeted Alpha Particle Therapy with an Actinium-225 Labelled Antibody for Carbonic Anhydrase IX. Chem. Sci. 2024, 15, 3372–3381. [Google Scholar] [CrossRef]
- Behling, K.; Maguire, W.F.; López Puebla, J.C.; Sprinkle, S.R.; Ruggiero, A.; O’Donoghue, J.; Gutin, P.H.; Scheinberg, D.A.; McDevitt, M.R. Vascular Targeted Radioimmunotherapy for the Treatment of Glioblastoma. J. Nucl. Med. 2016, 57, 1576–1582. [Google Scholar] [CrossRef]
- Fazel, J.; Rötzer, S.; Seidl, C.; Feuerecker, B.; Autenrieth, M.; Weirich, G.; Bruchertseifer, F.; Morgenstern, A.; Senekowitsch-Schmidtke, R. Fractionated Intravesical Radioimmunotherapy with 213Bi-Anti-EGFR-MAb Is Effective without Toxic Side-Effects in a Nude Mouse Model of Advanced Human Bladder Carcinoma. Cancer Biol. Ther. 2015, 16, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Allen, K.J.H.; Malo, M.E.; Helal, M.; Jiang, Z.; Smart, K.; Buhl, S.V.; Rickles, D.; Bryan, R.A.; Dadachova, E. Evaluation of Novel Highly Specific Antibodies to Cancer Testis Antigen Centrin-1 for Radioimmunoimaging and Radioimmunotherapy of Pancreatic Cancer. Cancer Med. 2019, 8, 5289–5300. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Allen, K.J.H.; Malo, M.E.; Rickles, D.; Dadachova, E. Evaluating the Combination of Radioimmunotherapy and Immunotherapy in a Melanoma Mouse Model. Int. J. Mol. Sci. 2020, 21, 773. [Google Scholar] [CrossRef] [PubMed]
- Revskaya, E.; Jiang, Z.; Morgenstern, A.; Bruchertseifer, F.; Sesay, M.; Walker, S.; Fuller, S.; Lebowitz, M.S.; Gravekamp, C.; Ghanbari, H.A.; et al. A Radiolabeled Fully Human Antibody to Human Aspartyl (Asparaginyl) β-Hydroxylase Is a Promising Agent for Imaging and Therapy of Metastatic Breast Cancer. Cancer Biother. Radiopharm. 2017, 32, 57–65. [Google Scholar] [CrossRef]
- Derrien, A.; Gouard, S.; Maurel, C.; Gaugler, M.-H.; Bruchertseifer, F.; Morgenstern, A.; Faivre-Chauvet, A.; Classe, J.-M.; Chérel, M. Therapeutic Efficacy of Alpha-RIT Using a 213Bi-Anti-HCD138 Antibody in a Mouse Model of Ovarian Peritoneal Carcinomatosis. Front. Med. 2015, 2, 88. [Google Scholar] [CrossRef]
- Fichou, N.; Gouard, S.; Maurel, C.; Barbet, J.; Ferrer, L.; Morgenstern, A.; Bruchertseifer, F.; Faivre-Chauvet, A.; Bigot-Corbel, E.; Davodeau, F.; et al. Single-Dose Anti-CD138 Radioimmunotherapy: Bismuth-213 Is More Efficient than Lutetium-177 for Treatment of Multiple Myeloma in a Preclinical Model. Front. Med. 2015, 2, 76. [Google Scholar] [CrossRef]
- Ménager, J.; Gorin, J.-B.; Maurel, C.; Drujont, L.; Gouard, S.; Louvet, C.; Chérel, M.; Faivre-Chauvet, A.; Morgenstern, A.; Bruchertseifer, F.; et al. Combining α-Radioimmunotherapy and Adoptive T Cell Therapy to Potentiate Tumor Destruction. PLoS ONE 2015, 10, e0130249. [Google Scholar] [CrossRef]
- Teiluf, K.; Seidl, C.; Blechert, B.; Gaertner, F.C.; Gilbertz, K.-P.; Fernandez, V.; Bassermann, F.; Endell, J.; Boxhammer, R.; Leclair, S.; et al. α-Radioimmunotherapy with 213Bi-Anti-CD38 Immunoconjugates Is Effective in a Mouse Model of Human Multiple Myeloma. Oncotarget 2015, 6, 4692–4703. [Google Scholar] [CrossRef]
- Adams, G.P.; Shaller, C.C.; Chappell, L.L.; Wu, C.; Horak, E.M.; Simmons, H.H.; Litwin, S.; Marks, J.D.; Weiner, L.M.; Brechbiel, M.W. Delivery of the α-Emitting Radioisotope Bismuth-213 to Solid Tumors via Single-Chain Fv and Diabody Molecules. Nucl. Med. Biol. 2000, 27, 339–346. [Google Scholar] [CrossRef]
- Green, D.J.; Shadman, M.; Jones, J.C.; Frayo, S.L.; Kenoyer, A.L.; Hylarides, M.D.; Hamlin, D.K.; Wilbur, D.S.; Balkan, E.R.; Lin, Y.; et al. Astatine-211 Conjugated to an Anti-CD20 Monoclonal Antibody Eradicates Disseminated B-Cell Lymphoma in a Mouse Model. Blood 2015, 125, 2111–2119. [Google Scholar] [CrossRef]
- Gouard, S.; Maurel, C.; Marionneau-Lambot, S.; Dansette, D.; Bailly, C.; Guérard, F.; Chouin, N.; Haddad, F.; Alliot, C.; Gaschet, J.; et al. Targeted-Alpha-Therapy Combining Astatine-211 and Anti-CD138 Antibody in a Preclinical Syngeneic Mouse Model of Multiple Myeloma Minimal Residual Disease. Cancers 2020, 12, 2721. [Google Scholar] [CrossRef]
- Bäck, T.A.; Jennbacken, K.; Hagberg Thulin, M.; Lindegren, S.; Jensen, H.; Olafsen, T.; Yazaki, P.J.; Palm, S.; Albertsson, P.; Damber, J.-E.; et al. Targeted Alpha Therapy with Astatine-211-Labeled Anti-PSCA A11 Minibody Shows Antitumor Efficacy in Prostate Cancer Xenografts and Bone Microtumors. EJNMMI Res. 2020, 10, 10. [Google Scholar] [CrossRef]
- Maaland, A.F.; Saidi, A.; Torgue, J.; Heyerdahl, H.; Stallons, T.A.R.; Kolstad, A.; Dahle, J. Targeted Alpha Therapy for Chronic Lymphocytic Leukaemia and Non-Hodgkin’s Lymphoma with the Anti-CD37 Radioimmunoconjugate 212Pb-NNV003. PLoS ONE 2020, 15, e0230526. [Google Scholar] [CrossRef] [PubMed]
- Actinium Pharmaceuticals Venetoclax and Lintuzumab-Ac225 in AML Patients. Available online: https://clinicaltrials.gov/study/NCT03867682?intr=Lintuzumab-Ac225&rank=2 (accessed on 5 June 2025).
