Artificial Intelligence in Small-Molecule Drug Discovery: A Critical Review of Methods, Applications, and Real-World Outcomes
Abstract
1. Introduction
Small Molecules in the Context of AI-Assisted Discovery
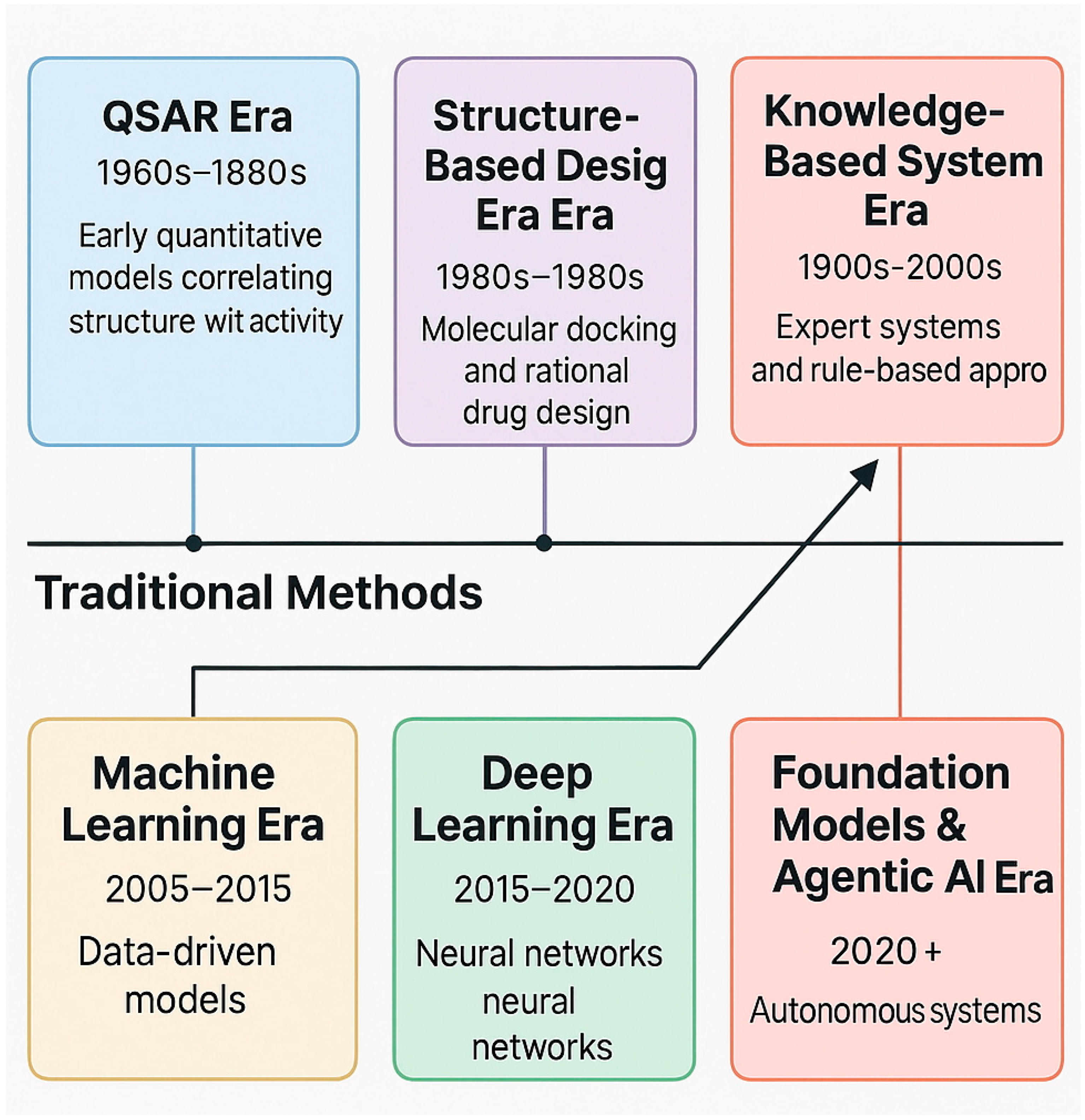
2. Historical Evolution of Computational Small-Molecule Discovery
3. Core Applications of AI in Small-Molecule Discovery
3.1. Target Identification and Validation
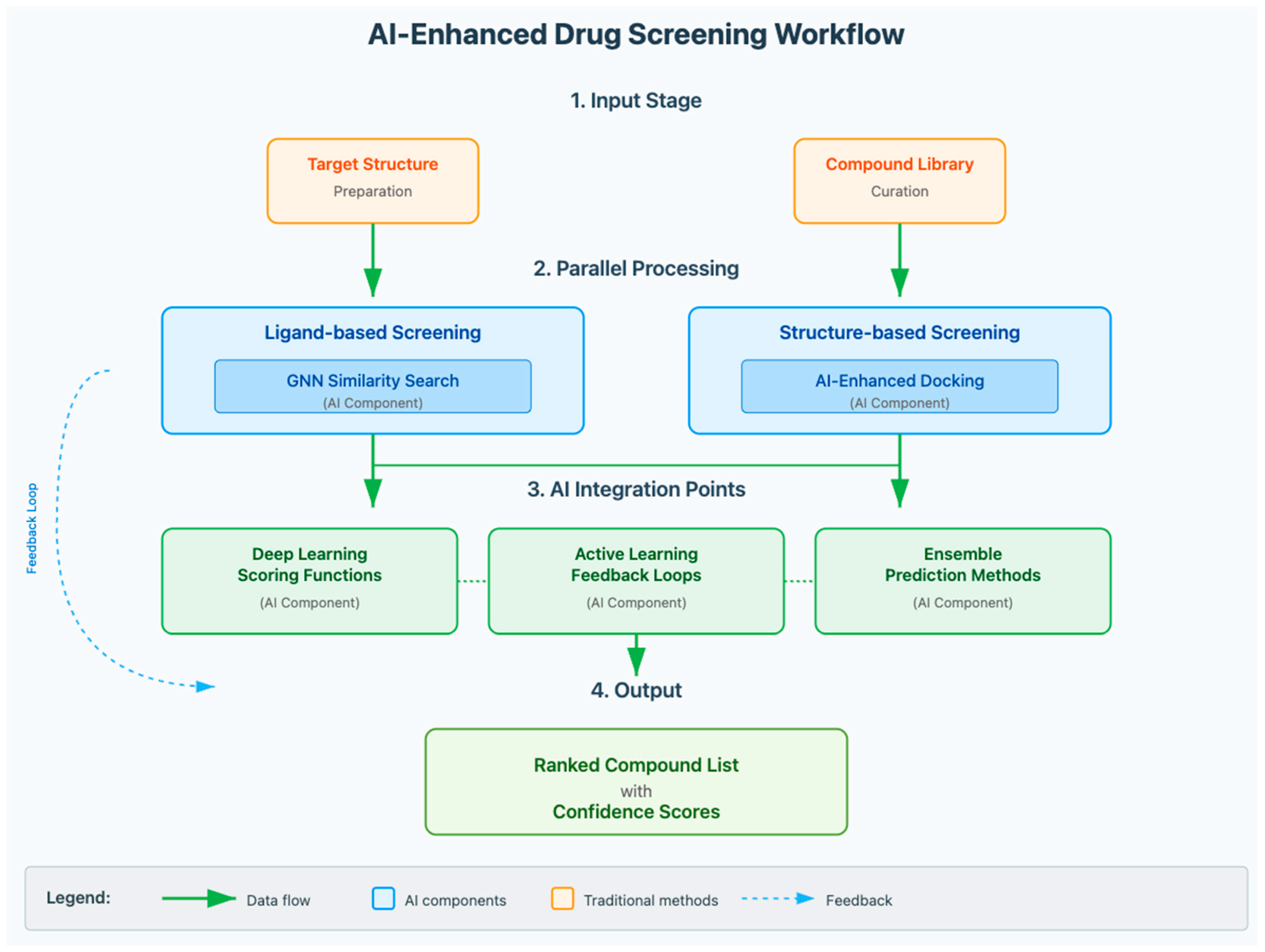
3.2. Hit Discovery and Virtual Screening
3.3. Lead Optimization
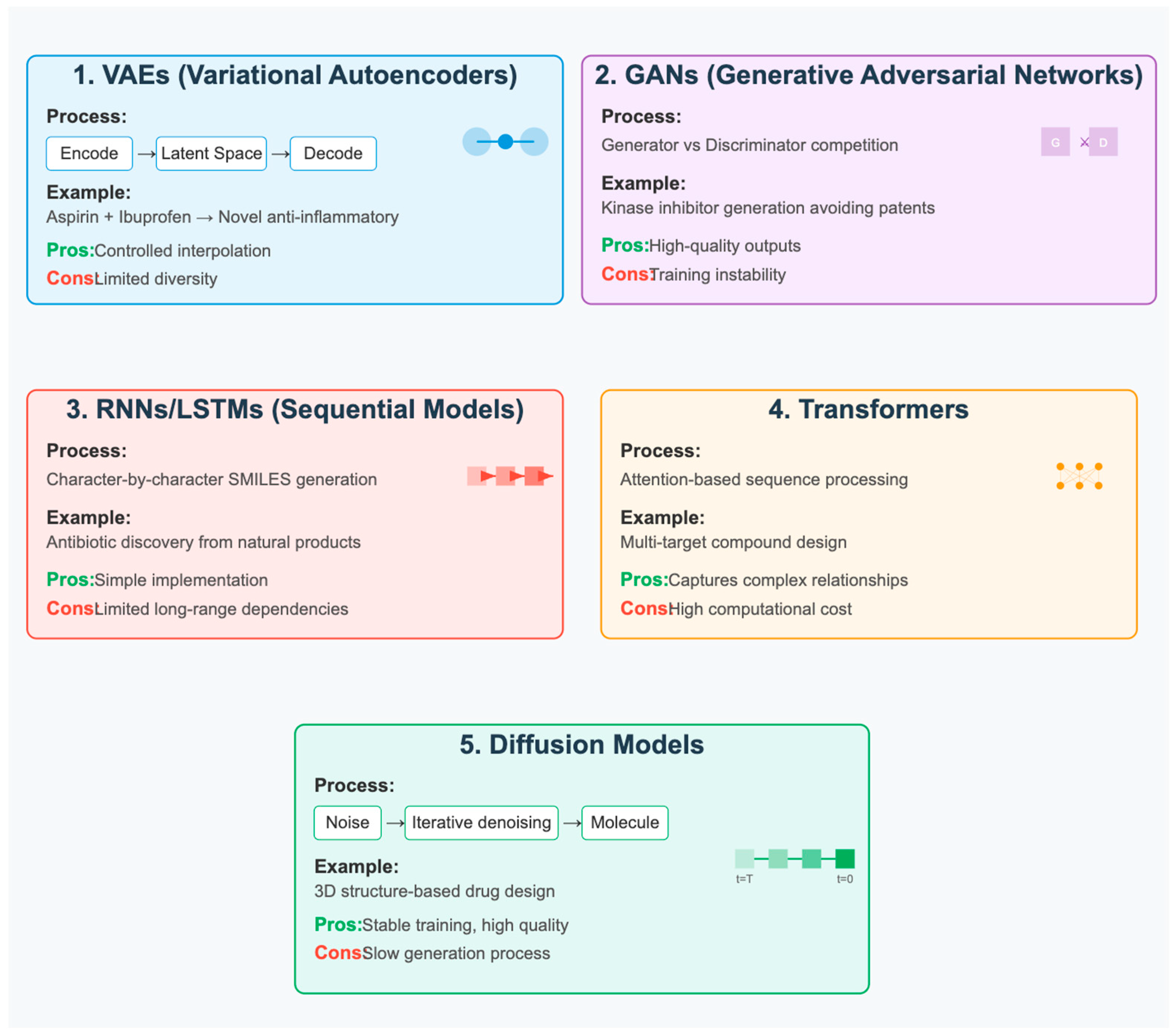
3.4. De Novo Small-Molecule Design
- Superior generation quality: They produce more chemically valid and diverse molecules
- Training stability: Unlike GANs, they don’t suffer from mode collapse or training instabilities
- 3D structure generation: Particularly effective for generating 3D molecular conformations
- Property control: Can incorporate property constraints during the generation process
3.5. Prediction of Small-Molecule Pharmacokinetics and Toxicity
4. AI-Discovered and AI-Assisted Small-Molecule Development: Success Stories and Lessons Learned
4.1. AI-Assisted Drug Repurposing: Baricitinib
4.2. AI-Discovered Clinical Candidates
4.3. Clinical Development Challenges and Lessons Learned
- The importance of high-quality training data that accurately represents the complexity of biological systems
- The need for experimental validation at each stage of the discovery process
- The value of hybrid approaches that combine AI predictions with human expertise
- The recognition that AI tools are most effective when integrated into existing workflows rather than replacing them entirely
5. Practical Case Studies
5.1. Scaffold Hopping Using Reinforcement Learning for Small-Molecule Kinase Inhibitor Discovery
5.2. Multi-Objective Optimization in Small-Molecule Lead Refinement Using Active Learning
5.3. AI-Enhanced High-Throughput Screening Triage for Antiviral Small-Molecule Discovery
6. Emerging Trends and Transformative Technologies
6.1. Foundation Models and Self-Supervised Learning
6.2. Computational Sustainability and Energy Considerations
6.3. Quantum Machine Learning and Molecular Simulation
6.4. Agentic AI and Autonomous Discovery Systems
- Autonomously read and synthesize scientific literature to identify drug targets
- Generate hypotheses about novel therapeutic mechanisms
- Design experimental protocols to test hypotheses
- Interpret experimental results and refine understanding
- Propose next steps in the discovery process
- Systems that combine target prediction, molecular design, and synthetic planning
- Platforms that can autonomously navigate patent landscapes
- AI agents that coordinate multiple specialized models for different tasks
- Decision-making systems that balance risk, cost, and potential reward
- Maintaining ethical oversight to prevent misuse
- Validating autonomous decisions against human expertise
- Managing the complexity of integrated multi-step processes
6.5. Automated Synthesis and Closed-Loop Discovery
6.6. Data Standardization and Collaborative Initiatives
7. Challenges and Limitations
8. Regulatory Evolution and Validation Frameworks
- Transparency: Clear documentation of model architecture, training data, and validation procedures
- Reproducibility: Ability to recreate model predictions using documented procedures
