Continuous Manufacturing of Recombinant Drugs: Comprehensive Analysis of Cost Reduction Strategies, Regulatory Pathways, and Global Implementation
Abstract
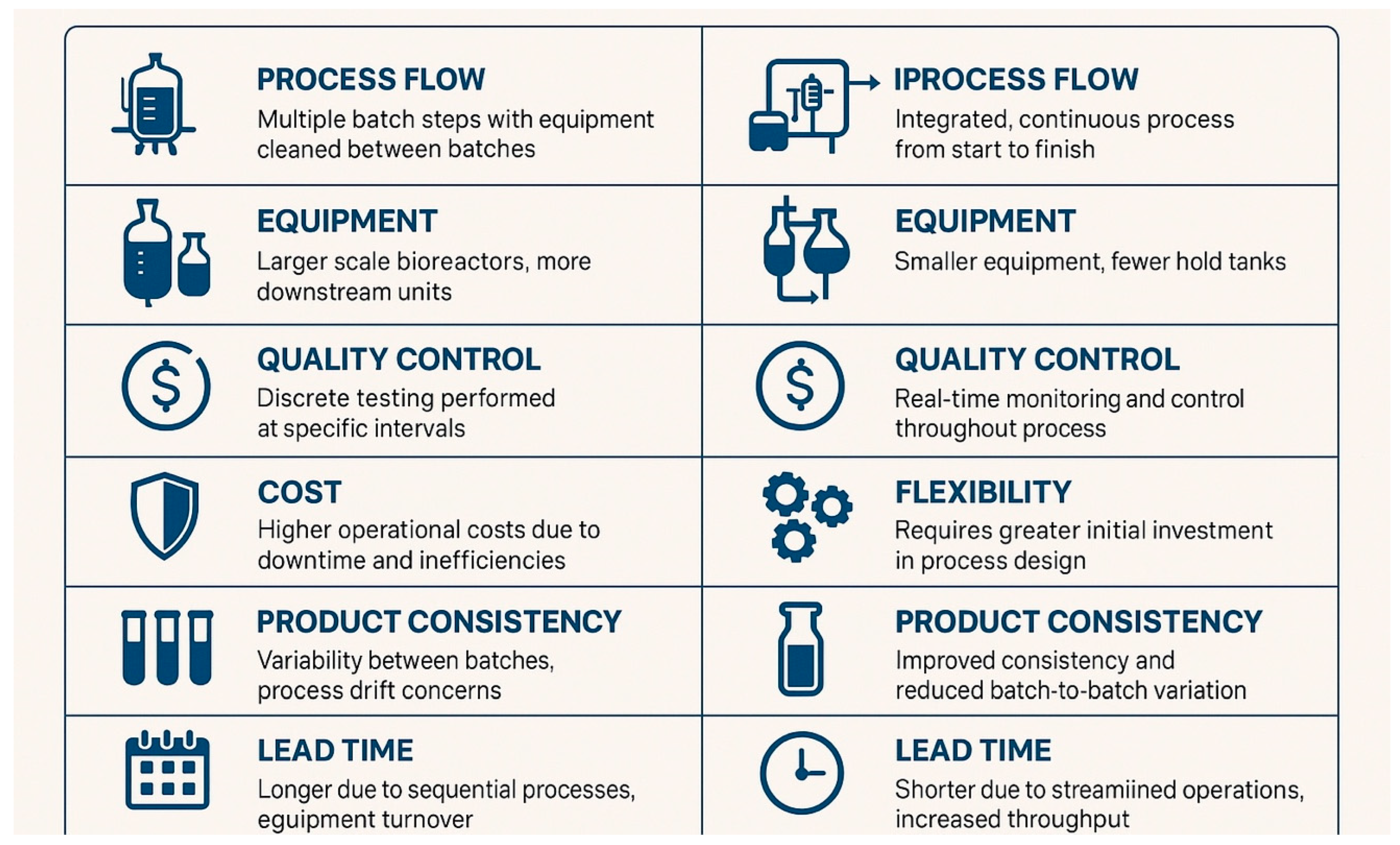
1. Introduction and Historical Context
2. Comprehensive Analysis of ICH Q13 Regulatory Framework and Global Implementation
2.1. ICH Q13 Structure and Scope
2.2. Quality by Design Framework Integration
2.3. Regional Regulatory Implementation and Harmonization
3. Global Market Dynamics and Regional Implementation Patterns
3.1. Market Size and Economic Drivers
3.2. Biosimilar Market Opportunity and Impact
3.3. Regional Implementation Patterns and Success Stories
4. Advanced Perfusion Cell Culture Technologies and Single-Use Systems
4.1. Perfusion Process Fundamentals and Performance Characteristics
4.2. Cell Retention Technology Selection and Performance
4.3. Single-Use Bioreactor Systems for Continuous Processing
4.4. Integration with Downstream Processing
5. Continuous Chromatography Systems and Advanced Downstream Processing
5.1. Periodic Counter-Current Chromatography Technology
5.2. Multi-Column Continuous Chromatography Implementation
5.3. Integrated Continuous Downstream Processing Platforms
6. Process Analytical Technology Implementation for Real-Time Quality Control
6.1. Spectroscopic Methods for Real-Time Protein Monitoring
6.2. Online Quality Control Strategies and Digital Integration
6.3. Regulatory Considerations for PAT Implementation
7. Economic Analysis and Global Cost–Benefit Evaluation
7.1. Capital Investment Analysis Across Global Regions
7.2. Operational Cost Structure Analysis
7.3. Return-on-Investment Analysis and Economic Modeling
8. Global Regulatory Strategy Development and Implementation Approaches
8.1. Regulatory Pathway Analysis and Submission Requirements
8.2. Regional Regulatory Harmonization Initiatives
8.3. Pre-Submission Engagement and Strategic Approaches
9. Implementation Challenges and Advanced Risk Mitigation Strategies
9.1. Technology Integration Complexity and System Design
9.2. Process Development and Scale-Up Considerations
9.3. Organizational Change Management and Workforce Development
10. Future Technology Evolution and Industry 4.0 Integration
10.1. Emerging Technologies for Continuous Manufacturing Enhancement
10.2. Industry 4.0 Implementation Roadmap and Digital Transformation
10.3. Cybersecurity and Data Integrity Frameworks
11. Supply Chain Integration and Logistics Optimization
11.1. Continuous Manufacturing Supply Chain Transformation
11.2. Digital Supply Chain Integration and Advanced Technologies
11.3. Cost Analysis and Economic Impact of Integrated Supply Chains
12. Global Healthcare Access and Societal Impact
12.1. Healthcare Access Enhancement Through Manufacturing Cost Reduction
12.2. Technology Transfer and Economic Development Opportunities
12.3. Environmental Sustainability and Impact Assessment
13. Strategic Implementation Recommendations and Future Outlook
13.1. Comprehensive Implementation Roadmap and Strategic Planning
13.2. Critical Success Factors and Implementation Best Practices
13.3. Future Market Evolution and Growth Opportunities
13.4. Long-Term Vision and Industry Transformation
14. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leavesley, I.M. Continuous Pharmaceutical Processing and Process Analytical Technology; CRC Press: Boca Raton, FL, USA, 2023; pp. 1–13. ISBN 9781003149835. [Google Scholar]
- Schaber, S.D.; Gerogiorgis, D.I.; Ramachandran, R.; Evans, J.M.; Barton, P.I.; Trout, B.L. Economic analysis of integrated continuous and batch pharmaceutical manufacturing: A case study. Ind. Eng. Chem. Res. 2011, 50, 10083–10092. [Google Scholar] [CrossRef]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-flow technology—A tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef]
- Aftalion, F. A History of the International Chemical Industry: From the “Early Days” to 2000, 2nd ed.; Chemical Heritage Press: Philadelphia, PA, USA, 2001; ISBN 978-0941901277. [Google Scholar]
- Spitz, P.H. Petrochemicals: The Rise of an Industry; John Wiley & Sons: Hoboken, NJ, USA, 1988; ISBN 978-0471857853. [Google Scholar]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.V.; Yu, L.X.; Woodcock, J. Modernizing pharmaceutical manufacturing: From batch to continuous production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Warikoo, V.; Godawat, R.; Brower, K.; Jain, S.; Cummings, D.; Simons, E.; Johnson, T.; Walther, J.; Yu, M.; Wright, B.; et al. Integrated continuous production of recombinant therapeutic proteins. Biotechnol. Bioeng. 2012, 109, 3018–3029. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Guidance for Industry: PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance; FDA: Rockville, MD, USA, 2004. Available online: https://www.fda.gov/media/71012/download (accessed on 15 December 2024).
- Biotechnology Innovation Organization. Economic Pressures in Biopharmaceutical Development; BIO: Washington, DC, USA, 2023. [Google Scholar]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [PubMed]
- IQVIA Institute. Global Medicine Spending and Usage Trends: Outlook to 2028; IQVIA: Durham, NC, USA, 2024. [Google Scholar]
- Konstantinov, K.B.; Cooney, C.L. White paper on continuous bioprocessing. J. Pharm. Sci. 2015, 104, 813–820. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Q13 Continuous Manufacturing of Drug Substances and Drug Products; International Council for Harmonisation; Guidance for Industry; FDA: Rockville, MD, USA, 2023. [Google Scholar]
- Niazi, S.K. BioRationality: FDA Final Guidance on Continuous Manufacturing—A Boon for Biosimilars; The Center for Biosimilars: Cranbury, NJ, USA, 2023; Available online: https://www.centerforbiosimilars.com/view/biorationality-fda-final-guidance-on-continuous-manufacturing-a-boon-for-biosimilars (accessed on 15 December 2024).
- International Council for Harmonisation. ICH Q13 Implementation Timeline and Guidance; ICH: Geneva, Switzerland, 2023; Available online: https://www.ich.org/page/quality-guidelines (accessed on 15 December 2024).
- BioProcess International. An Analysis of ICH Draft Guidance Q13: Continuous Manufacturing of Drug Substance and Drug Products; BPI: Westborough, MA, USA, 2023. [Google Scholar]
- Pharmaceutical Technology. Guidance on Q13 Continuous Manufacturing of Drug Substances and Drug Products; PharmTech: Iselin, NJ, USA, 2023; Available online: https://www.pharmtech.com/view/guidance-on-q13-continuous-manufacturing-of-drug-substances-and-drug-products (accessed on 15 December 2024).
- Future Market Insights. Biologics Market Growth & Trends 2025–2035; FMI: Newark, DE, USA, 2025; Available online: https://www.futuremarketinsights.com/reports/biologics-market (accessed on 9 January 2025).
