Excavating Precursors from Herb Pairs Polygala tenuifolia and Acori tatarinowii: Synthesis and Anticonvulsant Activity Evaluation of 3,4,5-Trimethoxycinnamic Acid (TMCA) Piperazine Amide Derivatives
Abstract
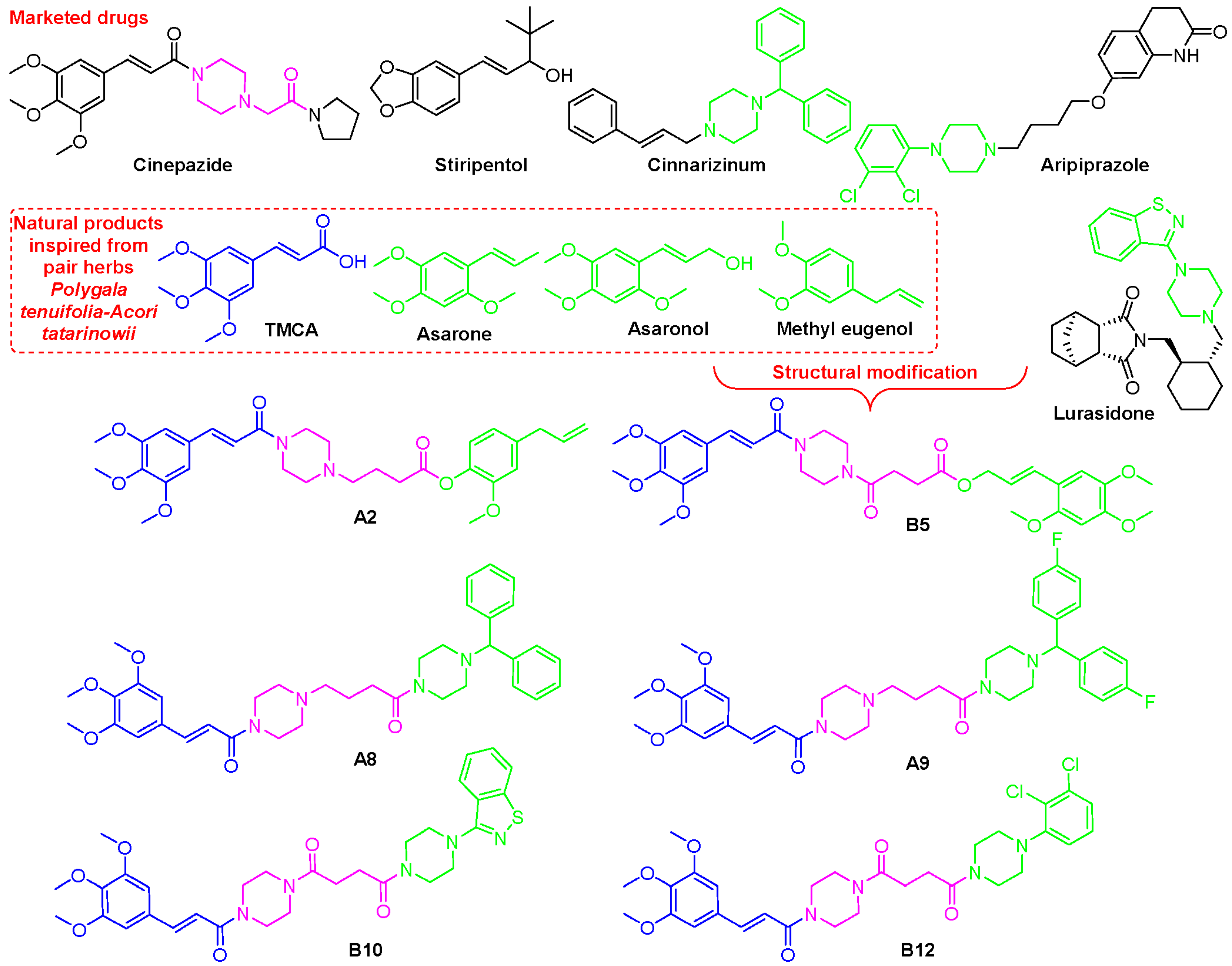
1. Introduction
2. Results
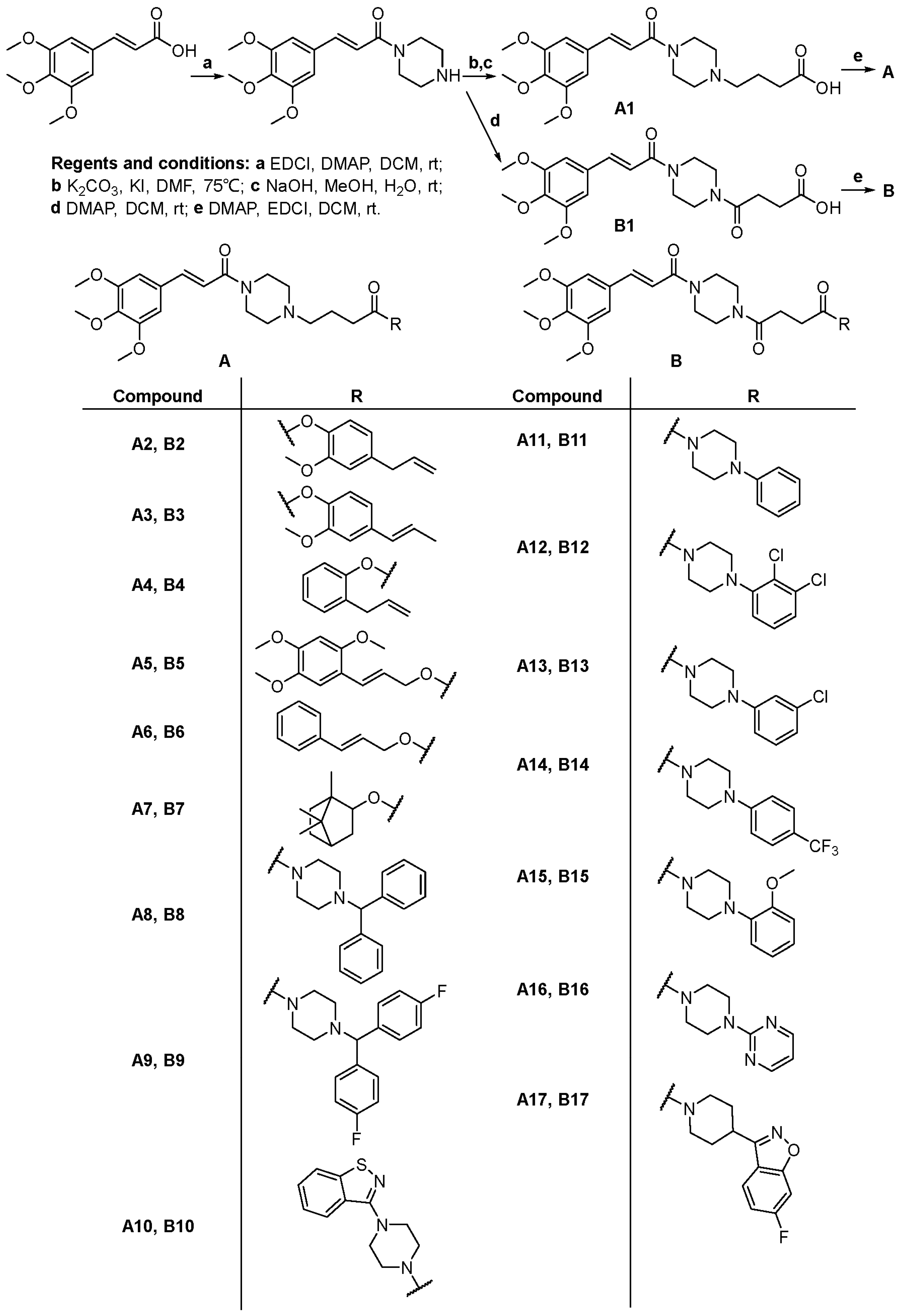
2.1. Chemistry
2.2. Pharmacology
2.2.1. Maximal Electroshock (MES) Test
2.2.2. Pentylenetetrazole (PTZ)-Induced Seizure Test
2.2.3. Acute Neurotoxicity Screening
2.2.4. Validation of Anti-Seizure Potential of B9 Through EEG
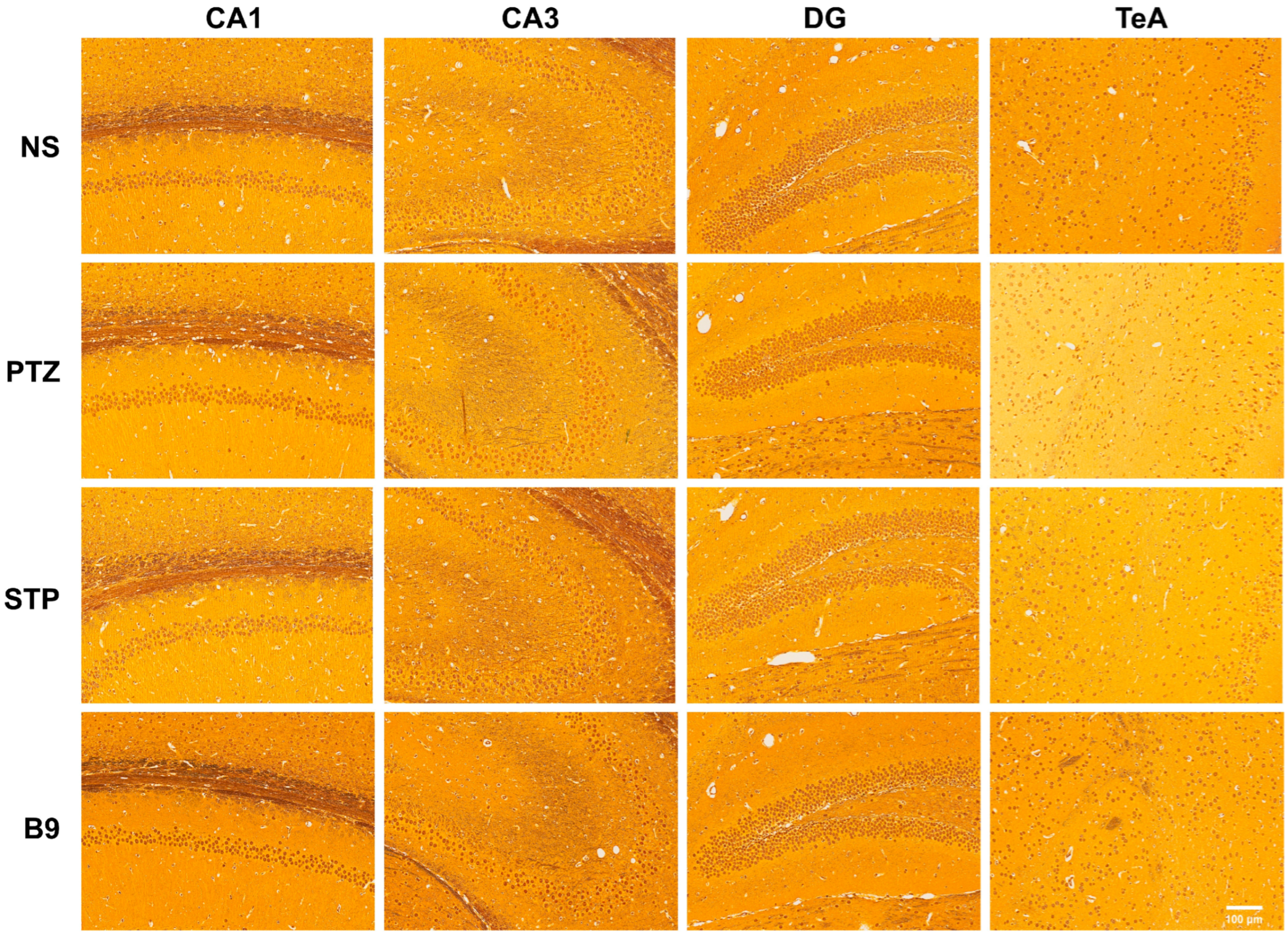
2.2.5. Silver Staining Results
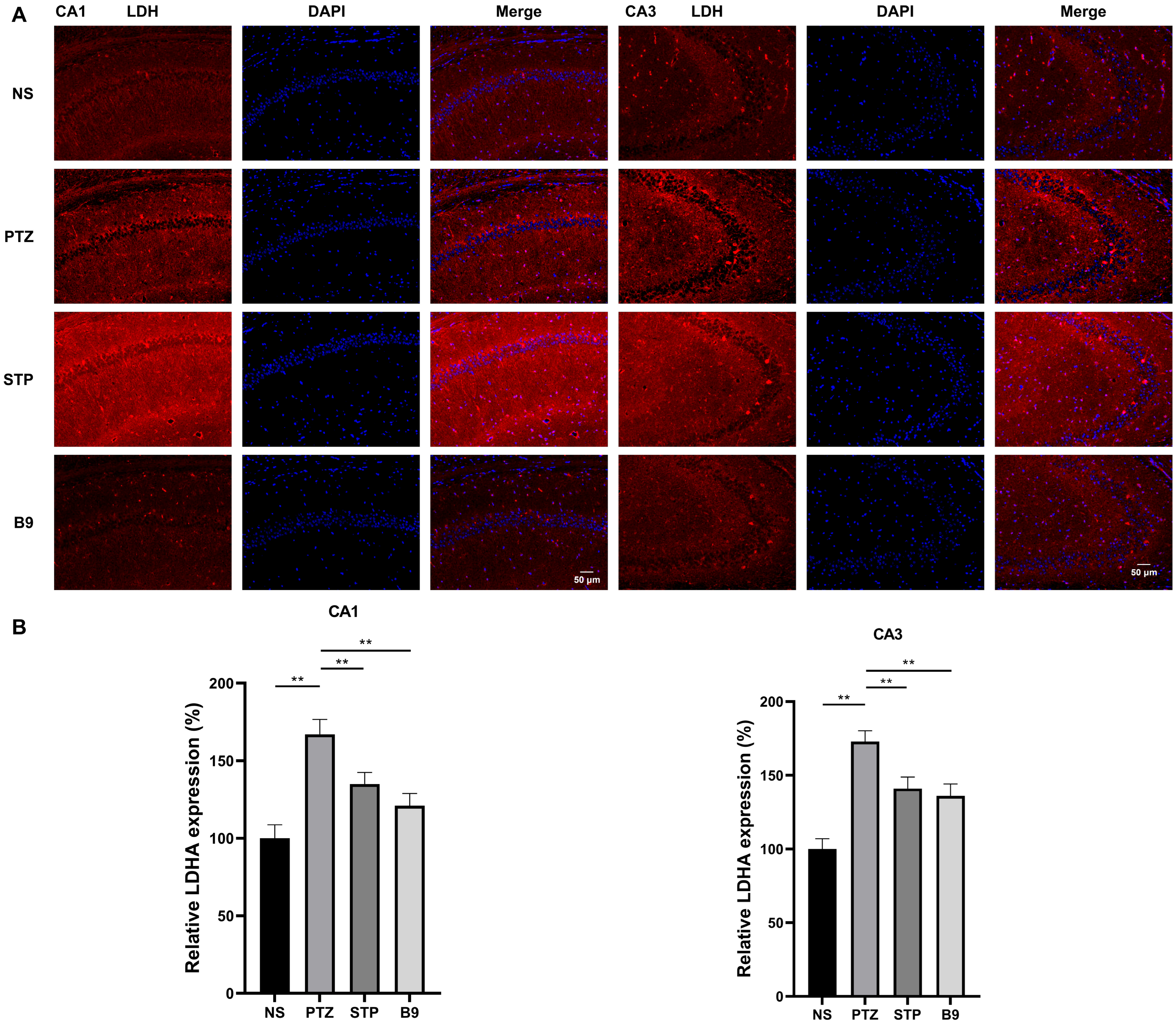
2.2.6. Immunofluorescence Staining
2.2.7. In Vitro LDH Assay
2.2.8. Molecular Docking Analysis
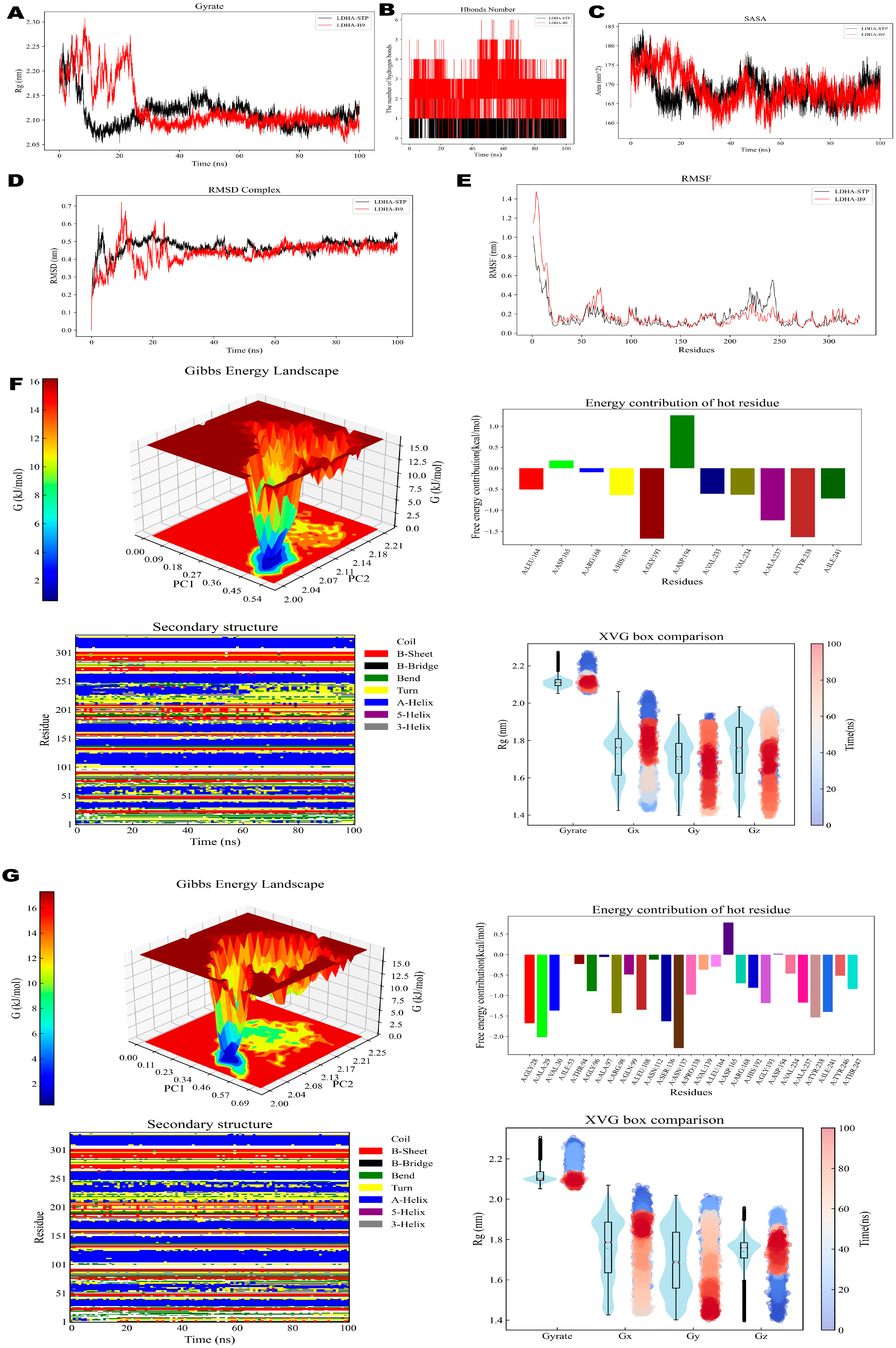
2.2.9. MD Simulation
2.2.10. In Silico ADMET Predictions
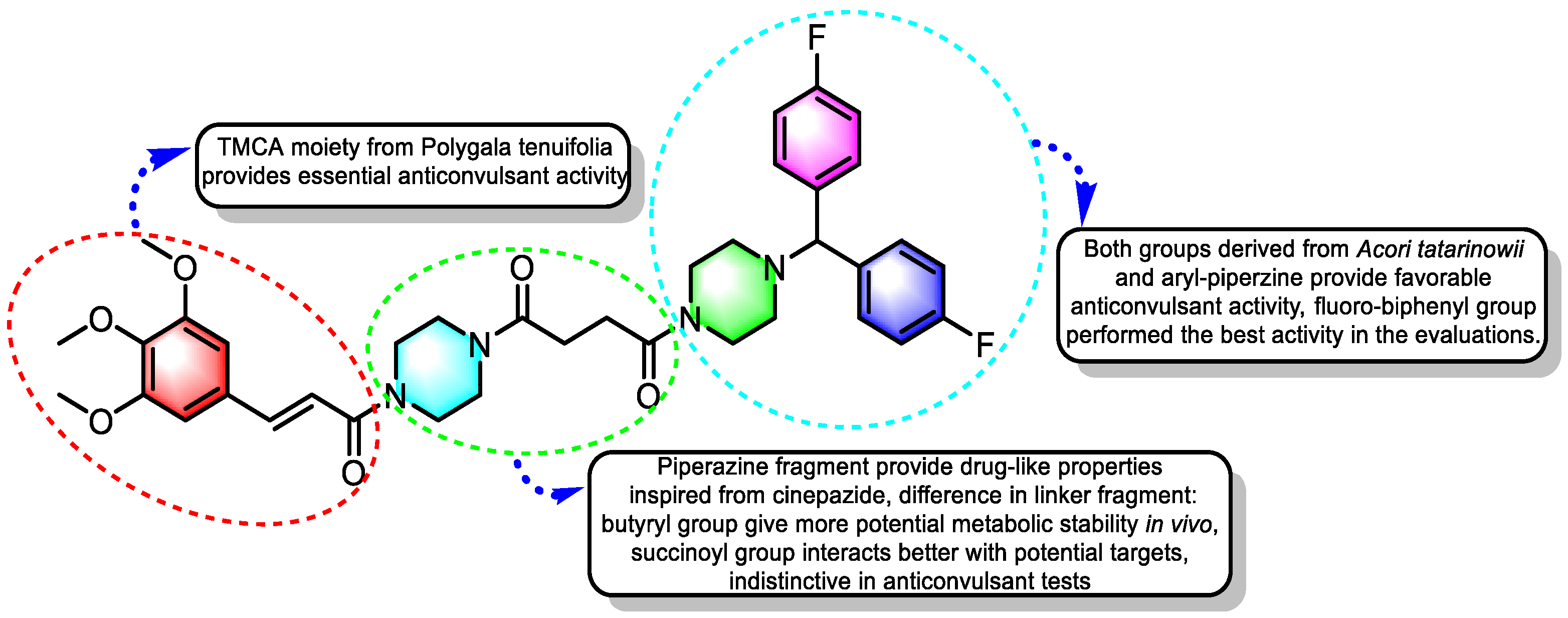
2.2.11. Structure–Activity Relationships (SAR)
3. Discussion
4. Materials and Methods
4.1. Chemistry
General Procedure for the Synthesis of TMCA Peptide Derivatives
4.2. Biological Activity
4.2.1. Animals and Experimental Conditions
4.2.2. Maximal Electroshock (MES) Test
4.2.3. Pentylenetetrazole (PTZ)-Induced Seizures
4.2.4. Acute Neurotoxicity Screening
4.2.5. Electroencephalogram (EEG)
4.2.6. Silver Staining
4.2.7. Immunofluorescence Staining
4.2.8. Statistical Analysis
4.2.9. In Vitro LDH Assay
4.2.10. Molecular Modeling
4.2.11. Molecular Dynamic (MD) Simulation
4.2.12. In Silico ADMET Predictions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.; He, X.; Ma, C.; Wu, S.; Cuan, Y.; Sun, Y.; Bai, Y.; Huang, L.; Chen, X.; Gao, T.; et al. Excavating anticonvulsant compounds from prescriptions of Traditional Chinese Medicine in the treatment of epilepsy. Am. J. Chin. Med. 2018, 46, 707–737. [Google Scholar] [CrossRef]
- Rapacz, A.; Rybka, S.; Obniska, J.; Jodłowska, A.; Góra, M.; Koczurkiewicz, P.; Pękala, E.; Siwek, A.; Filipek, B. Analgesic and antiallodynic activity of novel anticonvulsant agents derived from 3-benzhydryl-pyrrolidine-2,5-dione in mouse models of nociceptive and neuropathic pain. Eur. J. Pharmacol. 2020, 869, 172890. [Google Scholar] [CrossRef]
