Variations in Plasma Levels of Orally Administered Ivermectin Could Hamper Its Potential Drug Repositioning: Results of a Bioequivalence Study in Mexican Population
Abstract
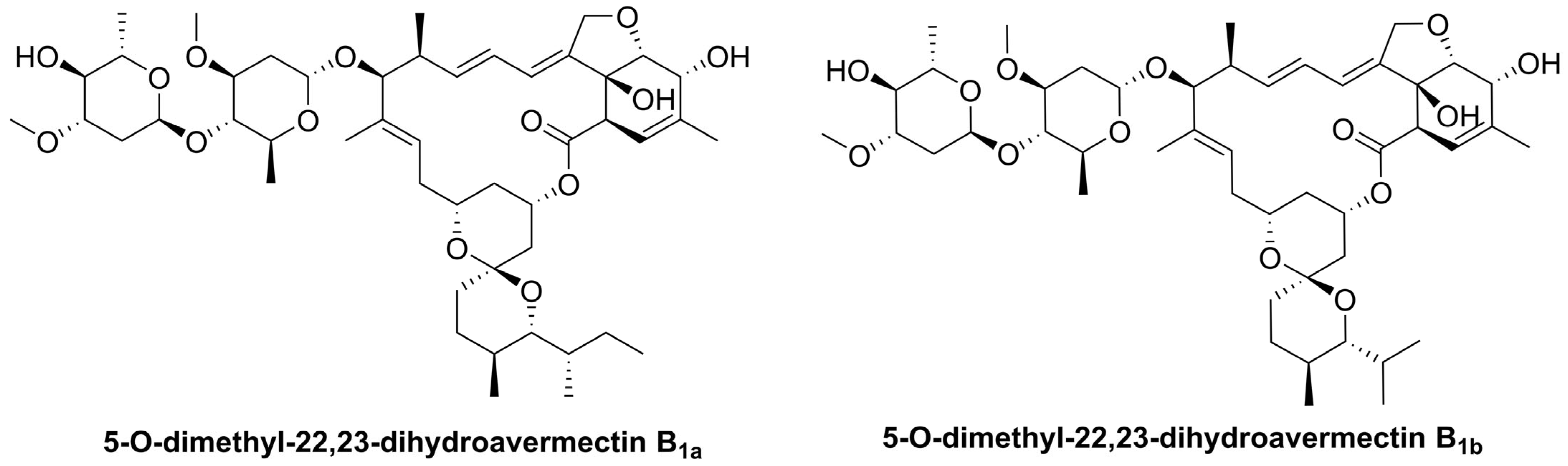
1. Introduction
2. Results
2.1. Subjects
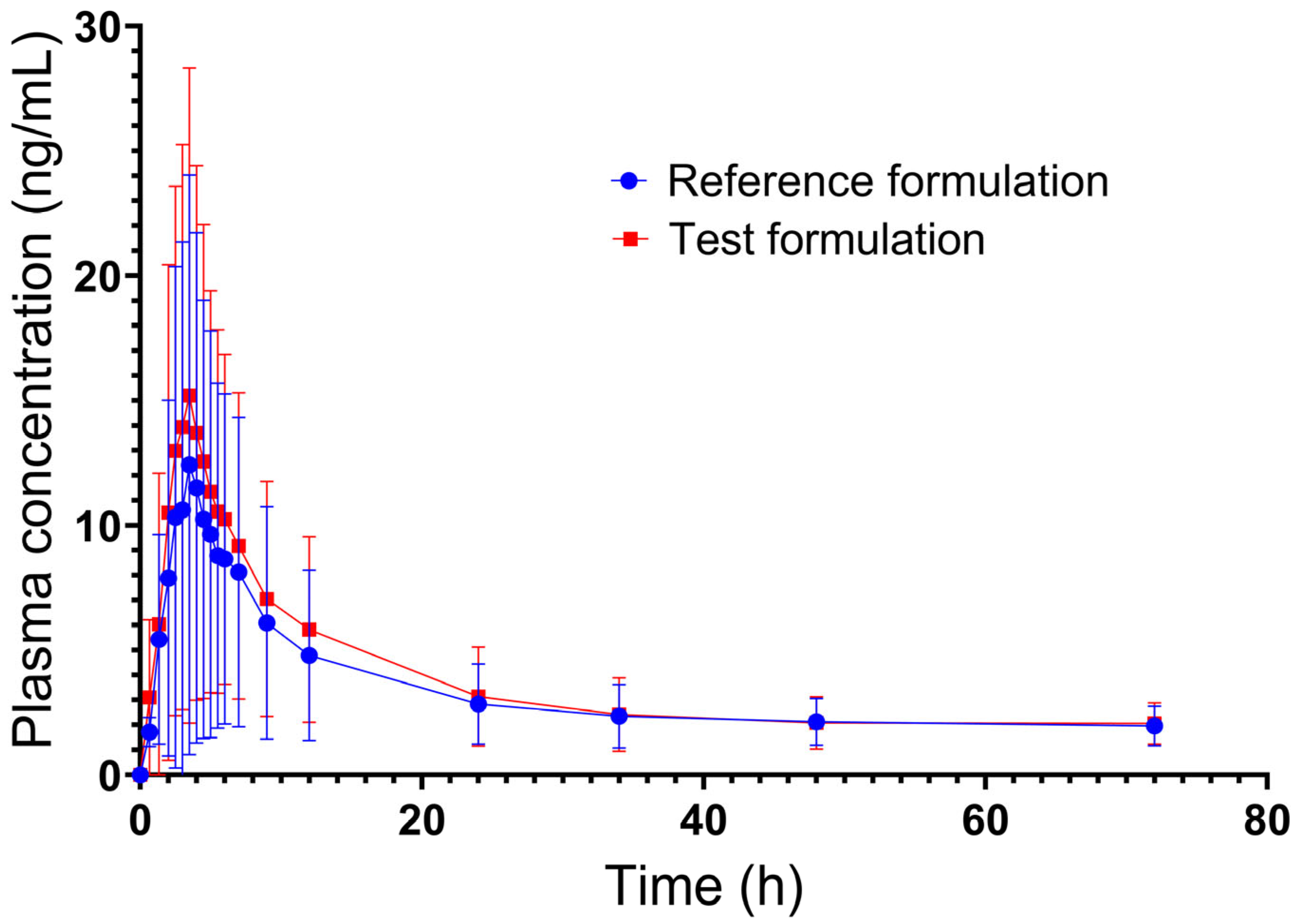
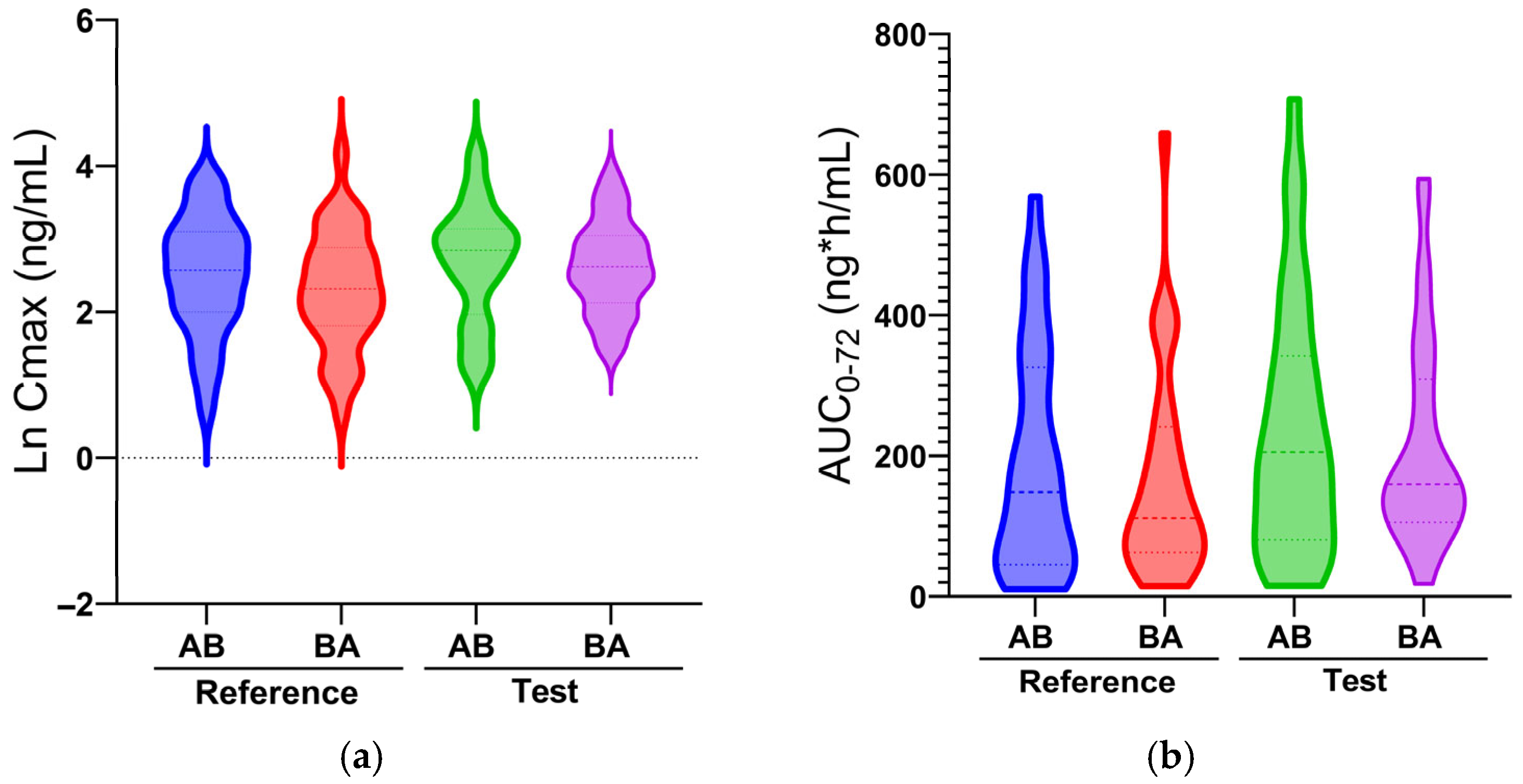
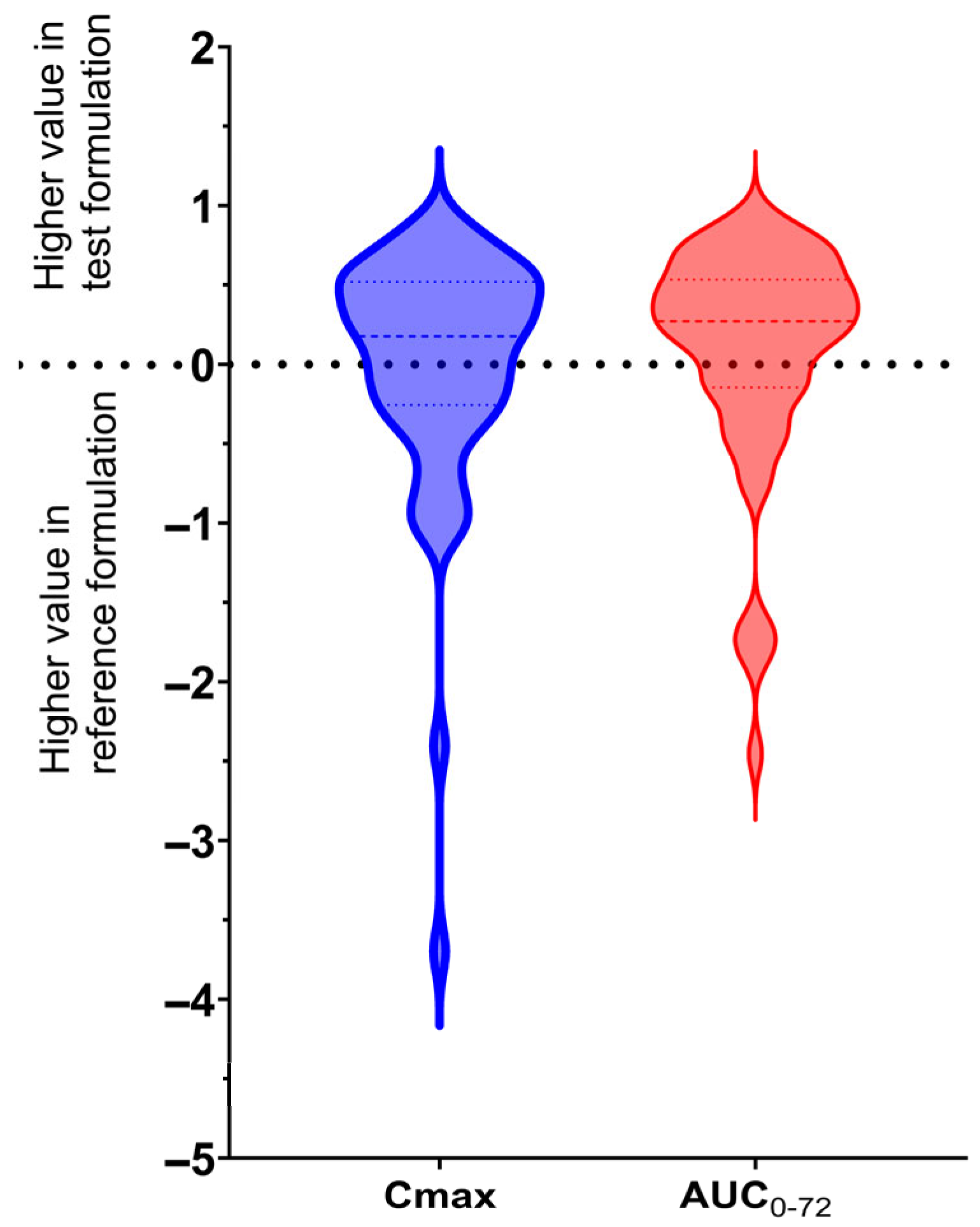
2.2. Pharmacokinetics
2.3. Safety
3. Discussion
4. Materials and Methods
4.1. Study Ethics
4.2. Study Drugs
4.3. Study Subjects
4.4. Study Design
4.5. Analysis Conditions
4.6. Pharmacokinetics and Bioequivalence Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaimo, S.; Pulvirenti, A. Network-Based Drug Repositioning: Approaches, Resources, and Research Directions BT-Computational Methods for Drug Repurposing. In Computational Methods for Drug Repurposing; Vanhaelen, Q., Ed.; Springer: New York, NY, USA, 2019; pp. 97–113. ISBN 978-1-4939-8955-3. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, Y. Teaching an old dog new tricks: Drug discovery by repositioning natural products and their derivatives. Drug Discov. Today 2022, 27, 1936–1944. [Google Scholar] [CrossRef]
- Wang, Y.; Aldahdooh, J.; Hu, Y.; Yang, H.; Vähä-Koskela, M.; Tang, J.; Tanoli, Z. DrugRepo: A novel approach to repurposing drugs based on chemical and genomic features. Sci. Rep. 2022, 12, 21116. [Google Scholar] [CrossRef]
- Ponce, S.A.; Green, A.; Strassle, P.D.; Nápoles, A.M. Positive and negative aspects of the COVID-19 pandemic among a diverse sample of US adults: An exploratory mixed-methods analysis of online survey data. BMC Public Health 2024, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Marcolino, M.S.; Meira, K.C.; Guimarães, N.S.; Motta, P.P.; Chagas, V.S.; Kelles, S.M.B.; de Sá, L.C.; Valacio, R.A.; Ziegelmann, P.K. Systematic review and meta-analysis of ivermectin for treatment of COVID-19: Evidence beyond the hype. BMC Infect. Dis. 2022, 22, 639. [Google Scholar] [CrossRef]