- Fusion Pharmaceuticals Inc. A Phase 1/2 Study of [225Ac]-FPI-1434 Injection. Available online: https://clinicaltrials.gov/study/NCT03746431?intr=FPI-1434&rank=1 (accessed on 5 June 2025).
- Weill Medical College of Cornell University Phase I Trial of 225Ac-J591 in Patients with MCRPC. Available online: https://clinicaltrials.gov/study/NCT03276572?intr=J591&rank=5 (accessed on 5 June 2025).
- Weill Medical College of Cornell University Re-Treatment 225Ac-J591 for MCRPC. Available online: https://clinicaltrials.gov/study/NCT04576871?intr=J591&rank=6 (accessed on 5 June 2025).
- Weill Medical College of Cornell Universit Fractionated and Multiple Dose 225Ac-J591 for Progressive MCRPC. Available online: https://clinicaltrials.gov/study/NCT04506567?intr=J591&rank=9 (accessed on 5 June 2025).
- City of Hope Medical Center Actinium 225 Labeled Anti-CEA Antibody (Ac225-DOTA-M5A) for the Treatment of CEA Producing Advanced or Metastatic Cancers. Available online: https://clinicaltrials.gov/study/NCT05204147 (accessed on 5 June 2025).
- City of Hope Medical Center 225Ac-DOTA-Anti-CD38 Daratumumab Monoclonal Antibody with Fludarabine, Melphalan and Total Marrow and Lymphoid Irradiation as Conditioning Treatment for Donor Stem Cell Transplant in Patients with High-Risk Acute Myeloid Leukemia, Acute Lymphoblastic Leukemia and Myelodysplastic Syndrome. Available online: https://clinicaltrials.gov/study/NCT06287944?intr=DOTA-daratumumab&rank=3 (accessed on 5 June 2025).
- Janssen Research & Development, L. A Study of JNJ-69086420, an Actinium-225-Labeled Antibody Targeting Human Kallikrein-2 (HK2) for Advanced Prostate Cancer. Available online: https://clinicaltrials.gov/study/NCT04644770?intr=DOTA-h11B6&rank=2 (accessed on 5 June 2025).
- Bayer A Study to Learn How Safe the Study Treatment Actinium-225-Macropa-Pelgifatamab (BAY3546828) Is, How It Affects the Body, How It Moves Into, Through and Out of the Body, and About Its Anticancer Activity in Men with Advanced Metastatic Castration-Resistant Prostate Cancer (MCRPC). Available online: https://clinicaltrials.gov/study/NCT06052306?intr=pelgifatamab&rank=1 (accessed on 5 June 2025).
- Cederkrantz, E.; Andersson, H.; Bernhardt, P.; Bäck, T.; Hultborn, R.; Jacobsson, L.; Jensen, H.; Lindegren, S.; Ljungberg, M.; Magnander, T.; et al. Absorbed Doses and Risk Estimates of 211At-MX35 F(Ab′)2 in Intraperitoneal Therapy of Ovarian Cancer Patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 569–576. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Gawęda, W.; Żelechowska-Matysiak, K.; Wawrowicz, K.; Bilewicz, A. Nanoparticles in Targeted Alpha Therapy. Nanomaterials 2020, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- Brühlmann, S.A.; Walther, M.; Blei, M.K.; Mamat, C.; Kopka, K.; Freudenberg, R.; Kreller, M. Scalability Study on [133La]LaCl3 Production with a Focus on Potential Clinical Applications. EJNMMI Radiopharm. Chem. 2024, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- White, J.M.; Escorcia, F.E.; Viola, N.T. Perspectives on Metals-Based Radioimmunotherapy (RIT): Moving Forward. Theranostics 2021, 11, 6293–6314. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Levy, M.Y.; Cicic, D.; Bergonio, G.; Berger, M. Trial in Progress: Phase I Study of Actinium-225 (225Ac)-Lintuzumab in Patients with Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2017, 17, S329–S330. [Google Scholar] [CrossRef]
- Chomet, M.; van Dongen, G.A.M.S.; Vugts, D.J. State of the Art in Radiolabeling of Antibodies with Common and Uncommon Radiometals for Preclinical and Clinical Immuno-PET. Bioconjug. Chem. 2021, 32, 1315–1330. [Google Scholar] [CrossRef]
- Dawicki, W.; Allen, K.J.H.; Jiao, R.; Malo, M.E.; Helal, M.; Berger, M.S.; Ludwig, D.L.; Dadachova, E. Daratumumab-225Actinium Conjugate Demonstrates Greatly Enhanced Antitumor Activity against Experimental Multiple Myeloma Tumors. Oncoimmunology 2019, 8, 1607673. [Google Scholar] [CrossRef]
