Physiological Conditions, Bioactive Ingredients, and Drugs Stimulating Non-Shivering Thermogenesis as a Promising Treatment Against Diabesity
Abstract
1. Introduction
2. Methods
3. White and Thermogenic Adipose Tissues
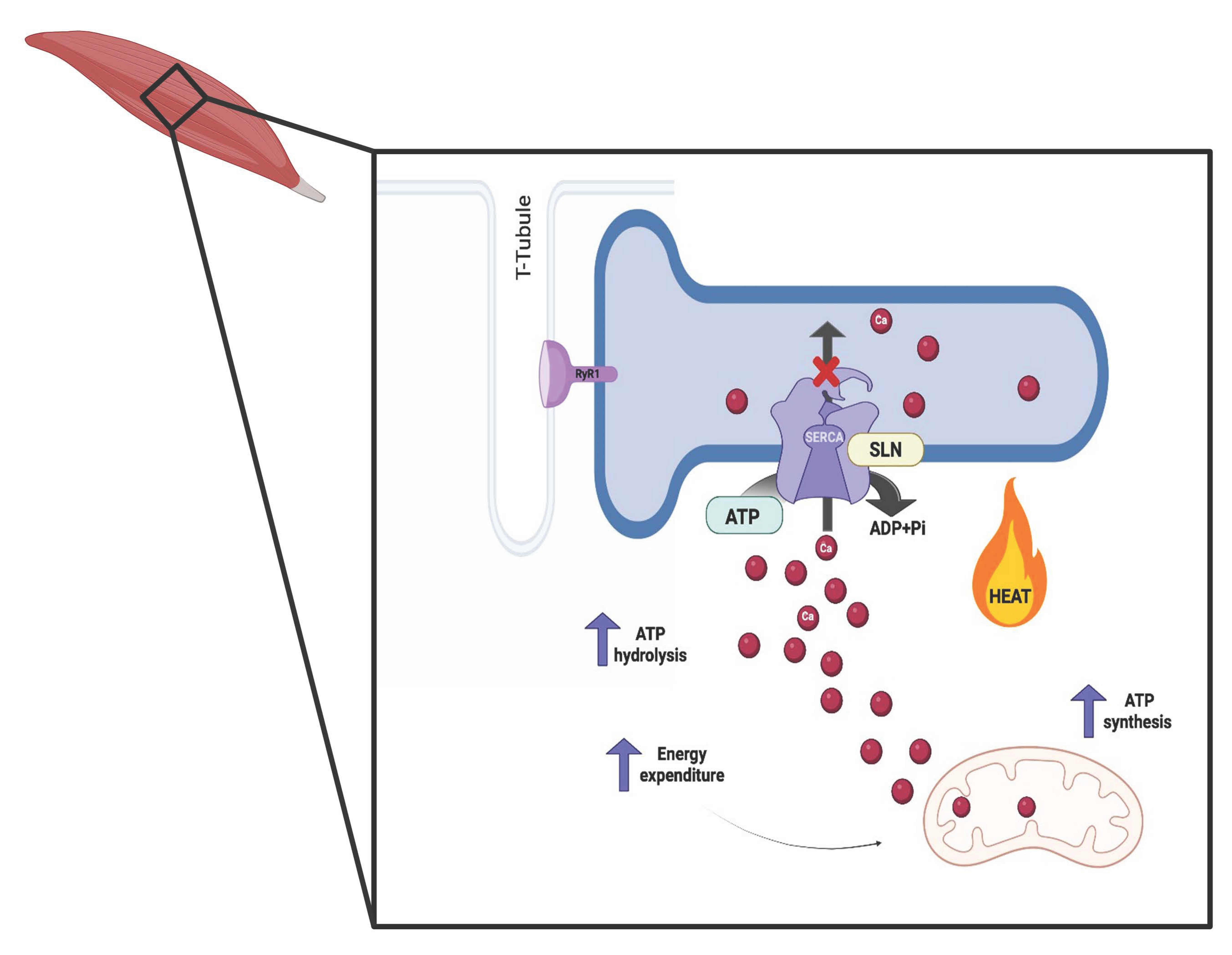
4. Skeletal Muscle and NST
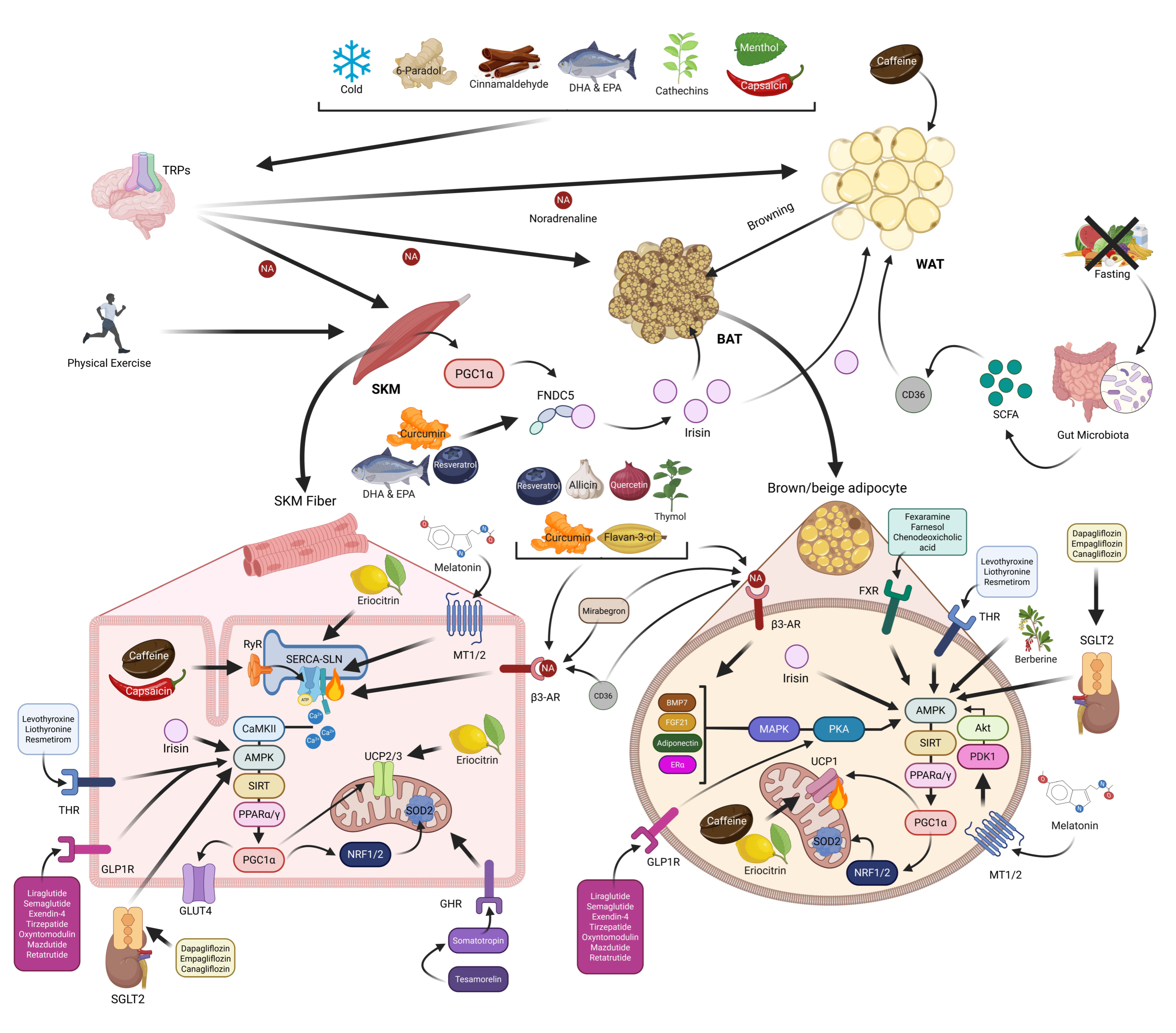
5. Physiological Conditions Stimulating NST
5.1. Cold Exposure
5.2. Physical Exercise
5.3. Fasting
6. Bioactive Ingredients and Their Role in Promoting NST
7. Drugs Stimulating NST
7.1. FDA-Approved Drugs
7.2. Melatonin
| Stimuli | Anti-Diabesity Effect | Mechanism | Molecular Pathway | References | |
|---|---|---|---|---|---|
| Physiological conditions | Cold exposure | ↑ Energy expenditure ↑ Insulin sensitivity | ↑ BAT activity and recruitment ↑ BAT & WAT UCP1 expression ↑ WAT browning & angiogenesiss ↑ SKM SLN expression ↓ SKM SERCA activity ↑ SKM mitochondrial function | TRPs/β3-ARs/PPARγ/PGC1α | [14,15,17,20] |
| Physical exercise | ↓ Insulin resistance ↑ Glucose sensitivity ↑ Lipid metabolism ↓ Fat mass | ↑ BAT activity and recruitment ↑ WAT browning & mitochondrial function ↑ WAT UCP1 expression ↓ vWAT adiposity ↑ SKM UCP3, SERCA1 & SLN expression ↑ SKM mitochondrial biogenesis and function | PGC1α/FNDC5/Irisin | [26,28,29,30] | |
| Fasting | ↓ Body weight ↑ Energy expenditure | ↑ BAT activation ↑ BAT & SKM energy expenditure ↑ WAT browning ↑ BAT & WAT UCP1 expression | SCFA (Gut microbiota)/CD36/ β3-ARs/SIRT6/PGC1α | [31,33,34,35,37,44] | |
| Bioactive ingredients | Capsaicin & Capsinoids 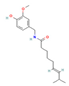 (Pepper) | ↓ Weight gain ↑ Energy expenditure ↓ Fat accumulation Improved glucose levels | ↑ BAT activity and recruitment ↑ WAT browning ↑ BAT & WAT UCP1 expression ↑ SKM UCP2/3, SERCA1/2 & RyR1/2 expression ↑ SKM SERCA activity & ATP hydrolysis | TRPV1/β3-ARs/CaMKII/AMPK/ SIRT1/PGC1α | [46,48] |
6-Paradol, 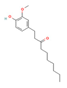 Gingerol 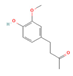 & Zingerone 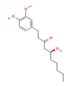 (Ginger) | ↓ Body weight ↓ Fat mass ↑ Energy expenditure | ↑ BAT activity & function ↑ vWAT, sWAT & white adipocytes (in vitro) browning ↑ BAT & WAT UCP1 expression ↓ WAT adipogenesis & ↑ lipolysis ↑ SKM mitochondrial biogenesis | TRPV1/β3-ARs/AMPK/SIRT1/ PPARα/PGC1α | [51,52,53,54] | |
Cinnamaldehyde 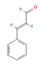 & Cinnamic acid  (Cinnamon) | ↓ Body weight ↑ Energy expenditure | ↑ Body temperature ↑ BAT activity & brown adipocytes (in vitro) function ↑ BAT mitochondrial ATP production ↑ vWAT, sWAT & white adipocytes (in vitro) browning ↑ BAT & WAT UCP1 expression ↓ Adipocytes size & lipogenesis (in vitro) | TRPA1/β3-ARs/FGF21/AMPK/ PPARγ/PGC1α | [55,56,57,59] | |
Curcumin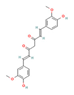 (Turmeric) | ↓ Body weight ↑ Energy expenditure ↑ Insulin sensitivity | ↑ BAT activation ↑ sWAT & white adipocytes (in vitro) browning ↑ BAT & sWAT UCP1 expression ↑ Muscle cells mitochondrial function & energy expenditure (in vitro) ↑ SKM SERCA1 expression ATP hydrolysis & fiber type composition modulation | FNDC5/Irisin/β3-ARs/AMPK/ PPARγ/PGC1α | [61,62,63] | |
Allicin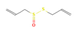 (Garlic) | ↓ Weight gain ↑ Energy expenditure Improved glucose homeostasis | ↑ BAT activation & fat oxidation ↑ WAT & white adipocytes (in vitro) browning ↑ BAT & sWAT UCP1 expression ↑ SKM UCP3 expression ↑ Lipolysis | β3-ARs/AMPK/SIRTs/ PPARα/PGC1α | [65] | |
Quercetin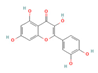 (Onion) | ↑ Weight loss ↑ Energy expenditure ↓ Fat accumulation Improved glucose homeostasis | ↑ BAT mass & function ↑ sWAT & white adipocytes (in vitro) browning ↑ BAT & WAT UCP1 expression ↑ Fat oxidation & ↓ adipogenesis ↑ SKM mitochondrial function & biogenesis SKM SERCA1/2 activity & function modulation SKM SERCAs conformational regulation ↑ SKM glucose uptake | β3-ARs/FGF21/PKA/AMPK/ SIRT1/PPARα/γ/PGC1α | [67,68,69] | |
Caffeine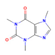 (Coffee) | ↑ Weight loss ↑ Energy expenditure | ↑ White adipocytes browning & UCP1 expression (in vitro) ↑ BAT UCP1 expression ↑ SKM UCP3 expression SKM RyR agonist & SERCA activity uncoupler ↑ Mitochondriogenesis | RyR/SERCA/PPARγ/PGC1α | [70,71,72] | |
Catechins 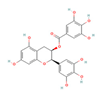 & Theaflavins 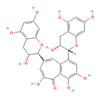 (Tea) | ↓ Body weight ↑ Energy expenditure | ↑ BAT activity & function ↑ BAT & WAT UCP1 expression ↑ WAT & white adipocytes (in vitro) browning =/↑ SKM UCP3 expression =SKM FNDC5/Irisin & SLN expression | TRPA/V1/β3-ARs/BMP7/FGF21/ Adiponectin/AMPK/SIRT1/ PPARγ/PGC1α | [73,74,76] | |
Flavan-3-ols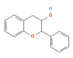 (Cocoa) | ↑ Energy expenditure | ↑ BAT activation & UCP1 expression ↑ SKM UCP3 expression ↑ Mitochondriogenesis | β3-ARs/AMPK/PGC1α | [77,78] | |
Berberine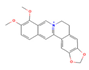 (Chinese goldthread, goldenseal) | ↓ Body weight ↑ Energy expenditure Improved glucose homeostasis | ↑ BAT activity ↑ sWAT & white adipocytes (in vitro) browning ↑ Brown adipocytes differentiation (in vitro) ↑ BAT & WAT UCP1 expression ↑ Mitochondrial biogenesis & function | AMPK/SIRT1/PPARγ/PGC1α | [79,80] | |
DHA 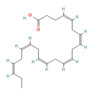 & EPA 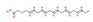 (Oily fish) | ↓ Weight gain ↑ Energy expenditure Improved glucose metabolism | ↑ BAT mass & activity ↑ WAT browning ↑ BAT, sWAT & brown and white adipocytes (in vitro) UCP1 expression ↑ Mitochondrial function & biogenesis ↑ Fat & glucose oxidation ↑ SKM SERCA activity uncoupling ↑ SKM SERCA2b & SLN expression ↑ SKM development | TRPV1/β3-ARs/FGF21/ Irisin/AMPK/SIRT1/PGC1α | [81,82,83,85,86] | |
Eriocitrin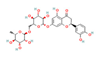 (Lemon) | ↑ Energy expenditure ↑ Insulin sensitivity | ↑ BAT UCP1 expression ↑ SKM UCP3, SERCA1/2 & SLN expression ↑ Fat oxidation | ? | [87] | |
Menthol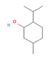 (Mint) | ↑ Weight loss ↑ Energy expenditure Improved glucose metabolism | ↑ BAT activity & fat oxidation ↑ WAT & white adipocyte (in vitro) browning ↑ BAT, WAT & brown and white adipocytes (in vitro) UCP1 expression ↑ Mitochondrial activity & metabolic rate ↑ SKM energy expenditure ↑ Skin temperature | TRPM8/β3-ARs/FGF21/ Calcium/PKA/AMPK/PGC1α | [88,89] | |