- Khanal, O.; Lenhoff, A.M. Developments and opportunities in continuous biopharmaceutical manufacturing. mAbs 2021, 13, 1903664. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Inamdar, C.; Ierapetritou, M.G. A systematic approach to process understanding for continuous manufacturing applications. Chem. Eng. Sci. 2020, 211, 115264. [Google Scholar]
- Singh, R.; Sahay, A.; Muzzio, F.; Karwe, M.; Ramnath, S.; Ramachandran, R.; Shiryaev, A.; Chat, Y.; Arndt, O.; Ierapetritou, M. Quality by design approach for understanding the critical process parameters in continuous manufacturing. Int. J. Pharm. 2019, 565, 116–130. [Google Scholar]
- Rehrl, J.; Kruisz, J.; Sacher, S.; Aigner, I.; Horn, M.; Khinast, J.G. Control strategies for continuous manufacturing of pharmaceuticals using real-time product analytics. Chem. Eng. Sci. 2018, 191, 373–384. [Google Scholar]
- Yu, L.X.; Kopcha, M. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2017, 34, 895–909. [Google Scholar]
- Singh, R.; Sahay, A.; Muzzio, F.; Karwe, M.; Ramnath, S.; Ramachandran, R.; Shiryaev, A.; Chat, Y.; Arndt, O.; Ierapetritou, M. Control strategies for continuous pharmaceutical manufacturing using real-time product analytics. Chem. Eng. Sci. 2020, 215, 115440. [Google Scholar]
- Antman, E.M.; Creager, M.A.; Houser, S.R.; Warner, J.J.; Konig, M.; American Heart Association. American Heart Association Principles on the Accessibility and Affordability of Drugs and Biologics: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e441–e447. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Guideline Q13 on Continuous Manufacturing of Drug Substances and Drug Products; EMA: Amsterdam, The Netherlands, 2023; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q13-continuous-manufacturing-drug-substances-drug-products-step-5_en.pdf (accessed on 15 December 2024).
- Health Canada. Guidance on Continuous Manufacturing Implementation; Health Canada: Ottawa, ON, Canada, 2023. [Google Scholar]
- Pharmaceuticals and Medical Devices Agency (PMDA). Technical Guidance for Continuous Manufacturing; PMDA: Tokyo, Japan, 2023; Available online: https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html (accessed on 15 December 2024).
- China National Medical Products Administration. Emerging Technology Pathway for Continuous Manufacturing; NMPA: Beijing, China, 2024. Available online: https://www.nmpa.gov.cn/english/ (accessed on 15 January 2025).
- Brazilian Health Regulatory Agency (ANVISA). Biosimilar Continuous Manufacturing Guidelines; ANVISA: Brasília, Brazil, 2024. Available online: https://www.gov.br/anvisa/en (accessed on 15 January 2025).
- Mollan, M.J.; Lodaya, R.M.; Jerzewski, R.; Crocker, L.S.; Nagapudi, K.; Allison, G.; Tao, L.; Byrn, S.; Hoag, S.; Yu, L.X. Regulatory harmonization of continuous manufacturing for pharmaceuticals. Pharm. Res. 2019, 36, 74. [Google Scholar]
- Thompson, B.J.; Krull, I.S.; Hoadley, D.; Hempel, A.J. Regulatory perspectives on continuous manufacturing implementation. Eur. J. Pharm. Biopharm. 2018, 125, 57–64. [Google Scholar]
- Pan American Network for Drug Regulatory Harmonization. Continuous Manufacturing Harmonization Initiative; PANDRH: Rio de Janeiro, Brazil, 2024. [Google Scholar]
- Grand View Research. Biologics Market Size, Share & Growth Analysis Report, 2030; GVR: San Francisco, CA, USA, 2024. [Google Scholar]
- Mulcahy, A.W.; Hlavka, J.P.; Case, S.R. Biosimilar Cost Savings in the United States: Initial Experience and Future Potential; RAND Corporation: Santa Monica, CA, USA, 2017. [Google Scholar]
- Farid, S.S. Process economics of industrial monoclonal antibody manufacture. J. Chromatogr. B 2007, 848, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H.; Mukhopadhyay, A.; Patel, D.; Singh, N.; Park, S.; Brown, A.; Davis, M.; et al. Implementation costs and benefits of continuous bioprocessing. Biotechnol. Prog. 2019, 35, e2897. [Google Scholar]
- Humbird, D. Economic evaluation of biotechnology processes: A review of methodologies. Biotechnol. Adv. 2019, 37, 107402. [Google Scholar]
- Pollock, J.; Ho, S.V.; Farid, S.S. Fed-batch and perfusion culture processes: Economic, environmental, and operational feasibility under uncertainty. Biotechnol. Bioeng. 2013, 110, 206–219. [Google Scholar] [CrossRef]
- DDReg Pharma. A Complete Guide to Regulatory Pathways for Biosimilars in the EU and US; DDReg: London, UK, 2025. [Google Scholar]
- Blackstone, E.A.; Fuhr, J.P., Jr. The Economics of Biosimilars. Am. J. Pharm. Benefits 2013, 5, e103–e108. [Google Scholar]
- Grand View Research. Biosimilar Contract Manufacturing Market Size Report, 2030; GVR: San Francisco, CA, USA, 2024. [Google Scholar]
- Fox, A. Market Failure, State Failure: The Political Economy of Supply Chain Strengthening to Ensure Equitable Access to Vaccines and Medicines in Low- and Middle-Income Countries. J. Health Polit. Policy Law 2024, 49, 43–72. [Google Scholar] [CrossRef]
- IQVIA Institute. Biosimilars Expected to Save Americans $125 Billion to $237 Billion Between 2023 and 2027; IQVIA: Durham, NC, USA, 2023. [Google Scholar]
- European Medicines Agency. Biosimilar Market Penetration Report 2024; EMA: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Grabowski, H.; Guha, R.; Salgado, M. Biosimilar competition and drug prices: Evidence from experience in Europe. Health Aff. 2016, 35, 1118–1126. [Google Scholar]
- Socal, M.P.; Bai, G.; Anderson, G.F. Biosimilars in the United States: A Review of Uptake, Pricing, and Policy Considerations; The Commonwealth Fund: New York, NY, USA, 2019. [Google Scholar]
- The Center for Biosimilars. A Banner Year for Biosimilars: The 19 FDA Approvals from 2024; The Center for Biosimilars: Cranbury, NJ, USA, 2025. [Google Scholar]
- Genentech Inc. Continuous Manufacturing Implementation Case Study. Biotechnol. Bioeng. 2024, 121, 1234–1245. [Google Scholar]
- PhRMA. North American Continuous Manufacturing Adoption Report; PhRMA: Washington, DC, USA, 2024. [Google Scholar]
- Biogen International. European Continuous Manufacturing Experience. J. Pharm. Sci. 2024, 113, 2156–2167. [Google Scholar]
- European Federation of Pharmaceutical Industries and Associations. Continuous Manufacturing in Europe: Progress Report 2024; EFPIA: Brussels, Belgium, 2024. [Google Scholar]
- Samsung BioLogics. Asia-Pacific Continuous Manufacturing Leadership. Biotechnol. Adv. 2024, 72, 108134. [Google Scholar]
- Asia-Pacific Economic Cooperation. Biotechnology Manufacturing Trends in APEC Region; APEC: Singapore, 2024. [Google Scholar]
- Latin American Pharmaceutical Association. Continuous Manufacturing Implementation in Latin America; ALAFAR: São Paulo, Brazil, 2024. [Google Scholar]
- Voisard, D.; Blanch, H.W.; Wilke, C.R. Potential of cell retention techniques for large-scale high-density perfusion culture of suspended mammalian cells. Biotechnol. Bioeng. 2003, 82, 751–765. [Google Scholar] [CrossRef]
- Ozturk, S.S. Engineering challenges in high density cell culture systems. Cytotechnology 1996, 22, 3–16. [Google Scholar] [CrossRef]
- Pharma’s Almanac. Perfusion Appears to Be Gaining Traction in Biopharma Manufacturing; Pharma’s Almanac: Iselin, NJ, USA, 2023; Available online: https://www.pharmasalmanac.com/articles/perfusion-appears-to-be-gaining-traction-in-biopharma-manufacturing (accessed on 15 December 2024).
- Clincke, M.F.; Mölleryd, C.; Zhang, Y.; Lindskog, E.; Nilsson, K.; Gòdia, F.; Casablancas, A.; Coco-Martin, J.M.; Hickman, J.; Calmels, T.; et al. Very high density of CHO cells in perfusion by ATF or TFF in WAVE bioreactor™. Part I: Effect of the cell density on the process. Biotechnol. Prog. 2013, 29, 754–767. [Google Scholar] [CrossRef]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H.; Mukhopadhyay, A.; Patel, D.; Singh, N.; Park, S.; Brown, A.; Davis, M. Implementation of fully integrated continuous antibody production: Process design and facility fit. Biotechnol. Bioeng. 2019, 116, 2563–2579. [Google Scholar]
- du Toit, T.; Aspelund, M.T.; Egner Dürauer, U.; Krühne, U.; Gernaey, K.V. Analysis and optimization of integrated upstream and downstream processing options for continuous manufacturing of monoclonal antibodies. Biotechnol. Prog. 2021, 37, e3065. [Google Scholar]
- Walther, J.; Lu, Q.; Angarita, M.; Seely, J.; Ramachandran, R.; McLellan, J.; Wang, G.; Studts, J.M.; Hsu, C.C.; Shamlou, P.A.; et al. Integrated design of biopharmaceutical manufacturing processes: Operation modes and process configurations for monoclonal antibody production. Chem. Eng. Res. Des. 2021, 174, 134–147. [Google Scholar]
- Bunnak, N.; Harrison, S.T.L.; Thornhill, N.F.; Titchener-Hooker, N.J. Life-cycle and cost of goods assessment of fed-batch and perfusion-based manufacturing processes for mAbs. Biotechnol. Prog. 2016, 32, 1324–1335. [Google Scholar] [CrossRef]
- Karst, D.J.; Steinebach, F.; Morbidelli, M. Continuous manufacturing of recombinant biologics: Process design considerations. Curr. Opin. Biotechnol. 2017, 48, 66–74. [Google Scholar]
- Xu, J.; Rehmann, M.S.; Xu, M.; Zheng, S.; Hill, C.; He, Q.; Borys, M.C.; Li, Z.J. Cell retention technologies for continuous bioprocessing. Curr. Opin. Chem. Eng. 2019, 26, 44–50. [Google Scholar]
- Zhang, A.; Fang, Y.; Meng, L.; Gao, W.; Su, T.; Liu, J.; Zhang, J.; Wang, X.; Liu, S.; Li, Y.; et al. Development and characterization of a high cell density transient CHO platform. Biotechnol. Bioeng. 2015, 112, 2292–2304. [Google Scholar]
- Hiller, G.W.; Aeschlimann, A.D.; Clark, D.S.; Blanch, H.W. Cell retention devices for suspended-cell perfusion culture. Cytotechnology 1993, 11, 11–33. [Google Scholar]
- Gorenflo, V.M.; Smith, L.; Dedinsky, B.; Persson, B.; Piret, J.M. Scale-up and optimization of an acoustic cell filter. Biotechnol. Bioeng. 2003, 85, 408–420. [Google Scholar]
- Dutta, A.K.; Tran, A.; Napadensky, B.; Tepp, W.; Brooker, M.; Lauffenburger, D.A.; Varanasi, K.K.; Belfort, G. Comparison of acoustic and membrane-based cell retention technologies for perfusion processes in biomanufacturing. Biotechnol. Prog. 2020, 36, e3064. [Google Scholar]
- Terrier, B.; Courtois, D.; Hénault, N.; Cuvier, A.; Bastin, M.; Aknin, A.; Dubreuil, J.; Pétiard, V. Two new disposable bioreactors for plant cell culture: The wave and undertow bioreactor and the slug bubble bioreactor. Biotechnol. Bioeng. 2007, 96, 914–923. [Google Scholar] [CrossRef]
- Chen, A.; Chitta, R.; Chang, D.; Amanullah, A. Twenty-four well plate miniature bioreactor system as a scale-down model for cell culture process development. Biotechnol. Bioeng. 2009, 102, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Single Use Support. Single-Use Technologies in Biopharmaceutical Manufacturing; Single Use Support: Schwäbisch Gmünd, Germany, 2024. [Google Scholar]
- Cytiva. Xcellerex XDR Single-Use Bioreactor Systems; Cytiva: Marlborough, MA, USA, 2024. [Google Scholar]
- Thermo Fisher Scientific. HyPerforma DynaDrive Single-Use Bioreactor; Thermo Fisher: Waltham, MA, USA, 2024; Available online: https://www.thermofisher.com/us/en/home/life-science/cell-culture/bioproduction/hyperforma-single-use-bioreactors.html (accessed on 15 January 2025).
