Network Pharmacology and Experimental Validation Identify Paeoniflorin as a Novel SRC-Targeted Therapy for Castration-Resistant Prostate Cancer
Abstract
1. Introduction
2. Results
2.1. Identification of Paeoniflorin Potential Targets in Prostate Cancer
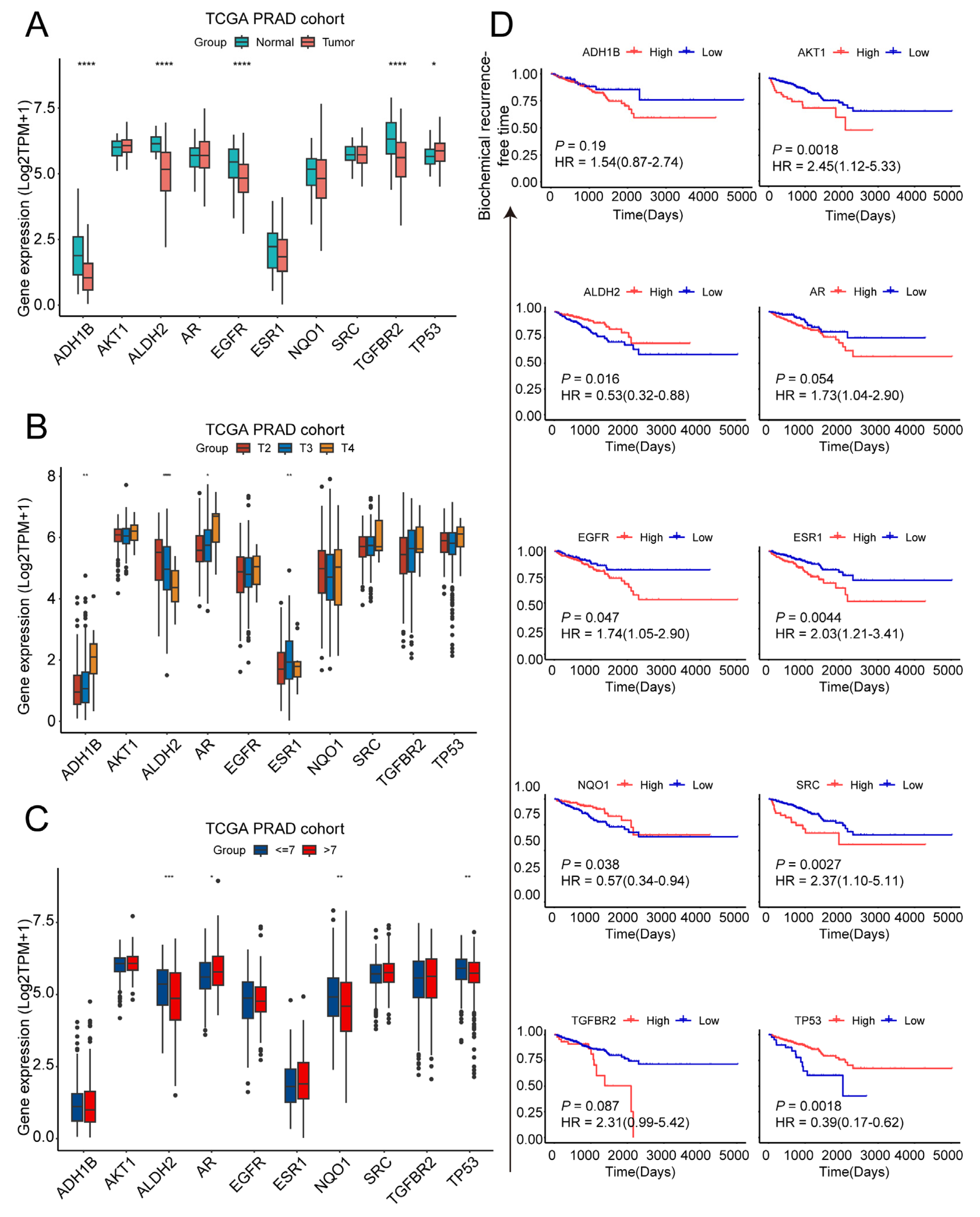
2.2. Exploration of Clinical Characteristics of Ten Target Genes in Prostate Cancer
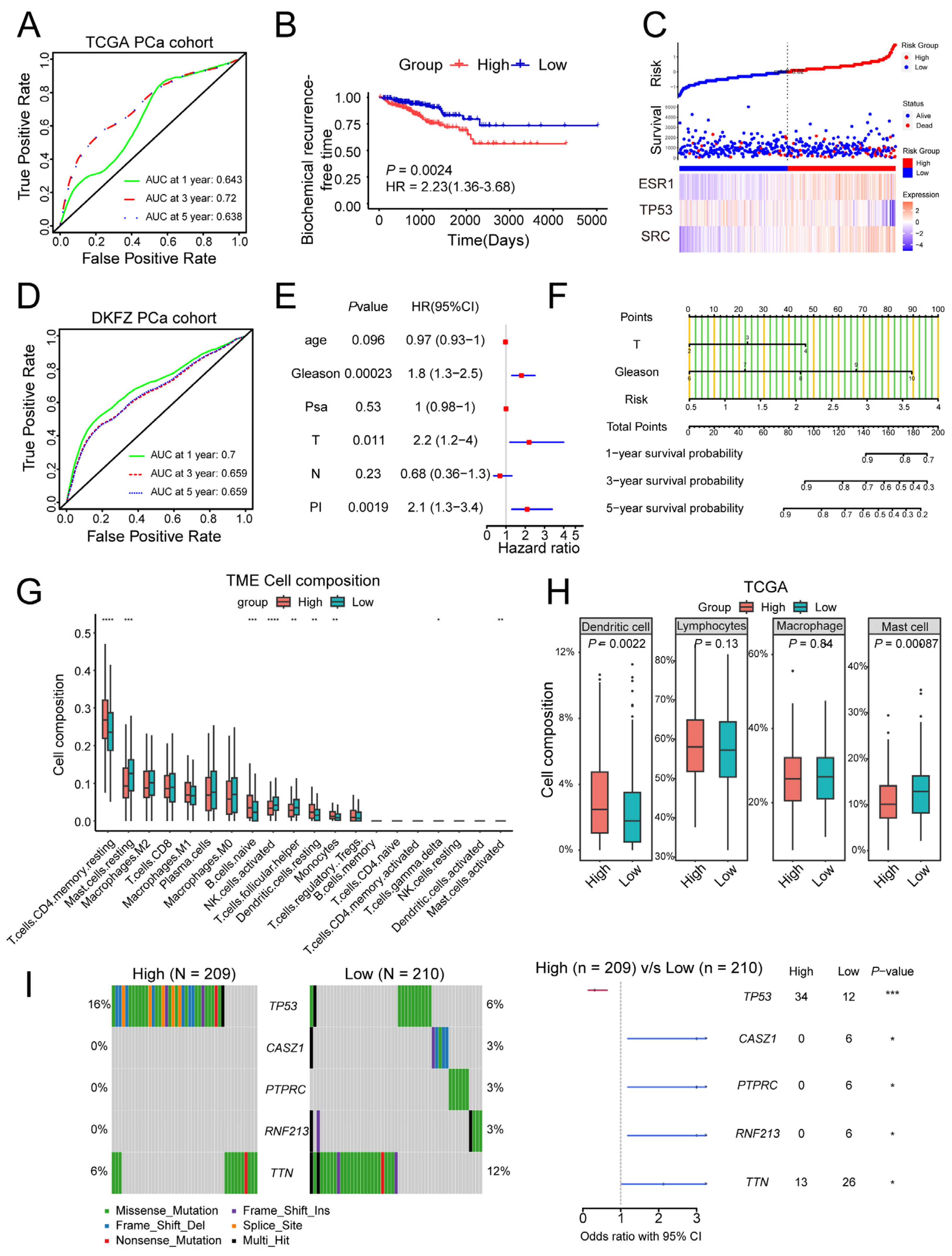
2.3. Establishment of a Prognosis Signature Based on Paeoniflorin Target Genes
2.4. Molecular Docking of Paeoniflorin Target Genes
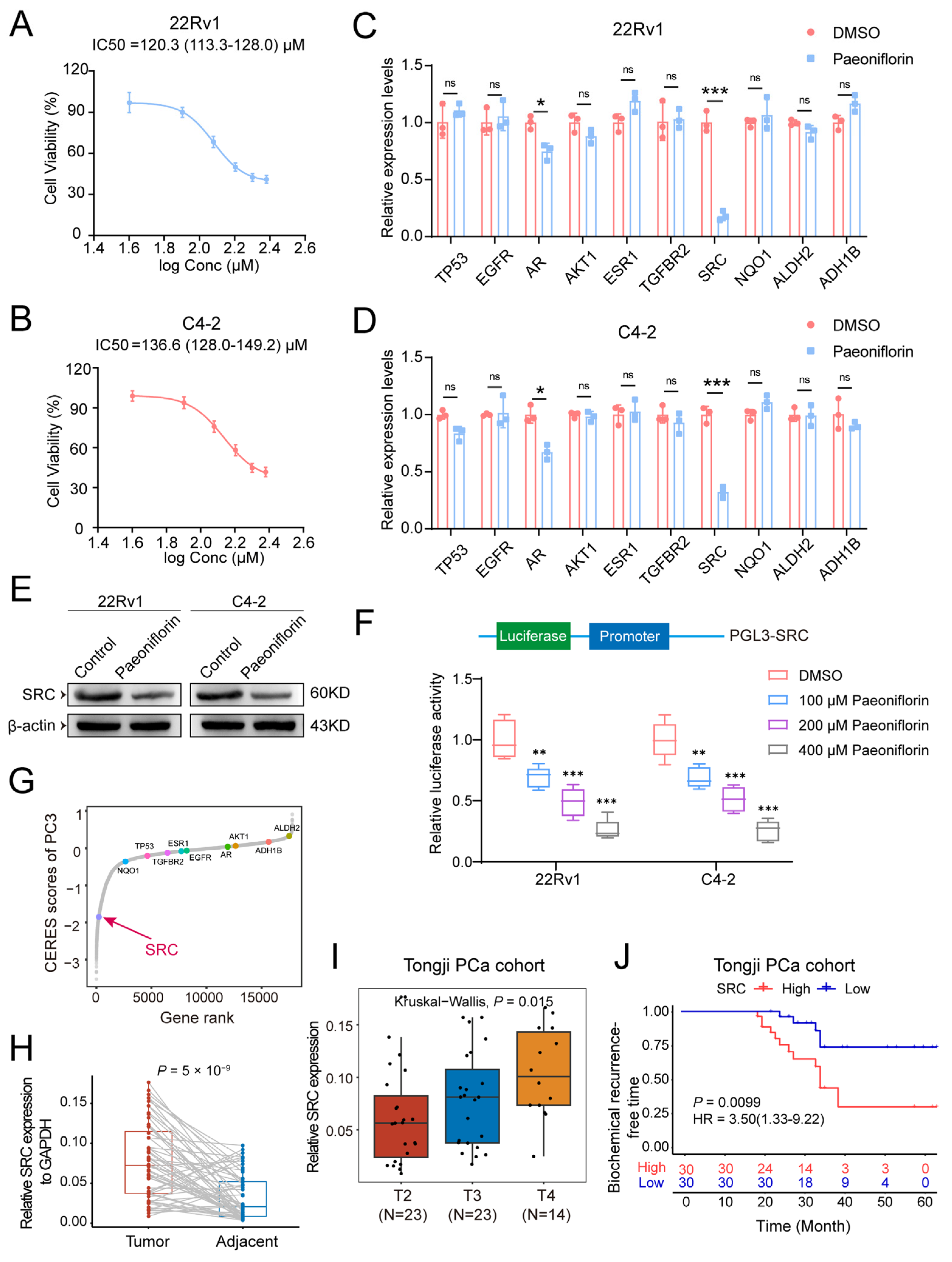
2.5. Paeoniflorin Reduces SRC Expression Levels in Prostate Cancer
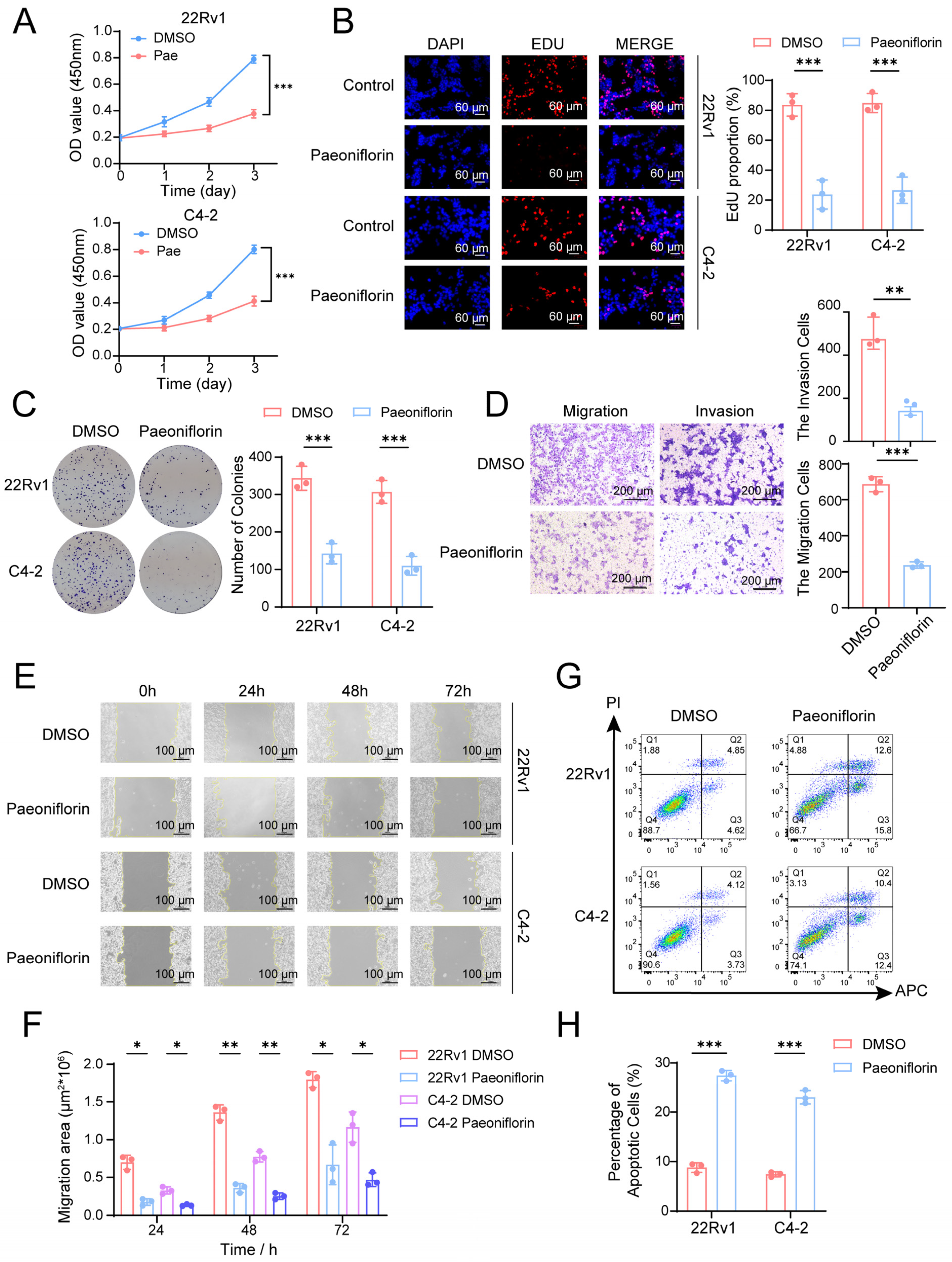
2.6. Paeoniflorin Effectively Inhibits CRPC Cell Proliferation and Metastasis and Induces Apoptosis
2.7. Paeoniflorin Has Therapeutic Effects on Patient-Derived Organoids and Subcutaneous Xenograft Tumors in Nude Mice
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Identification of Targets for Pae
4.3. GO and KEGG Enrichment Analysis
4.4. Establishment of Prognosis Signature
4.5. Molecular Docking
4.6. Cell Culture
4.7. Human PCa Tissues
4.8. Extraction of Protein and Western Blot
4.9. Luciferase Reporter Assay
4.10. RNA Isolation and Real-Time Quantitative PCR
4.11. Cell Counting Kit-8 (CCK-8) Assay
4.12. EdU Cell Proliferation Assay
4.13. Colony Formation Assay
4.14. Transwell Migration and Invasion Assay
4.15. Wound Healing Assay
4.16. Apoptosis Flow Cytometry Assay
4.17. Organoid Models
4.18. Experimental Animals
4.19. IHC and H&E Analysis
4.20. Survival Prognosis Analysis
4.21. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | Androgen deprivation therapy |
| BCR | Biochemical recurrence |
| CCK-8 | Cell counting Kit-8 |
| CRPC | Castration-resistant prostate cancer |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| Pae | Paeoniflorin |