- Robustness: Performance across diverse test sets and edge cases
- Continuous monitoring: Post-deployment surveillance for model drift and performance degradation
9. Outlook and Transformative Potential
9.1. Human-AI Collaboration Paradigms
9.2. Standardization and Benchmarking Initiatives
9.3. Timeline Transformation and Future Projections
- Foundation models that reduce training data requirements and enable rapid deployment to new targets
- Generative models, including diffusion models, that can explore vast chemical spaces efficiently
- Automated synthesis platforms that reduce synthesis bottlenecks
- Multi-task learning approaches that optimize multiple properties simultaneously
- Active learning strategies that minimize experimental requirements
- Increasingly sophisticated agentic AI systems
10. Practical Implementation Guidelines
10.1. Strategic Planning and Readiness Assessment
10.2. Data Infrastructure and Quality Management
- Audit existing datasets for quality, completeness, and consistency
- Implement data standardization procedures (FAIR principles)
- Invest in data curation and annotation capabilities
- Establish bias auditing procedures to ensure ethical compliance
- Develop secure cloud-based platforms for collaboration while maintaining IP protection
10.3. Technology Selection and Integration
- Start with simpler, well-understood approaches before adopting complex methods
- Evaluate vendor solutions for technical capabilities, integration requirements, and support
- Consider both commercial and open-source options
- Ensure compatibility with existing workflows and systems
11. Conclusions
Funding
Conflicts of Interest
References
- Niazi, S.K.; Mariam, Z. Artificial intelligence in drug development: Reshaping the therapeutic landscape. Ther. Adv. Drug. Saf. 2025, 16, 20420986251321704. [Google Scholar] [CrossRef]
- Niazi, S.K.; Mariam, Z. Computer-Aided Drug Design and Drug Discovery: A Prospective Analysis. Pharmaceuticals 2023, 17, 22. [Google Scholar] [CrossRef]
- Ren, F.; Aliper, A.; Chen, J.; Zhao, H.; Rao, S.; Kuppe, C.; Ozerov, I.V.; Zhang, M.; Witte, K.; Kruse, C.; et al. A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models. Nat. Biotechnol. 2025, 43, 63–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mullowney, M.W.; Duncan, K.R.; Elsayed, S.S.; Garg, N.; van der Hooft, J.J.J.; Martin, N.I.; Meijer, D.; Terlouw, B.R.; Biermann, F.; Blin, K.; et al. Artificial intelligence for natural product drug discovery. Nat. Rev. Drug Discov. 2023, 22, 895–916. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.K. The Coming of Age of AI/ML in Drug Discovery, Development, Clinical Testing, and Manufacturing: The FDA Perspectives. Drug Des. Devel. Ther. 2023, 17, 2691–2725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niazi, S.K. Regulatory Perspectives for AI/ML Implementation in Pharmaceutical GMP Environments. Pharmaceuticals 2025, 18, 901. [Google Scholar] [CrossRef]
- Singh, R.; Paxton, M.; Auclair, J. Regulating the AI-enabled ecosystem for human therapeutics. Commun. Med. 2025, 5, 181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [CrossRef]
- Jiménez-Luna, J.; Grisoni, F.; Schneider, G. Drug discovery with explainable artificial intelligence. Nat. Mach. Intell. 2020, 2, 573–584. [Google Scholar] [CrossRef]
- Urbina, F.; Lentzos, F.; Invernizzi, C.; Ekins, S. Dual use of artificial-intelligence-powered drug discovery. Nat. Mach. Intell. 2022, 4, 189–191. [Google Scholar] [CrossRef]