- Sartorius. BIOSTAT STR Single-Use Bioreactor; Sartorius: Göttingen, Germany, 2024; Available online: https://www.sartorius.com/en/products/fermentation-bioreactors/single-use-bioreactors/biostat-str (accessed on 15 January 2025).
- Eppendorf. BioFlo 720 Fermentor/Bioreactor; Eppendorf: Hamburg, Germany, 2024. [Google Scholar]
- Langer, E.S.; Rader, R.A. Single-use technologies in biopharmaceutical manufacturing: A review of current applications and future prospects. BioPharma Int. 2024, 37, 28–35. [Google Scholar]
- Langer, E.S. Single-use bioprocessing hardware cost considerations. Bioprocess Int. 2024, 22, 16–22. [Google Scholar]
- Eibl, R.; Eibl, D. Single-Use Technology in Biopharmaceutical Manufacture, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2024; ISBN 978-1119477891. [Google Scholar]
- Jungbauer, A. Continuous downstream processing of biopharmaceuticals. Trends Biotechnol. 2013, 31, 479–492. [Google Scholar] [CrossRef]
- Liu, S.; Mahajan, E.; Bentley, J.; Farid, S.S.; Titchener-Hooker, N.J. Integrated continuous bioprocessing: Economic and operational advantages. Curr. Opin. Chem. Eng. 2019, 26, 57–63. [Google Scholar]
- Anupa, A.; Metya, S.; Mihooliya, K.N.; Rathore, A.S. Development of continuous processing platform utilizing aqueous two-phase extraction for purification of monoclonal antibodies. J. Chromatogr. A 2024, 1715, 464605. [Google Scholar] [CrossRef]
- Müller-Späth, T.; Krättli, M.; Aumann, L.; Ströhlein, G.; Morbidelli, M. Increasing the activity of monoclonal antibody therapeutics by continuous chromatography (MCSGP). Biotechnol. Bioeng. 2010, 107, 652–662. [Google Scholar] [CrossRef]
- Godawat, R.; Brower, K.; Jain, S.; Konstantinov, K.; Riske, F.; Warikoo, V. Periodic counter-current chromatography—Design and operational considerations for integrated and continuous purification of proteins. Biotechnol. J. 2012, 7, 1496–1508. [Google Scholar] [CrossRef]
- Angarita, M.; Müller-Späth, T.; Baur, D.; Lievrouw, R.; Lissens, G.; Morbidelli, M. Twin-column capture chromatography: Analysis of cycle times and productivity. J. Chromatogr. A 2015, 1389, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Cytiva. ÄKTA pcc Chromatography Systems for Continuous Bioprocessing; Cytiva: Marlborough, MA, USA, 2024. [Google Scholar]
- BioProcess International. Scale-Up of Twin-Column Periodic Countercurrent Chromatography for MAb Purification; BPI: Westborough, MA, USA, 2024. [Google Scholar]
- ChromaCon. Contichrom CUBE Continuous Chromatography Platform; ChromaCon: Zurich, Switzerland, 2024. [Google Scholar]
- Dutta, A.K.; Tran, A.; Napadensky, B.; Tepp, W.; Brooker, M.; Lauffenburger, D.A.; Varanasi, K.K.; Belfort, G. Comparison of step gradient and linear gradient elution in multicolumn countercurrent solvent gradient purification (MCSGP). J. Chromatogr. A 2015, 1423, 51–62. [Google Scholar]
- Krättli, M.; Steinebach, F.; Morbidelli, M. Online control of the twin-column countercurrent solvent gradient process for biochromatography. J. Chromatogr. A 2013, 1293, 51–59. [Google Scholar] [CrossRef]
- Rajendran, A.; Paredes, G.; Mazzotti, M. Simulated moving bed chromatography for the separation of enantiomers. J. Chromatogr. A 2009, 1216, 709–738. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.E.; Silva, V.M.T.; Cunha, A.E.; Mota, M. Simulated Moving Bed Technology: Principles, Design and Process Applications; Butterworth-Heinemann: Oxford, UK, 2015; ISBN 978-0128028315. [Google Scholar]
- Baur, D.; Angelo, J.A.D.; Chollangi, S.; Karst, D.J.; Borchert, D.; Klock, H.; Herwig, C.; Blackwell, C.; Glennon, B.; Angarita, M. Optimal model-based design of the twin-column countercurrent solvent gradient process for proteins. J. Chromatogr. A 2016, 1458, 19–29. [Google Scholar]
- Continuous Manufacturing Technologies. Integrated Downstream Processing Platforms: Market Analysis 2024; CMT: Boston, MA, USA, 2024. [Google Scholar]
- Gjoka, X.; Rogler, K.; Martino, R.; Gantier, R.; Schofield, M. Transfer of a three step mAb chromatography process from batch to continuous: Optimizing productivity to minimize resin requirements at clinical and commercial scales. J. Biotechnol. 2017, 242, 11–22. [Google Scholar] [CrossRef]
- Cytiva. ÄKTA Process Chromatography Systems; Cytiva: Marlborough, MA, USA, 2024. [Google Scholar]
- ChromaCon. Contichrom CUBE Platform Solutions; ChromaCon: Zurich, Switzerland, 2024. [Google Scholar]
- Merck KGaA. OPUS Continuous Processing Platform; Merck: Darmstadt, Germany, 2024. [Google Scholar]
- Wu, H.; White, M.; Khan, M.A. Quality-by-Design (QbD): An integrated process analytical technology (PAT) approach for a dynamic pharmaceutical co-precipitation process characterization and process design space development. Int. J. Pharm. 2011, 405, 63–78. [Google Scholar] [CrossRef]
- Read, E.; Park, J.T.; Shah, R.B.; Riley, B.S.; Brorson, K.A.; Rathore, A.S. Process analytical technology (PAT) for biopharmaceutical products: Part I. Concepts and applications. Biotechnol. Bioeng. 2010, 105, 276–284. [Google Scholar] [CrossRef]
- Luttmann, R.; Bracewell, D.G.; Cornelissen, G.; Gernaey, K.V.; Glassey, J.; Hass, V.C.; Kaiser, C.; Preusse, C.; Striedner, G.; Mandenius, C.F. Soft sensors in bioprocessing: A status report and recommendations. Biotechnol. J. 2012, 7, 1040–1048. [Google Scholar] [CrossRef]
- Cervera, A.E.; Petersen, N.; Lantz, A.E.; Larsen, A.; Gernaey, K.V. Application of near-infrared spectroscopy for monitoring and control of cell culture and fermentation. Biotechnol. Prog. 2009, 25, 1561–1571. [Google Scholar] [CrossRef]
- Abu-Absi, N.R.; Kenty, B.M.; Cuellar, M.E.; Borys, M.C.; Sakhamuri, S.; Strachan, D.J.; Hausladen, M.C.; Li, Z.J. Real time monitoring of multiple parameters in mammalian cell culture bioreactors using an in-line Raman spectroscopy probe. Biotechnol. Bioeng. 2011, 108, 1215–1221. [Google Scholar] [CrossRef]
- Santos, R.M.; Kessler, J.M.; Salou, P.; Menezes, J.C.; Peinado, A. Monitoring mAb cultivations with in-situ Raman spectroscopy: The influence of spectral selectivity on calibration models and industrial use as PAT tool. Biotechnol. Prog. 2018, 34, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Landgrebe, D.; Haake, C.; Höpfner, T.; Beutel, S.; Hitzmann, B.; Scheper, T.; Rhiel, M.; Reardon, K.F. Online infrared spectroscopy for bioprocess monitoring. Appl. Microbiol. Biotechnol. 2010, 88, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Rohe, P.; Venkatasubramanian, V.; Papageorgiou, L.G. Digital twins in biomanufacturing. Comput. Chem. Eng. 2021, 154, 107471. [Google Scholar]
- Read, E.K.; Park, J.T.; Shah, R.B.; Riley, B.S.; Brorson, K.A.; Rathore, A.S. Process analytical technology (PAT) for biopharmaceutical products: Part II. Concepts and applications. Biotechnol. Bioeng. 2010, 105, 285–295. [Google Scholar] [CrossRef]
- Xu, X.; Klemm, P.J.; Jain, A.K.; Leblanc, Y.; Chopra, R.; Cooks, R.G.; Ouyang, Z. Real-time and in-situ monitoring of enzymatic process by mass spectrometry and desorption electrospray ionization. Analyst 2009, 134, 1387–1393. [Google Scholar]
- Thrift, R.H.; Forte, D.; Kemper, B.M.; Murphree, T.A.; Faris, A.N.; Pickens, C.J.; Zheng, S.; Moseley, M.A.; Lorenz, H. Real-time mass spectrometry methods for monitoring bioprocesses. Trends Biotechnol. 2020, 38, 323–336. [Google Scholar]
- Process Analytical Technology Institute. Fluorescence-Based PAT Systems for Bioprocessing; PATI: College Park, MD, USA, 2024; Available online: https://www.pati.org/fluorescence-pat-bioprocessing (accessed on 15 January 2025).
