Safety Considerations for Natural Products with Adaptogenic and Immunomodulating Activities
Abstract
1. Introduction
2. Results
2.1. Scoping Review
2.1.1. Single-Ingredient Products
Adverse Events Reported in Clinical Trials
Adverse Events Reported in Case Studies
Herb–Drug Interactions
2.1.2. Multi-Ingredient Products
Adverse Events Reported in Clinical Trials
Adverse Events in the Case Studies
2.2. Analysis of Reported Adverse Events from the Global ICSR Database VigiBase
2.2.1. Single-Ingredient Products
2.2.2. Multi-Ingredient Products
3. Discussion
3.1. Single-Ingredient Products
3.1.1. Adverse Events Reported in Clinical Trials from the Scoping Review
3.1.2. Serious Adverse Events Reported in Clinical Trials Retrieved in the Scoping Review
3.1.3. Adverse Events Reported in Case Studies Retrieved in the Scoping Review
3.1.4. Herb–Drug Interactions
3.1.5. Analysis of Reported Adverse Events from the Global ICSR Database VigiBase
3.2. Geographic and Population Trends of the ICSRs
3.3. Multi-Ingredient Products
Adverse Events Reported in Clinical Trials from the Scoping Review
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Scoping Review
- Inclusion: Clinical trials, case reports, and randomized controlled trials (RCTs) involving human subjects that specifically assess the safety or AEs of herbal products and fungi with presumed adaptogenic or immunomodulatory properties.
- Exclusion: Review articles; studies solely based on animal or in vitro models; papers without accessible full texts or without mention of AEs; and publications in languages other than Dutch or English.
4.2. Data Extraction and Analysis
4.2.1. Scoping Review
4.2.2. Global Individual Case Safety Reports Database (WHO-UMC) VigiBase
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE(s) | Adverse Event(s) |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| EMA | European Medicines Agency |
| EGCG | Epigallocatechin Gallate |
| FDA | Food and Drug Administration |
| GTE | Green Tea Extract |
| ICSR(s) | Individual Case Safety Report(s) |
| JBI | Joanna Briggs Institute |
| MAH(s) | Marketing Authorization Holder(s) |
| MRI | Magnetic Resonance Imaging |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews |
| PT(s) | Preferred Term(s) |
| RCT(s) | Randomized Controlled Trial(s) |
| RUCAM | Roussel Uclaf Causality Assessment Method |
| SAE(s) | Serious Adverse Event(s) |
| SOC(s) | System Organ Class(es) |
| TCM | Traditional Chinese Medicine |
| UMC | Uppsala Monitoring Centre |
| WHO | World Health Organization |
References
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens—History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef]
- Zebeaman, M.; Tadesse, M.G.; Bachheti, R.K.; Bachheti, A.; Gebeyhu, R.; Chaubey, K.K. Plants and Plant-Derived Molecules as Natural Immunomodulators. BioMed Res. Int. 2023, 2023, 7711297. [Google Scholar] [CrossRef]
- Adaptogenic Concept—Scientific Guideline|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/adaptogenic-concept-scientific-guideline (accessed on 18 September 2024).
- Panossian, A.; Wikman, G. Effects of Adaptogens on the Central Nervous System and the Molecular Mechanisms Associated with Their Stress—Protective Activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-Y.; He, Y.-F.; Li, L.; Meng, H.; Dong, Y.-M.; Yi, F.; Xiao, P.-G. A Preliminary Review of Studies on Adaptogens: Comparison of Their Bioactivity in TCM with That of Ginseng-like Herbs Used Worldwide. Chin. Med. 2018, 13, 57. [Google Scholar] [CrossRef]
- Rhodiolae Roseae Rhizoma et Radix—Herbal Medicinal Product|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/herbal/rhodiolae-roseae-rhizoma-et-radix (accessed on 18 September 2024).
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-Blind, Randomized, Placebo-Controlled Clinical Study. Cureus 2019, 11, e6466. [Google Scholar] [CrossRef] [PubMed]
- Ginseng Radix—Herbal Medicinal Product|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/herbal/ginseng-radix (accessed on 18 September 2024).
- Eleutherococci Radix—Herbal Medicinal Product|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/herbal/eleutherococci-radix (accessed on 18 September 2024).
- Echinaceae Purpureae Radix—Herbal Medicinal Product|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/herbal/echinaceae-purpureae-radix (accessed on 18 September 2024).
- Block, K.I.; Mead, M.N. Immune System Effects of Echinacea, Ginseng, and Astragalus: A Review. Integr. Cancer Ther. 2003, 2, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Kariman, K.; Mousavi, M.; Rengel, Z. Echinacea: Bioactive Compounds and Agronomy. Plants 2024, 13, 1235. [Google Scholar] [CrossRef]
- Kong, F.; Chen, T.; Li, X.; Jia, Y. The Current Application and Future Prospects of Astragalus Polysaccharide Combined with Cancer Immunotherapy: A Review. Front. Pharmacol. 2021, 12, 737674. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Liang, D.; Quan, X.; Gu, R.; Meng, Z.; Gan, H.; Wu, Z.; Sun, Y.; Liu, S.; et al. Pharmacological Effects of Astragaloside IV: A Review. Molecules 2023, 28, 6118. [Google Scholar] [CrossRef]
- Rasool, M.; Varalakshmi, P. Immunomodulatory Role of Withania somnifera Root Powder on Experimental Induced Inflammation: An In Vivo and In Vitro Study. Vascul. Pharmacol. 2006, 44, 406–410. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological Basis for the Role of Curcumin in Chronic Diseases: An Age-Old Spice with Modern Targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Wang, M.; Pan, J.; Xiang, W.; You, Z.; Zhang, Y.; Wang, J.; Zhang, A. β-Glucan: A Potent Adjuvant in Immunotherapy for Digestive Tract Tumors. Front. Immunol. 2024, 15, 1424261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory Effects of Edible and Medicinal Mushrooms and Their Bioactive Immunoregulatory Products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on Stress and the Stress- Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Curr. Neuropharmacol. 2021, 19, 1468–1495. [Google Scholar] [CrossRef]
- Ivanova Stojcheva, E.; Quintela, J.C. The Effectiveness of Rhodiola Rosea L. Preparations in Alleviating Various Aspects of Life-Stress Symptoms and Stress-Induced Conditions—Encouraging Clinical Evidence. Molecules 2022, 27, 3902. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C. The G115 Standardized Ginseng Extract: An Example for Safety, Efficacy, and Quality of an Herbal Medicine. J. Ginseng Res. 2020, 44, 179–193. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Serious Adverse Reaction. Available online: https://www.ema.europa.eu/en/glossary-terms/serious-adverse-reaction (accessed on 30 May 2025).
