Integrative Transcriptomic and Network Pharmacology Analysis Reveals Key Targets and Mechanisms of Moschus (musk) Against Viral Respiratory Tract Infections
Abstract
1. Introduction
2. Results
2.1. Screening of Active Compounds and Potential Targets
2.2. Retrieval Results of VRTI Targets
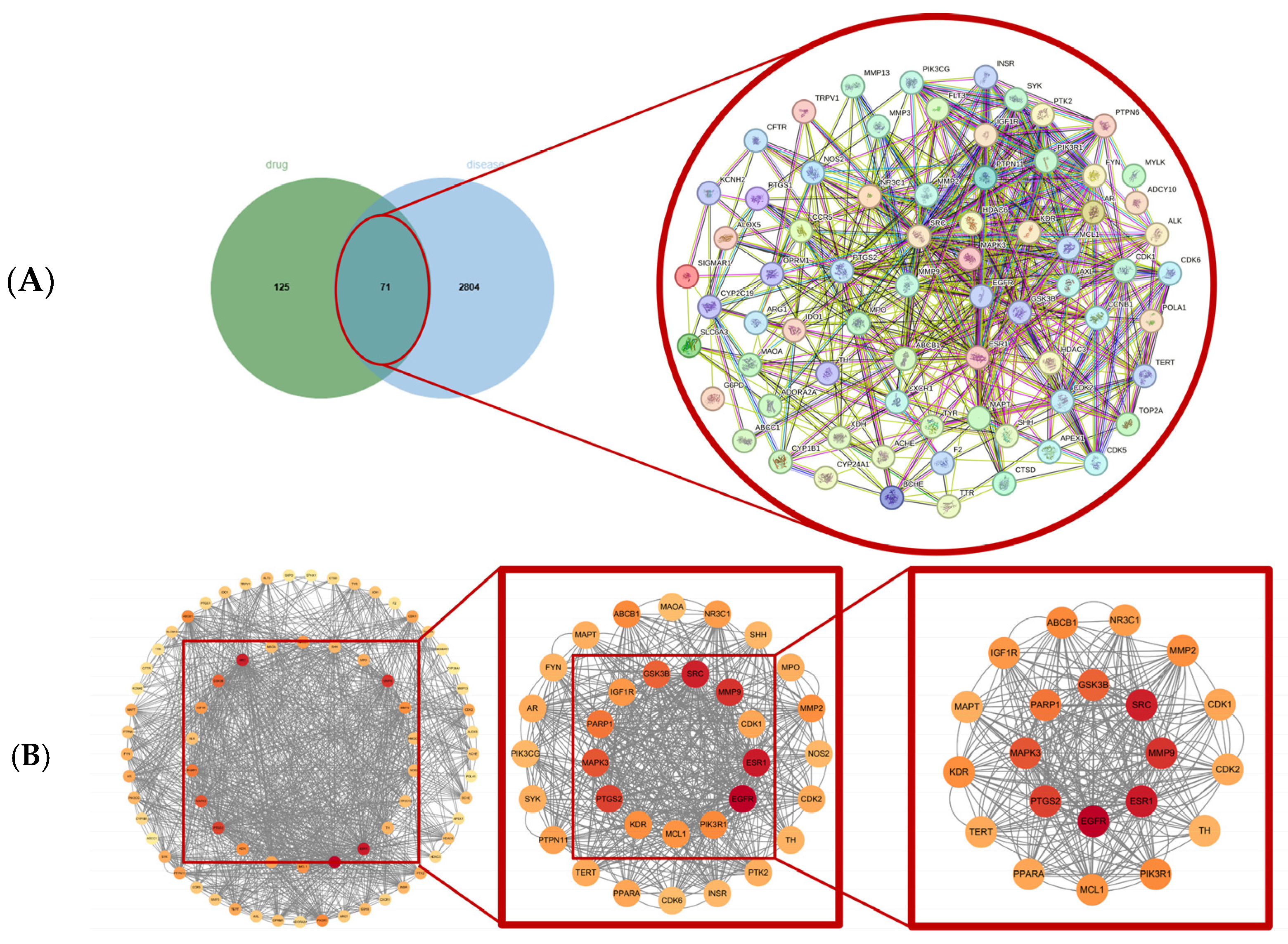
2.3. Screening of Core Drug–Disease Targets Based on PPI Network
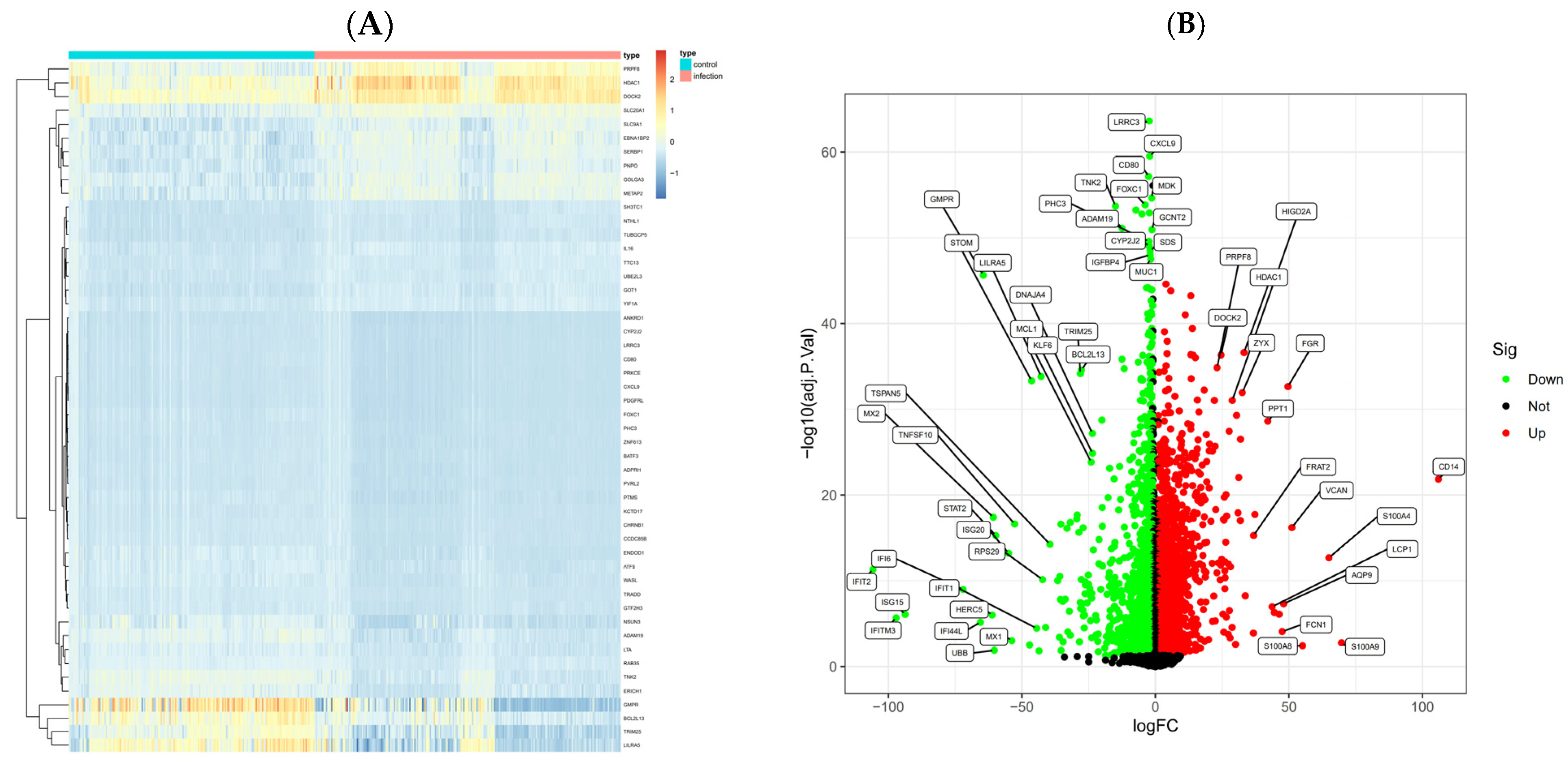
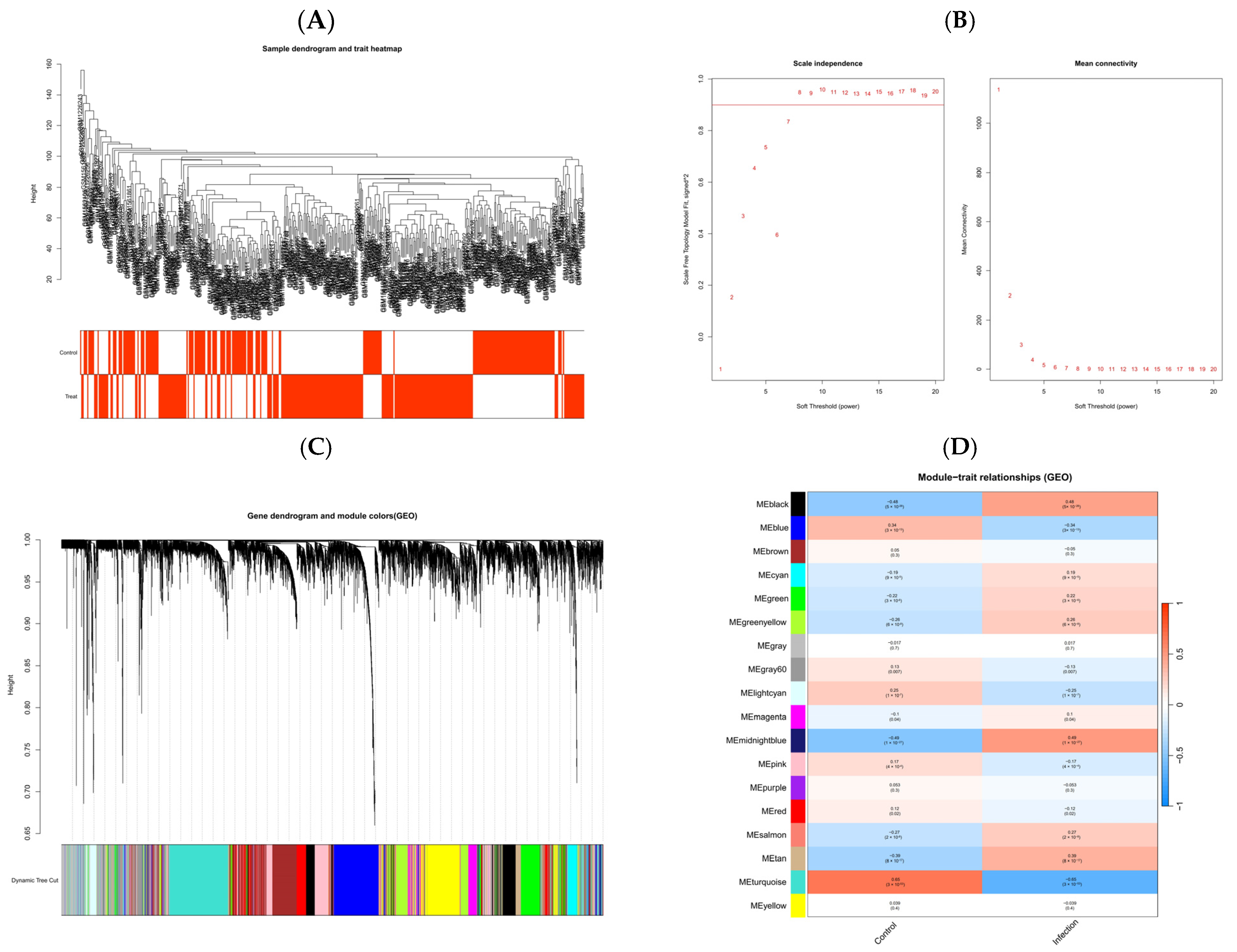
2.4. GEO Data Collection and DEG Analysis
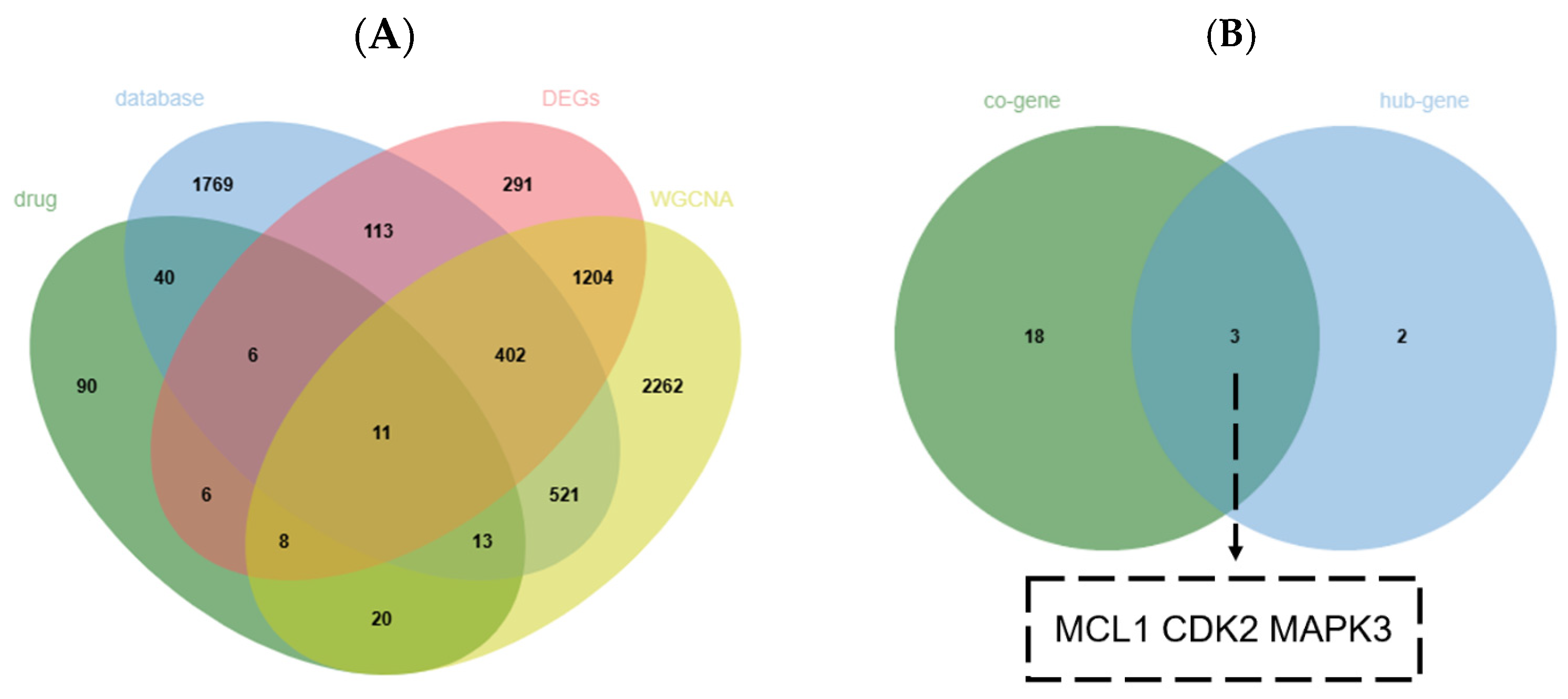
2.5. Screening of Key Genes
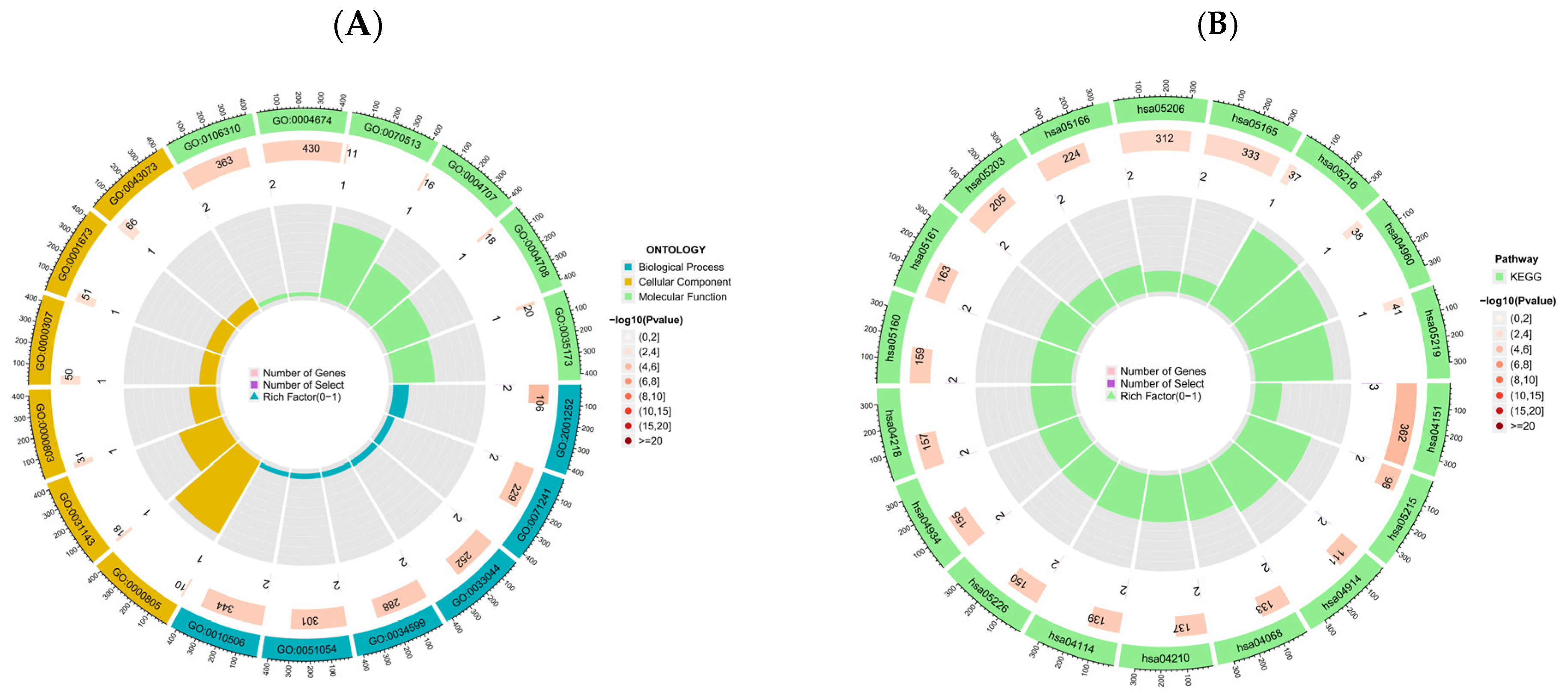
2.6. Gene Ontology and KEGG Pathway Analysis
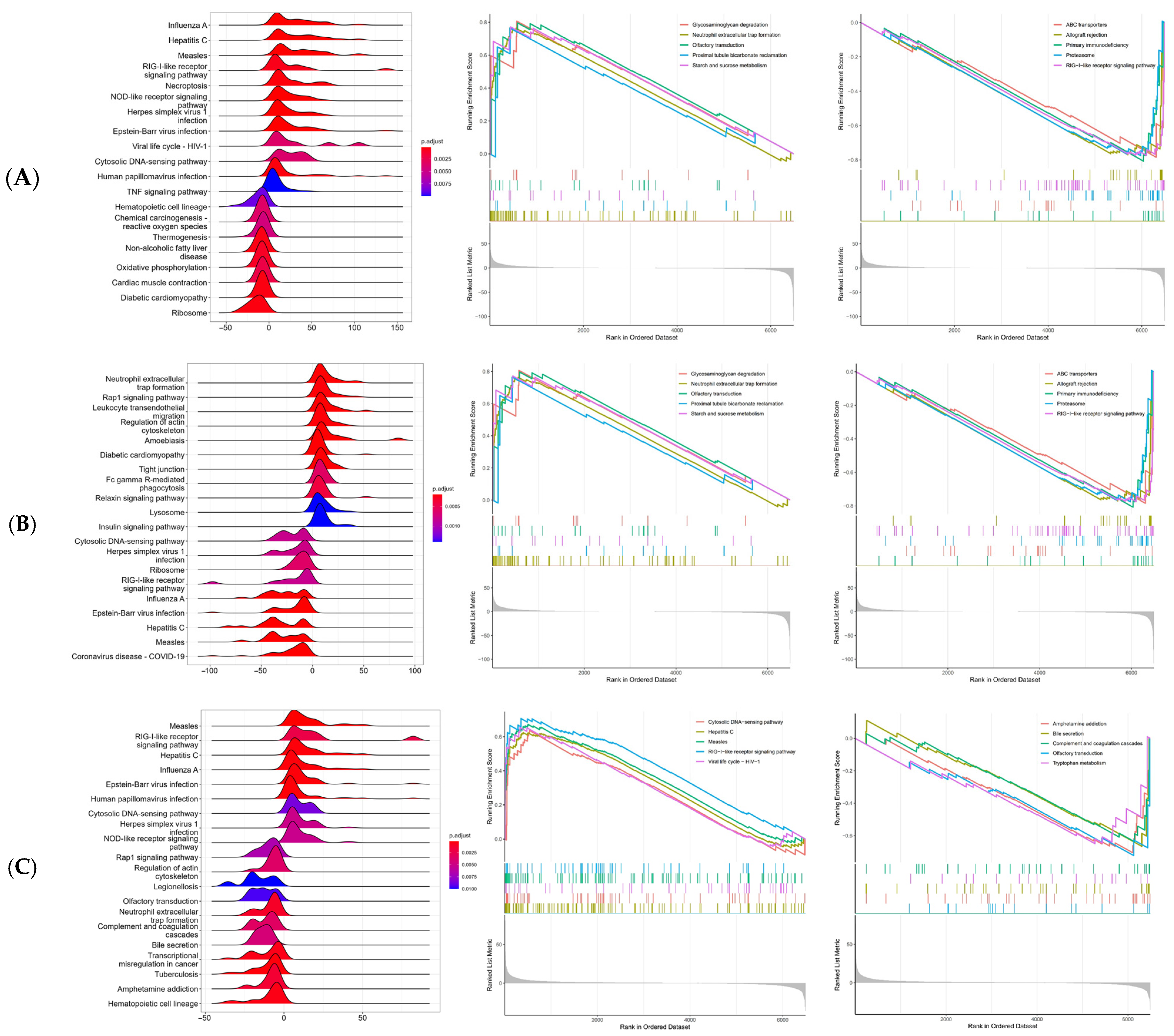
2.7. Single-Gene Gene Set Enrichment Analysis
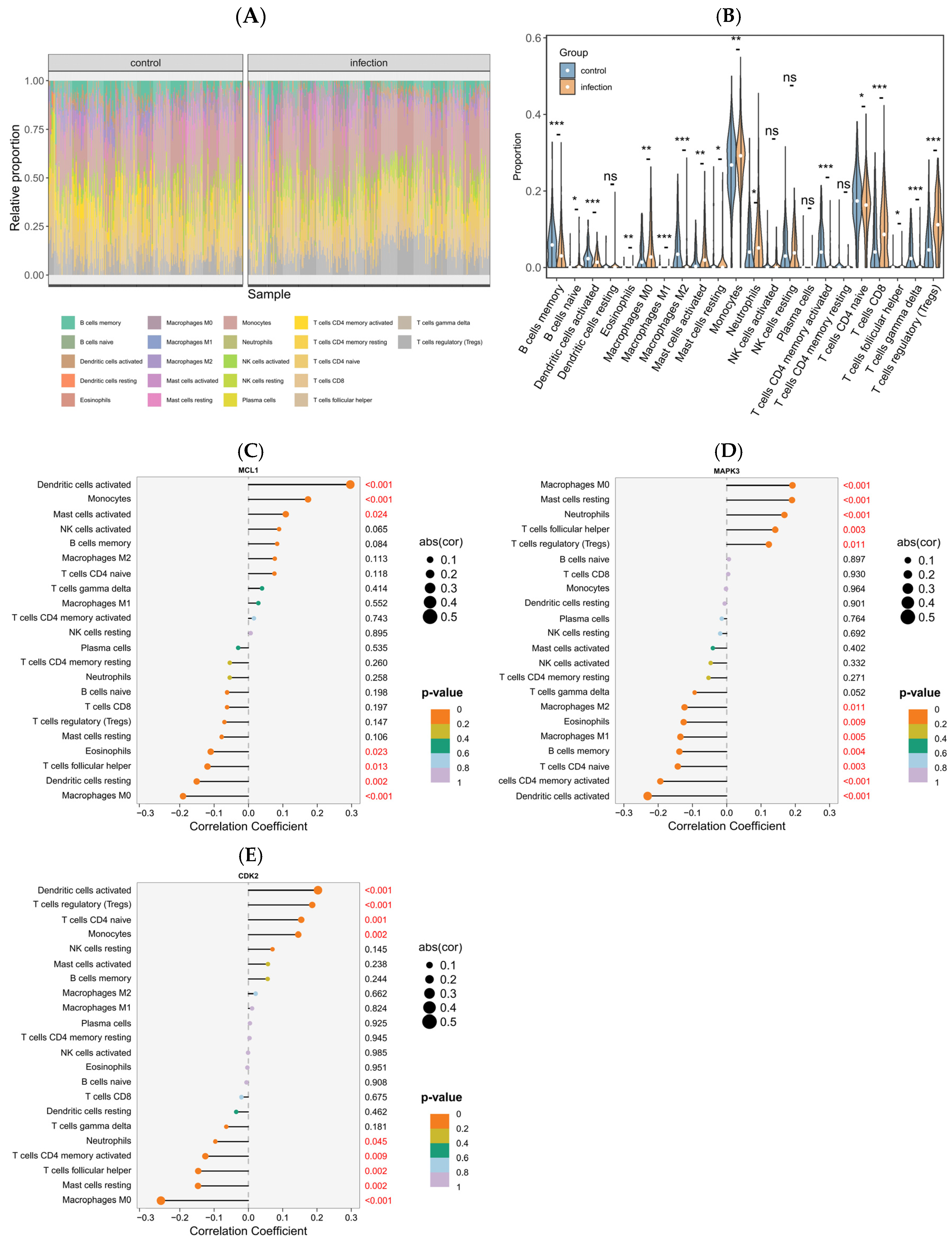
2.8. Immune Response Signatures and Associations with Key Genes
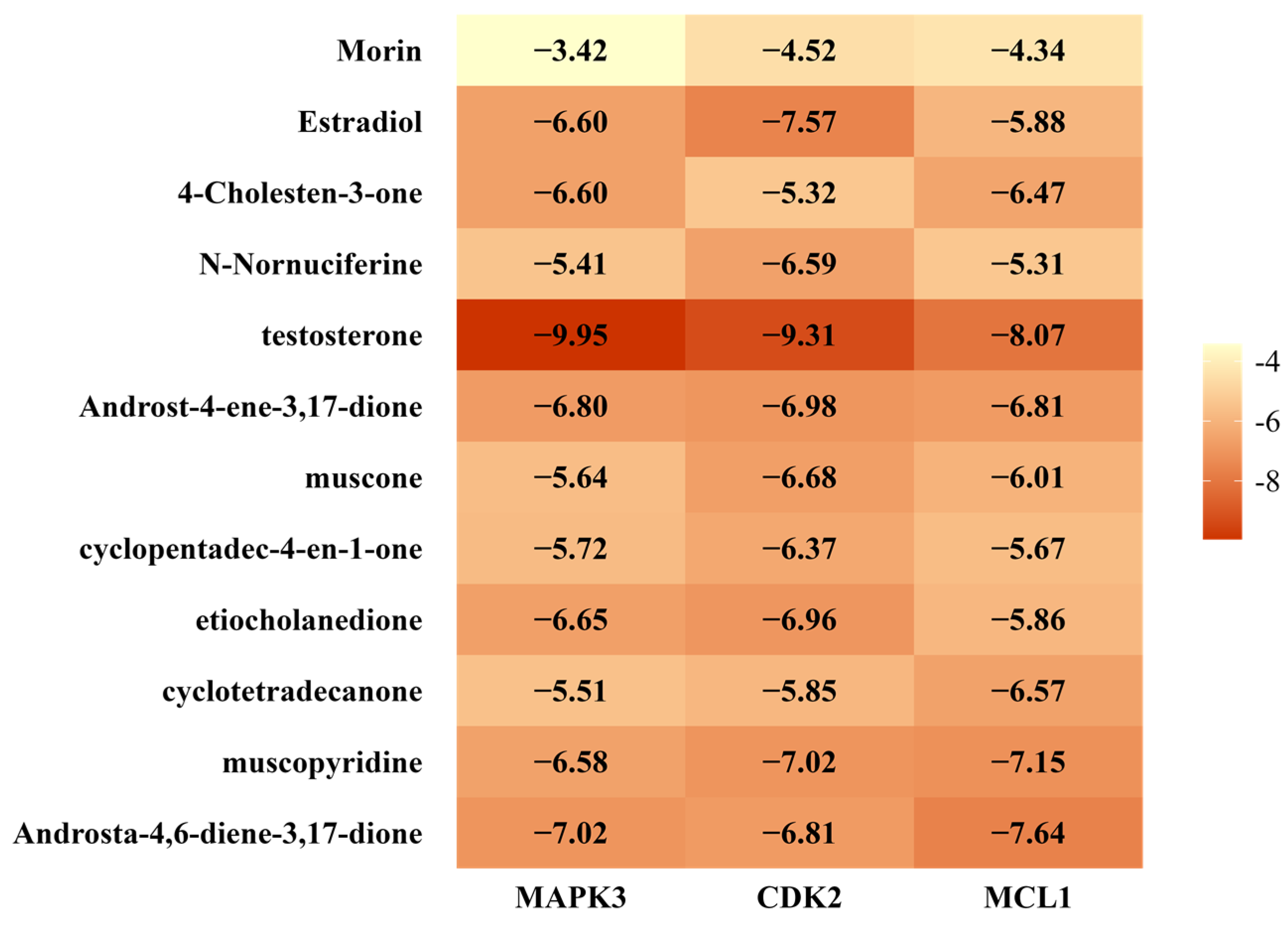
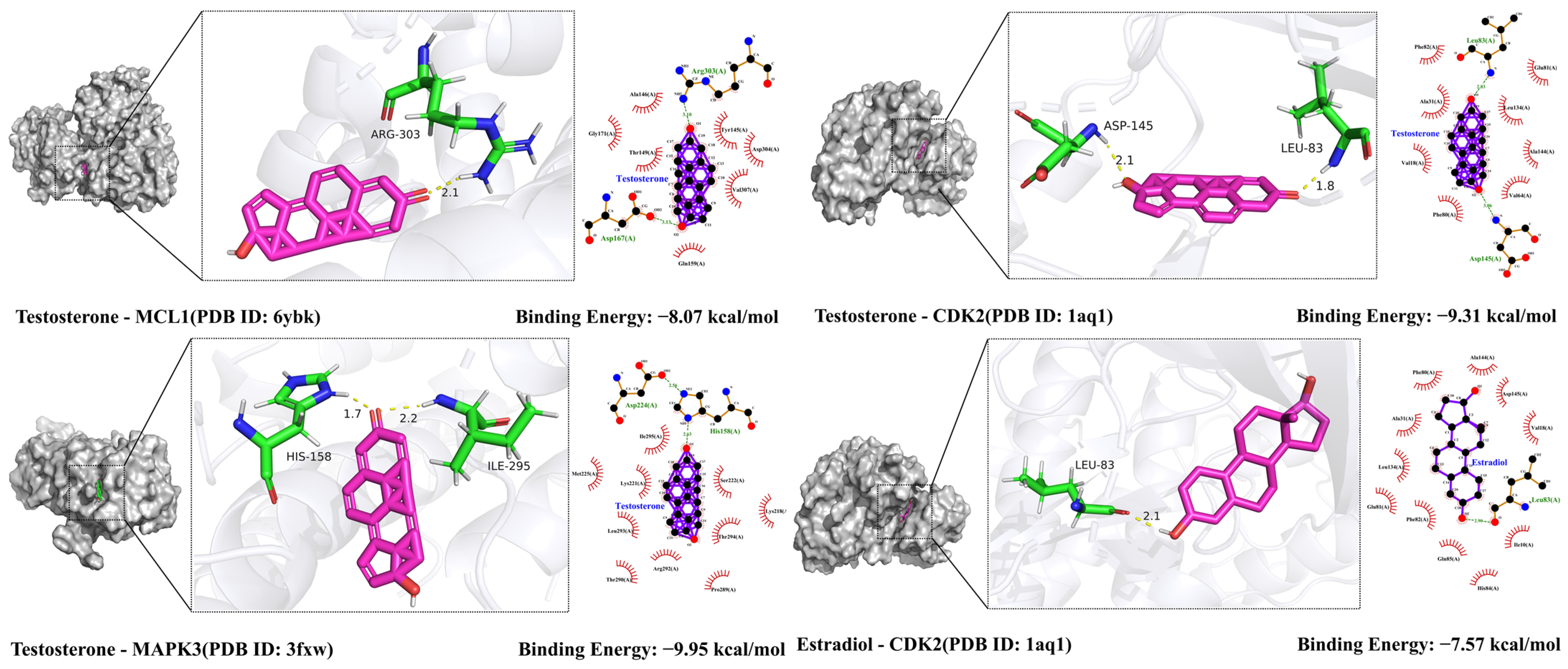
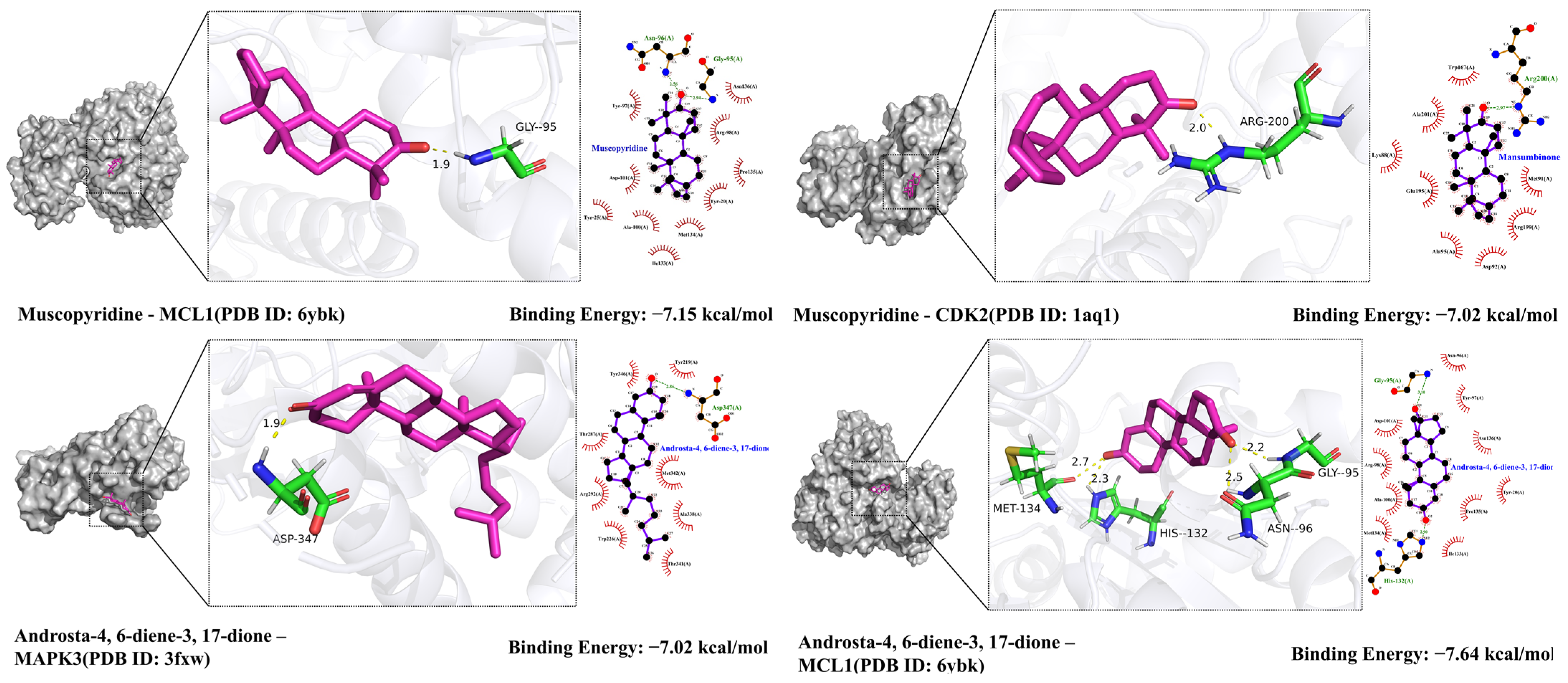
2.9. Molecular Docking Validation Results
3. Discussion
4. Materials and Methods
4.1. Identification of Active Compounds and Corresponding Targets
4.2. Retrieval of Genes Associated with Viral Respiratory Tract Infections
4.3. Construction of the Protein–Protein Interaction (PPI) Network and Screening of Core Targets
4.4. GEO-Based Differential Gene Expression Analysis
4.5. Weighted Gene Co-Expression Network Analysis
4.6. Enrichment Analysis
4.7. Immune Cell Infiltration Analysis
4.8. Molecular Docking Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VRTIs | Viral respiratory tract infections |
| SMILES | Simplified Molecular Input Line Entry System |
| PPI | Protein–protein interaction |
| BC | Betweenness Centrality |
| CC | Closeness Centrality |
| DC | Degree Centrality |
| EC | Eigenvector Centrality |
| LAC | Local Average Connectivity-based method |
| NC | Network Centrality |
| GEO | Gene Expression Omnibus |
| DEGs | Differentially expressed genes |
| WGCNA | Weighted gene co-expression network analysis |
| PBMCs | Peripheral blood mononuclear cells |
| GSEA | Gene Set Enrichment Analysis |
| Tregs | Regulatory T cells |
| MAPK3 | Mitogen-activated protein kinase 3 |
| ERK1 | Extracellular signal-regulated kinase 1 |
| AR | Androgen receptor |
References
- Clementi, N.; Ghosh, S.; De Santis, M.; Castelli, M.; Criscuolo, E.; Zanoni, I.; Clementi, M.; Mancini, N. Viral Respiratory Pathogens and Lung Injury. Clin. Microbiol. Rev. 2021, 34, e00103-20. [Google Scholar] [CrossRef]
- Kimura, H.; Yoshizumi, M.; Ishii, H.; Oishi, K.; Ryo, A. Cytokine Production and Signaling Pathways in Respiratory Virus Infection. Front. Microbiol. 2013, 4, 276. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Schwarze, J. Respiratory Viral Infections in Infants: Causes, Clinical Symptoms, Virology, and Immunology. Clin. Microbiol. Rev. 2010, 23, 74–98. [Google Scholar] [CrossRef]
- Message, S.D.; Johnston, S.L. The Immunology of Virus Infection in Asthma. Eur. Respir. J. 2001, 18, 1013–1025. [Google Scholar] [CrossRef]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef]
