Trimetazidine–Profen Hybrid Molecules: Synthesis, Chemical Characterization, and Biological Evaluation of Their Racemates
Abstract
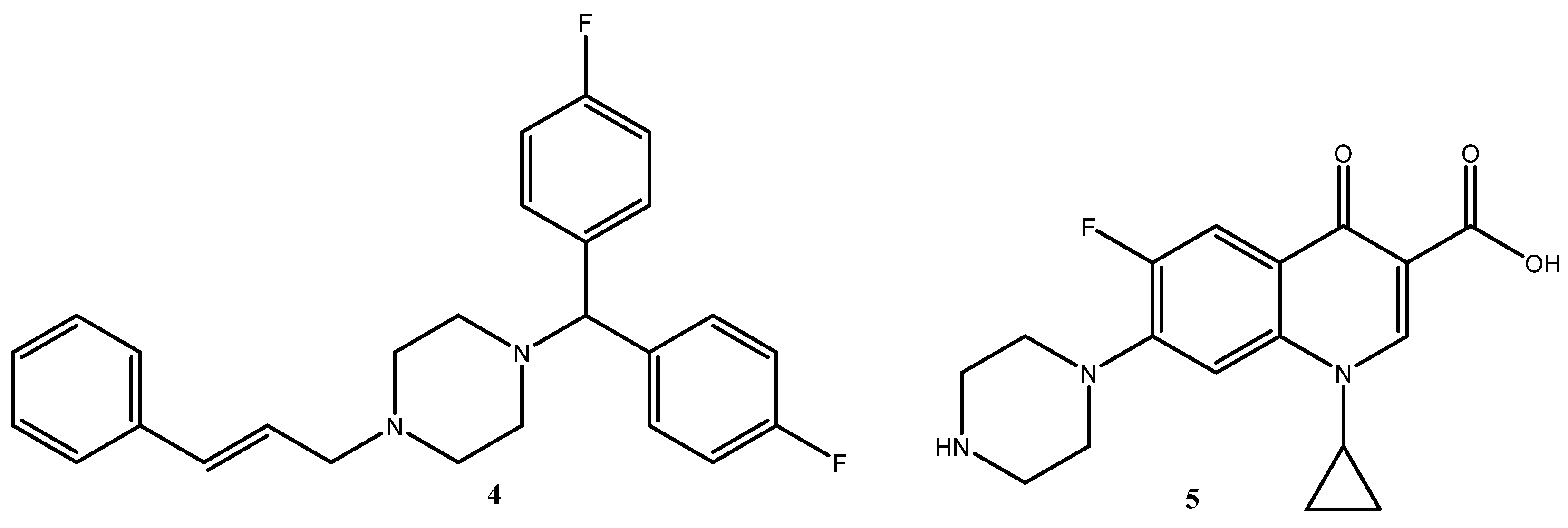
1. Introduction
2. Results and Discussion
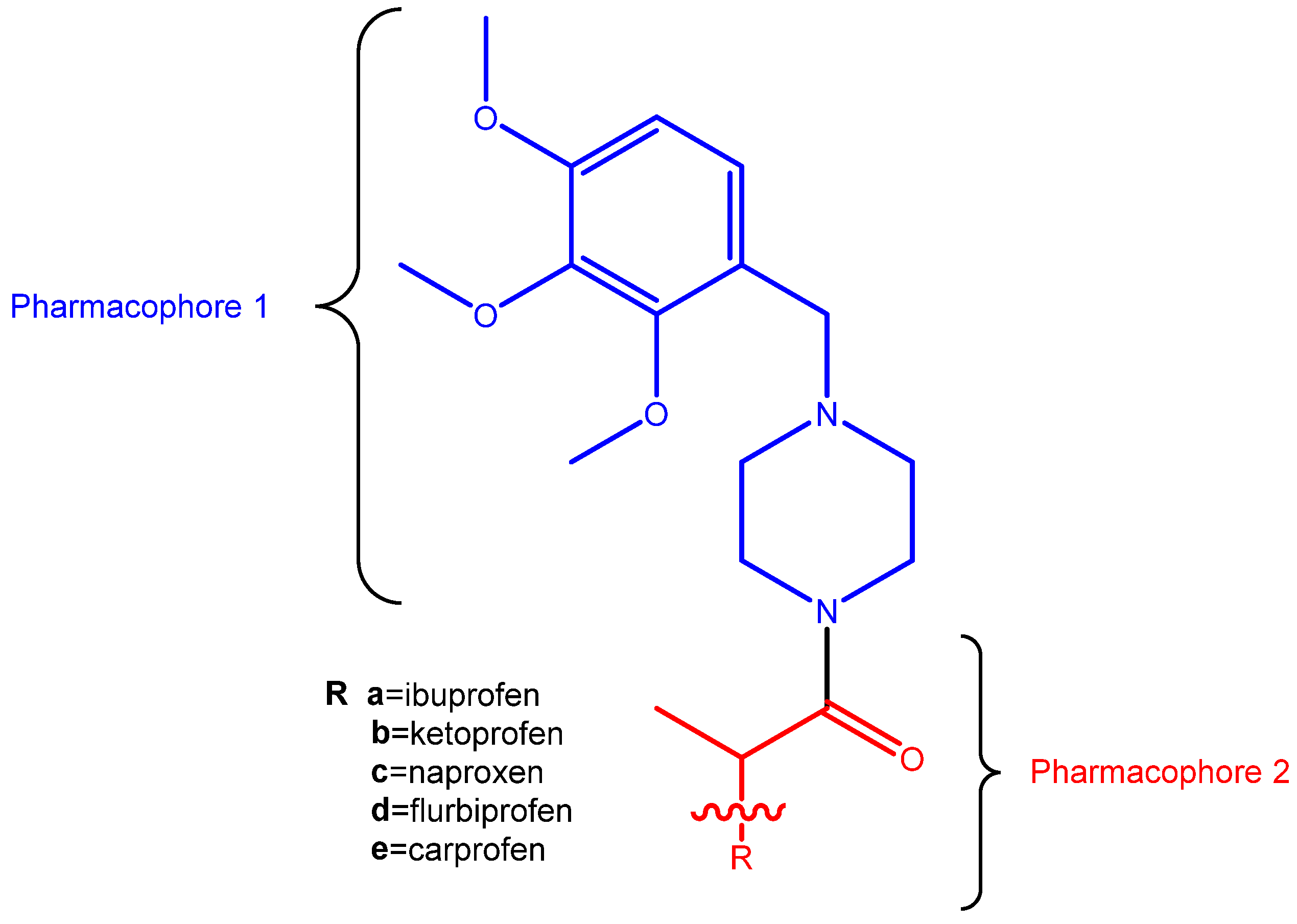
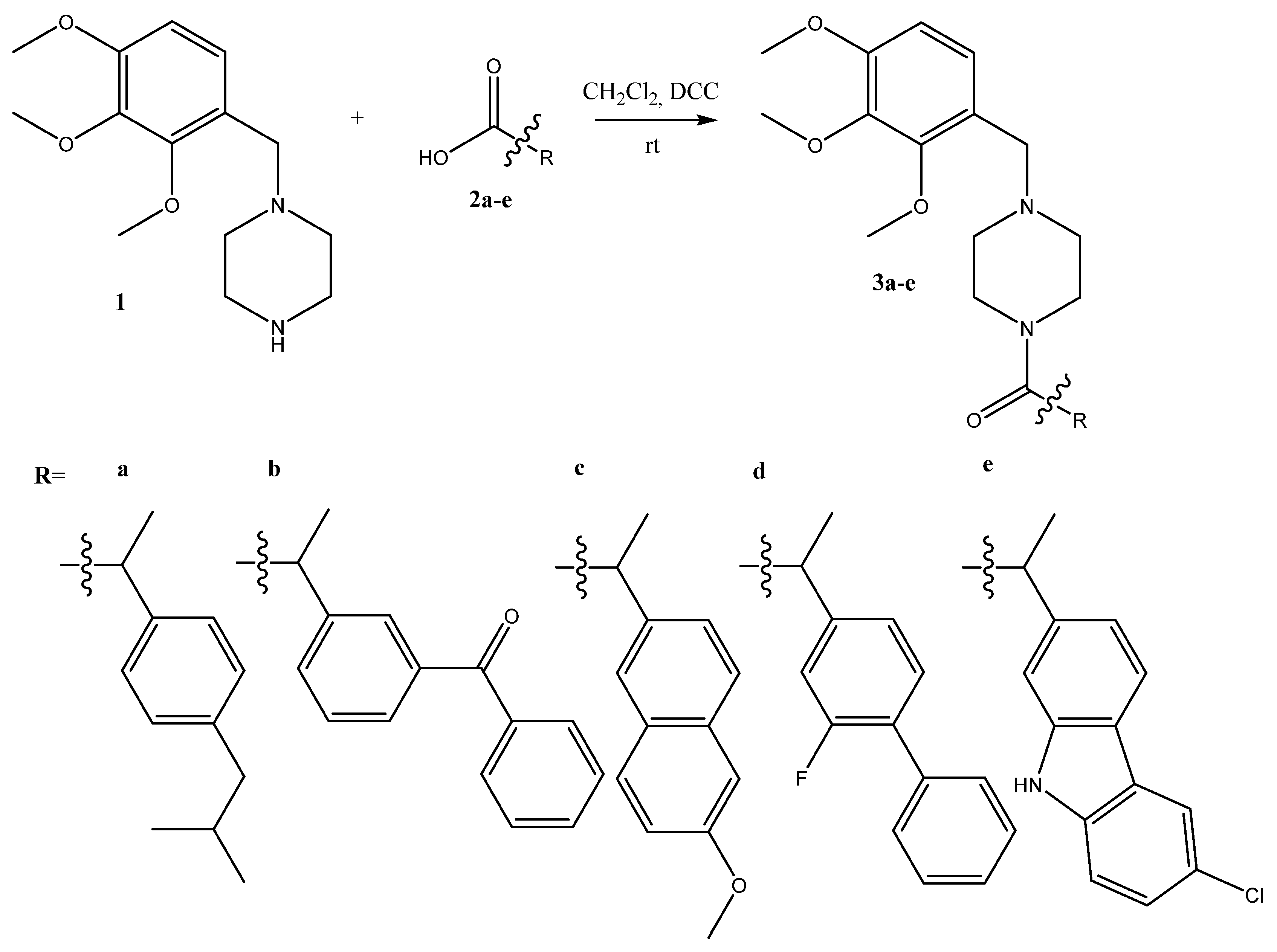
2.1. Synthesis
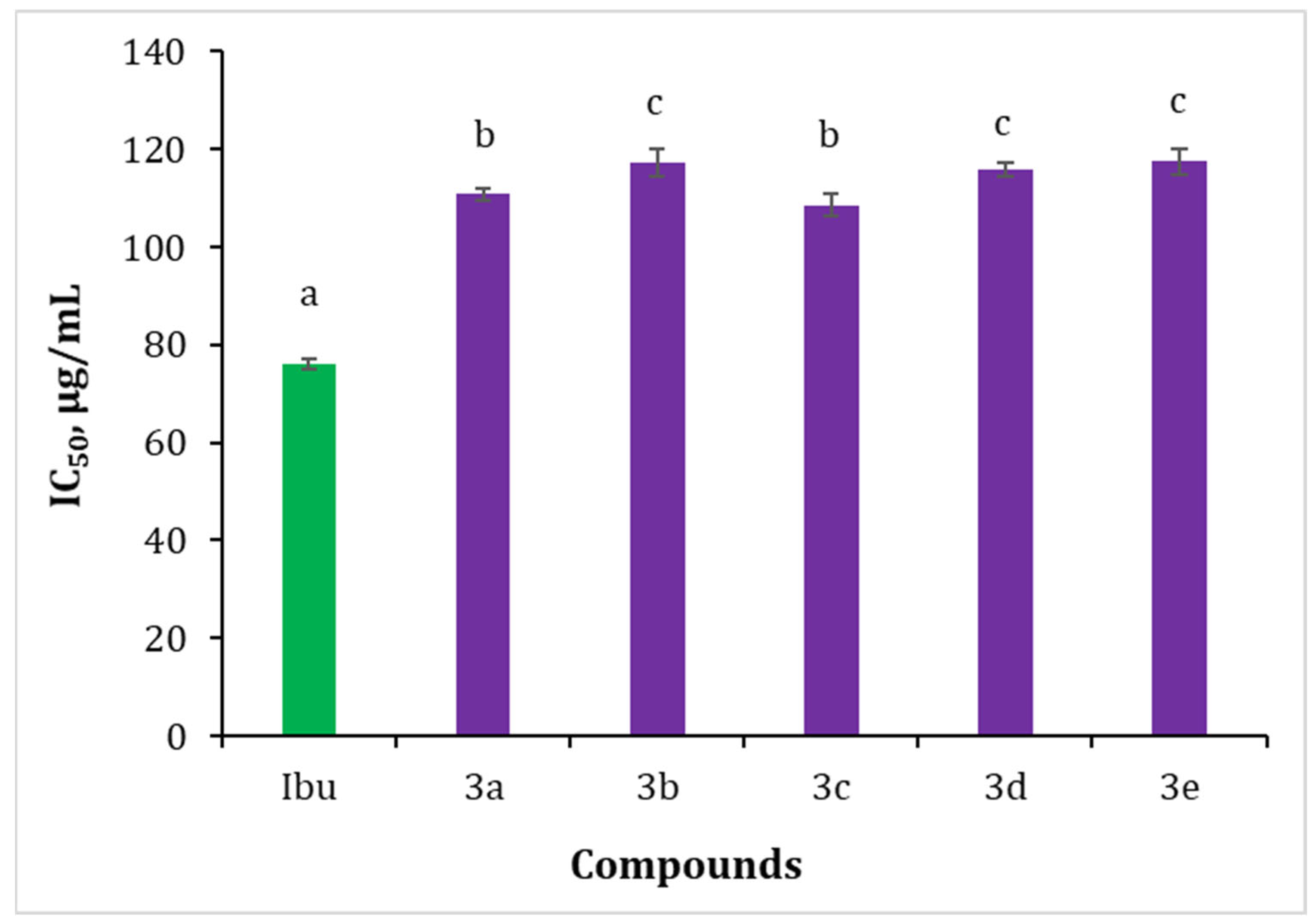
2.2. In Vitro Biological Evaluation
2.2.1. Hydrogen Peroxide Scavenging Activity (HPSA)
2.2.2. Hydroxyl Radical Scavenging Activity (HRSA)
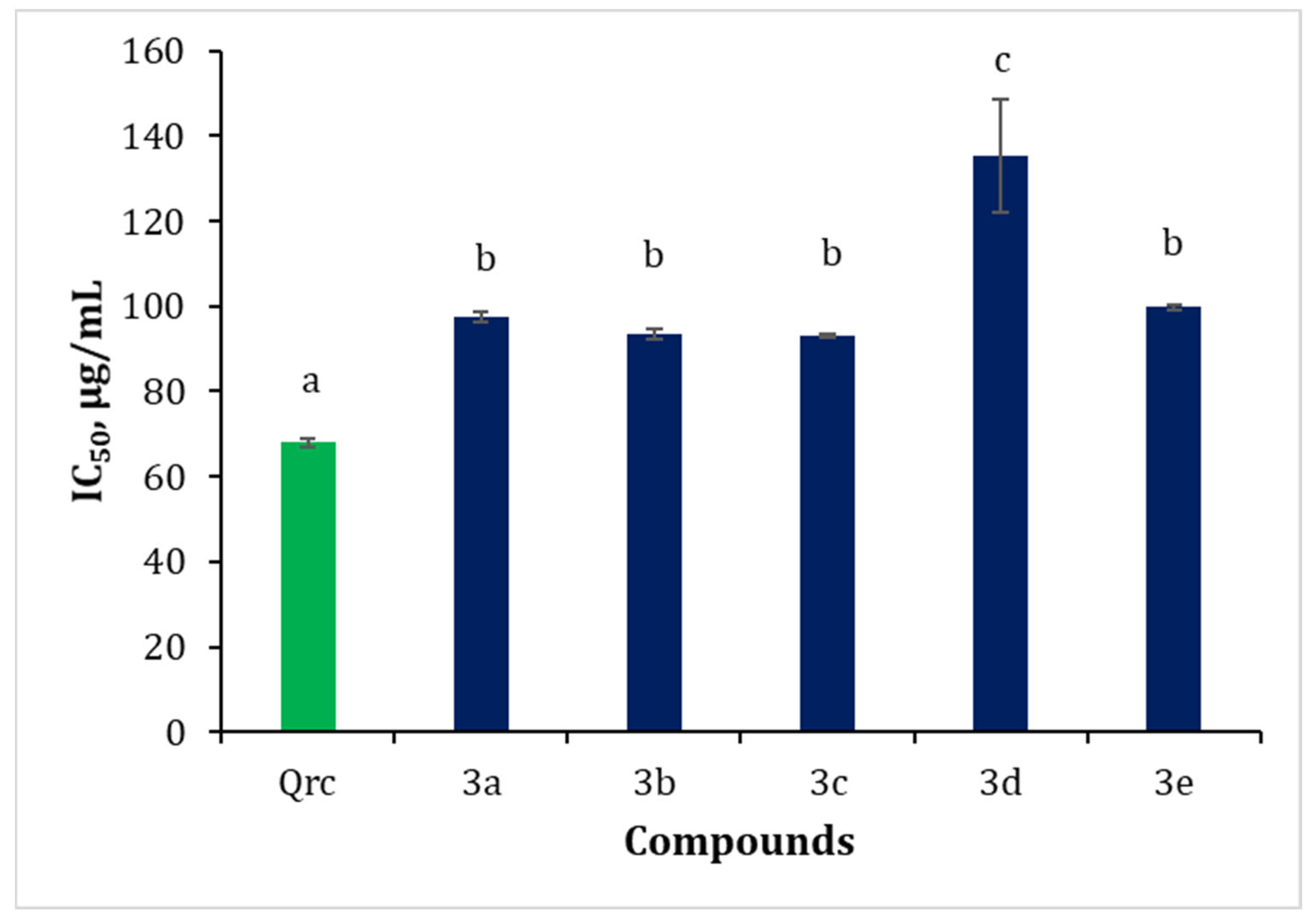
2.2.3. Inhibition of Albumin Denaturation (IAD)
2.2.4. Lipophilicity
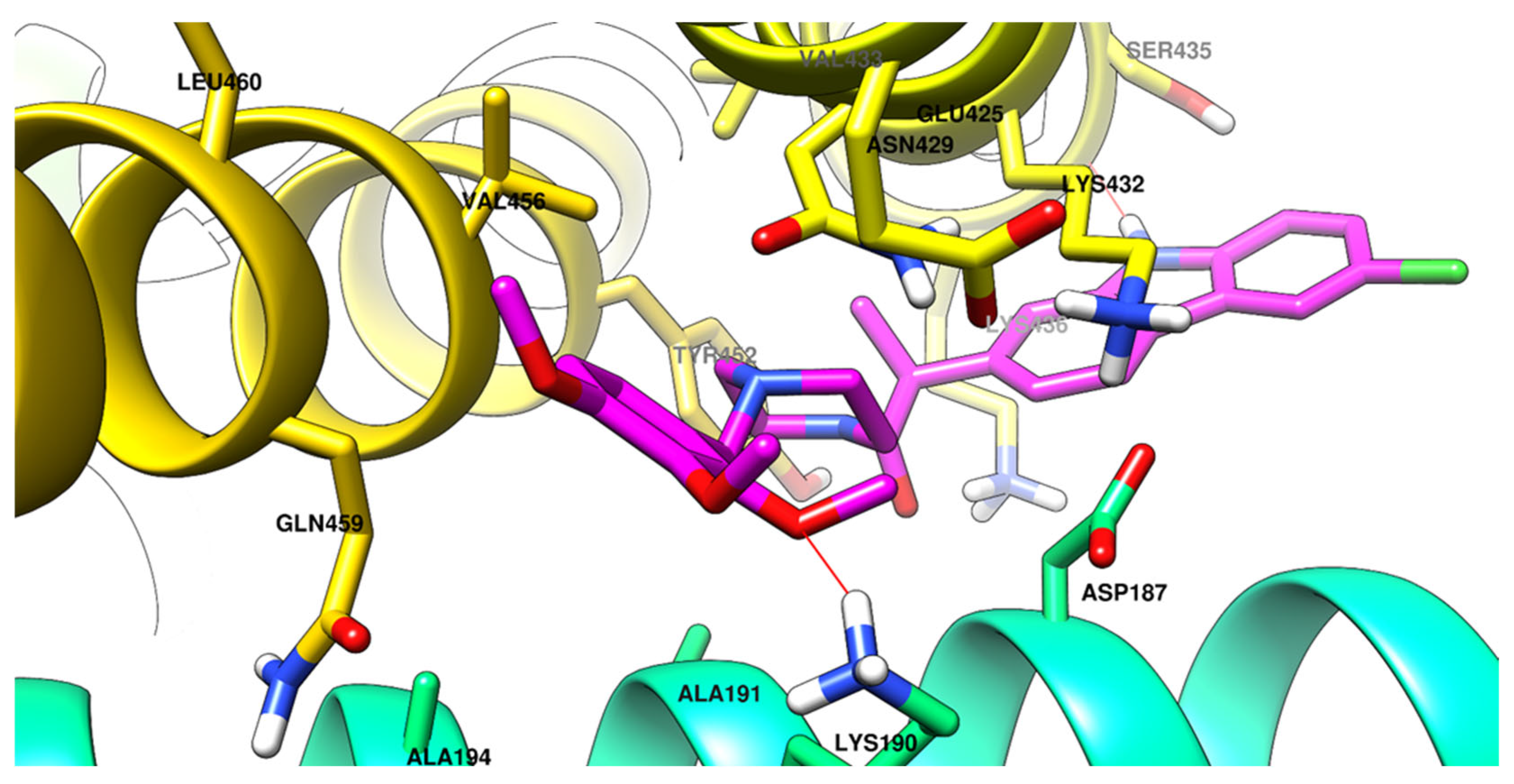
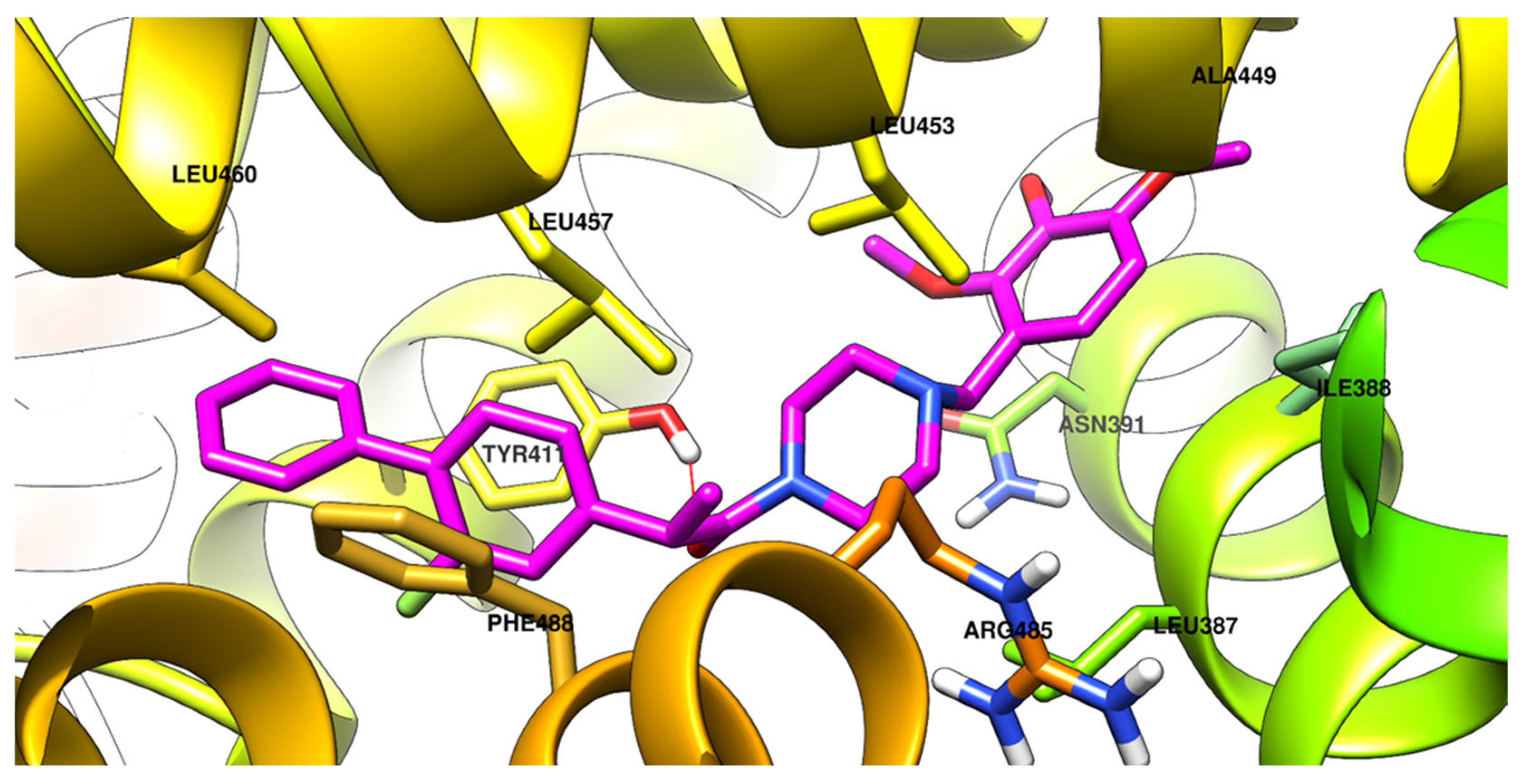
2.2.5. Molecular Docking
2.2.6. Molecular Dynamics
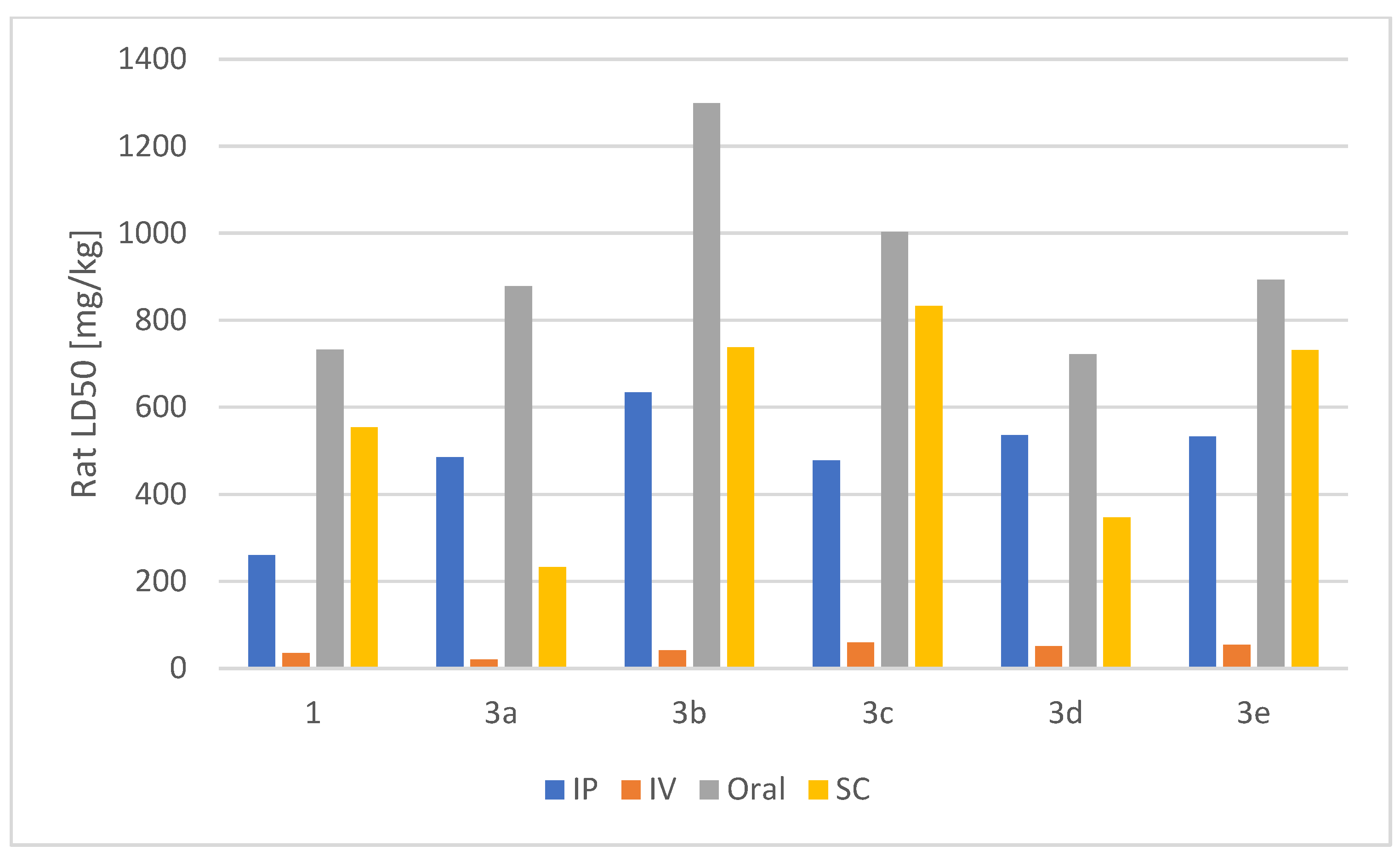
2.2.7. Acute Rat Toxicity Prediction Using GUSAR Software
3. Materials and Methods
3.1. General
3.2. Synthesis
- 3a 2-(4-isobutylphenyl)-1-(4-(2,3,4-trimethoxybenzyl)piperazin-1-yl)propan-1-one
- 3b 2-(3-benzoylphenyl)-1-(4-(2,3,4-trimethoxybenzyl)piperazin-1-yl)propan-1-one
- 3c 2-(6-methoxynaphthalen-2-yl)-1-(4-(2,3,4-trimethoxybenzyl)piperazin-1-yl)propan-1-one
- 3d 2-(2-fluoro-[1,1’-biphenyl]-4-yl)-1-(4-(2,3,4-trimethoxybenzyl)piperazin-1-yl)propan-1-one
- 3e 2-(6-chloro-9H-carbazol-2-yl)-1-(4-(2,3,4-trimethoxybenzyl)piperazin-1-yl)propan-1-one
3.3. Biological Activity
3.3.1. Hydrogen Peroxide Scavenging Activity (HPSA)
3.3.2. Hydroxyl Radical Scavenging Activity (HRSA)
3.3.3. Inhibition of Albumin Denaturation (IAD)
3.3.4. Molecular Docking
3.3.5. Molecular Dynamics Simulations
3.3.6. Determination of Lipophilicity as RM Values
3.3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, N.; Lei, J.; Liu, Q.; Huang, W.; Xiao, H.; Lei, H. The effectiveness of preoperative trimetazidine on myocardial preservation in coronary artery bypass graft patients: A systematic review and meta-analysis. Cardiology 2015, 131, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Khanra, S.; Reddy, P.; Giménez-Palomo, A.; Park, C.; Panizzutti, B.; McCallum, M.; Arumugham, S.; Umesh, S.; Debnath, M.; Das, B.; et al. Metabolic regulation to treat bipolar depression: Mechanisms and targeting by trimetazidine. Mol. Psychiatry 2023, 28, 3231–3242. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Peng, Y.; Hang, W.; Zhou, N.; Wang, D. Trimetazidine in Heart Failure. Front. Pharmacol. 2021, 11, 569132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, W.-Y.; Wang, Z.-H.; Tang, M.-X.; Wang, F.; Li, Y.; Zhong, M.; Zhang, Y.; Zhang, W. Early administration of trimetazidine attenuates diabetic cardiomyopathy in rats by alleviating fibrosis, reducing apoptosis and enhancing autophagy. J. Transl. Med. 2016, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, X.-J.; Shi, D.-Z. Effect of Trimetazidine in Patients Undergoing Percutaneous Coronary Intervention: A Meta-Analysis. PLoS ONE 2015, 10, e0137775. [Google Scholar] [CrossRef]
- Ferrari, R.; Ford, I.; Fox, K.; Challeton, J.; Correges, A.; Tendera, M.; Widimský, P.; Danchin, N. Efficacy and safety of trimetazidine after percutaneous coronary intervention (ATPCI): A randomised, double-blind, placebo-controlled trial. Lancet 2020, 396, 830–838. [Google Scholar] [CrossRef]
- Zou, H.; Zhu, X.-X.; Ding, Y.-H.; Jin, Q.-Y.; Qian, L.-Y.; Huang, D.-S.; Cen, X.-J. Trimetazidine in conditions other than coronary disease, old drug, new tricks? Int. J. Cardiol. 2017, 234, 1–6. [Google Scholar] [CrossRef]
- Coats, C.; Pavlou, M.; Watkinson, O.; Protonotarios, A.; Moss, L.; Hyland, R.; Rantell, K.; Pantazis, A.; Tome, M.; McKenna, W.; et al. Effect of Trimetazidine Dihydrochloride Therapy on Exercise Capacity in Patients With Nonobstructive Hypertrophic Cardiomyopathy: A Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 230–235. [Google Scholar] [CrossRef]
- Kamisah, Y.; Che Hassan, H.H. Role of Trimetazidine in Ameliorating Endothelial Dysfunction: A Review. Pharmaceuticals 2024, 17, 464. [Google Scholar] [CrossRef]