- Minnix, M.; Kujawski, M.; Poku, E.; Yazaki, P.J.; Wong, J.Y.; Shively, J.E. Improved Tumor Responses with Sequential Targeted α-Particles Followed by Interleukin 2 Immunocytokine Therapies in Treatment of CEA-Positive Breast and Colon Tumors in CEA Transgenic Mice. J. Nucl. Med. 2022, 63, 1859–1864. [Google Scholar] [CrossRef]
- Maguire, W.F.; McDevitt, M.R.; Smith-Jones, P.M.; Scheinberg, D.A. Efficient 1-Step Radiolabeling of Monoclonal Antibodies to High Specific Activity with 225Ac for α-Particle Radioimmunotherapy of Cancer. J. Nucl. Med. 2014, 55, 1492–1498. [Google Scholar] [CrossRef]
- Bobba, K.N.; Bidkar, A.P.; Meher, N.; Fong, C.; Wadhwa, A.; Dhrona, S.; Sorlin, A.; Bidlingmaier, S.; Shuere, B.; He, J.; et al. Evaluation of 134Ce/134La as a PET Imaging Theranostic Pair for 225Ac α-Radiotherapeutics. J. Nucl. Med. 2023, 64, 1076–1082. [Google Scholar] [CrossRef]
- Reissig, F.; Bauer, D.; Zarschler, K.; Novy, Z.; Bendova, K.; Ludik, M.-C.; Kopka, K.; Pietzsch, H.-J.; Petrik, M.; Mamat, C. Towards Targeted Alpha Therapy with Actinium-225: Chelators for Mild Condition Radiolabeling and Targeting PSMA—A Proof of Concept Study. Cancers 2021, 13, 1974. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Robertson, A.K.H.; Jermilova, U.; Zhang, C.; Yang, H.; Kunz, P.; Lassen, J.; Bratanovic, I.; Brown, V.; Southcott, L.; et al. Evaluation of Polydentate Picolinic Acid Chelating Ligands and an α-Melanocyte-Stimulating Hormone Derivative for Targeted Alpha Therapy Using ISOL-Produced 225Ac. EJNMMI Radiopharm. Chem. 2019, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. 2017, 56, 14712–14717. [Google Scholar] [CrossRef]
- Blei, M.K.; Waurick, L.; Reissig, F.; Kopka, K.; Stumpf, T.; Drobot, B.; Kretzschmar, J.; Mamat, C. Equilibrium Thermodynamics of Macropa Complexes with Selected Metal Isotopes of Radiopharmaceutical Interest. Inorg. Chem. 2023, 62, 20699–20709. [Google Scholar] [CrossRef] [PubMed]
- Merkx, R.I.J.; Rijpkema, M.; Franssen, G.M.; Kip, A.; Smeets, B.; Morgenstern, A.; Bruchertseifer, F.; Yan, E.; Wheatcroft, M.P.; Oosterwijk, E.; et al. Carbonic Anhydrase IX-Targeted α-Radionuclide Therapy with 225Ac Inhibits Tumor Growth in a Renal Cell Carcinoma Model. Pharmaceuticals 2022, 15, 570. [Google Scholar] [CrossRef]
- Mirzadeh, S.; Kumar, K.; Gansow, O.A. The Chemical Fate of 212 Bi-DOTA Formed by β-Decay of 212 Pb(DOTA)2-. Radiochim. Acta 1993, 60, 1–10. [Google Scholar] [CrossRef]
- Yong, K.; Brechbiel, M.W. Towards Translation of 212Pb as a Clinical Therapeutic; Getting the Lead In! Dalton Trans. 2011, 40, 6068. [Google Scholar] [CrossRef]
- Chappell, L.L.; Dadachova, E.; Milenic, D.E.; Garmestani, K.; Wu, C.; Brechbiel, M.W. Synthesis, Characterization, and Evaluation of a Novel Bifunctional Chelating Agent for the Lead Isotopes 203Pb and 212Pb. Nucl. Med. Biol. 2000, 27, 93–100. [Google Scholar] [CrossRef]
- McNeil, B.L.; Mastroianni, S.A.; McNeil, S.W.; Zeisler, S.; Kumlin, J.; Borjian, S.; McDonagh, A.W.; Cross, M.; Schaffer, P.; Ramogida, C.F. Optimized Production, Purification, and Radiolabeling of the 203Pb/212Pb Theranostic Pair for Nuclear Medicine. Sci. Rep. 2023, 13, 10623. [Google Scholar] [CrossRef]
- Metebi, A.; Kauffman, N.; Xu, L.; Singh, S.K.; Nayback, C.; Fan, J.; Johnson, N.; Diemer, J.; Grimm, T.; Zamiara, M.; et al. Pb-214/Bi-214-TCMC-Trastuzumab Inhibited Growth of Ovarian Cancer in Preclinical Mouse Models. Front. Chem. 2024, 11, 1322773. [Google Scholar] [CrossRef]
- Milenic, D.E.; Roselli, M.; Mirzadeh, S.; Pippin, C.G.; Gansow, O.A.; Colcher, D.; Brechbiel, M.W.; Schlom, J. In Vivo Evaluation of Bismuth-Labeled Monoclonal Antibody Comparing DTPA-Derived Bifunctional Chelates. Cancer Biother. Radiopharm. 2001, 16, 133–146. [Google Scholar] [CrossRef]