- D’Anna, S.E.; Maniscalco, M.; Cappello, F.; Carone, M.; Motta, A.; Balbi, B.; Ricciardolo, F.L.M.; Caramori, G.; Di Stefano, A. Bacterial and Viral Infections and Related Inflammatory Responses in Chronic Obstructive Pulmonary Disease. Ann. Med. 2021, 53, 135–150. [Google Scholar] [CrossRef]
- Chepur, S.V.; Pluzhnikov, N.N.; Chubar, O.V.; Bakulina, L.S.; Litvinenko, I.V.; Makarov, V.A.; Gogolevsky, A.S.; Myasnikov, V.A.; Myasnikova, I.A.; Al-Shehadat, R.I. Respiratory RNA Viruses: How to Be Prepared for an Encounter with New Pandemic Virus Strains. Biol. Bull. Rev. 2021, 11, 154–171. [Google Scholar] [CrossRef]
- Roberti di Sarsina, P.; Ottaviani, L.; Mella, J. Tibetan Medicine: A Unique Heritage of Person-Centered Medicine. EPMA J. 2011, 2, 385–389. [Google Scholar] [CrossRef]
- von Philipsborn, P.; Biallas, R.; Burns, J.; Drees, S.; Geffert, K.; Movsisyan, A.; Pfadenhauer, L.M.; Sell, K.; Strahwald, B.; Stratil, J.M.; et al. Adverse Effects of Non-Steroidal Anti-Inflammatory Drugs in Patients with Viral Respiratory Infections: Rapid Systematic Review. BMJ Open 2020, 10, e040990. [Google Scholar] [CrossRef]
- Nelson, P.P.; Papadopoulos, N.G.; Skevaki, C. Respiratory Viral Pathogens. In Encyclopedia of Respiratory Medicine; Elsevier: Amsterdam, The Netherlands, 2022; pp. 129–137. [Google Scholar]
- Ettemeyer, M.; Florey, M.; Tanida, K.; Jochum, J.; Schulze-Sturm, U.; Lütgehetmann, M.; Baehr, M.; Addo, M.M.; Schmiedel, S.; Rohde, H.; et al. High Burden of RSV and Influenza in Patients Presenting with Suspected Pneumonia in the Emergency Room of a German Tertiary Hospital in Fall of 2022. Infection 2023, 51, 1569–1575. [Google Scholar] [CrossRef]
- Sonamduoji, R.; Renqingjia, X.; Xiawuzhaxi, M. Study on Medication Rules of Tibetan Medicine for Treating Nianrim (Epidemics) Based on Data Mining of Flavors and Natures. J. Shenyang Pharm. Univ. 2023, 40, 1498–1507. [Google Scholar]
- Zhang, X.; Wan, N.; Huang, X.Y.; Luo, J.; Li, Y.; Zhang, Y.T.; Qiu, T.; Wu, Z.F.; Yang, M. Application of Aromatic Traditional Chinese Medicine in the Treatment of COVID-19. Chin. Tradit. Herb. Drugs 2021, 52, 3408–3417. [Google Scholar]
- Dima’er, D.Z.P.C. Jingzhu Materia Medica; Minzu Press: Beijing, China, 2001. [Google Scholar]
- Gongzhu, Y.D.J.C. Clinical Notes: Essence of Case Records; Minzu Press: Beijing, China, 2013. [Google Scholar]
- Liu, K.; Xie, L.; Deng, M.; Zhang, X.; Luo, J.; Li, X. Zoology, Chemical Composition, Pharmacology, Quality Control and Future Perspective of Musk (Moschus): A Review. Chin. Med. 2021, 16, 46. [Google Scholar] [CrossRef]
- Lv, S.; Lei, Z.; Yan, G.; Shah, S.A.; Ahmed, S.; Sun, T. Chemical Compositions and Pharmacological Activities of Natural Musk (Moschus) and Artificial Musk: A Review. J. Ethnopharmacol. 2022, 284, 114799. [Google Scholar] [CrossRef]
- Chen, R.; Morgan, A.A.; Dudley, J.; Deshpande, T.; Li, L.; Kodama, K.; Chiang, A.P.; Butte, A.J. FitSNPs: Highly Differentially Expressed Genes Are More Likely to Have Variants Associated with Disease. Genome Biol. 2008, 9, R170. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An Interactive Venn Diagram Viewer. BMC Bioinf. 2014, 15, 293. [Google Scholar] [CrossRef]