- Niazi, S.K. Molecular Biosimilarity—An AI-Driven Paradigm Shift. Int. J. Mol. Sci. 2022, 23, 10690. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [CrossRef]
- Gashaw, I.; Ellinghaus, P.; Sommer, A.; Asadullah, K. What makes a good drug target? Drug Discov. Today 2011, 16, 1037–1043. [Google Scholar] [CrossRef]
- Macarron, R.; Banks, M.N.; Bojanic, D.; Burns, D.J.; Cirovic, D.A.; Garyantes, T.; Green, D.V.; Hertzberg, R.P.; Janzen, W.P.; Paslay, J.W.; et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 2011, 10, 188–195. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef]
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–716. [Google Scholar] [CrossRef]
- Lo, Y.C.; Rensi, S.E.; Torng, W.; Altman, R.B. Machine learning in chemoinformatics and drug discovery. Drug Discov. Today 2018, 23, 1538–1546. [Google Scholar] [CrossRef]
- Chen, H.; Engkvist, O.; Wang, Y.; Olivecrona, M.; Blaschke, T. The rise of deep learning in drug discovery. Drug Discov. Today 2018, 23, 1241–1250. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Bender, A.; Cortes-Ciriano, I. Artificial intelligence in drug discovery: What is realistic, what are illusions? Drug Discov. Today 2021, 26, 1040–1052. [Google Scholar] [CrossRef]
- Goodfellow, I.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative adversarial nets. Adv. Neural Inf. Process Syst. 2014, 27, 2672–2680. Available online: https://proceedings.neurips.cc/paper_files/paper/2014/hash/f033ed80deb0234979a61f95710dbe25-Abstract.html (accessed on 3 August 2025).
- Bond-Taylor, S.; Leach, A.; Long, Y.; Willcocks, C.G. Deep generative models for molecular design: A review. Chem. Sci. 2021, 12, 14421–14432. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, S.; Chen, F.; Long, G.; Zhang, C.; Philip, S.Y. Comprehensive survey of graph neural networks. IEEE Trans. Neural Netw. Learn. Syst. 2020, 31, 1–21. [Google Scholar] [CrossRef]
- Zhou, J.; Cui, G.; Hu, S.D.; Zhang, Z.Y.; Yang, C.; Liu, Z.Y.; Wang, L.F.; Li, C.C.; Sun, M.S. Graph neural networks: A review of methods and applications. AI Open 2020, 1, 57–81. [Google Scholar] [CrossRef]
- Ragoza, M.; Hochuli, J.; Idrobo, E.; Sunseri, J.; Koes, D.R. Protein-ligand scoring with convolutional neural networks. J. Chem. Inf. Model 2017, 57, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.; Doerr, S.; Martínez-Rosell, G.; Rose, A.S.; De Fabritiis, G. DeepSite: Protein-binding site predictor using 3D-convolutional neural networks. Bioinformatics 2018, 34, 3036–3042. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef] [PubMed]
- Segler, M.H.; Kogej, T.; Tyrchan, C.; Waller, M.P. Generating focused molecule libraries for drug discovery with recurrent neural networks. ACS Cent. Sci. 2018, 4, 120–131. [Google Scholar] [CrossRef]
- Popova, M.; Isayev, O.; Tropsha, A. Deep reinforcement learning for de novo drug design. Sci. Adv. 2018, 4, eaap7885. [Google Scholar] [CrossRef]
- Hoogeboom, E.; Satorras, V.G.; Vignac, C.; Welling, M. Equivariant Diffusion for Molecule Generation in 3D. In Proceedings of the 39th International Conference on Machine Learning, Baltimore, MD, USA, 17–23 July 2022; PMLR: Cambridge, MA, USA, 2022; pp. 8867–8887. Available online: https://proceedings.mlr.press/v162/hoogeboom22a.html (accessed on 3 August 2025).
