Real-World Safety of Vedolizumab in Inflammatory Bowel Disease: A Retrospective Cohort Study Supported by FAERS Signal Analysis
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| CD | Crohn’s disease |
| CI | Confidence interval |
| EIM | Extraintestinal manifestation |
| FAERS | FDA Adverse Event Reporting System |
| HLGT | High level group term |
| HLT | High level term |
| IBD | Inflammatory bowel disease |
| ICH | International Council for Harmonization |
| IQR | Interquartile ratio |
| LLT | Lowest level term |
| LTS | Long-term safety |
| MAdCAM-1 | Mucosal vascular addressin cell adhesion molecule 1 |
| MedDRA | Medical Dictionary for Regulatory Activities |
| MSSO | Maintenance and support services organization |
| PML | Progressive multifocal leukoencephalopathy |
| PRR | Proportional reporting ratio |
| PT | Preferred term |
| PYs | Patient-years |
| ROR | Reporting odds ratio |
| RTI | Respiratory tract infections |
| SDR | Signal of disproportionate reporting |
| SOC | System organ class |
| TNF-α | Tumor necrosis factor-alpha |
| UC | Ulcerative colitis |
| URTI | Upper respiratory tract infections |
| USPI | U.S. Prescribing Information |
| VCAM-1 | Vascular cell adhesion protein 1 |
References
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of inflammatory bowel disease: A comprehensive review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Tang, H.J.; Bie, C.Q.; Guo, L.L.; Zhong, L.X.; Tang, S.H. Efficacy and safety of vedolizumab in the treatment of patients with inflammatory bowel disease: A systematic review and meta-analysis of randomized controlled trials. Exp. Ther. Med. 2023, 25, 298. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Arkkila, P.; Armuzzi, A.; Danese, S.; Guardiola, J.; Jahnsen, J.; Lees, C.; Louis, E.; Lukáš, M.; Reinisch, W.; et al. Comparative efficacy and safety of infliximab and vedolizumab therapy in patients with inflammatory bowel disease: A systematic review and meta-analysis. BMC Gastroenterol. 2022, 22, 291. [Google Scholar] [CrossRef] [PubMed]
- Soler, D.; Chapman, T.; Yang, L.-L.; Wyant, T.; Egan, R.; Fedyk, E.R. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J. Pharmacol. Exp. Ther. 2009, 330, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Wyant, T.; Fedyk, E.; Abhyankar, B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J. Crohns Colitis 2016, 10, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Hagan, M.; Cross, R.K. Safety of vedolizumab in the treatment of Crohn’s disease and ulcerative colitis. Expert. Opin. Drug Saf. 2015, 14, 1473–1479. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef]
- Sands, B.E.; Feagan, B.G.; Rutgeerts, P.; Colombel, J.F.; Sandborn, W.J.; Sy, R.; D’Haens, G.; Ben-Horin, S.; Xu, J.; Rosario, M.; et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014, 147, 618–627.e3. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef]
- Entyvio FDA Approval History. Available online: https://www.drugs.com/history/entyvio.html (accessed on 14 May 2025).
- Loftus, E.V.; Feagan, B.G.; Panaccione, R.; Colombel, J.F.; Sandborn, W.J.; Sands, B.E.; Danese, S.; D’Haens, G.; Rubin, D.T.; Shafran, I.; et al. Long-term safety of vedolizumab for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 52, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Panaccione, R.; Loftus, E.V.; Sankoh, S.; Fox, I.; et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.D.; Bhayat, F.; Blake, A.; Travis, S. The safety profile of vedolizumab in ulcerative colitis and crohn’s disease: 4 years of global post-marketing data. J. Crohns Colitis 2020, 14, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.S.; Ventimiglia, M.; Orlando, A. Effectiveness and Safety of Vedolizumab in Inflammatory Bowel Disease: A Comprehensive Meta-analysis of Observational Studies. J. Crohns Colitis 2023, 17, 1217–1227. [Google Scholar] [CrossRef]
- Feagan, B.G.; Bhayat, F.; Khalid, M.; Blake, A.; Travis, S.P.L. Respiratory tract infections in patients with inflammatory bowel disease: Safety analyses from vedolizumab clinical trials. J. Crohns Colitis 2018, 12, 905–919. [Google Scholar] [CrossRef]
- Marafini, I.; Troncone, E.; Rocchetti, I.; Monteleone, G. Respiratory tract infections in inflammatory bowel disease patients taking vedolizumab: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2020, 11, 585732. [Google Scholar] [CrossRef]
- Takeda Pharmaceuticals U.S.A. Inc. ENTYVIO (Vedolizumab) Injection, for Intravenous Use; U.S. Prescribing Information: Lexington, MA, USA, 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761359s000lbl.pdf (accessed on 14 May 2025).