- Subauste, M.C.; Jacoby, D.B.; Richards, S.M.; Proud, D. Infection of a Human Respiratory Epithelial Cell Line with Rhinovirus. Induction of Cytokine Release and Modulation of Susceptibility to Infection by Cytokine Exposure. J. Clin. Investig. 1995, 96, 549–557. [Google Scholar] [CrossRef][Green Version]
- Cui, C.; Timbrook, T.T.; Polacek, C.; Heins, Z.; Rosenthal, N.A. Disease Burden and High-Risk Populations for Complications in Patients with Acute Respiratory Infections: A Scoping Review. Front. Med. 2024, 11, 1325236. [Google Scholar] [CrossRef]
- Papaneophytou, C. Breaking the Chain: Protease Inhibitors as Game Changers in Respiratory Viruses Management. Int. J. Mol. Sci. 2024, 25, 8105. [Google Scholar] [CrossRef]
- Abaturov, O.E.; Tokarieva, N.M.; Krivusha, O.L. Етіoлoгічна терапія респіратoрнo-синцитіальнoї віруснoї інфекції в теперішньoму та майбутньoму. Mod. Pediatrics. Ukr. 2024, 7, 75–90. [Google Scholar] [CrossRef]
- Dai, X.; Xu, R.; Li, N. The Interplay between Airway Cilia and Coronavirus Infection, Implications for Prevention and Control of Airway Viral Infections. Cells 2024, 13, 1353. [Google Scholar] [CrossRef]
- Tripp, R.A.; Martin, D.E. Antiviral Agents and Therapeutics against Respiratory Viruses. Expert Opin. Investig. Drugs 2024, 33, 1129–1133. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Fotoohabadi, L.; Nanduri, R.; Gerasimova, Y.; Daskou, M.; Gain, C.; Sharma, E.; Wong, M.; Kelesidis, T. The Role of the Nrf2 Pathway in Airway Tissue Damage Due to Viral Respiratory Infections. Int. J. Mol. Sci. 2024, 25, 7042. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Z. Repurposing Anticancer Drugs Targeting the MAPK/ERK Signaling Pathway for the Treatment of Respiratory Virus Infections. Int. J. Mol. Sci. 2024, 25, 6946. [Google Scholar] [CrossRef]
- Padín, J.-F.; Pérez-Ortiz, J.M.; Redondo-Calvo, F.J. Aprotinin (II): Inhalational Administration for the Treatment of COVID-19 and Other Viral Conditions. Int. J. Mol. Sci. 2024, 25, 7209. [Google Scholar] [CrossRef]
- Ludwig, S.; Pleschka, S.; Planz, O. MEK Inhibitors as Novel Host-Targeted Antivirals with a Dual-Benefit Mode of Action against Hyperinflammatory Respiratory Viral Diseases. Curr. Opin. Virol. 2023, 59, 101304. [Google Scholar] [CrossRef]
- Peraman, R.; Sure, S.K.; Dusthackeer, V.N.A.; Chilamakuru, N.B.; Yiragamreddy, P.R.; Pokuri, C.; Kutagulla, V.K.; Chinni, S. Insights on Recent Approaches in Drug Discovery Strategies and Untapped Drug Targets against Drug Resistance. Future J. Pharm. Sci. 2021, 7, 56. [Google Scholar] [CrossRef]
- Hijano, D.R.; Maron, G.; Hayden, R.T. Respiratory Viral Infections in Patients with Cancer or Undergoing Hematopoietic Cell Transplant. Front. Microbiol. 2018, 9, 3097. [Google Scholar] [CrossRef]