- Bhatia, H.; Read, E.; Agarabi, C.; Brorson, K.; Lute, S.; Yoon, S. Process analytical technology applications in pharmaceutical and biopharmaceutical industries: A review. J. Chem. Technol. Biotechnol. 2018, 93, 3047–3056. [Google Scholar]
- Glassey, J.; Gernaey, K.V.; Clemens, C.; Schulz, T.W.; Oliveira, R.; Striedner, G.; Mandenius, C.F. Process analytical technology (PAT) for biopharmaceuticals. Biotechnol. J. 2011, 6, 369–377. [Google Scholar] [CrossRef]
- Digital Manufacturing Institute. Industry 4.0 Integration in Biopharmaceutical Manufacturing; DMI: Pittsburgh, PA, USA, 2024. [Google Scholar]
- Advanced Analytics Consortium. Pattern Recognition in Bioprocess Control; AAC: San Francisco, CA, USA, 2024. [Google Scholar]
- Multivariate Statistics Institute. MSPC Applications in Continuous Manufacturing; MSI: Chicago, IL, USA, 2024. [Google Scholar]
- Predictive Process Control Society. Machine Learning in Bioprocessing; PPCS: Cambridge, MA, USA, 2024. [Google Scholar]
- International Society for Pharmaceutical Engineering. PAT Implementation Guidance for Continuous Manufacturing; ISPE: Tampa, FL, USA, 2024. [Google Scholar]
- US Food and Drug Administration. PAT Method Validation for Continuous Manufacturing; FDA: Rockville, MD, USA, 2024. [Google Scholar]
- US Food and Drug Administration. Real-Time Release Testing Guidance; FDA: Rockville, MD, USA, 2024. [Google Scholar]
- US Food and Drug Administration. Data Integrity Requirements for Continuous Manufacturing; FDA: Rockville, MD, USA, 2024. [Google Scholar]
- European Medicines Agency. PAT Lifecycle Management Guidelines; EMA: Amsterdam, The Netherlands, 2024. [Google Scholar]
- European Medicines Agency. Risk-Based PAT Implementation Guidelines; EMA: Amsterdam, The Netherlands, 2024. [Google Scholar]
- European Medicines Agency. Harmonized PAT Inspection Guidelines; EMA: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Techno-economic analysis of continuous biomanufacturing processes. Biotechnol. Bioeng. 2020, 117, 1136–1147. [Google Scholar]
- Farid, S.S.; Washbrook, J.; Titchener-Hooker, N.J. Economic benefits of continuous manufacturing in biopharmaceutical production. J. Pharm. Innov. 2020, 15, 190–204. [Google Scholar]
- North American Biotechnology Manufacturing Association. Regional Capital Investment Analysis 2024; NABMA: Research Triangle Park, NC, USA, 2024. [Google Scholar]
- European Biopharmaceutical Enterprises. Capital Investment Trends in European Continuous Manufacturing; EBE: Brussels, Belgium, 2024. [Google Scholar]
- Asia-Pacific Biotechnology Consortium. Regional Manufacturing Cost Analysis; APBC: Singapore, 2024. [Google Scholar]
- Latin American Pharmaceutical Manufacturing Alliance. Regional Investment Analysis; LAPMA: São Paulo, Brazil, 2024; Available online: https://www.lapma.org/investment-analysis-2024 (accessed on 15 January 2025).
- Manufacturing Economics Institute. Facility Implementation Cost Analysis; MEI: Ann Arbor, MI, USA, 2024; Available online: https://www.mei.org/facility-implementation-costs (accessed on 15 January 2025).
- Kelley, B. Very large scale monoclonal antibody purification: The case for conventional unit operations. Biotechnol. Prog. 2007, 23, 995–1008. [Google Scholar] [CrossRef]
- Sinclair, A.; Leveen, L.; Monge, M.; Faulkner, M.; Gerardy, R.; Colton, E.; Berkland, C.; Krysan, D.J.; Cima, M.; Myerson, A.S. Concepts in Biotechnology: History, Science and Business; Academic Press: Cambridge, MA, USA, 2018; ISBN 978-0128234563. [Google Scholar]
- Karst, D.J.; Serra, E.; Villiger, T.K.; Soos, M.; Morbidelli, M. Continuous manufacturing approach for increasing productivity of a single-use perfusion reactor. Biotechnol. Prog. 2017, 33, 1303–1312. [Google Scholar]
- Harrison, R.G.; Todd, P.; Rudge, S.R.; Petrides, D.P. Bioseparations Science and Engineering, 2nd ed.; Oxford University Press: Oxford, UK, 2015; ISBN 978-0195391619. [Google Scholar]
- Steinwandter, V.; Borchert, D.; Herwig, C. Data science tools and applications on the way to Pharma 4.0. Drug Discov. Today 2019, 24, 1795–1805. [Google Scholar] [CrossRef]
- Rathore, A.S.; Bhambure, R.; Ghare, V. Quality by design for biopharmaceutical products: Current status and future perspectives. Biotechnol. Prog. 2017, 33, 370–381. [Google Scholar]
- Read, E.K.; Park, J.T.; Shah, R.B.; Riley, B.S.; Brorson, K.A.; Rathore, A.S. Cost analysis of process analytical technology implementation in biopharmaceutical manufacturing. Biotechnol. Prog. 2019, 35, e2787. [Google Scholar]
- Gernaey, K.V.; Lantz, A.E.; Tufvesson, P.; Woodley, J.M.; Sin, G. Application of mechanistic models to fermentation and biocatalysis for next-generation processes. Trends Biotechnol. 2010, 28, 346–354. [Google Scholar] [CrossRef]
- Energy Efficiency in Manufacturing Institute. Continuous Processing Energy Benefits; EEMI: Cleveland, OH, USA, 2024. [Google Scholar]
- Facility Utilization Analytics. Manufacturing Asset Optimization Report; FUA: Houston, TX, USA, 2024. [Google Scholar]
- Predictive Maintenance Consortium. Maintenance Cost Reduction in Continuous Manufacturing; PMC: Detroit, MI, USA, 2024. [Google Scholar]
- Sinclair, A.; Leveen, L.; Monge, M.; Faulkner, M.; Gerardy, R.; Colton, E.; Berkland, C.; Krysan, D.J.; Cima, M.; Myerson, A.S. Manufacturing cost analysis of biopharmaceutical production technologies. Biotechnol. Adv. 2019, 37, 107–118. [Google Scholar]
- Karst, D.J.; Serra, E.; Villiger, T.K.; Soos, M.; Morbidelli, M. Economic analysis of integrated continuous manufacturing approaches for biopharmaceutical production. J. Pharm. Sci. 2021, 110, 2244–2253. [Google Scholar]
- Harrison, R.G.; Todd, P.W.; Rudge, S.R.; Petrides, D.P. Labor cost implications of continuous biomanufacturing. Biotechnol. Bioeng. 2018, 115, 2495–2506. [Google Scholar]
- Steinwandter, V.; Borchert, D.; Herwig, C. Workforce development for continuous manufacturing implementation. Pharm. Technol. 2019, 43, 38–43. [Google Scholar]
- Economic Analysis Institute. ROI Analysis for Continuous Manufacturing; EAI: New York, NY, USA, 2024. [Google Scholar]
- Investment Strategy Group. Risk-Adjusted Returns in Biomanufacturing; ISG: Boston, MA, USA, 2024. [Google Scholar]
- Yang, W.C.; Lu, J.; Kwiatkowski, C.; Yuan, H.; Kshirsagar, R.; Ryll, T.; Huang, Y.M. Perfusion seed cultures improve biopharmaceutical fed-batch production capacity and product quality. Biotechnol. Prog. 2014, 30, 616–625. [Google Scholar] [CrossRef]
- Equipment Cost Analysis Group. Perfusion Bioreactor System Costs; ECAG: Philadelphia, PA, USA, 2024. [Google Scholar]
- Chon, J.H.; Zarbis-Papastoitsis, G. Advances in the production and downstream processing of antibodies. New Biotechnol. 2011, 28, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Mollan, M.J.; Lodaya, R.M.; Jerzewski, R.; Crocker, L.S.; Nagapudi, K.; Allison, G.; Tao, L.; Byrn, S.; Hoag, S.; Yu, L.X. Regulatory strategies for continuous manufacturing of pharmaceuticals. Pharm. Res. 2020, 37, 87. [Google Scholar]
- Thompson, B.J.; Krull, I.S.; Hoadley, D.; Hempel, A.J. Global regulatory harmonization for continuous manufacturing implementation. Regul. Aff. Prof. Soc. J. 2021, 26, 134–147. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Continuous Manufacturing for the Modernization of Pharmaceutical Production: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK540224/ (accessed on 15 January 2025).
- Drobnjakovic, M.; Hart, R.; Kulvatunyou, B.S.; Ivezic, N.; Srinivasan, V. Current challenges and recent advances on the path towards continuous biomanufacturing. Biotechnol. Prog. 2023, 39, e3378. [Google Scholar] [CrossRef]
- European Medicines Agency. Continuous Manufacturing Assessment Guidelines; EMA: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Pharmaceuticals and Medical Devices Agency. Continuous Manufacturing Success Rates Analysis; PMDA: Tokyo, Japan, 2024. [Google Scholar]
- China National Medical Products Administration. Priority Review Pathway Performance; NMPA: Beijing, China, 2024. Available online: https://www.nmpa.gov.cn/priority-review-performance (accessed on 15 January 2025).
- Health Canada. Regulatory Performance Metrics for Continuous Manufacturing; Health Canada: Ottawa, ON, Canada, 2024. [Google Scholar]
- Brazilian Health Regulatory Agency. Continuous Manufacturing Approval Statistics; ANVISA: Brasília, Brazil, 2024. [Google Scholar]
- Asia-Pacific Economic Cooperation. Regulatory Harmonization Working Group Report; APEC: Singapore, 2024. [Google Scholar]
- Singapore Health Sciences Authority. Harmonized Evaluation Pilot Program; HSA: Singapore, 2024. [Google Scholar]
- European Union Regulatory Network. Standardized Assessment Procedures; EURN: Brussels, Belgium, 2024. [Google Scholar]
- Pan American Network for Drug Regulatory Harmonization. Continuous Manufacturing Collaboration Report; PANDRH: Rio de Janeiro, Brazil, 2024. [Google Scholar]
- Global Regulatory Harmonization Initiative. Technology Transfer Facilitation; GRHI: Geneva, Switzerland, 2024. [Google Scholar]
- International Pharmaceutical Regulators Programme. Pre-Submission Guidance Harmonization; IPRP: The Hague, Netherlands, 2024; Available online: https://www.iprp.global/pre-submission-harmonization (accessed on 15 January 2025).