- Zheng, S.; Du, Y.; Peng, Q.; Fan, X.; Li, J.; Chen, M. Trimetazidine Protects Against Atherosclerosis by Changing Energy Charge and Oxidative Stress. Med. Sci. Monit. 2018, 23, 8459–8468. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, L.; Luo, Y.; Li, S.; Zheng, Z.; Dong, R.; Zhu, J.; Liu, J. Trimetazidine improves exercise tolerance in patients with ischemic heart disease: A meta-analysis. Herz 2016, 41, 514–522. [Google Scholar] [CrossRef]
- Tsioufis, K.; Andrikopoulos, G.; Manolis, A. Trimetazidine and Cardioprotection: Facts and Perspectives. Angiology 2014, 66, 204–210. [Google Scholar] [CrossRef]
- Mahmoud, N.; Elbaset, M.; Mohawed, O.; Ahmed, H. Trimetazidine attenuates neuroinflammation via TLR4/NF-KB pathway inhibition in lipopolysaccharides-induced dementia in rats. J. Appl. Pharm. Sci. 2024, 14, 125–142. [Google Scholar] [CrossRef]
- Guarini, G.; Huqi, A.; Morrone, D.; Capozza, P.; Marzilli, M. Trimetazidine and Other Metabolic Modifiers. Eur. Cardiol. Rev. 2018, 13, 104–111. [Google Scholar] [CrossRef]
- Marzilli, M.; Vinereanu, D.; Lopaschuk, G.; Chen, Y.; Dalal, J.; Danchin, N.; Etriby, E.; Ferrari, R.; Gowdak, L.; Lopatin, Y.; et al. Trimetazidine in cardiovascular medicine. Int. J. Cardiol. 2019, 293, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Bovenkamp, A.; Geurkink, K.; Oosterveer, F.; de Man, F.; Kok, W.; Bronzwaer, P.; Allaart, C.; Nederveen, A.; Rossum, A.; Bakermans, A.; et al. Trimetazidine in heart failure with preserved ejection fraction: A randomized controlled cross-over trial. ESC Heart Fail 2023, 10, 2998–3010. [Google Scholar] [CrossRef]
- Kallistratos, M.; Poulimenos, L.; Giannitsi, S.; Tsinivizov, P.; Manolis, A. Trimetazidine in the Prevention of Tissue Ischemic Conditions. Angiology 2019, 70, 291–298. [Google Scholar] [CrossRef]
