SGLT2 Inhibitors in MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease) Associated with Sustained Hepatic Benefits, Besides the Cardiometabolic
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Demographics and Comorbidities (Table 1)
2.3. Laboratory Parameters (Table 1)
2.4. Clinical Outcomes
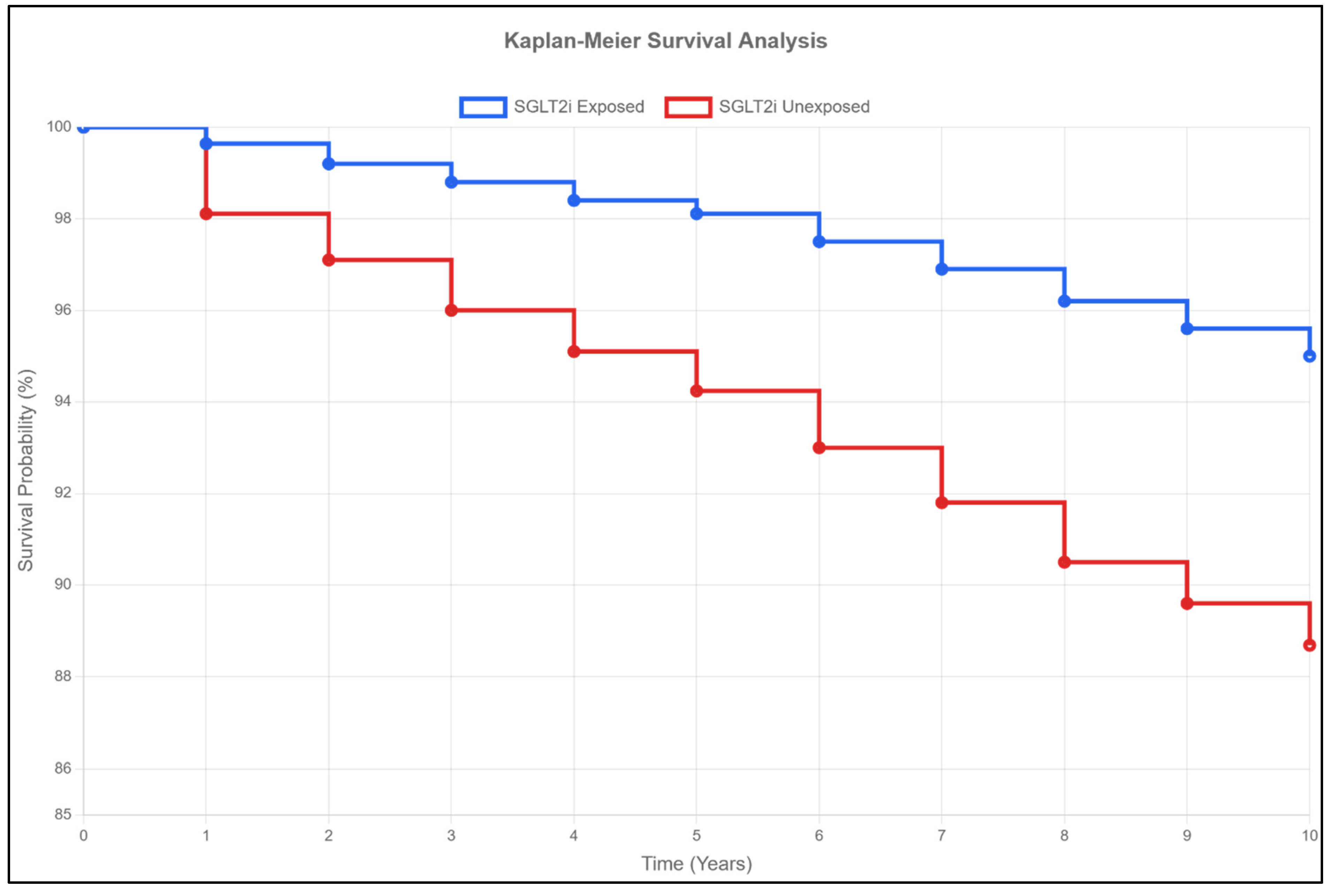
2.5. Mortality and Survival (Table 2, Figure 1)
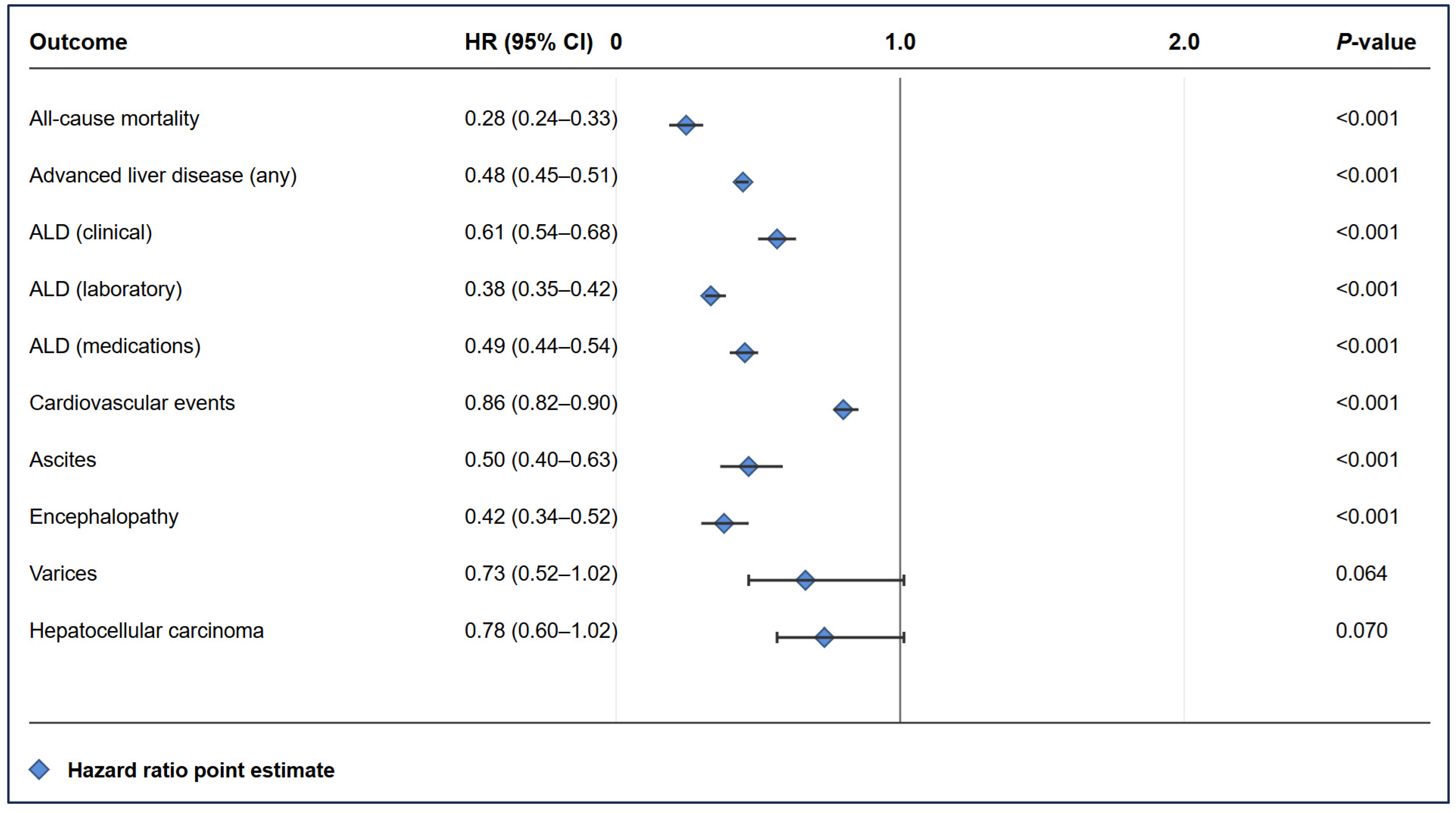