- Šimeček, J.; Hermann, P.; Seidl, C.; Bruchertseifer, F.; Morgenstern, A.; Wester, H.-J.; Notni, J. Efficient Formation of Inert Bi-213 Chelates by Tetraphosphorus Acid Analogues of DOTA: Towards Improved Alpha-Therapeutics. EJNMMI Res. 2018, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Autenrieth, M.E.; Seidl, C.; Bruchertseifer, F.; Horn, T.; Kurtz, F.; Feuerecker, B.; D’Alessandria, C.; Pfob, C.; Nekolla, S.; Apostolidis, C.; et al. Treatment of Carcinoma in Situ of the Urinary Bladder with an Alpha-Emitter Immunoconjugate Targeting the Epidermal Growth Factor Receptor: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, M.R.; Finn, R.D.; Sgouros, G.; Ma, D.; Scheinberg, D.A. An 225Ac/213Bi Generator System for Therapeutic Clinical Applications: Construction and Operation. App Radiat. Isot. 1999, 50, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Vanermen, M.; Ligeour, M.; Oliveira, M.-C.; Gestin, J.-F.; Elvas, F.; Navarro, L.; Guérard, F. Astatine-211 Radiolabelling Chemistry: From Basics to Advanced Biological Applications. EJNMMI Radiopharm. Chem. 2024, 9, 69. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Reardon, D.A.; Akabani, G.; Coleman, R.E.; Friedman, A.H.; Friedman, H.S.; McLendon, R.E.; Wong, T.Z.; Bigner, D.D. Clinical Experience with α-Particle–Emitting 211At: Treatment of Recurrent Brain Tumor Patients with 211At-Labeled Chimeric Antitenascin Monoclonal Antibody 81C6. J. Nucl. Med. 2008, 49, 30–38. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Pozzi, O.R.; Choi, J.; Zhao, X.-G.; Murphy, S.; Zalutsky, M.R. Labeling Monoclonal Antibody with α-Emitting 211At at High Activity Levels via a Tin Precursor. Cancer Biother. Radiopharm. 2020, 35, 511–519. [Google Scholar] [CrossRef]
- Lindegren, S.; Frost, S.; Bäck, T.; Haglund, E.; Elgqvist, J.; Jensen, H. Direct Procedure for the Production of 211At-Labeled Antibodies with an ε-Lysyl-3-(Trimethylstannyl)Benzamide Immunoconjugate. J. Nucl. Med. 2008, 49, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wilson, J.J.; Orvig, C.; Li, Y.; Wilbur, D.S.; Ramogida, C.F.; Radchenko, V.; Schaffer, P. Harnessing α-Emitting Radionuclides for Therapy: Radiolabeling Method Review. J. Nucl. Med. 2022, 63, 5–13. [Google Scholar] [CrossRef]
- Pallares, R.M.; Abergel, R.J. Development of Radiopharmaceuticals for Targeted Alpha Therapy: Where Do We Stand? Front. Med. 2022, 9, 1020188. [Google Scholar] [CrossRef] [PubMed]
- Xenaki, K.T.; Oliveira, S.; van Bergen en Henegouwen, P.M.P. Antibody or Antibody Fragments: Implications for Molecular Imaging and Targeted Therapy of Solid Tumors. Front. Immunol. 2017, 8, 1287. [Google Scholar] [CrossRef]
- Pandit-Taskar, N. Targeted Radioimmunotherapy and Theranostics with Alpha Emitters. J. Med. Imaging Radiat. Sci. 2019, 50, S41–S44. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.; Bhatavdekar, O.; Salerno, D.; Josefsson, A.; Pacheco-Torres, J.; Bhujwalla, Z.M.; Gabrielson, K.L.; Sgouros, G.; Sofou, S. Combination of Carriers with Complementary Intratumoral Microdistributions of Delivered α-Particles May Realize the Promise for 225Ac in Large, Solid Tumors. J. Nucl. Med. 2022, 63, 1223–1230. [Google Scholar] [CrossRef]
- Poty, S.; Carter, L.M.; Mandleywala, K.; Membreno, R.; Abdel-Atti, D.; Ragupathi, A.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. Leveraging Bioorthogonal Click Chemistry to Improve 225Ac-Radioimmunotherapy of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 868–880. [Google Scholar] [CrossRef]
- Aguirre, N.; Veach, D.R.; Cercek, A.; Cheal, S.M.; Larson, S.M.; Nash, G.M.; Cheung, N.-K.V. Radioimmunotherapy for Peritoneal Carcinomatosis: Preclinical Proof of Concept to Clinical Translation. Cell Rep. Med. 2025, 6, 102040. [Google Scholar] [CrossRef]
- Robinson, M.K.; Shaller, C.; Garmestani, K.; Plascjak, P.S.; Hodge, K.M.; Yuan, Q.-A.; Marks, J.D.; Waldmann, T.A.; Brechbiel, M.W.; Adams, G.P. Effective Treatment of Established Human Breast Tumor Xenografts in Immunodeficient Mice with a Single Dose of the α-Emitting Radioisotope Astatine-211 Conjugated to Anti-HER2/Neu Diabodies. Clin. Cancer Res. 2008, 14, 875–882. [Google Scholar] [CrossRef]
- Shah, M.A.; Zhang, X.; Rossin, R.; Robillard, M.S.; Fisher, D.R.; Bueltmann, T.; Hoeben, F.J.M.; Quinn, T.P. Metal-Free Cycloaddition Chemistry Driven Pretargeted Radioimmunotherapy Using α-Particle Radiation. Bioconjug Chem. 2017, 28, 3007–3015. [Google Scholar] [CrossRef]