| PCa | Prostate cancer |
| PPI | Protein–protein interaction |
| TCGA | The Cancer Genome Atlas |
| TCM | Traditional Chinese medicine |
References
- Qiu, H.; Cao, S.; Xu, R. Cancer incidence, mortality, and burden in China: A time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun. 2021, 41, 1037–1048. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar]
- Teo, M.Y.; Rathkopf, D.E.; Kantoff, P. Treatment of Advanced Prostate Cancer. Annu. Rev. Med. 2019, 70, 479–499. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.F.; Jiang, W.P.; Basavaraj, P.; Huang, S.Y.; Ruangsai, P.; Wu, J.B.; Huang, G.J.; Huang, W.C. Cell suspension culture extract of Eriobotrya japonica attenuates growth and induces apoptosis in prostate cancer cells via targeting SREBP-1/FASN-driven metabolism and AR. Phytomed. Int. J. Phytother. Phytopharm. 2021, 93, 153806. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.H.; Sung, K.W.; Pham, T.M.; Najy, A.J.; Zamiri, A.; Jang, H.; Mun, S.R.; Kim, S.; Kwon, H.K.; Son, Y.S.; et al. An Autophagy-Targeting Chimera Induces Degradation of Androgen Receptor Mutants and AR-v7 in Castration-Resistant Prostate Cancer. Cancer Res. 2025, 85, 342–359. [Google Scholar] [CrossRef] [PubMed]
- Rodarte, K.E.; Nir Heyman, S.; Guo, L.; Flores, L.; Savage, T.K.; Villarreal, J.; Deng, S.; Xu, L.; Shah, R.B.; Oliver, T.G.; et al. Neuroendocrine Differentiation in Prostate Cancer Requires ASCL1. Cancer Res. 2024, 84, 3522–3537. [Google Scholar] [CrossRef]
- Cai, M.; Song, X.L.; Li, X.A.; Chen, M.; Guo, J.; Yang, D.H.; Chen, Z.; Zhao, S.C. Current therapy and drug resistance in metastatic castration-resistant prostate cancer. Drug Resist. Updates 2023, 68, 100962. [Google Scholar] [CrossRef]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Zhang, L.; Troccoli, C.I.; Mateo-Victoriano, B.; Misiara Lincheta, L.; Jackson, E.; Shu, P.; Plastini, T.; Tao, W.; Kwon, D.; Chen, X.S.; et al. Stimulating Soluble Guanylyl Cyclase with the Clinical Agonist Riociguat Restrains the Development and Progression of Castration-Resistant Prostate Cancer. Cancer Res. 2025, 85, 134–153. [Google Scholar] [CrossRef]