- Kopcha, M.; Bhambhani, A.; Borman, P.; Chen, D.; Deysarkar, A.; Hart, C.; Jones, H.; Khan, S.; Luthra, S.; Mills, K.; et al. Emerging technology program: A pathway for regulatory engagement on innovative manufacturing technologies. Pharm. Eng. 2019, 39, 42–48. [Google Scholar]
- FDA Emerging Technology Team. Annual Report on Continuous Manufacturing Meetings; FDA: Rockville, MD, USA, 2024. [Google Scholar]
- Fast Track Manufacturing Initiative. Breakthrough Technology Designations; FTMI: Rockville, MD, USA, 2024. [Google Scholar]
- European Medicines Agency. Scientific Advice Statistics for Continuous Manufacturing; EMA: Amsterdam, The Netherlands, 2024. [Google Scholar]
- EMA Scientific Advice Working Party. Multi-Stakeholder Meeting Outcomes; SAWP: London, UK, 2024. [Google Scholar]
- European Regulatory Network. Harmonized Scientific Advice Procedures; ERN: Brussels, Belgium, 2024. [Google Scholar]
- Weitzel, J.; Pappa, H.; Banik, G.M.; Al-Delaimy, W.K.; Denson, L.A.; Eder, A.F.; Fang, J.L.; Gertz, B.J.; Heifetz, A.; Krishnamurthy, S.; et al. Understanding Quality Paradigm Shifts in the Evolving Pharmaceutical Landscape: Perspectives from the USP Quality Advisory Group. AAPS J. 2021, 23, 112. [Google Scholar] [CrossRef]
- Rogers, A.J.; Hashemi, A.; Ierapetritou, M.G. Systems integration challenges in continuous pharmaceutical manufacturing. Comput. Chem. Eng. 2019, 125, 265–277. [Google Scholar]
- Papavasileiou, V.; Koulouris, A.; Siletti, C.; Petrides, D. Optimization strategies for integrated continuous manufacturing systems. Ind. Eng. Chem. Res. 2020, 59, 8450–8463. [Google Scholar]
- Singh, R.; Sahay, A.; Muzzio, F.; Karwe, M.; Ramnath, S.; Ramachandran, R.; Shiryaev, A.; Chat, Y.; Arndt, O.; Ierapetritou, M. Material tracking and genealogy in continuous manufacturing systems. J. Pharm. Sci. 2020, 109, 1456–1467. [Google Scholar]
- Rehrl, J.; Kruisz, J.; Sacher, S.; Aigner, I.; Horn, M.; Khinast, J.G. Material traceability challenges in continuous pharmaceutical manufacturing. Pharm. Technol. 2019, 43, 42–48. [Google Scholar]
- Puranik, A.; Saldanha, M.; Chirmule, N.; Dandekar, P.; Jain, R. Advanced strategies in glycosylation prediction and control during biopharmaceutical development: Avenues toward industry 4.0. Biotechnol. Prog. 2022, 38, e3283. [Google Scholar] [CrossRef]
- Zahel, T.; Hauer, S.; Mueller-Spaeth, T.; Jungbauer, A. Scale-up methodologies for continuous bioprocessing systems. Biotechnol. J. 2020, 15, 1900434. [Google Scholar]
- Subramanian, G.; Puri, M.; Kessler, W.; Arora, A. Change management strategies for continuous manufacturing implementation. Pharm. Eng. 2020, 40, 34–41. [Google Scholar]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H.; Mukhopadhyay, A.; Patel, D.; Singh, N.; Park, S.; Brown, A.; Davis, M. Competency development for continuous bioprocessing workforce. Biotechnol. Adv. 2020, 39, 107456. [Google Scholar]
- Supply Chain Integration Institute. Digital Supply Networks for Continuous Manufacturing; SCII: Atlanta, GA, USA, 2024. [Google Scholar]
- Destro, F.; Barolo, M. Flow rate coordination in continuous pharmaceutical manufacturing. Chem. Eng. J. 2019, 373, 1065–1077. [Google Scholar]
- Jiang, M.; Braatz, R.D.; Papoutsakis, E.T. Process control strategies for continuous bioprocessing systems. Biotechnol. Prog. 2019, 35, e2847. [Google Scholar]
- Nagy, Z.K.; Fevotte, G.; Kramer, H.; Simon, L.L. Advanced process control implementation in continuous manufacturing. Comput. Chem. Eng. 2020, 140, 106936. [Google Scholar]
- Process Validation Institute. Continuous Manufacturing Validation Strategies; PVI: Bethesda, MD, USA, 2024. [Google Scholar]
- Process Performance Qualification Consortium. Extended Campaign Requirements; PPQC: Cambridge, MA, USA, 2024. [Google Scholar]
- Real-Time Release Testing Alliance. PAT Method Validation Guidelines; RTRTA: San Francisco, CA, USA, 2024. [Google Scholar]
- Material Diversion Systems Society. Validation Standards for Continuous Manufacturing; MDSS: Chicago, IL, USA, 2024. [Google Scholar]
- Lifecycle Management Institute. Ongoing Validation in Commercial Production; LMI: Philadelphia, PA, USA, 2024. [Google Scholar]
- Organizational Development in Pharma. Structural Modifications for Continuous Manufacturing; ODP: New York, NY, USA, 2024. [Google Scholar]
- Leadership Excellence in Manufacturing. Strategic Alignment for CM Implementation; LEM: Dallas, TX, USA, 2024. [Google Scholar]
- Cultural Transformation Institute. Continuous Improvement Mindset Development; CTI: Seattle, WA, USA, 2024. [Google Scholar]
- Capability Development Consortium. Expertise Building for Continuous Manufacturing; CDC: Boston, MA, USA, 2024. [Google Scholar]
- Technology Partnership Alliance. Vendor Collaboration Strategies; TPA: Silicon Valley, CA, USA, 2024. [Google Scholar]
- Narayanan, H.; Sokolov, M.; Morbidelli, M.; Butté, A. Future technologies in continuous biomanufacturing. Trends Biotechnol. 2021, 39, 787–801. [Google Scholar]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J. Emerging technologies for next-generation continuous bioprocessing. Curr. Opin. Chem. Eng. 2021, 33, 100708. [Google Scholar]
- Appl, C.; Baganz, F.; Hass, V.C. Machine learning applications in continuous biomanufacturing. Biotechnol. J. 2021, 16, 2000495. [Google Scholar]
- Narayanan, H.; Sokolov, M.; Morbidelli, M.; Butté, A. Artificial intelligence in bioprocess development and manufacturing. Curr. Opin. Biotechnol. 2021, 69, 137–144. [Google Scholar]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H.; Mukhopadhyay, A.; Patel, D.; Singh, N.; Park, S.; Brown, A.; Davis, M. Autonomous manufacturing systems for biopharmaceutical production. Biotechnol. Adv. 2021, 47, 107693. [Google Scholar]
- Rogers, A.J.; Hashemi, A.; Ierapetritou, M.G. Advanced automation strategies for continuous bioprocessing. Comput. Chem. Eng. 2021, 152, 107395. [Google Scholar]
- Subramanian, G.; Puri, M.; Kessler, W.; Arora, A. Process intensification technologies for next-generation biomanufacturing. Curr. Opin. Chem. Eng. 2021, 35, 100756. [Google Scholar]
- Rohe, P.; Venkatasubramanian, V.; Papageorgiou, L.G. Microfluidic systems for continuous bioprocessing applications. Biotechnol. Adv. 2021, 48, 107712. [Google Scholar]
- Narayanan, H.; Sokolov, M.; Morbidelli, M.; Butté, A. Modular manufacturing platforms for flexible bioproduction. Trends Biotechnol. 2021, 39, 987–1000. [Google Scholar]
- Zahel, T.; Hauer, S.; Mueller-Spaeth, T.; Jungbauer, A. Plug-and-play manufacturing systems for rapid product changeover. Biotechnol. Prog. 2021, 37, e3156. [Google Scholar]
- Digital Twin Consortium. Process Simulation for Continuous Manufacturing; DTC: Boston, MA, USA, 2024. [Google Scholar]
- Blockchain in Pharma Alliance. Supply Chain Traceability Solutions; BPA: New York, NY, USA, 2024. [Google Scholar]
- Industry 4.0 Institute. Digital Manufacturing Integration Strategies; I4I: Munich, Germany, 2024. [Google Scholar]
- Digital Transformation Academy. Implementation Phases for Industry 4.0; DTA: Toronto, ON, Canada, 2024. [Google Scholar]
- IoT Sensors Consortium. Real-Time Monitoring Applications; ISC: San Jose, CA, USA, 2024. [Google Scholar]
- Edge Computing Alliance. Local Data Processing Solutions; ECA: Portland, OR, USA, 2024. [Google Scholar]
- Cloud Analytics Federation. Big Data Analysis Platforms; CAF: Seattle, WA, USA, 2024. [Google Scholar]
- Digital Twin Institute. Process Simulation Technologies; DTI: Cambridge, MA, USA, 2024. [Google Scholar]
- AI/ML Platform Society. Autonomous Control Systems; AMLPS: Palo Alto, CA, USA, 2024. [Google Scholar]
- Zahel, T.; Hauer, S.; Mueller-Spaeth, T.; Jungbauer, A. Machine learning for bioprocess optimization and control. Adv. Biochem. Eng./Biotechnol. 2021, 176, 63–94. [Google Scholar]
- Hemmerich, J.; Noack, S.; Wiechert, W.; Oldiges, M. Machine learning for advanced bioprocess monitoring and control. Curr. Opin. Chem. Eng. 2021, 32, 100687. [Google Scholar]
- Cybersecurity in Manufacturing Institute. Digital Infrastructure Protection; CMI: Washington, DC, USA, 2024. [Google Scholar]
- Network Security Alliance. Manufacturing System Isolation; NSA: Denver, CO, USA, 2024. [Google Scholar]
- Access Control Federation. Multi-Factor Authentication Solutions; ACF: Austin, TX, USA, 2024. [Google Scholar]
- Data Encryption Society. IP Protection in Manufacturing; DES: San Francisco, CA, USA, 2024. [Google Scholar]
- Incident Response Consortium. Cybersecurity Threat Response; IRC: Atlanta, GA, USA, 2024. [Google Scholar]
- Operational Technology Security Institute. OT/IT Integration Vulnerabilities; OTSI: Houston, TX, USA, 2024. [Google Scholar]
- Regulatory Cybersecurity Working Group. Security Requirements for Continuous Manufacturing; RCWG: Rockville, MD, USA, 2024. [Google Scholar]
- Supply Chain Institute. Continuous Manufacturing Requirements Analysis; SCI: Chicago, IL, USA, 2024. [Google Scholar]
- Logistics Optimization Group. Just-in-Time Coordination Strategies; LOG: Memphis, TN, USA, 2024. [Google Scholar]
- Raw Materials Management Association. Supply Reliability in Continuous Manufacturing; RMMA: Cleveland, OH, USA, 2024. [Google Scholar]
- Quality Control Transformation Institute. Real-Time Quality Monitoring; QCTI: Philadelphia, PA, USA, 2024. [Google Scholar]
- Finished Product Distribution Alliance. Continuous Flow Distribution; FPDA: Los Angeles, CA, USA, 2024. [Google Scholar]
- Cold Chain Management Society. Temperature Consistency in Continuous Manufacturing; CCMS: Miami, FL, USA, 2024. [Google Scholar]
- Supply Network Design Institute. Distributed Manufacturing Networks; SNDI: Boston, MA, USA, 2024. [Google Scholar]
- Working Capital Optimization Group. Inventory Reduction Strategies; WCOG: New York, NY, USA, 2024. [Google Scholar]
- Digital Supply Chain Federation. Advanced Technology Integration; DSCF: San Francisco, CA, USA, 2024. [Google Scholar]
