Assessing the Comparability of Degradation Profiles Between Biosimilar and Originator Anti-VEGF Monoclonal Antibodies Under Thermal Stress
Abstract
1. Introduction
2. Results
2.1. Method Development and Validation of CE-SDS
2.1.1. Specificity
2.1.2. Linearity
2.1.3. Accuracy
2.1.4. Precision (Repeatability and Intermediate Precision)
2.1.5. Limit of Quantitation (LOQ)
2.1.6. Range
2.1.7. Robustness
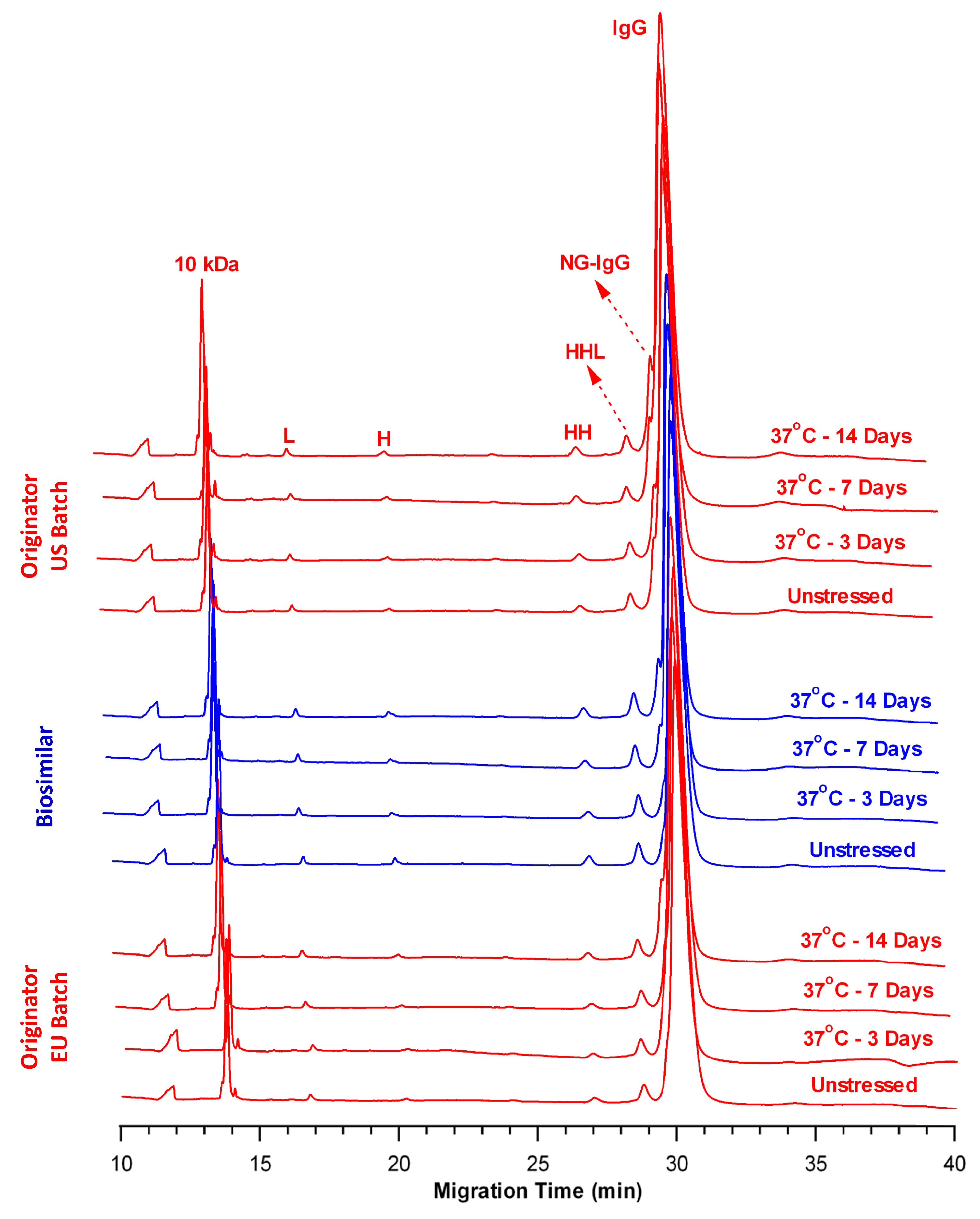
2.2. Comparison of Fragmentation Profiles at 37 °C
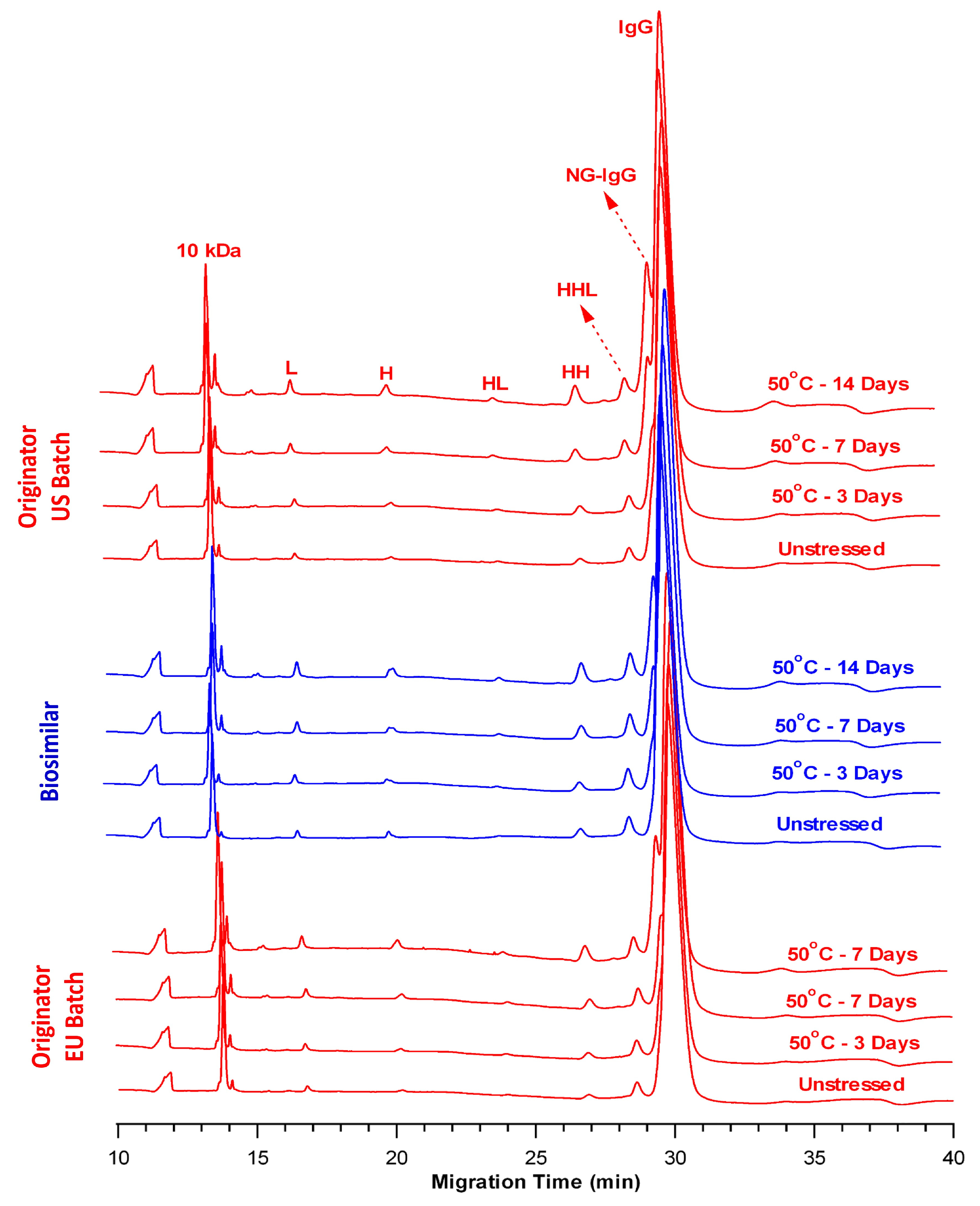
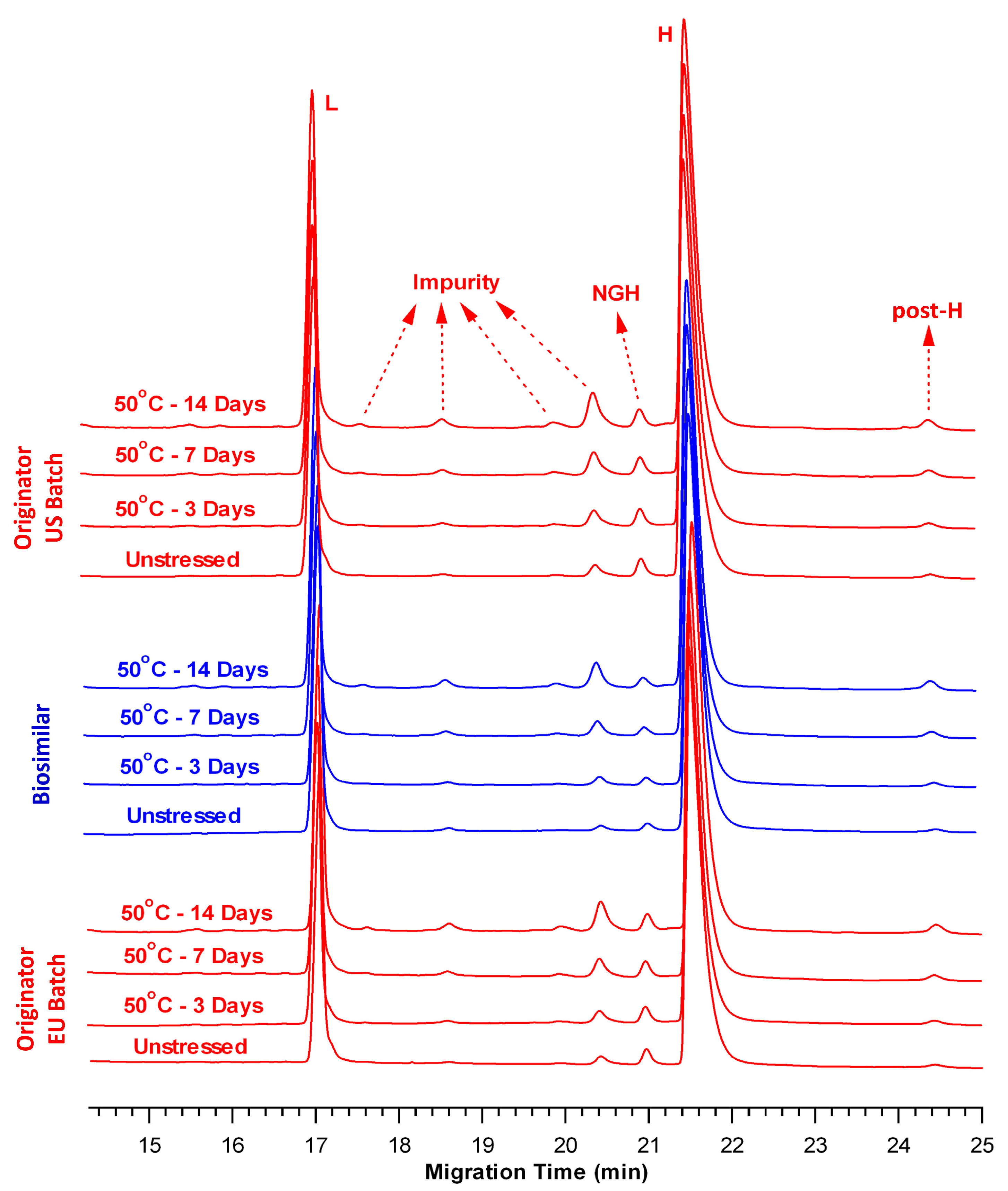
2.3. Comparison of Fragmentation Profiles at 50 °C
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Thermally Stressed Samples
4.3. CE-SDS
4.4. SE-UPLC
4.5. LC-MS/MS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BGE | Background Electrolyte Solution |
| BME | 2-mercaptoethanol |
| BS | Biosimilar |
| CE-SDS | Capillary Electrophoresis–Sodium Dodecyl Sulfate |
| CHO | Chinese Hamster Ovary |
| CQA | Critical Quality Attribute |
| DTT | Dithiothreitol |
| E | Glutamic Acid |
| H | Heavy Chain |
| HHL | Heavy–Heavy–Light Chain |