- Królicki, L.; Kunikowska, J.; Bruchertseifer, F.; Koziara, H.; Królicki, B.; Jakuciński, M.; Pawlak, D.; Rola, R.; Morgenstern, A.; Rosiak, E.; et al. 225Ac- and 213Bi-Substance P Analogues for Glioma Therapy. Semin. Nucl. Med. 2020, 50, 141–151. [Google Scholar] [CrossRef]
- Andersson, H.; Cederkrantz, E.; Bäck, T.; Divgi, C.; Elgqvist, J.; Himmelman, J.; Horvath, G.; Jacobsson, L.; Jensen, H.; Lindegren, S.; et al. Intraperitoneal α-Particle Radioimmunotherapy of Ovarian Cancer Patients: Pharmacokinetics and Dosimetry of 211At-MX35 F(Ab′)2—A Phase I Study. J. Nucl. Med. 2009, 50, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.B.; Wilson, J.; Andersson, J.D.; Wuest, F. Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications. Pharmaceuticals 2023, 16, 1622. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, S.; Liu, J.; Jiang, D.; Wei, W. Antibody Theranostics in Precision Medicine. Med 2023, 4, 69–74. [Google Scholar] [CrossRef]
- Seo, Y. Quantitative Imaging of Alpha-Emitting Therapeutic Radiopharmaceuticals. Nucl. Med. Mol. Imaging 2019, 53, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Allen, K.J.H.; Malo, M.E.; Yilmaz, O.; Wilson, J.; Nelson, B.J.B.; Wuest, F.; Dadachova, E. A Theranostic Approach to Imaging and Treating Melanoma with 203Pb/212Pb-Labeled Antibody Targeting Melanin. Cancers 2023, 15, 3856. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Longtine, M.; Fears, A.; Benabdallah, N.; Unnerstall, R.; Johnston, H.; Shim, K.; Hasson, A.; Zhang, H.; Ulmert, D.; et al. Evaluation of Candidate Theranostics for 227Th/89Zr Paired Radioimmunotherapy of Lymphoma. J. Nucl. Med. 2023, 64, 1062–1068. [Google Scholar] [CrossRef]
- Watabe, T.; Kabayama, K.; Naka, S.; Yamamoto, R.; Kaneda, K.; Serada, S.; Ooe, K.; Toyoshima, A.; Wang, Y.; Haba, H.; et al. Immuno-PET and Targeted α-Therapy Using Anti–Glypican-1 Antibody Labeled with 89Zr or 211At: A Theranostic Approach for Pancreatic Ductal Adenocarcinoma. J. Nucl. Med. 2023, 64, 1949–1955. [Google Scholar] [CrossRef]
- Kondo, M.; Cai, Z.; Chan, C.; Forkan, N.; Reilly, R.M. [225Ac]Ac- and [111In]In-DOTA-Trastuzumab Theranostic Pair: Cellular Dosimetry and Cytotoxicity in Vitro and Tumour and Normal Tissue Uptake in Vivo in NRG Mice with HER2-Positive Human Breast Cancer Xenografts. EJNMMI Radiopharm. Chem. 2023, 8, 24. [Google Scholar] [CrossRef]
- Kelly, V.J.; Wu, S.; Gottumukkala, V.; Coelho, R.; Palmer, K.; Nair, S.; Erick, T.; Puri, R.; Ilovich, O.; Mukherjee, P. Preclinical Evaluation of an 111In/225Ac Theranostic Targeting Transformed MUC1 for Triple Negative Breast Cancer. Theranostics 2020, 10, 6946–6958. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Carter, L.M.; Atmane, M.I.; De Gregorio, R.; Michel, A.; Kaminsky, S.; Monette, S.; Li, M.; Schultz, M.K.; Lewis, J.S. 212Pb-Pretargeted Theranostics for Pancreatic Cancer. J. Nucl. Med. 2024, 65, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kanagasundaram, T.; Sun, Y.; Lee, K.K.; MacMillan, S.N.; Brugarolas, P.; Wilson, J.J. Fluorine-18 Incorporation and Radiometal Coordination in Macropa Ligands for PET Imaging and Targeted Alpha Therapy. Chem. Commun. 2024, 60, 11940–11943. [Google Scholar] [CrossRef]
- Jang, A.; Kendi, A.T.; Johnson, G.B.; Halfdanarson, T.R.; Sartor, O. Targeted Alpha-Particle Therapy: A Review of Current Trials. Int. J. Mol. Sci. 2023, 24, 11626. [Google Scholar] [CrossRef]
- Dekempeneer, Y.; Keyaerts, M.; Krasniqi, A.; Puttemans, J.; Muyldermans, S.; Lahoutte, T.; D’huyvetter, M.; Devoogdt, N. Targeted Alpha Therapy Using Short-Lived Alpha-Particles and the Promise of Nanobodies as Targeting Vehicle. Expert. Opin. Biol. Ther. 2016, 16, 1035–1047. [Google Scholar] [CrossRef]
- Hurley, K.; Cao, M.; Huang, H.; Wang, Y. Targeted Alpha Therapy (TAT) with Single-Domain Antibodies (Nanobodies). Cancers 2023, 15, 3493. [Google Scholar] [CrossRef]