Thymol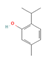 (Thyme) | ? | ↑ White adipocytes browning & UCP1 expression (in vitro) ↑ Mitochondriogenesis (in vitro) | β3-ARs/PKA/AMPK | [91] | |
Resveratrol, 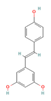 Proanthocyanidin  & Anthocyanin 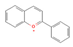 (Grapes & Berries) | ↓ Body weight ↑ Energy expenditure ↓ Fat mass ↓ Insulin resistance Improved glycemic and lipid profile | ↑ BAT activity & recruitment ↑ WAT & white adipocytes (in vitro) browning ↑ BAT, WAT & brown and white adipocytes (in vitro) UCP1 expression ↑ Fat oxidation & ↓ vWAT adipogenesis ↑ SKM UCP3 expression ↑ SKM glucose uptake ↑ SKM, BAT, WAT, brown and white adipocytes (in vitro) mitochondrial dynamic & function | β3-ARs/BMP7/FNDC5/Irisin/ERα/ AMPK/SIRT1/3/PPARα/γ/PGC1α | [92,93,95,96,97,98,99,100] | |
| Drugs | β3-AR agonists Mirabegron 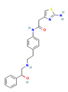 | ↑ Weight loss ↑ Energy expenditure ↑ Insulin sensitivity and secretion Improved lipid profile and glucose homeostasis | ↑ BAT mass & activity ↑ WAT & white adipocytes (in vitro) browning & UCP1 expression | β3-ARs/PGC1α | [102] |
| THR agonist Levothyroxine 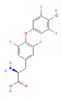 Liothyronine 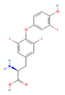 Resmetirom 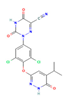 | ↓ Body weight ↑ Energy expenditure Improved lipid profile | ↑ BAT activity & function ↑ WAT browning ↑ BAT & WAT UCP1 expression | THRB/AMPK | [106] | |
| FXR agonist Fexaramine 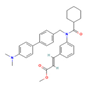 Farnesol  CDCA  | ↑ Energy expenditure | ↑ BAT mitochondriogenesis & fat oxidation ↑ WAT browning ↑ BAT, WAT & white and brown adipocytes (in vitro) UCP1 expression Adipogenesis modulation | FXR/AMPK/PPARγ/PGC1α | [109,110] | |
| Growth Hormone Tesamorelin 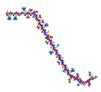 | ↓ Fat mass Improved lipid profile, glucose tolerance and insulin sensitivity | =/↑ BAT activity =/↑ WAT browning | GHR | [114] | |
| GLP1R agonists Liraglutide  Semaglutide 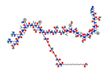 Exedin-4 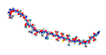 GLP1R/GIPR dual agonist Tirzepatide  GLP1R/GR dual agonist Oxyntomodulin  Mazdutide  GLP1R/GR/GIPR triple agonist Retatrutide  | ↑ Weight loss ↑ Energy expenditure ↓ Food intake ↓ Fat mass Improved insulin sensitivity and secretion, and glycemic control | ↑ BAT activity & function ↑ WAT & white adipocytes (in vitro) browning ↑ BAT, WAT & white adipocytes (in vitro) UCP1 expression ↑ SKM mitochondrial function & thermogenic genes expression | GLP1R/PKA/AMPK/SIRT1/PGC1α | [115,116,118] | |
| SGLT2 Inhibitors Dapagliflozin 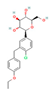 Empagliflozin 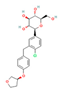 Canagliflozin 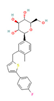 | ↓ Body weight ↑ Energy expenditure ↓ Fat mass Improved lipid profile, glucose homeostasis and insulin sensitivity | ↑ BAT activity & WAT browning ↑ BAT & WAT UCP1 expression ↑ SKM fat oxidation | SGLT2/AMPK/SIRT1 | [125] | |
Melatonin | ↑ Energy expenditure ↓ Body weight ↓ Visceral fat mass Improved lipid profile, glucose homeostasis and insulin sensitivity | ↑ Body temperature ↑ BAT activity, mass & function ↑ sWAT browning ↑ BAT & WAT UCP1 expression ↑ SKM SERCA activity & expression ↑ SKM SLN expression ↑ BAT, WAT & SKM mitochondrial quality, function and biogenesis ↑ Mitochondrial membrane integrity & dynamics ↓ Organellar stress & apoptosis ↓ Fat accumulation, oxidative stress & low-grade inflammation ↑ Lipid metabolism Improved SKM fiber composition, microbiota dysbiosis, metabolism control and plasticity & energy balance over 24 h | MT1/2/PDK1/Akt/CaMKII/AMPK/ SIRT1/3/PGC1α/NRF1/2/SOD2 | [134,137,138,139,140,141,142,143,144,150,151,153,154,155,156] | |
| Stimuli | Type of Study | Anti-Diabesity Effect | Mechanism | References | |
|---|---|---|---|---|---|
| Physiological conditions | Cold exposure | Clinical Trial | ↑ Energy expenditure ↑ Insulin sensitivity | ↑ BAT activity and recruitment ↑ sWAT UCP1 expression & mitochondrial function ↑ SKM GLUT4 expression | [18,19,21] |
| Physical exercise | Observational Human study | ↓ Insulin resistance ↑ Glucose sensitivity ↑ Lipid metabolism ↓ Visceral fat mass | ↑ BAT activation ↑ WAT browning ↑ BAT & WAT UCP1 expression ↑ SKM UCP3 expression | [24,25,27] | |
| Fasting | Clinical Trial | ↓ Body weight ↑ Energy expenditure | = sWAT UCP1 expression | [32,33] | |
| In vivo Human study | Improved metabolic health | ↑ SKM UCP3 expression | [45] | ||
| Bioactive ingredients | Capsaicin & Capsinoids 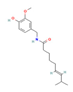 (Pepper) | Clinical Trial | ↓ Weight gain ↑ Energy expenditure ↓ Abdominal fat accumulation Restored glucose levels | ↑ BAT activity ↑ Fat oxidation | [47] |
6-Paradol 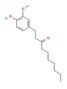 (Grain of Paradise) | Clinical Trial | ↓ Body weight ↓ Visceral fat mass ↑ Energy expenditure | ↑ BAT activation and recruitment | [49,50] | |
Cinnamaldehyde 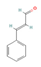 & Cinnamic acid  (Cinnamon) | In vitro Human study | ↑ Metabolic response | ↑ Adipocytes browning & UCP1 expression ↑ Fat oxidation | [57] | |
| Clinical Trial | ↓ Body weight ↑ Energy expenditure | ↑ Facial skin temperature | [58] | ||
Curcumin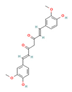 (Turmeric) | Clinical Trial | ↓ Body weight ↓ Waist/hip circumference ↑ Energy expenditure ↑ Insulin sensitivity | ↓ Fat mass & anthropometric measurements | [60] | |
Allicin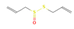 (Garlic) | In vitro Human study | ↓ Weight gain ↑ Energy expenditure Improved glucose tolerance | ↑ White adipocytes browning & UCP1 expression | [66] | |
Caffeine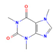 (Coffee) | In vitro & in vivo Human study | ↑ Weight loss ↑ Energy expenditure | ↑ BAT activity & function ↑ Adipocytes browning & UCP1 expression = SKM UCP3 expression | [71] | |
Catechins 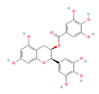 (Tea) | Clinical Trial | ↓ Body weight ↑ Energy expenditure | ↑ BAT activation and recruitment | [75] | |
