Effect of Drying Methods on Bioactivity of Pyrostegia venusta Extracts: Antioxidant Assays, Cytotoxicity, and Computational Approaches
Abstract
1. Introduction
2. Results
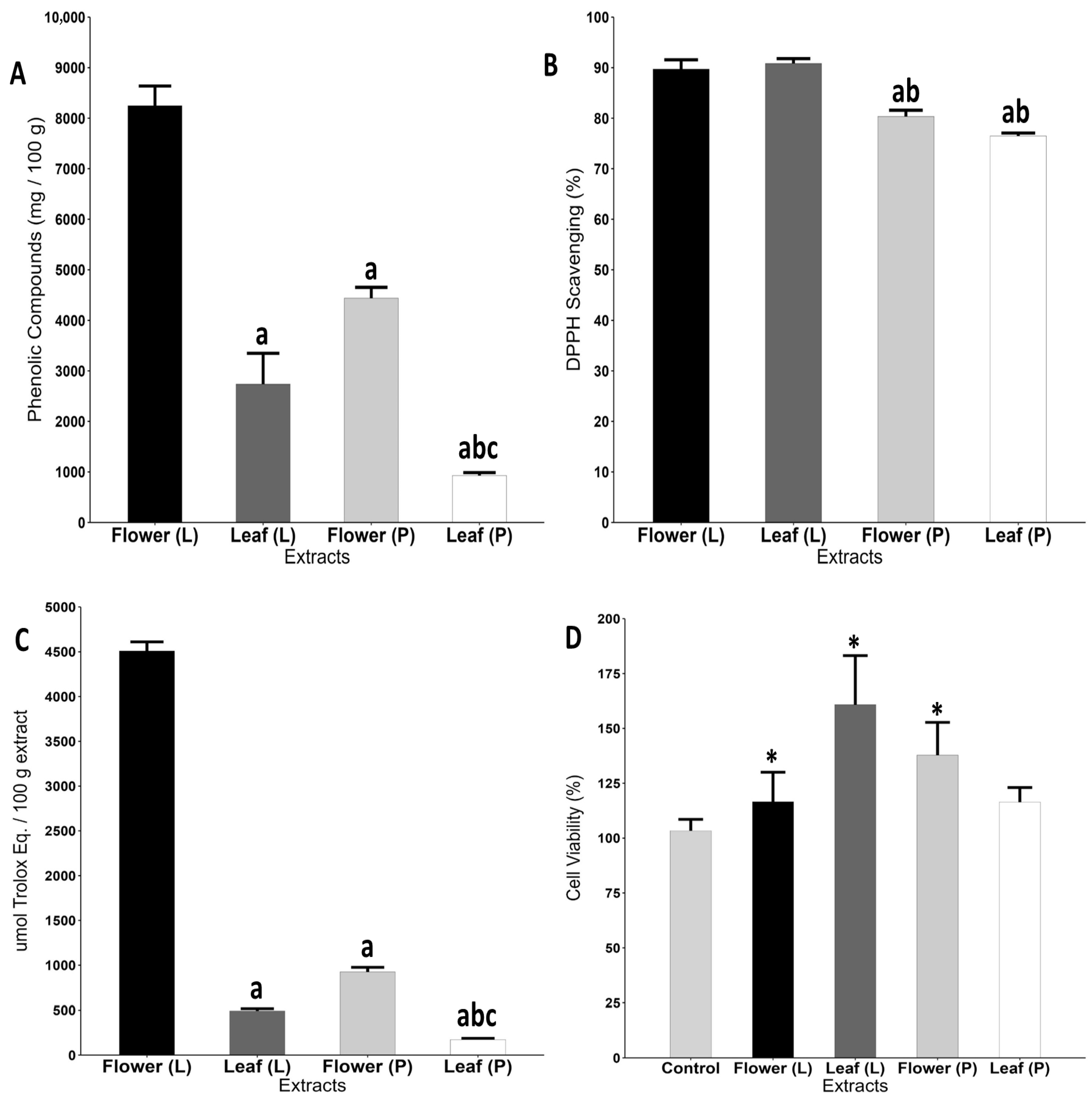
2.1. Freeze-Dried Flower Extract Exhibited Higher Concentration of Phenolic Compounds, Antioxidant Capacity, and Enhanced HaCaT Cell Viability
2.2. UHPLC Analysis of Phytochemical Compound Profiles
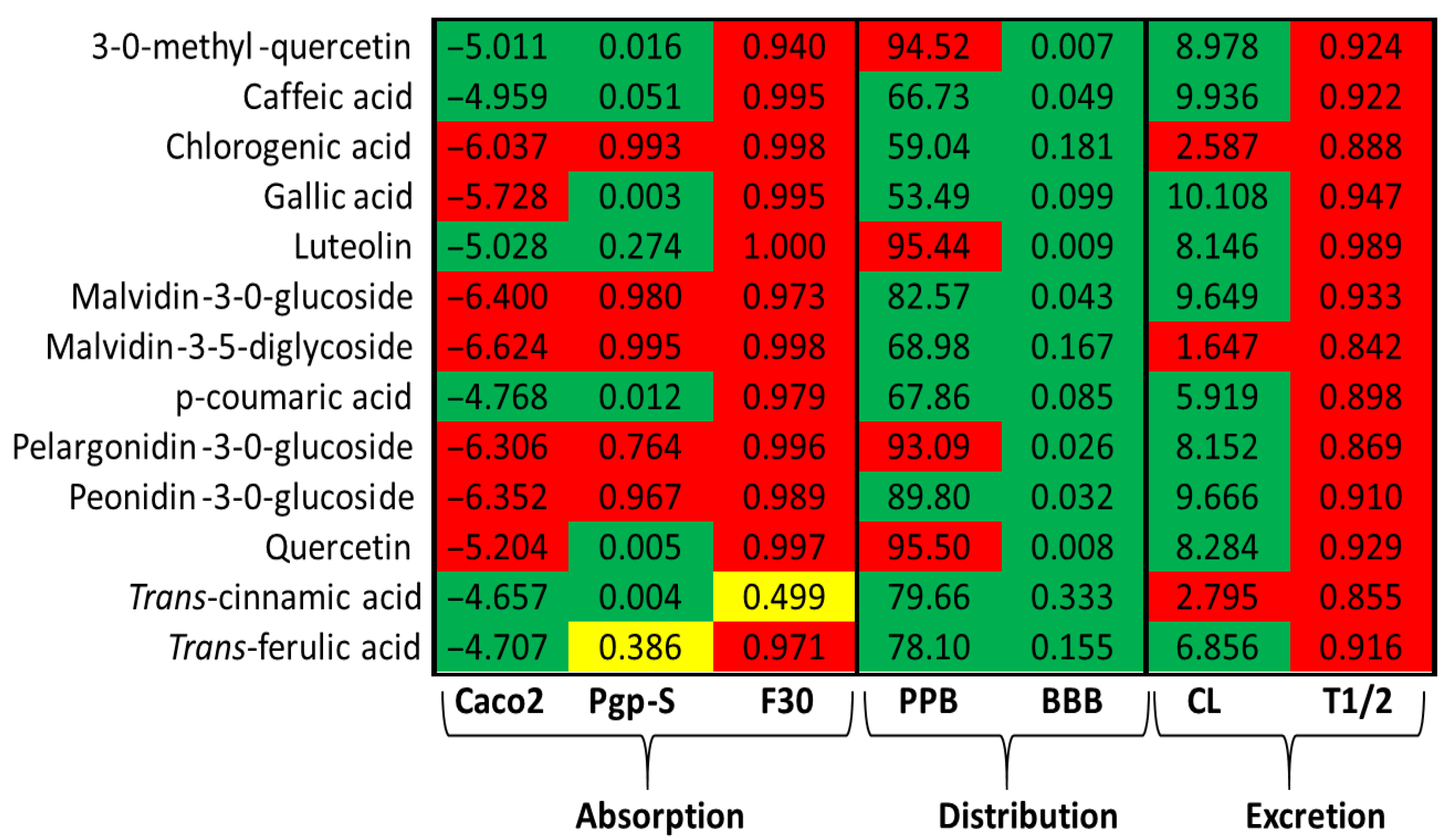
2.3. Structural and Biological Properties of Phenolic Compounds and Their Predicted Toxicity
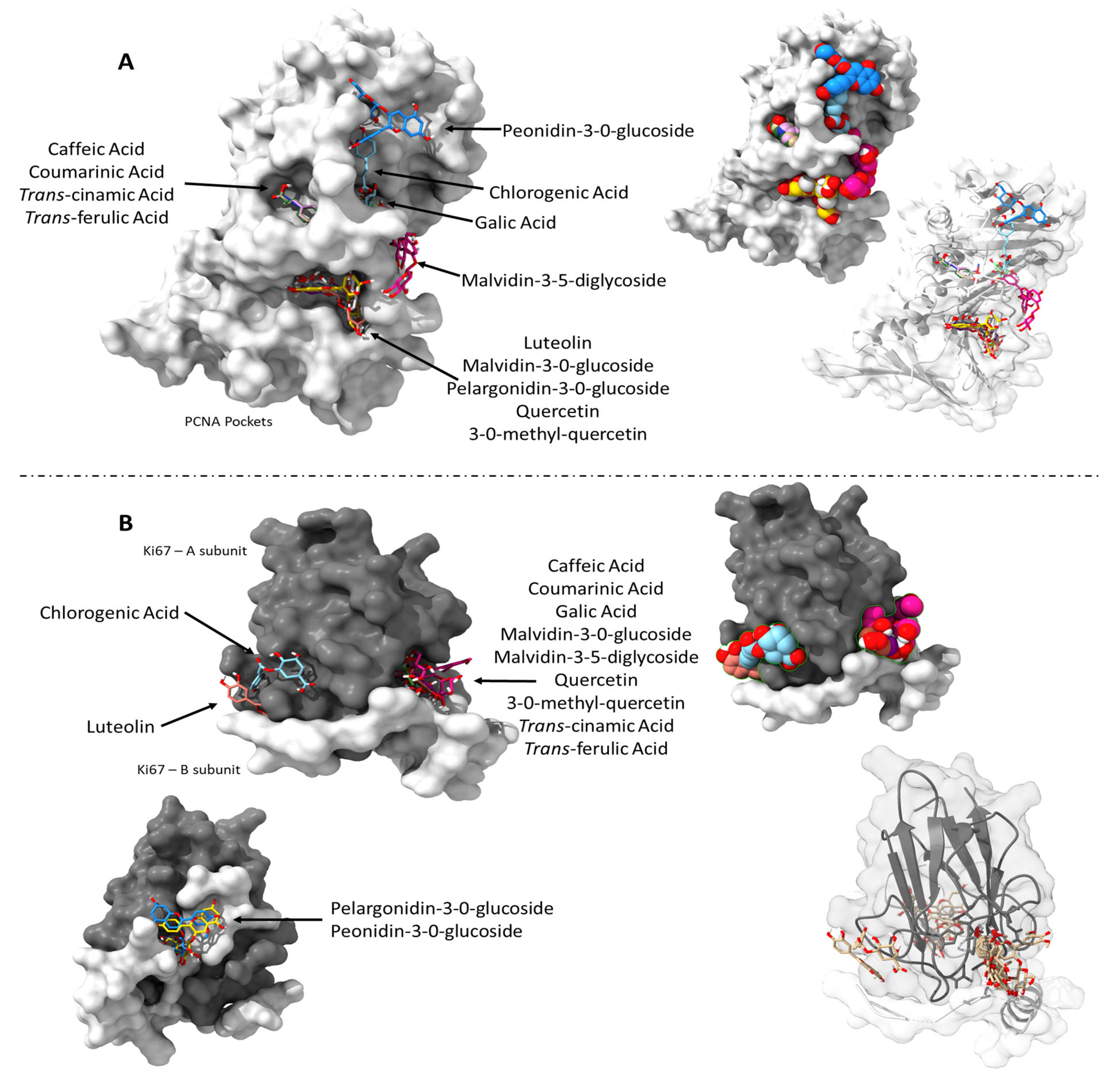
2.4. Phenolic Compounds Interacted with Cell-Cycle Proteins
3. Discussion
4. Materials and Methods
4.1. Material Collection and Production of Hot-Air Oven- and Freeze-Dried Extracts
4.2. Preparation of Aqueous Extracts from Leaves and Flowers
4.3. Determination of Total Phenolic Compounds
4.4. Determination of Antioxidant Capacity by the DPPH Method
4.5. Total Antioxidant Activity by the Iron Reduction Method—FRAP
4.6. Cell Viability Assay
4.7. Profile of Phenolic Compounds by Ultra-High-Performance Liquid Chromatography (UHPLC)
4.8. ADMET Predictions
4.9. In Silico Molecular Blind Docking
4.10. Statistical Analysis
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamang, S.; Singh, A.; Bussmann, R.W.; Shukla, V.; Nautiyal, M.C.; Tamang, S.; Singh, A.; Bussmann, R.W.; Shukla, V.; Nautiyal, M.C. Ethno-Medicinal Plants of Tribal People: A Case Study in Pakyong Subdivision of East Sikkim, India. Acta Ecol. Sin. 2023, 43, 34–46. [Google Scholar] [CrossRef]
- John, O.O.; Amarachi, I.S.; Chinazom, A.P.; Adaeze, E.; Kale, M.B.; Umare, M.D.; Upaganlawar, A.B. Phytotherapy: A Promising Approach for the Treatment of Alzheimer’s Disease. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100030. [Google Scholar] [CrossRef]
- Chávez-Castillo, M.; Ortega, Á.; Duran, P.; Pirela, D.; Marquina, M.; Cano, C.; Salazar, J.; Gonzalez, M.C.; Bermúdez, V.; Rojas-Quintero, J.; et al. Phytotherapy for Cardiovascular Disease: A Bench-to-Bedside Approach. Curr. Pharm. Des. 2020, 26, 4410–4429. [Google Scholar] [CrossRef]
- Chávez-Castillo, M.; Nuñez, V.; Rojas, M.; Ortega, Á.; Durán, P.; Pirela, D.; Marquina, M.; Cano, C.; Chacín, M.; Velasco, M.; et al. Exploring Phytotherapeutic Alternatives for Obesity, Insulin Resistance and Diabetes Mellitus. Curr. Pharm. Des. 2020, 26, 4430–4443. [Google Scholar] [CrossRef]
- Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Winker, M.; Gründemann, C. Phytotherapy in Integrative Oncology-An Update of Promising Treatment Options. Molecules 2022, 27, 3209. [Google Scholar] [CrossRef]
- Da Fonseca, L.R.; de Rodrigues, R.A.; de Ramos, A.S.; Da Cruz, J.D.; Ferreira, J.L.P.; de Silva, J.R.A.; Amaral, A.C.F. Herbal Medicinal Products from Passiflora for Anxiety: An Unexploited Potential. Sci. World J. 2020, 2020, 6598434. [Google Scholar] [CrossRef]
- Bhat, M.P.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Dupadahalli, K.; Rudrappa, M.; Shivapoojar Basavarajappa, D.; Sathyanarayana Swamy, P.; Perumal, K.; Nayaka, S. Characterization, Antimicrobial Activity and Anticancer Activity of Pyrostegia venusta Leaf Extract-Synthesized Silver Nanoparticles against COS-7 Cell Line. Appl. Nanosci. 2023, 13, 2303–2314. [Google Scholar] [CrossRef]
- Angelo, P.M.; Jorge, N. Phenolic Compounds in Foods—A Brief Review. Rev. Inst. Adolfo Lutz 2007, 66, 1–9. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Pammi, S.S.S.; Suresh, B.; Giri, A. Antioxidant Potential of Medicinal Plants. J. Crop Sci. Biotechnol. 2023, 26, 13–26. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Zuchetto, M.; Oliveira, C.F.; Paula, C.S.; Duarte, A.F.S.; Miguel, M.D.; Miguel, O.G. Effect of Different Extraction Techniques on the Yield, Antioxidant Activity, Total, and Profile by Hplc-Dad of Dicksonia Sellowiana (Presl.). Hook, Dicksoniaceae. Rev. Bras. de Plantas Med. 2016, 18, 230–239. [Google Scholar] [CrossRef]
- Freitas, B.A.G.; Ribeiro, J.S.; Viana, E.B.M.; de Souza, C.C.E.; Zanuto, M.E. Main Drying Methods Used to Obtain Soluble Fruit Pulp Powders: A Review. Braz. Appl. Sci. Rev. 2022, 6, 1588–1620. [Google Scholar] [CrossRef]
- Tinoi, J.; Rakariyatham, N.; Deming, R.L. Determination of Major Carotenoid Constituents in Petal Extracts of Eight Selected Flowering Plants in the North of Thailand. Chiang Mai J. Sci. 2006, 33, 327–334. [Google Scholar]
- Pereira, A.M.S.; Hernandes, C.; Pereira, S.I.V.; Bertoni, B.W.; França, S.C.; Pereira, P.S.; Taleb-Contini, S.H. Evaluation of Anticandidal and Antioxidant Activities of Phenolic Compounds from Pyrostegia venusta (Ker Gawl.) Miers. Chem. Biol. Interact. 2014, 224, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Shewale, V.D.; Agrawal, H.S.; Chaudhari, R.N. Comparative Phytochemical Investigation and Determination of Flavonoids, Alkaloid and Antioxidant Activity of Leaves, Stem and Flower Extract of Pyrostegia Venusta. Int. J. Res. Trends Innov. 2023, 8, 66–71. [Google Scholar]