- Pintér, D.; Kovács, M.; Harmat, M.; Juhász, A.; Janszky, J.; Kovács, N. Trimetazidine and parkinsonism: A prospective study. Park. Relat. Disord. 2019, 62, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Dy, A.; Limjoco, L.; Jamora, R. Trimetazidine-Induced Parkinsonism: A Systematic Review. Front. Neurol. 2020, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Noreen, S.; Asim, S.; Liaqat, Z.; Shaheen, M.; Ibrahim, H. A comprehensive review on the synthesis of substituted piperazine and its novel bio-medicinal applications. Chem. Inorg. Mater. 2024, 2, 100041. [Google Scholar] [CrossRef]
- Kutala, V.; Khan, M.; Mandal, R.; Ganesan, L.; Tridandapani, S.; Kalai, T.; Hideg, K.; Kuppusamy, P. Attenuation of Myocardial Ischemia-Reperfusion Injury by Trimetazidine Derivatives Functionalized with Antioxidant Properties. J. Pharmacol. Exp. Ther. 2006, 317, 921–928. [Google Scholar] [CrossRef]
- Eyilcim, Ö.; Günay, F.; Ng, Y.; Açan, Ö.; Turgut, Z.; Günkara, Ö. Design, Synthesis, Biological Evaluation and Molecular Docking Studies of a New Series of Maleimide Derivatives. ChemistryOpen 2024, 13, e2020400058. [Google Scholar] [CrossRef]
- Haley, R.; Recum, H. Localized and targeted delivery of NSAIDs for treatment of inflammation: A review. Exp. Biol. Med. 2019, 244, 433–444. [Google Scholar] [CrossRef]
- Al-Otaibi, N. Characterization of non-steroidal anti-inflammatory drugs in tablets by direct analysis in real time/time of flight/mass spectrometry (dart/tof/ms) without sample preparation. Int. J. Res. 2017, 5, 191–199. [Google Scholar] [CrossRef]
- Bhushan, I.; Saraswat, R.; Gupta, P.; Shah, B. Enantioselective resolution of 2-arylpropionic acid derivatives employing immobilization of lipase from Bacillus subtilis strain Kakrayal_1 (BSK-L). Bioresour. Technol. 2018, 269, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Siódmiak, J.; Siódmiak, T.; Tarczykowska, A.; Czirson, K.; Dulęba, J.; Marszall, M. Metabolic chiral inversion of 2-arylpropionic acid derivatives (profens). Med. Res. J. 