Berberine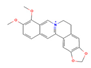 (Chinese goldthread, goldenseal) | In vitro & in vivo Human study | ↓ Body weight ↑ Energy expenditure Improved glucose homeostasis Restored metabolic health | ↑ BAT mass & function ↑ BAT recruitment & brown adipogenesis | [80] | |
EPA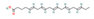 (Oily fish) | In vitro & in vivo Human study | ↓ Weight gain ↑ Energy expenditure Improved glucose metabolism | ↑ Subcutaneous white adipocytes browning = SKM SERCA activity & function | [84] | |
Menthol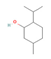 (Mint) | Clinical Trial | ↑ Weight loss ↑ Energy expenditure Improved glucose metabolism | ↑ Skin temperature & metabolic rate | [90] | |
Resveratrol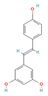 (Grapes & Berries) | In vitro & in vivo Human study | ↓ Body weight ↑ Energy expenditure ↓ Visceral fat mass ↓ Insulin resistance Improved glycemic and lipid profile | ↑ sWAT & white adipocytes browning ↑ sWAT & white adipocytes UCP1 expression ↑ SERCA activity and expression & calcium modulation | [93,94] | |
| Drugs | β3-AR agonists Mirabegron 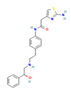 | Clinical Trial | ↑ Weight loss ↑ Energy expenditure ↑ Insulin sensitivity and secretion Improved lipid profile and glucose homeostasis | ↑ BAT mass & activity ↑ BAT energy expenditure & metabolic rate ↑ sWAT browning & UCP1 expression ↑ SKM PGC1α expression ↑ SKM oxidative fibers ↑ Supraclavicular skin temperature | [19,103,104,105] |
| THR agonist Levothyroxine 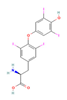 Liothyronine 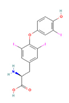 Resmetirom 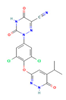 | In vitro & in vivo Human study | Improved metabolism homeostasis | ↑ BAT activity & function ↑ Adipocytes browning & UCP1 expression ↑ SKM glucose uptake & function ↑ SKM SERCA activity and expression SKM fiber composition modulation ↑ Mitochondrial biogenesis & oxidative metabolism | [106,107] | |
| Clinical Trial | ↓ Body weight ↑ Energy expenditure Improved lipid profile | ↑ Body temperature | [108] | ||
| FXR agonist Farnesol  CDCA 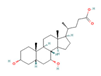 | In vitro Human study | ↓ Fat accumulation ↑ Metabolic response | ↑ Adipocytes browning & UCP1 expression | [110] | |
| Clinical Trial | ↑ Energy expenditure | ↑ BAT activity & function | [111] | ||
| Growth Hormone Tesamorelin  | Clinical Trial | ↑ Energy expenditure ↓ BMI, waist circumference ↓ Visceral fat mass Improved lipid profile, glucose tolerance and insulin sensitivity | ↑ SKM mitochondrial function & phosphocreatine recovery | [112,113] | |
| GLP1R agonists Liraglutide  Semaglutide  GLP1R/GIPR dual agonist Tirzepatide  GLP1R/GR dual agonist Oxyntomodulin  Mazdutide  GLP1R/GR/GIPR triple agonist Retatrutide 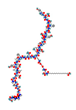 | In vitro Human study | ↓ Fat accumulation | ↑ Adipocytes browning ↑ Lipid metabolism | [119] | |
| Clinical Trial | ↑ Weight loss ↑ Energy expenditure ↓ BMI, waist circumference, and food intake ↓ Visceral fat mass Improved insulin sensitivity and secretion, and glycemic control | ↑ Supraclavicular & neck BAT activity ↑ Lipid metabolism | [116,117,120,121,122,123,124] | ||
| SGLT2 Inhibitors Dapagliflozin 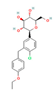 Empagliflozin 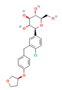 Canagliflozin 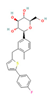 | Clinical Trial | ↓ Body weight ↑ Energy expenditure ↓ Waist circumference, waist-to-height ratio ↓ Fat mass Improved lipid profile, glucose homeostasis and insulin sensitivity | ↑ SKM fat oxidative metabolic rate Improved respiratory exchange ratio | [126,127] | |
Melatonin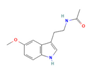 | Clinical Trial | ↑ Energy expenditure ↓/=/↑ Body weight ↓/= BMI & waist and hip circumference ↓ Visceral fat mass Improved lipid profile, glucose homeostasis and insulin sensitivity | ↑ BAT activity, mass & function ↓ Oxidative stress | [131,132,145] | |
8. Limitations of the Study
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| Akt | Protein kinase B |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| ATP | Adenosine triphosphate |
| β3-AR | β3 adrenergic receptor |
| BAT | Brown adipose tissue |
| bAT | Beige adipose tissue |
| BMP7 | Bone morphogenetic protein 7 |
| Ca2+ | Calcium |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CD36 | Cluster of differentiation 36 |
| DHA | Docosahexaenoic acid |
| DKO | Double knockout |
| EPA | Eicosapentaenoic acid |
| ERα | Estrogen receptor α |
| FGF21 | Fibroblast growth factor 21 |
| FNDC5 | Fibronectin type III domain-containing protein 5 |
| FXR | Farnesoid X receptor |
| GH | Growth hormone |
| GHR | Growth hormone receptor |
| GIPR | Glucose-dependent insulinotropic polypeptide receptor |
| GLP-1 | Glucagon-like peptide 1 |
| GLUT4 | Glucose transporter type 4 |
| GP | Grain of Paradise |
| GPx | Glutathione peroxidase |
| GR | Glucagon receptor |
| HFD | High-fat diet |
| IF | Intermittent fasting |
| KO | Knockout |
| mPTP | Mitochondrial permeability transition pore |
| MT | Melatonin receptor |
| NRF1/2 | Nuclear respiratory factor 1/2 |
| NST | Non-shivering thermogenesis |
| OXPHOS | Oxidative phosphorylation |
| PGC1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PKA | Protein kinase A |
| PPAR | Peroxisome proliferator-activated receptor |
| PRDM16 | PR/SET domain 16 |
| PUFA | Polyunsaturated fatty acid |
| RyR | Ryanodine receptor |
| SCFA | Short-chain fatty acids |
| SERCA | Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase |
| SGLT2 | Sodium-glucose linked transporter 2 |
| SIRT | Sirtuin |
| SKM | Skeletal muscle |
| SLN | Sarcolipin |
| SOD2 | Superoxide dismutase 2 |
| sWAT | Subcutaneous white adipose tissue |
| T2DM | Type 2 diabetes mellitus |
| T3 | Triiodothyronine |
| TH | Thyroid hormone |
| THR | Thyroid hormone receptor |
| TRP | Transient receptor potential |
| TRPA1 | Transient receptor potential A1 |
| TRPM8 | Transient receptor potential cation channel melastatin 8 |
| TRPV1 | Transient receptor potential vanilloid 1 |
| UCP | Uncoupling protein |
| vWAT | Visceral white adipose tissue |
| WAT | White adipose tissue |
References
- Hummasti, S.; Hotamisligil, G.S. Endoplasmic Reticulum Stress and Inflammation in Obesity and Diabetes. Circ. Res. 2010, 107, 579–591. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 21 March 2025).