- Reker, D.; Schneider, G. Active-learning strategies in computer-assisted drug discovery. Drug Discov. Today 2015, 20, 458–465. [Google Scholar] [CrossRef]
- Brown, N.; Fiscato, M.; Segler, M.H.; Vaucher, A.C. GuacaMol: Benchmarking models for de novo molecular design. J. Chem. Inf. Model 2019, 59, 1096–1108. [Google Scholar] [CrossRef]
- Lakhan, S.E. The Agentic Era: Why Biopharma Must Embrace Artificial Intelligence That Acts, Not Just Informs. Cureus 2025, 17, e83390. [Google Scholar] [CrossRef]
- Hansch, C.; Maloney, P.P.; Fujita, T.; Muir, R.M. Correlation of biological activity of phenoxyacetic acids with Hammett substituent constants and partition coefficients. Nature 1962, 194, 178–180. [Google Scholar] [CrossRef]
- Maggiora, G.M. On outliers and activity cliffs—Why QSAR often disappoints. J. Chem. Inf. Model 2006, 46, 1535. [Google Scholar] [CrossRef] [PubMed]
- Verlinde, C.L.; Hol, W.G. Structure-based drug design: Progress, results and challenges. Structure 1994, 2, 577–587. [Google Scholar] [CrossRef]
- Kuntz, I.D.; Blaney, J.M.; Oatley, S.J.; Langridge, R.; Ferrin, T.E. A geometric approach to macromolecule-ligand interactions. J. Mol. Biol. 1982, 161, 269–288. [Google Scholar] [CrossRef]
- Burke, M.D.; Schreiber, S.L. A planning strategy for diversity-oriented synthesis. Angew. Chem. Int. Ed. 2004, 43, 46–58. [Google Scholar] [CrossRef]
- Yang, S.Y. Pharmacophore modeling and applications in drug discovery: Challenges and recent advances. Drug Discov. Today 2010, 15, 444–450. [Google Scholar] [CrossRef]
- Corey, E.J.; Wipke, W.T. Computer-assisted design of complex organic syntheses. Science 1969, 166, 178–192. [Google Scholar] [CrossRef]
- Todd, M.H. Computer-aided organic synthesis. Chem. Soc. Rev. 2005, 34, 247–266. [Google Scholar] [CrossRef]
- Vapnik, V.N. The Nature of Statistical Learning Theory; Springer: New York, NY, YSA, 1995; ISBN 978-0-387-94559-0. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Mayr, A.; Klambauer, G.; Unterthiner, T.; Hochreiter, S. DeepTox: Toxicity prediction using deep learning. Front. Environ. Sci. 2016, 3, 80. [Google Scholar] [CrossRef]
- Ma, J.; Sheridan, R.P.; Liaw, A.; Dahl, G.E.; Svetnik, V. Deep neural nets as a method for quantitative structure-activity relationships. J. Chem. Inf. Model 2015, 55, 263–274. [Google Scholar] [CrossRef]
- Duvenaud, D.; Maclaurin, D.; Aguilera-Iparraguirre, J.; Gómez-Bombarelli, R.; Hirzel, T.; Aspuru-Guzik, A. Convolutional networks on graphs for learning molecular fingerprints. Adv. Neural Inf. Process Syst. 2015, 28, 2224–2232. Available online: https://papers.nips.cc/paper/2015/hash/f9be311e65d81a9ad8150a60844bb94c-Abstract.html (accessed on 3 August 2025).
- Kearnes, S.; McCloskey, K.; Berndl, M.; Pande, V.; Riley, P. Molecular graph convolutions: Moving beyond fingerprints. J. Comput. Aided Mol. Des. 2016, 30, 595–608. [Google Scholar] [CrossRef]
- Schwaller, P.; Laino, T.; Gaudin, T.; Bolgar, P.; Hunter, C.A.; Bekas, C.; Lee, A.A. Molecular transformer: A model for uncertainty-calibrated chemical reaction prediction. ACS Cent. Sci. 2019, 5, 1572–1583. [Google Scholar] [CrossRef]
- Wallach, I.; Dzamba, M.; Heifets, A. AtomNet: A Deep Convolutional Neural Network for Bioactivity Prediction in Structure-Based Drug Discovery. arXiv 2015, arXiv:1510.02855. Available online: https://arxiv.org/abs/1510.02855 (accessed on 3 August 2025).
- Bommasani, R.; Hudson, D.A.; Adeli, E.; Altman, R.; Arora, S.; von Arx, S.; Bernstein, M.S.; Bohg, J.; Bosselut, A.; Brunskill, E.; et al. On the Opportunities and Risks of Foundation Models. arXiv 2021, arXiv:2108.07258. Available online: https://arxiv.org/abs/2108.07258 (accessed on 3 August 2025).
- Zeng, X.; Lin, Y.; He, Y.; Lv, L.; Min, X.; Rodriguez-Paton, A. Deep learning for multi-omics data integration in cancer research. Brief Bioinform. 2019, 20, 1708–1721. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine: Opportunities and challenges. Nat. Med. 2022, 28, 16–22. [Google Scholar] [CrossRef]
- Baker, M.; Bode, K.; Cheetham, T.; Del Carratore, F. Text mining in drug discovery: Applications and opportunities. Drug Discov. Today 2017, 22, 271–279. [Google Scholar] [CrossRef]
- Chen, Y.; Elenee Argentinis, J.D.; Weber, G. IBM Watson: How cognitive computing can be applied to big data challenges in life sciences research. Clin. Ther. 2016, 38, 688–701. [Google Scholar] [CrossRef]
- Zitnik, M.; Agrawal, M.; Leskovec, J. Machine learning for integrating data in biology and medicine: Principles, practice, and opportunities. Inf. Fusion. 2018, 50, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Himmelstein, D.S.; Lizee, A.; Hessler, C.; Brueggeman, L.; Chen, S.L.; Hadley, D.; Green, A.; Khankhanian, P.; Baranzini, S.E. Systematic integration of biomedical knowledge prioritizes drugs for repurposing. eLife 2017, 6, e26726. [Google Scholar] [CrossRef]
- Lavecchia, A.; Di Giovanni, C. Virtual screening strategies in drug discovery: A critical review. Curr. Med. Chem. 2013, 20, 2839–2860. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.D.; Gaieb, Z.; Chiu, M.; Yang, H.; Shao, C.; Walters, W.P. Deep learning models for virtual screening: A critical review. Curr. Opin. Struct. Biol. 2020, 63, 82–92. [Google Scholar] [CrossRef]
- Su, M.; Yang, Q.; Du, Y.; Feng, G.; Liu, Z.; Li, Y. Comparative assessment of scoring functions: The CASF-2016 update. J. Chem. Inf. Model 2019, 59, 895–913. [Google Scholar] [CrossRef]
- Lim, J.; Ryu, S.; Park, K.; Choe, Y.J.; Ham, J.; Kim, W.Y. Predicting drug-target interaction using a novel graph neural network with 3D structure-embedded graph representation. J. Chem. Inf. Model 2019, 59, 3981–3988. [Google Scholar] [CrossRef] [PubMed]
- Gilmer, J.; Schoenholz, S.S.; Riley, P.F.; Vinyals, O.; Dahl, G.E. Neural Message Passing for Quantum Chemistry. In Proceedings of the 34th International Conference on Machine Learning, Sydney, Australia, 6–11 August 2017; PMLR: Cambridge, MA, USA, 2017. pp. 1263–1272. Available online: https://proceedings.mlr.press/v70/gilmer17a.html (accessed on 3 August 2025).
