In Vitro Evaluation of Annona muricata Leaf Infusion as a Modulator of Antineoplastic Drug-Induced Cytotoxicity in Cancer Cell Lines
Abstract
1. Introduction
2. Results
2.1. AM Infusion in Combination with First Line ADs
2.2. AM Infusion in Combination with AD vs. Healthy Cell Lines
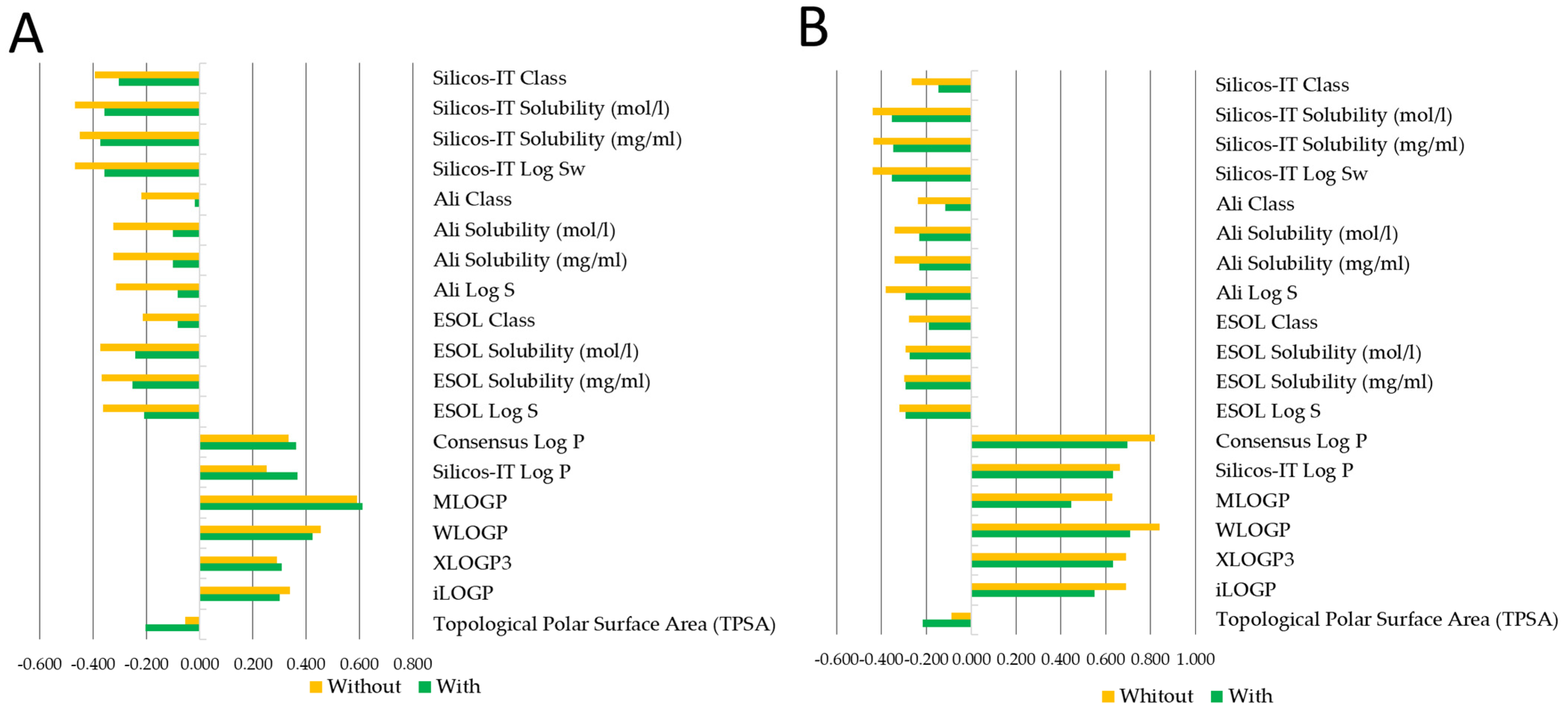
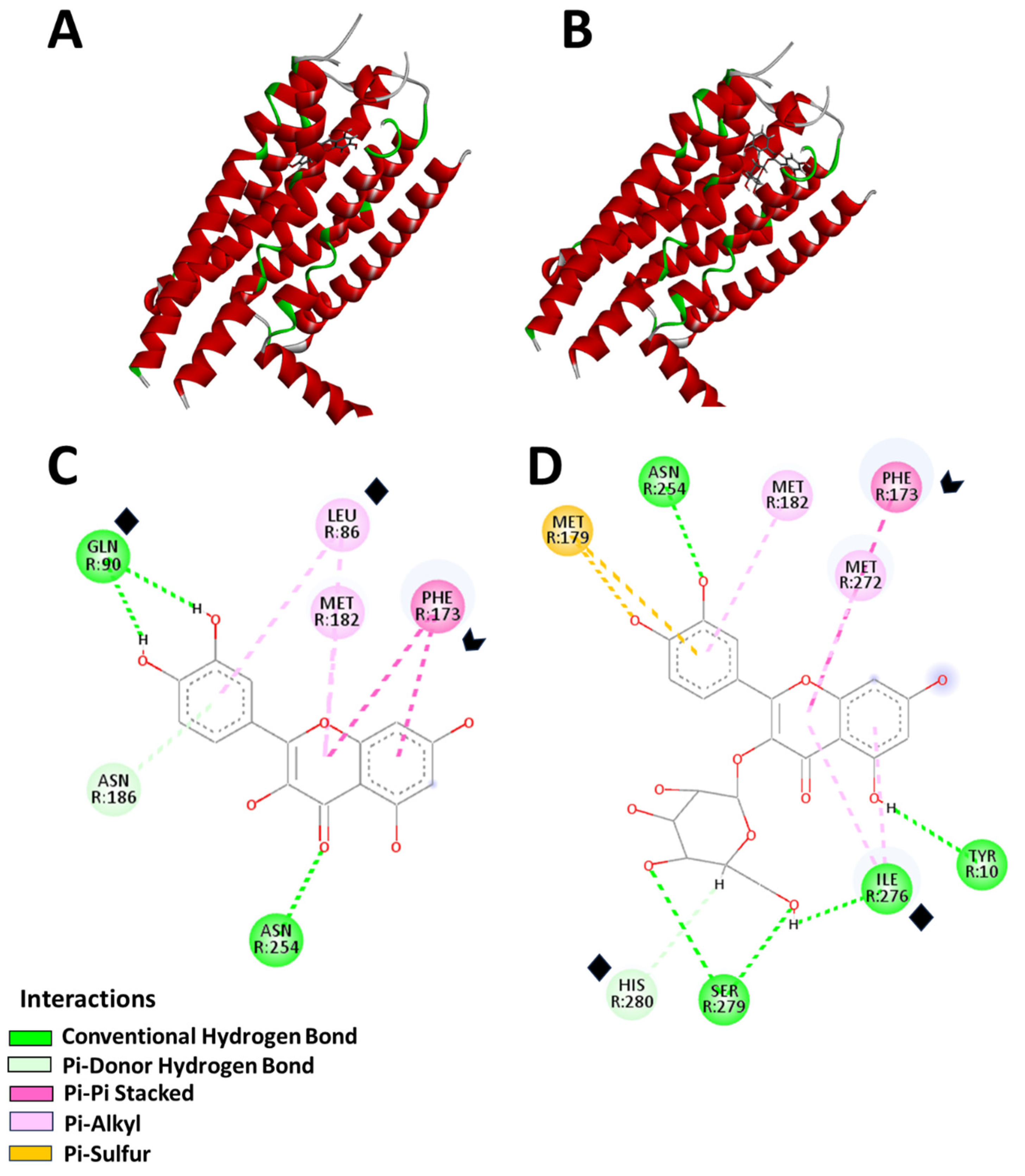
2.3. AM Infusion with ADs from an In Silico Point of View
3. Discussion
4. Materials and Methods
4.1. AM Infusion Preparation
4.2. Reagents and Treatments
4.3. In Vitro Evaluation
4.3.1. Isolation of Lymphocytes from Human Peripheral Blood and Cell Viability Test
4.3.2. Establishment of Primary Culture of Mouse Lung Fibroblasts
4.4. In Silico Analysis
4.5. Molecular Docking
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zubaidi, S.N.; Nani, H.M.; Kamal, M.S.A.; Qayyum, T.A.; Maarof, S.; Afzan, A.; Misnan, N.M.; Hamezah, H.S.; Baharum, S.N.; Mediani, A. Annona muricata: Comprehensive Review on the Ethnomedicinal, Phytochemistry, and Pharmacological Aspects Focusing on Antidiabetic Properties. Life 2023, 13, 353. [Google Scholar] [CrossRef]
- Hernandez-Fuentes, G.A.; Delgado-Enciso, O.G.; Larios-Cedeño, E.G.; Sánchez-Galindo, J.M.; Ceballos-Magaña, S.G.; Pineda-Urbina, K.; Alcalá-Pérez, M.A.; Magaña-Vergara, N.E.; Delgado-Enciso, J.; Díaz-Llerenas, U.; et al. Comparative Analysis of Infusions and Ethanolic Extracts of Annona muricata Leaves from Colima, Mexico: Phytochemical Profile and Antioxidant Activity. Life 2024, 14, 1702. [Google Scholar] [CrossRef]
- Fuentes, L.M.H.; González, E.M.; Magaña, M.d.L.G.; Esparza, L.M.A.; González, Y.N.; Villagrán, Z.; Torres, S.G.; Monreal, J.J.V.; Flores, D.A.M. Current Situation and Perspectives of Fruit Annonaceae in Mexico: Biological and Agronomic Importance and Bioactive Properties. Plants 2021, 11, 7. [Google Scholar] [CrossRef]
- Riley-Saldaña, C.A.; Cruz-Ortega, M.D.R.; Vázquez, M.M.; De-La-Cruz-Chacón, I.; Castro-Moreno, M.; González-Esquinca, A.R. Acetogenins and Alkaloids during the Initial Development of Annona muricata L. (Annonaceae). Z. Fur Naturforschung Sect. C J. Biosci. 2017, 72, 497–506. [Google Scholar] [CrossRef]
- Leboeuf, M.; Legueut, C.; Cavé, A.; Desconclois, J.; Forgacs, P.; Jacquemin, H. Alkaloids of Annonaceae. XXIX. Alkaloids of Annona muricata. Planta Med. 1981, 42, 37–44. [Google Scholar] [CrossRef]
- Huynh, N.V.; Nguyen Huu, D.M.; Huynh, N.T.; Chau, D.H.; Nguyen, C.D.; Nguyen Truong, Q.D.; Mai, D.T.; Dang, P.H. Anonazepine, a New Alkaloid from the Leaves of Annona muricata (Annonaceae). Z. Fur Naturforschung Sect. C J. Biosci. 2023, 78, 247–251. [Google Scholar] [CrossRef]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A Comprehensive Review on Its Traditional Medicinal Uses, Phytochemicals, Pharmacological Activities, Mechanisms of Action and Toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef]