- Shu, F.; Liu, H.; Chen, X.; Liu, Y.; Zhou, J.; Tang, L.; Cao, W.; Yang, S.; Long, Y.; Li, R.; et al. m6A Modification Promotes EMT and Metastasis of Castration-Resistant Prostate Cancer by Upregulating NFIB. Cancer Res. 2024, 84, 1947–1962. [Google Scholar] [CrossRef]
- Efferth, T.; Oesch, F. Repurposing of plant alkaloids for cancer therapy: Pharmacology and toxicology. Semin. Cancer Biol. 2021, 68, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, S.; Wang, N.; Zheng, Y.; Zhou, J.; Hong, M.; Chen, Z.; Wang, S.; Wang, Z.; Xiang, S. Icariin inhibits prostate cancer bone metastasis and destruction via suppressing TAM/CCL5-mediated osteoclastogenesis. Phytomed. Int. J. Phytother. Phytopharm. 2023, 120, 155076. [Google Scholar] [CrossRef] [PubMed]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, Y.; Liu, X.; Wu, Z.; Li, J.; Ma, Z. Roles of plant-derived bioactive compounds and related microRNAs in cancer therapy. Phytother. Res. 2021, 35, 1176–1186. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, S.A.; Jung, H.; Kim, E.; Kim, Y.K.; Kim, S.; Kim, J.; Lee, Y.; Jo, M.K.; Woo, J.; et al. Novel insights into paclitaxel’s role on tumor-associated macrophages in enhancing PD-1 blockade in breast cancer treatment. J. Immunother. Cancer 2024, 12, e008864. [Google Scholar] [CrossRef]
- Chu, B.; Deng, H.; Niu, T.; Qu, Y.; Qian, Z. Stimulus-Responsive Nano-Prodrug Strategies for Cancer Therapy: A Focus on Camptothecin Delivery. Small Methods 2024, 8, e2301271. [Google Scholar] [CrossRef]