- Zhu, H.L.; Wan, J.B.; Wang, Y.T.; Li, B.C.; Xiang, C.; He, J.; Li, P. Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia 2014, 55, 3–16. [Google Scholar] [CrossRef]
- Bian, X.; Zhang, Y.; Huang, B.; Wang, X.; Wang, G.; Zhu, Y.; Liu, Y.; Liang, J.; Jia, Y.; Wang, K. Natural product incarvillateine aggravates epileptic seizures by inhibiting GABAA currents. Eur. J. Pharmacol. 2019, 858, 172496. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.M.; Piao, J.H.; Xu, X.L.; Zhu, L.; Yang, L.; Lin, F.L.; Chen, J.; Jiang, J.G. Chinese medicines with sedative-hypnotic effects and their active components. Sleep. Med. Rev. 2016, 29, 108–118. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, Y.; Chen, X.; Wu, S.; He, X.; Bai, Y.; Sun, Y.; Zheng, X. Design, synthesis and biological evaluation of (E)-3-(3,4,5-trimethoxyphenyl) acrylic acid (TMCA) amide derivatives as anticonvulsant and sedative agents. Med. Chem. Res. 2018, 27, 2387–2396. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, Y.; Xie, J.; Chen, X.; He, X.; Sun, Y.; Bai, Y.; Zhang, Y.; Wu, S.; Zheng, X. Excavating precursors from the traditional Chinese herb Polygala tenuifolia and Gastrodia elata: Synthesis, anticonvulsant activity evaluation of 3,4,5-trimethoxycinnamic acid (TMCA) ester derivatives. Bioorg. Chem. 2019, 88, 102832–102840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Song, H.; Xie, J.; Liu, T.; Zhao, X.; Chen, X.; He, X.; Wu, S.; Zhang, Y.; Zheng, X. Research progress in the biological activities of 3,4,5-trimethoxycinnamic acid (TMCA) derivatives. Eur. J. Med. Chem. 2019, 173, 213–227. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wei, X.D.; Chen, C.R. 3,4,5-Trimethoxycinnamic acid, one of the constituents of Polygalae Radix exerts anti-seizure effects by modulating GABAAergic systems in mice. J. Pharmacol. Sci. 2016, 131, 1–5. [Google Scholar] [CrossRef]
- Jung, J.C.; Moon, S.; Min, D.; Park, W.K.; Jung, M.; Oh, S. Synthesis and evaluation of a series of 3,4,5-trimethoxycinnamic acid derivatives as potential antinarcotic agents. Chem. Biol. Drug Des. 2013, 81, 389–398. [Google Scholar] [CrossRef]