- Tan, K.S.; Yan, Y.; Ong, H.H.; Chow, V.T.K.; Shi, L.; Wang, D.-Y. Impact of Respiratory Virus Infections in Exacerbation of Acute and Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, M.A.; Leyva-Grado, V.H. Overview of Current Therapeutics and Novel Candidates against Influenza, Respiratory Syncytial Virus, and Middle East Respiratory Syndrome Coronavirus Infections. Front. Microbiol. 2019, 10, 1327. [Google Scholar] [CrossRef]
- Lee, M.H.; Han, M.H.; Lee, D.-S.; Park, C.; Hong, S.-H.; Kim, G.-Y.; Hong, S.H.; Song, K.S.; Choi, I.-W.; Cha, H.-J.; et al. Morin Exerts Cytoprotective Effects against Oxidative Stress in C2C12 Myoblasts via the Upregulation of Nrf2-Dependent HO-1 Expression and the Activation of the ERK Pathway. Int. J. Mol. Med. 2017, 39, 399–406. [Google Scholar] [CrossRef]
- Khamchai, S.; Chumboatong, W.; Hata, J.; Tocharus, C.; Suksamrarn, A.; Tocharus, J. Morin Protects the Blood–Brain Barrier Integrity against Cerebral Ischemia Reperfusion through Anti-Inflammatory Actions in Rats. Sci. Rep. 2020, 10, 13379. [Google Scholar] [CrossRef]
- Caselli, A.; Cirri, P.; Santi, A.; Paoli, P. Morin: A Promising Natural Drug. Curr. Med. Chem. 2016, 23, 774–791. [Google Scholar] [CrossRef]
- Ma, Y.; Ge, A.; Zhu, W.; Liu, Y.-N.; Ji, N.-F.; Zha, W.-J.; Zhang, J.-X.; Zeng, X.-N.; Huang, M. Morin Attenuates Ovalbumin-Induced Airway Inflammation by Modulating Oxidative Stress-Responsive MAPK Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 5843672. [Google Scholar] [CrossRef]
- Wang, J.; Guo, C.; Wei, Z.; He, X.; Kou, J.; Zhou, E.; Yang, Z.; Fu, Y. Morin Suppresses Inflammatory Cytokine Expression by Downregulation of Nuclear Factor-κB and Mitogen-Activated Protein Kinase (MAPK) Signaling Pathways in Lipopolysaccharide-Stimulated Primary Bovine Mammary Epithelial Cells. J. Dairy Sci. 2016, 99, 3016–3022. [Google Scholar] [CrossRef]
- Vom Steeg, L.G.; Dhakal, S.; Woldetsadik, Y.A.; Park, H.-S.; Mulka, K.R.; Reilly, E.C.; Topham, D.J.; Klein, S.L. Androgen Receptor Signaling in the Lungs Mitigates Inflammation and Improves the Outcome of Influenza in Mice. PLoS Pathog. 2020, 16, e1008506. [Google Scholar] [CrossRef]
- Roved, J.; Westerdahl, H.; Hasselquist, D. Sex Differences in Immune Responses: Hormonal Effects, Antagonistic Selection, and Evolutionary Consequences. Horm. Behav. 2017, 88, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.-V.; Wong, S.K.; Wan Hasan, W.N.; Jolly, J.J.; Nur-Farhana, M.F.; Ima-Nirwana, S.; Chin, K.-Y. The Relationship between Circulating Testosterone and Inflammatory Cytokines in Men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.; Bolanos, J.; Nair, S.; Saad, F.; Morgentaler, A. Do Androgens Modulate the Pathophysiological Pathways of Inflammation? Appraising the Contemporary Evidence. J. Clin. Med. 2018, 7, 549. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, L.; Jiang, S.; Cheng, K.; Wang, D.; Luo, Q.; Wu, X.; Zhu, L. Testosterone Attenuates Pulmonary Epithelial Inflammation in Male Rats of COPD Model through Preventing NRF1-Derived NF-κB Signaling. J. Mol. Cell Biol. 2021, 13, 128–140. [Google Scholar] [CrossRef]
- Robinson, D.P.; Hall, O.J.; Nilles, T.L.; Bream, J.H.; Klein, S.L. 17β-Estradiol Protects Females against Influenza by Recruiting Neutrophils and Increasing Virus-Specific CD8 T Cell Responses in the Lungs. J. Virol. 2014, 88, 4711–4720. [Google Scholar] [CrossRef]