- Asghar, S.; Khan, I.U.; Salman, S.; Khalid, S.H.; Ashfaq, R.; Vandamme, T.F. Plant-derived nanotherapeutic systems to counter the overgrowing threat of resistant microbes and biofilms. Adv. Drug. Deliv. Rev. 2021, 179, 114019. [Google Scholar] [CrossRef]
- Ma, W.; Ren, H.; Meng, X.; Liu, S.; Du, K.; Fang, S.; Chang, Y. A review of the ethnopharmacology, phytochemistry, pharmacology, pharmacokinetics and quality control of Paeonia lactiflora Pall. J. Ethnopharmacol. 2024, 335, 118616. [Google Scholar] [CrossRef]
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef]
- Ahmed, I.; Yang, S.H.; Ogden, S.; Zhang, W.; Li, Y.; Sharrocks, A.D. eRNA profiling uncovers the enhancer landscape of oesophageal adenocarcinoma and reveals new deregulated pathways. eLife 2023, 12, e80840. [Google Scholar] [CrossRef]
- Li, H.; Sun, X.; Zhang, J.; Sun, Y.; Huo, R.; Li, H.; Zhai, T.; Shen, B.; Zhang, M.; Li, N. Paeoniflorin ameliorates symptoms of experimental Sjogren’s syndrome associated with down-regulating Cyr61 expression. Int. Immunopharmacol. 2016, 30, 27–35. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhao, W.; Chen, X.; Shen, B.; Wang, T.; Wu, C.; Rong, X. Deciphering the action mechanism of paeoniflorin in suppressing pancreatic cancer: A network pharmacology study and experimental validation. Front. Pharmacol. 2022, 13, 1032282. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, D.; Jiang, C.; Jin, Q.; Zhang, J. Paeoniflorin inhibits hepatocellular carcinoma growth by reducing PD-L1 expression. Biomed. Pharmacother. 2023, 166, 115317. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W. Paeoniflorin Induces ER Stress-Mediated Apoptotic Cell Death by Generating Nox4-Derived ROS under Radiation in Gastric Cancer. Nutrients 2023, 15, 5092. [Google Scholar] [CrossRef]
- Zhai, J.; Guo, Y. Paeoniflorin attenuates cardiac dysfunction in endotoxemic mice via the inhibition of nuclear factor-κB. Biomed. Pharmacother. 2016, 80, 200–206. [Google Scholar] [CrossRef]