- Ramos, A.L.C.C.; Mazzinghy, A.C.D.C.; Correia, V.T.d.V.; Nunes, B.V.; Ribeiro, L.V.; Silva, V.D.M.; Weichert, R.F.; de Paula, A.C.C.F.F.; de Sousa, I.M.N.; Ferreira, R.M.d.S.B.; et al. An Integrative Approach to the Flavonoid Profile in Some Plants’ Parts of the Annona Genus. Plants 2022, 11, 2855. [Google Scholar] [CrossRef]
- Amjad, E.; Sokouti, B.; Asnaashari, S. A Systematic Review of Anti-Cancer Roles and Mechanisms of Kaempferol as a Natural Compound. Cancer Cell Int. 2022, 22, 260. [Google Scholar] [CrossRef]
- Hernández-Fuentes, G.A.; Delgado-Enciso, I.; Enríquez-Maldonado, I.G.; Delgado-Machuca, J.J.; Zaizar-Fregoso, S.A.; Hernandez-Rangel, A.E.; Garcia-Casillas, A.C.; Guzman-Esquivel, J.; Rodriguez-Sanchez, I.P.; Martinez-Fierro, M.L.; et al. Antitumor Effects of Annopurpuricin A, an Acetogenin from the Roots of Annona purpurea. Rev. Bras. Farmacogn. 2023, 34, 111–121. [Google Scholar] [CrossRef]
- Ji, X.D.; Melman, N.; Jacobson, K.A. Interactions of Flavonoids and Other Phytochemicals with Adenosine Receptors. J. Med. Chem. 1996, 39, 781–788. [Google Scholar] [CrossRef]
- Fishman, P.; Bar-Yehuda, S.; Barer, F.; Madi, L.; Multani, A.S.; Pathak, S. The A3 Adenosine Receptor as a New Target for Cancer Therapy and Chemoprotection. Exp. Cell Res. 2001, 269, 230–236. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Buccioni, M. Current Understanding of the Role of Adenosine Receptors in Cancer. Molecules 2024, 29, 3501. [Google Scholar] [CrossRef]
- Karton, Y.; Jiang, J.; Ji, X.; Melman, N.; Olah, M.E.; Stiles, G.L.; Jacobson, K.A. Synthesis and Biological Activities of Flavonoid Derivatives as A3 Adenosine Receptor Antagonists. J. Med. Chem. 1996, 39, 2293–2301. [Google Scholar] [CrossRef]
- Lira-Ortiz, R.; Cortés-Cruz, M.A.; López-Guzmán, G.G.; Palomino-Hermosillo, Y.A.; Sandoval-Padilla, I.; Ochoa-Jiménez, V.A.; Sánchez-Herrera, L.M.; Balois-Morales, R.; Berumen-Varela, G. Diversidad Génetica de Poblaciones de Guanábana (Annona muricata L.) en Nayarit, México Mediante Marcadores SSR y SRAP. Acta Biol. Colomb. 2021, 27, 104–112. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Majumder, A.; Banik, A. Tea and Its Phytochemicals: Hidden Health Benefits & Modulation of Signaling Cascade by Phytochemicals. Food Chem. 2022, 371, 131098. [Google Scholar] [CrossRef]
- Poswal, F.S.; Russell, G.; Mackonochie, M.; MacLennan, E.; Adukwu, E.C.; Rolfe, V. Herbal Teas and Their Health Benefits: A Scoping Review. Plant Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef]
- Tomou, E.M.; Peppa, E.; Trichopoulou, A. Consumption of Herbal Infusions/Decoctions and Tea in Greece: A Planeterranean Perspective on the Results of Hydria Survey. J. Transl. Med. 2023, 21, 899. [Google Scholar] [CrossRef]
- Sousa, A.C.; Pádua, I.; Gonçalves, V.M.F.; Ribeiro, C.; Leal, S. Exploring Tea and Herbal Infusions Consumption Patterns and Behaviours: The Case of Portuguese Consumers. Heliyon 2024, 10, e28779. [Google Scholar] [CrossRef]