- Roy, P.; Amdekar, S.; Kumar, A.; Singh, V. Preliminary Study of the Antioxidant Properties of Flowers and Roots of Pyrostegia venusta (Ker Gawl) Miers. BMC Complement. Altern. Med. 2011, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Balestra, A.C.; Sandy, C.M.; Ramalho, F.; Júnior, A.A.J.; Contini, S.H.T.; Crevelin, E.J.; Carmona, F.; Pereira, A.M.S.; Borges, M.C. Aqueous Pyrostegia venusta (Ker Gawl.) Miers Extract Attenuates Allergen-Induced Asthma in a Mouse Model via an Antioxidant Mechanism. J. Asthma 2021, 58, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Viel, A.M.; Silva, L.P.; Martins, G.R.; Urtremari, B.; Sekiya, A.; Dokkedal, A.L.; Souza, E.B.; Camargo, I.C.C.; Silva, R.M.G. Toxicological, genotoxic and antioxidant potential of Pyrostegia venusta. Biosci. J. 2019, 35, 570–585. [Google Scholar] [CrossRef]
- Ali Redha, A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, T.; Shi, M.; Wei, Y.; Huang, X.; Shen, J.; Zhang, X.; Xie, Z.; Huang, P.; Yuan, K.; et al. Polyphenols: Natural Food Grade Biomolecules for Treating Neurodegenerative Diseases from a Multi-Target Perspective. Front. Nutr. 2023, 10, 1139558. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Baris, Y.; Taylor, M.R.G.; Aria, V.; Yeeles, J.T.P. Fast and Efficient DNA Replication with Purified Human Proteins. Nature 2022, 606, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Younis, R.L.; El-Gohary, R.M.; Ghalwash, A.A.; Hegab, I.I.; Ghabrial, M.M.; Aboshanady, A.M.; Mostafa, R.A.; El-Azeem, A.H.A.; Farghal, E.E.; Belal, A.A.E.; et al. Luteolin Mitigates D-Galactose-Induced Brain Ageing in Rats: SIRT1-Mediated Neuroprotection. Neurochem. Res. 2024, 49, 2803–2820. [Google Scholar] [CrossRef]
- Hardebeck, S.; Schreiber, S.; Adick, A.; Langer, K.; Jose, J. A FRET-Based Assay for the Identification of PCNA Inhibitors. Int. J. Mol. Sci. 2023, 24, 11858. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Goh, N.Y.; Mohamad Razif, M.F.; Yap, Y.H.Y.; Ng, C.L.; Fung, S.Y. In Silico Analysis and Characterization of Medicinal Mushroom Cystathionine Beta-Synthase as an Angiotensin Converting Enzyme (ACE) Inhibitory Protein. Comput. Biol. Chem. 2022, 96, 107620. [Google Scholar] [CrossRef]