- Low, Z.Y.; Yip, A.J.W.; Lal, S.K. Repositioning Ivermectin for COVID-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2022, 1868, 166294. [Google Scholar] [CrossRef] [PubMed]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Chaccour, C.; Casellas, A.; Blanco-Di Matteo, A.; Pineda, I.; Fernandez-Montero, A.; Ruiz-Castillo, P.; Richardson, M.-A.; Rodríguez-Mateos, M.; Jordán-Iborra, C.; Brew, J.; et al. The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial. eClinicalMedicine 2021, 32, 100720. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.; Silva, E.A.S.M.; Silva, D.C.M.; Thabane, L.; Milagres, A.C.; Ferreira, T.S.; dos Santos, C.V.Q.; Campos, V.H.S.; Nogueira, A.M.R.; de Almeida, A.P.F.G.; et al. Effect of Early Treatment with Ivermectin among Patients with COVID-19. N. Engl. J. Med. 2022, 386, 1721–1731. [Google Scholar] [CrossRef]
- Naggie, S.; Boulware, D.R.; Lindsell, C.J.; Stewart, T.G.; Slandzicki, A.J.; Lim, S.C.; Cohen, J.; Kavtaradze, D.; Amon, A.P.; Gabriel, A.; et al. Effect of Higher-Dose Ivermectin for 6 Days vs Placebo on Time to Sustained Recovery in Outpatients with COVID-19: A Randomized Clinical Trial. JAMA 2023, 329, 888–897. [Google Scholar] [CrossRef]
- Song, Z.; Shi, S.; Zhang, Y. Ivermectin for treatment of COVID-19: A systematic review and meta-analysis. Heliyon 2024, 10, e27647. [Google Scholar] [CrossRef]
- Crump, A. Ivermectin: Enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef]