- Hu, W.; Liu, B.; Gomes, J.; Zitnik, M.; Liang, P.; Pande, V. Strategies for Pre-Training Graph Neural Networks. In Proceedings of the International Conference on Learning Representations, Addis Ababa, Ethiopia, 30 April 2020; Available online: https://openreview.net/forum?id=HJlWWJSFDH (accessed on 3 August 2025).
- Dietterich, T.G. Ensemble Methods in Machine Learning. In Proceedings of the International Workshop on Multiple Classifier Systems, Cagliari, Italy, 21–23 June 2000; pp. 1–15. [Google Scholar] [CrossRef]
- Yang, K.; Swanson, K.; Jin, W.; Coley, C.; Eiden, P.; Gao, H.; Guzman-Perez, A.; Hopper, T.; Kelley, B.; Mathea, M.; et al. Analyzing learned molecular representations for property prediction. J. Chem. Inf. Model 2019, 59, 3370–3388. [Google Scholar] [CrossRef]
- Ramsundar, B.; Eastman, P.; Walters, P.; Pande, V.; Leswing, K.; Wu, Z. Deep Learning for the Life Sciences: Applying Deep Learning to Genomics, Microscopy, Drug Discovery, and More; O’Reilly Media: Sebastopol, CA, USA, 2019; ISBN 978-1492039839. [Google Scholar]
- Proudfoot, J.R. Drugs, leads, and drug-likeness: An analysis of some recently launched drugs. Bioorg. Med. Chem. Lett. 2002, 12, 1647–1650. [Google Scholar] [CrossRef]
- Olivecrona, M.; Blaschke, T.; Engkvist, O.; Chen, H. Molecular de-novo design through deep reinforcement learning. J. Cheminform. 2017, 9, 51. [Google Scholar] [CrossRef]
- Zhou, Z.; Kearnes, S.; Li, L.; Zare, R.N.; Riley, P. Optimization of molecules via deep reinforcement learning. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 2717. [Google Scholar] [CrossRef]
- Mayr, A.; Klambauer, G.; Unterthiner, T.; Steijaert, M.; Wegner, J.K.; Ceulemans, H.; Clevert, D.A.; Hochreiter, S. Large-scale comparison of machine learning methods for drug target prediction on ChEMBL. Chem. Sci. 2018, 9, 5441–5451. [Google Scholar] [CrossRef]
- Pan, S.J.; Yang, Q. A survey on transfer learning. IEEE Trans. Knowl. Data Eng. 2010, 22, 1345–1359. [Google Scholar] [CrossRef]
- Zhang, O.; Lin, H.; Zhang, H.; Zhao, H.; Huang, Y.; Hsieh, C.Y.; Pan, P.; Hou, T. Deep Lead Optimization: Leveraging Generative AI for Structural Modification. J. Am. Chem. Soc. 2024, 146, 31357–31370. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Fechner, U. Computer-based de novo design of drug-like molecules. Nat. Rev. Drug Discov. 2005, 4, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bombarelli, R.; Wei, J.N.; Duvenaud, D.; Hernández-Lobato, J.M.; Sánchez-Lengeling, B.; Sheberla, D. Automatic chemical design using a data-driven continuous representation of molecules. ACS Cent. Sci. 2018, 4, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kingma, D.P.; Welling, M. Auto-encoding Variational Bayes. arXiv 2013, arXiv:1312.6114. Available online: https://arxiv.org/abs/1312.6114 (accessed on 3 August 2025).
- Guimaraes, G.L.; Sanchez-Lengeling, B.; Outeiral, C.; Farias, P.L.C.; Aspuru-Guzik, A. Objective-Reinforced Generative Adversarial Networks (ORGAN) for Sequence Generation Models. arXiv 2017, arXiv:1705.10843. Available online: https://arxiv.org/abs/1705.10843 (accessed on 3 August 2025).
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, L.; Polosukhin, L. Attention is all you need. Adv. Neural Inf. Process Syst. 2017, 30, 5998–6008. Available online: https://papers.nips.cc/paper/2017/hash/3f5ee243547dee91fbd053c1c4a845aa-Abstract.html (accessed on 3 August 2025).
- Zhang, P.; Baker, D.; Song, M.; Bi, J. Unraveling the potential of diffusion models in small-molecule generation. Drug Discov. Today 2025, 30, 104413. [Google Scholar] [CrossRef]
- Settles, B. Active Learning Literature Survey; Technical Report 1648; University of Wisconsin-Madison Computer Sciences: Madison, WI, USA, 2009; Available online: https://minds.wisconsin.edu/handle/1793/60660 (accessed on 3 August 2025).
- Deb, K.; Pratap, A.; Agarwal, S.; Meyarivan, T. A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Trans. Evol. Comput. 2002, 6, 182–197. [Google Scholar] [CrossRef]
- Bradshaw, J.; Paige, B.; Kusner, M.J.; Segler, M.; Hernández-Lobato, J.M. A generative model for electron paths. Chem. Sci. 2019, 10, 1656–1666. [Google Scholar]
- Coley, C.W.; Barzilay, R.; Jaakkola, T.S.; Green, W.H.; Jensen, K.F. A graph-convolutional neural network model for the prediction of chemical reactivity. Chem. Sci. 2019, 10, 370–377. [Google Scholar] [CrossRef]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ramsundar, B.; Feinberg, E.N.; Gomes, J.; Geniesse, C.; Pappu, A.S. MoleculeNet: A benchmark for molecular machine learning. Chem. Sci. 2018, 9, 513–530. [Google Scholar] [CrossRef]
- Holzinger, A.; Biemann, C.; Pattichis, C.S.; Kell, D.B. What do We Need to Build Explainable AI Systems for the Medical Domain? arXiv 2017, arXiv:1712.09923. Available online: https://arxiv.org/abs/1712.09923 (accessed on 3 August 2025).