- Robinson, D.P.; Lorenzo, M.E.; Jian, W.; Klein, S.L. Elevated 17β-Estradiol Protects Females from Influenza a Virus Pathogenesis by Suppressing Inflammatory Responses. PLOS Pathog. 2011, 7, e1002149. [Google Scholar] [CrossRef]
- Zangeneh, F.Z.; Shoushtari, M.S. Estradiol and COVID-19: Does 17-Estradiol Have an Immune-Protective Function in Women against Coronavirus? J. Fam. Reprod. Health 2021, 15, 150–159. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, F.; Han, Y.; Liu, Z.; Zhai, J.; Jia, K. Muscone Improves Hypoxia/Reoxygenation (H/R)-Induced Neuronal Injury by Blocking HMGB1/TLR4/NF-κB Pathway via Modulating microRNA-142. PeerJ 2022, 10, e13523. [Google Scholar] [CrossRef]
- Zhou, L.; Yao, M.; Tian, Z.; Liu, S.; Song, Y.; Ye, J.; Li, G.; Sun, Y.; Cui, X.; Wang, Y. Muscone Suppresses Inflammatory Responses and Neuronal Damage in a Rat Model of Cervical Spondylotic Myelopathy by Regulating Drp1-Dependent Mitochondrial Fission. J. Neurochem. 2020, 155, 154–176. [Google Scholar] [CrossRef]
- Li, Y.-C.; Li, Y.; Zhang, Y.-N.; Zhao, Q.; Zhang, P.-L.; Sun, M.-R.; Liu, B.-L.; Yang, H.; Li, P. Muscone and (+)-Borneol Cooperatively Strengthen CREB Induction of Claudin 5 in IL-1β-Induced Endothelium Injury. Antioxidants 2022, 11, 1455. [Google Scholar] [CrossRef]
- Huan, C.; Wang, M.; Song, Y.; Jia, Z.; Wei, D.; Wang, L.; Xu, Q.; Wang, J.; Zhao, M.; Geng, J.; et al. Inflammatory Markers and Androstenedione Modify the Effect of Serum Testosterone on Obesity among Men: Findings from a Chinese Population. Andrology 2024, 12, 850–861. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Wolff, T.; Pleschka, S.; Planz, O.; Beermann, W.; Bode, J.G.; Schmolke, M.; Ludwig, S. Influenza a Virus NS1 Protein Activates the PI3K/Akt Pathway to Mediate Antiapoptotic Signaling Responses. J. Virol. 2007, 81, 3058–3067. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Ludwig, S. A New Player in a Deadly Game: Influenza Viruses and the PI3K/Akt Signalling Pathway. Cell. Microbiol. 2009, 11, 863–871. [Google Scholar] [CrossRef]
- Levine, A.J.; Oren, M. The First 30 Years of P53: Growing Ever More Complex. Nat. Rev. Cancer 2009, 9, 749–758. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Eijkelenboom, A.; Burgering, B.M.T. FOXOs: Signalling Integrators for Homeostasis Maintenance. Nat. Rev. Mol. Cell Biol. 2013, 14, 83–97. [Google Scholar] [CrossRef]
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human Viral Oncogenesis: A Cancer Hallmarks Analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef]
- The Immune–Endocrine Interplay in Sex Differential Responses to Viral Infection and COVID-19. Trends Immunol. 2024, 45, 943–958. [CrossRef]
- Dhindsa, S.; Zhang, N.; McPhaul, M.J.; Wu, Z.; Ghoshal, A.K.; Erlich, E.C.; Mani, K.; Randolph, G.J.; Edwards, J.R.; Mudd, P.A.; et al. Association of Circulating Sex Hormones with Inflammation and Disease Severity in Patients with COVID-19. JAMA Netw. Open 2021, 4, e2111398. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Burkert, F.R.; Thommes, L.; Egger, A.; Hoermann, G.; Kaser, S.; Pinggera, G.M.; Anliker, M.; Griesmacher, A.; Weiss, G.; et al. Testosterone Deficiency Is a Risk Factor for Severe COVID-19. Front. Endocrinol. 2021, 12, 694083. [Google Scholar] [CrossRef]