- Talevi, A.; Bellera, C.L. Challenges and opportunities with drug repurposing: Finding strategies to find alternative uses of therapeutics. Expert Opin. Drug Discov. 2020, 15, 397–401. [Google Scholar] [CrossRef]
- Jourdan, J.-P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef]
- Seo, J.I.; Jin, G.; Yoo, H.H. Pharmacokinetic considerations for enhancing drug repurposing opportunities of anthelmintics: Niclosamide as a case study. Biomed. Pharmacother. 2024, 173, 116394. [Google Scholar] [CrossRef] [PubMed]
- González Canga, A.; Sahagún Prieto, A.M.; Diez Liébana, M.J.; Fernández Martínez, N.; Sierra Vega, M.; García Vieitez, J.J. The Pharmacokinetics and Interactions of Ivermectin in Humans—A Mini-review. AAPS J. 2008, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- González Canga, A.; Sahagún Prieto, A.M.; José Diez Liébana, M.; Martínez, N.F.; Vega, M.S.; Vieitez, J.J.G. The pharmacokinetics and metabolism of ivermectin in domestic animal species. Vet. J. 2009, 179, 25–37. [Google Scholar] [CrossRef]
- Duthaler, U.; Suenderhauf, C.; Karlsson, M.O.; Hussner, J.; Meyer zu Schwabedissen, H.; Krähenbühl, S.; Hammann, F. Population pharmacokinetics of oral ivermectin in venous plasma and dried blood spots in healthy volunteers. Br. J. Clin. Pharmacol. 2019, 85, 626–633. [Google Scholar] [CrossRef]
- Duthaler, U.; Leisegang, R.; Karlsson, M.O.; Krähenbühl, S.; Hammann, F. The effect of food on the pharmacokinetics of oral ivermectin. J. Antimicrob. Chemother. 2020, 75, 438–440. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.A.F.; van Oudtshoorn, J.; Tam, A.; González, L.C.A.; Aurela, E.G.; Potthast, H.; Mettke, K.; Kuribayashi, R.; Shimojo, K.; Kasuga, M.; et al. The bioequivalence study design recommendations for immediate-release solid oral dosage forms in the international pharmaceutical regulators programme participating regulators and organisations: Differences and commonalities. J. Pharm. Pharm. Sci. 2024, 27, 12398. [Google Scholar] [CrossRef]