- Lan, M.J.; Yao, D.F.; Zhu, L.L.; Zhou, Q. The Rate of Infusion Represents an Important Aspect of Administering Anticancer Agents. Risk Manag. Healthc. Policy 2023, 16, 2531–2541. [Google Scholar] [CrossRef]
- Hernandez-Fuentes, G.A.; Gómez-Bueno, J.d.D.; Pérez-Santos, V.M.; Valle-Capitaine, I.J.; Villaseñor-Gonzalez, P.M.; Hernández-Zamorano, C.J.; Silva-Vázquez, C.G.; de la Cruz-Ruiz, M.; Diaz-Martinez, J.; Garza-Veloz, I.; et al. Comparing Perspectives on Traditional and Complementary Medicine Use in Oncology: Insights from Healthcare Professionals and Oncology Patients in Western Mexico. Curr. Oncol. 2025, 32, 71. [Google Scholar] [CrossRef]
- Allard, B.; Allard, D.; Buisseret, L.; Stagg, J. The Adenosine Pathway in Immuno-Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 611–629. [Google Scholar] [CrossRef]
- Feoktistov, I.; Ryzhov, S.; Zhong, H.; Goldstein, A.E.; Matafonov, A.; Zeng, D.; Biaggioni, I. Hypoxia Modulates Adenosine Receptors in Human Endothelial and Smooth Muscle Cells toward an A2B Angiogenic Phenotype. Hypertension 2004, 44, 649–654. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Cai, H.; Xu, Y.; Guo, S.; He, X.; Sun, J.; Li, X.; Li, C.; Yin, W.; Cheng, X.; Jiang, H.; et al. Structures of Adenosine Receptor A2BR Bound to Endogenous and Synthetic Agonists. Cell Discov. 2022, 8, 140. [Google Scholar] [CrossRef]
- Koussémou, M.; Lorenz, K.; Klotz, K.N. The A2B Adenosine Receptor in MDA-MB-231 Breast Cancer Cells Diminishes ERK1/2 Phosphorylation by Activation of MAPK-Phosphatase-1. PLoS ONE 2018, 13, e0202914. [Google Scholar] [CrossRef]
- Wang, K.; Ning, S.; Zhang, S.; Jiang, M.; Huang, Y.; Pei, H.; Li, M.; Tan, F. Extracellular Matrix Stiffness Regulates Colorectal Cancer Progression via HSF4. J. Exp. Clin. Cancer Res. 2025, 44, 30. [Google Scholar] [CrossRef]
- Ng, C.X.; Affendi, M.M.; Chong, P.P.; Lee, S.H. The Potential of Plant-Derived Extracts and Compounds to Augment Anticancer Effects of Chemotherapeutic Drugs. Nutr. Cancer 2022, 74, 3058–3076. [Google Scholar] [CrossRef]
- Mollaei, M.; Hassan, Z.M.; Khorshidi, F.; Langroudi, L. Chemotherapeutic Drugs: Cell Death- and Resistance-Related Signaling Pathways. Are they really as smart as the tumor cells? Transl. Oncol. 2021, 14, 101056. [Google Scholar] [CrossRef]
- Nayak, A.; Hegde, K. A Comprehensive Review on the Miracle Nature of Annona muricata Linn. RGUHS J. Pharm. Sci. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- AL-Ishaq, R.K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Enzymatic Metabolism of Flavonoids by Gut Microbiota and Its Impact on Gastrointestinal Cancer. Cancers 2021, 13, 3934. [Google Scholar] [CrossRef]