- Palmer, R.; Dimairo, M.; Latimer, N.; Cross, E.; Brady, M.; Enderby, P.; Bowen, A.; Julious, S.; Harrison, M.; Alshreef, A.; et al. Definitions of Adverse Events and Serious Adverse Events and Categories of Serious Adverse Event Results. In Computerised Speech and Language Therapy or Attention Control Added to Usual Care for People with Long-Term Post-Stroke Aphasia: The Big CACTUS Three-Arm RCT; NIHR Journals Library: Southampton, UK, 2020. [Google Scholar]
- Bupparenoo, P.; Pakchotanon, R.; Narongroeknawin, P.; Asavatanabodee, P.; Chaiamnuay, S. Effect of Curcumin on Serum Urate in Asymptomatic Hyperuricemia: A Randomized Placebo-Controlled Trial. J. Diet. Suppl. 2021, 18, 248–260. [Google Scholar] [CrossRef]
- Selvi, M.; Mohan Mv, R.; Bethapudi, B.; Mundkinajeddu, D.; Kumari, S. Safety of NR-INF-02, an Extract of Curcuma longa Containing Turmerosaccharides, in Healthy Volunteers: A Randomized, Open-Label Clinical Trial. Altern. Ther. Health Med. 2022, 28, 116–123. [Google Scholar]
- Chainani-Wu, N.; Madden, E.; Lozada-Nur, F.; Silverman, S. High-Dose Curcuminoids Are Efficacious in the Reduction in Symptoms and Signs of Oral Lichen Planus. J. Am. Acad. Dermatol. 2012, 66, 752–760. [Google Scholar] [CrossRef]
- Ariyasriwatana, C.; Phoolcharoen, N.; Oranratanaphan, S.; Worasethsin, P. Efficacy of Curcuminoids in Managing Postoperative Pain after Total Laparoscopic Hysterectomy: A Randomized Controlled, Open-Label Trial. Complement. Med. Res. 2022, 29, 223–227. [Google Scholar] [CrossRef]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and Efficacy of Curcumin versus Diclofenac in Knee Osteoarthritis: A Randomized Open-Label Parallel-Arm Study. Trials 2019, 20, 214. [Google Scholar] [CrossRef]
- Carrion-Gutierrez, M.; Ramirez-Bosca, A.; Navarro-Lopez, V.; Martinez-Andres, A.; Asín-Llorca, M.; Bernd, A.; Horga de la Parte, J.F. Effects of Curcuma Extract and Visible Light on Adults with Plaque Psoriasis. Eur. J. Dermatol. 2015, 25, 240–246. [Google Scholar] [CrossRef]
- Panahi, Y.; Ghanei, M.; Bashiri, S.; Hajihashemi, A.; Sahebkar, A. Short-Term Curcuminoid Supplementation for Chronic Pulmonary Complications Due to Sulfur Mustard Intoxication: Positive Results of a Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2014, 65, 567–573. [Google Scholar] [CrossRef]
- Samadian, F.; Dalili, N.; Poor-reza Gholi, F.; Fattah, M.; Malih, N.; Nafar, M.; Firoozan, A.; Ahmadpoor, P.; Samavat, S.; Ziaie, S. Evaluation of Curcumin’s Effect on Inflammation in Hemodialysis Patients. Clin. Nutr. ESPEN 2017, 22, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients with Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Ha, K.-C.; Choi, E.-K.; Jung, S.-Y.; Kim, M.-G.; Kwon, D.-Y.; Yang, H.-J.; Kim, M.-J.; Kang, H.-J.; Back, H.-I.; et al. The Effectiveness of Fermented Turmeric Powder in Subjects with Elevated Alanine Transaminase Levels: A Randomised Controlled Study. BMC Complement. Altern. Med. 2013, 13, 58. [Google Scholar] [CrossRef]
- Henrotin, Y.; Malaise, M.; Wittoek, R.; de Vlam, K.; Brasseur, J.-P.; Luyten, F.P.; Jiangang, Q.; Van den Berghe, M.; Uhoda, R.; Bentin, J.; et al. Bio-Optimized Curcuma longa Extract Is Efficient on Knee Osteoarthritis Pain: A Double-Blind Multicenter Randomized Placebo Controlled Three-Arm Study. Arthritis Res. Ther. 2019, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jones, G.; Winzenberg, T.; Cai, G.; Laslett, L.L.; Aitken, D.; Hopper, I.; Singh, A.; Jones, R.; Fripp, J.; et al. Effectiveness of Curcuma longa Extract for the Treatment of Symptoms and Effusion–Synovitis of Knee Osteoarthritis. Ann. Intern. Med. 2020, 173, 861–869. [Google Scholar] [CrossRef]
- Madhu, K.; Chanda, K.; Saji, M.J. Safety and Efficacy of Curcuma longa Extract in the Treatment of Painful Knee Osteoarthritis: A Randomized Placebo-Controlled Trial. Inflammopharmacology 2013, 21, 129–136. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and Safety of Curcuma domestica Extracts Compared with Ibuprofen in Patients with Knee Osteoarthritis: A Multicenter Study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Thanakhumtorn, S.; Chinswangwatanakul, P.; Wattanamongkonsil, L.; Thamlikitkul, V. Efficacy and Safety of Curcuma domestica Extracts in Patients with Knee Osteoarthritis. J. Altern. Complement. Med. 2009, 15, 891–897. [Google Scholar] [CrossRef]
- Raj, J.P.; Venkatachalam, S.; Racha, P.; Bhaskaran, S.; Amaravati, R.S. Effect of Turmacin Supplementation on Joint Discomfort and Functional Outcome among Healthy Participants—A Randomized Placebo-Controlled Trial. Complement. Ther. Med. 2020, 53, 102522. [Google Scholar] [CrossRef] [PubMed]
- Sawangroj, N.; Budkaew, J.; Chumworathayi, B. Efficacy of Curcuma longa in Treatment of Postprandial Distress Syndrome: An Open-Label Randomized-Controlled Trial. F1000Research 2019, 8, 1827. [Google Scholar] [CrossRef]
- Bahraini, P.; Rajabi, M.; Mansouri, P.; Sarafian, G.; Chalangari, R.; Azizian, Z. Turmeric Tonic as a Treatment in Scalp Psoriasis: A Randomized Placebo-control Clinical Trial. J. Cosmet. Dermatol. 2018, 17, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Sanmukhani, J.; Satodiya, V.; Trivedi, J.; Patel, T.; Tiwari, D.; Panchal, B.; Goel, A.; Tripathi, C.B. Efficacy and Safety of Curcumin in Major Depressive Disorder: A Randomized Controlled Trial. Phytother. Res. 2014, 28, 579–585. [Google Scholar] [CrossRef]
- Singhal, S.; Hasan, N.; Nirmal, K.; Chawla, R.; Chawla, S.; Kalra, B.S.; Dhal, A. Bioavailable Turmeric Extract for Knee Osteoarthritis: A Randomized, Non-Inferiority Trial versus Paracetamol. Trials 2021, 22, 105. [Google Scholar] [CrossRef]
- Lal, B.; Kapoor, A.K.; Asthana, O.P.; Agrawal, P.K.; Prasad, R.; Kumar, P.; Srimal, R.C. Efficacy of Curcumin in the Management of Chronic Anterior Uveitis. Phytother. Res. 1999, 13, 318–322. [Google Scholar] [CrossRef]
- Bayet-Robert, M.; Kwiatowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.-A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I Dose Escalation Trial of Docetaxel plus Curcumin in Patients with Advanced and Metastatic Breast Cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef]
- Irving, G.R.B.; Howells, L.M.; Sale, S.; Kralj-Hans, I.; Atkin, W.S.; Clark, S.K.; Britton, R.G.; Jones, D.J.L.; Scott, E.N.; Berry, D.P.; et al. Prolonged Biologically Active Colonic Tissue Levels of Curcumin Achieved After Oral Administration—A Clinical Pilot Study Including Assessment of Patient Acceptability. Cancer Prev. Res. 2013, 6, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Fança-Berthon, P.; Tenon, M.; Bouter-Banon, S.L.; Manfré, A.; Maudet, C.; Dion, A.; Chevallier, H.; Laval, J.; van Breemen, R.B. Pharmacokinetics of a Single Dose of Turmeric Curcuminoids Depends on Formulation: Results of a Human Crossover Study. J. Nutr. 2021, 151, 1802–1816. [Google Scholar] [CrossRef]