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, C.; Li, L.; Li, L. Skeletal Muscle Non-Shivering Thermogenesis as an Attractive Strategy to Combat Obesity. Life Sci. 2021, 269, 119024. [Google Scholar] [CrossRef]
- Betz, M.J.; Enerbäck, S. Targeting Thermogenesis in Brown Fat and Muscle to Treat Obesity and Metabolic Disease. Nat. Rev. Endocrinol. 2018, 14, 77–87. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, J.; Dai, H.; Duan, Y.; An, Y.; Shi, L.; Lv, Y.; Li, H.; Wang, C.; Ma, Q.; et al. Brown and Beige Adipose Tissue: A Novel Therapeutic Strategy for Obesity and Type 2 Diabetes Mellitus. Adipocyte 2021, 10, 48–65. [Google Scholar] [CrossRef]
- Hamdy, O.; Porramatikul, S.; Al-Ozairi, E. Metabolic Obesity: The Paradox between Visceral and Subcutaneous Fat. Curr. Diabetes Rev. 2006, 2, 367–373. [Google Scholar] [CrossRef]
- Harms, M.; Seale, P. Brown and Beige Fat: Development, Function and Therapeutic Potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Wang, Q.A.; Song, A.; Vishvanath, L.; Busbuso, N.C.; Scherer, P.E.; Gupta, R.K. Cellular Origins of Beige Fat Cells Revisited. Diabetes 2019, 68, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Porter, C. Quantification of UCP1 Function in Human Brown Adipose Tissue. Adipocyte 2017, 6, 167–174. [Google Scholar] [CrossRef]
- Periasamy, M.; Herrera, J.L.; Reis, F.C.G. Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism. Diabetes Metab. J. 2017, 41, 327–336. [Google Scholar] [CrossRef]
- Periasamy, M.; Maurya, S.K.; Sahoo, S.K.; Singh, S.; Reis, F.C.G.; Bal, N.C. Role of SERCA Pump in Muscle Thermogenesis and Metabolism. Compr. Physiol. 2017, 7, 879–890. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Martinez-Canton, M.; Gelabert-Rebato, M.; Martin-Rincon, M.; de Pablos-Velasco, P.; Holmberg, H.C.; Calbet, J.A.L. Sarcolipin Expression in Human Skeletal Muscle: Influence of Energy Balance and Exercise. Scand. J. Med. Sci. Sports 2020, 30, 408–420. [Google Scholar] [CrossRef]
- Bal, N.C.; Maurya, S.K.; Pani, S.; Sethy, C.; Banerjee, A.; Das, S.; Patnaik, S.; Kundu, C.N. Mild Cold Induced Thermogenesis: Are BAT and Skeletal Muscle Synergistic Partners? Biosci. Rep. 2017, 37, BSR20171087. [Google Scholar] [CrossRef] [PubMed]
- Leiria, L.O.; Wang, C.H.; Lynes, M.D.; Yang, K.; Shamsi, F.; Sato, M.; Sugimoto, S.; Chen, E.Y.; Bussberg, V.; Narain, N.R.; et al. 12-Lipoxygenase Regulates Cold Adaptation and Glucose Metabolism by Producing the Omega-3 Lipid 12-HEPE from Brown Fat. Cell Metab. 2019, 30, P768–P783. [Google Scholar] [CrossRef]
- Sun, W.; Luo, Y.; Zhang, F.; Tang, S.; Zhu, T. Involvement of TRP Channels in Adipocyte Thermogenesis: An Update. Front. Cell Dev. Biol. 2021, 9, 686173. [Google Scholar] [CrossRef]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-Induced Activation of Brown Adipose Tissue and Adipose Angiogenesis in Mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef]
- Van Der Lans, A.A.J.J.; Hoeks, J.; Brans, B.; Vijgen, G.H.E.J.; Visser, M.G.W.; Vosselman, M.J.; Hansen, J.; Jörgensen, J.A.; Wu, J.; Mottaghy, F.M.; et al. Cold Acclimation Recruits Human Brown Fat and Increases Nonshivering Thermogenesis. J. Clin. Investig. 2013, 123, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Finlin, B.S.; Memetimin, H.; Confides, A.L.; Kasza, I.; Zhu, B.; Vekaria, H.J.; Harfmann, B.; Jones, K.A.; Johnson, Z.R.; Westgate, P.M.; et al. Human Adipose Beiging in Response to Cold and Mirabegron. JCI Insight 2018, 3, e121510. [Google Scholar] [CrossRef] [PubMed]
- Pant, M.; Bal, N.C.; Periasamy, M. Cold Adaptation Overrides Developmental Regulation of Sarcolipin Expression in Mice Skeletal Muscle: SOS for Muscle-Based Thermogenesis? J. Exp. Biol. 2015, 218, 2321–2325. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, M.J.W.; Hoeks, J.; Brans, B.; Van Der Lans, A.A.J.J.; Schaart, G.; Van Den Driessche, J.J.; Jörgensen, J.A.; Boekschoten, M.V.; Hesselink, M.K.C.; Havekes, B.; et al. Short-Term Cold Acclimation Improves Insulin Sensitivity in Patients with Type 2 Diabetes Mellitus. Nat. Med. 2015, 21, 863–865. [Google Scholar] [CrossRef]
- Rowland, L.A.; Bal, N.C.; Kozak, L.P.; Periasamy, M. Uncoupling Protein 1 and Sarcolipin Are Required to Maintain Optimal Thermogenesis, and Loss of Both Systems Compromises Survival of Mice under Cold Stress. J. Biol. Chem. 2015, 290, 12282–12289. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Hofmann, T.; Goebel-Stengel, M.; Elbelt, U.; Kobelt, P.; Klapp, B.F. Circulating Levels of Irisin in Patients with Anorexia Nervosa and Different Stages of Obesit—Correlation with Body Mass Index. Peptides 2013, 39, 125–130. [Google Scholar] [CrossRef]
- Summermatter, S.; Shui, G.; Maag, D.; Santos, G.; Wenk, M.R.; Handschin, C. PGC-1α Improves Glucose Homeostasis in Skeletal Muscle in an Activity-Dependent Manner. Diabetes 2013, 62, 85–95. [Google Scholar] [CrossRef]
- Bonfante, I.L.P.; Monfort-Pires, M.; Duft, R.G.; da Silva Mateus, K.C.; de Lima Júnior, J.C.; dos Santos Trombeta, J.C.; Finardi, E.A.R.; Brunelli, D.T.; Morari, J.; de Lima, J.A.B.; et al. Combined Training Increases Thermogenic Fat Activity in Patients with Overweight and Type 2 Diabetes. Int. J. Obes. 2022, 46, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Hao, X.; Sun, Y.; Zhao, Y.; Wang, Y.; Cao, X.; Gong, Z.; Ji, S.; Lu, J.; Yan, Y.; et al. Exercise-Inducible Circulating Extracellular Vesicle Irisin Promotes Browning and the Thermogenic Program in White Adipose Tissue. Acta Physiol. 2024, 240, e14103. [Google Scholar] [CrossRef]
- Gheit, R.E.A.E.; Younis, R.L.; El-Saka, M.H.; Emam, M.N.; Soliman, N.A.; El-Sayed, R.M.; Hafez, Y.M.; AbuoHashish, N.A.; Radwan, D.A.; Khaled, H.E.; et al. Irisin Improves Adiposity and Exercise Tolerance in a Rat Model of Postmenopausal Obesity through Enhancing Adipo-Myocyte Thermogenesis. J. Physiol. Biochem. 2022, 78, 897–913. [Google Scholar] [CrossRef]
- Tekin, S.; Erden, Y.; Ozyalin, F.; Onalan, E.E.; Cigremis, Y.; Colak, C.; Tekedereli, I.; Sandal, S. Central Irisin Administration Suppresses Thyroid Hormone Production but Increases Energy Consumption in Rats. Neurosci. Lett. 2018, 674, 136–141. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, Y.H.; Son, J.E.; Lee, J.H.; Kim, S.; Choe, M.S.; Moon, J.H.; Zhong, J.; Fu, K.; Lenglin, F.; et al. Intermittent Fasting Promotes Adipose Thermogenesis and Metabolic Homeostasis via VEGF-Mediated Alternative Activation of Macrophage. Cell Res. 2017, 27, 1309–1326. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-Hour Time Restricted Feeding on Body Weight and Metabolic Disease Risk Factors in Obese Adults: A Pilot Study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 801. [Google Scholar] [CrossRef]
- Putri, M.; Syamsunarno, M.R.A.A.; Iso, T.; Yamaguchi, A.; Hanaoka, H.; Sunaga, H.; Koitabashi, N.; Matsui, H.; Yamazaki, C.; Kameo, S.; et al. CD36 Is Indispensable for Thermogenesis under Conditions of Fasting and Cold Stress. Biochem. Biophys. Res. Commun. 2015, 457, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Wei, Q.; Zhao, F.; Zhang, C. Short-Term Fasting Reshapes Fat Tissue. Endocr. J. 2021, 68, 387–398. [Google Scholar] [CrossRef]
- Wu, D.; Wang, H.; Xie, L.; Hu, F. Cross-Talk Between Gut Microbiota and Adipose Tissues in Obesity and Related Metabolic Diseases. Front. Endocrinol. 2022, 13, 908868. [Google Scholar] [CrossRef]
- Liu, B.; Page, A.J.; Hutchison, A.T.; Wittert, G.A.; Heilbronn, L.K. Intermittent Fasting Increases Energy Expenditure and Promotes Adipose Tissue Browning in Mice. Nutrition 2019, 66, 38–43. [Google Scholar] [CrossRef]
- Hu, X.; Xia, K.; Dai, M.; Han, X.; Yuan, P.; Liu, J.; Liu, S.; Jia, F.; Chen, J.; Jiang, F.; et al. Intermittent Fasting Modulates the Intestinal Microbiota and Improves Obesity and Host Energy Metabolism. NPJ Biofilms Microbiomes 2023, 9, 19. [Google Scholar] [CrossRef]
- Jyoti; Dey, P. Mechanisms and Implications of the Gut Microbial Modulation of Intestinal Metabolic Processes. NPJ Metab. Health Dis. 2025, 3, 24. [Google Scholar] [CrossRef]