| HMW | High Molecular Weight |
| IAM | Iodoacetamide |
| ICH | International Council for Harmonization |
| L | Light Chain |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| LMW | Low Molecular Weight |
| LOQ | Limit of Quantitation |
| mAb | Monoclonal Antibody |
| N | Asparagine |
| NGH | Non-Glycosylated Heavy Chain |
| NG-IgG | Non-Glycosylated Immunoglobulin G |
| nrCE-SDS | Non-reducing Capillary Electrophoresis of Sodium Dodecyl Sulfate |
| NRS | Non-reducible species |
| OR | Originator |
| OR-EU | Originator-European Union |
| OR-US | Originator-United States |
| R2 | Correlation Coefficient |
| rCE-SDS | Reducing Capillary Electrophoresis of Sodium Dodecyl Sulfate |
| RSB | Reducing Sample Buffer |
| RSD | Relative Standard Deviation |
| S/N | Signal-to-Noise |
| SD | Standard Deviation |
| SE-UPLC | Size-Exclusion Ultra-Performance Liquid Chromatography |
| TCA | Time-Corrected Area |
| USP | United States Pharmacopoeia |
| VEGF | Vascular Endothelial Growth Factor |
References
- Sänger-van de Griend, C.E. CE-SDS method development, validation, and best practice-an overview. Electrophoresis 2019, 40, 2361–2374. [Google Scholar] [CrossRef]
- Rustandi, R.R.; Washabaugh, M.W.; Wang, Y. Applications of CE SDS gel in development of biopharmaceutical antibody-based products. Electrophoresis 2008, 29, 3612–3620. [Google Scholar] [CrossRef]
- Michels, D.A.; Parker, M.; Salas-Solano, O. Quantitative impurity analysis of monoclonal antibody size heterogeneity by CE-LIF: Example of development and validation through a quality-by-design framework. Electrophoresis 2012, 33, 815–826. [Google Scholar] [CrossRef]
- Zhang, J.G.; Burmana, S.; Gunturia, S.; Foley, J.P. Method development and validation of capillary sodium dodecyl sulfate gel electrophoresis for the characterization of a monoclonal antibody. J. Pharm. Biomed. Anal. 2010, 53, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Esterman, A.L.; Katiyar, A.; Krishnamurthy, G. Implementation of USP antibody standard for system suitability in capillary electrophoresis sodium dodecyl sulfate (CE-SDS) for release and stability methods. J. Pharm. Biomed. Anal. 2016, 128, 447–454. [Google Scholar] [CrossRef]
- Wagner, E.; Colas, O.; Chenu, S.; Goyon, A.; Murisier, A.; Cianferani, S.; François, Y.; Fekete, S.; Guillarme, D.; D’Atri, V.; et al. Determination of size variants by CE-SDS for approved therapeutic antibodies: Key implications of subclasses and light chain specificities. J. Pharm. Biomed. Anal. 2020, 184, 113166. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Lin, J. Advantages of CE-SDS over SDS-PAGE in mAb purity analysis. Anal. Methods 2012, 4, 1637–1642. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, J.J.; Liu, S. Protein separation by capillary gel electrophoresis: A review. Anal. Chim. Acta 2012, 709, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Gahoual, R.; Beck, A.; Leize-Wagner, E.; Francois, Y.N. Cutting-edge capillary electrophoresis characterization of monoclonal antibodies and related products. J. Chromatogr. B 2016, 1032, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Analytical Procedures for Recombinant Therapeutic Monoclonal Antibodies; United States Pharmacopeia General Chapter <129>; United States Pharmacopeia: Rockville, MD, USA, 2024.