- Dudek, A.; Szulc, N.; Pawlak, A.; Strugała-Danak, P.; Krawczyk-Łebek, A.; Perz, M.; Kostrzewa-Susłow, E.; Pruchnik, H. Structural Investigation of Interactions between Halogenated Flavonoids and the Lipid Membrane along with Their Role as Cytotoxic Agents. Sci. Rep. 2024, 14, 10561. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane Interactions: A Protective Role of Flavonoids at the Membrane Surface? J. Immunol. Res. 2005, 12, 19–25. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Influence of Membrane Lipid Composition on Flavonoid–Membrane Interactions: Implications on Their Biological Activity. Prog. Lipid Res. 2015, 58, 1–13. [Google Scholar] [CrossRef]
- Luo, P.; Feng, X.; Liu, S.; Jiang, Y. Traditional Uses, Phytochemistry, Pharmacology and Toxicology of Ruta graveolens L.: A Critical Review and Future Perspectives. Drug Des. Dev. Ther. 2024, 18, 6459–6485. [Google Scholar] [CrossRef]
- Lin, X.; Liao, Y.; Chen, X.; Long, D.; Yu, T.; Shen, F. Regulation of Oncoprotein 18/Stathmin Signaling by ERK Concerns the Resistance to Taxol in Nonsmall Cell Lung Cancer Cells. Cancer Biother. Radiopharm. 2016, 31, 37–43. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, J.; Li, H.; Zhao, D.; Liu, Z.; Zhu, L.; Zhang, Z.; Peng, W. Quercetin: A Promising Therapy for Diabetic Encephalopathy through Inhibition of Hippocampal Ferroptosis. Phytomedicine 2024, 126, 154887. [Google Scholar] [CrossRef]
- Asif, M.; Naqvi, S.A.R.; Sherazi, T.A.; Ahmad, M.; Zahoor, A.F.; Shahzad, S.A.; Hussain, Z.; Mahmood, H.; Mahmood, N. Antioxidant, Antibacterial and Antiproliferative Activities of Pumpkin (Cucurbit) Peel and Puree Extracts—An in Vitro Study. Pak. J. Pharm. Sci. 2017, 30, 1327–1334. [Google Scholar]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy, 1st ed.; StatPearls Publishing LLC.: St. Petersburg, FL, USA, 2025; Volume 1. [Google Scholar]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Choudhuri, S.; Klaassen, C.D. Structure, Function, Expression, Genomic Organization, and Single Nucleotide Polymorphisms of Human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) Efflux Transporters. Int. J. Toxicol. 2006, 25, 231–259. [Google Scholar] [CrossRef]
- Jaber, B.L.; Cendoroglo, M.; Balakrishnan, V.S.; Perianayagam, M.C.; King, A.J.; Pereira, B.J.G. Apoptosis of Leukocytes: Basic Concepts and Implications in Uremia. Kidney Int. 2001, 59, S197–S205. [Google Scholar] [CrossRef]
- Shahneh, F.Z.; Valiyari, S.; Baradaran, B.; Abdolalizadeh, J.; Bandehagh, A.; Azadmehr, A.; Hajiaghaee, R. Inhibitory and Cytotoxic Activities of Salvia officinalis L. Extract on human lymphoma and leukemia cells by induction of apoptosis. Adv. Pharm. Bull. 2013, 3, 51–55. [Google Scholar] [CrossRef]
- Chennamadhavuni, A.; Lyengar, V.; Mukkamalla, S.K.R.; Shimanovsky, A. Leukemia; StatPearls Publishing LLC.: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Zhamanbayeva, G.T.; Aralbayeva, A.N.; Murzakhmetova, M.K.; Tuleukhanov, S.T.; Danilenko, M. Cooperative Antiproliferative and Differentiation-Enhancing Activity of Medicinal Plant Extracts in Acute Myeloid Leukemia Cells. Biomed. Pharmacother. 2016, 82, 80–89. [Google Scholar] [CrossRef]