- Arrieta, A.B.; Díaz-Rodríguez, N.; Del Ser, J.; Bennetot, A.; Tabik, S.; Barbado, A. Explainable Artificial Intelligence (XAI): Concepts, taxonomies, opportunities and challenges toward responsible AI. Inf. Fusion 2020, 58, 82–115. [Google Scholar] [CrossRef]
- Bahdanau, D.; Cho, K.; Bengio, Y. Neural Machine Translation by Jointly Learning to Align and Translate. arXiv 2014, arXiv:1409.0473. Available online: https://arxiv.org/abs/1409.0473 (accessed on 3 August 2025).
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process Syst. 2017, 30, 4765–4774. Available online: https://papers.nips.cc/paper/2017/hash/8a20a8621978632d76c43dfd28b67767-Abstract.html (accessed on 3 August 2025).
- Sorkun, M.C.; Khetan, A.; Er, S. AqSolDB, a curated reference set of aqueous solubility and octanol/water partition coefficient data. Sci. Data 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Wang, N.N.; Dong, J.; Deng, Y.H.; Zhu, M.F.; Wen, M.; Yao, Z.J.; Lu, A.P.; Wang, J.B.; Cao, D.S. ADME Properties Evaluation in Drug Discovery: Prediction of Caco-2 Cell Permeability Using a Combination of NSGA-II and Boosting. J. Chem. Inf. Model. 2016, 56, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Vijay, V.; Shi, Q.; Liu, Z.; Fang, H.; Tong, W. DILIrank: The largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov. Today 2016, 21, 648–653. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, D.; Liu, X.; Zhong, F.; Wan, X.; Li, X.; Li, Z.; Luo, X.; Chen, K.; Jiang, H.; et al. Pushing the boundaries of molecular representation for drug discovery with graph attention mechanisms. J. Med. Chem. 2020, 63, 8749–8769. [Google Scholar] [CrossRef]
- Dang, N.L.; Hughes, T.B.; Miller, G.P.; Swamidass, S.J. In silico ADMET profiling: Progress and challenges. Future Med. Chem. 2020, 12, 1991–2008. [Google Scholar] [CrossRef]
- Watanabe, R.; Esaki, T.; Kawashima, H.; Natsume-Kitatani, Y.; Nagao, C.; Ohashi, R.; Mizuguchi, K. Predicting Fraction Unbound in Human Plasma from Chemical Structure: Improved Accuracy in the Low Value Ranges. Mol. Pharm. 2018, 15, 5302–5311. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Kim, H.U.; Lee, S.Y. Deep learning improves prediction of drug-drug and drug-food interactions. Proc. Natl. Acad. Sci. USA 2018, 115, E4304–E4311. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Rawling, M.; Savory, E.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackermann, Z.; et al. A deep learning approach to antibiotic discovery. Cell 2020, 180, 688–702. [Google Scholar] [CrossRef]
- Hopkins, A.L. Exscientia progress report: AI-designed molecules in the clinic. Drug Discov. Today 2022, 27, 1903–1905. [Google Scholar] [CrossRef]
- Zhavoronkov, A.; Ivanenkov, Y.A.; Aliper, A.; Veselov, M.S.; Aladinskiy, V.A.; Aladinskaya, A.V.; Terentiev, V.A.; Polykovskiy, D.A.; Kuznetsov, M.D.; Asadulaev, A.; et al. Deep learning enables rapid identification of potent DDR1 kinase inhibitors. Nat. Biotechnol. 2019, 37, 1038–1040. [Google Scholar] [CrossRef]
- Jayatunga, M.K.; Xie, W.; Ruder, L.; Schulze, U.; Meier, C. AI in small-molecule drug discovery: A coming wave? Nat. Rev. Drug Discov. 2022, 21, 175–176. [Google Scholar] [CrossRef]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef]
- Gholap, A.D.; Uddin, M.J.; Faiyazuddin, M.; Omri, A.; Gowri, S.; Khalid, M. Advances in artificial intelligence for drug delivery and development: A comprehensive review. Comput. Biol. Med. 2024, 178, 108702. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, F.M.; Gray, N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug Discov. 2018, 17, 353–377. [Google Scholar] [CrossRef]
- Cohen, S.; Fleischmann, R. Kinase inhibitors: A new approach to rheumatoid arthritis treatment. Curr. Opin. Rheumatol. 2010, 22, 330–335. [Google Scholar] [CrossRef]
- Amin, M.; Martínez-Heras, E.; Ontaneda, D. Artificial Intelligence and Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2024, 24, 233–243. [Google Scholar] [CrossRef]
- Bar-Or, A.; Calkwood, J.C.; Chognot, C.; Evershed, J.; Fox, E.J.; Herman, A.; Manfrini, M.; McNamara, J.; Robertson, D.S.; Stokmaier, D.; et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis. Neurology 2020, 95, e1999–e2008. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Mullard, A. The drug-maker’s guide to the galaxy. Nature 2017, 549, 445–447. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Schneider, P.; Walters, W.P.; Plowright, A.T.; Sieroka, N.; Listgarten, J.; Goodnow, R.A.; Fisher, J.; Jansen, J.M.; Duca, J.S.; Rush, T.S.; et al. Rethinking drug design in the artificial intelligence era. Nat. Rev. Drug Discov. 2020, 19, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Mouchlis, V.D.; Afantitis, A.; Serra, A.; Fratello, M.; Papadiamantis, A.G.; Aidinis, V.; Lynch, I.; Greco, D.; Melagraki, G. Advances in de Novo Drug Design: From Conventional to Machine Learning Methods. Int. J. Mol. Sci. 2021, 22, 1676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reymond, J.L. The chemical space project. Acc. Chem. Res. 2015, 48, 722–730. [Google Scholar] [CrossRef]
- Coley, C.W.; Rogers, L.; Green, W.H.; Jensen, K.F. SCScore: Synthetic complexity learned from a reaction corpus. J. Chem. Inf. Model 2018, 58, 252–261. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.; Wang, Y.; Sun, H.; Huang, J. Self-supervised learning for molecular representations. Nat. Mach. Intell. 2022, 4, 279–287. [Google Scholar] [CrossRef]
- Irwin, J.J.; Gaskins, G.; Sterling, T.; Mysinger, M.M.; Keiser, M.J. ChemBERTa: Large-scale self-supervised pretraining for molecular property prediction. J. Chem. Inf. Model 2022, 62, 6–14. [Google Scholar] [CrossRef]
- Strubell, E.; Ganesh, A.; McCallum, A. Energy and policy considerations for deep learning in NLP. arXiv 2019, arXiv:1906.02243. Available online: https://arxiv.org/abs/1906.02243 (accessed on 3 August 2025).
- Hinton, G.; Vinyals, O.; Dean, J. Distilling the knowledge in a neural network. arXiv 2015, arXiv:1503.02531. Available online: https://arxiv.org/abs/1503.02531 (accessed on 3 August 2025).