- Alameddine, Z.; Abi Melhem, R.; Dimachkie, R.; Rabah, H.; Chehab, H.; El Khoury, M.; Qaqish, F.; Stefanov, D.; El-Sayegh, S. Risk of nephrolithiasis in patients with inflammatory bowel disease receiving biologic treatment. J. Clin. Med. 2023, 12, 6114. [Google Scholar] [CrossRef]
- Ganji-Arjenaki, M.; Nasri, H.; Rafieian-Kopaei, M. Nephrolithiasis as a common urinary system manifestation of inflammatory bowel diseases; a clinical review and meta-analysis. J. Nephropathol. 2017, 6, 264–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Yuan, L. Investigating the causal relationship between inflammatory bowel disease and simple appendicitis using Mendelian randomization. Sci. Rep. 2024, 14, 23617. [Google Scholar] [CrossRef]
- Bye, W.A.; Jairath, V.; Travis, S.P.L. Systematic review: The safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 46, 3–15. [Google Scholar] [CrossRef]
- Schreiber, S.; Dignass, A.; Peyrin-Biroulet, L.; Hather, G.; Demuth, D.; Mosli, M.; Curtis, R.; Khalid, J.M.; Loftus, E.V. Systematic review with meta-analysis: Real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J. Gastroenterol. 2018, 53, 1048–1064. [Google Scholar] [CrossRef] [PubMed]
- Card, T.; Ungaro, R.; Bhayat, F.; Blake, A.; Hantsbarger, G.; Travis, S. Vedolizumab use is not associated with increased malignancy incidence: GEMINI LTS study results and post-marketing data. Aliment. Pharmacol. Ther. 2020, 51, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Vedamurthy, A.; Gangasani, N.; Ananthakrishnan, A.N. Vedolizumab or tumor necrosis factor antagonist use and risk of new or recurrent cancer in patients with inflammatory bowel disease with prior malignancy: A retrospective cohort study. Clin. Gastroenterol. Hepatol. 2022, 20, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lightner, A.L.; McKenna, N.P.; Tse, C.S.; Raffals, L.E.; Loftus, E.V.; Mathis, K.L. Postoperative outcomes in vedolizumab-treated Crohn’s disease patients undergoing major abdominal operations. Aliment. Pharmacol. Ther. 2018, 47, 573–580. [Google Scholar] [CrossRef]
- Poylin, V.Y.; Serrato, J.C.; Pastrana Del Valle, J.; Feuerstein, J.D. Vedolizumab does not increase perioperative surgical complications in patients with inflammatory bowel disease, cohort study. Intest. Res. 2022, 20, 72–77. [Google Scholar] [CrossRef]
- Marković, S.; Kralj, Đ.; Svorcan, P.; Knežević Ivanovski, T.; Odanović, O.; Obradović, S.; Homšek, A.; Jovanović, M.; Savić, R.; Vučićević, K.M. Vedolizumab Clearance as a Surrogate Marker for Remission in Inflammatory Bowel Disease Patients: Insights from Real-World Pharmacokinetics. Pharmaceutics 2024, 16, 1629. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for Industry and Investigators, Safety Reporting Requirements for INDs and BA/BE Studies; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2012.
- Yajnik, V.; Khan, N.; Dubinsky, M.; Axler, J.; James, A.; Abhyankar, B.; Lasch, K. Efficacy and safety of vedolizumab in ulcerative colitis and Crohn’s disease patients stratified by age. Adv. Ther. 2017, 34, 542–559. [Google Scholar] [CrossRef]
- Medical Dictionary for Regulatory Activities. Available online: https://www.ich.org/page/meddra (accessed on 27 December 2024).