- Georgiou, N.; Mavromoustakos, T.; Tzeli, D. Docking, MD Simulations, and DFT Calculations: Assessing W254’s Function and Sartan Binding in Furin. Curr. Issues Mol. Biol. 2024, 46, 8226–8238. [Google Scholar] [CrossRef]
- Chigurupati, S.; Al-Murikhy, A.; Almahmoud, S.A.; Almoshari, Y.; Saber Ahmed, A.; Vijayabalan, S.; Ghazi Felemban, S.; Raj Palanimuthu, V. Molecular Docking of Phenolic Compounds and Screening of Antioxidant and Antidiabetic Potential of Moringa Oleifera Ethanolic Leaves Extract from Qassim Region, Saudi Arabia. Saudi J. Biol. Sci. 2022, 29, 854–859. [Google Scholar] [CrossRef]
- Gaidhani, K.A.; Harwalkar, M.; Bhambere, D.; Nirgude, P.S. Lyophilization/Freeze Drying—A Review. World J. Pharm. Res. 2015, 4, 516. [Google Scholar]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.B.; Medeiros, A.C.M.; Duarte, M.C.T.; Ruiz, A.L.T.G.; Kolb, R.M.; Frei, F.; Santos, C.D. Avaliação Do Potencial Alelopático, Atividade Antimicrobiana e Antioxidante Dos Extratos Orgânicos Das Folhas de Pyrostegia venusta (Ker Gawl.) Miers (Bignoniaceae). Rev. Bras. de Plantas Med. 2011, 13, 447–455. [Google Scholar] [CrossRef]
- Rajashekar, C.B. Dual Role of Plant Phenolic Compounds as Antioxidants and Prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. P-Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Gomez-Gomez, H.A.; Borges, C.V.; Minatel, I.O.; Luvizon, A.C.; Lima, G.P.P. Health Benefits of Dietary Phenolic Compounds and Biogenic Amines. Ref. Ser. Phytochem. 2019, 3–27. [Google Scholar] [CrossRef]
- Aijaz, M.; Keserwani, N.; Yusuf, M.; Ansari, N.H.; Ushal, R.; Kalia, P. Chemical, Biological, and Pharmacological Prospects of Caffeic Acid. Biointerface Res. Appl. Chem. 2023, 13, 324. [Google Scholar] [CrossRef]
- de Oliveira Silva, E.; Batista, R. Ferulic Acid and Naturally Occurring Compounds Bearing a Feruloyl Moiety: A Review on Their Structures, Occurrence, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 64, 4–17. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Elhady, S.S.; Ahmed, H.A.; Badr, J.M.; Noor, A.O.; Ahmed, S.A.; Nafie, M.S. Chemical Profiling, Antioxidant, Cytotoxic Activities and Molecular Docking Simulation of Carrichtera Annua DC. (Cruciferae). Antioxidants 2020, 9, 1286. [Google Scholar] [CrossRef]
- Hassan, I.; Gul, S.; Zaman, A.; Zafar, E.; Khan, M. Molecular Docking-Aided Identification of Natural Bioactive Molecules as Potential Cancer Cell Proliferation Inhibitors. Futur. Biotechnol. 2024, 4, 25–30. [Google Scholar] [CrossRef]