- Schwartz, R.; Dodge, J.; Smith, N.A.; Etzioni, O. Green AI. Commun. ACM 2020, 63, 54–63. [Google Scholar] [CrossRef]
- Cao, Y.; Romero, J.; Olson, J.P.; Degroote, M.; Johnson, P.D.; Kieferová, M.; Kivlichan, I.D.; Menke, T.; Peropadre, B.; Sawaya, N.P.D.; et al. Quantum chemistry in the age of quantum computing. Chem. Rev. 2019, 119, 10856–10915. [Google Scholar] [CrossRef] [PubMed]
- McClean, J.R.; Romero, J.; Babbush, R.; Aspuru-Guzik, A. The variational quantum eigensolver: A new paradigm for quantum chemistry. Phys. Rev. X 2016, 6, 031007. [Google Scholar] [CrossRef]
- Biamonte, J.; Wittek, P.; Pancotti, N.; Rebentrost, P.; Wiebe, N.; Lloyd, S. Quantum machine learning. Nature 2017, 549, 195–202. [Google Scholar] [CrossRef]
- Jacobsen, A.; de Miranda Azevedo, R.; Juty, N.; Batista, D.; Coles, S.; Cornet, R. FAIR principles: Interpretations and implementation. Data Intell. 2020, 2, 10–29. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Sato, Y.; Odagiri, H. MELLODDY: Federated learning for drug discovery. Nat. Mach. Intell. 2021, 3, 837–838. [Google Scholar] [CrossRef]
- Carvalho-Silva, D.; Pierleoni, A.; Pignatelli, M.; Ong, C.; Fumis, L.; Karamanis, N.; Carmona, M.; Faulconbridge, A.; Hercules, A.; McAuley, E.; et al. Open Targets Platform: New developments and updates two years on. Nucleic Acids Res. 2019, 47, D1056–D1065. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Fu, T.; Gao, W.; Zhao, Y.; Roohani, Y.; Leskovec, J. Therapeutics Data Commons: Machine learning datasets and tasks for drug discovery and development. arXiv 2021, arXiv:2102.09548. Available online: https://arxiv.org/abs/2102.09548 (accessed on 3 August 2025).
- Fourches, D.; Muratov, E.; Tropsha, A. Trust, but verify: On the importance of chemical structure curation in cheminformatics and QSAR modeling research. J. Chem. Inf. Model 2010, 50, 1189–1204. [Google Scholar] [CrossRef]
- Sheridan, R.P. Time to change how we do QSAR. J. Comput. Aided Mol. Des. 2013, 27, 847–855. [Google Scholar] [CrossRef]
- Ekins, S.; Puhl, A.C.; Zorn, K.M.; Lane, T.R.; Russo, D.P.; Klein, J.J.; Hickey, A.J.; Clark, A.M. Exploiting machine learning for end-to-end drug discovery and development. Nat. Mater. 2019, 18, 435–441. [Google Scholar] [CrossRef]
- Williams, K.; Bilsland, E.; Sparkes, A.; Aubrey, W.; Young, M.; Soldatova, L.N.; De Grave, K.; Ramon, J.; de Clare, M.; Sirawaraporn, W.; et al. Cheaper faster drug development validated by the repositioning of drugs against neglected tropical diseases. J. R. Soc. Interface 2015, 12, 20141289. [Google Scholar] [CrossRef]
- Torng, W.; Altman, R.B. Graph convolutional neural networks for predicting drug-target interactions. J. Chem. Inf. Model 2019, 59, 4131–4149. [Google Scholar] [CrossRef]
- Marshall, S.F.; Burghaus, R.; Cosson, V.; Cheung, S.Y.; Chenel, M.; DellaPasqua, O.; Frey, N.; Hamrén, B.; Harnisch, L.; Ivanow, F.; et al. Good Practices in Model-Informed Drug Discovery and Development: Practice, Application, and Documentation. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 93–122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Méndez-Lucio, O.; Baillif, B.; Clevert, D.A.; Rouquié, D.; Wichard, J. De novo generation of hit-like molecules from gene expression signatures using artificial intelligence. Nat. Commun. 2020, 11, 10. [Google Scholar] [CrossRef]
- McDermott, M.B.; Wang, S.; Marinsek, N.; Ranganath, R.; Foschini, L.; Ghassemi, M. Reproducibility in machine learning for health research: Still a ways to go. Sci. Transl. Med. 2021, 13, eabb1655. [Google Scholar] [CrossRef]
- Arús-Pous, J.; Johansson, S.V.; Prykhodko, O.; Bjerrum, E.J.; Tyrchan, C.; Reymond, J.L.; Chen, H.; Engkvist, O. Randomized SMILES strings improve the quality of molecular generative models. J. Cheminform. 2019, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Reardon, S. Rise of robot chemists sparks debate over AI patents. Nature 2019, 574, 155–156. [Google Scholar] [CrossRef]
- Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 2018, 17, 97–113. [Google Scholar] [CrossRef]
- Obermeyer, Z.; Powers, B.; Vogeli, C.; Mullainathan, S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 2019, 366, 447–453. [Google Scholar] [CrossRef]
- Eichler, H.G.; Baird, L.G.; Barker, R.; Bloechl-Daum, B.; Børlum-Kristensen, F.; Brown, J.; Chua, R.; Del Signore, S.; Dugan, U.; Ferguson, J.; et al. From adaptive licensing to adaptive pathways: Delivering a flexible life-span approach to bring new drugs to patients. Clin. Pharmacol. Ther. 2015, 97, 234–246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rashidi, P.; Bihorac, A.; Rashidi, H.; Johnson, J.A.; Tighe, P.J. Digital twins: The convergence of physical and virtual in healthcare. Nat. Biotechnol. 2019, 37, 1395–1397. [Google Scholar] [CrossRef]
- Mak, K.K.; Pichika, M.R. Artificial intelligence in drug development: Present status and future prospects. Drug Discov. Today 2019, 24, 773–780. [Google Scholar] [CrossRef]
- Fleming, N. How artificial intelligence is changing drug discovery. Nature 2018, 557, S55–S57. [Google Scholar] [CrossRef]
| Method Category | Traditional Approach | AI-Enhanced Approach | Typical Performance (AUC) | Dataset Size | Computational Requirements | Key Advantages | Limitations | Key References |
|---|---|---|---|---|---|---|---|---|
| Ligand-based similarity | Tanimoto coefficient, 2D fingerprints | Graph neural networks, learned embeddings | 0.65–0.75 vs. 0.70–0.80 | 103–104 compounds | Low-Medium | Fast, interpretable | Limited to known chemotypes | [37,63,64] |
| Structure-based docking | Glide, AutoDock | CNN scoring functions, DeepDocking | 0.70–0.80 vs. 0.72–0.82 | 106–108 compounds | High | Physics-based, broad coverage | Target flexibility challenges | [60,61,62] |
| Pharmacophore modeling | Manual feature definition | AI-learned pharmacophores | 0.68–0.78 vs. 0.72–0.82 | 103–105 compounds | Medium | Mechanism insights | Feature engineering dependent | [41,67] |
| Machine learning QSAR | Random forests, SVM | Deep neural networks, transformers | 0.75–0.85 vs. 0.78–0.88 | 104–106 compounds | Medium-High | Pattern recognition | Black box nature | [44,45,47,50] |