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Hong, J.; Lee, Y.; Lee, C.; Eo, S.; Kim, S.; Lee, N.; Park, J.; Park, S.; Seo, D.; Jeong, M.; et al. Physicochemical and biological characterization of SB2, a biosimilar of Remicade® (infliximab). mAbs 2017, 9, 365–383. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, J.; Bae, J.S.; Kim, Y.J.; Kang, H.A.; Kim, S.H.; Lee, S.J.; Lim, K.J.; Lee, J.W.; Jung, S.K.; et al. Analytical similarity assessment of rituximab biosimilar CT-P10 to reference medicinal product. mAbs 2018, 10, 380–396. [Google Scholar] [CrossRef] [PubMed]
- Hutterer, K.M.; Polozova, A.; Kuhns, S.; McBride, H.J.; Cao, X.; Liu, J. Assessing analytical and functional similarity of proposed Amgen biosimilar ABP 980 to trastuzumab. BioDrugs 2019, 33, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Hawe, A.; Wiggenhorn, M.; van de Weert, M.; Garbe, J.H.; Mahler, H.C.; Jiskoot, W. Forced degradation of therapeutic proteins. J. Pharm. Sci. 2012, 101, 895–913. [Google Scholar] [CrossRef] [PubMed]
- Nowak, C.; Cheung, J.K.; Dellatore, S.M.; Katiyar, A.; Bhat, R.; Sun, J.; Ponniah, G.; Neill, A.; Mason, B.; Beck, A.; et al. Forced degradation of recombinant monoclonal antibodies: A practical guide. mAbs 2017, 9, 1217–1230. [Google Scholar] [CrossRef]
- Moussa, E.M.; Panchal, J.P.; Moorthy, B.S.; Blum, J.S.; Joubert, M.K.; Narhi, L.O.; Topp, E.M. Immunogenicity of therapeutic protein aggregates. J. Pharm. Sci. 2016, 105, 417–430. [Google Scholar] [CrossRef]
- Eon-Duval, A.; Broly, H.; Gleixner, R. Quality attributes of recombinant therapeutic proteins: An assessment of impact on safety and efficacy as part of a quality by design development approach. Biotechnol. Prog. 2012, 28, 608–622. [Google Scholar] [CrossRef]
- Tamizi, E.; Jouyban, A. Forced degradation studies of biopharmaceuticals: Selection of stress conditions. Eur. J. Pharm. Biopharm. 2016, 98, 26–46. [Google Scholar] [CrossRef]
- Liu, H.; Gaza-Bulseco, G.; Sun, J. Characterization of the stability of a fully human monoclonal IgG after prolonged incubation at elevated temperature. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 837, 35–43. [Google Scholar] [CrossRef]
- Hawe, A.; Kasper, J.C.; Friess, W.; Jiskoot, W. Structural properties of monoclonal antibody aggregates induced by freeze-thawing and thermal stress. Eur. J. Pharm. Sci. 2009, 38, 79–87. [Google Scholar] [CrossRef]
- Kamerzell, T.J.; Li, M.; Arora, S.; Ji, J.A.; Wang, Y.J. The relative rate of immunoglobulin gamma 1 fragmentation. J. Pharm. Sci. 2011, 100, 1341–1349. [Google Scholar] [CrossRef]
- Duhamel, L.; Gu, Y.; Barnett, G.; Tao, Y.; Voronov, S.; Ding, J.; Mussa, N.; Li, Z.J. Therapeutic protein purity and fragmented species characterization by capillary electrophoresis sodium dodecyl sulfate using systematic hybrid cleavage and forced degradation. Anal. Bioanal. Chem. 2019, 411, 5617–5629. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Kobayashi, N.; Yabuta, M.; Ohara, M.; Naito, T.; Kubo, T.; Otsuka, K. Identification and characterization of a thermally cleaved fragment of monoclonal antibody-a detected by sodium dodecyl sulfate-capillary gel electrophoresis. J. Pharm. Biomed. Anal. 2017, 140, 98–104. [Google Scholar] [CrossRef]