- Hylander, B.L.; Repasky, E.A. Temperature as a Modulator of the Gut Microbiome: What Are the Implications and Opportunities for Thermal Medicine? Int. J. Hyperth. 2019, 36, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, L.; Li, M.; Lam, S.M.; Wang, G.; Wu, Y.; Zhang, H.; Niu, C.; Zhang, X.; Liu, X.; et al. Microbiota Depletion Impairs Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. Cell Rep. 2019, 26, 2720–2737.e5. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Liu, G.; Wang, S.; Zong, G.; Zhang, X.; Pan, L.; Han, J. Intermittent Fasting Improves Insulin Resistance by Modulating the Gut Microbiota and Bile Acid Metabolism in Diet-Induced Obesity. Mol. Nutr. Food Res. 2024, 68, e2400451. [Google Scholar] [CrossRef] [PubMed]
- Riedl, R.A.; Burnett, C.M.L.; Pearson, N.A.; Reho, J.J.; Mokadem, M.; Edwards, R.A.; Kindel, T.L.; Kirby, J.R.; Grobe, J.L. Gut Microbiota Represent a Major Thermogenic Biomass. Function 2021, 2, zqab019. [Google Scholar] [CrossRef]
- Wu, D.; Bang, I.H.; Park, B.H.; Bae, E.J. Loss of Sirt6 in Adipocytes Impairs the Ability of Adipose Tissue to Adapt to Intermittent Fasting. Exp. Mol. Med. 2021, 53, 1298–1306. [Google Scholar] [CrossRef]
- Tunstall, R.J.; Mehan, K.A.; Hargreaves, M.; Spriet, L.L.; Cameron-Smith, D. Fasting Activates the Gene Expression of UCP3 Independent of Genes Necessary for Lipid Transport and Oxidation in Skeletal Muscle. Biochem. Biophys. Res. Commun. 2002, 294, 301–308. [Google Scholar] [CrossRef]
- Abdillah, A.M.; Lee, J.Y.; Lee, Y.R.; Yun, J.W. Modulatory Roles of Capsaicin on Thermogenesis in C2C12 Myoblasts and the Skeletal Muscle of Mice. Chem. Biol. Interact. 2025, 407, 111380. [Google Scholar] [CrossRef]
- Snitker, S.; Fujishima, Y.; Shen, H.; Ott, S.; Pi-Sunyer, X.; Furuhata, Y.; Sato, H.; Takahashi, M. Effects of Novel Capsinoid Treatment on Fatness and Energy Metabolism in Humans: Possible Pharmacogenetic Implications. Am. J. Clin. Nutr. 2009, 89, 45–50. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin Induces Browning of White Adipose Tissue and Counters Obesity by Activating TRPV1 Channel-Dependent Mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef]
- Sugita, J.; Yoneshiro, T.; Hatano, T.; Aita, S.; Ikemoto, T.; Uchiwa, H.; Iwanaga, T.; Kameya, T.; Kawai, Y.; Saito, M. Grains of Paradise (Aframomum melegueta) Extract Activates Brown Adipose Tissue and Increases Whole-Body Energy Expenditure in Men. Br. J. Nutr. 2013, 110, 733–738. [Google Scholar] [CrossRef]
- Sugita, J.; Yoneshiro, T.; Sugishima, Y.; Ikemoto, T.; Uchiwa, H.; Suzuki, I.; Saito, M. Daily Ingestion of Grains of Paradise (Aframomum melegueta) Extract Increases Whole-Body Energy Expenditure and Decreases Visceral Fat in Humans. J. Nutr. Sci. Vitaminol. 2014, 60, 22–27. [Google Scholar] [CrossRef]
- Kim, S.P.; Jeong, I.; Kang, N.; Kim, M.; Kim, O.K. Black Ginger Extract Suppresses Fat Accumulation by Regulating Lipid Metabolism in High-Fat Diet-Fed Mice. J. Med. Food 2024, 27, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Wang, P.; Hu, X.; Chen, F. Ginger Prevents Obesity through Regulation of Energy Metabolism and Activation of Browning in High-Fat Diet-Induced Obese Mice. J. Nutr. Biochem. 2019, 70, 105–115. [Google Scholar] [CrossRef]
- Li, X.; Yao, Y.; Yu, C.; Wei, T.; Xi, Q.; Li, J.; Chen, F.; Deng, Z.Y.; Luo, T. Modulation of PPARα-Thermogenesis Gut Microbiota Interactions in Obese Mice Administrated with Zingerone. J. Sci. Food Agric. 2023, 103, 3065–3076. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, S.; Wu, J.; Sun, X.; Shen, Z.; Dong, J.; Huang, J. Promotion of Mitochondrial Biogenesis via Activation of AMPK-PGC1ɑ Signaling Pathway by Ginger (Zingiber Officinale Roscoe) Extract, and Its Major Active Component 6-Gingerol. J. Food Sci. 2019, 84, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.G.O.; Boechat, S.K.; Romão, J.S.; Kuhnert, L.R.B.; Pazos-Moura, C.C.; Oliveira, K.J. Cinnamaldehyde Treatment during Adolescence Improves White and Brown Adipose Tissue Metabolism in a Male Rat Model of Early Obesity. Food Funct. 2022, 13, 3405–3418. [Google Scholar] [CrossRef]
- Zuo, J.; Zhao, D.; Yu, N.; Fang, X.; Mu, Q.; Ma, Y.; Mo, F.; Wu, R.; Ma, R.; Wang, L.; et al. Cinnamaldehyde Ameliorates Diet-Induced Obesity in Mice by Inducing Browning of White Adipose Tissue. Cell. Physiol. Biochem. 2017, 42, 1514–1525. [Google Scholar] [CrossRef]
- Jiang, J.; Emont, M.P.; Jun, H.; Qiao, X.; Liao, J.; Kim, D.I.; Wu, J. Cinnamaldehyde Induces Fat Cell-Autonomous Thermogenesis and Metabolic Reprogramming. Metabolism 2017, 77, 58–64. [Google Scholar] [CrossRef]
- Michlig, S.; Merlini, J.M.; Beaumont, M.; Ledda, M.; Tavenard, A.; Mukherjee, R.; Camacho, S.; Le Coutre, J. Effects of TRP Channel Agonist Ingestion on Metabolism and Autonomic Nervous System in a Randomized Clinical Trial of Healthy Subjects. Sci. Rep. 2016, 6, 20795. [Google Scholar] [CrossRef]
- Kang, N.H.; Mukherjee, S.; Yun, J.W. Trans-Cinnamic Acid Stimulates White Fat Browning and Activates Brown Adipocytes. Nutrients 2019, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Dipierro, F.; Bressan, A.; Ranaldi, D.; Rapacioli, G.; Giacomelli, L.; Bertuccioli, A. Potential Role of Bioavailable Curcumin in Weight Loss and Omental Adipose Tissue Decrease: Preliminary Data of a Randomized, Controlled Trial in Overweight People with Metabolic Syndrome. Preliminary Study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4195–4202. [Google Scholar]
- Bilmen, J.G.; Khan, S.Z.; Javed, M.U.H.; Michelangeli, F. Inhibition of the SERCA Ca2+ Pumps by Curcumin. Curcumin Putatively Stabilizes the Interaction between the Nucleotide-Binding and Phosphorylation Domains in the Absence of ATP. Eur. J. Biochem. 2001, 268, 6318–6327. [Google Scholar] [CrossRef]
- Zou, T.; Li, S.; Wang, B.; Wang, Z.; Liu, Y.; You, J. Curcumin Improves Insulin Sensitivity and Increases Energy Expenditure in High-Fat-Diet-Induced Obese Mice Associated with Activation of FNDC5/Irisin. Nutrition 2021, 90, 111263. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Ye, Z.; Xu, C.; Zhang, M.; Ruan, B.; Wei, M.; Jiang, Y.; Zhang, Y.; Wang, L.; et al. Curcumin Promotes Browning of White Adipose Tissue in a Norepinephrine-Dependent Way. Biochem. Biophys. Res. Commun. 2015, 466, 247–253. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.; Sheng, Y.; Xu, J.; Yang, C.; Zheng, S.; Liu, J.; Li, H.; Ge, J.; Yang, M.; et al. Allicin Regulates Energy Homeostasis through Brown Adipose Tissue. iScience 2020, 23, 101113. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, I.H.; Kim, C.T.; Kim, Y. Reduction of Body Weight by Dietary Garlic Is Associated with an Increase in Uncoupling Protein MRNA Expression and Activation of AMP-Activated Protein Kinase in Diet-Induced Obese Mice. J. Nutr. 2011, 141, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Baek, S.H.; Bang, K.S.; Kim, N.H.; Takimoto, K. Fermented Garlic Extract Increases Oxygen Consumption and UCP-1 MRNA Expression in Human Adipose-Derived Stem Cells. Cell J. 2019, 21, 357–362. [Google Scholar] [CrossRef]
- Choi, H.; Kim, C.S.; Yu, R. Quercetin Upregulates Uncoupling Protein 1 in White/Brown Adipose Tissues through Sympathetic Stimulation. J. Obes. Metab. Syndr. 2018, 27, 102–109. [Google Scholar] [CrossRef]
- Lee, S.G.; Parks, J.S.; Kang, H.W. Quercetin, a Functional Compound of Onion Peel, Remodels White Adipocytes to Brown-like Adipocytes. J. Nutr. Biochem. 2017, 42, 62–71. [Google Scholar] [CrossRef]
- Rezbarikova, P.; Viskupicova, J.; Majekova, M.; Horakova, L. Interaction of Quercetin and Its Derivatives with Ca2+-ATPase from Sarcoplasmic Reticulum: Kinetic and Molecular Modeling Studies. Gen. Physiol. Biophys. 2023, 42, 457–468. [Google Scholar] [CrossRef]
- Kogure, A.; Sakane, N.; Takakura, Y.; Umekawa, T.; Yoshioka, K.; Nishino, H.; Yamamoto, T.; Kawada, T.; Yoshikawa, T.; Yoshida, T. Effects of Caffeine on the Uncoupling Protein Family in Obese Yellow KK Mice. Clin. Exp. Pharmacol. Physiol. 2002, 29, 391–394. [Google Scholar] [CrossRef]
