Study on the Excretion of a New Antihypertensive Drug 221s (2,9) in Rats
Abstract
1. Introduction
2. Results
2.1. Method Development
2.2. Method Validation
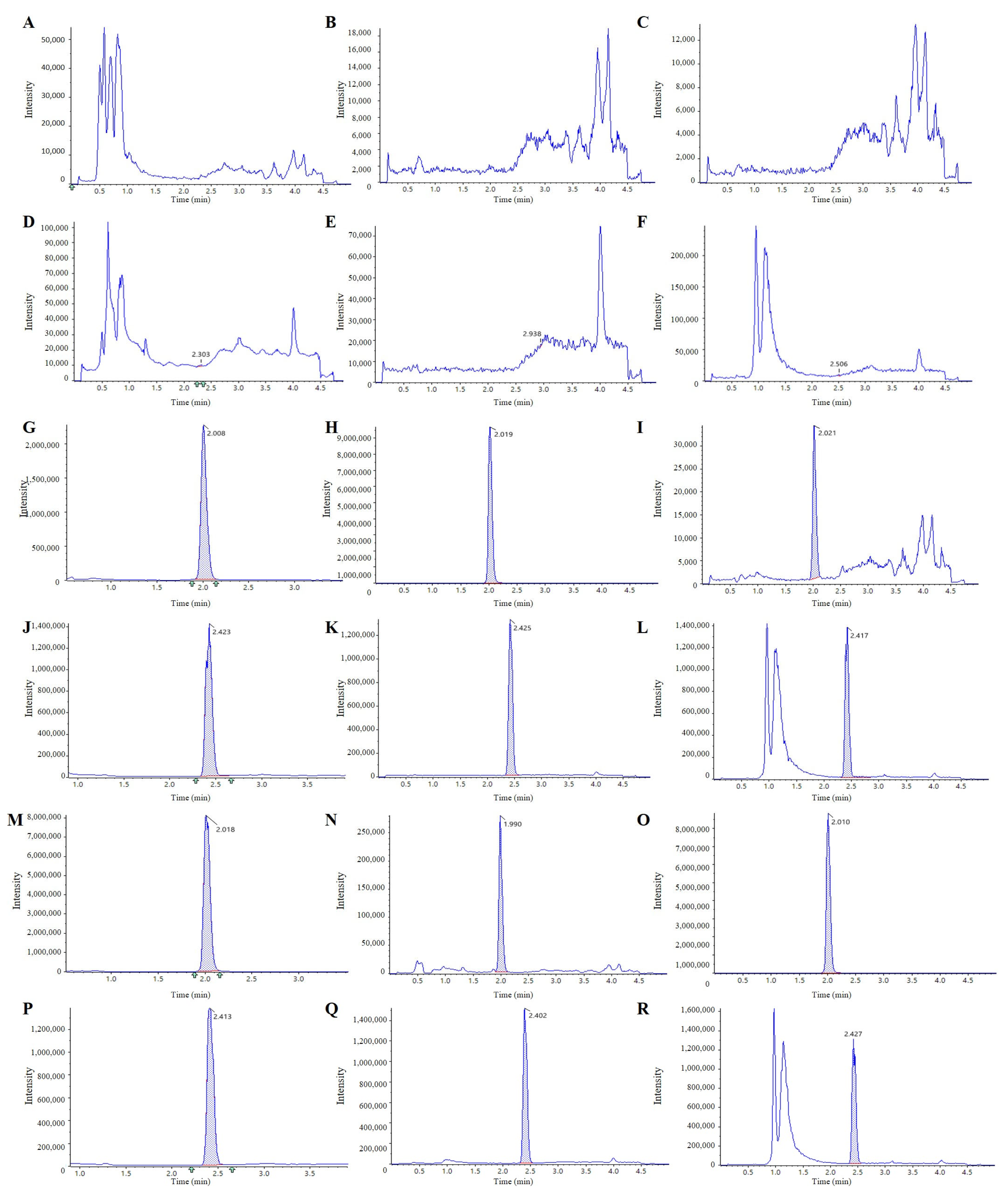
2.2.1. Selectivity and Specific
2.2.2. Linearity and LLOQ
2.2.3. Precision and Accuracy
2.2.4. Extraction Recovery and Matrix Effect
2.2.5. Stability
2.3. Excretion Study
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. UPLC-MS/MS Instrument and Conditions
4.3. Preparation of Calibration Standards and Quality Control Samples
4.4. Method Validation
4.4.1. Extraction Recovery and Matrix Effect
4.4.2. Linearity and LLOQ
4.4.3. Precision and Accuracy
4.4.4. Extraction Recovery and Matrix Effect
4.4.5. Stability
4.5. Excretion Study
4.5.1. Collection of Urine, Fecal, and Bile Samples
4.5.2. Pretreatment of Urine, Fecal, and Bile Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parodi, R.; Brandani, L.; Romero, C.; Klein, M. Resistant hypertension: Diagnosis, evaluation, and treatment practical approach. Eur. J. Intern. Med. 2024, 123, 23–28. [Google Scholar] [CrossRef]
- Al Ghorani, H.; Gotzinger, F.; Bohm, M.; Mahfoud, F. Arterial hypertension—Clinical trials update 2021. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 21–31. [Google Scholar] [CrossRef]
- Lauder, L.; Mahfoud, F.; Azizi, M.; Bhatt, D.L.; Ewen, S.; Kario, K.; Parati, G.; Rossignol, P.; Schlaich, M.P.; Teo, K.K.; et al. Hypertension management in patients with cardiovascular comorbidities. Eur. Heart J. 2023, 44, 2066–2077. [Google Scholar] [CrossRef]
- Carey, R.M.; Moran, A.E.; Whelton, P.K. Treatment of Hypertension: A Review. JAMA 2022, 328, 1849–1861. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Rysz-Gorzynska, M.; Gluba-Brzozka, A. Pharmacogenomics of Hypertension Treatment. Int. J. Mol. Sci. 2020, 21, 4709. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, A.; Cisse, M.S.; Rovito, P.F.; Puente, M. Angiotensin-Converting Enzyme (ACE) Inhibitor-Induced Angioedema of the Small Bowel: A Diagnostic Dilemma. J. Am. Board. Fam. Med. 2023, 36, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Wu, Z.; Wang, Z.; Chen, L.; Liu, B.; Lu, M.; Shen, N. Association between angiotensin-converting enzyme inhibitor-induced cough and the risk of lung cancer: A Mendelian randomization study. Front. Pharmacol. 2023, 14, 1267924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, X.; Fan, T.; Li, Z.; Zhang, Y.; Zheng, J. A novel drug discovery strategy inspired by traditional medicine philosophies. Science 2015, 347, S38–S40. [Google Scholar]