- Muñoz, J.; Ballester, M.R.; Antonijoan, R.M.; Gich, I.; Rodríguez, M.; Colli, E.; Gold, S.; Krolewiecki, A.J. Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PLoS Negl. Trop. Dis. 2018, 12, e0006020. [Google Scholar] [CrossRef]
- Guzzo, C.A.; Furtek, C.I.; Porras, A.G.; Chen, C.; Tipping, R.; Clineschmidt, C.M.; Sciberras, D.G.; Hsieh, J.Y.-K.; Lasseter, K.C. Safety, Tolerability, and Pharmacokinetics of Escalating High Doses of Ivermectin in Healthy Adult Subjects. J. Clin. Pharmacol. 2002, 42, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Algorta, J.; Krolewiecki, A.; Pinto, F.; Gold, S.; Muñoz, J. Pharmacokinetic Characterization and Comparative Bioavailability of an Innovative Orodispersible Fixed-Dose Combination of Ivermectin and Albendazole: A Single Dose, Open Label, Sequence Randomized, Crossover Clinical Trial in Healthy Volunteers. Front. Pharmacol. 2022, 13, 914886. [Google Scholar] [CrossRef]
- Chaccour, C.; Hammann, F.; Rabinovich, N.R. Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety. Malar. J. 2017, 16, 161. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Polli, J.E. Prediction of positive food effect: Bioavailability enhancement of BCS class II drugs. Int. J. Pharm. 2016, 506, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Shim, S.; Yee, J.; Choi, K.H.; Gwak, H.S. Effects of CYP3A4*22 polymorphism on trough concentration of tacrolimus in kidney transplantation: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1201083. [Google Scholar] [CrossRef]