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L.; Dehghanzadeh, G.-R. Oral Supplementation of Turmeric Decreases Proteinuria, Hematuria, and Systolic Blood Pressure in Patients Suffering From Relapsing or Refractory Lupus Nephritis: A Randomized and Placebo-Controlled Study. J. Ren. Nutr. 2012, 22, 50–57. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose Escalation of a Curcuminoid Formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Thanawala, S.; Shah, R.; Alluri, K.V.; Somepalli, V.; Vaze, S.; Upadhyay, V. Comparative Bioavailability of Curcuminoids from a Water-Dispersible High Curcuminoid Turmeric Extract against a Generic Turmeric Extract: A Randomized, Cross-over, Comparative, Pharmacokinetic Study. J. Pharm. Pharmacol. 2021, 73, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Ghaisas, S.; Vaidya, A.; Vaidya, R.; Kamat, D.V.; Bhagwat, A.N.; Bhide, S. Early Human Safety Study of Turmeric Oil (Curcuma longa Oil) Administered Orally in Healthy Volunteers. J. Assoc. Physicians India 2003, 51, 1055–1060. [Google Scholar]
- Pakfetrat, M.; Akmali, M.; Malekmakan, L.; Dabaghimanesh, M.; Khorsand, M. Role of Turmeric in Oxidative Modulation in End-stage Renal Disease Patients. Hemodial. Int. 2015, 19, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Pakfetrat, M.; Basiri, F.; Malekmakan, L.; Roozbeh, J. Effects of Turmeric on Uremic Pruritus in End Stage Renal Disease Patients: A Double-Blind Randomized Clinical Trial. J. Nephrol. 2014, 27, 203–207. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Clark, A.K.; Notay, M.; Sivamani, R.K. Randomized Controlled Pilot Study of Dietary Supplementation with Turmeric or Herbal Combination Tablets on Skin Barrier Function in Healthy Subjects. J. Med. Food 2018, 21, 1260–1265. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Pourang, A.; Clark, A.K.; Burney, W.; Sivamani, R.K. Dietary Supplementation with Turmeric Polyherbal Formulation Decreases Facial Redness: A Randomized Double-Blind Controlled Pilot Study. J. Integr. Med. 2019, 17, 20–23. [Google Scholar] [CrossRef]
- Taylor, J.A.; Weber, W.; Standish, L.; Quinn, H.; Goesling, J.; McGann, M.; Calabrese, C. Efficacy and Safety of Echinacea in Treating Upper Respiratory Tract Infections in Children: A Randomized Controlled Trial. JAMA 2003, 290, 2824. [Google Scholar] [CrossRef]
- Fried, M.W. Effect of Silymarin (Milk Thistle) on Liver Disease in Patients with Chronic Hepatitis C Unsuccessfully Treated with Interferon Therapy: A Randomized Controlled Trial. JAMA 2012, 308, 274. [Google Scholar] [CrossRef]
- Dostal, A.M.; Samavat, H.; Bedell, S.; Torkelson, C.; Wang, R.; Swenson, K.; Le, C.; Wu, A.H.; Ursin, G.; Yuan, J.-M.; et al. The Safety of Green Tea Extract Supplementation in Postmenopausal Women at Risk for Breast Cancer: Results of the Minnesota Green Tea Trial. Food Chem. Toxicol. 2015, 83, 26–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Xie, J. Torsade de Pointes Caused by a Compound Licorice Tablet. Int. Heart J. 2024, 65, 23–609. [Google Scholar] [CrossRef]
- Shintani, S.; Murase, H.; Tsukagoshi, H.; Shiigai, T. Glycyrrhizin (Licorice)-Induced Hypokalemic Myopathy. Eur. Neurol. 1992, 32, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Johns, C. Glycyrrhizic Acid Toxicity Caused by Consumption of Licorice Candy Cigars. Can. J. Emerg. Med. 2009, 11, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Yanagawa, T.; Watanabe, K. Risk Factors for Pseudoaldosteronism with Rhabdomyolysis Caused by Consumption of Drugs Containing Licorice and Differences Between Incidence of These Conditions in Japan and Other Countries: Case Report and Literature Review. J. Altern. Complement. Med. 2014, 20, 516–520. [Google Scholar] [CrossRef]
- Barrella, M.; Lauria, G.; Quatrale, R.; Paolino, E. Hypokaliemic Rhabdomyolysis Associated with Liquorice Ingestion: Report of an Atypical Case. Ital. J. Neurol. Sci. 1997, 18, 217–220. [Google Scholar] [CrossRef]
- Albermann, M.E.; Musshoff, F.; Hagemeier, L.; Madea, B. Determination of Glycyrrhetic Acid after Consumption of Liquorice and Application to a Fatality. Forensic Sci. Int. 2010, 197, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Francini-Pesenti, F.; Puato, M.; Piccoli, A.; Brocadello, F. Liquorice-induced Hypokalaemia and Water Retention in the Absence of Hypertension. Phytother. Res. 2008, 22, 563–565. [Google Scholar] [CrossRef]
- Caradonna, P.; Gentiloni, N.; Servidei, S.; Perrone, G.A.; Greco, A.V.; Russo, M.A. Acute Myopathy Associated with Chronic Licorice Ingestion: Reversible Loss of Myoadenylate Deaminase Activity. Ultrastruct. Pathol. 1992, 16, 529–535. [Google Scholar] [CrossRef]
- Costa, M.L.; Rodrigues, J.A.; Azevedo, J.; Vasconcelos, V.; Eiras, E.; Campos, M.G. Hepatotoxicity Induced by Paclitaxel Interaction with Turmeric in Association with a Microcystin from a Contaminated Dietary Supplement. Toxicon 2018, 150, 207–211. [Google Scholar] [CrossRef]
- Suhail, F.K.; Masood, U.; Sharma, A.; John, S.; Dhamoon, A. Turmeric Supplement Induced Hepatotoxicity: A Rare Complication of a Poorly Regulated Substance. Clin. Toxicol. 2020, 58, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.; Hair, C.; Beswick, L. A Rare Case of Turmeric-induced Hepatotoxicity. Intern. Med. J. 2020, 50, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Arzallus, T.; Izagirre, A.; Castiella, A.; Torrente, S.; Garmendia, M.; Zapata, E.M. Drug Induced Autoimmune Hepatitis after Turmeric Intake. Gastroenterol. Hepatol. 2023, 46, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Lukefahr, A.L.; McEvoy, S.; Alfafara, C.; Funk, J.L. Drug-Induced Autoimmune Hepatitis Associated with Turmeric Dietary Supplement Use. BMJ Case Rep. 2018, 2018, bcr-2018-224611. [Google Scholar] [CrossRef]
- Javaid, A.; Bonkovsky, H.L. Hepatotoxicity Due to Extracts of Chinese Green Tea (Camellia sinensis): A Growing Concern. J. Hepatol. 2006, 45, 334–335. [Google Scholar] [CrossRef]
- Bonkovsky, H.L. Hepatotoxicity Associated with Supplements Containing Chinese Green Tea (Camellia sinensis). Ann. Intern. Med. 2006, 144, 68–71. [Google Scholar] [CrossRef]
- Jimenez-Saenz, M.; Del Carmen Martinez-Sanchez, M. Acute Hepatitis Associated with the Use of Green Tea Infusions. J. Hepatol. 2006, 44, 616–617. [Google Scholar] [CrossRef]
- Vanstraelen, S.; Rahier, J.; Geubel, A.P. Jaundice as a Misadventure of a Green Tea (Camellia sinensis) Lover: A Case Report. Acta Gastro-Enterol. Belg. 2008, 71, 409–412. [Google Scholar]
- Gloro, R.; Hourmand-Ollivier, I.; Mosquet, B.; Mosquet, L.; Rousselot, P.; Salam, E.; Piquet, M.-A.; Dao, T. Fulminant Hepatitis during Self-Medication with Hydroalcoholic Extract of Green Tea. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1135–1137. [Google Scholar] [CrossRef]