2.6. Cardiovascular Events (Table 2)
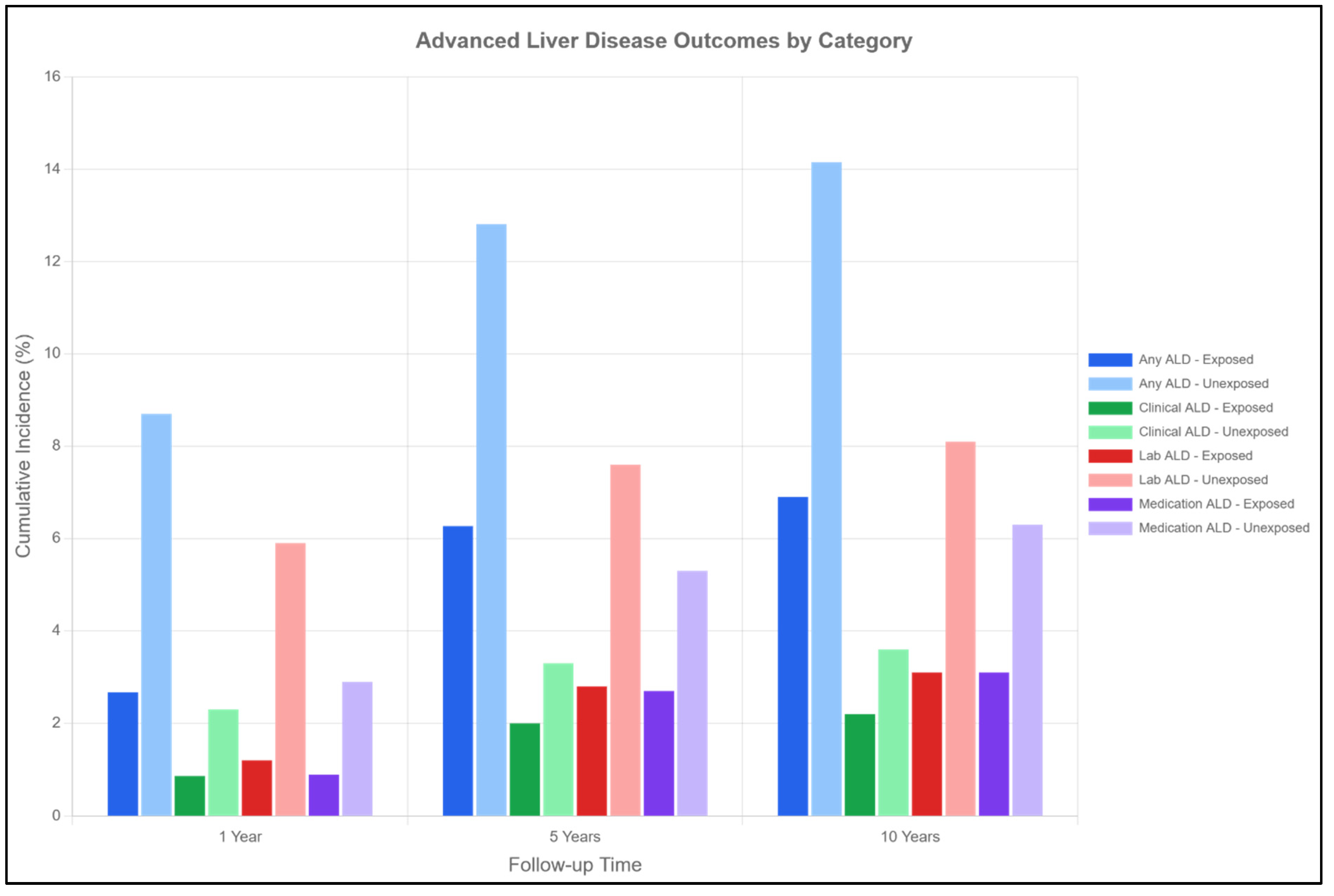
2.7. Advanced Liver Disease (Table 2, Figure 3)
- Clinical ALD: 2.20% vs. 3.60% at 10 years (HR 0.61, 95% CI 0.54–0.68).