- Ozeki, T.; Nagahama, M.; Fujita, K.; Suzuki, A.; Sugino, K.; Ito, K.; Miura, M. Influence of CYP3A4/5 and ABC transporter polymorphisms on lenvatinib plasma trough concentrations in Japanese patients with thyroid cancer. Sci. Rep. 2019, 9, 5404. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Chen, H.; Wang, F. Influence of ABCB1 Gene Polymorphism on Rivaroxaban Blood Concentration and Hemorrhagic Events in Patients With Atrial Fibrillation. Front. Pharmacol. 2021, 12, 639854. [Google Scholar] [CrossRef]
- Reyes-Hernández, O.D.; Lares-Asseff, I.; Sosa-Macias, M.; Vega, L.; Albores, A.; Elizondo, G. A Comparative Study of CYP3A4 Polymorphisms in Mexican Amerindian and Mestizo Populations. Pharmacology 2007, 81, 97–103. [Google Scholar] [CrossRef]
- Favela-Mendoza, A.F.; Rangel-Villalobos, H.; Fricke-Galindo, I.; Ortega-Vázquez, A.; Martínez-Cortés, G.; López-López, M. Genetic variability among Mexican Mestizo and Amerindian populations based on three ABCB1 polymorphisms. Mol. Biol. Rep. 2018, 45, 2525–2533. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-L.; Duong, V.-A.; Maeng, H.-J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef]
- Constantinides, P.P.; Wasan, K.M. Lipid Formulation Strategies for Enhancing Intestinal Transport and Absorption of P-Glycoprotein (P-gp) Substrate Drugs: In vitro/In vivo Case Studies. J. Pharm. Sci. 2007, 96, 235–248. [Google Scholar] [CrossRef]
- Maji, I.; Mahajan, S.; Sriram, A.; Medtiya, P.; Vasave, R.; Khatri, D.K.; Kumar, R.; Singh, S.B.; Madan, J.; Singh, P.K. Solid self emulsifying drug delivery system: Superior mode for oral delivery of hydrophobic cargos. J. Control. Release 2021, 337, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Halder, J.; Pradhan, D.; Kar, B.; Ghosh, G.; Rath, G. Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102494. [Google Scholar] [CrossRef]