- Velickovic, K.; Wayne, D.; Leija, H.A.L.; Bloor, I.; Morris, D.E.; Law, J.; Budge, H.; Sacks, H.; Symonds, M.E.; Sottile, V. Caffeine Exposure Induces Browning Features in Adipose Tissue in Vitro and in Vivo. Sci. Rep. 2019, 9, 9104. [Google Scholar] [CrossRef]
- Martins, B.C.; Soares, A.C.; Martins, F.F.; Resende, A.d.C.; Inada, K.O.P.; Souza-Mello, V.; Nunes, N.M.; Daleprane, J.B. Coffee Consumption Prevents Obesity-Related Comorbidities and Attenuates Brown Adipose Tissue Whitening in High-Fat Diet-Fed Mice. J. Nutr. Biochem. 2023, 117, 109336. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Peng, W.; Sheng, J.; Zi, C.; Wu, X. EGCG Suppresses Adipogenesis and Promotes Browning of 3T3-L1 Cells by Inhibiting Notch1 Expression. Molecules 2024, 29, 2555. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Chien, Y.W.; Liang, C.T.; Chan, C.H.; Fan, M.H.; Huang, H.Y. Green Tea Extract Induces Genes Related to Browning of White Adipose Tissue and Limits Weight-Gain in High Energy Diet-Fed Rat. Food Nutr. Res. 2017, 61, 1347480. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Matsushita, M.; Hibi, M.; Tone, H.; Takeshita, M.; Yasunaga, K.; Katsuragi, Y.; Kameya, T.; Sugie, H.; Saito, M. Tea Catechin and Caffeine Activate Brown Adipose Tissue and Increase Cold-Induced Thermogenic Capacity in Humans. Am. J. Clin. Nutr. 2017, 105, 873–881. [Google Scholar] [CrossRef]
- Kudo, N.; Arai, Y.; Suhara, Y.; Ishii, T.; Nakayama, T.; Osakabe, N. A Single Oral Administration of Theaflavins Increases Energy Expenditure and the Expression of Metabolic Genes. PLoS ONE 2015, 10, e0137809. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Nakagawa, Y.; Mikome, K.; Yamamoto, H.; Osakabe, N. Enhancement of Energy Expenditure Following a Single Oral Dose of Flavan-3-Ols Associated with an Increase in Catecholamine Secretion. PLoS ONE 2014, 9, e112180. [Google Scholar] [CrossRef]
- Kamio, N.; Suzuki, T.; Watanabe, Y.; Suhara, Y.; Osakabe, N. A Single Oral Dose of Flavan-3-Ols Enhances Energy Expenditure by Sympathetic Nerve Stimulation in Mice. Free Radic. Biol. Med. 2016, 91, 256–263. [Google Scholar] [CrossRef]
- Ağaçdiken, A.A.; Göktaş, Z. Berberine-Induced Browning and Energy Metabolism: Mechanisms and Implications. PeerJ 2025, 13, e18924. [Google Scholar] [CrossRef]
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine Promotes the Recruitment and Activation of Brown Adipose Tissue in Mice and Humans. Cell Death Dis. 2019, 10, 468. [Google Scholar] [CrossRef]
- Fajardo, V.A.; Bombardier, E.; Irvine, T.; Metherel, A.H.; Stark, K.D.; Duhamel, T.; Rush, J.W.E.; Green, H.J.; Tupling, A.R. Dietary Docosahexaenoic Acid Supplementation Reduces SERCA Ca2+ Transport Efficiency in Rat Skeletal Muscle. Chem. Phys. Lipids 2015, 187, 56–61. [Google Scholar] [CrossRef]
- Bargut, T.C.L.; Martins, F.F.; Santos, L.P.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Administration of Eicosapentaenoic and Docosahexaenoic Acids May Improve the Remodeling and Browning in Subcutaneous White Adipose Tissue and Thermogenic Markers in Brown Adipose Tissue in Mice. Mol. Cell Endocrinol. 2019, 482, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Pahlavani, M.; Ramalingam, L.; Jayarathne, S.; Andrade, J.; Scoggin, S.; Festuccia, W.T.; Kalupahana, N.S.; Moustaid-Moussa, N. Temperature-Dependent Effects of Eicosapentaenoic Acid (EPA) on Browning of Subcutaneous Adipose Tissue in UCP1 Knockout Male Mice. Int. J. Mol. Sci. 2023, 24, 8708. [Google Scholar] [CrossRef] [PubMed]
- Laiglesia, L.M.; Lorente-Cebrián, S.; Prieto-Hontoria, P.L.; Fernández-Galilea, M.; Ribeiro, S.M.R.; Sáinz, N.; Martínez, J.A.; Moreno-Aliaga, M.J. Eicosapentaenoic Acid Promotes Mitochondrial Biogenesis and Beige-like Features in Subcutaneous Adipocytes from Overweight Subjects. J. Nutr. Biochem. 2016, 37, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Goto, T.; Yu, R.; Uchida, K.; Tominaga, M.; Kano, Y.; Takahashi, N.; Kawada, T. Fish Oil Intake Induces UCP1 Upregulation in Brown and White Adipose Tissue via the Sympathetic Nervous System. Sci. Rep. 2015, 5, 18013. [Google Scholar] [CrossRef]
- Wei, W.; Yu, S.; Zeng, H.; Tan, W.; Hu, M.; Huang, J.; Li, X.; Mao, L. Docosahexaenoic and Eicosapentaenoic Acids Promote the Accumulation of Browning-Related Myokines via Calcium Signaling in Insulin-Resistant Mice. J. Nutr. 2024, 154, 1271–1281. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Choi, M.S. Eriocitrin Improves Adiposity and Related Metabolic Disorders in High-Fat Diet-Induced Obese Mice. J. Med. Food 2020, 23, 233–241. [Google Scholar] [CrossRef]
- Ma, S.; Yu, H.; Zhao, Z.; Luo, Z.; Chen, J.; Ni, Y.; Jin, R.; Ma, L.; Wang, P.; Zhu, Z.; et al. Activation of the Cold-Sensing TRPM8 Channel Triggers UCP1-Dependent Thermogenesis and Prevents Obesity. J. Mol. Cell Biol. 2012, 4, 88–96. [Google Scholar] [CrossRef]
- Sankina, P.; Lal, R.; Khare, P.; von Hörsten, S.; Fester, L.; Aggarwal, V.; Zimmermann, K.; Bishnoi, M. Topical Menthol, a Pharmacological Cold Mimic, Induces Cold Sensitivity, Adaptive Thermogenesis and Brown Adipose Tissue Activation in Mice. Diabetes Obes. Metab. 2024, 26, 4329–4345. [Google Scholar] [CrossRef]
- Valente, A.; Carrillo, A.E.; Tzatzarakis, M.N.; Vakonaki, E.; Tsatsakis, A.M.; Kenny, G.P.; Koutedakis, Y.; Jamurtas, A.Z.; Flouris, A.D. The Absorption and Metabolism of a Single L-Menthol Oral versus Skin Administration: Effects on Thermogenesis and Metabolic Rate. Food Chem. Toxicol. 2015, 86, 262–273. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.W.; Yu, R.; Yun, J.W. Monoterpene Phenolic Compound Thymol Promotes Browning of 3T3-L1 Adipocytes. Eur. J. Nutr. 2017, 56, 2329–2341. [Google Scholar] [CrossRef]
- Abedi-Taleb, E.; Vahabi, Z.; Sekhavati-Moghadam, E.; Khedmat, L.; Jazayeri, S.; Saboor-Yaraghi, A.A. Upregulation of FNDC5 Gene Expression in C2C12 Cells after Single and Combined Treatments of Resveratrol and ATRA. Lipids Health Dis. 2019, 18, 181. [Google Scholar] [CrossRef]
- Andrade, J.M.O.; Barcala-Jorge, A.S.; Batista-Jorge, G.C.; Paraíso, A.F.; de Freitas, K.M.; Lelis, D.d.F.; Guimarães, A.L.S.; de Paula, A.M.B.; Santos, S.H.S. Effect of Resveratrol on Expression of Genes Involved Thermogenesis in Mice and Humans. Biomed. Pharmacother. 2019, 112, 108634. [Google Scholar] [CrossRef]
- Aguirre, L.; Fernández-Quintela, A.; Arias, N.; Portillo, M.P. Resveratrol: Anti-Obesity Mechanisms of Action. Molecules 2014, 19, 18632–18655. [Google Scholar] [CrossRef]
- Sreekumar, S.; Gangaraj, K.P.; Kiran, M.S. Modulation of Angiogenic Switch in Reprogramming Browning and Lipid Metabolism in White Adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159423. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Han, X.; Tan, H.; Huang, W.; You, Y.; Zhan, J. Blueberry Extract Improves Obesity through Regulation of the Gut Microbiota and Bile Acids via Pathways Involving FXR and TGR5. iScience 2019, 19, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Wang, B.; Yang, Q.; de Avila, J.M.; Zhu, M.J.; You, J.; Chen, D.; Du, M. Raspberry Promotes Brown and Beige Adipocyte Development in Mice Fed High-Fat Diet through Activation of AMP-Activated Protein Kinase (AMPK) A1. J. Nutr. Biochem. 2018, 55, 157–164. [Google Scholar] [CrossRef]
- Pajuelo, D.; Díaz, S.; Quesada, H.; Fernández-Iglesias, A.; Mulero, M.; Arola-Arnal, A.; Salvadó, M.J.; Bladé, C.; Arola, L. Acute Administration of Grape Seed Proanthocyanidin Extract Modulates Energetic Metabolism in Skeletal Muscle and BAT Mitochondria. J. Agric. Food Chem. 2011, 59, 4279–4287. [Google Scholar] [CrossRef]
- Du, H.; Wang, Q.; Li, T.; Ren, D.; Yang, X. Grape Seed Proanthocyanidins Reduced the Overweight of C57BL/6J Mice through Modulating Adipose Thermogenesis and Gut Microbiota. Food Funct. 2021, 12, 8467–8477. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Mukherjee, S.; Yun, J.W. Anthocyanin Oligomers Stimulate Browning in 3T3-L1 White Adipocytes via Activation of the Β3-Adrenergic Receptor and ERK Signaling Pathway. Phytother. Res. 2021, 35, 6281–6294. [Google Scholar] [CrossRef]