- Verhelst, X.; Burvenich, P.; Van Sassenbroeck, D.; Gabriel, C.; Lootens, M.; Baert, D. Acute Hepatitis after Treatment for Hair Loss with Oral Green Tea Extracts (Camellia sinensis). Acta Gastro-Enterol. Belg. 2009, 72, 262–264. [Google Scholar]
- Lugg, S.T.; Braganza Menezes, D.; Gompertz, S. Chinese Green Tea and Acute Hepatitis: A Rare yet Recurring Theme. BMJ Case Rep. 2015, 2015, bcr2014208534. [Google Scholar] [CrossRef]
- Gavrić, A.; Ribnikar, M.; Šmid, L.; Luzar, B.; Štabuc, B. Fat Burner–Induced Acute Liver Injury: Case Series of Four Patients. Nutrition 2018, 47, 110–114. [Google Scholar] [CrossRef]
- Patel, S.S. Green Tea Extract: A Potential Cause of Acute Liver Failure. World J. Gastroenterol. 2013, 19, 5174. [Google Scholar] [CrossRef]
- Molinari, M.; Watt, K.D.S.; Kruszyna, T.; Nelson, R.; Walsh, M.; Huang, W.-Y.; Nashan, B.; Peltekian, K. Acute Liver Failure Induced by Green Tea Extracts: Case Report and Review of the Literature. Liver Transpl. 2006, 12, 1892–1895. [Google Scholar] [CrossRef]
- Pillukat, M.H.; Bester, C.; Hensel, A.; Lechtenberg, M.; Petereit, F.; Beckebaum, S.; Müller, K.-M.; Schmidt, H.H.J. Concentrated Green Tea Extract Induces Severe Acute Hepatitis in a 63-Year-Old Woman—A Case Report with Pharmaceutical Analysis. J. Ethnopharmacol. 2014, 155, 165–170. [Google Scholar] [CrossRef]
- Vázquez-Fernández, P.; Garayoa-Roca, A.; Añón-Rodríguez, R.; Cabezas-Macián, M.; Serra-Desfilis, M.Á.; Mora-Miguel, F. Aloe vera: Not Always so Beneficial in Patients with Chronic Liver Disease. Rev. Esp. Enfermedades Dig. 2013, 105, 434–435. [Google Scholar] [CrossRef]
- Lee, A.; Chui, P.T.; Aun, C.S.; Gin, T.; Lau, A.S. Possible Interaction Between Sevoflurane and Aloe vera. Ann. Pharmacother. 2004, 38, 1651–1654. [Google Scholar] [CrossRef] [PubMed]
- Hervás-García, J.V.; Montané, E.; Serrado-Iglesias, A.; Ramo-Tello, C. Hepatitis Tóxica Tras Tratamiento Concomitante Con Interferón Beta y Aloe vera En Un Paciente Con Esclerosis Múltiple: A Propósito de Un Caso. Neurología 2017, 32, 546–547. [Google Scholar] [CrossRef] [PubMed]
- Parlati, L.; Voican, C.S.; Perlemuter, K.; Perlemuter, G. Aloe vera-Induced Acute Liver Injury: A Case Report and Literature Review. Clin. Res. Hepatol. Gastroenterol. 2017, 41, e39–e42. [Google Scholar] [CrossRef]
- Lee, J.; Lee, M.S.; Nam, K.W. Acute Toxic Hepatitis Caused by an Aloe vera Preparation in a Young Patient: A Case Report with a Literature Review. Korean J. Gastroenterol. 2014, 64, 54. [Google Scholar] [CrossRef]
- Bottenberg, M.M.; Wall, G.C.; Harvey, R.L.; Habib, S. Oral Aloe vera-Induced Hepatitis. Ann. Pharmacother. 2007, 41, 1740–1743. [Google Scholar] [CrossRef]
- Morrow, D.M.; Rapaport, M.J.; Strick, R.A. Hypersensitivity to Aloe. Arch. Dermatol. 1980, 116, 1064–1065. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.N.; Kim, D.J.; Kim, Y.M.; Kim, B.H.; Sohn, K.M.; Choi, M.J.; Choi, Y.H. Aloe-Induced Toxic Hepatitis. J. Korean Med. Sci. 2010, 25, 492. [Google Scholar] [CrossRef] [PubMed]
- Finall, A.I.; McIntosh, S.A.; Thompson, W.D. Subcutaneous Inflammation Mimicking Metastatic Malignancy Induced by Injection of Mistletoe Extract. BMJ 2006, 333, 1293–1294. [Google Scholar] [CrossRef]
- Casetti, F.; Rafei-Shamsabadi, D.; Müller, S. Grade II-anaphylaxis after Subcutaneous Injection of Mistletoe Extract. Contact Dermat. 2021, 85, 462–465. [Google Scholar] [CrossRef]
- Hutt, N.; Kopferschmitt-Kubler, M.C.; Cabalion, J.; Purohit, A.; Alt, M.; Pauli, G. Anaphylactic Reactions after Therapeutic Injection of Mistletoe (Viscum album L.). Allergol. Immunopathol. 2001, 29, 201–203. [Google Scholar] [CrossRef]
- Harvey, J.; Colin-Jones, D.G. Mistletoe Hepatitis. BMJ 1981, 282, 186–187. [Google Scholar] [CrossRef]
- von Schoen-Angerer, T.; Wilkens, J.; Kienle, G.S.; Kiene, H.; Vagedes, J. High-Dose Viscum album Extract Treatment in the Prevention of Recurrent Bladder Cancer: A Retrospective Case Series. Perm. J. 2015, 19, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Lee Soon, S.; Crawford, R.I. Recurrent Erythema Nodosum Associated with Echinacea Herbal Therapy. J. Am. Acad. Dermatol. 2001, 44, 298–299. [Google Scholar] [CrossRef]
- Mullins, R.J.; Heddle, R. Adverse Reactions Associated with Echinacea: The Australian Experience. Ann. Allergy. Asthma. Immunol. 2002, 88, 42–51. [Google Scholar] [CrossRef]
- Aalto-Korte, K.; Susitaival, P.; Kaminska, R.; Mäkinen-Kiljunen, S. Occupational Protein Contact Dermatitis from Shiitake Mushroom and Demonstration of Shiitake-specific Immunoglobulin E. Contact Dermat. 2005, 53, 211–213. [Google Scholar] [CrossRef]
- Tarvainen, K.; Salonen, J.-P.; Kanerva, L.; Estlander, T.; Keskinen, H.; Rantanen, T. Allergy and Toxicodermia from Shiitake Mushrooms. J. Am. Acad. Dermatol. 1991, 24, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Ampere, A.; Delhaes, L.; Soots, J.; Bart, F.; Wallaert, B. Hypersensitivity Pneumonitis Induced by Shiitake Mushroom Spores. Med. Mycol. 2012, 50, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Ching, D.; Wood, B.A.; Tiwari, S.; Chan, J.; Harvey, N.T. Histological Features of Flagellate Erythema. Am. J. Dermatopathol. 2019, 41, 410–421. [Google Scholar] [CrossRef]
- Corazza, M.; Zauli, S.; Ricci, M.; Borghi, A.; Pedriali, M.; Mantovani, L.; Virgili, A. Shiitake Dermatitis: Toxic or Allergic Reaction? J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1449–1451. [Google Scholar] [CrossRef]
- Balasuriya, A.; Goel, A. Shiitake Flagellate Dermatitis (Toxicoderma): A Case Report. Natl. Med. J. India 2021, 34, 161. [Google Scholar] [CrossRef]
- de Mendonça, C.N.; Silva, P.M.C.E.; Avelleira, J.C.R.; Nishimori, F.S.; de Freire Cassia, F. Shiitake Dermatitis. An. Bras. Dermatol. 2015, 90, 276–278. [Google Scholar] [CrossRef]
- Heer, R.S.; Patel, N.B.; Mandal, A.K.J.; Lewis, F.; Missouris, C.G. Not a Fungi to Be with: Shiitake Mushroom Flagellate Dermatitis. Am. J. Emerg. Med. 2020, 38, 412.e1–412.e2. [Google Scholar] [CrossRef]
- Chu, E.Y.; Anand, D.; Dawn, A.; Elenitsas, R.; Adler, D.J. Shiitake Dermatitis: A Report of 3 Cases and Review of the Literature. Cutis 2013, 91, 287–290. [Google Scholar]
- Mulhall, J.; Elseth, A.; Perdue, J.; Pomerantz, H.; Brown, B.; Frasca, D. Flagellate Dermatitis After Ingestion of Shiitake Mushrooms in a Healthy Female Living in the Southeastern United States. J. Emerg. Med. 2020, 59, e13–e15. [Google Scholar] [CrossRef]
- Kopp, T.; Mastan, P.; Mothes, N.; Tzaneva, S.; Stingl, G.; Tanew, A. Systemic Allergic Contact Dermatitis Due to Consumption of Raw Shiitake Mushroom. Clin. Exp. Dermatol. 2009, 34, e910–e913. [Google Scholar] [CrossRef] [PubMed]
- Sharp, O.; Waseem, S.; Wong, K.Y. A Garlic Burn. BMJ Case Rep. 2018, 2018, bcr-2018-226027. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Che, J.; Song, J.; Duan, X.; Yang, J. Topical Garlic Treatment for Verruca Plana Triggers Koebner Phenomenon: A Case Report. J. Cosmet. Dermatol. 2023, 22, 913–915. [Google Scholar] [CrossRef]