- Zhang, C.; Du, Q.Y.; Chen, L.D.; Wu, W.H.; Liao, S.Y.; Yu, L.H.; Liang, X.T. Design, synthesis and evaluation of novel tacrine-multialkoxybenzene hybrids as multi-targeted compounds against Alzheimer’s disease. Eur. J. Med. Chem. 2016, 116, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ganesh, T.; Du, Y.; Quan, Y.; Serrano, G.; Qui, M.; Speigel, I.; Rojas, A.; Lelutiu, N.; Dingledine, R. Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc. Natl. Acad. Sci. USA 2012, 109, 3149–3154. [Google Scholar] [CrossRef]
- Bai, Y.; He, X.; Bai, Y.; Sun, Y.; Zhao, Z.; Chen, X.; Li, B.; Xie, J.; Li, Y.; Jia, P.; et al. Polygala tenuifolia-Acori tatarinowii herbal pair as an inspiration for substituted cinnamic alpha-asaronol esters: Design, synthesis, anticonvulsant activity, and inhibition of lactate dehydrogenase study. Eur. J. Med. Chem. 2019, 183, 111650. [Google Scholar] [CrossRef]
- Machnik, P.; Biazar, N.; Schuster, S. Recordings in an integrating central neuron reveal the mode of action of isoeugenol. Commun. Biol. 2023, 6, 309. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Jing, B.; Chen, Z.; Shi, H.; Zheng, Y.; Chang, S.; Sun, J.; Zhao, G. α-Asarone attenuates chronic sciatica by inhibiting peripheral sensitization and promoting neural repair. Phytother. Res. PTR 2023, 37, 151–162. [Google Scholar] [CrossRef]
- Zhao, Z.; Yue, J.; Ji, X.; Nian, M.; Kang, K.; Qiao, H.; Zheng, X. Research progress in biological activities of succinimide derivatives. Bioorg. Chem. 2021, 108, 104557. [Google Scholar] [CrossRef]
- López-Rodríguez, M.L.; Morcillo, M.J.; Fernández, E.; Porras, E.; Orensanz, L.; Beneytez, M.E.; Manzanares, J.; Fuentes, J.A. Synthesis and structure-activity relationships of a new model of arylpiperazines. 5. Study of the physicochemical influence of the pharmacophore on 5-HT(1a)/alpha(1)-adrenergic receptor affinity: Synthesis of a new derivative with mixed 5-HT(1a)/d(2) antagonist properties. J. Med. Chem. 2001, 44, 186–197. [Google Scholar] [PubMed]