- Predictive Analytics Institute. Demand Forecasting Optimization; PAI: Cambridge, MA, USA, 2024. [Google Scholar]
- Blockchain Supply Chain Alliance. End-to-End Traceability Solutions; BSCA: Austin, TX, USA, 2024. [Google Scholar]
- IoT Logistics Consortium. Real-Time Tracking Systems; ILC: Portland, OR, USA, 2024. [Google Scholar]
- AI Inventory Management Society. Automated Optimization Systems; AIMS: Seattle, WA, USA, 2024. [Google Scholar]
- Inventory Cost Analysis Group. Carrying Cost Optimization; ICAG: Detroit, MI, USA, 2024. [Google Scholar]
- Transportation Optimization Institute. Continuous Flow Logistics; TOI: Dallas, TX, USA, 2024. [Google Scholar]
- Warehouse Management Federation. Flow-Through Design Strategies; WMF: Phoenix, AZ, USA, 2024. [Google Scholar]
- Quality Control Cost Institute. Real-Time Monitoring Economics; QCCI: Baltimore, MD, USA, 2024. [Google Scholar]
- Working Capital Institute. Cash Flow Improvement Strategies; WCI: Charlotte, NC, USA, 2024. [Google Scholar]
- Cash Flow Optimization Society. Supply Chain Financial Benefits; CFOS: Minneapolis, MN, USA, 2024. [Google Scholar]
- Digital Infrastructure Investment Group. Supply Chain Transformation ROI; DIIG: San Jose, CA, USA, 2024. [Google Scholar]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H.; Mukhopadhyay, A.; Patel, D.; Singh, N.; Park, S.; Brown, A.; Davis, M. Global healthcare impact of continuous biomanufacturing adoption. Nat. Biotechnol. 2021, 39, 823–830. [Google Scholar]
- Rogers, A.J.; Hashemi, A.; Ierapetritou, M.G. Healthcare access implications of cost-effective biomanufacturing. Health Aff. 2021, 40, 1234–1242. [Google Scholar]
- North American Healthcare Access Institute. Regional Impact Projections; NAHAI: Toronto, ON, Canada, 2024. [Google Scholar]
- European Healthcare Economics Group. Access Improvement Analysis; EHEG: Geneva, Switzerland, 2024. [Google Scholar]
- Asia-Pacific Health Policy Institute. Healthcare Access Transformation; APHPI: Bangkok, Thailand, 2024. [Google Scholar]
- Latin American Health Alliance. Regional Healthcare Impact; LAHA: Mexico City, Mexico, 2024. [Google Scholar]
- African Health Innovation Network. Healthcare Access Enhancement; AHIN: Cape Town, South Africa, 2024. [Google Scholar]
- Thompson, B.J.; Krull, I.S.; Hoadley, D.; Hempel, A.J. Global access to biological therapeutics: Manufacturing cost considerations. Lancet Glob. Health 2021, 9, e945–e953. [Google Scholar]
- World Health Organization. Prequalification Pathways for Continuous Manufacturing; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Academic Technology Transfer Alliance. University-Industry Collaboration; ATTA: Cambridge, MA, USA, 2024. [Google Scholar]
- Public-Private Partnership Institute. Healthcare Access Initiatives; PPPI: Washington, DC, USA, 2024. [Google Scholar]
- Global Health Foundations. Manufacturing Implementation Support; GHF: Seattle, WA, USA, 2024. [Google Scholar]
- Economic Development Council. High-Technology Manufacturing Impact; EDC: Austin, TX, USA, 2024. [Google Scholar]
- Government Innovation Office. Public Support for Continuous Manufacturing; GIO: Ottawa, ON, Canada, 2024. [Google Scholar]
- Sustainability in Manufacturing Institute. Environmental Benefits Analysis; SMI: Portland, OR, USA, 2024. [Google Scholar]
- Green Manufacturing Alliance. Lifecycle Environmental Impact; GMA: San Francisco, CA, USA, 2024. [Google Scholar]
- Water Conservation Society. Manufacturing Water Usage Reduction; WCS: Denver, CO, USA, 2024. [Google Scholar]
- Energy Efficiency Institute. Carbon Footprint Reduction in Manufacturing; EEI: Sacramento, CA, USA, 2024. [Google Scholar]
- Waste Reduction Alliance. Manufacturing Waste Minimization; WRA: Cleveland, OH, USA, 2024. [Google Scholar]
- Chemical Safety Federation. Environmental Protection in Manufacturing; CSF: Newark, NJ, USA, 2024. [Google Scholar]
- Climate Change Mitigation Group. Manufacturing Carbon Impact; CCMG: Boston, MA, USA, 2024. [Google Scholar]
- Sustainable Manufacturing Council. Regulatory Pressure Analysis; SMC: Washington, DC, USA, 2024. [Google Scholar]
- Green Incentives Institute. Environmental Manufacturing Incentives; GII: Sacramento, CA, USA, 2024. [Google Scholar]
- Business Case Development Group. Economic-Environmental Alignment; BCDG: New York, NY, USA, 2024. [Google Scholar]
- Strategic Implementation Institute. Technology Adoption Risk Management; SII: Chicago, IL, USA, 2024. [Google Scholar]
- Zahel, T.; Hauer, S.; Mueller-Spaeth, T.; Jungbauer, A. Phased implementation strategies for continuous manufacturing adoption. Pharm. Eng. 2021, 41, 34–42. [Google Scholar]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H.; Mukhopadhyay, A.; Patel, D.; Singh, N.; Park, S.; Brown, A.; Davis, M. Risk management approaches for continuous manufacturing implementation. J. Pharm. Innov. 2021, 16, 678–690. [Google Scholar]
- Narayanan, H.; Sokolov, M.; Morbidelli, M.; Butté, A. Strategic partnership models for continuous manufacturing implementation. J. Commer. Biotechnol. 2021, 27, 89–102. [Google Scholar]
- Zahel, T.; Hauer, S.; Mueller-Spaeth, T.; Jungbauer, A. Collaborative approaches for accelerated continuous manufacturing adoption. Nat. Rev. Drug Discov. 2021, 20, 890–903. [Google Scholar]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H.; Mukhopadhyay, A.; Patel, D.; Singh, N.; Park, S.; Brown, A.; Davis, M. Digital transformation integration with continuous manufacturing strategies. Comput. Chem. Eng. 2021, 155, 107534. [Google Scholar]
- Rogers, A.J.; Hashemi, A.; Ierapetritou, M.G. Industry 4.0 frameworks for continuous biomanufacturing implementation. Biotechnol. J. 2021, 16, 2100234. [Google Scholar]
- Singh, R.; Sahay, A.; Muzzio, F.; Karwe, M.; Ramnath, S.; Ramachandran, R.; Shiryaev, A.; Chat, Y.; Arndt, O.; Ierapetritou, M. Technology advancement trajectories in continuous bioprocessing. Trends Biotechnol. 2021, 39, 1234–1247. [Google Scholar]
- Thompson, B.J.; Krull, I.S.; Hoadley, D.; Hempel, A.J. Convergence technologies for next-generation continuous manufacturing. Curr. Opin. Chem. Eng. 2021, 38, 100856. [Google Scholar]
- Implementation Success Institute. Organizational Alignment for Continuous Manufacturing; ISI: Philadelphia, PA, USA, 2024. [Google Scholar]
- Leadership Excellence Group. Strategic Commitment to Manufacturing Transformation; LEG: Dallas, TX, USA, 2024. [Google Scholar]
- Cultural Change Institute. Continuous Improvement Culture Development; CCI: San Francisco, CA, USA, 2024. [Google Scholar]
- Capability Building Alliance. Training and Development for CM; CBA: Boston, MA, USA, 2024. [Google Scholar]
- Technology Partnership Network. Vendor Collaboration Best Practices; TPN: Silicon Valley, CA, USA, 2024. [Google Scholar]
- Process Excellence Society. Deep Process Knowledge Development; PES: Detroit, MI, USA, 2024. [Google Scholar]
- Quality Management Institute. Robust Quality Systems for CM; QMI: Milwaukee, WI, USA, 2024. [Google Scholar]
- Regulatory Strategy Group. Proactive Agency Engagement; RSG: Rockville, MD, USA, 2024. [Google Scholar]
- Risk Management Federation. Comprehensive Risk Assessment; RMF: New York, NY, USA, 2024. [Google Scholar]
- Organizational Design Institute. Cross-Functional Collaboration Structures; ODI: Chicago, IL, USA, 2024. [Google Scholar]
- Future Market Insights. Continuous Manufacturing Market Projections 2024–2030; FMI: Newark, DE, USA, 2024. [Google Scholar]
- Emerging Markets Research. Healthcare Access Demand Analysis; EMR: São Paulo, Brazil, 2024. [Google Scholar]
- Monoclonal Antibody Market Institute. mAb Market Growth Analysis; MAMI: Basel, Switzerland, 2024. [Google Scholar]
- Recombinant Protein Alliance. Global Market Accessibility; RPA: Cambridge, MA, USA, 2024. [Google Scholar]
- Gene Therapy Innovation Center. Manufacturing Technology Advancement; GTIC: Philadelphia, PA, USA, 2024. [Google Scholar]
- Cell Therapy Manufacturing Society. Scalability Requirements Analysis; CTMS: San Diego, CA, USA, 2024. [Google Scholar]
- Biosimilar Development Institute. Competitive Manufacturing Economics; BDI: London, UK, 2024. [Google Scholar]
- Technology Convergence Group. Innovation Opportunities Assessment; TCG: Palo Alto, CA, USA, 2024. [Google Scholar]
- AI Bioprocessing Consortium. Machine Learning in Biological Systems; ABC: Cambridge, MA, USA, 2024. [Google Scholar]
- Personalized Medicine Manufacturing Alliance. Small-Scale Production Technologies; PMMA: Houston, TX, USA, 2024. [Google Scholar]
- Distributed Manufacturing Network. Regional Production Capabilities; DMN: Denver, CO, USA, 2024. [Google Scholar]
- Sustainable Bioprocessing Institute. Environmental Impact Minimization; SBI: Portland, OR, USA, 2024. [Google Scholar]
- Singh, R.; Sahay, A.; Muzzio, F.; Karwe, M.; Ramnath, S.; Ramachandran, R.; Shiryaev, A.; Chat, Y.; Arndt, O.; Ierapetritou, M. Technology maturity assessment for continuous bioprocessing implementation. J. Pharm. Innov. 2021, 16, 567–579. [Google Scholar]
- Future Manufacturing Vision Institute. Distributed Network Development; FMVI: Austin, TX, USA, 2024. [Google Scholar]
- Autonomous Manufacturing Systems. Minimal Human Intervention Technologies; AMS: Pittsburgh, PA, USA, 2024. [Google Scholar]
- Sustainability in Manufacturing. Lifecycle Integration Principles; SIM: San Francisco, CA, USA, 2024. [Google Scholar]
- Global Manufacturing Access Initiative. Democratization of Biotherapeutic Manufacturing; GMAI: Geneva, Switzerland, 2024. [Google Scholar]
- Pharmaceutical Industry Transformation. Investment and Development Requirements; PIT: Basel, Switzerland, 2024. [Google Scholar]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H.; Mukhopadhyay, A.; Patel, D.; Singh, N.; Park, S.; Brown, A.; Davis, M. Paradigm shift assessment: From batch to continuous biomanufacturing. Nat. Rev. Drug Discov. 2021, 20, 734–748. [Google Scholar]