- Laboratory-defined ALD: 3.10% vs. 8.10% at 10 years (HR 0.38, 95% CI 0.35–0.42).
- ALD requiring medications: 3.10% vs. 6.30% at 10 years (HR 0.49, 95% CI 0.44–0.54).
2.8. Specific Liver Complications (Table 2)
- Ascites: 0.39% vs. 0.78% at 10 years (HR 0.50, 95% CI 0.40–0.63, p < 0.001).
- Encephalopathy: 0.45% vs. 1.15% at 10 years (HR 0.42, 95% CI 0.34–0.52, p < 0.001).
- Varices: 0.25% vs. 0.35% at 10 years (HR 0.73, 95% CI 0.52–1.02, p = 0.064).
2.9. Metabolic Profile (Table 2)
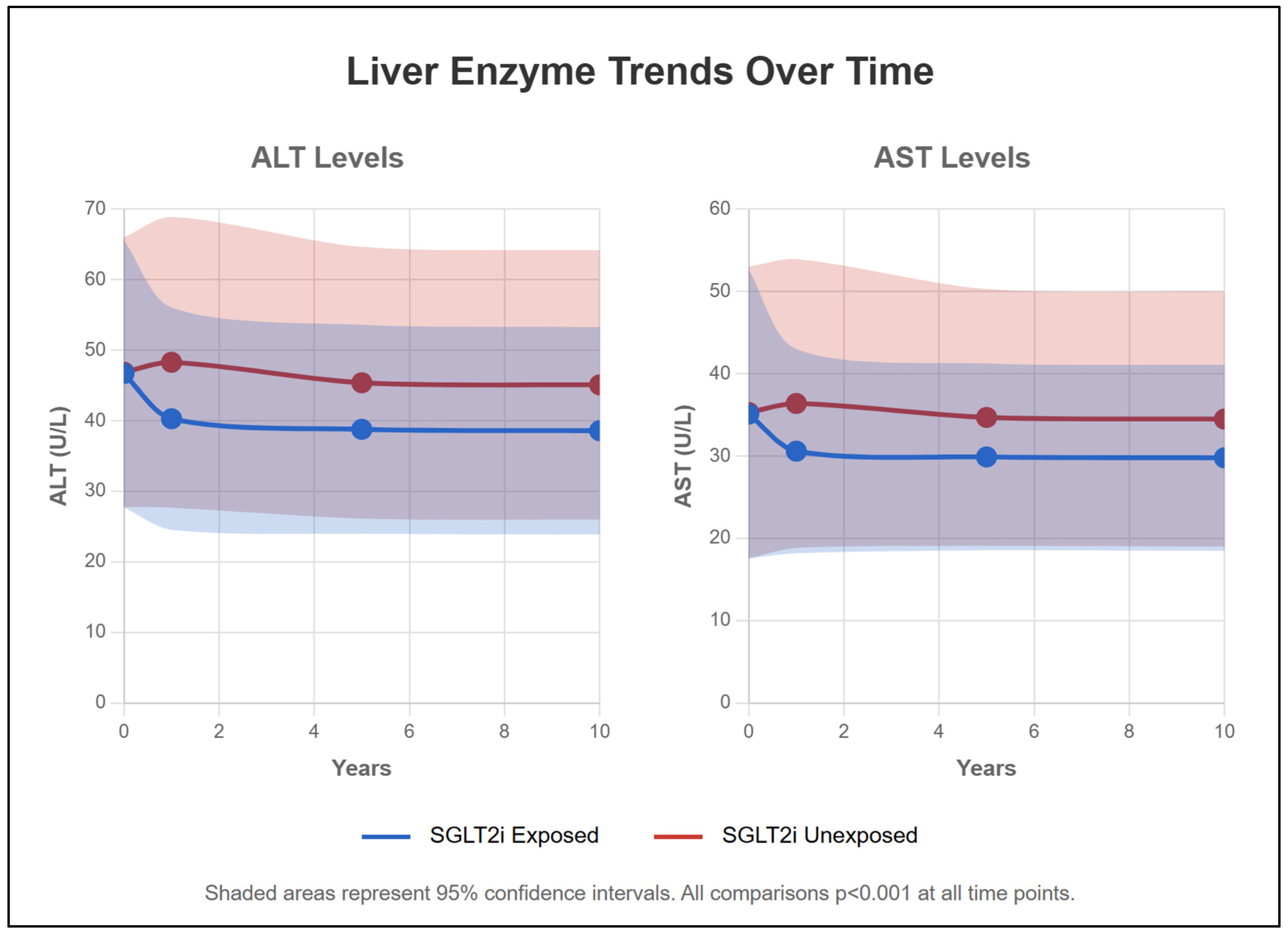
2.10. Liver Function Parameters (Table 2, Figure 4)
3. Discussion
4. Materials and Methods
4.1. Data Source
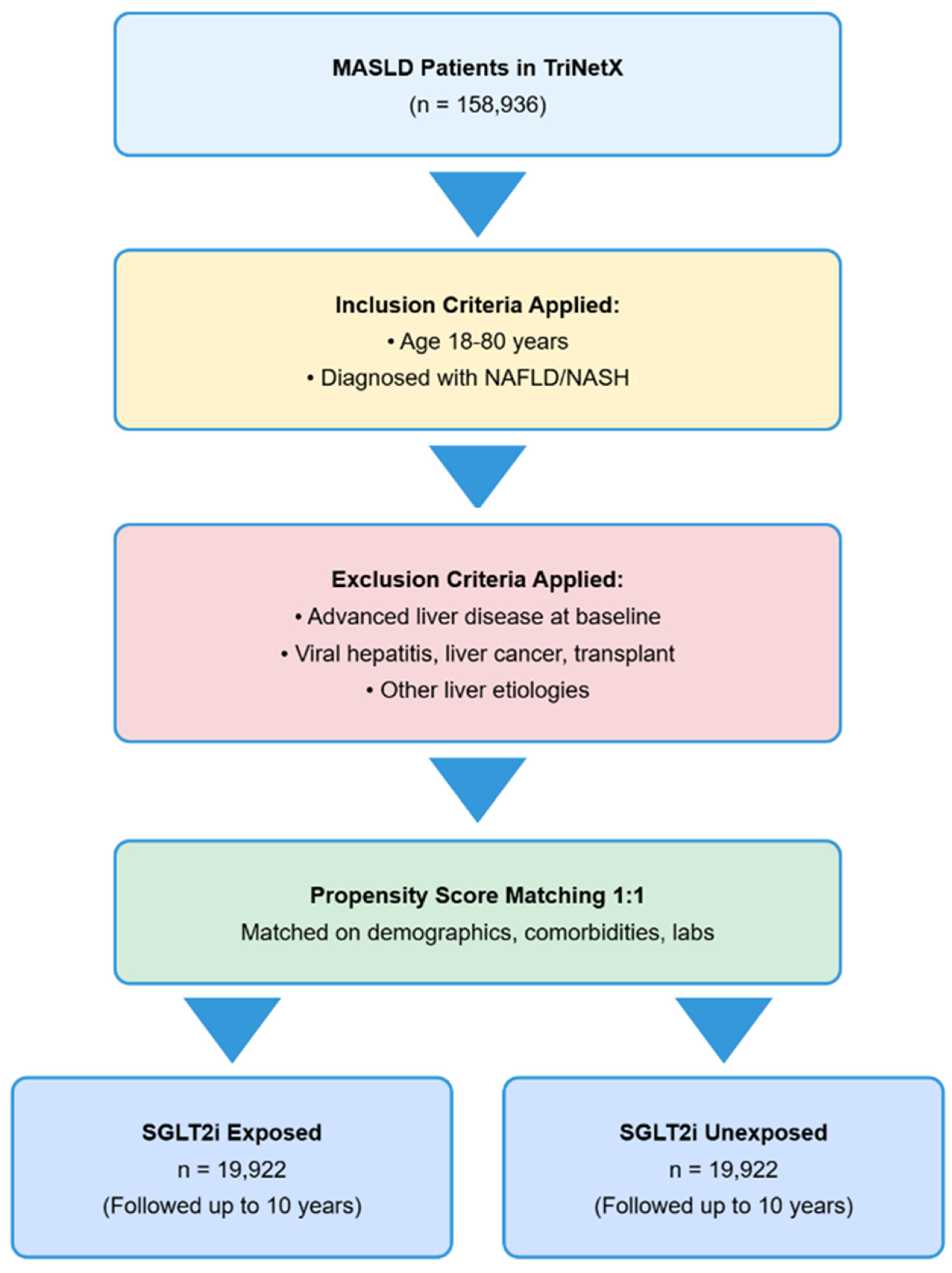
4.2. Study Population and Cohort Definitions
4.3. Exclusion Criteria
4.4. Propensity Score Matching
4.5. Outcomes
- Primary outcomes: all-cause mortality and overall survival.