- Giraud, C.; Manceau, S.; Treluyer, J.-M. ABC Transporters in Human Lymphocytes: Expression, Activity and Role, Modulating Factors and Consequences for Antiretroviral Therapies. Expert Opin. Drug Metab. Toxicol. 2010, 6, 571–589. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Universidad Nacional Autónoma de México (UNAM) Guanábana. Biblioteca Digital de la Medicina Tradicional Mexicana. Available online: http://www.medicinatradicionalmexicana.unam.mx/ (accessed on 4 January 2025).
- Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D. Efecto de la Digestión en la Biodisponibilidad de Péptidos con Actividad Biológica. Rev. Chil. Nutr. 2010, 37, 386–391. [Google Scholar] [CrossRef]
- Truba, J.; Stanisławska, I.; Walasek, M.; Wieczorkowska, W.; Woliński, K.; Buchholz, T.; Melzig, M.F.; Czerwińska, M.E. Inhibition of Digestive Enzymes and Antioxidant Activity of Extracts from Fruits of Cornus alba, Cornus sanguinea subsp. Hungarica and Cornus florida—A Comparative Study. Plants 2020, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Oshimori, N.; Fuchs, E. Paracrine TGF-β Signaling Counterbalances BMP-Mediated Repression in Hair Follicle Stem Cell Activation. Cell Stem Cell 2012, 10, 63–75. [Google Scholar] [CrossRef]
- Brüning, A.; Runnebaum, I.B. CAR Is a Cell–Cell Adhesion Protein in Human Cancer Cells and Is Expressionally Modulated by Dexamethasone, TNFα, and TGFβ. Gene Ther. 2003, 10, 198–205. [Google Scholar] [CrossRef]
- Panjehpour, M.; Castro, M.; Klotz, K. Human Breast Cancer Cell Line MDA-MB-231 Expresses Endogenous A2B Adenosine Receptors Mediating a Ca2+ Signal. Br. J. Pharmacol. 2005, 145, 211–218. [Google Scholar] [CrossRef] [PubMed]
- You, C.P.; Leung, M.H.; Tsang, W.C.; Khoo, U.S.; Tsoi, H. Androgen Receptor as an Emerging Feasible Biomarker for Breast Cancer. Biomolecules 2022, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Afzal, O.; Altamimi, A.S.A.; Almalki, W.H.; Kazmi, I.; Al-Abbasi, F.A.; Alzarea, S.I.; Makeen, H.A.; Albratty, M. Potential Role of Nutraceuticals via Targeting a Wnt/β-Catenin and NF-ΚB Pathway in Treatment of Osteoarthritis. J. Food Biochem. 2022, 46, e14427. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, J.; Wang, Z.; Peng, Y.; Zeng, L. Comprehensive Pan-Cancer Analysis Reveals ENC1 as a Promising Prognostic Biomarker for Tumor Microenvironment and Therapeutic Responses. Sci. Rep. 2024, 14, 25331. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Akula, R.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Herbario Nacional de México (MEXU). Annona muricata L. 1358521. Available online: https://datosabiertos.unam.mx/IBUNAM:MEXU:1358521 (accessed on 3 December 2024).