- Baruah, I.; Kashyap, C.; Guha, A.K.; Borgohain, G. Insights into the Interaction between Polyphenols and β-Lactoglobulin through Molecular Docking, MD Simulation, and QM/MM Approaches. ACS Omega 2022, 7, 23083–23095. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Prottoy, N.I.; Araf, Y.; Hossain, S.; Sarkar, B.; Saha, A. Molecular Docking and Pharmacological Property Analysis of Phytochemicals from Clitoria ternatea as Potent Inhibitors of Cell Cycle Checkpoint Proteins in the Cyclin/CDK Pathway in Cancer Cells. Comput. Mol. Biosci. 2019, 9, 81–94. [Google Scholar] [CrossRef]
- Stompor-Gorący, M.; Machaczka, M. Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. Int. J. Mol. Sci. 2021, 22, 12889. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Upadhyay, T.K.; Giri, P.P. Therapeutic Efficacy of Caffeic Acid Phenethyl Ester in Cancer Therapy: An Updated Review. Chem. Biol. Drug Des. 2023, 102, 201–216. [Google Scholar] [CrossRef]
- Li, H.; Wilhelm, M.; Baumbach, C.M.; Hacker, M.C.; Szardenings, M.; Rischka, K.; Koenig, A.; Schulz-Kornas, E.; Fuchs, F.; Simon, J.C.; et al. Laccase-Treated Polystyrene Surfaces with Caffeic Acid, Dopamine, and L-3,4-Dihydroxyphenylalanine Substrates Facilitate the Proliferation of Melanocytes and Embryonal Carcinoma Cells NTERA-2. Int. J. Mol. Sci. 2024, 25, 5927. [Google Scholar] [CrossRef]
- Houghton, P. Synergy and Polyvalence: Paradigms to Explain the Activity of Herbal Products. In Evaluation of Herbal Medicinal Products; Pharmaceutical Press: London, UK, 2009; Volume 85, p. 94. [Google Scholar]
- Williamson, E.M. Synergy and Other Interactions in Phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole Plant Extracts versus Single Compounds for the Treatment of Malaria: Synergy and Positive Interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef]
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.H.; Zhao, J.; Zhang, W.D.; Ma, B.L. Synergistic Mechanisms of Constituents in Herbal Extracts during Intestinal Absorption: Focus on Natural Occurring Nanoparticles. Pharmaceutics 2020, 12, 128. [Google Scholar] [CrossRef]