- Żmudzka, E.; Lustyk, K.; Siwek, A.; Wolak, M.; Gałuszka, A.; Jaśkowska, J.; Kołaczkowski, M.; Sapa, J.; Pytka, K. Novel Arylpiperazine Derivatives of Salicylamide with α(1)-Adrenolytic Properties Showed Antiarrhythmic and Hypotensive Properties in Rats. Int. J. Mol. Sci. 2022, 24, 923. [Google Scholar] [CrossRef]
- Verrotti, A.; Lattanzi, S.; Brigo, F.; Zaccara, G. Pharmacodynamic interactions of antiepileptic drugs: From bench to clinical practice. Epilepsy Behav. EB 2020, 104, 106939. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.; Li, Z.; Xu, C.; Wang, Y.; Chen, Z. Diverse nature of interictal oscillations: EEG-based biomarkers in epilepsy. Neurobiol. Dis. 2023, 177, 105999. [Google Scholar] [CrossRef]
- Boudreau, A.; Purkey, H.E.; Hitz, A.; Robarge, K.; Peterson, D.; Labadie, S.; Kwong, M.; Hong, R.; Gao, M.; Del Nagro, C.; et al. Metabolic plasticity underpins innate and acquired resistance to LDHA inhibition. Nat. Chem. Biol. 2016, 12, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lei, M.; Zhao, Z.; Wu, S.; Zheng, X.; Qiao, H.; Yang, X. Multi-targeted pharmacological properties of cinnamyl piperazine derivatives: A comprehensive review. Med. Chem. Res. 2025, 34, 19–44. [Google Scholar] [CrossRef]
- Schwabe, K.; Ebert, U. Animal Models of Epilepsy. In Animal Models of Neuropsychiatric Diseases; Imperial College Press: London, UK; World Scientific Publishing Co.: Singapore, 2006; pp. 75–117. [Google Scholar]
- Wang, T.; Phillips, E.M.; Dalby, S.M.; Sirota, E.; Axnanda, S.; Shultz, C.S.; Patel, P.; Waldman, J.H.; Alwedi, E.; Wang, X.; et al. Manufacturing Process Development for Belzutifan, Part 5: A Streamlined Fluorination-Dynamic Kinetic Resolution Process. Org. Process Res. Dev. 2022, 26, 543–550. [Google Scholar] [CrossRef]
- Sang, Z.; Wang, K.; Zhang, P.; Shi, J.; Liu, W.; Tan, Z. Design, synthesis, in-silico and biological evaluation of novel chalcone derivatives as multi-function agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 180, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Swinyard, E.A. Laboratory evaluation of antiepileptic drugs. Review of laboratory methods. Epilepsia 1969, 10, 107–119. [Google Scholar] [CrossRef]