| Ensemble methods | Consensus scoring | Multi-task deep learning | 0.80–0.90 vs. 0.83–0.92 | 105–107 compounds | High | Robust performance | Computational complexity | [66,68] |
| ADMET Property | Model Architecture | Dataset Size | Performance Metric | Performance Value | Data Source | Key References |
|---|---|---|---|---|---|---|
| Aqueous solubility | Graph CNN | 9982 compounds | R2 | 0.77 | AqSolDB | [93] |
| Lipophilicity (LogP) | Transformer | 14,050 compounds | MAE | 0.54 log units | ChEMBL | [50,94] |
| Permeability (Caco-2) | Multi-task DNN | 906 compounds | R2 | 0.71 | Literature compilation | [94] |
| Blood-brain barrier | Graph attention | 1975 compounds | AUC | 0.91 | BBBP dataset | [67] |
| Hepatotoxicity | Deep neural network | 1254 compounds | Balanced accuracy | 0.79 | DILIrank | [95] |
| hERG cardiotoxicity | Graph neural network | 13,445 compounds | AUC | 0.94 | ChEMBL | [96] |
| Metabolic stability | Ensemble methods | 2896 compounds | R2 | 0.68 | Proprietary pharma data | [97] |
| Plasma binding | Random forest + DNN | 1797 compounds | R2 | 0.74 | Multiple sources | [98] |
| Oral bioavailability | Multi-task learning | 1020 compounds | AUC | 0.75 | Literature/patents | [47,68] |
| Half-life | LSTM + molecular descriptors | 1352 compounds | R2 | 0.62 | DrugBank + literature | [99] |
| Drug Name | Company | Indication | AI Application | Development Stage | Timeline Reduction | Key Innovation | Outcome | Key References |
|---|---|---|---|---|---|---|---|---|
| AI-Assisted Repurposing | ||||||||
| Baricitinib | Benevolent AI/Eli Lilly | COVID-19, RA | AI literature mining and target network analysis for repurposing | Approved | 3 months for new indication identification | Rapid pandemic response through repurposing | Approved | [100,101,106] |
| AI-Designed De Novo | ||||||||
| DSP-1181 | Exscientia | Obsessive-compulsive disorder | AI-driven small-molecule design | Phase I completed, discontinued | 12 months vs. 4–6 years | First AI-designed small-molecule in trials | Discontinued (2022) | [103,107] |
| Halicin | MIT/Broad Institute | Antibiotic-resistant infections | Deep learning virtual screening | Preclinical | N/A (novel mechanism) | Novel antibiotic mechanism identification | Preclinical | [102] |
| ISM001-055 (rentosertib) | Insilico Medicine | Idiopathic pulmonary fibrosis | Integrated AI platform | Phase IIa completed | 18 months vs. 6+ years | End-to-end AI small-molecule discovery | Positive Phase IIa (2025) | [3,104] |
| AI-Assisted Optimization | ||||||||
| EXS-21546 | Exscientia | Inflammatory diseases | AI-guided small-molecule optimization | Preclinical | ~24 months vs. 5+ years | Complex small-molecule target | Ongoing | [108,109] |
| ATM-3507 | Atomwise | Multiple sclerosis | Virtual screening platform | Phase I | ~36 months vs. 6+ years | Previously challenging target | Ongoing | [110,111] |
| DSP-0038 | Exscientia | Alzheimer’s disease | AI-designed | Phase I | 13 months of design | Precision-designed molecule | Ongoing | [112] |
| IAMA-6 | Iktos/Almirall | Dermatology | Generative AI design | Preclinical | 21 months | Novel scaffold generation | Ongoing | [113] |
| BEN-2293 | BenevolentAI | Atopic dermatitis | AI target discovery | Phase I | ~30 months | Novel target identification | Ongoing | [114] |
| Challenge Category | Specific Issues | Current Impact | Mitigation Strategies | Future Research Directions | Key References |
|---|---|---|---|---|---|
| Data Quality | Experimental bias, missing values, protocol inconsistencies | High—limits model reliability | Standardized assay protocols, data curation pipelines, and uncertainty quantification | Automated data quality assessment, federated learning | [131,134] |
| Model Interpretability | Black box predictions, lack of mechanistic insights | Medium—regulatory concerns | SHAP values, attention mechanisms, surrogate models | Inherently interpretable architectures, causal inference | [89,90,92] |
| Generalizability | Poor performance on novel scaffolds | High—limits applicability | Transfer learning, domain adaptation, meta-learning | Foundation models, few-shot learning | [132,135] |
| Regulatory Acceptance | Unclear validation requirements | Medium—slows adoption | Early regulatory engagement, model documentation | AI-specific guidance documents, digital twins | [133,136] |
| Integration Challenges | Workflow compatibility, skill gaps | High organizational barriers | Change management, training programs, and hybrid teams | Automated workflows, user-friendly interfaces | [137,138] |
| Reproducibility | Inconsistent benchmarking, code availability | Medium—scientific validity | Standardized benchmarks, open-source software | Community-driven evaluation platforms | [139,140] |
| Computational Resources | High training costs, infrastructure requirements | Medium—limits accessibility | Cloud platforms, model compression, and knowledge distillation | Edge computing, efficient architectures | [121,122,123] |
| Intellectual Property | Algorithm patentability, data ownership | Low—legal uncertainties | Clear IP strategies, collaborative frameworks | Open science initiatives, pre-competitive consortia | [141,142] |
| Ethical Biases | Underrepresentation in datasets, equity issues | High—societal impact | Diverse data collection, bias audits | Ethical AI frameworks, inclusive design | [54,143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niazi, S.K. Artificial Intelligence in Small-Molecule Drug Discovery: A Critical Review of Methods, Applications, and Real-World Outcomes. Pharmaceuticals 2025, 18, 1271. https://doi.org/10.3390/ph18091271
Niazi SK. Artificial Intelligence in Small-Molecule Drug Discovery: A Critical Review of Methods, Applications, and Real-World Outcomes. Pharmaceuticals. 2025; 18(9):1271. https://doi.org/10.3390/ph18091271
Chicago/Turabian StyleNiazi, Sarfaraz K. 2025. "Artificial Intelligence in Small-Molecule Drug Discovery: A Critical Review of Methods, Applications, and Real-World Outcomes" Pharmaceuticals 18, no. 9: 1271. https://doi.org/10.3390/ph18091271
APA StyleNiazi, S. K. (2025). Artificial Intelligence in Small-Molecule Drug Discovery: A Critical Review of Methods, Applications, and Real-World Outcomes. Pharmaceuticals, 18(9), 1271. https://doi.org/10.3390/ph18091271