- Beas-Guzmán, O.F.; Cabrera-Licona, A.; Hernández-Fuentes, G.A.; Ceballos-Magaña, S.G.; Guzmán-Esquivel, J.; De-León-Zaragoza, L.; Ramírez-Flores, M.; Diaz-Martinez, J.; Garza-Veloz, I.; Martínez-Fierro, M.L.; et al. Ethanolic Extract of Averrhoa carambola Leaf Has an Anticancer Activity on Triple-Negative Breast Cancer Cells: An In Vitro Study. Pharmaceutics 2024, 17, 2. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A. Use of Multiple Assay Endpoints to Investigate the Effects of Incubation Time, Dose of Toxin, and Plating Density in Cell-Based Cytotoxicity Assays. Assay Drug Dev. Technol. 2004, 2, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Angulo, J.; Hernández-Fuentes, G.A.; Parra-Delgado, H.; Olvera-Valdéz, M.; Padilla-Martínez, I.; Cabrera-Licona, A.; Espinosa-Gil, A.; Delgado-Enciso, I.; Martínez-Martínez, F. Design, Synthesis, Biological and in Silico Evaluation of 3-carboxy-coumarin Sulfonamides as Potential Antiproliferative Agents Targeting HDAC6. Biomed. Rep. 2024, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- de Aluja, A.S. Animales de laboratorio y la Norma Oficial Mexicana (NOM-062-ZOO-1999) [Laboratory animals and official Mexican norms (NOM-062-ZOO-1999)]. Gac. Medica Mex. 2002, 138, 295–298. [Google Scholar]
- Saxena, A.K.; Gupta, A.K.; Bhatia, K.S. Physicochemical Significance of ChemDraw and Dragon Computed Parameters: Correlation Studies in the Sets with Aliphatic and Aromatic Substituents. J. Math. Chem. 2024, 62, 2430–2455. [Google Scholar] [CrossRef]
- Dearden, J.C. Prediction of physicochemical properties. In Computational Toxicology. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 929, pp. 93–138. [Google Scholar]
- Rosner, B. Fundamentals of Biostatistics/Bernard Rosner, 7th ed.; Brooks/Cole, Cengage Learning: Boston, MA, USA, 2011; Volume 1. [Google Scholar]
- Dudley, W.N.; Benuzillo, J.G.; Carrico, M.S. SPSS and SAS Programming for the Testing of Mediation Models. Nurs. Res. 2004, 53, 59–62. [Google Scholar] [CrossRef] [PubMed]