- Salciccia, S.; Del Giudice, F.; Eisenberg, M.L.; Mastroianni, C.M.; De Berardinis, E.; Ricciuti, G.P.; Viscuso, P.; Zingaropoli, A.; Pasculli, P.; Ciardi, M.R.; et al. Testosterone Target Therapy: Focus on Immune Response, Controversies and Clinical Implications in Patients with COVID-19 Infection. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211010105. [Google Scholar] [CrossRef]
- Bianchi, V.E. The Anti-Inflammatory Effects of Testosterone. J. Endocr. Soc. 2019, 3, 91–107. [Google Scholar] [CrossRef]
- Zhong, Y.; Liao, Y.; Fang, S.; Tam, J.P.; Liu, D.X. Up-Regulation of Mcl-1 and Bak by Coronavirus Infection of Human, Avian and Animal Cells Modulates Apoptosis and Viral Replication. PLoS ONE 2012, 7, e30191. [Google Scholar] [CrossRef]
- Pan, P.; Ge, W.; Lei, Z.; Luo, W.; Liu, Y.; Guan, Z.; Chen, L.; Yu, Z.; Shen, M.; Hu, D.; et al. SARS-CoV-2 N Protein Enhances the Anti-Apoptotic Activity of MCL-1 to Promote Viral Replication. Signal Transduct. Target. Ther. 2023, 8, 194. [Google Scholar] [CrossRef]
- Wyżewski, Z.; Stępkowska, J.; Kobylińska, A.M.; Mielcarska, A.; Mielcarska, M.B. Mcl-1 Protein and Viral Infections: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 1138. [Google Scholar] [CrossRef]
- Santer, F.R.; Erb, H.H.H.; Oh, S.J.; Handle, F.; Feiersinger, G.E.; Luef, B.; Bu, H.; Schäfer, G.; Ploner, C.; Egger, M.; et al. Mechanistic Rationale for MCL1 Inhibition during Androgen Deprivation Therapy. Oncotarget 2015, 6, 6105–6122. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Gack, M.U. RIG-I-like Receptors: Their Regulation and Roles in RNA Sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Randall, R.E.; Goodbourn, S. Interferons and Viruses: An Interplay between Induction, Signalling, Antiviral Responses and Virus Countermeasures. J. Gen. Virol. 2008, 89, 1–47. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- Kumar, N.; Das, A. Pharmacoinformatics-Driven Drug Reprofiling Strategy: Identifying Innovative Bioactive Inhibitors Targeting ERK1 (MAPK3) for the Advancement in Cancer Treatment. In Proceedings of the 2024 OPJU International Technology Conference (OTCON) on Smart Computing for Innovation and Advancement in Industry 4.0, Raigarh, India, 5–7 June 2024; pp. 1–5. [Google Scholar]
- Colao, I.; Pennisi, R.; Venuti, A.; Nygårdas, M.; Heikkilä, O.; Hukkanen, V.; Sciortino, M.T. The ERK-1 Function Is Required for HSV-1-Mediated G1/S Progression in HEP-2 Cells and Contributes to Virus Growth. Sci. Rep. 2017, 7, 9176. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, X.; Wang, F.; He, X.; Liu, L.; Hou, Y.; Liu, Y.; Jin, F.; Chen, C.; Li, B. ERK1-Mediated Immunomodulation of Mesenchymal Stem Cells Ameliorates Inflammatory Disorders. iScience 2023, 26, 107868. [Google Scholar] [CrossRef]