- Parish, R.A.; McIntire, S.; Heimbach, D.M. Garlic Burns: A Naturopathic Remedy Gone Awry. Pediatr. Emerg. Care 1987, 3, 258–260. [Google Scholar] [CrossRef]
- Sisson, D.; Balmer, C. A Chemical Burn from a Garlic Poultice Applied to the Face to Treat Toothache: A Case Report. Prim. Dent. J. 2014, 3, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Strickler, L.; Harjo, T. Burns Due to Application of Raw Garlic to the Feet as a Home Remedy for Fever. Pediatr. Emerg. Care 2019, 35, e234–e235. [Google Scholar] [CrossRef]
- Eming, S.A.; Piontek, J.O.; Hunzelmann, N.; Rasokat, H.; Scharffetter-Kachanek, K. Severe Toxic Contact Dermatitis Caused by Garlic. Br. J. Dermatol. 1999, 141, 391–392. [Google Scholar] [CrossRef]
- Kaçar, C.K.; Kılıç, E.T.; Akelma, H.; Uzundere, O.; Kaydu, A.; Gökçek, E. Medical Folk Remedy: Two Cases of Garlic Burns. J. Burn. Care Res. 2019, 40, 133–135. [Google Scholar] [CrossRef]
- Jappe, U. Garlic-Related Dermatoses: Case Report and Review of the Literature. Am. J. Contact Dermat. 1999, 10, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, N. Neem Oil Poisoning as a Cause of Toxic Encephalopathy in an Infant. Indian J. Pediatr. 2014, 81, 955. [Google Scholar] [CrossRef]
- Lai, S.M.; Lim, K.W.; Cheng, H.K. Margosa Oil Poisoning as a Cause of Toxic Encephalopathy. Singap. Med. J. 1990, 31, 463–465. [Google Scholar]
- Suresha, A.R.; Rajesh, P.; Anil Raj, K.S.; Torgal, R. A Rare Case of Toxic Optic Neuropathy Secondary to Consumption of Neem Oil. Indian J. Ophthalmol. 2014, 62, 337. [Google Scholar] [CrossRef]
- Iyyadurai, R.; Surekha, V.; Sathyendra, S.; Paul Wilson, B.; Gopinath, K.G. Azadirachtin Poisoning: A Case Report. Clin. Toxicol. 2010, 48, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.; Murali, A.; Balakrishnan, R.; Narasimhan, D. “Herbal Remedy Is Natural and Safe”—Truth or Myth? J. Assoc. Physicians India 2013, 61, 848–850. [Google Scholar]
- Lee, S.; Lee, H.Y.; Park, Y.; Ko, E.J.; Ban, T.H.; Chung, B.H.; Lee, H.S.; Yang, C.W. Development of End Stage Renal Disease after Long-Term Ingestion of Chaga Mushroom: Case Report and Review of Literatures. J. Korean Med. Sci. 2020, 35, e122. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, Y.; Paek, J.H.; Park, W.Y.; Han, S.; Sin, H.; Jin, K. Chaga Mushroom-Induced Oxalate Nephropathy That Clinically Manifested as Nephrotic Syndrome. Medicine 2022, 101, e28997. [Google Scholar] [CrossRef]
- Bae, W.; Kim, S.; Choi, J.; Lee, T.W.; Bae, E.; Jang, H.N.; Jung, S.; Lee, S.; Chang, S.-H.; Park, D.J. Acute Interstitial Nephritis Associated with Ingesting a Momordica charantia Extract. Medicine 2021, 100, e26606. [Google Scholar] [CrossRef]
- Dega, H.; Laporte, J.-L.; Francès, C.; Herson, S.; Chosidow, O. Ginseng as a cause for Stevens-Johnson syndrome? Lancet 1996, 347, 1344. [Google Scholar] [CrossRef]
- Maskatia, Z.K.; Baker, K. Hypereosinophilia Associated with Echinacea Use. South. Med. J. 2010, 103, 1173–1174. [Google Scholar] [CrossRef] [PubMed]
- Acquarulo, B.; Tandon, P.; Macica, C.M. Suspected Cholinergic Toxicity Due to Cevimeline Hydrochloride and Bacopa monnieri Interaction: A Case Report. J. Med. Case Rep. 2022, 16, 253. [Google Scholar] [CrossRef]
- Ragsdell, J.E.; Tynes, B.E.; Tynes, L.L. A Possible Role for Uncaria tomentosa (Cat’s claw) in a Case of Serotonin Syndrome. Prim. Care Companion CNS Disord. 2021, 23, 21cr02937. [Google Scholar] [CrossRef]
- Cosentino, C.; Torres, L. Reversible Worsening of Parkinson Disease Motor Symptoms After Oral Intake of Uncaria tomentosa (Cat’s claw). Clin. Neuropharmacol. 2008, 31, 293–294. [Google Scholar] [CrossRef]
- Hilepo, J.N.; Bellucci, A.G.; Mossey, R.T. Acute Renal Failure Caused by ‘Cat’s Claw’ Herbal Remedy in a Patient with Systemic Lupus Erythematosus. Nephron 1997, 77, 361. [Google Scholar] [CrossRef]
- McRae, S. Elevated Serum Digoxin Levels in a Patient Taking Digoxin and Siberian Ginseng. CMAJ 1996, 155, 293–295. [Google Scholar]
- Hatton, M.N.; Desai, K.; Le, D.; Vu, A. Excessive Postextraction Bleeding Associated with Cordyceps Sinensis: A Case Report and Review of Select Traditional Medicines Used by Vietnamese People Living in the United States. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 494–500. [Google Scholar] [CrossRef]
- Rombolà, L.; Scuteri, D.; Marilisa, S.; Watanabe, C.; Morrone, L.A.; Bagetta, G.; Corasaniti, M.T. Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance. Life 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Teraki, Y.; Shiohara, T. Propolis-Induced Granulomatous Contact Dermatitis Accompanied by Marked Lymphadenopathy. Br. J. Dermatol. 2001, 144, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Callejo, A.; Armentia, A.; Lombardero, M.; Asensio, T. Propolis, a New Bee-Related Allergen. Allergy 2001, 56, 579. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.; Griggs, J.; De Padova, M.P.; Tosti, A. What’s the “Buzz” about Propolis? Propolis-induced Systemic Contact Dermatitis. Contact Dermat. 2019, 80, 65–67. [Google Scholar] [CrossRef]
- Silvani, S.; Spettoli, E.; Stacul, F.; Tosti, A. Contact Dermatitis in Psoriasis Due to Propolis. Contact Dermat. 1997, 37, 48–49. [Google Scholar] [CrossRef]
- Bellegrandi, S.; D’Offizi, G.; Ansotegui, I.J.; Ferrara, R.; Scala, E.; Paganelli, R. Propolis Allergy in an HIV-Positive Patient. J. Am. Acad. Dermatol. 1996, 35, 644. [Google Scholar] [CrossRef]
- Brailo, V.; Boras, V.V.; Alajbeg, I.; Juras, V. Delayed Contact Sensitivity on the Lips and Oral Mucosa Due to Propolis-Case Report. Med. Oral Patol. Oral Cir. Bucal. 2006, 11, E303–E304. [Google Scholar]
- Ramien, M.L.; Pratt, M.D. Fixed Drug Eruption to Ingested Propolis. Dermatitis 2012, 23, 173–175. [Google Scholar] [CrossRef]
- Li, Y.-J.; Lin, J.-L.; Yang, C.-W.; Yu, C.-C. Acute Renal Failure Induced by a Brazilian Variety of Propolis. Am. J. Kidney Dis. 2005, 46, e125–e129. [Google Scholar] [CrossRef] [PubMed]
- Angelini, G.; Vena, G.A.; Meneghini, C.L. Psoriasis and Contact Allergy to Propolis. Contact Dermat. 1987, 17, 251–253. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.B.; Pien, L. Exercise-Induced Anaphylaxis Associated with the Use of Bee Pollen. Ann. Allergy Asthma. Immunol. 2019, 122, 118–119. [Google Scholar] [CrossRef]
- Tremlett, H.; Fu, P.; Yoshida, E.; Hashimoto, S. Symptomatic Liver Injury (Hepatotoxicity) Associated with Administration of Complementary and Alternative Products (Ayurveda-AP-Mag Capsules®) in a Beta-Interferon-Treated Multiple Sclerosis Patient. Eur. J. Neurol. 2011, 18, e78–e79. [Google Scholar] [CrossRef]
- Gilbert, J.D.; Musgrave, I.F.; Hoban, C.; Byard, R.W. Lethal Hepatocellular Necrosis Associated with Herbal Polypharmacy in a Patient with Chronic Hepatitis B Infection. Forensic Sci. Int. 2014, 241, 138–140. [Google Scholar] [CrossRef]