- de Freitas Pereira, T.; de Carvalho, R.A.; Yoshida, C.M.P.; dos Santos Garcia, R.H.; Veiga-Santos, P.; Agneis, M.L.G.; Seiva, F.R.F.; Martelli, S.M.; Vilela, D.M.; Garcia, V.A.d.S. Production and characterization of ora-pro-nóbis and agar-agar based edible leathers (Snack-films): A new plant-based food option. J. Indian Chem. Soc. 2025, 102, 101936. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Garcia, V.A.d.S.; Borges, J.G.; Maciel, V.B.V.; Mazalli, M.R.; Lapa-Guimaraes, J.d.G.; Vanin, F.M.; de Carvalho, R.A. Gelatin/Starch Orally Disintegrating Films as a Promising System for Vitamin C Delivery. Food Hydrocoll. 2018, 79, 127–135. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Martins, R.C.; Leonel, S.; Souza, J.M.A.; Lima, G.P.P.; Leonel, M.; Putti, F.F.; Monteiro, G.C.; Züge, P.G.Ü.; Napoleão, G.M.; Figueira, R.; et al. Profile of Bioactive Compounds in Orange Juice Related to the Combination of Different Scion/Rootstocks, Packaging and Storage. Horticulturae 2023, 9, 347. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Byeon, I.J.L.; Li, H.; Song, H.; Gronenborn, A.M.; Tsai, M.D. Sequential Phosphorylation and Multisite Interactions Characterize Specific Target Recognition by the FHA Domain of Ki67. Nat. Struct. Mol. Biol. 2005, 12, 987–993. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for Structure Building and Analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef] [PubMed]

| Quantification (µg/mg) | |||||
|---|---|---|---|---|---|
| Rt | λmax | Tentative Identification | Leaves | Flowers | p-Value |
| 11.33 | 360 | 3-O-methyl-quercetin | 0.0292 ± 0.0004 | 0.5867 ± 0.0006 | p < 0.0001 |
| 8.24 | 320 | Caffeic acid | 3.0728 ± 0.0105 | 0.1721 ± 0.0024 | p < 0.0001 |
| 7.15 | 320 | Chlorogenic acid | 3.5582 ± 0.0213 | 2.1584 ± 0.0198 | p < 0.0001 |
| 5.83 | 270 | Gallic acid | 0.0983 ± 0.0012 | 0.0215 ± 0.0015 | p > 0.9999 |
| 11.47 | 360 | Luteolin | 0.2030 ± 0.0012 | 0.0404 ± 0.0014 | p = 0.6951 |
| 8.71 | 520 | Malvidin-3-O-glucoside | 0.1752 ± 0.0538 | 0.5577 ± 0.2117 | p = 0.0001 |
| 8.54 | 520 | Malvidin-3-5-diglycoside | 0.1016 ± 0.0329 | 0.3752 ± 0.1426 | p = 0.0174 |
| 9.39 | 320 | p-coumaric acid | 1.3098 ± 0.0248 | 33.8937 ± 0.0466 | p < 0.0001 |
| 8.14 | 520 | Pelargonidin-3-O-glucoside | 0.0952 ± 0.0215 | 0.0901 ± 0.0112 | p > 0.9999 |
| 8.67 | 520 | Peonidin-3-O-glucoside | 0.1048 ± 0.0346 | 0.3194 ± 0.1215 | p = 0.1460 |
| 11.57 | 360 | Quercetin | 0.5228 ± 0.0052 | 0.3078 ± 0.0008 | p = 0.1441 |
| 12.65 | 270 | Trans-cinnamic acid | 0.004 ± 0.0001 | 0.2340 ± 1.1636 | p = 0.0868 |
| 9.74 | 320 | Trans-ferulic acid | 1.7463 ± 0.0156 | 1.7756 ± 0.0279 | p > 0.9999 |