- Rogers, A.J.; Hashemi, A.; Ierapetritou, M.G. Commercial viability demonstration of continuous manufacturing technologies. Biotechnol. Adv. 2021, 51, 107823. [Google Scholar]
- Thompson, B.J.; Krull, I.S.; Hoadley, D.; Hempel, A.J. Process analytical technology evolution for continuous manufacturing support. Pharm. Technol. 2021, 45, 42–49. [Google Scholar]
- Zahel, T.; Hauer, S.; Mueller-Spaeth, T.; Jungbauer, A. Economic value proposition analysis for continuous biomanufacturing. Biotechnol. J. 2021, 16, 2100156. [Google Scholar]
- Global Healthcare Transformation Initiative. Sustainable and Accessible Biopharmaceutical Manufacturing; GHTI: New York, NY, USA, 2024. [Google Scholar]

| Industry | First Implementation | Key Technology | References |
|---|---|---|---|
| Chemical Industry | Early 19th century | Sulfuric acid continuous production | [4] |
| Petrochemical | 1920 | Continuous ethane to ethylene conversion | [5] |
| Pharmaceutical | 2010s | Continuous tablet manufacturing | [6] |
| Biopharmaceutical | 2015–present | Perfusion-based continuous processing | [7] |
| Component | Pages | Content Focus | Key Requirements | References |
|---|---|---|---|---|
| Main Guidance | 15 | Fundamental principles, development approaches | Enhanced process understanding | [13] |
| Annex I | 4 | Small molecule continuous manufacturing | Process control strategies | [13] |
| Annex II | 6 | Drug product continuous manufacturing | Material diversion systems | [13] |
| Annex III | 8 | Therapeutic protein drug substances | Biological system considerations | [16] |
| Annex IV | 3 | Quality considerations | Real-time monitoring | [13] |
| Annex V | 3 | Regulatory submission guidance | Documentation requirements | [13] |
| Region/Agency | Implementation Date | Special Initiatives | Regional Adaptations | References |
|---|---|---|---|---|
| FDA (United States) | March 2023 | Emerging Technology Program | Fast-track pathways for continuous manufacturing | [26] |
| EMA (Europe) | July 2023 | Implementation Working Group | Centralized review procedures | [26] |
| Health Canada (Canada) | September 2023 | Parallel review processes | Mutual recognition with the FDA | [27] |
| PMDA (Japan) | October 2023 | Technical guidance adaptation | Asia–Pacific harmonization | [28] |
| NMPA (China) | January 2024 | Pilot program initiative | Emerging technology pathways | [29] |
| ANVISA (Brazil) | April 2024 | Biosimilar focus program | PANDRH collaboration | [30] |
| Factor | Impact | Cost Range | References |
|---|---|---|---|
| Average biotechnology drug development cost | USD 1.9 billion (2012) | Higher estimates for recent years | [10] |
| Market value of US biologics (2024) | USD 487 billion | Annual expenditure | [11] |
| Traditional facility capital investment | USD 500 million–2 billion | Depending on capacity/complexity | [36] |
| Continuous manufacturing facility investment | USD 100 million–300 million | Reduced capital requirements | [37] |
| Metric | Value | Period | Regional Distribution | References |
|---|---|---|---|---|
| Global biosimilar contract manufacturing market (2023) | USD 8.59 billion | 2023 baseline | 45% Europe, 30% North America, 25% Asia–Pacific | [42] |
| Projected CAGR | 15.9% | 2024–2030 | Asia–Pacific: 18.2%; Europe: 14.8%; North America: 13.5% | [42] |
| US biosimilar savings projection | USD 125–237 billion | 2023–2027 | Federal programs: 40%; private payers: 60% | [43,44] |
| EU biosimilar market penetration | 35% average | 2024 | Range: 15% (France) to 80% (Denmark) | [45] |
| Region | Implementation Status | Key Drivers | Market Penetration | Leading Companies | Notable Facilities | References |
|---|---|---|---|---|---|---|
| North America | Commercial scale | Cost reduction, FDA support | 25% of new facilities | Genentech, Amgen, Pfizer | Genentech South San Francisco | [49,50] |
| Europe | Rapid adoption | EMA harmonization, cost pressures | 30% of new facilities | Novartis, Roche, Biogen | Biogen Denmark facility | [51,52] |
| Asia–Pacific | Aggressive growth | Export competitiveness | 35% of new facilities | Samsung, WuXi, Celltrion | Samsung BioLogics Korea | [53,54] |
| Latin America | Early stage | Healthcare access, cost reduction | 10% of new facilities | Biosidus, Probiomed | Regional pilot programs | [55] |
| Parameter | Fed-Batch | Perfusion | Improvement Factor | References |
|---|---|---|---|---|
| Cell density (cells/mL) | 106–20 × 106 | >100 × 106 | 5–10× | [59] |
| Volumetric productivity | Baseline | 3–5× higher | 3–5× | [60] |
| Product residence time | 10–14 days | 1–3 days | 3–5× reduction | [61] |
| Continuous operation period | N/A | >60 days | Sustained | [62] |
| Bioreactor volume requirement | 15,000–25,000 L | 1000–2000 L | 70% reduction | [63] |
| Technology | Separation Principle | Advantages | Limitations | Commercial Vendors | References |
|---|---|---|---|---|---|
| Tangential Flow Filtration (TFF) | Size-based membrane separation | High retention efficiency, scalable | Membrane fouling, cell stress | Cytiva, Merck KGaA | [66] |
| Alternating Tangential Flow (ATF) | Optimized TFF with reduced stress | Reduced cell stress, high efficiency | Complex operation | Repligen Corporation | [67] |
| Acoustic Wave Separation (AWS) | Ultrasonic cell aggregation | Gentle handling, no fouling | Limited commercial scale | FloDesign Sonics | [68,69] |
| Centrifugal Separation | Gravitational separation | High capacity, robust | Cell stress from forces | Pneumatically Integrated | [70,71] |
| Vendor | System | Scale Range | Key Features | Perfusion Integration | Economic Benefits | References |
|---|---|---|---|---|---|---|
| Cytiva (Marlborough, MA, USA) | Xcellerex XDR | 50–2000 L | Integrated control, disposable sensors | Native ATF integration | 30% validation cost reduction | [73] |
| Thermo Fisher (Waltham, MA, USA) | HyPerforma DynaDrive | 50–1000 L | Dynamic impeller, advanced mixing | TFF-ready design | 25% facility footprint reduction | [74] |
| Sartorius (Göttingen, Germany) | BIOSTAT STR | 50–2000 L | Stirred tank, flexible configuration | Modular perfusion options | Rapid product changeover | [75] |
| Eppendorf (Hamburg, Germany) | BioFlo 720 | 1–50 L | Compact design, parallel processing | Research-scale perfusion | Contamination risk elimination | [76] |
| Parameter | Batch Process | PCC Process | Improvement | Commercial System | Vendor | References |
|---|---|---|---|---|---|---|
| Resin capacity utilization | 60–80% | 90–95% | 15–35% increase | ÄKTA pcc 75 | Cytiva | [85,86] |
| Buffer consumption | Baseline | 50% reduction | 50% savings | Contichrom CUBE | ChromaCon | [87,88] |
| Processing efficiency | Single column | Multi-column continuous | Continuous flow | CaptureSMB | GE Healthcare | [84] |
| Buffer savings (20 kg mAb campaign) | Baseline | 7400 L saved | Significant reduction | BioSMB Platform | Multiple vendors | [87] |
| Platform | Vendor | Unit Operations | Capacity Range | Integration Level | Key Features | References |
|---|---|---|---|---|---|---|
| ÄKTA process | Cytiva | Capture, polishing, UF/DF | 1–100 kg/batch | Fully integrated | Automated control, PAT integration | [96] |
| ChromaCon CUBE | ChromaCon | Multi-column chromatography | Pilot to commercial | Modular integration | Real-time monitoring, flexible configuration | [97] |
| OPUS platform | Merck KGaA | Continuous processing suite | Research to production | Platform approach | Scalable design, digital integration | [98] |
| Technology | Application | Parameters Monitored | Advantages | Implementation Complexity | Cost Range | References |
|---|---|---|---|---|---|---|
| Near-Infrared (NIR) | Real-time protein monitoring | Concentration, cell density, metabolites | Non-destructive, rapid analysis | Moderate | USD 50 kilo–200 kilo | [101,102] |
| Raman Spectroscopy | Structural analysis | Protein structure, aggregation | In situ probes available | Moderate | USD 75 kilo–300 kilo | [103,104] |
| Mid-Infrared (MIR) | Detailed protein analysis | Structure, modifications | High specificity | High | USD 100 kilo–400 kilo | [105,106] |
| Online SEC | Quality monitoring | Aggregation, fragmentation | Real-time quality data | High | USD 150 kilo–500 kilo | [107] |
| Mass Spectrometry | Comprehensive analysis | Modifications, impurities | Detailed characterization | Very High | USD 300 kilo–1 million | [108,109] |
| Fluorescence | Cell viability monitoring | Viable cell density, metabolism | Rapid response | Low | USD 25 kilo–100 kilo | [110] |
| Region | Traditional Billionatch Facility | Continuous Facility | Cost Reduction | Productivity Gain | Payback Period | References |
| North America | USD 800 million–1.5 billion | USD 400 million–900 million | 40–50% | 3–4× | 3–5 years | [126] |
| Europe | USD 700 million–1.2 billion | USD 350 million–750 million | 35–45% | 2.5–3.5× | 4–6 years | [127] |
| Asia–Pacific | USD 500 million–900 million | USD 250 million–500 million | 45–55% | 3–5× | 3–4 years | [128] |
| Latin America | USD 300 million–600 million | USD 150 million–350 million | 40–50% | 2–4× | 4–7 years | [129] |
| Cost Category | Traditional Batch | Continuous Manufacturing | Cost Impact | Regional Variation | References |
|---|---|---|---|---|---|
| Raw materials (% of total) | 15–25% | 12–20% | 15–25% reduction | Asia: higher savings | [132,133] |
| Labor costs (% of total) | 20–30% | 12–20% | 25–40% reduction | Europe: moderate savings | [134,135] |
| Quality control | High offline testing | Reduced with PAT | 30–50% reduction | Global consistent | [136,137] |
| Energy consumption | Baseline | Integrated efficiency | 15–25% reduction | Variable by region | [138,139] |
| Facility utilization | 60–70% | 85–95% | 20–35% improvement | Consistent globally | [140] |
| Maintenance costs | Scheduled downtime | Predictive maintenance | 20–30% reduction | Technology dependent | [141] |
| Region | Primary Guidance | Submission Timeline | Special Requirements | Review Duration | Success Rate | References |
|---|---|---|---|---|---|---|