- Teschke, R.; Bahre, R. Severe Hepatotoxicity by Indian Ayurvedic Herbal Products: A Structured Causality Assessment. Ann. Hepatol. 2009, 8, 258–266. [Google Scholar] [CrossRef]
- Koenig, G.; Callipari, C.; Smereck, J.A. Acute Liver Injury After Long-Term Herbal “Liver Cleansing” and “Sleep Aid” Supplement Use. J. Emerg. Med. 2021, 60, 610–614. [Google Scholar] [CrossRef]
- Karousatos, C.M.; Lee, J.K.; Braxton, D.R.; Fong, T.-L. Case Series and Review of Ayurvedic Medication Induced Liver Injury. BMC Complement. Med. Ther. 2021, 21, 91. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.; Ahn, J.H.; Suk, H.J. Liver Injury Induced by Herbal Extracts Containing Mistletoe and Kudzu. J. Altern. Complement. Med. 2015, 21, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Encarnación, E.; Ríos, G.; Muñoz-Mirabal, A.; Vilá, L.M. Euforia-Induced Acute Hepatitis in a Patient with Scleroderma. BMJ Case Rep. 2012, 2012, bcr2012006907. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rashid, A.M.; Abd Ghani, F.; Inche Mat, L.N.; Lim, C.T.S. Herbal Medication Triggering Lupus Nephritis—A Case Report. BMC Complement. Med. Ther. 2020, 20, 163. [Google Scholar] [CrossRef]
- Palanisamy, A.; Haller, C.; Olson, K.R. Photosensitivity Reaction in a Woman Using an Herbal Supplement Containing Ginseng, Goldenseal, and Bee Pollen. J. Toxicol. Clin. Toxicol. 2003, 41, 865–867. [Google Scholar] [CrossRef]
- Yadav, P.; Stigall, K.; Johnson, H.E.; Rayapati, A.O.; Chopra, N. Functional Foods: How Functional Are They? A Case Report of Supplement-Induced Psychosis. Int. J. Psychiatry Med. 2016, 51, 479–485. [Google Scholar] [CrossRef]
- Yigit, M.; Cevik, E. A Rare Cause of Pulmonary Embolism: Panax. Am. J. Emerg. Med. 2015, 33, 311.e1–311.e2. [Google Scholar] [CrossRef]
- Gerontakos, S.E.; Casteleijn, D.; Shikov, A.N.; Wardle, J. A Critical Review to Identify the Domains Used to Measure the Effect and Outcome of Adaptogenic Herbal Medicines. Yale J. Biol. Med. 2020, 93, 327–346. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Eleutherococcus Senticosus (Rupr. et Maxim.) Maxim., Radix; European Medicines Agency: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Center for Food Safety and Applied Nutrition (CFSAN). Lone Star Botanicals Inc.—MARCS-CMS 659735—6 November 2023. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/lone-star-botanicals-inc-659735-11062023 (accessed on 19 May 2025).
- Center for Food Safety and Applied Nutrition (CFSAN). Red Moon Herbs—MARCS-CMS 607640—18 November 2020. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/red-moon-herbs-607640-11182020 (accessed on 19 May 2025).
- Acosta, L.; Byham-Gray, L.; Kurzer, M.; Samavat, H. Hepatotoxicity with High-Dose Green Tea Extract: Effect of Catechol-O-Methyltransferase and Uridine 5′-Diphospho-Glucuronosyltransferase 1A4 Genotypes. J. Diet. Suppl. 2023, 20, 850–869. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of High Oral Dose (−)-Epigallocatechin-3-Gallate in Mice. Food Chem. Toxicol. 2010, 48, 409–416. [Google Scholar] [CrossRef]
- Makino, T. Exploration for the Real Causative Agents of Licorice-Induced Pseudoaldosteronism. J. Nat. Med. 2021, 75, 275–283. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Aloe vera: A Review of Toxicity and Adverse Clinical Effects. J. Environ. Sci. Health Part. C 2016, 34, 77–96. [Google Scholar] [CrossRef]
- Herbal Medicinal Products|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/human-regulatory-overview/herbal-medicinal-products (accessed on 19 May 2025).
- Nguyen, A.H.; Gonzaga, M.I.; Lim, V.M.; Adler, M.J.; Mitkov, M.V.; Cappel, M.A. Clinical Features of Shiitake Dermatitis: A Systematic Review. Int. J. Dermatol. 2017, 56, 610–616. [Google Scholar] [CrossRef]
- Eubanks, B.N.; Linabury, J.F.; Kumetz, E.A. Shiitake Dermatitis and Potential Implications for Military Readiness. Mil. Med. 2023, 188, 3285–3288. [Google Scholar] [CrossRef]
- Baltazar, P.; De Melo Junior, A.F.; Fonseca, N.M.; Lança, M.B.; Faria, A.; Sequeira, C.O.; Teixeira-Santos, L.; Monteiro, E.C.; Campos Pinheiro, L.; Calado, J.; et al. Oxalate (Dys)Metabolism: Person-to-Person Variability, Kidney and Cardiometabolic Toxicity. Genes 2023, 14, 1719. [Google Scholar] [CrossRef] [PubMed]
- Appiahopong, R.; Commandeur, J.; Vanvugtlussenburg, B.; Vermeulen, N. Inhibition of Human Recombinant Cytochrome P450s by Curcumin and Curcumin Decomposition Products. Toxicology 2007, 235, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-W.; Huang, C.-Y.; Yang, S.-Y.; Peng, Y.-H.; Yu, C.-P.; Chao, P.-D.L.; Hou, Y.-C. Oral Intake of Curcumin Markedly Activated CYP 3A4: In Vivo and Ex-Vivo Studies. Sci. Rep. 2014, 4, 6587. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Qi, H.; Pan, P.; Hou, T.; Li, J.; He, G.; Zhang, H. Pathway-Dependent Inhibition of Paclitaxel Hydroxylation by Kinase Inhibitors and Assessment of Drug–Drug Interaction Potentials. Drug Metab. Dispos. 2014, 42, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Wu, S.; Actor, J.; Olsen, M.; Wells, A.; Datta, P. Effect of Asian and Siberian Ginseng on Serum Digoxin Measurement by Five Digoxin Immunoassays: Significant Variation in Digoxin-Like Immunoreactivity Among Commercial Ginsengs. Am. J. Clin. Pathol. 2003, 119, 298–303. [Google Scholar] [CrossRef]
- Mathur, D.; Goyal, K.; Koul, V.; Anand, A. The Molecular Links of Re-Emerging Therapy: A Review of Evidence of Brahmi (Bacopa monniera). Front. Pharmacol. 2016, 7, 44. [Google Scholar] [CrossRef]
- Walker, E.A.; Pellegrini, M.V. Bacopa monnieri. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Jurgensen, S.; Dalbo, S.; Angers, P.; Santos, A.; Ribeirodovalle, R. Involvement of 5-HT Receptors in the Antinociceptive Effect of Uncaria tomentosa. Pharmacol. Biochem. Behav. 2005, 81, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Luo, L.; Dressel, W.; Shadier, G.; Krumbiegel, D.; Schmidtke, P.; Zepp, F.; Meyer, C.U. Cordycepin Is an Immunoregulatory Active Ingredient of Cordyceps sinensis. Am. J. Chin. Med. 2008, 36, 967–980. [Google Scholar] [CrossRef]
- Cho, H.-J.; Cho, J.Y.; Rhee, M.H.; Park, H.-J. Cordycepin (3′-Deoxyadenosine) Inhibits Human Platelet Aggregation in a Cyclic AMP- and Cyclic GMP-Dependent Manner. Eur. J. Pharmacol. 2007, 558, 43–51. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F.F. Ganoderma Lucidum (Lingzhi or Reishi): A Medicinal Mushroom. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; ISBN 978-1-4398-0713-2. [Google Scholar]
- Stjernberg, L.; Berglund, J.; Halling, A. Age and Gender Effect on the Use of Herbal Medicine Products and Food Supplements among the Elderly. Scand. J. Prim. Health Care 2006, 24, 50–55. [Google Scholar] [CrossRef]
- Zucker, I.; Prendergast, B.J. Sex Differences in Pharmacokinetics Predict Adverse Drug Reactions in Women. Biol. Sex. Differ. 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- The EndNote Team. EndNote, version 21; Clarivate: Philadelphia, PA, USA, 2013.