- Xiao-Hui, Z.; Pu, J.; Ya-Jun, B. Research strategy for Combination of Traditional Chinese Medicine Molecular Chemistry based on the traditional theory of ‘Jun-Chen-Zuo-Shi’. J. Northwest Univ. 2015, 45, 405–411. [Google Scholar]
- Qin, B.; Chen, Y.; Yang, K.; Wang, R.; Yu, L.; Wang, N.; Liu, S. An Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS) Method for Qualifying DAPB in Rat Plasma and Application to Pharmacokinetic Studies. Molecules 2024, 29, 541. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.; Wang, N.; Tan, X.; Yixin, Z. The study on acute toxicity test of anti-hypertensive new drug proline Danshensu borneol ester on mice. Chin. J. Pharmacovigil. 2023, 20, 754–757. [Google Scholar] [CrossRef]
- Qin, B.; Yu, L.; Wang, R.; Tang, Y.; Chen, Y.; Wang, N.; Zhang, Y.; Tan, X.; Yang, K.; Zhang, B.; et al. Chemical Synthesis, Safety and Efficacy of Antihypertensive Candidate Drug 221s (2,9). Molecules 2023, 28, 4975. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Chu, X.; Di, L.; Gao, W.; Guo, Y.; Liu, X.; Lu, C.; Mao, J.; Shen, H.; Tang, H.; et al. Recent advances in the translation of drug metabolism and pharmacokinetics science for drug discovery and development. Acta Pharm. Sin. B 2022, 12, 2751–2777. [Google Scholar] [CrossRef]
- He, M.M.; Zhu, S.X.; Cannon, J.R.; Christensen, J.K.; Duggal, R.; Gunduz, M.; Hilgendorf, C.; Hughes, A.; Kekessie, I.; Kullmann, M.; et al. Metabolism and Excretion of Therapeutic Peptides: Current Industry Practices, Perspectives, and Recommendations. Drug Metab. Dispos. 2023, 51, 1436–1450. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.V.; Tayara, H.; Chong, K.T. Artificial Intelligence in Drug Metabolism and Excretion Prediction: Recent Advances, Challenges, and Future Perspectives. Pharmaceutics 2023, 15, 1260. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xue, M.; He, Y.; Yin, H.; Yang, C.; Zhong, D.; Zeng, H.; Zheng, Y.; Diao, X. Absorption, Distribution, Metabolism, and Excretion of [(14)C]BS1801, a Selenium-Containing Drug Candidate, in Rats. Molecules 2023, 28, 8102. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, D.; Wu, J.; Ou, Y.; Mu, C.; Han, B.; Zhang, Q. Improved blood-brain barrier distribution: Effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J. Ethnopharmacol. 2015, 162, 270–277. [Google Scholar] [CrossRef]
- Chan, K.; Chui, S.H.; Wong, D.Y.; Ha, W.Y.; Chan, C.L.; Wong, R.N. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci. 2004, 75, 3157–3171. [Google Scholar] [CrossRef]
- Du, X.; Tao, Q.; Du, H.; Zhao, Z.; Dong, Y.; He, S.; Shao, R.; Wang, Y.; Han, W.; Wang, X.; et al. Tengdan Capsule Prevents Hypertensive Kidney Damage in SHR by Inhibiting Periostin-Mediated Renal Fibrosis. Front. Pharmacol. 2021, 12, 638298. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, K.; Xia, Y.; Wang, Y.; Wang, A.; Zhao, Y. Danshensu Attenuated Epithelial-Mesenchymal Transformation and Chemoresistance of Colon Cancer Cells Induced by Platelets. Front. Biosci. 2022, 27, 160. [Google Scholar] [CrossRef]
- Jia, S.; Chen, Q.; Wu, J.; Yao, X.; Shao, J.; Cheng, X.; Zhang, C.; Cen, D.; Wang, Y.; Shen, Z.; et al. Danshensu derivative ADTM ameliorates CCl4-induced acute liver injury in mice through inhibiting oxidative stress and apoptosis. Pathology 2021, 228, 153656. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.F.; Yeung, J.H.; Chan, K.M.; Or, P.M. Relaxant effects of danshen aqueous extract and its constituent danshensu on rat coronary artery are mediated by inhibition of calcium channels. Vasc. Pharmacol. 2007, 46, 271–277. [Google Scholar] [CrossRef] [PubMed]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58 (Suppl. S10), S10–S25. [Google Scholar] [CrossRef] [PubMed]
- Cerny, M.A.; Spracklin, D.K.; Obach, R.S. Human Absorption, Distribution, Metabolism, and Excretion Studies: Origins, Innovations, and Importance. Drug Metab. Dispos. 2023, 51, 647–656. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, W. Drug metabolism in drug discovery and development. Acta Pharm. Sin. B 2018, 8, 721–732. [Google Scholar] [CrossRef]
- Benet, L.Z.; Bowman, C.M.; Koleske, M.L.; Rinaldi, C.L.; Sodhi, J.K. Understanding drug-drug interaction and pharmacogenomic changes in pharmacokinetics for metabolized drugs. J. Pharmacokinet. Pharmacodyn. 2019, 46, 155–163. [Google Scholar] [CrossRef]