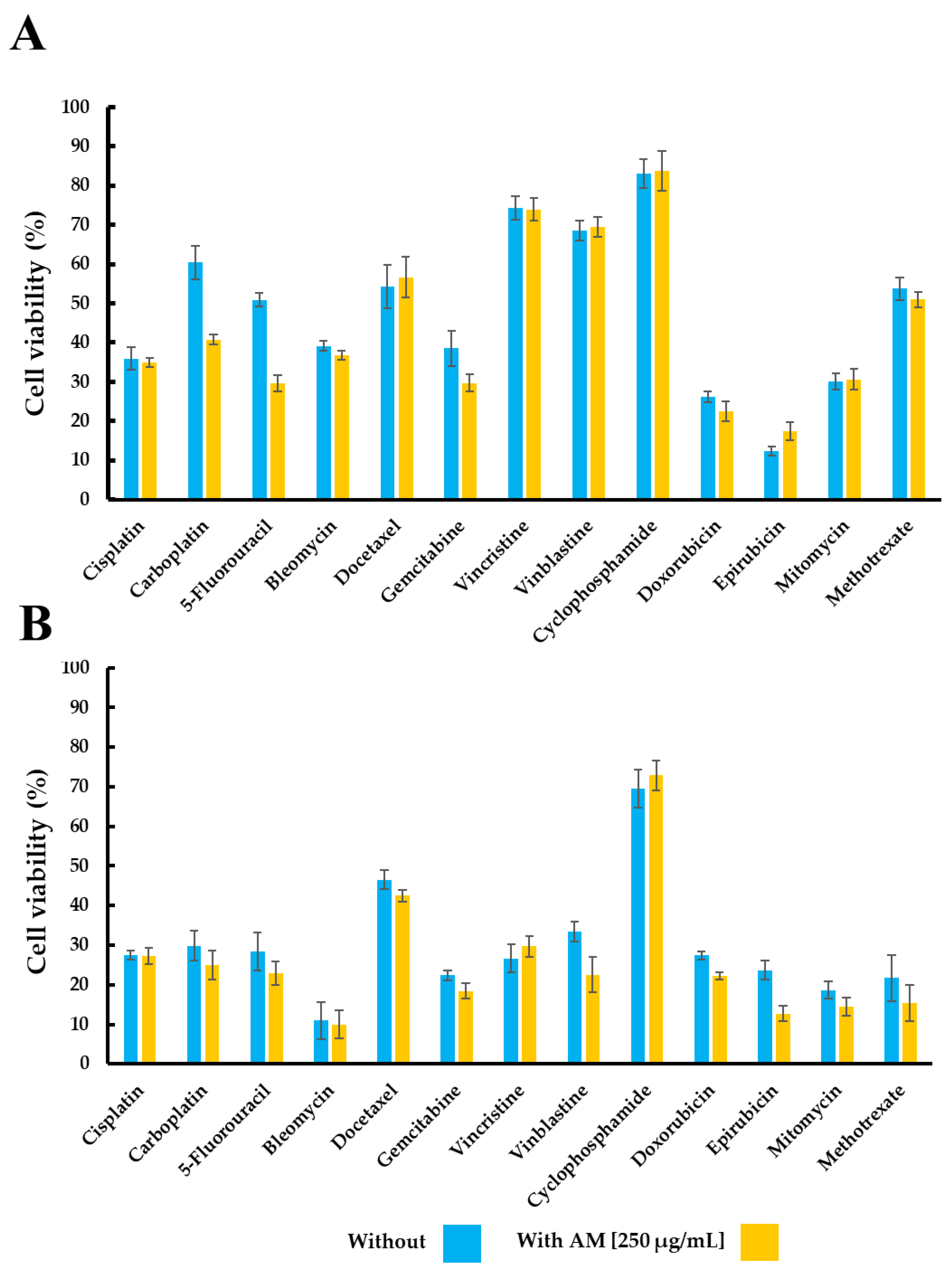
| Treatment | Cell Viability (%) | |||
|---|---|---|---|---|
| TC-1 | MDA-MB-231 | |||
| Without | With | Without | With | |
| Annona muricata infusion [250 µg/mL] | 97.51 ± 2.53 | 70.27 ± 10.41 | ||
| Cisplatin | 35.95 ± 2.98 | 34.92 ± 1.26 | 27.48 ± 1.06 | 27.20 ± 2.08 |
| Carboplatin | 60.45 ± 7.23 | 40.76 ± 1.34 | 29.83 ± 3.80 | 25.03 ± 3.65 |
| 5-Fluoracil | 50.88 ± 1.69 | 29.67 ± 2.09 | 28.43 ± 4.87 | 22.93 ± 2.87 |
| Bleomycin | 39.12 ± 1.28 | 36.76 ± 1.05 | 10.94 ± 4.68 | 9.99 ± 3.50 |
| Docetaxel | 54.27 ± 5.55 | 56.69 ± 5.25 | 46.51 ± 2.37 | 42.47 ± 1.47 |
| Gemcitabine | 38.56 ± 4.49 | 29.73 ± 2.26 | 22.38 ± 1.21 | 18.40 ± 1.90 |
| Vincristine | 74.34 ± 3.05 | 73.97 ± 2.87 | 26.69 ± 3.47 | 29.73 ± 2.66 |
| Vinblastine | 68.55 ± 2.58 | 69.40 ± 2.55 | 33.42 ± 2.56 | 22.57 ± 4.54 |
| Cyclophosphamide | 83.10 ± 3.74 | 83.76 ± 5.15 | 69.58 ± 4.79 | 72.91 ± 3.80 |
| Doxorubicin | 26.15 ± 1.44 | 22.48 ± 2.61 | 27.38 ± 1.06 | 22.27 ± 0.84 |
| Epirubicin | 12.28 ± 1.16 | 17.18 ± 2.32 | 23.72 ± 2.34 | 12.69 ± 1.94 |
| Mitomycin | 30.15 ± 2.04 | 30.66 ± 2.54 | 18.69 ± 2.13 | 14.46 ± 2.20 |
| Methotrexate | 53.77 ± 2.84 | 51.00 ± 1.98 | 21.69 ± 5.90 | 15.49 ± 4.57 |
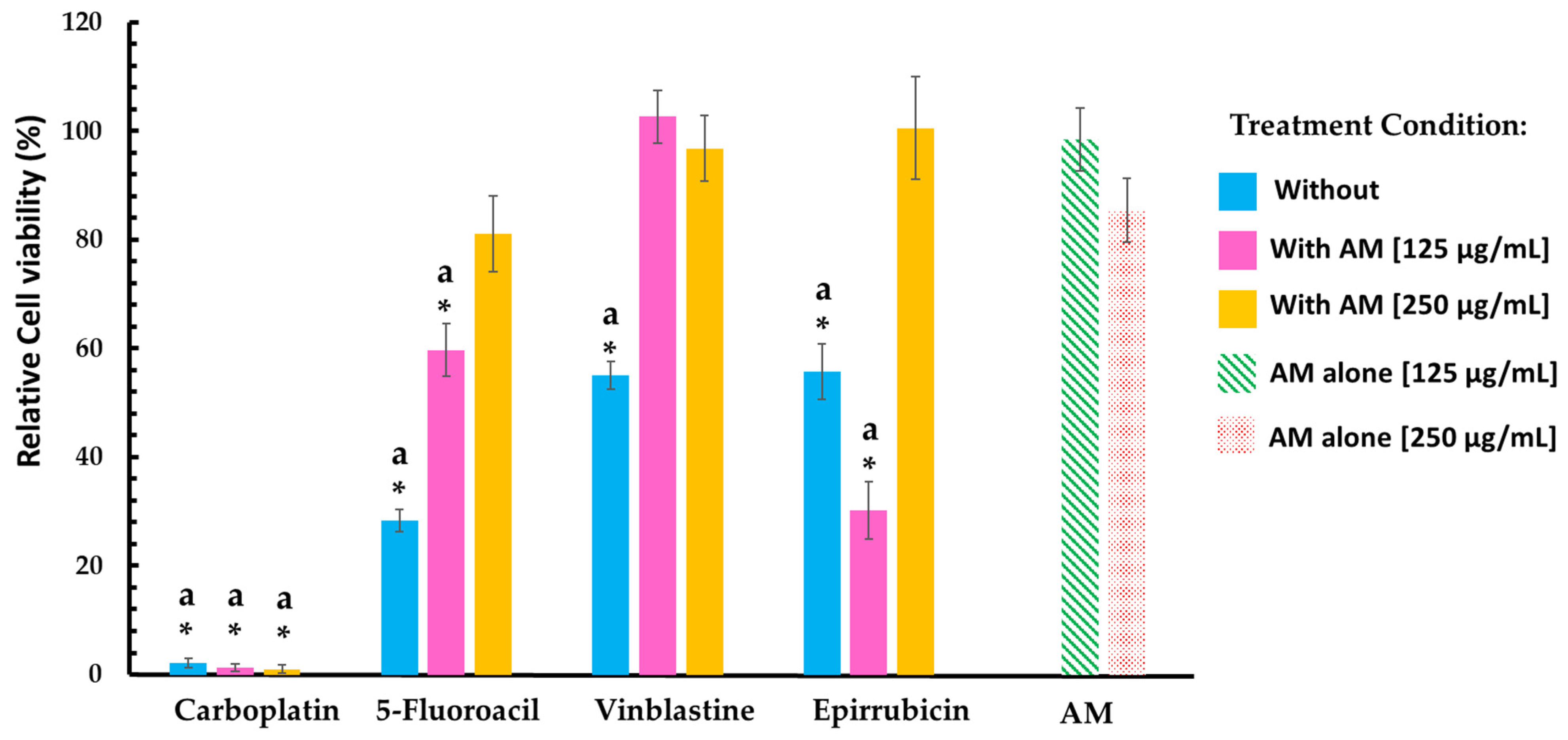
| Treatment | Cell Viability (%) | ||
|---|---|---|---|
| Without | With AM Infusion | ||
| 125 μg/mL | 250 μg/mL | ||
| Leukocytes | |||
| Annona muricata infusion | 61.34 ± 9.54 | 31.81 ± 6.61 | |
| 5-Fluoracil | 90.37 ± 5.48 | 62.39 ± 9.80 | 46.18 ± 12.35 |
| Carboplatin | 84.29 ± 4.42 | 68.49 ± 6.14 | 42.13 ± 7.32 |
| Epirubicin | 86.51 ± 1.80 | 28.28 ± 5.97 | 18.54 ± 3.62 |
| Vinblastine | 90.44 ± 1.96 | 30.91 ± 9.46 | 15.58 ± 4.47 |
| Murine Lung Cell Line | |||
| Annona muricata infusion | 98.42 ± 5.83 | 85.37 ± 5.88 | |
| 5-Fluoracil | 28.40 ± 2.03 | 59.72 ± 4.87 | 81.04 ± 6.98 |
| Carboplatin | 2.22 ± 0.82 | 1.38 ± 0.75 | 1.22 ± 0.80 |