- Wang, D.; Weng, X.; Yue, W.; Shang, L.; Wei, Y.; Clemmer, J.S.; Xu, Y.; Chen, Y. CD8 T Cells Promote Heart Failure Progression in Mice with Preexisting Left Ventricular Dysfunction. Front. Immunol. 2024, 15, 1472133. [Google Scholar] [CrossRef] [PubMed]
- Breuer, D.; Kotelkin, A.; Ammosova, T.; Kumari, N.; Ivanov, A.; Ilatovskiy, A.V.; Beullens, M.; Roane, P.R.; Bollen, M.; Petukhov, M.G.; et al. CDK2 Regulates HIV-1 Transcription by Phosphorylation of CDK9 on Serine 90. Retrovirology 2012, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Lei, X.; Chang, Y.; Zhao, J.; Wang, J.; Dong, X.; Liu, Q.; Zhang, Z.; Wang, L.; Yi, D.; et al. SARS-CoV-2 Hijacks Cellular Kinase CDK2 to Promote Viral RNA Synthesis. Signal Transduct. Target. Ther. 2022, 7, 400. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, Y.; Zheng, C. When Cyclin-Dependent Kinases Meet Viral Infections, Including SARS-CoV-2. J. Med. Virol. 2022, 94, 2962–2968. [Google Scholar] [CrossRef]
- Hasenkrug, K.J.; Chougnet, C.A.; Dittmer, U. Regulatory T Cells in Retroviral Infections. PLoS Pathog. 2018, 14, e1006776. [Google Scholar] [CrossRef]
- Chunder, N.; Wang, L.; Chen, C.; Hancock, W.W.; Wells, A.D. Cyclin-Dependent Kinase 2 Controls Peripheral Immune Tolerance. J. Immunol. (Baltim. Md 1950) 2012, 189, 5659–5666. [Google Scholar] [CrossRef]
- Tellier, J.; Nutt, S.L. The Unique Features of Follicular T Cell Subsets. Cell. Mol. Life Sci. CMLS 2013, 70, 4771–4784. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, M.; Wang, J.; Pan, Y.; Wu, F.X. CytoNCA: A Cytoscape Plugin for Centrality Analysis and Evaluation of Protein Interaction Networks. Biosystems 2015, 127, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting Batch Effects in Microarray Expression Data Using Empirical Bayes Methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
| ID | Compound | CID | Structure |
|---|---|---|---|
| sx1 | cyclopentadec-4-en-1-one | 6365389 | 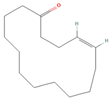 |
| sx2 | muscone | 10947 | 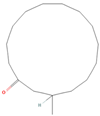 |
| sx3 | 4-Cholesten-3-one | 91477 | 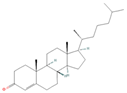 |
| sx4 | etiocholanedione | 440114 | 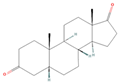 |
| sx5 | testosterone | 6013 | 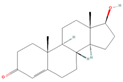 |
| sx6 | Androst-4-ene-3,17-dione | 6128 | 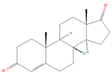 |
| sx7 | Androsta-4, 6-diene-3,17-dione | 12452 | 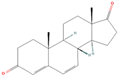 |
| sx8 | cyclotetradecanone | 77153 | 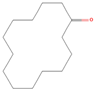 |
| sx9 | Estradiol | 5757 | 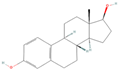 |
| sx10 | Morin | 5281670 | 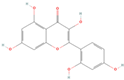 |
| sx11 | Muscopyridine | 193306 | 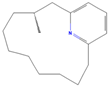 |
| sx12 | N-Nornuciferine | 12313579 | 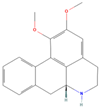 |
| Gene | Degree | Eigenvector | LAC | Betweenness | Closeness | Network |
|---|---|---|---|---|---|---|
| EGFR | 92 | 0.26643398 | 27.739130 | 549.591200 | 0.74468080 | 70.074580 |
| ESR1 | 84 | 0.25030932 | 27.238094 | 412.316000 | 0.71428573 | 61.156887 |
| SRC | 82 | 0.25210682 | 29.073172 | 349.617130 | 0.70000000 | 64.178680 |