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; Shi, Q.; Li, H.; Guo, J.; Ouyang, J.; Jia, X.; Zhang, L.; You, S.; Qin, B. Engineering of Saccharomyces pastorianus old yellow enzyme 1 for the synthesis of pharmacologically active (S)-profen derivatives. Mol. Catal. 2021, 507, 111568. [Google Scholar] [CrossRef]
- Lushchak, V. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Salla, S.; Sunkara, R.; Ogutu, S.; Walker, L.; Verghese, M. Antioxidant activity of papaya seed extracts against H2O2 induced oxidative stress in HepG2 cells. LWT-Food Sci. Technol. 2016, 66, 293–297. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Kadouh, H.; Zhou, K. Phenolic compounds and antioxidant properties of gooseberry as affected by in vitro digestion. LWT-Food Sci. Technol. 2013, 51, 417–422. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Wang, Y.-M.; Zhang, B.; Deng, S.-G. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutterdge, J. Free Radicals in Biology and Medicine, 5th ed.; Clarendon Press: Oxford, UK, 2015; p. 346. [Google Scholar] [CrossRef]
- Bektaşoğlu, B.; Esin Çelik, S.; Özyürek, M.; Güçlü, K.; Apak, R. Novel hydroxyl radical scavenging antioxidant activity assay for water-soluble antioxidants using a modified CUPRAC method. Biochem. Biophys. Res. Commun. 2006, 345, 1194–1200. [Google Scholar] [CrossRef]
- Bohnert, T.; Prakash, C. ADME Profiling in Drug Discovery and Development: An Overview; Encyclopedia of Drug Metabolism and Interactions; Part III. General Principles of ADME. Drug Transporters and Other Mechanisms of Transport; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Dwivedi, P.; Tekade, R.; Jain, N. Nanoparticulate carrier mediated intranasal delivery of insulin for the restoration of memory signaling in alzheimer’s disease. Curr. Nanosci. 2013, 9, 46–55. [Google Scholar] [CrossRef]