- FDA’s Adverse Event Reporting System (FAERS). 2024. Available online: https://www.fda.gov/drugs/surveillance/fdas-adverse-event-reporting-system-faers (accessed on 24 December 2024).
- OpenVigil 2.1, OpenVigil—A Pharmacovigilance Data Analysis Tool. 2025. Available online: https://openvigil.sourceforge.net (accessed on 14 May 2025).
- van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.; Lindquist, M.; Orre, R.; Egberts, A.C. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef]
- Cutroneo, P.M.; Sartori, D.; Tuccori, M.; Crisafulli, S.; Battini, V.; Carnovale, C.; Rafaniello, C.; Capuano, A.; Poluzzi, E.; Moretti, U. Conducting and interpreting disproportionality analyses derived from spontaneous reporting systems. Front. Drug Saf. Regul. 2024, 3, 1323057. [Google Scholar] [CrossRef]
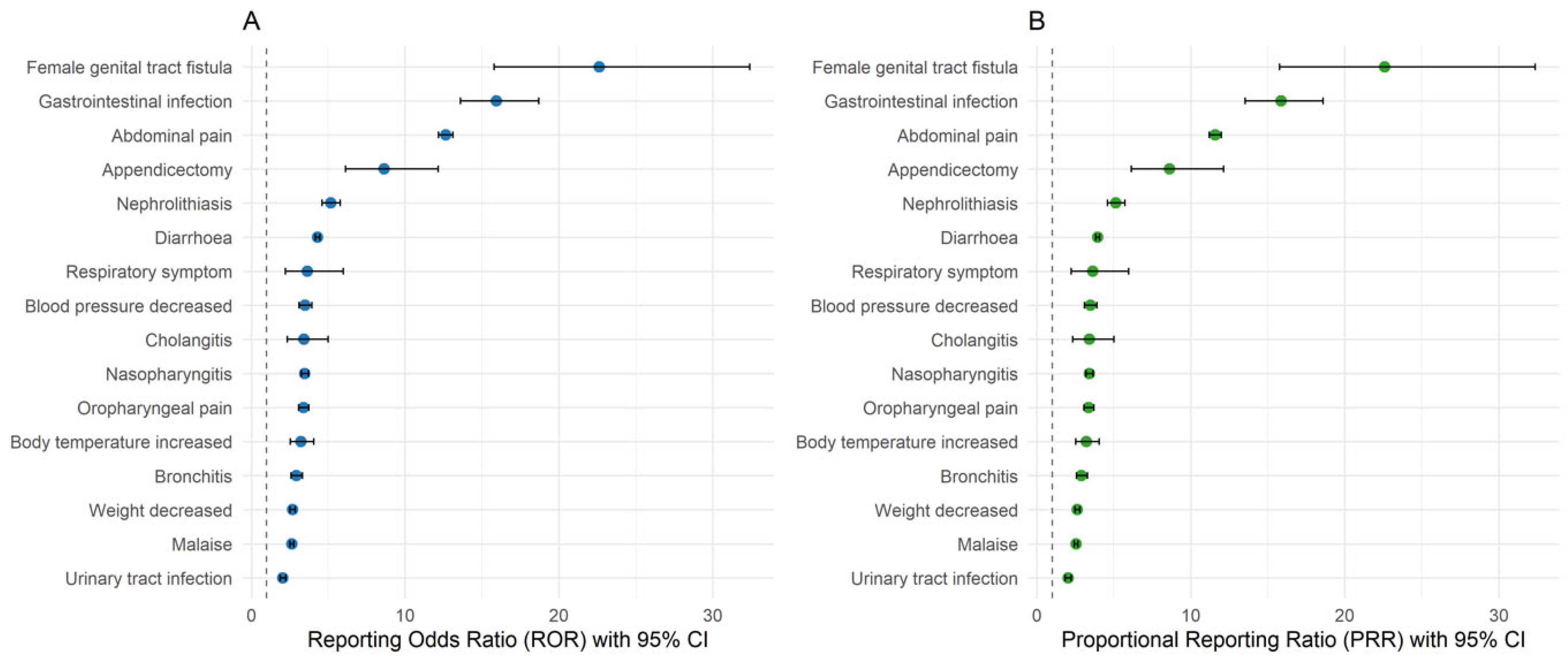
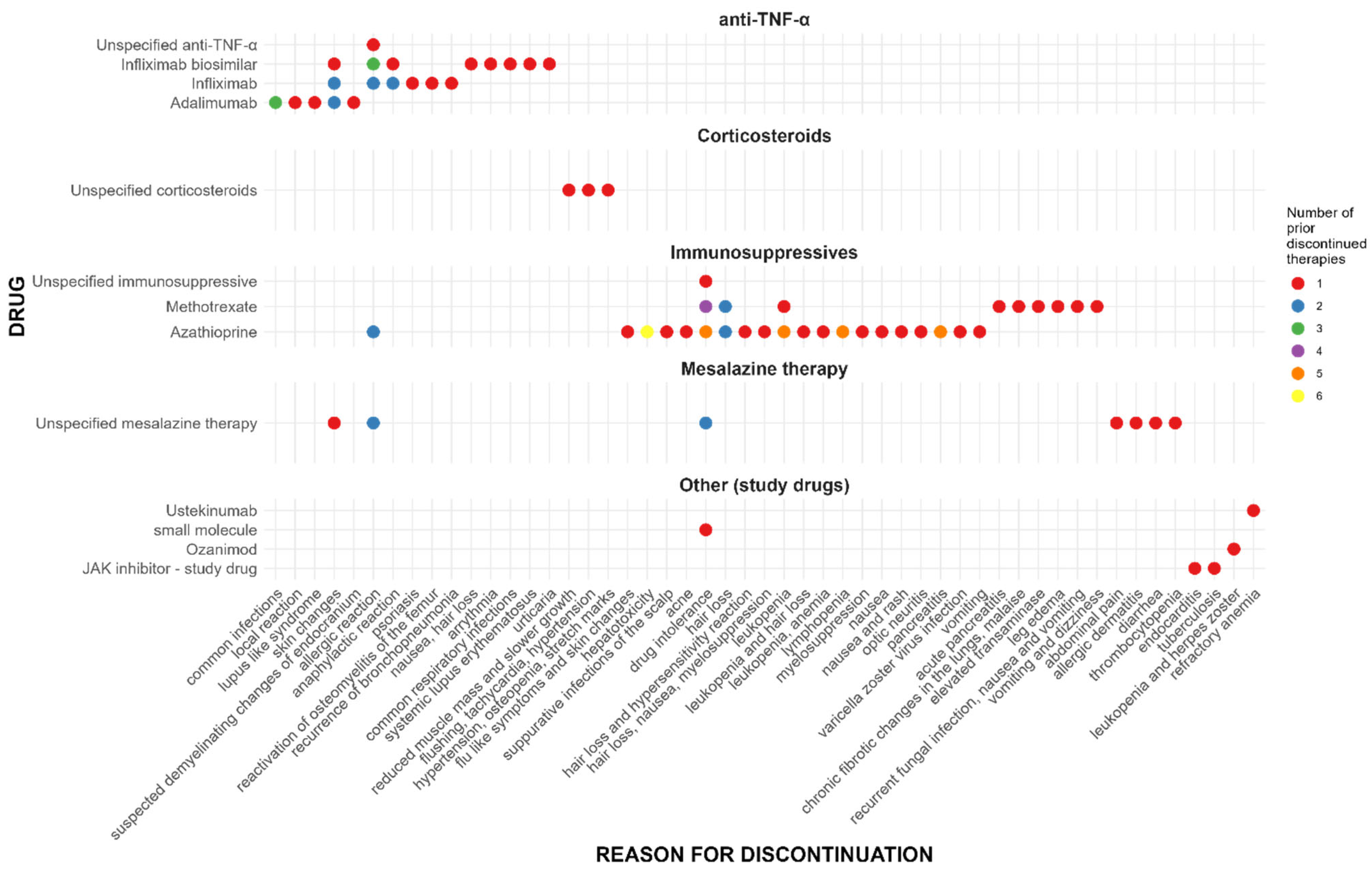
| Variable | Value |
|---|---|
| Age at time of vedolizumab initiation, median (IQR) | 48 (33.5–66) |
| ≥65 years of age, n (%) | 29 (27.1) |
| Male gender, n (%) | 54 (50.5) |
| CD, n (%) | 45 (42.1) |
| UC, n (%) | 62 (57.9) |
| EIM present at baseline, n (%) | 42 (39.2) |
| Age at time of IBD diagnosis, median (IQR) | 38 (22–53) |
| Years from diagnosis to vedolizumab initiation, median (IQR) | 9 (4–17) |
| Vedolizumab exposure (days), median (IQR) | 605 (209–860) |
| Prior therapy | |
| Previous immunosuppressive therapy, n (%) | 87 (81.3) |
| Previous exposure to anti-TNF-α agents, n (%) | 46 (43.0) |
| Previous corticosteroid therapy, n (%) | 81 (75.7) |
| Concomitant therapy at baseline | |
| Concomitant corticosteroids, n (%) | 22 (20.6) |
| Concomitant immunosuppressive therapy, n (%) | 26 (24.3) |