- Lassmann, M.; Eberlein, U. Targeted Alpha-Particle Therapy: Imaging, Dosimetry, and Radiation Protection. Ann. ICRP 2018, 47, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, M.; Nosske, D. Dosimetry of 223Ra-Chloride: Dose to Normal Organs and Tissues. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 207–212. [Google Scholar] [CrossRef]


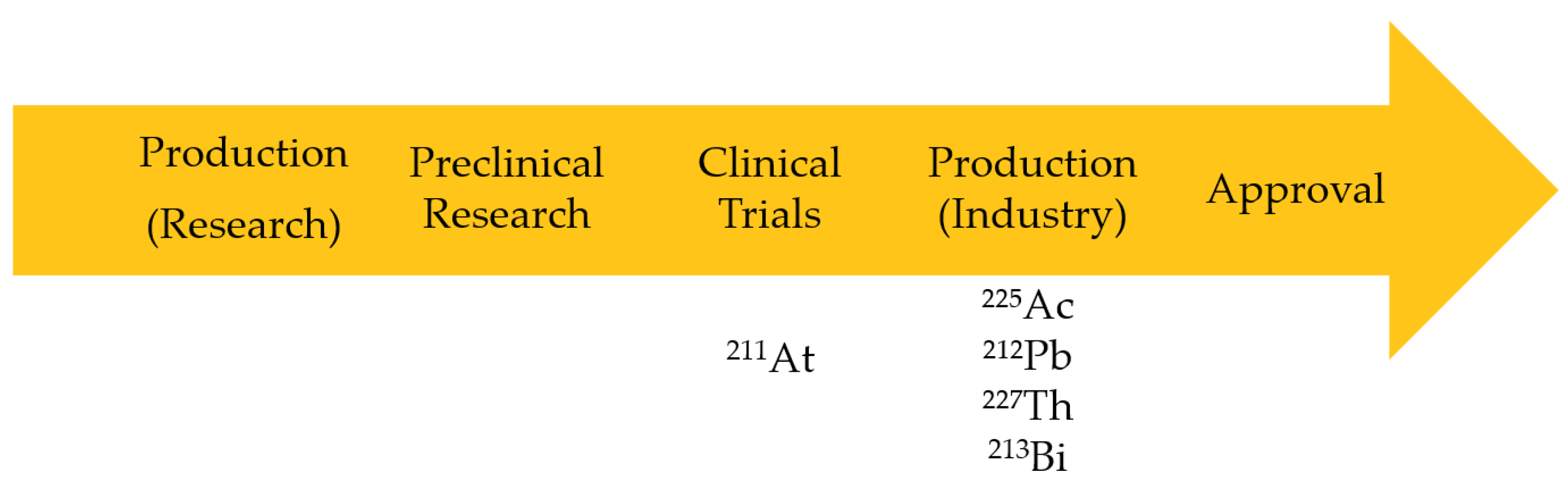
| Isotope | Production Cost | Availability |
|---|---|---|
| 225Ac | High | Limited (1:42; production: demand) but expanding |
| 213Bi | High | Limited |
| 227Th | Low | Available |
| 211At | Low | Limited to a few centers |
| 212Pb | Low | Available |
| TAT Agent | Target; Mouse Model | Findings | Route and Activity | Ref. |
|---|---|---|---|---|
| [225Ac]Ac-DOTA-BC8 | CD45; multiple myeloma | Selective killing of CD45+ and safe, targeted conditioning for bone marrow transplants. | N/A; a single dose of 300 nCi | [67] |
| [225Ac]Ac-DOTA-2Rs15d sdAb | HER2; HER2-expressing cancer | Significantly extending survival compared to control and to trastuzumab alone. Renal toxicity is found. | i.v.; 3 × 85 kBq | [68] |
| [225Ac]Ac-DOTA-HuM195/lintuzumab + venetoclax | CD33; acute myeloid leukemia | Combination showed superior tumor control and significantly prolonged survival in venetoclax-resistant. | 7.4 kBq (i.p.) + 200 mg/kg venetoclax (orally) | [27] |
| [225Ac]Ac-DOTA-hu11B6 | hK2; prostate cancer | Single high dose is better. No treatment-related toxicity observed. | i.v.; single high dose 22 kBq or 2 × ~11.1 kBq (300 nCi) spaced 4.5 months | [25,69] |
| [225Ac]Ac-DOTA-PKU525 | FAP; cancer-associated fibroblast | Significant tumor inhibition. No weight loss or organ damage. | i.v.; single dose ~11.1 kBq (300 nCi) | [26] |
| [225Ac]Ac-DOTAylated-huCC49 | TAG-72; ovarian cancer | Single high dose extended survival more than 3-fold over control. It is similar to multidose. No weight loss and no organ toxicity. | i.v.; single high dose 7.4 kBq or multi-dose 1.85 kBq followed by 5 × 0.7 kBq 5 weekly dose | [70] |
| [225Ac]Ac-anti-HER2/anti-DOTA IgG-scFv BsAb (pretargeted radioimmunotherapy) | HER2; ovarian cancer | Both single and double cycles prolonged survival. RBE-weighted dose per cycle: tumor is 56.9 Gy and kidneys are 16.1 Gy. | i.p; 37 kBq or 2 × 37 kBq (spaced 1 week). | [71] |
| [225Ac]Ac-DOTA-OTSA101 | FZD10; synovial sarcoma | Reduced tumor volume and prolonged survival. 60% of mice receiving a single 7.4 kBq dose achieved a complete response with no tumor recurrence. | i.v.; 7.4 kBq | [72] |
| [225Ac]Ac-DOTA-hu5A10 | FcRn; prostate cancer | Sustained tumor control. 7/18 complete remissions. | i.v.; ~11.1 kBq (300 nCi) | [73] |
| [225Ac]Ac(MacropaSq-hG250) | Carbonic anhydrase IX; renal cell carcinoma | Specific tumor targeting, significant tumor inhibition and DNA damage. Reduced kidney toxicity compared to DOTA-based variants. Fast, stable room-temperature labeling of antibodies with 225Ac. | i.v.; 14.8 kBq | [74] |
| [225Ac]Ac-E4G10 | Cadherin; glioblastoma | Increase overall survival. | i.v.; 11.1 kBq (300 nCi) | [75] |
| [213Bi]Bi-CHX-A″-DTPA-anti-EGFR-mAb | EGFR; bladder carcinoma | Both dosing regimens yielded potent antitumor effects with durable responses in over 30% of subjects. No normal tissue toxicity. | i.p; 2 × 0.93 MBq or 3 × 0.46 MBq | [76] |
| [213Bi]Bi-CHX-A″-69-11 antibodies | CETN1; pancreatic ductal adenocarcinoma | Significant tumor suppression. No weight loss or organ damage observed. | i.p.; no specified | [77] |
| [213Bi]Bi-CHX-A″-h8C3 antibody + anti-PD-1 | Melanin; melanoma | The combination showed significant tumor control and prolonged survival. No weight loss or over toxicity. | i.p.; ~0.46–0.93 MBq (1–2 doses) | [78] |
| [213Bi]Bi-CHX-A″-DTPA-MX35-mAb | NaPi2b; ovarian cancer | The tumor-free rates for low and high doses are 55% and 78%, respectively. No weight loss, stable WBC/platelet. | i.p.; 3 MBq/mL (~10 µg) or 9 MBq/mL (~30 µg) | [31] |
| [213Bi]Bi-DTPA-PAN-622-mAb | HAAH; breast cancer | Significant inhibition of primary tumor growth. | i.p.; 150 μCi | [79] |
| [213Bi]Bi-CHX-A″-DTPA-Anti-hCD138 antibody | CD138; ovarian cancer | Single i.p. injections of both 7.4 and 11.1 MBq doses significantly prolong survival. | i.p.; 7.4 MBq or 11.1 MBq | [80] |