- Van De Schoot, R.; De Bruin, J.; Schram, R.; Zahedi, P.; De Boer, J.; Weijdema, F.; Kramer, B.; Huijts, M.; Hoogerwerf, M.; Ferdinands, G.; et al. An Open Source Machine Learning Framework for Efficient and Transparent Systematic Reviews. Nat. Mach. Intell. 2021, 3, 125–133. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Scoping Reviews. In JBI Manual for Evidence Synthesis; Aromataris, E., Lockwood, C., Porritt, K., Pilla, B., Jordan, Z., Eds.; JBI: Adelaide, Australia, 2024; ISBN 978-0-6488488-2-0. [Google Scholar]
- CIOMS Cumulative Pharmacovigilance GLOSSARY Version 1.0. Available online: https://cioms.ch/wp-content/uploads/2021/03/CIOMS-Cumulative-PV-Glossary-v1.0.pdf (accessed on 7 July 2025).

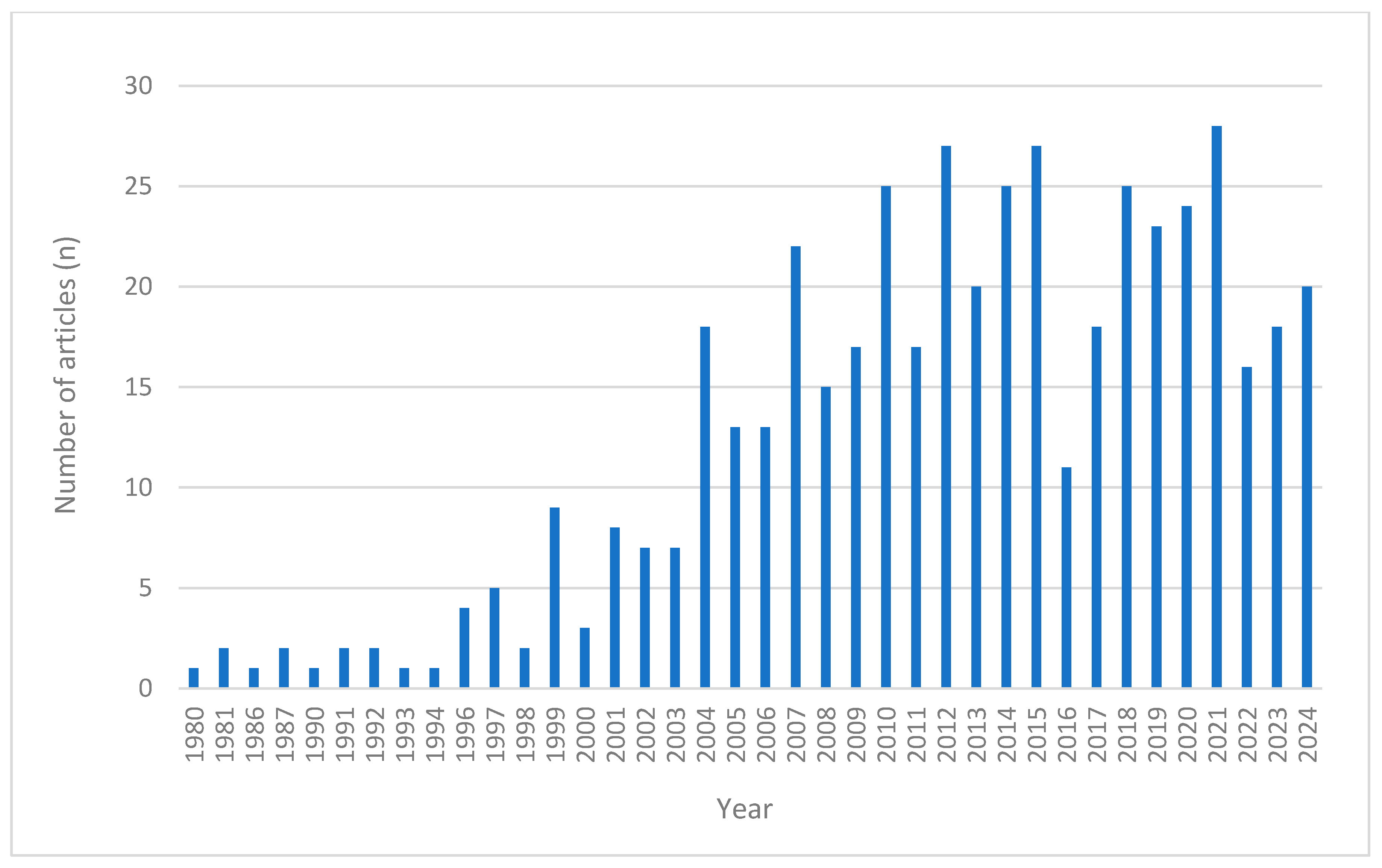
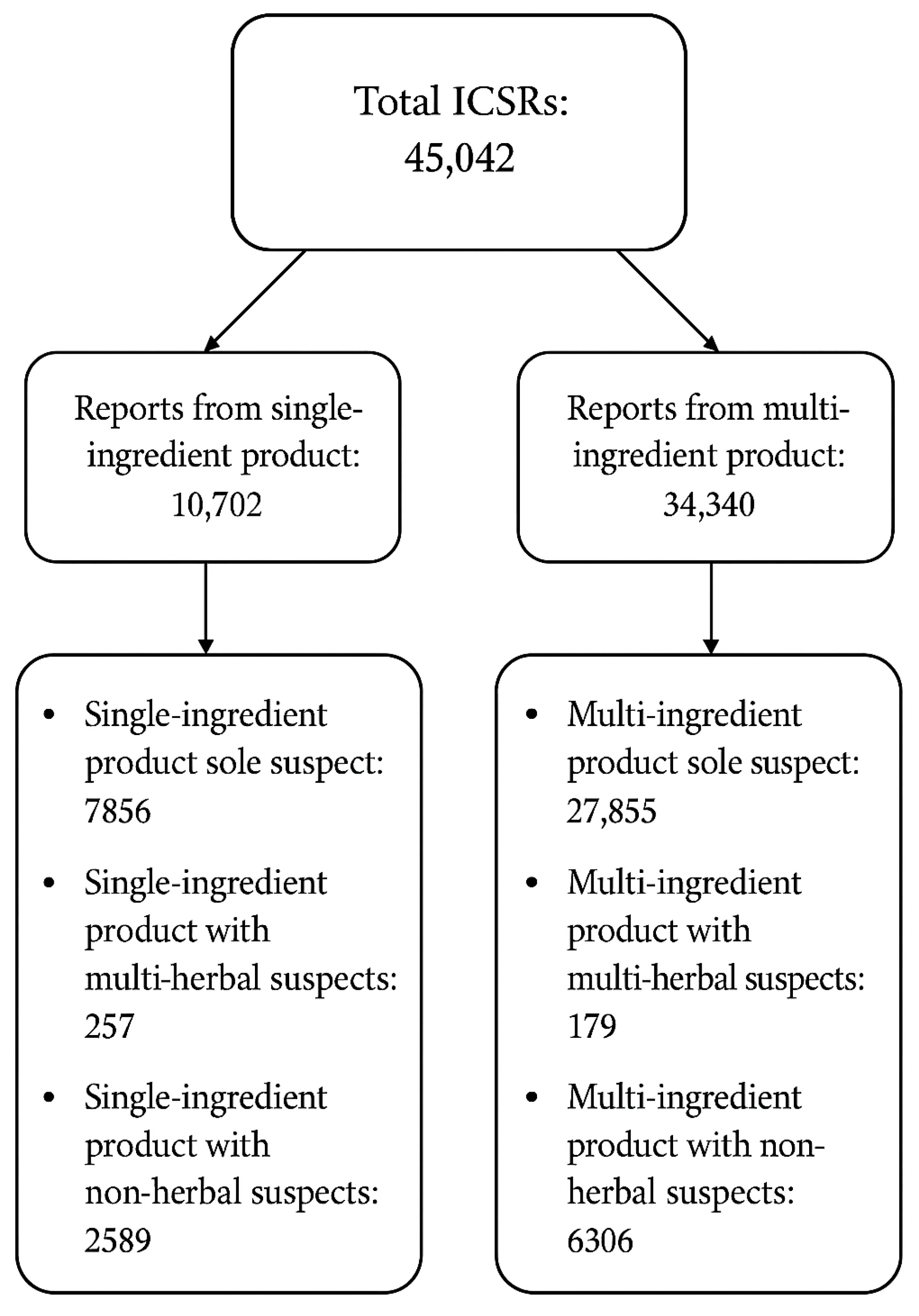
| Type of Study | Number of Studies with Single-Ingredient Products (n) | Number of Studies with Multi-Ingredient Products (n) |
|---|---|---|
| Non-randomized clinical trial | 49 | 7 |
| Randomized clinical trial | 236 | 47 |
| Case studies | 119 | 21 |
| Total | 404 | 75 |
| Natural Product | Number of Studies with Single-Ingredient Products (n) | Number of Studies with Multi-Ingredient Products (n) | Total (n) and % * |
|---|---|---|---|
| Curcuma longa L. | 35 | 4 | 39 (11.5%) |
| Panax ginseng C.A. Meyer | 24 | 1 | 25 (7.4%) |
| Zingiber officinale Roscoe | 18 | 6 | 24 (7.1%) |
| Withania somnifera (L.) Dunal | 18 | 4 | 22 (6.5%) |
| Viscum album L. | 21 | 0 | 21 (6.2%) |
| Silybum marianum (L.) Gaertn. | 16 | 5 | 21 (6.2%) |
| Pelargonium sidoides DC. | 15 | 0 | 15 (4.4%) |
| Echinacea purpurea (L.) Moench | 7 | 5 | 12 (3.5%) |
| Aloe vera (L.) Burm.f. | 9 | 1 | 10 (2.9%) |
| Rhodiola rosea L. | 8 | 1 | 9 (2.7%) |
| Natural Product | Number of Case Studies (n) and % * |
|---|---|
| Glycyrrhiza glabra L. | 15 (11.5%) |
| Camellia sinensis (L.) Kuntze | 11 (8.5%) |
| Lentinula edodes (Berk.) Pegl. | 11 (8.5%) |
| Panax ginseng C.A. Meyer | 10 (7.7%) |
| Allium sativum L. | 9 (6.9%) |
| Aloe vera (L.) Burm.f. | 8 (6.2%) |
| Curcuma longa L. | 8 (6.2%) |
| Viscum album L. | 6 (4.6%) |
| Azadirachta indica A. Juss. | 5 (3.8%) |
| Morinda citrifolia L. | 5 (3.8%) |
| Age Group | Number of ICSRs (n) and % * | Number of ICSRs of Single-Ingredient Products (n) | Number of ICSRs of Multi-Ingredient Products (n) |
|---|---|---|---|
| 0–27 days | 54 (0.1%) | 25 | 29 |
| 28 days to 23 months | 174 (0.4%) | 97 | 77 |
| 2–11 years | 607 (1.3%) | 377 | 230 |
| 12–17 years | 468 (1.0%) | 214 | 254 |
| 18–44 years | 7830 (17.4%) | 2466 | 5364 |
| 45–64 years | 15,922 (35.3%) | 3537 | 12,385 |
| 65–74 years | 8249 (18.3%) | 1465 | 6784 |
| ≥75 years | 6641 (14.7%) | 903 | 5738 |
| Unknown | 5097 (11.3%) | 1618 | 3479 |
| Sex | |||
| Female | 26,233 (58.2%) | 6354 | 19,879 |
| Male | 17,625 (39.1%) | 4002 | 13,623 |
| Unknown | 1184 (2.6%) | 346 | 838 |
| WHO Region | Number of ICSRs (n) and % of Single-Ingredient Products | Number of ICSRs (n) and % of Multi-Ingredient Products |
|---|---|---|
| Western Pacific Region | 4786 (44.7%) | 29,319 (85.4%) |
| European Region | 3597 (33.6%) | 3219 (9.4%) |
| Region of the Americas | 1052 (9.8%) | 951 (2.8%) |