- Cha, E.; Fong, L. Immunotherapy for prostate cancer: Biology and therapeutic approaches. J. Clin. Oncol. 2011, 29, 3677–3685. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Buonerba, C.; Kantoff, P.W. Immunotherapy for the treatment of prostate cancer. Nat. Rev. Clin. Oncol. 2011, 8, 551–561. [Google Scholar] [CrossRef]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major enhancements for small-molecule docking with Attracting Cavities and AutoDock Vina. Nucleic Acids. Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Polak, R.; Zhang, E.T.; Kuo, C.J. Cancer organoids 2.0: Modelling the complexity of the tumour immune microenvironment. Nat. Rev. Cancer 2024, 24, 523–539. [Google Scholar] [CrossRef]
- LeSavage, B.L.; Suhar, R.A.; Broguiere, N.; Lutolf, M.P.; Heilshorn, S.C. Next-generation cancer organoids. Nat. Mater. 2022, 21, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 2020, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Corsini, N.S.; Knoblich, J.A. Human organoids: New strategies and methods for analyzing human development and disease. Cell 2022, 185, 2756–2769. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef]
- Wang, L.Y.; Hung, C.L.; Wang, T.C.; Hsu, H.C.; Kung, H.J.; Lin, K.H. PROTACs as Therapeutic Modalities for Drug Discovery in Castration-Resistant Prostate Cancer. Annu. Rev. Pharmacol. Toxicol. 2024, 65, 375–396. [Google Scholar] [CrossRef]
- Liu, R.J.; Xu, Z.P.; Huang, X.; Xu, B.; Chen, M. Yin Yang 1 promotes the neuroendocrine differentiation of prostate cancer cells via the non-canonical WNT pathway (FYN/STAT3). Clin. Transl. Med. 2023, 13, e1422. [Google Scholar] [CrossRef]