| [213Bi]Bi-DOTA-9E7.4 | CD138; multiple myeloma | Median survival increased to 80 days vs. 37 days in control group and 54 days in 18.5 MBq [177Lu] Lu-DOTA-9E7.4. ~45% of mice were cured, exhibiting long-term complete remission. | i.v.; 3.7 MBq | [81] |
| [213Bi]Bi-CHX-A″-DTPA-anti-CD138-mAb + adoptive T cell therapy | CD138; multiple myeloma | The combination achieved a significant tumor growth delay compared to either treatment alone. | i.v.; 3.7 MBq followed by 5 × 106 T cells injection after 24 h. | [82] |
| [213Bi]Bi-DTPA-anti-CD38-mAb | CD38; multiple myeloma | Dramatic tumor suppression and significantly extended survival. | i.v.; 6 × 1.85 MBq | [83] |
| [213Bi]Bi-CHX-A″-DTPA-C6.5K-A scFv and diabody | HER2; ovarian cancer | The 0.3 µCi dose of scFv resulted in a significant reduction in tumor growth rate compared to controls. Acceptable toxicity levels. However, it was not antigen specific. Diabody conjugates did not significantly inhibit tumor growth compared to controls. | i.v.; diabody: 0.64, 0.35, and 0.15 µCi scFv: 1.1, 0.6, and 0.3 µCi | [84] |
| [211At]At-anti-CD123-mAb | CD123; leukemia | Decreased tumor burdens and substantially prolonged survival. | i.v.; 40 µCi | [47] |
| [211At]At-OKT10 | CD38; multiple myeloma | Sustained remission and long-term survival (>150 days) for 50% to 80% of treated mice. | i.v.; 24 to 45 µCi | [48] |
| [211At]At-CA12.10C12 + total body irradiation (TBI) | CD45; aplastic anemia and hemoglobinopathy | The combination was successful in abrogating graft rejection in 86% of dogs in this presensitization model. | i.v.; 0.188 mCi/kg (7 MBq) on day-3, and TBI followed by marrow grafts on day 0. | [50] |
| [211At]At-1F5-B10 | CD20; minimal residual disease lymphoma | Complete eradication of disseminated lymphoma in treated mice, with no detectable disease at 90 days post-treatment. [211At]At-1F5-B10 demonstrated superior therapeutic efficacy compared to its 131I-labeled counterpart. | i.v.; up to 0.5 mCi/kg | [85] |
| [211At]At-9E7.4 | CD 138; multiple myeloma minimal residual | The 740 kBq significantly prolonged survival, with about 65% of mice surviving at 150 days post-treatment. | i.v.; 370, 555, 740, and 1100 kBq | [86] |
| [211At]At-Mel-14 F(ab′)2 | Chondroitin sulfate proteoglycan; gliomas | Specifically localized to human glioma xenografts in mice. Good tumor uptake and retention. | i.v.; N/A | [51] |
| [211At]At-A11 | PSCA; prostate cancer or bone microtumors | Lower doses showed efficacy with minimal toxicity. | i.v.; 0.3 to 1.0 MBq | [87] |
| [212Pb]Pb-TCMC-rituximab | CD20; non-Hodgkin lymphoma | Significantly prolonged median survival compared to controls. Toxicity was dose-dependent; lethal effects occurred at doses exceeding 740 kBq. At 277.5 kBq, the treatment was well tolerated with minimal hematological toxicity. | i.v.; 277.5 kBq | [57] |
| [212Pb]Pb-TCMC-daratumumab | CD38; multiple myeloma | Efficacy at 277.5 kBq without toxic effects. | i.v.; 185kBq or 277.5 kBq | [58] |
| [212Pb]Pb-TCMC-YS5 | CD46; prostate cancer | 0.74 MBq effectively and safely inhibited tumor growth and enhanced survival. | i.v.; 0.74 MBq | [59] |
| [212Pb]Pb-TCMC-NNV003 | CD37; chronic lymphocytic leukemia and non-Hodgkin lymphoma | Daudi model (CB17 SCID): 67–91% survival at 28 weeks post-cell injection. MEC-2 model (R2G2): 30–90% survival at study endpoint (~21 weeks). Mild/transient hematology effects; no major organ toxicity. | i.v.; 185–555 kBq | [88] |
| TAT Agent | Target, Indication | Route and Activity | Status | Findings | Ref. |
|---|---|---|---|---|---|
| [225Ac]Ac-DOTA-HuM195/lintuzumab + venetoclax | CD33; acute myeloid leukemia | i.v.; 18.5 or 9.25 kBq/kg on day 5 (4 cycles) + Venetoclax on day 1–21 (12 cycles) | Phase I/II, recruiting (2020) | Recruiting, not yet reported. | [89] |
| [225Ac]Ac-FPI-1434 | IGF-1R; advanced solid tumours | N/A; dose is per cohort assignment. | Phase I/II, recruiting (2019) | Recruiting, not yet reported | [90] |