| Concomitant corticosteroids and immunosuppressive therapy, n (%) | 5 (4.7) |
| Number of comorbidities per patient, median (IQR) | 1 (0–2) |
| Age at time of vedolizumab initiation, median (IQR) | 48 (33.5–66) |
| MedDRA SOC/MedDRA PT | Count of MedDRA PT | Incidence/100 PYs |
|---|---|---|
| Renal and urinary disorders | 4 | 2.46 |
| Nephrolithiasis | 4 | 2.46 |
| Blood and lymphatic system disorders | 1 | 0.61 |
| Thrombocytopenia | 1 | 0.61 |
| Cardiac disorders | 1 | 0.61 |
| Cardiac failure | 1 | 0.61 |
| Gastrointestinal disorders | 7 | 4.30 |
| Abdominal pain | 1 | 0.61 |
| Coeliac artery stenosis | 1 | 0.61 |
| Diarrhea | 1 | 0.61 |
| Nausea | 2 | 1.23 |
| Stomatitis | 1 | 0.61 |
| Vomiting | 1 | 0.61 |
| General disorders and administration site conditions | 5 | 3.07 |
| Malaise | 1 | 0.61 |
| Oedema peripheral | 2 | 1.23 |
| Asthenia | 1 | 0.61 |
| Chest pain | 1 | 0.61 |
| Hepatobiliary disorders | 2 | 1.23 |
| Cholangitis | 1 | 0.61 |
| Hepatic cytolysis | 1 | 0.61 |
| Immune system disorders | 2 | 1.23 |
| Drug hypersensitivity | 1 | 0.61 |
| Hypersensitivity | 1 | 0.61 |
| Infections and infestations | 39 | 23.95 |
| Bronchitis | 1 | 0.61 |
| Conjunctivitis | 1 | 0.61 |
| COVID-19 | 23 | 14.13 |
| COVID-19 pneumonia | 4 | 2.46 |
| Gastrointestinal infection | 2 | 1.23 |
| Herpes zoster | 1 | 0.61 |
| Nasopharyngitis | 3 | 1.84 |
| Sialadenitis | 1 | 0.61 |
| Upper respiratory tract infection | 1 | 0.61 |
| Urinary tract infection | 2 | 1.23 |
| Injury, poisoning, and procedural complications | 3 | 1.84 |
| Exposure during pregnancy | 1 | 0.61 |
| Fall | 1 | 0.61 |
| Joint injury | 1 | 0.61 |
| Investigations | 6 | 3.69 |
| Blood alkaline phosphatase increased | 1 | 0.61 |
| Blood creatinine increased | 1 | 0.61 |