| South-East Asia Region | 1013 (9.5%) | 707 (2.1%) |
| Eastern Mediterranean Region | 220 (2.1%) | 90 (0.3%) |
| African Region | 34 (0.3%) | 54 (0.2%) |
| Total | 10,702 | 34,340 |
| Natural Product | Number of Reports (n) and % |
|---|---|
| Ganoderma lucidum (Curtis) P. Karst | 1529 (19.4%) |
| Viscum album L. | 1358 (17.3%) |
| Silybum marianum (L.) Gaertn. | 1103 (14.0%) |
| Pelargonium sidoides DC. | 749 (9.5%) |
| Andrographis paniculata (Burm.f.) Wall. ex Nees | 545 (6.9%) |
| Rhodiola rosea L. | 484 (6.1%) |
| Salvia miltiorrhiza Bunge | 472 (6.0% |
| Curcuma longa L. | 318 (4.0%) |
| Echinacea purpurea (L.) Moench | 295 (3.8%) |
| Propolis from Apis mellifera | 126 (1.6%) |
| Seriousness of Reaction | Frequency (n) and % * | Impact of the Serious Reaction | Frequency (n) | Top Three Most Frequently Reported | Frequency (n) and % * |
|---|---|---|---|---|---|
| Serious | 1242 (15.8%) | Caused/Prolonged Hospitalization | 249 | Ganoderma lucidum | 435 (35.0%) |
| Caused/Prolonged Hospitalization, Other | 25 | Pelargonium sidoides | 171 (13.8%) | ||
| Death | 8 | Salvia miltiorrhiza | 133 (10.7%) | ||
| Death, Caused/Prolonged Hospitalization, Disabling/Incapacitating | 1 | ||||
| Disabling/Incapacitating | 10 | ||||
| Disabling/Incapacitating, Other | 5 | ||||
| Life-Threatening | 35 | ||||
| Life-Threatening, Caused/Prolonged Hospitalization | 25 | ||||
| Life-Threatening, Caused/Prolonged Hospitalization, Disabling/Incapacitating | 1 | ||||
| Life-Threatening, Caused/Prolonged Hospitalization, Other | 5 | ||||
| Life-Threatening, Other | 3 | ||||
| Not serious | 5236 (66.6%) | Unknown | 7209 | ||
| Unknown | 1377 (17.5%) | Other | 279 |
| Natural Product | Number of Reports (n) |
|---|---|
| Salvia miltiorrhiza Bunge | 18,623 |
| Glycyrrhiza glabra L. | 1853 |
| Echinacea purpurea (L.) Moench | 1536 |
| Zingiber officinale Roscoe | 1378 |
| Panax ginseng C.A. Meyer | 1253 |
| Achyranthes bidentata Blume | 988 |
| Silybum marianum (L.) Gaertn | 980 |
| Andrographis paniculata (Burm.f.) Wall. ex Nees | 836 |
| Terminalia chebula Retz. | 528 |
| Allium sativum L. | 504 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.J.W.; Woerdenbag, H.J.; Ekhart, C.; Vitalone, A.; van Hunsel, F.P.A.M. Safety Considerations for Natural Products with Adaptogenic and Immunomodulating Activities. Pharmaceuticals 2025, 18, 1208. https://doi.org/10.3390/ph18081208
Liang CJW, Woerdenbag HJ, Ekhart C, Vitalone A, van Hunsel FPAM. Safety Considerations for Natural Products with Adaptogenic and Immunomodulating Activities. Pharmaceuticals. 2025; 18(8):1208. https://doi.org/10.3390/ph18081208
Chicago/Turabian StyleLiang, Chen Jia Wen, Herman J. Woerdenbag, Corine Ekhart, Annabella Vitalone, and Florence P. A. M. van Hunsel. 2025. "Safety Considerations for Natural Products with Adaptogenic and Immunomodulating Activities" Pharmaceuticals 18, no. 8: 1208. https://doi.org/10.3390/ph18081208
APA StyleLiang, C. J. W., Woerdenbag, H. J., Ekhart, C., Vitalone, A., & van Hunsel, F. P. A. M. (2025). Safety Considerations for Natural Products with Adaptogenic and Immunomodulating Activities. Pharmaceuticals, 18(8), 1208. https://doi.org/10.3390/ph18081208