- Genchi, V.A.; Palma, G.; Sorice, G.P.; D’Oria, R.; Caccioppoli, C.; Marrano, N.; Biondi, G.; Caruso, I.; Cignarelli, A.; Natalicchio, A.; et al. Pharmacological Modulation of Adaptive Thermogenesis: New Clues for Obesity Management? J. Endocrinol. Investig. 2023, 46, 2213–2236. [Google Scholar] [CrossRef]
- Hao, L.; Scott, S.; Abbasi, M.; Zu, Y.; Khan, M.S.H.; Yang, Y.; Wu, D.; Zhao, L.; Wang, S. Beneficial Metabolic Effects of Mirabegron In Vitro and in High-Fat Diet-Induced Obese Mice. J. Pharmacol. Exp. Ther. 2019, 369, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Elía, E.F.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of Human Brown Adipose Tissue by a Β3-Adrenergic Receptor Agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic Mirabegron Treatment Increases Human Brown Fat, HDL Cholesterol, and Insulin Sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef]
- Ma, L.; Xiong, L.; Huang, G. Effects of Mirabegron on Brown Adipose Tissue and Metabolism in Humans: A Systematic Review and Meta-Analysis. Eur. J. Clin. Pharmacol. 2024, 80, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Lee, J.Y.; Takahashi, N.; Yasubuchi, M.; Kim, Y.I.; Hashizaki, H.; Kim, M.J.; Sakamoto, T.; Goto, T.; Kawada, T. Triiodothyronine Induces UCP-1 Expression and Mitochondrial Biogenesis in Human Adipocytes. Am. J. Physiol. Cell Physiol. 2012, 302, C463–C472. [Google Scholar] [CrossRef]
- Grover, G.; Mellstrom, K.; Malm, J. Therapeutic Potential for Thyroid Hormone Receptor-Beta Selective Agonists for Treating Obesity, Hyperlipidemia and Diabetes. Curr. Vasc. Pharmacol. 2007, 5, 141–154. [Google Scholar] [CrossRef]
- Fang, S.; Suh, J.M.; Reilly, S.M.; Yu, E.; Osborn, O.; Lackey, D.; Yoshihara, E.; Perino, A.; Jacinto, S.; Lukasheva, Y.; et al. Intestinal FXR Agonism Promotes Adipose Tissue Browning and Reduces Obesity and Insulin Resistance. Nat. Med. 2015, 21, 159–165. [Google Scholar] [CrossRef]
- Kim, H.L.; Jung, Y.; Park, J.; Youn, D.H.; Kang, J.; Lim, S.; Lee, B.S.; Jeong, M.Y.; Choe, S.K.; Park, R.; et al. Farnesol Has an Anti-Obesity Effect in High-Fat Diet-Induced Obese Mice and Induces the Development of Beige Adipocytes in Human Adipose Tissue Derived-Mesenchymal Stem Cells. Front. Pharmacol. 2017, 8, 654. [Google Scholar] [CrossRef]
- Broeders, E.P.M.; Nascimento, E.B.M.; Havekes, B.; Brans, B.; Roumans, K.H.M.; Tailleux, A.; Schaart, G.; Kouach, M.; Charton, J.; Deprez, B.; et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 2015, 22, 418–426. [Google Scholar] [CrossRef]
- Beauregard, C.; Utz, A.L.; Schaub, A.E.; Nachtigall, L.; Biller, B.M.K.; Miller, K.K.; Klibanski, A. Growth Hormone Decreases Visceral Fat and Improves Cardiovascular Risk Markers in Women with Hypopituitarism: A Randomized, Placebo-Controlled Study. J. Clin. Endocrinol. Metab. 2008, 93, 2063–2071. [Google Scholar] [CrossRef]
- Makimura, H.; Murphy, C.A.; Feldpausch, M.N.; Grinspoon, S.K. The Effects of Tesamorelin on Phosphocreatine Recovery in Obese Subjects with Reduced GH. J. Clin. Endocrinol. Metab. 2014, 99, 338–343. [Google Scholar] [CrossRef]
- de Winne, C.; Pascual, F.L.; Lopez-Vicchi, F.; Etcheverry-Boneo, L.; Mendez-Garcia, L.F.; Ornstein, A.M.; Lacau-Mengido, I.M.; Sorianello, E.; Becu-Villalobos, D. Neuroendocrine Control of Brown Adipocyte Function by Prolactin and Growth Hormone. J. Neuroendocrinol. 2024, 36, e13248. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Lin, B.; Zheng, X.; Chen, Z.; Cao, H.; Xu, H.; Liang, H.; Weng, J. GLP-1 Receptor Agonist Promotes Brown Remodelling in Mouse White Adipose Tissue through SIRT1. Diabetologia 2016, 59, 1059–1069. [Google Scholar] [CrossRef]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J.; et al. GLP-1 Agonism Stimulates Brown Adipose Tissue Thermogenesis and Browning through Hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults with Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.F.; Marinho, T.S.; Cardoso, L.E.M.; Barbosa-da-Silva, S.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Semaglutide (GLP-1 Receptor Agonist) Stimulates Browning on Subcutaneous Fat Adipocytes and Mitigates Inflammation and Endoplasmic Reticulum Stress in Visceral Fat Adipocytes of Obese Mice. Cell Biochem. Funct. 2022, 40, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, X.; Guo, Y.; Liu, L.; Li, H.; Tan, J.; Feng, W.; Guan, H.; Cao, X.; Xiao, H.; et al. Glucose-Dependent Insulinotropic Polypeptide Modifies Adipose Plasticity and Promotes Beige Adipogenesis of Human Omental Adipose-Derived Stem Cells. FASEB J. 2021, 35, e21534. [Google Scholar] [CrossRef]
- Look, M.; Dunn, J.P.; Kushner, R.F.; Cao, D.; Harris, C.; Gibble, T.H.; Stefanski, A.; Griffin, R. Body Composition Changes during Weight Reduction with Tirzepatide in the SURMOUNT-1 Study of Adults with Obesity or Overweight. Diabetes Obes. Metab. 2025, 27, 2720–2729. [Google Scholar] [CrossRef]
- Heise, T.; Devries, J.H.; Urva, S.; Li, J.; Pratt, E.J.; Thomas, M.K.; Mather, K.J.; Karanikas, C.A.; Dunn, J.; Haupt, A.; et al. Tirzepatide Reduces Appetite, Energy Intake, and Fat Mass in People with Type 2 Diabetes. Diabetes Care 2023, 46, 998–1004. [Google Scholar] [CrossRef]
- Wynne, K.; Park, A.J.; Small, C.J.; Meeran, K.; Ghatei, M.A.; Frost, G.S.; Bloom, S.R. Oxyntomodulin Increases Energy Expenditure in Addition to Decreasing Energy Intake in Overweight and Obese Humans: A Randomised Controlled Trial. Int. J. Obes. 2006, 30, 1729–1736. [Google Scholar] [CrossRef]
- Ji, L.; Jiang, H.; Cheng, Z.; Qiu, W.; Liao, L.; Zhang, Y.; Li, X.; Pang, S.; Zhang, L.; Chen, L.; et al. A Phase 2 Randomised Controlled Trial of Mazdutide in Chinese Overweight Adults or Adults with Obesity. Nat. Commun. 2023, 14, 8289. [Google Scholar] [CrossRef]
- Rosenstock, J.; Frias, J.; Jastreboff, A.M.; Du, Y.; Lou, J.; Gurbuz, S.; Thomas, M.K.; Hartman, M.L.; Haupt, A.; Milicevic, Z.; et al. Retatrutide, a GIP, GLP-1 and Glucagon Receptor Agonist, for People with Type 2 Diabetes: A Randomised, Double-Blind, Placebo and Active-Controlled, Parallel-Group, Phase 2 Trial Conducted in the USA. Lancet 2023, 402, 529–544. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, M.; Lee, J.Y.; Bae, J.; Shin, E.; Lee, Y.H.; Lee, B.W.; Kang, E.S.; Cha, B.S. Ipragliflozin, an SGLT2 Inhibitor, Ameliorates High-Fat Diet-Induced Metabolic Changes by Upregulating Energy Expenditure through Activation of the AMPK/SIRT1 Pathway. Diabetes Metab. J. 2021, 45, 921–932. [Google Scholar] [CrossRef]
- Op den Kamp, Y.J.M.; de Ligt, M.; Dautzenberg, B.; Kornips, E.; Esterline, R.; Hesselink, M.K.C.; Hoeks, J.; Schrauwen-Hinderling, V.B.; Havekes, B.; Oscarsson, J.; et al. Effects of the SGLT2 Inhibitor Dapagliflozin on Energy Metabolism in Patients with Type 2 Diabetes: A Randomized, Double-Blind Crossover Trial. Diabetes Care 2021, 44, 1334–1343. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, Y.; Wang, R.; Xu, Y.; Ji, H.; Zhao, Y. Effect of SGLT-2 Inhibitors on Body Composition in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2022, 17, e0279889. [Google Scholar] [CrossRef]
- Navarro-Alarcón, M.; Ruiz-Ojeda, F.J.; Blanca-Herrera, R.M.; A-Serrano, M.M.; Acuña-Castroviejo, D.; Fernández-Vázquez, G.; Agil, A. Melatonin and Metabolic Regulation: A Review. Food Funct. 2014, 5, 2806–2832. [Google Scholar] [CrossRef]
- Karamitri, A.; Jockers, R. Melatonin in Type 2 Diabetes Mellitus and Obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, Energy Metabolism, and Obesity: A Review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M. Melatonin Supplementation and Anthropometric Indicators of Obesity: A Systematic Review and Meta-Analysis. Nutrition 2021, 91–92, 111399. [Google Scholar] [CrossRef]