| Blood pressure decreased | 1 | 0.61 |
| Body temperature increased | 2 | 1.23 |
| Weight decreased | 1 | 0.61 |
| Metabolism and nutrition disorders | 2 | 1.23 |
| Hyperlipidemia | 1 | 0.61 |
| Hyperproteinemia | 1 | 0.61 |
| Neoplasms benign, malignant, and unspecified (incl cysts and polyps) | 1 | 0.61 |
| Lipoma | 1 | 0.61 |
| Nervous system disorders | 4 | 2.46 |
| Cerebral small vessel ischemic disease | 1 | 0.61 |
| Paresthesia | 1 | 0.61 |
| Sciatica | 1 | 0.61 |
| Tremor | 1 | 0.61 |
| Reproductive system and breast disorders | 2 | 1.23 |
| Female genital tract fistula | 1 | 0.61 |
| Hematospermia | 1 | 0.61 |
| Respiratory, thoracic, and mediastinal disorders | 4 | 2.46 |
| Oropharyngeal pain | 1 | 0.61 |
| Pleural effusion | 1 | 0.61 |
| Respiratory failure | 1 | 0.61 |
| Respiratory symptom | 1 | 0.61 |
| Skin and subcutaneous tissue disorders | 7 | 4.30 |
| Alopecia | 1 | 0.61 |
| Eczema | 1 | 0.61 |
| Erythema | 2 | 1.23 |
| Hair growth abnormal | 1 | 0.61 |
| Pruritus | 1 | 0.61 |
| Skin plaque | 1 | 0.61 |
| Surgical and medical procedures | 1 | 0.61 |
| Appendicectomy | 1 | 0.61 |
| Vascular disorders | 1 | 0.61 |
| Thrombosis | 1 | 0.61 |
| Total | 92 | 56.51 |
| Study Data | FAERS Data | ||||||
|---|---|---|---|---|---|---|---|
| MedDRA PT | n | Incidence/100 PYs | N | ROR (95% CI) | PRR (95% CI) | Chi Square | Listedness per USPI |
| Anemia | 6 | 3.68 | 382 | 1.465 (1.326, 1.620) * | 1.468 (1.328, 1.623) | 56.606 | Unlisted |
| Iridocyclitis | 1 | 0.61 | 6 | 1.063 (0.477, 2.370) | 1.063 (0.477, 2.37) | 0.004 | Unlisted |
| Arthralgia ** | 21 | 12.90 | 1587 | 2.776 (2.646, 2.914) * | 2.793 (2.662, 2.931) * | 1838.365 | Listed |
| Total | 28 | 17.20 | |||||