- Liver-related outcomes: advanced liver disease (ALD) was defined according to clinical diagnoses (e.g., portal hypertension, ascites, varices, hepatic encephalopathy), laboratory abnormalities (e.g., thrombocytopenia, hyperbilirubinemia, hypoalbuminemia, hyperammonemia), or dispensed medications used specifically for cirrhosis (e.g., propranolol, lactulose, rifaximin, spironolactone).
- Cardiovascular events: myocardial infarction, stroke, atrial fibrillation, and heart failure.
- Metabolic parameters: LDL, HDL, triglycerides, HbA1c, BMI.
- Disease progression: changes in liver enzymes, liver synthetic function, and development of liver-related complications.
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. TriNetX Definitions and Codes
- Inclusion Criteria
- Age: Between 18 and 80 years (TNX:9074).
- MASLD diagnosis:
- ○
- Nonalcoholic steatohepatitis (UMLS:ICD10CM:K75.81).
- ○
- Fatty liver (UMLS:ICD10CM:K76.0).
- SGLT2 inhibitor exposure:
- ○
- Empagliflozin (NLM:RXNORM:1545653).
- ○
- Dapagliflozin (NLM:RXNORM:1488564).
- ○
- Canagliflozin (NLM:RXNORM:1373458).
- ○
- Ertugliflozin (NLM:RXNORM:1992672).
- Exclusion Criteria
- Advanced liver disease at baseline: No anticoagulants, esophageal varices, ascites, encephalopathy, medications for liver complications, hepatomegaly, liver fibrosis/cirrhosis, or abnormal laboratory values prior to index date.
- Liver cancer/transplantation: No liver transplantation procedures, liver cell carcinoma, or liver transplant status.
- Viral hepatitis: No hepatitis B or C diagnosis.
- Other liver etiologies: No other causes of liver disease (primary sclerosing cholangitis, primary biliary cirrhosis, hemochromatosis, etc.).
Appendix A.2. Outcome Definitions
- Clinical Outcomes
- Mortality: Deceased status.