- Momeni, S.N.; Masoud, S.A.; Banafshe, H.R. Inhibitory effects of chronic administration of vitamin D-3 on pentylenetetrazole-induced seizures in mice. Epilepsy Res. 2019, 149, 76–82. [Google Scholar] [CrossRef]
- Dunham, N.W.; Miya, T.S.; Edwards, L.D. The pharmacological activity of a series of basic esters of mono- and dialkylmalonic acids. J. Am. Pharm. Assoc. 1957, 46, 64–66. [Google Scholar] [CrossRef]
- Rasool, N.; Razzaq, Z.; Gul Khan, S.; Javaid, S.; Akhtar, N.; Mahmood, S.; Christensen, J.B.; Ali Altaf, A.; Muhammad Muneeb Anjum, S.; Alqahtani, F.; et al. A facile synthesis of 1,3,4-oxadiazole-based carbamothioate molecules: Antiseizure potential, EEG evaluation and in-silico docking studies. Arab. J. Chem. 2023, 16, 104610. [Google Scholar] [CrossRef]
- Song, Y.; Gao, M.; Wei, B.; Huang, X.; Yang, Z.; Zou, J.; Guo, Y. Mitochondrial ferritin alleviates ferroptosis in a kainic acid-induced mouse epilepsy model by regulating iron homeostasis: Involvement of nuclear factor erythroid 2-related factor 2. Cns Neurosci. Ther. 2024, 30, e14663. [Google Scholar] [CrossRef]
- Diaz, I.; Salido, S.; Nogueras, M.; Cobo, J. Synthesis of Ethyl Pyrimidine-Quinolincarboxylates Selected from Virtual Screening as Enhanced Lactate Dehydrogenase (LDH) Inhibitors. Int. J. Mol. Sci. 2024, 25, 9744. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, G.; Meng, Y.; Tian, J.; Chen, X.; Shen, M.; Li, Y.; Li, B.; Gao, C.; Wu, S.; et al. Synthesis and anti-tyrosinase mechanism of the substituted vanillyl cinnamate analogues. Bioorg. Chem. 2019, 93, 103316. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. AMDock: A versatile graphical tool for assisting molecular docking with Autodock Vina and Autodock4. Biol. Direct 2020, 15, 12. [Google Scholar] [CrossRef]