- O’ Sullivan, J.; Blake, K.; Berntgen, M.; Salmonson, T.; Welink, J.; on behalf of the Pharmacokinetics Working Party. Overview of the European Medicines Agency’s Development of Product-Specific Bioequivalence Guidelines. Clin. Pharmacol. Ther. 2018, 104, 539–545. [Google Scholar] [CrossRef]
- Alshehri, A.; Chhonker, Y.S.; Bala, V.; Edi, C.; Bjerum, C.M.; Koudou, B.G.; John, L.N.; Mitjà, O.; Marks, M.; King, C.L.; et al. Population pharmacokinetic model of ivermectin in mass drug administration against lymphatic filariasis. PLoS Negl. Trop. Dis. 2023, 17, e0011319. [Google Scholar] [CrossRef]
- Modi, N.B.; Wang, B.; Hu, W.T.; Gupta, S.K. Effect of food on the pharmacokinetics of osmotic controlled-release methylphenidate HCl in healthy subjects. Biopharm. Drug Dispos. 2000, 21, 23–31. [Google Scholar] [CrossRef]
- Banerjee, S.; Shankar, K.R.; Prasad Y, R. Formulation development and systematic optimization of stabilized ziprasidone hydrochloride capsules devoid of any food effect. Pharm. Dev. Technol. 2016, 21, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Gera, S.; Sampathi, S.; Maddukuri, S.; Dodoala, S.; Junnuthula, V.; Dyawanapelly, S. Therapeutic Potential of Naringenin Nanosuspension: In Vitro and In Vivo Anti-Osteoporotic Studies. Pharmaceutics 2022, 14, 1449. [Google Scholar] [CrossRef] [PubMed]
- AboulFotouh, K.; Allam, A.A.; El-Badry, M.; El-Sayed, A.M. Role of self-emulsifying drug delivery systems in optimizing the oral delivery of hydrophilic macromolecules and reducing interindividual variability. Colloids Surf. B Biointerfaces 2018, 167, 82–92. [Google Scholar] [CrossRef]
- Rangaraj, N.; Sampathi, S.; Junnuthula, V.; Kolimi, P.; Mandati, P.; Narala, S.; Nyavanandi, D.; Dyawanapelly, S. Fast-Fed Variability: Insights into Drug Delivery, Molecular Manifestations, and Regulatory Aspects. Pharmaceutics 2022, 14, 1807. [Google Scholar] [CrossRef]