- Vlasak, J.; Ionescu, R. Fragmentation of monoclonal antibodies. mAbs 2011, 3, 253–263. [Google Scholar] [CrossRef]
- Dada, O.O.; Rao, R.; Jones, N.; Jaya, N.; Salas-Solano, O. Comparison of SEC and CE-SDS methods for monitoring hinge fragmentation in IgG1 monoclonal antibodies. J. Pharm. Biomed. Anal. 2017, 145, 91–97. [Google Scholar] [CrossRef]
- Salas-Solano, O.; Tomlinson, B.; Du, S.; Parker, M.; Strahan, A.; Ma, S. Optimization and validation of a quantitative capillary electrophoresis sodium dodecyl sulfate method for quality control and stability monitoring of monoclonal antibodies. Anal. Chem. 2006, 78, 6583–6594. [Google Scholar] [CrossRef]
- Lacher, N.A.; Roberts, R.K.; He, Y.; Cargill, H.; Kearns, K.M.; Holovics, H.; Ruesch, M.N. Development, validation, and implementation of capillary gel electrophoresis as a replacement for SDS-PAGE for purity analysis of IgG2 mAbs. J. Sep. Sci. 2010, 33, 218–227. [Google Scholar] [CrossRef]
- Li, M.; Yu, C.; Wang, W.; Wu, G.; Wang, L. Interlaboratory method validation of capillary electrophoresis sodium dodecyl sulfate (CE-SDS) methodology for analysis of mAbs. Electrophoresis 2021, 42, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation (ICH). ICH Q2R2: Validation of Analytical Procedures Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 21 April 2025).
- Jaccoulet, E.; Daniel, T.; Prognon, P.; Caudron, E. Forced degradation of monoclonal antibodies after compounding: Impact on routine hospital quality control. J. Pharm. Sci. 2019, 108, 3252–3261. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, M.R.; Randolph, T.W.; Volkin, D.B.; Schöneich, C.; Carpenter, J.F.; Crommelin, D.J.; Jiskoot, W. Postproduction handling and administration of protein pharmaceuticals and potential instability issues. J. Pharm. Sci. 2018, 107, 2013–2019. [Google Scholar] [CrossRef]
- Song, X.; Tian, H.; Zhang, G. Application of capillary electrophoresis-sodium dodecyl sulfate in assessing the purity of monoclonal antibody biopharmaceuticals. J. Liq. Chromatogr. Relat. Technol. 2024, 47, 44–54. [Google Scholar] [CrossRef]
- Seo, N.; Polozova, A.; Zhang, M.; Yates, Z.; Cao, S.; Li, H.; Kuhns, S.; Maher, G.; McBride, H.J.; Liu, J. Analytical and functional similarity of Amgen biosimilar ABP 215 to bevacizumab. mAbs 2018, 10, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xu, G.; He, W.; Yu, C.; Huang, W.; Zheng, S.; Kang, D.; Xie, M.H.; Cao, X.; Wang, L.; et al. Comparability strategy and demonstration for post-approval production cell line change of a bevacizumab biosimilar IBI305. Antib. Ther. 2023, 6, 194–210. [Google Scholar] [CrossRef]