- Haque, M.A.; Marathakam, A.; Rana, R.; Almehmadi, S.J.; Tambe, V.B.; Charde, M.S.; Islam, F.; Siddiqui, F.A.; Culletta, G.; Almerico, A.M.; et al. Fighting Antibiotic Resistance: New Pyrimidine-Clubbed Benzimidazole Derivatives as Potential DHFR Inhibitors. Molecules 2023, 28, 501. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Almatary, A.M.; Al-Sanea, M.M.; Nasr, E.E.; Haikal, A.; Thompson, G.S.; Abood, A.; Ibrahim, M.A.A.; Elgazar, A.A.; Hamdi, A. Triazole-linked thiazolidinedione-Benzothiazole hybrids: Design and biological evaluation as AChE inhibitors. Bioorg. Chem. 2025, 157, 108295. [Google Scholar] [CrossRef] [PubMed]


| Com. | 0.5 h | 1 h | 2 h | 3 h | Com. | 0.5 h | 1 h | 2 h | 3 h |
|---|---|---|---|---|---|---|---|---|---|
| Ctrl | 0/4 a | 0/4 | 0/4 | 0/4 | |||||
| STP | 4/4 | 2/4 | 1/4 | 0/4 | |||||
| A1 | 1/4 | 1/4 | 0/4 | 0/4 | B1 | 1/4 | 1/4 | 0/4 | 0/4 |
| A2 | 2/4 | 2/4 | 1/4 | 0/4 | B2 | 3/4 | 3/4 | 1/4 | 0/4 |
| A3 | 4/4 | 3/4 | 2/4 | 1/4 | B3 | 3/4 | 3/4 | 1/4 | 0/4 |
| A4 | 2/4 | 2/4 | 1/4 | 0/4 | B4 | 3/4 | 2/4 | 2/4 | 0/4 |
| A5 | 3/4 | 3/4 | 2/4 | 0/4 | B5 | 3/4 | 3/4 | 2/4 | 0/4 |
| A6 | 2/4 | 2/4 | 1/4 | 0/4 | B6 | 2/4 | 1/4 | 1/4 | 0/4 |
| A7 | 3/4 | 2/4 | 1/4 | 0/4 | B7 | 3/4 | 2/4 | 1/4 | 0/4 |
| A8 | 3/4 | 2/4 | 1/4 | 0/4 | B8 | 3/4 | 3/4 | 1/4 | 0/4 |
| A9 | 4/4 | 3/4 | 2/4 | 0/4 | B9 | 4/4 | 3/4 | 1/4 | 0/4 |
| A10 | 3/4 | 1/4 | 1/4 | 0/4 | B10 | 3/4 | 3/4 | 1/4 | 0/4 |
| A11 | 1/4 | 1/4 | 1/4 | 0/4 | B11 | 2/4 | 2/4 | 1/4 | 0/4 |
| A12 | 4/4 | 3/4 | 3/4 | 1/4 | B12 | 4/4 | 3/4 | 1/4 | 1/4 |
| A13 | 1/4 | 1/4 | 0/4 | 0/4 | B13 | 2/4 | 1/4 | 0/4 | 0/4 |
| A14 | 4/4 | 4/4 | 3/4 | 1/4 | B14 | 3/4 | 3/4 | 2/4 | 0/4 |
| A15 | 3/4 | 3/4 | 1/4 | 0/4 | B15 | 3/4 | 3/4 | 1/4 | 0/4 |
| A16 | 3/4 | 3/4 | 2/4 | 0/4 | B16 | 3/4 | 2/4 | 2/4 | 0/4 |
| A17 | 2/4 | 2/4 | 1/4 | 0/4 | B17 | 2/4 | 2/4 | 1/4 | 0/4 |
| Com. | MES ED50 | SCPTZ | TD50 | PI a (MES) | |
|---|---|---|---|---|---|
| Protective Ratio (100 mg/kg) | Latent Time (100 mg/kg, s) | ||||
| NS | - | 0/4 | 150.8 ± 16.3 | - | - |
| STP b | 277 (1182) | 2/4 | 269.5 ± 19.8 ** | 161.7 (146.2–256.2) | 1.41 |
| A3 | 73.31 (41.96–92.14) | 1/4 | 222.0 ± 14.7 ** | 443.42 (327.60–626.48) | 6.05 |
| A9 | 37.46 (16.51–78.97) | 1/4 | 204.5 ± 12.6 ** | 304.79 (188.99–474.43) | 8.14 |