- Wang, X.; Fang, G.; Pang, Y. Chinese Medicines in the Treatment of Prostate Cancer: From Formulas to Extracts and Compounds. Nutrients 2018, 10, 283. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Chen, C.; Tong, X.; Li, Y.; Yang, K.; Lv, H.; Xu, J.; Qin, L. Holo-lactoferrin: The link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics 2021, 11, 3167–3182. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, C.; Gu, X.; Wang, X.; Zhang, G.; Fan, M.; Zhao, Y.; Liu, X.; Zhang, X. Paeoniflorin alleviated muscle atrophy in cancer cachexia through inhibiting TLR4/NF-κB signaling and activating AKT/mTOR signaling. Toxicol. Appl. Pharmacol. 2024, 484, 116846. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, L.; Du, Y.; Ye, M.; Lu, D.; Huang, S.; Zhao, J.; Tibenda, J.J.; Meng, F.; Nan, Y. Network pharmacology and in vitro experiments to investigate the anti-gastric cancer effects of paeoniflorin through the RAS/MAPK signaling pathway. Discov. Oncol. 2024, 15, 659. [Google Scholar] [CrossRef]
- Mayer, E.L.; Krop, I.E. Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin. Cancer Res. 2010, 16, 3526–3532. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Xia, L.; Zhang, X.Y.; Chen, Q.; Li, X.; Mou, Y.; Wang, T.; Zhang, Y.N. The multifaceted mechanisms of Paeoniflorin in the treatment of tumors: State-of-the-Art. Biomed. Pharmacother. 2022, 149, 112800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S. Modulating Bcl-2 family proteins and caspase-3 in induction of apoptosis by paeoniflorin in human cervical cancer cells. Phytother. Res. 2011, 25, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, Y.; Cui, J.; Xiao, T.; Jiang, D. Paeoniflorin inhibits proliferation and invasion of breast cancer cells through suppressing Notch-1 signaling pathway. Biomed. Pharmacother. 2016, 78, 197–203. [Google Scholar] [CrossRef]
- Esposito, A.; Ferraresi, A.; Vallino, L.; Garavaglia, B.; Dhanasekaran, D.N.; Isidoro, C. Three-Dimensional In Vitro Cell Cultures as a Feasible and Promising Alternative to Two-Dimensional and Animal Models in Cancer Research. Int. J. Biol. Sci. 2024, 20, 5293–5311. [Google Scholar] [CrossRef]