| Measure of Disproportionality | Formula for Calculation | Statistical Threshold for SDR |
|---|---|---|
| Reporting Odds Ratio (ROR) | lower bound 95% CI > 1; number of reports ≥ 3 | |
| Proportional Reporting Ratio (PRR) | PRR ≥ 2; Chi-square ≥ 4; number of reports ≥ 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milašinović, B.; Vezmar Kovačević, S.; Marković, S.; Jovanović, M.; Knežević Ivanovski, T.; Kralj, Đ.; Svorcan, P.; Miljković, B.; Vučićević, K. Real-World Safety of Vedolizumab in Inflammatory Bowel Disease: A Retrospective Cohort Study Supported by FAERS Signal Analysis. Pharmaceuticals 2025, 18, 1127. https://doi.org/10.3390/ph18081127
Milašinović B, Vezmar Kovačević S, Marković S, Jovanović M, Knežević Ivanovski T, Kralj Đ, Svorcan P, Miljković B, Vučićević K. Real-World Safety of Vedolizumab in Inflammatory Bowel Disease: A Retrospective Cohort Study Supported by FAERS Signal Analysis. Pharmaceuticals. 2025; 18(8):1127. https://doi.org/10.3390/ph18081127
Chicago/Turabian StyleMilašinović, Bojana, Sandra Vezmar Kovačević, Srđan Marković, Marija Jovanović, Tamara Knežević Ivanovski, Đorđe Kralj, Petar Svorcan, Branislava Miljković, and Katarina Vučićević. 2025. "Real-World Safety of Vedolizumab in Inflammatory Bowel Disease: A Retrospective Cohort Study Supported by FAERS Signal Analysis" Pharmaceuticals 18, no. 8: 1127. https://doi.org/10.3390/ph18081127
APA StyleMilašinović, B., Vezmar Kovačević, S., Marković, S., Jovanović, M., Knežević Ivanovski, T., Kralj, Đ., Svorcan, P., Miljković, B., & Vučićević, K. (2025). Real-World Safety of Vedolizumab in Inflammatory Bowel Disease: A Retrospective Cohort Study Supported by FAERS Signal Analysis. Pharmaceuticals, 18(8), 1127. https://doi.org/10.3390/ph18081127