- Murteira, S.; Millier, A.; Ghezaiel, Z.; Lamure, M. Drug Reformulations and Repositioning in the Pharmaceutical Industry and Their Impact on Market Access: Regulatory Implications. J. Mark. Access Health Policy 2014, 2, 22813. [Google Scholar] [CrossRef]
- Mittal, N.; Mittal, R. Repurposing old molecules for new indications: Defining pillars of success from lessons in the past. Eur. J. Pharmacol. 2021, 912, 174569. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, A.; Virkel, G.; Sallovitz, J.; Sutra, J.F.; Galtier, P.; Alvinerie, M.; Lanusse, C. Comparative distribution of ivermectin and doramectin to parasite location tissues in cattle. Vet. Parasitol. 2000, 87, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Gardon, J.; Boussinesq, M.; Kamgno, J.; Gardon-Wendel, N.; Demanga-Ngangue; Duke, B.O.L. Effects of standard and high doses of ivermectin on adult worms of Onchocerca volvulus: A randomised controlled trial. Lancet 2002, 360, 203–210. [Google Scholar] [CrossRef] [PubMed]
- COFEPRIS. Norma Oficial Mexicana NOM-177-SSA1-2013; Que Establece las Pruebas y Procedimientos Para Demostrar Que un Medicamento es Intercambiable. Requisitos a Que Deben Sujetarse los Terceros Autorizados Que Realicen las Pruebas de Intercambiabilidad. Requisitos Para Realizar los Estudios de Biocomparabilidad. Requisitos a Que Deben Sujetarse los Terceros Autorizados, Centros de Investigación o Instituciones Hospitalarias Que Realicen las Pruebas de Biocomparabilidad; Diario Oficial de la Federación (DOF). Published on 20 September 2013. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5314833&fecha=20/09/2013#gsc.tab=0 (accessed on 15 March 2021).