| [225Ac]Ac-J591 | PSMA; mCRPC | i.v.; 65 or 50 kBq/kg | Early phase I, active, not recruiting (2020) | Not yet reported | [28,91,92,93] |
| i.v.; single dose every 6 weeks × 4 | Phase I/II, suspended (2020) | Not yet reported | |||
| i.v.; 13.3–93.3 or 0.36–2.52 kBq/kg on day 1 | Phase I, completed (2017) | Dose-limiting toxicity was 80 KBq/kg and the recommended phase II dose was 93.3 KBq/kg | |||
| [225Ac]Ac-DOTA-M5A | CEA; positive colorectal cancer | i.v.; over 25 min on day 1, dose is per cohort assignment. | Phase I, recruiting (2022) | Not yet reported | [94] |
| [225Ac]Ac-DOTA-daratumumab + fludarabine + melphalan + total marrow and lymphoid irradiation (TMLI) | CD38; high-risk myeloid leukemia, acute lymphoblastic leukemia, and myelodysplastic syndrome | i.v.; injection on day 15. The dose is per cohort assignment. TMLI BID on days −8 to −5, fludarabine IV on days −4 to −2, and melphalan IV on day −2, followed by HCT on day 0. | Phase I, recruiting (2024) | Not yet reported | [95] |
| [225Ac]Ac-DOTA-hu11B6 | hK2; advanced prostate cancer | i.v.; one or multiple doses. The dose levels will be escalated based on the dose-limiting toxicities. | Phase I, recruiting (2020) | Not yet reported | [96] |
| [225Ac]Ac-macropa-pelgifatamab | PSMA; mCRPC | i.v. | Phase I, recruiting (2023) | Not yet reported | [97] |
| [213Bi]Bi-CHX-A”-DTPA-HuM195/lintuzumab | CD33; acute myeloid leukemia | i.v; 18.5, 27.75, 37, and 46.25 kBq/kg | Phase I/II, completed (2001) | MTD = 37MBq/kg. Treatment-related deaths occurred = 10% of those who received the MTD. | [32] |
| [227Th]Th-corixetan-anetumab | Mesothelin; malignant pleural epithelioid, malignant peritoneal epithelioid, and ovarian cancer | i.v.; 1.5 MBq | Phase I, completed (2018) | Not yet reported | [42] |
| [211At]At-MX35 F(ab′)2 | 95-kDa plasma membrane sodium-dependent phosphate transporter protein 2b (NaPi2b); ovarian | i.p. infusion; dose escalation up to 215 MBq/L (5MBq/kg) | Early phase I, completed (2005) | i.p. administration is possible to achieve therapeutic absorbed doses without significant toxicity. | [98] |
| [211At]At-OKT10-B10 + fludarabine | CD38; high-risk multiple myeloma | i.v. | Phase I, not yet recruiting (2024) | Not yet reported | [49] |
| [212Pb]Pb-TCMC-trastuzumab | HER2; HER2-expressing malignancies in the peritoneal cavity | i.p.; dose escalation up to 40 MBq | Phase I, completed (2011) | MTD = 27 MBq/m2 = 0.9 MBq/kg | [61] |
| [227Th]227Th-epratuzumab | CD22; relapsed/refractory CD22-positive non-Hodgkin lymphoma | i.v.; dose up to 4.6 MBq | Phase I, completed (2015) | Tolerated dose up to 4.6 MBq (10 mg antibody) without reading MTD. Safe and tolerated in patients with R/R-NHL. | [38] |
| [227Th]Th-corixetan-anetumab | Mesothelin; solid tumors known to express mesothelin | i.v.; start at 1.5 MBq and increase in steps of 1.0 or 1.5 MBq | Phase I, Completed (2018) | Not yet reported | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palangka, C.R.A.P.; Mahendra, I.; Ritawidya, R.; Kondo, N.; Nakajima, T. Alpha Particle Emitter Radiolabeled Antibodies in Cancer Therapy: Current Status, Challenges, and Future Prospects. Pharmaceuticals 2025, 18, 1316. https://doi.org/10.3390/ph18091316
Palangka CRAP, Mahendra I, Ritawidya R, Kondo N, Nakajima T. Alpha Particle Emitter Radiolabeled Antibodies in Cancer Therapy: Current Status, Challenges, and Future Prospects. Pharmaceuticals. 2025; 18(9):1316. https://doi.org/10.3390/ph18091316
Chicago/Turabian StylePalangka, Citra R. A. P., Isa Mahendra, Rien Ritawidya, Naoya Kondo, and Takahito Nakajima. 2025. "Alpha Particle Emitter Radiolabeled Antibodies in Cancer Therapy: Current Status, Challenges, and Future Prospects" Pharmaceuticals 18, no. 9: 1316. https://doi.org/10.3390/ph18091316
APA StylePalangka, C. R. A. P., Mahendra, I., Ritawidya, R., Kondo, N., & Nakajima, T. (2025). Alpha Particle Emitter Radiolabeled Antibodies in Cancer Therapy: Current Status, Challenges, and Future Prospects. Pharmaceuticals, 18(9), 1316. https://doi.org/10.3390/ph18091316