- Yue, Z.X.; Gu, Y.X.; Yan, T.C.; Liu, F.M.; Cao, J.; Ye, L.H. Phase Ⅰ and phase Ⅱ metabolic studies of Citrus flavonoids based on electrochemical simulation and in vitro methods by EC-Q-TOF/MS and HPLC-Q-TOF/MS. Food Chem. 2022, 380, 132202. [Google Scholar] [CrossRef]
- Lan, W.; Bian, L.; Zhao, X.; Jia, P.; Meng, X.; Wu, Y.; Wang, S.; Liao, S.; Yu, J.; Zheng, X. Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry for Identification of In Vitro and In Vivo Metabolites of Bornyl Gallate in Rats. J. Anal. Methods Chem. 2013, 2013, 473649. [Google Scholar] [CrossRef]
- Di, L. An update on the importance of plasma protein binding in drug discovery and development. Expert. Opin. Drug Discov. 2021, 16, 1453–1465. [Google Scholar] [CrossRef]


| Normial Conc. (ng/mL) | Intra-Day | Inter-Day | |||
|---|---|---|---|---|---|
| Accuracy (RE, %) | Precision (RSD, %) | Accuracy (RE, %) | Precision (RSD, %) | ||
| Urine | 2 | −6.8 | 9.1 | 3.5 | 9.5 |
| 10 | 2.4 | 6.3 | −6.4 | 7.4 | |
| 500 | 2.9 | 8.4 | −3.7 | 4.8 | |
| 1600 | 3.8 | 5.2 | 4.1 | 6.2 | |
| 2000 | 1.9 | 5.7 | 1.9 | 2.6 | |
| Feces | 2 | −8.6 | 9.4 | −5.3 | 3.6 |
| 10 | −6.4 | 5.9 | 4.5 | 4.7 | |
| 500 | 3.4 | 5.7 | 4.4 | 3.2 | |
| 1600 | 4.1 | 2.7 | 4.9 | 8.6 | |
| 2000 | −4.5 | 3.2 | 2.7 | 1.3 | |
| Bile | 2 | −9.7 | 7.5 | −7.4 | 10.5 |
| 10 | −7.5 | 8.4 | −4.9 | 8.4 | |
| 500 | 6.4 | 4.9 | 5.4 | 5.6 | |
| 1600 | −3.8 | 5.1 | 6.8 | 7.2 | |
| 2000 | 3.1 | 2.6 | 3.3 | 2.5 | |
| Spiked Conc. (ng/mL) | Matrix Effect (Mean ± SD, %) | RSD (%) | |
|---|---|---|---|
| Urine | 10 | 98.21 ± 7.51 | 7.6 |
| 1000 | 101.65 ± 5.24 | 5.2 | |
| 1600 | 96.84 ± 8.35 | 8.6 | |
| Feces | 10 | 99.14 ± 5.34 | 5.4 |
| 1000 | 97.55 ± 4.28 | 4.4 | |
| 1600 | 103.27 ± 4.13 | 4.0 | |
| Bile | 10 | 95.48 ± 6.22 | 6.5 |
| 1000 | 102.47 ± 3.15 | 3.1 | |
| 1600 | 104.56 ± 1.68 | 1.6 |
| Spiked Conc. (ng/mL) | Matrix Effect (Mean ± SD, %) | RSD (%) | |
|---|---|---|---|
| Urine | 10 | 89.15 ± 3.58 | 4.0 |
| 1000 | 94.26 ± 5.49 | 5.8 | |
| 1600 | 100.52 ± 6.47 | 6.4 | |
| Feces | 10 | 88.41 ± 10.44 | 11.8 |
| 1000 | 99.75 ± 6.43 | 6.4 | |
| 1600 | 97.46 ± 5.28 | 5.4 | |