| Reference | Test | |||||
|---|---|---|---|---|---|---|
| Cmax (ng/mL) | AUC0–72 (ng/mL·h) | Tmax (h) | Cmax (ng/mL) | AUC0–72 (ng/mL·h) | Tmax (h) | |
| Mean | 14.89 | 181.80 | 3.97 | 17.92 | 226.62 | 3.82 |
| Standard deviation | 12.07 | 152.95 | 1.24 | 12.99 | 166.57 | 1.31 |
| Minimum | 1.88 | 10.24 | 2.00 | 3.19 | 15.11 | 2.00 |
| Median | 11.28 | 134.90 | 3.50 | 14.88 | 167.90 | 3.50 |
| Maximum | 64.56 | 658.97 | 7.00 | 61.90 | 707.52 | 9.00 |
| CV (%) | 81.10 | 84.10 | 31.30 | 72.50 | 73.50 | 34.40 |
| Geometric mean | 11.02 | 119.23 | 3.80 | 14.07 | 165.89 | 3.63 |
| Parameter | Ratio (%) | Classic Confidence Interval (CI) | BEq Criteria | Schuirmann’s One-Sided Double t-Test | BEq Criteria | TOST 1 Potency |
|---|---|---|---|---|---|---|
| Ln Cmax | 127.70 | (109.53–148.89) | (80.00–125.00%) | p (θ < 80%) = 0.00 p (θ < 125%) = 0.59 | p < 0.05 | 0.03 |
| Ln AUC0–72 | 139.14 | (117.48–164.79) | (80.00–125.00%) | p (θ < 80%) = 0.00 p (θ < 125%) = 0.85 | p < 0.05 | <0.01 |
| Demographic Characteristic | Mean | Std. Dev. | CV% | Range |
|---|---|---|---|---|
| Age (years) | 29.98 | 9.56 | 31.88 | 18–52 |
| Weight (kg) | 62.79 | 10.10 | 16.08 | 41.3–88.4 |
| Height (cm) | 162 | 0.09 | 5.7 | 146–186 |
| Body-mass index (BMI, kg/m2) | 23.75 | 2.41 | 10.14 | 18.15–26.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Puente, E.; Ramos-Mundo, C.; Flores-Pérez, E.I.; Vergara-Castañeda, A.; Reyes-Grajeda, J.P.; Medina-Reyes, L.J.; Ruiz-Olmedo, M.I.; Loza-Mejía, M.A. Variations in Plasma Levels of Orally Administered Ivermectin Could Hamper Its Potential Drug Repositioning: Results of a Bioequivalence Study in Mexican Population. Pharmaceuticals 2025, 18, 1193. https://doi.org/10.3390/ph18081193
de la Puente E, Ramos-Mundo C, Flores-Pérez EI, Vergara-Castañeda A, Reyes-Grajeda JP, Medina-Reyes LJ, Ruiz-Olmedo MI, Loza-Mejía MA. Variations in Plasma Levels of Orally Administered Ivermectin Could Hamper Its Potential Drug Repositioning: Results of a Bioequivalence Study in Mexican Population. Pharmaceuticals. 2025; 18(8):1193. https://doi.org/10.3390/ph18081193
Chicago/Turabian Stylede la Puente, Ernesto, Carlos Ramos-Mundo, Elena I. Flores-Pérez, Arely Vergara-Castañeda, Juan Pablo Reyes-Grajeda, Liz J. Medina-Reyes, María Isabel Ruiz-Olmedo, and Marco A. Loza-Mejía. 2025. "Variations in Plasma Levels of Orally Administered Ivermectin Could Hamper Its Potential Drug Repositioning: Results of a Bioequivalence Study in Mexican Population" Pharmaceuticals 18, no. 8: 1193. https://doi.org/10.3390/ph18081193
APA Stylede la Puente, E., Ramos-Mundo, C., Flores-Pérez, E. I., Vergara-Castañeda, A., Reyes-Grajeda, J. P., Medina-Reyes, L. J., Ruiz-Olmedo, M. I., & Loza-Mejía, M. A. (2025). Variations in Plasma Levels of Orally Administered Ivermectin Could Hamper Its Potential Drug Repositioning: Results of a Bioequivalence Study in Mexican Population. Pharmaceuticals, 18(8), 1193. https://doi.org/10.3390/ph18081193