- Liu, J.; Eris, T.; Li, C.; Cao, S.; Kuhns, S. Assessing analytical similarity of proposed Amgen biosimilar ABP 501 to adalimumab. BioDrugs 2016, 30, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Saleem, R.; Cantin, G.; Wikström, M.; Botton, G.; Kuhns, S.; McBride, H.J.; Liu, J. Analytical and functional similarity assessment of ABP 710, a biosimilar to infliximab reference product. Pharm. Res. 2020, 37, 114. [Google Scholar] [CrossRef] [PubMed]
- Tous, G.I.; Wei, Z.; Feng, J.; Bilbulian, S.; Bowen, S.; Smith, J.; Strouse, R.; McGeehan, P.; Casas-Finet, J.; Schenerman, M.A. Characterization of a novel modification to monoclonal antibodies: Thioether cross-link of heavy and light chains. Anal. Chem. 2005, 77, 2675–2682. [Google Scholar] [CrossRef]
- Bansal, R.; Dash, R.; Rathore, A.S. Impact of mAb aggregation on its biological activity: Rituximab as a case study. J. Pharm. Sci. 2020, 109, 2684–2698. [Google Scholar] [CrossRef]
- Sreenivasan, S.; Schöneich, C.; Rathore, A.S. Aggregation of therapeutic monoclonal antibodies due to thermal and air/liquid interfacial agitation stress: Occurrence, stability assessment strategies, aggregation mechanism, influencing factors, and ways to enhance stability. Int. J. Pharm. 2024, 666, 124735. [Google Scholar] [CrossRef]
- Chelius, D.; Jing, K.; Lueras, A.; Rehder, D.S.; Dillon, T.M.; Vizel, A.; Rajan, R.S.; Li, T.; Treuheit, M.J.; Bondarenko, P.V. Formation of pyroglutamic acid from N-terminal glutamic acid in immunoglobulin gamma antibodies. Anal. Chem. 2006, 78, 2370–2376. [Google Scholar] [CrossRef]
- Vlasak, J.; Bussat, M.C.; Wang, S.; Wagner-Rousset, E.; Schaefer, M.; Klinguer-Hamour, C.; Kirchmeier, M.; Corvaia, N.; Ionescu, R.; Beck, A. Identification and characterization of asparagine deamidation in the light chain CDR1 of a humanized IgG1 antibody. Anal. Biochem. 2009, 392, 145–154. [Google Scholar] [CrossRef]
- Chelius, D.; Rehder, D.S.; Bondarenko, P.V. Identification and characterization of deamidation sites in the conserved regions of human immunoglobulin gamma antibodies. Anal. Chem. 2005, 77, 6004–6011. [Google Scholar] [CrossRef] [PubMed]

| Validation Parameters | nrCE-SDS | rCE-SDS | ||||
|---|---|---|---|---|---|---|
| Intact IgG | Total LMW | L | H | Total Impurity | ||
| Specificity | No interference | No interference | ||||
| Linearity | R2 = 0.99 | R2 = 0.99 | R2 = 0.99 | R2 = 0.99 | R2 = 0.98 | |
| Accuracy | 90–116% | 91–128% | 87–109% | 86–109% | 85–114% | |
| Precision | Repeatability | RSD = 2.0% | RSD = 1.8% | RSD = 2.4% | RSD = 2.4% | RSD = 4.5% |
| Intermediate Precision | RSD = 0.1% | RSD = 0.6% | RSD = 1.0% | RSD = 0.5% | RSD = 2.2% | |
| Limit of Quantitation | 0.8% | 0.6% | ||||
| Range | 1.25–15.0 mg/mL | 0.158–15.0 mg/mL | ||||
| Robustness | Complies | |||||
| Sample Name | Time Point | Total LMW (%) | Intact IgG (%) |
|---|---|---|---|
| OR-US | Control | 6.11 ± 0.05 | 93.89 ± 0.04 |
| Day 3 | 7.26 ± 0.02 | 92.73 ± 0.02 | |
| Day 7 | 8.39 ± 0.12 | 91.61 ± 0.13 | |
| Day 14 | 10.72 ± 0.02 | 89.28 ± 0.02 | |
| BS | Control | 5.52 ± 0.05 | 94.48 ± 0.04 |
| Day 3 | 6.02 ± 0.02 | 93.98 ± 0.02 | |
| Day 7 | 7.21 ± 0.12 | 92.79 ± 0.12 | |