| Compound | M.W (g/mol) | H-Bond Acceptor | H-Bond Donor | logD | TPSA (Å) | Linpiski Rule |
|---|---|---|---|---|---|---|
| 3-O-methyl-quercetin | 316.26 | 7 | 4 | 1.75 | 120.36 | Yes |
| Caffeic acid | 180.16 | 4 | 3 | 0.93 | 77.76 | Yes |
| Chlorogenic acid | 354.31 | 9 | 6 | −0.39 | 164.75 | Yes |
| Gallic acid | 170.12 | 5 | 4 | 0.21 | 97.99 | Yes |
| Luteolin | 286.24 | 6 | 4 | 1.73 | 111.13 | Yes |
| Malvidin-3-O-glucoside | 493.44 | 12 | 7 | −0.90 | 191.67 | No |
| Malvidin-3-5-diglycoside | 655.58 | 17 | 10 | −2.86 | 270.82 | No |
| p-coumaric acid | 164.16 | 3 | 2 | 1.26 | 57.53 | Yes |
| Pelargonidin-3-O-glucoside | 433.39 | 10 | 7 | −0.84 | 173.21 | Yes |
| Peonidin-3-O-glucoside | 463.41 | 11 | 7 | −0.69 | 182.44 | No |
| Quercetin | 302.24 | 7 | 5 | 1.23 | 131.36 | Yes |
| Trans-cinnamic acid | 148.16 | 2 | 1 | 1.79 | 37.30 | Yes |
| Trans-ferulic acid | 194.18 | 4 | 2 | 1.36 | 66.76 | Yes |
| Compound | Parameters | |||||
|---|---|---|---|---|---|---|
| LD50 (g/kg) | Hepato. | Carcino. | Immuno. | Muta. | Nutr. | |
| 3-0-methyl-quercetin | 5.00 | 0.72-I | 0.55-A | 0.50-A | 0.61-I | 0.54-A |
| Caffeic acid | 2.98 | 0.57-I | 0.78-A | 0.50-I | 0.98-I | 0.77-I |
| Chlorogenic acid | 5.00 | 0.72-I | 0.68-I | 0.99-A | 0.93-I | 0.64-I |
| Gallic acid | 2.00 | 0.61-I | 0.56-A | 0.99-I | 0.94-I | 0.83-I |
| Luteolin | 3.91 | 0.69-I | 0.68-A | 0.97-I | 0.51-A | 0.63-A |
| Malvidin-3-0-glucoside | 5.00 | 0.81- I | 0.89-I | 0.95-A | 0.74-I | 0.51-I |
| Malvidin-3-5-diglycoside | 5.00 | 0.78-I | 0.87-I | 0.94-A | 0.73-I | 0.52-I |
| p-coumaric acid | 2.85 | 0.51-I | 0.5-A | 0.91-I | 0.93-I | 0.89-I |
| Pelargonidin-3-0-glucoside | 5.00 | 0.76-I | 0.86-I | 0.56-I | 0.72-I | 0.53-A |
| Peonidin-3-0-glucoside | 5.00 | 0.82-I | 0.87-I | 0.89-A | 0.65-I | 0.51-I |
| Quercetin | 0.16 | 0.69-I | 0.68-A | 0.87-I | 0.51-A | 0.63-A |
| Trans-cinnamic acid | 2.5 | 0.54-A | 0.82-I | 0.95-I | 0.96-I | 0.92-I |
| Trans-ferulic acid | 1.77 | 0.51-I | 0.61-I | 0.91-A | 0.96-I | 0.82-I |
| Polyphenol (Ligand) | PCNA | Ki-67 | ||
|---|---|---|---|---|
| Binding Affinity | Inhibition Constant | Binding Affinity | Inhibition Constant | |
| 3-0-methyl-quercetin | −10.48 Kcal/mol | 20.94 nM | −10.23 Kcal/mol | 31.72 nM |
| Caffeic acid | −7.64 Kcal/mol | 2.50 uM | −8.15 Kcal/mol | 1.06 uM |
| Chlorogenic acid | −11.25 Kcal/mol | 5.67 nM | −6.50 Kcal/mol | 11.11 uM |