- Pauli, C.; Hopkins, B.D.; Prandi, D.; Shaw, R.; Fedrizzi, T.; Sboner, A.; Sailer, V.; Augello, M.; Puca, L.; Rosati, R.; et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017, 7, 462–477. [Google Scholar] [CrossRef]
- Phillips, R. Innovation: Organoids-a better model for prostate cancer. Nat. Rev. Urol. 2014, 11, 604. [Google Scholar] [CrossRef]
- Huang, L.; Holtzinger, A.; Jagan, I.; BeGora, M.; Lohse, I.; Ngai, N.; Nostro, C.; Wang, R.; Muthuswamy, L.B.; Crawford, H.C.; et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med. 2015, 21, 1364–1371. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Rappaport, N.; Twik, M.; Plaschkes, I.; Nudel, R.; Iny Stein, T.; Levitt, J.; Gershoni, M.; Morrey, C.P.; Safran, M.; Lancet, D. MalaCards: An amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic. Acids. Res. 2016, 45, D877–D887. [Google Scholar] [CrossRef]
- Amberger, J.S.; Hamosh, A. Searching Online Mendelian Inheritance in Man (OMIM): A Knowledgebase of Human Genes and Genetic Phenotypes. Curr. Protoc. Bioinform. 2017, 58, 1–2. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.; Feng, B.; Yu, S.; Wang, Q.; Zhu, F.; Zhu, H. Multivariate Regression Models for Predicting the Prognosis of Luxation Injuries of Permanent Teeth: Periodontal and Pulp Analyses; Dental Traumatology: Official Publication of International Association for Dental Traumatology: San Diego, CA, USA, 2024. [Google Scholar]
- Guan, Y.; Zheng, L.; Wang, Y.; Xu, X.; Zhu, Y. Risk factors for death or serious adverse events within 3-month after surgery in frail older persons with hip fractures and development and validation of a risk prediction model: Based on LASSO-logistic regression. BMC Geriatr. 2025, 25, 463. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Mayakonda, A.; Lin, D.C.; Assenov, Y.; Plass, C.; Koeffler, H.P. Maftools: Efficient and comprehensive analysis of somatic variants in cancer. Genome. Res. 2018, 28, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Ormö, M.; Cubitt, A.B.; Kallio, K.; Gross, L.A.; Tsien, R.Y.; Remington, S.J. Crystal structure of the Aequorea victoria green fluorescent protein. Science 1996, 273, 1392–1395. [Google Scholar] [CrossRef]
- Hou, X.; Du, J.; Zhang, J.; Du, L.; Fang, H.; Li, M. How to improve docking accuracy of AutoDock4.2: A case study using different electrostatic potentials. J. Chem. Inf. Model. 2013, 53, 188–200. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.-Y.; Zhang, J.-B.; Peng, Y.-Z.; Liu, M.-C.; Ma, S.-Y.; Zhou, Y.; Wang, Z.-H.; Ma, S. Network Pharmacology and Experimental Validation Identify Paeoniflorin as a Novel SRC-Targeted Therapy for Castration-Resistant Prostate Cancer. Pharmaceuticals 2025, 18, 1241. https://doi.org/10.3390/ph18081241
Xu M-Y, Zhang J-B, Peng Y-Z, Liu M-C, Ma S-Y, Zhou Y, Wang Z-H, Ma S. Network Pharmacology and Experimental Validation Identify Paeoniflorin as a Novel SRC-Targeted Therapy for Castration-Resistant Prostate Cancer. Pharmaceuticals. 2025; 18(8):1241. https://doi.org/10.3390/ph18081241
Chicago/Turabian StyleXu, Meng-Yao, Jun-Biao Zhang, Yu-Zheng Peng, Mei-Cheng Liu, Si-Yang Ma, Ye Zhou, Zhi-Hua Wang, and Sheng Ma. 2025. "Network Pharmacology and Experimental Validation Identify Paeoniflorin as a Novel SRC-Targeted Therapy for Castration-Resistant Prostate Cancer" Pharmaceuticals 18, no. 8: 1241. https://doi.org/10.3390/ph18081241
APA StyleXu, M.-Y., Zhang, J.-B., Peng, Y.-Z., Liu, M.-C., Ma, S.-Y., Zhou, Y., Wang, Z.-H., & Ma, S. (2025). Network Pharmacology and Experimental Validation Identify Paeoniflorin as a Novel SRC-Targeted Therapy for Castration-Resistant Prostate Cancer. Pharmaceuticals, 18(8), 1241. https://doi.org/10.3390/ph18081241