| B9 | 23.58 (10.33–71.72) | 3/4 | 274.3 ± 17.2 ** | 235.04 (107.18–373.21) | 9.97 |
| A12 | 54.71 (29.90–85.28) | 0/4 | 161.5 ± 24.0 | 284.38 (173.91–448.98) | 5.20 |
| B12 | 60.27 (38.71–84.89) | 1/4 | 230.3 ± 17.4 ** | 417.16 (293.63–614.75) | 6.92 |
| A14 | 52.39 (28.48–83.43) | 2/4 | 233.5 ± 18.3 ** | 358.26 (231.95–528.49) | 6.84 |
| Com. | Binding Affinity (Kcal/mol) | Key Interactions | LDH Inhibition Ratio (0.1 mM) |
|---|---|---|---|
| STP | −6.5 | ARG 168 (H-bond), TYR 238 (Pi-Sigma), GLY 193 (Amide-Pi Stacked), VAL 234, VAL 237 (Pi-Alkyl), VAL 233, HIS 192 (Carbon Hydrogen Bond) | 46.1 ± 6.2% |
| A3 | −6.3 | ASN 137 (H-bond), TYR 238 (Pi-Pi T-shaped), SER 136, VAL 135, THR 94 (Carbon Hydrogen Bond) | 37.6 ± 8.1% |
| A9 | −8.5 | ASN 137 (H-bond), VAL 90, ILE241, ILE 251 (Pi-Alkyl), GLY96, ALA 97, GLN 99 (Halogen), ARG 98, VAL 135, HIS 192 (Carbon Hydrogen Bond) | 52.6 ± 8.5% |
| B9 | −9.3 | VAL 30, ALA 29, ARG 98 (H-bond), TYR 238 (Pi-Sigma), VAL 233 (Halogen), GLY 193 (Amide-Pi Stacked), THR 247, GLY 28, VAL 234 (Carbon Hydrogen Bond) | 54.9 ± 9.7% |
| A12 | −7.6 | GLN 99 (H-bond), ILE 241, ILE 251, VAL 30 (Alkyl) | 41.9 ± 9.2% |
| B12 | −7.7 | ARG 98 (H-bond), ALA 237, VAL 30, ILE 251 (Alkyl) | 44.3 ± 6.5% |
| A14 | −6.9 | ASN 137 (H-bond), VAL 30, ILE 251, ILE 241 (Alkyl), SER 136, HIS 192, GLY 96, GLY 28 (Carbon Hydrogen Bond) | 42.6 ± 7.3% |
| Contribution Components | LDH-STP | LDH-B9 |
|---|---|---|
| ΔVDWAALS | −29.93 ± 0.04 | −72.55 ± 1.86 |
| ΔEelec | −4.87 ± 0.01 | −19.91 ± 0.77 |
| ΔEGB | 19.63 ± 0.14 | 43.11 ± 1.25 |
| ΔEsurf | −3.99 ± 0.01 | −9.09 ± 0.19 |
| ΔGgas | −34.81 ± 0.04 | −92.47 ± 2.02 |
| ΔGsolvation | 15.65 ± 0.14 | 34.02 ± 1.26 |
| ΔTotal | −19.16 ± 0.15 | −58.45 ± 2.38 |
| Category | Property | ADMET Lab 3 | VNN-ADMET | PreADMET | PkCSM | Consensus |
|---|---|---|---|---|---|---|
| Absorption | Water solubility | - | 389.712 mg/L | −4.246 log mol/L | Low | |
| Caco-2 permeability | −5.454 log unit | - | 52.768 nm/s | 0.856 log Papp in 10−6 cm/s | Moderate | |