| Gallic acid | −7.70 Kcal/mol | 2.25 uM | −7.52 Kcal/mol | 3.07 uM |
| Luteolin | −5.66 Kcal/mol | 70.82 uM | −6.06 Kcal/mol | 35.92 uM |
| Malvidin-3-0-glucoside | −12.09 Kcal/mol | 1.37 nM | −10.39 Kcal/mol | 24.10 nM |
| Malvidin-3-5-diglycoside | −9.21 Kcal/mol | 177.22 nM | −11.20 Kcal/mol | 6.16 nM |
| p-coumaric acid | −7.54 Kcal/mol | 3.00 uM | −7.43 Kcal/mol | 3.59 uM |
| Pelargonidin-3-0-glucoside | −11.55 Kcal/mol | 3.41 nM | −10.20 Kcal/mol | 33.57 nM |
| Peonidin-3-0-glucoside | −11.25 Kcal/mol | 5.64 nM | −10.47 Kcal/mol | 21.23 nM |
| Quercetin | −10.17 Kcal/mol | 35.3 nM | −9.66 Kcal/mol | 82.32 nM |
| Trans-cinnamic acid | −5.57 Kcal/mol | 83.04 uM | −5.08 Kcal/mol | 189.35 uM |
| Trans-ferulic acid | −8.16 Kcal/mol | 1.05 uM | −7.84 Kcal/mol | 1.79 uM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, M.C.; Bertini, L.; Szmaruk, J.E.; de Almeida, M.R.; Agneis, M.L.G.; Cesário, R.C.; Caputo, W.L.; da Costa, C.L.; Garcia, V.A.d.S.; Seiva, F.R.F. Effect of Drying Methods on Bioactivity of Pyrostegia venusta Extracts: Antioxidant Assays, Cytotoxicity, and Computational Approaches. Pharmaceuticals 2025, 18, 1315. https://doi.org/10.3390/ph18091315
de Souza MC, Bertini L, Szmaruk JE, de Almeida MR, Agneis MLG, Cesário RC, Caputo WL, da Costa CL, Garcia VAdS, Seiva FRF. Effect of Drying Methods on Bioactivity of Pyrostegia venusta Extracts: Antioxidant Assays, Cytotoxicity, and Computational Approaches. Pharmaceuticals. 2025; 18(9):1315. https://doi.org/10.3390/ph18091315
Chicago/Turabian Stylede Souza, Milena Cremer, Letícia Bertini, Julia Estrella Szmaruk, Matheus Ribas de Almeida, Maria Luisa G. Agneis, Roberta Carvalho Cesário, Wesley Ladeira Caputo, Christiane Luciana da Costa, Vitor Augusto dos Santos Garcia, and Fábio R. F. Seiva. 2025. "Effect of Drying Methods on Bioactivity of Pyrostegia venusta Extracts: Antioxidant Assays, Cytotoxicity, and Computational Approaches" Pharmaceuticals 18, no. 9: 1315. https://doi.org/10.3390/ph18091315
APA Stylede Souza, M. C., Bertini, L., Szmaruk, J. E., de Almeida, M. R., Agneis, M. L. G., Cesário, R. C., Caputo, W. L., da Costa, C. L., Garcia, V. A. d. S., & Seiva, F. R. F. (2025). Effect of Drying Methods on Bioactivity of Pyrostegia venusta Extracts: Antioxidant Assays, Cytotoxicity, and Computational Approaches. Pharmaceuticals, 18(9), 1315. https://doi.org/10.3390/ph18091315