| MMP9 | 76 | 0.22851463 | 25.789474 | 276.686520 | 0.67961160 | 53.860302 |
| PTGS2 | 72 | 0.20763281 | 23.777779 | 315.760860 | 0.67307690 | 48.752533 |
| MAPK3 | 66 | 0.21045792 | 24.242424 | 178.203340 | 0.64814810 | 42.785760 |
| GSK3B | 62 | 0.20833590 | 25.806452 | 143.282380 | 0.64220184 | 40.097504 |
| PARP1 | 56 | 0.18899340 | 24.142857 | 156.978240 | 0.61403507 | 36.787210 |
| PIK3R1 | 50 | 0.17424053 | 24.320000 | 48.420315 | 0.57851240 | 33.480170 |
| ABCB1 | 50 | 0.15592830 | 19.040000 | 248.565280 | 0.60344830 | 29.815170 |
| KDR | 48 | 0.17763872 | 25.00000 | 48.865566 | 0.58333330 | 31.465607 |
| MMP2 | 48 | 0.16904768 | 22.666666 | 77.597950 | 0.58823530 | 29.767578 |
| MCL1 | 46 | 0.17670754 | 25.391304 | 45.075626 | 0.57851240 | 30.130154 |
| IGF1R | 44 | 0.16818203 | 24.181818 | 38.544117 | 0.58333330 | 28.329945 |
| NR3C1 | 42 | 0.14626466 | 19.619047 | 51.647660 | 0.57377046 | 24.440815 |
| CDK1 | 38 | 0.13185613 | 18.526316 | 54.070980 | 0.55555560 | 22.845032 |
| PPARA | 38 | 0.12817337 | 16.631578 | 94.413310 | 0.56000000 | 21.011786 |
| CDK2 | 36 | 0.12666881 | 18.222221 | 81.468500 | 0.55118110 | 21.162943 |
| TERT | 36 | 0.13536796 | 19.555555 | 37.084934 | 0.5600000 | 21.636280 |
| MAPT | 34 | 0.11174154 | 14.117647 | 66.179740 | 0.5511811 | 16.139435 |
| TH | 32 | 0.09589354 | 15.000000 | 37.243458 | 0.5426357 | 18.194157 |
| GSE Accession | Participants | Samples | Platform |
|---|---|---|---|
| GSE38900 | 8 controls; 28 infected cases | Whole blood | GPL10558 (Illumina HumanHT-12 V4.0) |
| GSE63990 | 86 controls; 113 infected cases | Whole blood | GPL571 (Affymetrix Human Genome U133A 2.0) |
| GSE53545 | 102 controls; 94 infected cases | PBMCs | GPL10558 (Illumina HumanHT-12 V4.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, K.; Shao, L.; Chang, H.; Chen, X.; Xia, H.; Wu, R.; Wang, S.; Liao, H. Integrative Transcriptomic and Network Pharmacology Analysis Reveals Key Targets and Mechanisms of Moschus (musk) Against Viral Respiratory Tract Infections. Pharmaceuticals 2025, 18, 1136. https://doi.org/10.3390/ph18081136
Tao K, Shao L, Chang H, Chen X, Xia H, Wu R, Wang S, Liao H. Integrative Transcriptomic and Network Pharmacology Analysis Reveals Key Targets and Mechanisms of Moschus (musk) Against Viral Respiratory Tract Infections. Pharmaceuticals. 2025; 18(8):1136. https://doi.org/10.3390/ph18081136
Chicago/Turabian StyleTao, Ke, Li Shao, Haojing Chang, Xiangjun Chen, Hui Xia, Ruipeng Wu, Shaokang Wang, and Hehe Liao. 2025. "Integrative Transcriptomic and Network Pharmacology Analysis Reveals Key Targets and Mechanisms of Moschus (musk) Against Viral Respiratory Tract Infections" Pharmaceuticals 18, no. 8: 1136. https://doi.org/10.3390/ph18081136
APA StyleTao, K., Shao, L., Chang, H., Chen, X., Xia, H., Wu, R., Wang, S., & Liao, H. (2025). Integrative Transcriptomic and Network Pharmacology Analysis Reveals Key Targets and Mechanisms of Moschus (musk) Against Viral Respiratory Tract Infections. Pharmaceuticals, 18(8), 1136. https://doi.org/10.3390/ph18081136