| Human intestinal absorption (%) | - | - | 97.683 | 65.214 | High | |
| Skin permeability (log Kp) | - | - | −2.563 | −2.735 | Low | |
| P-gp inhibitor | Yes | No | Yes | Yes | Yes | |
| Distribution | VDss (human, log L/kg) | - | - | - | 0.674 | Low |
| BBB permeability (log BB) | Yes | - | Yes | −1.872 | Yes | |
| Metabolism | CYP1A2 inhibitor | No | Yes | - | No | Partial |
| CYP2C9 inhibitor | Yes | No | Yes | Yes | Yes | |
| CYP2C19 inhibitor | Yes | No | No | No | No | |
| CYP2D6 inhibitor | Yes | No | No | No | No | |
| CYP3A4 inhibitor | Yes | No | Yes | Yes | Yes | |
| Excretion | Total clearance (log ml/min/kg) | - | - | - | 0.367 | Low |
| Toxicity | AMES toxicity | No | - | No | No | No |
| hERG blocker | Yes | Yes | Medium risk | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Lei, M.; Wang, Y.; Bai, Y.; Qiao, H. Excavating Precursors from Herb Pairs Polygala tenuifolia and Acori tatarinowii: Synthesis and Anticonvulsant Activity Evaluation of 3,4,5-Trimethoxycinnamic Acid (TMCA) Piperazine Amide Derivatives. Pharmaceuticals 2025, 18, 1312. https://doi.org/10.3390/ph18091312
Zhao Z, Lei M, Wang Y, Bai Y, Qiao H. Excavating Precursors from Herb Pairs Polygala tenuifolia and Acori tatarinowii: Synthesis and Anticonvulsant Activity Evaluation of 3,4,5-Trimethoxycinnamic Acid (TMCA) Piperazine Amide Derivatives. Pharmaceuticals. 2025; 18(9):1312. https://doi.org/10.3390/ph18091312
Chicago/Turabian StyleZhao, Zefeng, Mengchen Lei, Yongqi Wang, Yujun Bai, and Haifa Qiao. 2025. "Excavating Precursors from Herb Pairs Polygala tenuifolia and Acori tatarinowii: Synthesis and Anticonvulsant Activity Evaluation of 3,4,5-Trimethoxycinnamic Acid (TMCA) Piperazine Amide Derivatives" Pharmaceuticals 18, no. 9: 1312. https://doi.org/10.3390/ph18091312
APA StyleZhao, Z., Lei, M., Wang, Y., Bai, Y., & Qiao, H. (2025). Excavating Precursors from Herb Pairs Polygala tenuifolia and Acori tatarinowii: Synthesis and Anticonvulsant Activity Evaluation of 3,4,5-Trimethoxycinnamic Acid (TMCA) Piperazine Amide Derivatives. Pharmaceuticals, 18(9), 1312. https://doi.org/10.3390/ph18091312