- Vajdi, M.; Moeinolsadat, S.; Noshadi, N.; Pourteymour Fard Tabrizi, F.; Khajeh, M.; Abbasalizad-Farhangi, M.; Alipour, B. Effect of Melatonin Supplementation on Body Composition and Blood Pressure in Adults: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trial. Heliyon 2024, 10, e34604. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Mechanisms of Melatonin in Obesity: A Review. Int. J. Mol. Sci. 2021, 23, 218. [Google Scholar] [CrossRef]
- Salagre, D.; Navarro-Alarcón, M.; Villalón-Mir, M.; Alcázar-Navarrete, B.; Gómez-Moreno, G.; Tamimi, F.; Agil, A. Chronic Melatonin Treatment Improves Obesity by Inducing Uncoupling of Skeletal Muscle SERCA-SLN Mediated by CaMKII/AMPK/PGC1α Pathway and Mitochondrial Biogenesis in Female and Male Zücker Diabetic Fatty Rats. Biomed. Pharmacother. 2024, 172, 116314. [Google Scholar] [CrossRef]
- Amaral, F.G.D.; Andrade-Silva, J.; Kuwabara, W.M.T.; Cipolla-Neto, J. New Insights into the Function of Melatonin and Its Role in Metabolic Disturbances. Expert. Rev. Endocrinol. Metab. 2019, 14, 293–300. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Vigo, D.E. Melatonin, Mitochondria, and the Metabolic Syndrome. Cell. Mol. Life Sci. 2017, 74, 3941–3954. [Google Scholar] [CrossRef]
- Agil, A.; Navarro-Alarcon, M.; Ali, F.A.Z.; Albrakati, A.; Salagre, D.; Campoy, C.; Elmahallawy, E.K. Melatonin Enhances the Mitochondrial Functionality of Brown Adipose Tissue in Obese-Diabetic Rats. Antioxidants 2021, 10, 1482. [Google Scholar] [CrossRef]
- Fernández Vázquez, G.; Reiter, R.J.; Agil, A. Melatonin Increases Brown Adipose Tissue Mass and Function in Zücker Diabetic Fatty Rats: Implications for Obesity Control. J. Pineal Res. 2018, 64, e12472. [Google Scholar] [CrossRef]
- Jiménez-Aranda, A.; Fernández-Vázquez, G.; Campos, D.; Tassi, M.; Velasco-Perez, L.; Tan, D.X.; Reiter, R.J.; Agil, A. Melatonin Induces Browning of Inguinal White Adipose Tissue in Zucker Diabetic Fatty Rats. J. Pineal Res. 2013, 55, 416–423. [Google Scholar] [CrossRef]
- Agil, A.; Rosado, I.; Ruiz, R.; Figueroa, A.; Zen, N.; Fernández-Vázquez, G. Melatonin Improves Glucose Homeostasis in Young Zucker Diabetic Fatty Rats. J. Pineal Res. 2012, 52, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Navarro-Alarcõn, M.; Ruiz, R.; Abuhamadah, S.; El-Mir, M.Y.; Vázquez, G.F. Beneficial Effects of Melatonin on Obesity and Lipid Profile in Young Zucker Diabetic Fatty Rats. J. Pineal Res. 2011, 50, 207–212. [Google Scholar] [CrossRef]
- Salagre, D.; Bajit, H.; Fernández-Vázquez, G.; Dwairy, M.; Garzón, I.; Haro-López, R.; Agil, A. Melatonin Induces Fiber Switching by Improvement of Mitochondrial Oxidative Capacity and Function via NRF2/RCAN/MEF2 in the Vastus Lateralis Muscle from Both Sex Zücker Diabetic Fatty Rats. Free Radic. Biol. Med. 2025, 227, 322–335. [Google Scholar] [CrossRef]
- Agil, A.; Reiter, R.J.; Jiménez-Aranda, A.; Ibán-Arias, R.; Navarro-Alarcón, M.; Marchal, J.A.; Adem, A.; Fernández-Vázquez, G. Melatonin Ameliorates Low-Grade Inflammation and Oxidative Stress in Young Zucker Diabetic Fatty Rats. J. Pineal Res. 2013, 54, 381–388. [Google Scholar] [CrossRef]
- Jimenéz-Aranda, A.; Fernández-Vázquez, G.; Mohammad A-Serrano, M.; Reiter, R.J.; Agil, A. Melatonin Improves Mitochondrial Function in Inguinal White Adipose Tissue of Zücker Diabetic Fatty Rats. J. Pineal Res. 2014, 57, 103–109. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Bernatoniene, J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Protective Role of Melatonin in Mitochondrial Dysfunction and Related Disorders. Arch. Toxicol. 2015, 89, 923–939. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the Electron Transport Chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef] [PubMed]
- Salagre, D.; Raya Álvarez, E.; Cendan, C.M.; Aouichat, S.; Agil, A. Melatonin Improves Skeletal Muscle Structure and Oxidative Phenotype by Regulating Mitochondrial Dynamics and Autophagy in Zücker Diabetic Fatty Rat. Antioxidants 2023, 12, 1499. [Google Scholar] [CrossRef]
- Xu, L.; Li, D.; Li, H.; Zhang, O.; Huang, Y.; Shao, H.; Wang, Y.; Cai, S.; Zhu, Y.; Jin, S.; et al. Suppression of Obesity by Melatonin through Increasing Energy Expenditure and Accelerating Lipolysis in Mice Fed a High-Fat Diet. Nutr. Diabetes 2022, 12, 42. [Google Scholar] [CrossRef]
- de Almeida Chuffa, L.G.; Seiva, F.R.F.; Silveira, H.S.; Cesário, R.C.; da Silva Tonon, K.; Simão, V.A.; Zuccari, D.A.P.C.; Reiter, R.J. Melatonin Regulates Endoplasmic Reticulum Stress in Diverse Pathophysiological Contexts: A Comprehensive Mechanistic Review. J. Cell Physiol. 2024, 239, e31383. [Google Scholar] [CrossRef]
- Fernández-Mateos, P.; Cano-Barquilla, P.; Jiménez-Ortega, V.; Virto, L.; Pérez-Miguelsanz, J.; Esquifino, A.I. Effect of Melatonin on Redox Enzymes Daily Gene Expression in Perirenal and Subcutaneous Adipose Tissue of a Diet Induced Obesity Model. Int. J. Mol. Sci. 2023, 24, 960. [Google Scholar] [CrossRef]
- Wang, L.; McFadden, J.W.; Yang, G.; Zhu, H.; Lian, H.; Fu, T.; Sun, Y.; Gao, T.; Li, M. Effect of Melatonin on Visceral Fat Deposition, Lipid Metabolism and Hepatic Lipo-Metabolic Gene Expression in Male Rats. J. Anim. Physiol. Anim. Nutr. 2021, 105, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Salagre, D.; Navarro-Alarcón, M.; González, L.G.; Elrayess, M.A.; Villalón-Mir, M.; Haro-López, R.; Agil, A. Melatonin Ameliorates Organellar Calcium Homeostasis, Improving Endoplasmic Reticulum Stress-Mediated Apoptosis in the Vastus Lateralis Muscle of Both Sexes of Obese Diabetic Rats. Antioxidants 2024, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wang, C.; Yang, G.; Maimaiti, D.; Hou, M.; Liu, H.; Yang, H.; Chen, X.; Xu, Y.; He, F. Enhancement of Mitochondrial Energy Metabolism by Melatonin Promotes Vascularized Skeletal Muscle Regeneration in a Volumetric Muscle Loss Model. Free Radic. Biol. Med. 2024, 210, 146–157. [Google Scholar] [CrossRef] [PubMed]
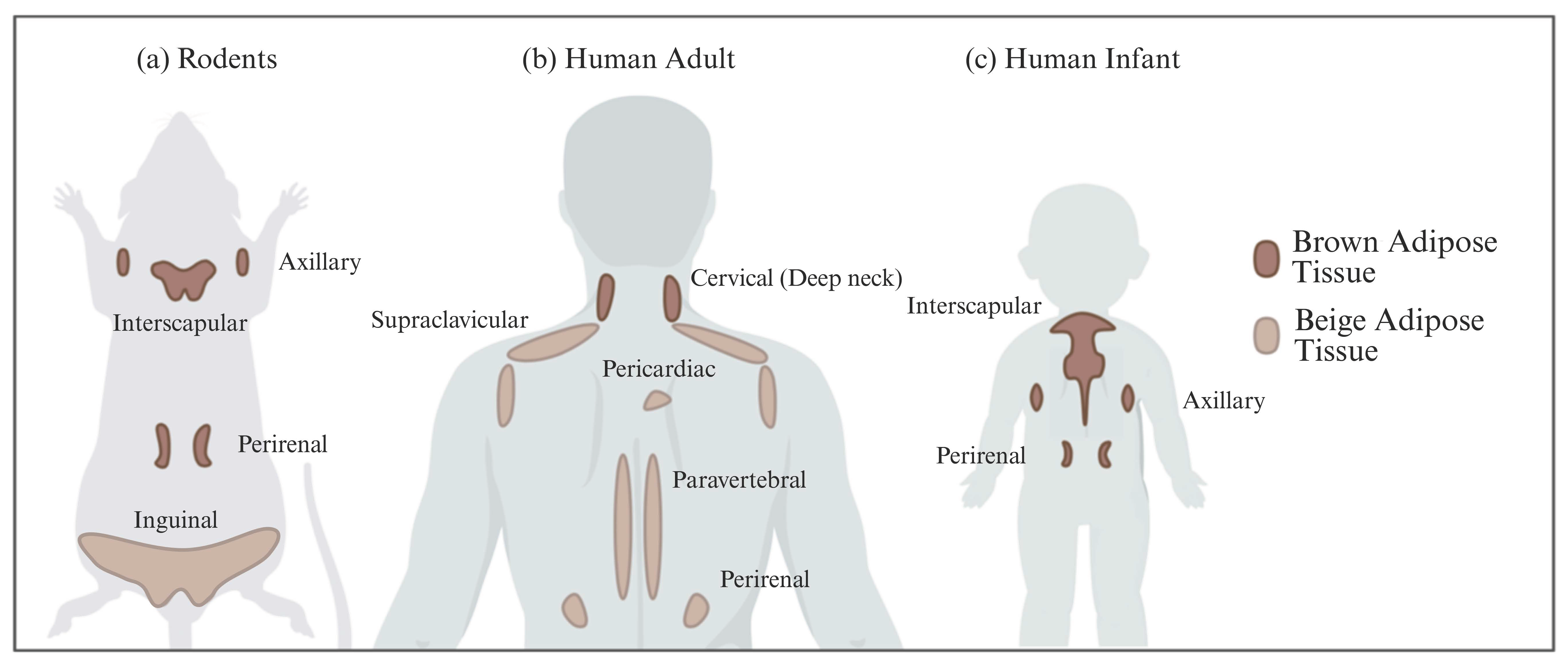
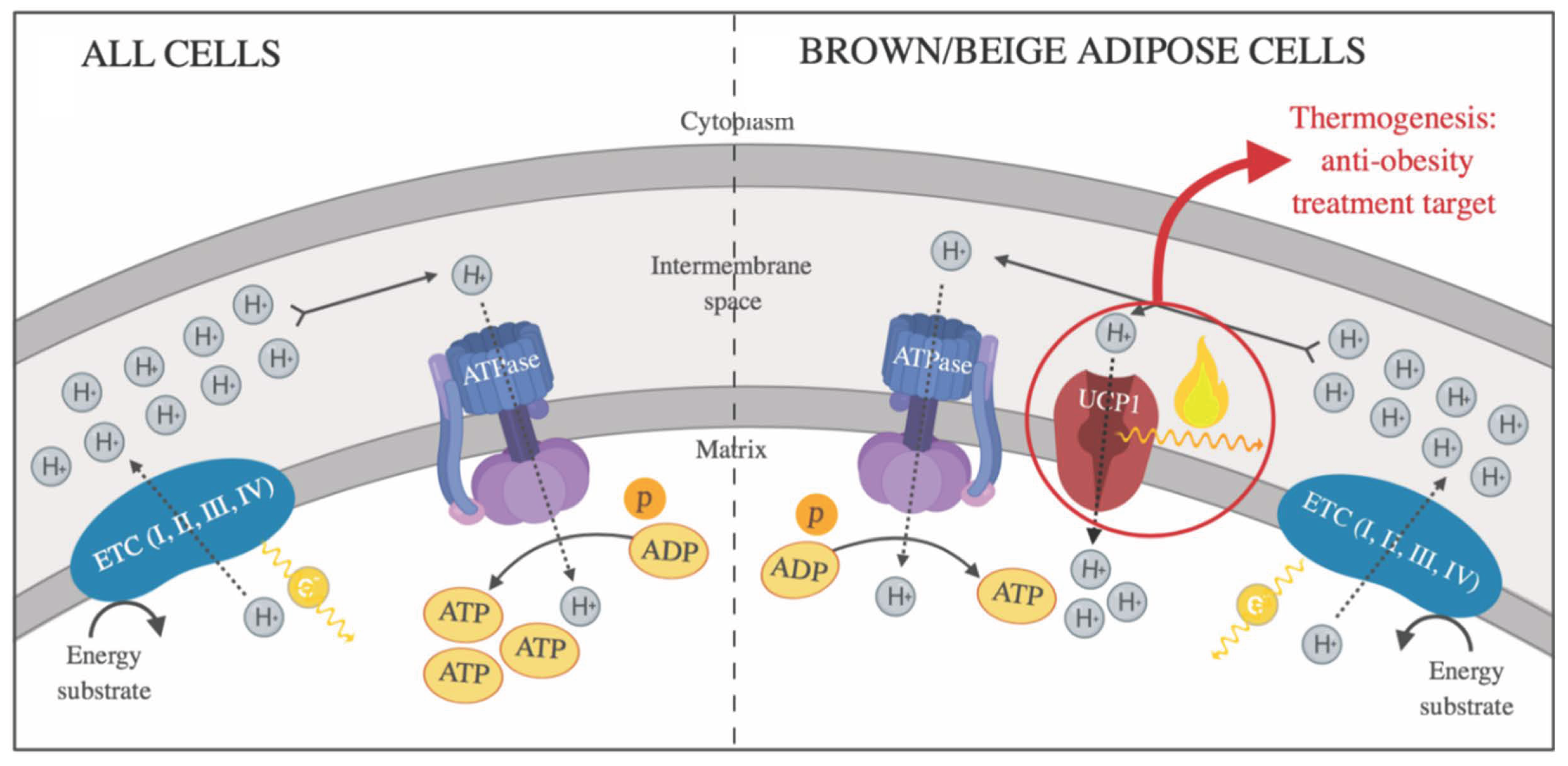
| Characteristics | White Adipose Tissue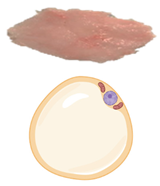 | Brown Adipose Tissue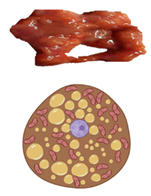 | Beige Adipose Tissue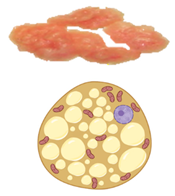 | |
|---|---|---|---|---|
| Lipid morphology | Single large droplet | Small multilocular droplets | Small/Medium multilocular droplets | |
| Mitochondrial content | Low | Very high | High | |
| UCP1 protein expression | − | +++ | ++ | |
| Vascularity | Low | Abundant | High | |
| Location | Rodents & human infants | Intra-abdominal (Visceral) Subcutaneous | Interscapular Axillary Perirenal | Inguinal (rodents) |
| Adult humans | Intra-abdominal (Visceral) Subcutaneous | Cervical (Deep neck) | Supraclavicular Pericardiac Paravertebral Perirenal | |
| Function | Energy storage as triglycerides | Heat production (Non-shivering thermogenesis) | Thermogenic potential | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salagre, D.; Ayala-Mosqueda, C.V.; Aouichat, S.; Agil, A. Physiological Conditions, Bioactive Ingredients, and Drugs Stimulating Non-Shivering Thermogenesis as a Promising Treatment Against Diabesity. Pharmaceuticals 2025, 18, 1247. https://doi.org/10.3390/ph18091247
Salagre D, Ayala-Mosqueda CV, Aouichat S, Agil A. Physiological Conditions, Bioactive Ingredients, and Drugs Stimulating Non-Shivering Thermogenesis as a Promising Treatment Against Diabesity. Pharmaceuticals. 2025; 18(9):1247. https://doi.org/10.3390/ph18091247
Chicago/Turabian StyleSalagre, Diego, Ciskey V. Ayala-Mosqueda, Samira Aouichat, and Ahmad Agil. 2025. "Physiological Conditions, Bioactive Ingredients, and Drugs Stimulating Non-Shivering Thermogenesis as a Promising Treatment Against Diabesity" Pharmaceuticals 18, no. 9: 1247. https://doi.org/10.3390/ph18091247
APA StyleSalagre, D., Ayala-Mosqueda, C. V., Aouichat, S., & Agil, A. (2025). Physiological Conditions, Bioactive Ingredients, and Drugs Stimulating Non-Shivering Thermogenesis as a Promising Treatment Against Diabesity. Pharmaceuticals, 18(9), 1247. https://doi.org/10.3390/ph18091247