- Bergström, C.A. Computational models to predict aqueous drug solubility, permeability and intestinal absorption. Expert Opin. Drug Metab. Tox. 2005, 1, 613–627. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D. Synthesis and pharmacochemical evaluation of novel aryl-acetic acid inhibitors of lipoxygenase, antioxidants, and anti-inflammatory agents. Bioorg. Med. Chem. 2007, 15, 5819–5827. [Google Scholar] [CrossRef]
- Koch, G.; Engstrom, A.; Taechalertpaisarn, J.; Faris, J.; Ono, S.; Naylor, M.; Lokey, S. Chromatographic Determination of Permeability-Relevant Lipophilicity Facilitates Rapid Analysis of Macrocyclic Peptide Scaffolds. J. Med. Chem. 2024, 67, 19612–19622. [Google Scholar] [CrossRef]
- Barseem, A.; Belal, F.; Mabrouk, M.; Hammad, S.; Ahmed, H. Probing the potential toxicity of trimetazidine by characterizing its interaction with human serum albumin. Methods Appl. Fluoresc. 2023, 11, 045003. [Google Scholar] [CrossRef]
- Chrusciel, P.; Rysz, J.; Banach, M. Defining the Role of Trimetazidine in the Treatment of Cardiovascular Disorders: Some Insights on Its Role in Heart Failure and Peripheral Artery Disease. Drugs 2014, 74, 971–980. [Google Scholar] [CrossRef]
- Onay-Besikci, A.; Ozkan, S. Trimetazidine revisited: A comprehensive review of the pharmacological effects and analytical techniques for the determination of trimetazidine. Cardiovasc. Ther. 2008, 26, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Sohail, R.; Mathew, M.; Patel, K.; Reddy, S.; Haider, Z.; Naria, M.; Habib, A.; Abdin, Z.; Chaudhry, W.; Akbar, A. Effects of Non-steroidal Anti-inflammatory Drugs (NSAIDs) and Gastroprotective NSAIDs on the Gastrointestinal Tract: A Narrative Review. Cureus 2023, 15, e37080. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S. Nonsteroidal Anti-Inflammatory Drugs in Animals. Merck Manual Veterinary Manual. Available online: https://www.merckvetmanual.com/pharmacology/inflammation/nonsteroidal-anti-inflammatory-drugs-in-animals (accessed on 13 August 2025).