| Bile | 10 | 88.39 ± 6.34 | 7.2 |
| 1000 | 96.47 ± 3.44 | 3.6 | |
| 1600 | 99.25 ± 4.12 | 4.2 |
| Stability Conditions | Spiked Conc. (10 ng/mL) | Spiked Conc. (1000 ng/mL) | Spiked Conc. (1600 ng/mL) | ||||
|---|---|---|---|---|---|---|---|
| RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | ||
| Urine | Room temperature for 2 h | −12.5 | 7.8 | −1.5 | 3.3 | 3.4 | 4.0 |
| Three freeze/thaw cycles | −9.4 | 6.6 | −6.4 | 8.7 | −2.6 | 3.9 | |
| −80 °C for 30 days | −8.7 | 5.3 | −5.4 | 7.2 | −5.5 | 6.7 | |
| Autosampler rack (4 °C) for 24 h | −7.3 | 4.5 | −6.3 | 6.8 | 3.9 | 3.4 | |
| Dilution capability (factor: 5) | −12.8 | 3.4 | −9.4 | 9.4 | −7.4 | 8.1 | |
| Feces | Room temperature for 2 h | −6.7 | 4.8 | −3.6 | 6.6 | −4.7 | 5.5 |
| Three freeze/thaw cycles | −5.3 | 3.5 | −4.8 | 4.9 | −3.2 | 4.6 | |
| −80 °C for 30 days | −6.4 | 4.1 | −5.3 | 5.7 | 2.8 | 3.7 | |
| Autosampler rack (4 °C) for 24 h | −4.9 | 5.8 | −2.2 | 3.4 | 4.6 | 4.2 | |
| Dilution capability (factor: 5) | −3.7 | 2.7 | −3.7 | 4.1 | −7.6 | 9.1 | |
| Bile | Room temperature for 2 h | −5.2 | 6.2 | −4.5 | 6.5 | 5.1 | 5.8 |
| Three freeze/thaw cycles | −8.4 | 7.2 | −4.1 | 5.7 | 4.2 | 6.2 | |
| −80 °C for 30 days | −3.1 | 3.1 | 1.5 | 1.2 | 3.4 | 4.3 | |
| Autosampler rack (4 °C) for 24 h | −4.4 | 3.8 | −3.2 | 4.1 | −3.8 | 3.7 | |
| Dilution capability (factor: 5) | −5.6 | 7.6 | 2.4 | 3.3 | −2.8 | 1.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, K.; Liu, S.; Yu, L.; Wang, R.; Qin, B. Study on the Excretion of a New Antihypertensive Drug 221s (2,9) in Rats. Pharmaceuticals 2025, 18, 1138. https://doi.org/10.3390/ph18081138
Chen Y, Yang K, Liu S, Yu L, Wang R, Qin B. Study on the Excretion of a New Antihypertensive Drug 221s (2,9) in Rats. Pharmaceuticals. 2025; 18(8):1138. https://doi.org/10.3390/ph18081138
Chicago/Turabian StyleChen, Yunmei, Kuan Yang, Shaojing Liu, Lili Yu, Rong Wang, and Bei Qin. 2025. "Study on the Excretion of a New Antihypertensive Drug 221s (2,9) in Rats" Pharmaceuticals 18, no. 8: 1138. https://doi.org/10.3390/ph18081138
APA StyleChen, Y., Yang, K., Liu, S., Yu, L., Wang, R., & Qin, B. (2025). Study on the Excretion of a New Antihypertensive Drug 221s (2,9) in Rats. Pharmaceuticals, 18(8), 1138. https://doi.org/10.3390/ph18081138