| Epirubicin | 55.78 ± 5.10 | 90.24 ± 5.83 | 100.5 ± 9.42 |
| Vinblastine | 55.07 ± 2.60 | 102.6 ± 4.81 | 96.77 ± 5.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Licona, A.; Hernández-Fuentes, G.A.; Pineda-Urbina, K.; Hernández-Rangel, A.E.; Alcalá-Pérez, M.A.; Diaz-Martinez, J.; Díaz-Llerenas, U.; Guzmán-Esquivel, J.; Montesinos-López, O.A.; Casarez-Price, J.C.; et al. In Vitro Evaluation of Annona muricata Leaf Infusion as a Modulator of Antineoplastic Drug-Induced Cytotoxicity in Cancer Cell Lines. Pharmaceuticals 2025, 18, 1177. https://doi.org/10.3390/ph18081177
Cabrera-Licona A, Hernández-Fuentes GA, Pineda-Urbina K, Hernández-Rangel AE, Alcalá-Pérez MA, Diaz-Martinez J, Díaz-Llerenas U, Guzmán-Esquivel J, Montesinos-López OA, Casarez-Price JC, et al. In Vitro Evaluation of Annona muricata Leaf Infusion as a Modulator of Antineoplastic Drug-Induced Cytotoxicity in Cancer Cell Lines. Pharmaceuticals. 2025; 18(8):1177. https://doi.org/10.3390/ph18081177
Chicago/Turabian StyleCabrera-Licona, Ariana, Gustavo A. Hernández-Fuentes, Kayim Pineda-Urbina, Alejandra E. Hernández-Rangel, Mario A. Alcalá-Pérez, Janet Diaz-Martinez, Uriel Díaz-Llerenas, José Guzmán-Esquivel, Osval A. Montesinos-López, Juan C. Casarez-Price, and et al. 2025. "In Vitro Evaluation of Annona muricata Leaf Infusion as a Modulator of Antineoplastic Drug-Induced Cytotoxicity in Cancer Cell Lines" Pharmaceuticals 18, no. 8: 1177. https://doi.org/10.3390/ph18081177
APA StyleCabrera-Licona, A., Hernández-Fuentes, G. A., Pineda-Urbina, K., Hernández-Rangel, A. E., Alcalá-Pérez, M. A., Diaz-Martinez, J., Díaz-Llerenas, U., Guzmán-Esquivel, J., Montesinos-López, O. A., Casarez-Price, J. C., Del-Toro-Equihua, M., Zaizar-Fregoso, S. A., Gamez-Bayardo, S., Beas-Guzmán, O. F., & Delgado-Enciso, I. (2025). In Vitro Evaluation of Annona muricata Leaf Infusion as a Modulator of Antineoplastic Drug-Induced Cytotoxicity in Cancer Cell Lines. Pharmaceuticals, 18(8), 1177. https://doi.org/10.3390/ph18081177