| Day 14 | 8.80 ± 0.06 | 91.19 ± 0.05 | |
| OR-EU | Control | 5.34 ± 0.05 | 94.66 ± 0.05 |
| Day 3 | 6.10 ± 0.03 | 93.90 ± 0.02 | |
| Day 7 | 7.56 ± 0.10 | 92.44 ± 0.10 | |
| Day 14 | 8.33 ± 0.04 | 91.66 ± 0.04 |
| Sample Name | Time Point | Total Impurity (%) | L + H (%) |
|---|---|---|---|
| OR-US | Control | 2.95 ± 0.10 | 97.05 ± 0.10 |
| Day 3 | 3.15 ± 0.04 | 96.85 ± 0.04 | |
| Day 7 | 3.36 ± 0.09 | 96.64 ± 0.09 | |
| Day 14 | 4.03 ± 0.13 | 95.97 ± 0.13 | |
| BS | Control | 1.56 ± 0.03 | 98.44 ± 0.03 |
| Day 3 | 1.85 ± 0.03 | 98.15 ± 0.03 | |
| Day 7 | 2.62 ± 0.02 | 97.38 ± 0.02 | |
| Day 14 | 3.20 ± 0.08 | 96.80 ± 0.08 | |
| OR-EU | Control | 2.21 ± 0.08 | 97.79 ± 0.08 |
| Day 3 | 3.12 ± 0.03 | 96.88 ± 0.03 | |
| Day 7 | 3.19 ± 0.03 | 96.81 ± 0.03 | |
| Day 14 | 3.98 ± 0.02 | 96.02 ± 0.02 |
| Sample Name | Time Point | Total LMW (%) | Intact IgG (%) |
|---|---|---|---|
| OR-US | Control | 6.11 ± 0.05 | 93.89 ± 0.04 |
| Day 3 | 9.65 ± 0.03 | 90.35 ± 0.03 | |
| Day 7 | 14.17 ± 0.02 | 85.83 ± 0.02 | |
| Day 14 | 21.61 ± 0.13 | 78.39 ± 0.13 | |
| BS | Control | 5.52 ± 0.05 | 94.48 ± 0.04 |
| Day 3 | 9.40 ± 0.05 | 90.60 ± 0.05 | |
| Day 7 | 13.75 ± 0.11 | 86.25 ± 0.11 | |
| Day 14 | 19.71 ± 0.15 | 80.29 ± 0.15 | |
| OR-EU | Control | 5.34 ± 0.05 | 94.66 ± 0.05 |
| Day 3 | 9.18 ± 0.05 | 90.82 ± 0.05 | |
| Day 7 | 13.61 ± 0.03 | 86.39 ± 0.03 | |
| Day 14 | 20.00 ± 0.02 | 80.00 ± 0.02 |
| Sample Name | Time Point | Total Impurity (%) | L + H (%) |
|---|---|---|---|
| OR-US | Control | 2.95 ± 0.10 | 97.05 ± 0.10 |
| Day 3 | 4.88 ± 0.06 | 95.12 ± 0.06 | |
| Day 7 | 6.41 ± 0.10 | 93.59 ± 0.10 | |
| Day 14 | 8.06 ± 0.05 | 91.94 ± 0.05 | |
| BS | Control | 1.56 ± 0.03 | 98.44 ± 0.03 |
| Day 3 | 2.95 ± 0.06 | 97.05 ± 0.06 | |
| Day 7 | 4.52 ± 0.10 | 95.48 ± 0.10 | |
| Day 14 | 5.56 ± 0.13 | 94.44 ± 0.13 | |
| OR-EU | Control | 2.21 ± 0.08 | 97.79 ± 0.08 |
| Day 3 | 4.03 ± 0.02 | 95.97 ± 0.02 | |
| Day 7 | 5.83 ± 0.08 | 94.17 ± 0.08 | |
| Day 14 | 7.09 ± 0.09 | 92.91 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pamukcu, C.; Atik, A.E. Assessing the Comparability of Degradation Profiles Between Biosimilar and Originator Anti-VEGF Monoclonal Antibodies Under Thermal Stress. Pharmaceuticals 2025, 18, 1267. https://doi.org/10.3390/ph18091267
Pamukcu C, Atik AE. Assessing the Comparability of Degradation Profiles Between Biosimilar and Originator Anti-VEGF Monoclonal Antibodies Under Thermal Stress. Pharmaceuticals. 2025; 18(9):1267. https://doi.org/10.3390/ph18091267
Chicago/Turabian StylePamukcu, Ceren, and Ahmet Emin Atik. 2025. "Assessing the Comparability of Degradation Profiles Between Biosimilar and Originator Anti-VEGF Monoclonal Antibodies Under Thermal Stress" Pharmaceuticals 18, no. 9: 1267. https://doi.org/10.3390/ph18091267
APA StylePamukcu, C., & Atik, A. E. (2025). Assessing the Comparability of Degradation Profiles Between Biosimilar and Originator Anti-VEGF Monoclonal Antibodies Under Thermal Stress. Pharmaceuticals, 18(9), 1267. https://doi.org/10.3390/ph18091267