- Buseman, M.; Blong, A.; Walton, R. Successful management of severe carprofen toxicity with manual therapeutic plasma exchange in a dog. J. Vet. Emerg. Crit. Care 2022, 32, 675–679. [Google Scholar] [CrossRef]
- Lagunin, A.; Zakharov, A.; Filimonov, D.; Poroikov, V. QSAR modelling of rat acute toxicity on the basis of PASS prediction. Mol. Inform. 2011, 30, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Manolov, S.; Ivanov, I.; Bojilov, D. Synthesis of New 1,2,3,4-Tetrahydroquinoline Hybrid of Ibuprofen and Its Biological Evaluation. Molbank 2022, 2022, M1350. [Google Scholar] [CrossRef]
- Luo, H.Y.; Wang, B.; Yu, C.G.; Qu, Y.L.; Su, C.L. Evaluation of antioxidant activities of five selected brown seaweeds from China. J. Med. Plant Res. 2010, 4, 2557–2565. [Google Scholar] [CrossRef]
- Manolov, S.; Ivanov, I.; Bojilov, D. Microwave-assisted synthesis of 1,2,3,4-tetrahydroisoquinoline sulfonamide derivatives and their biological evaluation. J. Serb. Chem. Soc. 2021, 86, 139–151. [Google Scholar] [CrossRef]
- Dimitrova, D.; Manolov, S.; Bojilov, D.; Ivanov, I.; Nedialkov, P. (±)-R,S-2-Chloro-N-(4-methyl-2-oxo-2H-chromen-7-yl)-2-phenylacetamide. Molbank 2024, 2024, M1830. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J Comput Chem 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Makeneni, S.; Thieker, D.F.; Woods, R.J. Applying Pose Clustering and MD Simulations To Eliminate False Positives in Molecular Docking. J. Chem. Inf. Model. 2018, 58, 605–614. [Google Scholar] [CrossRef]
- Manolov, S.; Bojilov, D.; Ivanov, I.; Marc, G.; Bataklieva, N.; Oniga, S.; Oniga, O.; Nedialkov, P. Synthesis, Molecular Docking, Molecular Dynamics Studies, and In Vitro Biological Evaluation of New Biofunctional Ketoprofen Derivatives with Different N-Containing Heterocycles. Processes 2023, 11, 1837. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Borlan, R.; Stoia, D.; Gaina, L.; Campu, A.; Marc, G.; Perde-Schrepler, M.; Silion, M.; Maniu, D.; Focsan, M.; Astilean, S. Fluorescent Phthalocyanine-Encapsulated Bovine Serum Albumin Nanoparticles: Their Deployment as Therapeutic Agents in the NIR Region. Molecules 2021, 26, 4679. [Google Scholar] [CrossRef] [PubMed]
- Ascoli, G.A.; Domenici, E.; Bertucci, C. Drug binding to human serum albumin: Abridged review of results obtained with high-performance liquid chromatography and circular dichroism. Chirality 2006, 18, 667–679. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Liang, H. Interactive Association of Drugs Binding to Human Serum Albumin. Int. J. Mol. Sci. 2014, 15, 3580–3595. [Google Scholar] [CrossRef]
- Nishi, K.; Yamasaki, K.; Otagiri, M. Serum Albumin, Lipid and Drug Binding. Subcell. Biochem. 2020, 94, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Czub, M.; Stewart, A.; Shabalin, I.; Minor, W. Organism-specific differences in the binding of ketoprofen to serum albumin. IUCrJ 2022, 9, 551–561. [Google Scholar] [CrossRef]
- Zsila, F. Subdomain IB Is the Third Major Drug Binding Region of Human Serum Albumin: Toward the Three-Sites Model. Mol. Pharm. 2013, 10, 1668–1682. [Google Scholar] [CrossRef]
- Mishra, V.; Heath, R.J. Structural and Biochemical Features of Human Serum Albumin Essential for Eukaryotic Cell Culture. Int. J. Mol. Sci. 2021, 22, 8411. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, H.S.; Niu, X.D. Molecular dynamics simulations reveal insight into key structural elements of aaptamines as sortase inhibitors with free energy calculations. Chem. Phys. Lett. 2013, 585, 171–177. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, Z.; Wang, D.; Guan, S.; Han, W. Molecular Dynamics Simulations of Acylpeptide Hydrolase Bound to Chlorpyrifosmethyl Oxon and Dichlorvos. Int. J. Mol. Sci. 2015, 16, 6217–6234. [Google Scholar] [CrossRef] [PubMed]
- Crișan, O.; Marc, G.; Nastasă, C.; Oniga, S.D.; Vlase, L.; Pîrnău, A.; Oniga, O. Synthesis And In Silico Approaches Of New Symmetric Bis-Thiazolidine-2,4-Diones As Ras And Raf Oncoproteins Inhibitors. Farmacia 2023, 71, 254–263. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]







| Compound | Enantiomer | Cleft | Sudlow I | Sudlow II | Site III |
|---|---|---|---|---|---|
| 3a | R | −9.2 | −8.2 | −10.1 | −10.4 |
| S | −9.7 | −8.2 | −10.2 | −10.1 | |
| 3b | R | −10.0 | −9.4 | −11.0 | −12.0 |
| S | −10.5 | −9.1 | −11.4 | −11.8 | |
| 3c | R | −9.8 | −8.5 | −10.4 | −11.2 |
| S | −10.3 | −8.5 | −11.2 | −11.5 | |
| 3d | R | −9.9 | −8.7 | −11.1 | −10.4 |