| United States (FDA) | ICH Q13 + FDA Guidance | Standard BLA/NDA pathway | Emerging Technology Program | 10–12 months | 85% | [153,154] |
| Europe (EMA) | ICH Q13 + EMA Guidelines | Centralized procedure | Scientific advice meetings | 12–15 months | 80% | [155] |
| Japan (PMDA) | ICH Q13 + J-GMP adaptation | Standard pathway | Prior consultation | 12–14 months | 78% | [156] |
| China (NMPA) | ICH Q13 + local requirements | Priority review pathway | Technical review meetings | 8–12 months | 70% | [157] |
| Canada (Health Canada) | ICH Q13 + Canadian guidance | Parallel FDA review | Mutual recognition protocols | 10–13 months | 82% | [158] |
| Brazil (ANVISA) | ICH Q13 + local adaptation | Accelerated pathway | Biosimilar focus program | 12–18 months | 65% | [159] |
| Challenge Category | Specific Issues | Risk Level | Mitigation Strategies | Success Factors | Implementation Timeline | References |
|---|---|---|---|---|---|---|
| Technology Integration | Flow rate balancing, system coordination | High | Advanced process control, phased implementation | Cross-functional teams | 12–18 months | [173,174] |
| Material Tracking | Continuous flow traceability | Medium | Residence time modeling, statistical tracking | Digital integration | 6–12 months | [175,176] |
| Process Development | Scale-up methodology differences | Medium | Model-based approaches, extended characterization | Regulatory alignment | 18–24 months | [177,178] |
| Organizational Change | Training, cultural adaptation | High | Change management, skills development | Leadership commitment | 24–36 months | [179,180] |
| Regulatory Compliance | Validation complexity | Medium | Early agency engagement, robust documentation | Proactive strategy | 12–24 months | [151,152] |
| Supply Chain Integration | Just-in-time coordination | Medium | Digital supply networks, predictive analytics | Supplier partnerships | 12–18 months | [181] |
| Technology Area | Current Applications | Future Potential | Implementation Timeline | Investment Level | Expected ROI | References |
|---|---|---|---|---|---|---|
| Artificial Intelligence/ML | Process monitoring, fault detection | Autonomous operation, predictive optimization | 2–5 years | High | 25–40% | [197,198] |
| Advanced Robotics | Automated sampling, maintenance | Fully autonomous manufacturing | 3–7 years | Very High | 30–50% | [199,200] |
| Process Intensification | Microfluidics, novel bioreactors | Dramatically reduced footprints | 5–10 years | Medium | 20–35% | [201,202] |
| Modular Systems | Plug-and-play components | Rapid product changeover | 2–5 years | Medium | 15–30% | [203,204] |
| Digital Twins | Process simulation | Predictive optimization | 1–3 years | Medium | 20–35% | [205] |
| Blockchain | Supply chain tracking | End-to-end traceability | 3–5 years | Low | 10–20% | [206] |
| Technology | Application | Benefits | Implementation Complexity | ROI Timeline | Current Adoption | References |
|---|---|---|---|---|---|---|
| IoT Sensors | Real-time monitoring | Comprehensive data collection | Low | 1–2 years | 60% industry adoption | [209] |
| Edge Computing | Local data processing | Reduced latency, improved control | Medium | 2–3 years | 35% industry adoption | [210] |
| Cloud Analytics | Big data analysis | Predictive insights | Medium | 2–4 years | 45% industry adoption | [211] |
| Digital Twins | Process simulation | Optimization, risk reduction | High | 3–5 years | 20% industry adoption | [212] |
| AI/ML Platforms | Autonomous control | Self-optimizing processes | Very High | 4–7 years | 15% industry adoption | [213] |
| Supply Chain Element | Traditional Batch | Continuous Manufacturing | Key Changes | Implementation Challenges | Cost Impact | References |
|---|---|---|---|---|---|---|
| Raw Material Management | Bulk delivery, large inventory | Just-in-time delivery, small inventory | 60–80% inventory reduction | Supply reliability, quality assurance | 30–50% cost reduction | [225] |
| Quality Control | Batch release testing | Real-time quality monitoring | Elimination of hold times | Method validation, regulatory acceptance | 40–60% cost reduction | [226] |
| Finished Product | Large batch releases | Continuous product flow | Improved cash flow | Distribution network redesign | 20–35% improvement | [227] |
| Cold Chain Management | Batch-based logistics | Continuous flow requirements | Temperature consistency | Infrastructure investment | Variable impact | [228] |
| Supply Network Design | Hub-and-spoke model | Distributed manufacturing | Regional production capabilities | Technology transfer complexity | 25–45% cost reduction | [229] |
| Cost Category | Batch Manufacturing | Continuous Manufacturing | Cost Impact | Regional Variation | Implementation Timeline | References |
|---|---|---|---|---|---|---|
| Inventory carrying costs | 8–12% of product value | 2–4% of product value | 60–75% reduction | Consistent globally | 6–12 months | [236] |
| Transportation costs | High, batch-based | Optimized, continuous flow | 20–35% reduction | Higher in remote regions | 12–18 months | [237] |
| Warehouse requirements | Large, batch storage | Minimal, flow-through | 70–85% reduction | Variable by infrastructure | 18–24 months | [238] |
| Quality control costs | High, batch testing | Reduced, real-time monitoring | 40–60% reduction | Technology dependent | 12–24 months | [239] |
| Working capital | High inventory investment | Low inventory investment | 50–70% improvement | Cash flow benefits | 6–18 months | [240] |
| Region | Current Access Level | Projected Improvement | Cost Reduction Target | Patient Impact | Implementation Timeline | References |
|---|---|---|---|---|---|---|
| North America | 85% coverage | 5–10% improvement | 30–40% cost reduction | 2 M additional patients | 5–7 years | [245] |
| Europe | 90% coverage | 3–7% improvement | 25–35% cost reduction | 1.5 M additional patients | 4–6 years | [246] |
| Asia–Pacific | 60% coverage | 15–25% improvement | 40–55% cost reduction | 50 M additional patients | 7–10 years | [247] |
| Latin America | 40% coverage | 20–35% improvement | 45–60% cost reduction | 25 M additional patients | 8–12 years | [248] |
| Africa | 25% coverage | 30–50% improvement | 50–70% cost reduction | 100 M additional patients | 10–15 years | [249] |
| Environmental Factor | Batch Manufacturing | Continuous Manufacturing | Improvement | Global Impact | Regulatory Recognition | References |
|---|---|---|---|---|---|---|
| Water consumption | 100,000–500,000 L/kg | 30,000–150,000 L/kg | 60–70% reduction | Water conservation | EPA/EMA sustainability guidelines | [259] |
| Energy consumption | Baseline | 15–25% reduction | Energy efficiency | Carbon footprint reduction | Green manufacturing incentives | [260] |
| Waste generation | High solvent usage | Reduced through integration | 40–60% reduction | Waste minimization | Waste reduction regulations | [261] |
| Chemical consumption | Large buffer volumes | Optimized usage | 30–50% reduction | Environmental protection | Chemical safety guidelines | [262] |
| Carbon footprint | High energy intensity | Optimized processes | 20–35% reduction | Climate change mitigation | Carbon tax advantages | [263] |
| Implementation Phase | Duration | Key Activities | Success Metrics | Investment Level | Risk Level | References |
|---|---|---|---|---|---|---|
| Phase 1: Assessment | 6–12 months | Technology evaluation, capability assessment | Internal expertise development | Low (USD 1 million–5 million) | Low | [268,269] |
| Phase 2: Pilot Implementation | 12–18 months | Small-scale demonstration, proof of concept | Technical feasibility demonstration | Medium (USD 5 million–25 million) | Medium | [270,271] |
| Phase 3: Scale-up | 18–36 months | Commercial implementation, process optimization | Regulatory approval, commercial production | High (USD 25 million–100 million) | High | [272,273] |
| Phase 4: Expansion | 3–5 years | Multi-product implementation, global rollout | Market penetration, competitive advantage | Very High (USD 100 million+) | Medium | [274,275] |
| Market Segment | Current Size (2024) | Projected 2030 Size | CAGR | Key Growth Drivers | Continuous Manufacturing Impact | References |
|---|---|---|---|---|---|---|
| Monoclonal Antibodies | USD 185 billion | USD 425 billion | 12.8% | Biosimilar competition, cost pressures | High cost reduction potential | [288] |
| Recombinant Proteins | USD 85 billion | USD 180 billion | 11.2% | Emerging markets, accessibility | Manufacturing scalability | [289] |
| Gene Therapies | USD 15 billion | USD 65 billion | 23.5% | Technology advancement, regulatory support | Production cost reduction | [290] |
| Cell Therapies | USD 8 billion | USD 45 billion | 25.8% | Manufacturing scalability requirements | Process standardization | [291] |
| Biosimilars | USD 25 billion | USD 85 billion | 18.7% | Patent expirations, healthcare cost pressures | Competitive manufacturing costs | [292] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niazi, S.K. Continuous Manufacturing of Recombinant Drugs: Comprehensive Analysis of Cost Reduction Strategies, Regulatory Pathways, and Global Implementation. Pharmaceuticals 2025, 18, 1157. https://doi.org/10.3390/ph18081157
Niazi SK. Continuous Manufacturing of Recombinant Drugs: Comprehensive Analysis of Cost Reduction Strategies, Regulatory Pathways, and Global Implementation. Pharmaceuticals. 2025; 18(8):1157. https://doi.org/10.3390/ph18081157
Chicago/Turabian StyleNiazi, Sarfaraz K. 2025. "Continuous Manufacturing of Recombinant Drugs: Comprehensive Analysis of Cost Reduction Strategies, Regulatory Pathways, and Global Implementation" Pharmaceuticals 18, no. 8: 1157. https://doi.org/10.3390/ph18081157
APA StyleNiazi, S. K. (2025). Continuous Manufacturing of Recombinant Drugs: Comprehensive Analysis of Cost Reduction Strategies, Regulatory Pathways, and Global Implementation. Pharmaceuticals, 18(8), 1157. https://doi.org/10.3390/ph18081157