| S | −10.5 | −8.7 | −11.5 | −11.0 | |
| 3e | R | −10.2 | −8.9 | −10.5 | −11.2 |
| S | −10.7 | −8.0 | −10.8 | −10.3 |
| Compound | Enantiomer | Cleft | Sudlow I | Sudlow II | Site III | ||||
|---|---|---|---|---|---|---|---|---|---|
| ΔG | %C | ΔG | %C | ΔG | %C | ΔG | %C | ||
| 3a | R | −8.56 | 26 | −8.59 | 2 | −10.45 | 38 | −11.70 | 48 |
| S | −9.17 | 36 | −7.56 | 4 | −10.04 | 24 | −10.98 | 24 | |
| 3b | R | −9.26 | 16 | −9.33 | 6 | −11.41 | 28 | −12.53 | 22 |
| S | −10.22 | 28 | −8.48 | 6 | −11.39 | 10 | −12.03 | 24 | |
| 3c | R | −9.42 | 14 | −7.63 | 6 | −11.46 | 44 | −11.38 | 44 |
| S | −9.68 | 32 | −8.44 | 8 | −11.44 | 36 | −11.28 | 54 | |
| 3d | R | −9.05 | 20 | −7.90 | 4 | −11.67 | 48 | −11.41 | 32 |
| S | −9.73 | 32 | −8.52 | 4 | −11.80 | 26 | −11.33 | 36 | |
| 3e | R | −10.00 | 26 | −8.41 | 2 | −11.96 | 20 | −12.08 | 44 |
| S | −9.72 | 24 | −8.86 | 8 | −11.86 | 10 | −12.21 | 16 | |
| System Evaluated | Cleft | Sudlow I | Sudlow II | Site III |
|---|---|---|---|---|
| apo + 3a[R] | - | - | - | 0.49 |
| apo + 3a[S] | - | - | 0.29 | - |
| apo + 3b[R] | - | - | - | 0.44 |
| apo + 3b[S] | - | - | - | 0.32 |
| apo + 3c[R] | - | - | 0.28 b | 0.76 a |
| apo + 3c[S] | - | - | 0.32 b | 0.92 a |
| apo + 3d[R] | - | - | 0.26 | - |
| apo + 3d[S] | - | - | 0.23 | - |
| apo + 3e[R] | - | - | 0.26 b | 0.92 a |
| apo + 3e[S] | - | - | 0.33 | - |
| System Evaluated | Cleft | Sudlow I | Sudlow II | Site III |
|---|---|---|---|---|
| apo + 3a[R] | - | - | - | 0.35 |
| apo + 3a[S] | - | - | 0.42 | - |
| apo + 3b[R] | - | - | - | 0.19 |
| apo + 3b[S] | - | - | - | 0.19 |
| apo + 3c[R] | - | - | 0.17 | unstable complex |
| apo + 3c[S] | - | - | 0.22 | unstable complex |
| apo + 3d[R] | - | - | 0.29 | - |
| apo + 3d[S] | - | - | 0.22 | - |
| apo + 3e[R] | - | - | 0.19 | unstable complex |
| apo + 3e[S] | - | - | 0.19 | - |
| apo | 0.38 | |||
| System Evaluated | Cleft | Sudlow I | Sudlow II | Site III |
|---|---|---|---|---|
| apo + 3a[R] | - | - | - | 2.74 |
| apo + 3a[S] | - | - | 2.73 | - |
| apo + 3b[R] | - | - | - | 2.81 |
| apo + 3b[S] | - | - | - | 2.77 |
| apo + 3c[R] | - | - | 2.72 | unstable complex |
| apo + 3c[S] | - | - | 2.77 | unstable complex |
| apo + 3d[R] | - | - | 2.79 | - |
| apo + 3d[S] | - | - | 2.75 | - |
| apo + 3e[R] | - | - | 2.76 | unstable complex |
| apo + 3e[S] | - | - | 2.76 | - |
| apo | 2.78 | |||
| System Evaluated | Cleft | Sudlow I | Sudlow II | Site III |
|---|---|---|---|---|
| apo + 3a[R] | - | - | - | 0.91 |
| apo + 3a[S] | - | - | 0.66 | - |
| apo + 3b[R] | - | - | - | 2.27 |
| apo + 3b[S] | - | - | - | 0.08 |
| apo + 3c[R] | - | - | 0.29 | unstable complex |
| apo + 3c[S] | - | - | 0.37 | unstable complex |
| apo + 3d[R] | - | - | 0.00 | - |
| apo + 3d[S] | - | - | 0.61 | - |
| apo + 3e[R] | - | - | 0.00 | unstable complex |
| apo + 3e[S] | - | - | 0.98 | - |
| Compound | Rat LD50 [mg/kg] | |||
|---|---|---|---|---|
| IP | IV | Oral | SC | |
| 1 | 259.84a | 35.053a | 732.44a | 553.64a |
| 3a | 485.14a | 20.353a | 878.24a | 232.34b |
| 3b | 634.45a | 41.534a | 12994a | 738.14a |
| 3c | 477.64a | 58.814a | 10034a | 833.24a |
| 3d | 535.65a | 50.744a | 7224a | 346.84a |
| 3e | 532.75b | 53.574a | 892.84a | 7314a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrova, D.; Manolov, S.; Ivanov, I.; Bojilov, D.; Dimova, N.; Marc, G.; Oniga, S.; Oniga, O. Trimetazidine–Profen Hybrid Molecules: Synthesis, Chemical Characterization, and Biological Evaluation of Their Racemates. Pharmaceuticals 2025, 18, 1251. https://doi.org/10.3390/ph18091251
Dimitrova D, Manolov S, Ivanov I, Bojilov D, Dimova N, Marc G, Oniga S, Oniga O. Trimetazidine–Profen Hybrid Molecules: Synthesis, Chemical Characterization, and Biological Evaluation of Their Racemates. Pharmaceuticals. 2025; 18(9):1251. https://doi.org/10.3390/ph18091251
Chicago/Turabian StyleDimitrova, Diyana, Stanimir Manolov, Iliyan Ivanov, Dimitar Bojilov, Nikol Dimova, Gabriel Marc, Smaranda Oniga, and Ovidiu Oniga. 2025. "Trimetazidine–Profen Hybrid Molecules: Synthesis, Chemical Characterization, and Biological Evaluation of Their Racemates" Pharmaceuticals 18, no. 9: 1251. https://doi.org/10.3390/ph18091251
APA StyleDimitrova, D., Manolov, S., Ivanov, I., Bojilov, D., Dimova, N., Marc, G., Oniga, S., & Oniga, O. (2025). Trimetazidine–Profen Hybrid Molecules: Synthesis, Chemical Characterization, and Biological Evaluation of Their Racemates. Pharmaceuticals, 18(9), 1251. https://doi.org/10.3390/ph18091251