- Cardiovascular events: Cerebrovascular disease (ICD10:I60-I69), myocardial infarction (ICD10:I21-I24), heart failure (ICD10:I50), or atrial fibrillation/flutter (ICD10:I48).
- Advanced liver disease (clinical): Portal hypertension (ICD10:K76.6), ascites (ICD10:R18), encephalopathy (ICD10:G93.40), hepatorenal syndrome (ICD10:K76.7), hepatopulmonary syndrome (ICD10:K76.81), spontaneous bacterial peritonitis (ICD10:K65.2), varices (ICD10:I85, I86.4), liver failure (ICD10:K72.9), or cirrhosis (ICD10:K74.6).
- Advanced liver disease (laboratory): Platelets ≤ 100 × 103/μL (TNX:9020), bilirubin ≥ 2 mg/dL (TNX:9050), albumin ≤ 2.8 g/dL (TNX:9045), or ammonia ≥ 50 μmol/L (TNX:LG4629-4).
- Advanced liver disease (medications): Neomycin (RXNORM:7299), rifaximin (RXNORM:35619), propranolol (RXNORM:8787), carvedilol (RXNORM:20352), or high-dose spironolactone (RXNORM:9997).
- Laboratory Outcomes
- Liver inflammatory markers: ALT ≥ 50 U/L (TNX:9044), AST ≥ 50 U/L (TNX:9047), ALP ≥ 80 U/L (TNX:9046), or GGT ≥ 80 U/L (TNX:9051).
- Synthetic impairment: Bilirubin ≥ 2 mg/dL (TNX:9050), albumin ≤ 2.8 g/dL (TNX:9045), or INR ≥ 1.7 (TNX:9032).
- Poor metabolic markers: LDL ≥ 130 mg/dL (TNX:9002), HDL ≤ 40 mg/dL (TNX:9001), triglycerides ≥ 200 mg/dL (TNX:9004), or HbA1c ≥ 8% (TNX:9037).
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Paik, J.M.; Golabi, P.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Changes in the global burden of chronic liver diseases from 2012 to 2017: The growing impact of NAFLD. Hepatology 2020, 72, 1605–1616. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Bugianesi, E.; Lenzi, M.; McCullough, A.J.; Natale, S.; Forlani, G.; Melchionda, N. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes 2001, 50, 1844–1850. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015, 149, 367–378. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Chan, J.C.H.; Chan, M.C.Y. SGLT2 inhibitors: The next blockbuster multifaceted drug? Medicina 2023, 59, 388. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Norton, L.; Abdul-Ghani, M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat. Rev. Nephrol. 2017, 13, 11–26. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Jojima, T.; Tomotsune, T.; Iijima, T.; Akimoto, K.; Suzuki, K.; Aso, Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetol. Metab. Syndr. 2016, 8, 45. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef]
- Chehrehgosha, H.; Sohrabi, M.R.; Ismail-Beigi, F.; Malek, M.; Reza Babaei, M.; Zamani, F.; Ajdarkosh, H.; Khoonsari, M.; Fallah, A.E.; Khamseh, M.E. Empagliflozin improves liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease and type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Diabetes Ther. 2021, 12, 843–861. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, X.; Guo, L.; Li, J.; Li, L. Effect of SGLT2 inhibitors on type 2 diabetes mellitus with non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Front. Endocrinol. 2021, 12, 635556. [Google Scholar] [CrossRef]
- Zhou, P.; Tan, Y.; Hao, Z.; Xu, W.; Zhou, X.; Yu, J. Effects of SGLT2 inhibitors on hepatic fibrosis and steatosis: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1144838. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-world evidence—What is it and what can it tell us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Tahara, A.; Takasu, T. SGLT2 inhibitor ipragliflozin alone and combined with pioglitazone prevents progression of nonalcoholic steatohepatitis in a type 2 diabetes rodent model. Physiol. Rep. 2019, 7, e14286. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Huang, W.; Han, Y.; Tan, H.; An, M.; Xiang, Q.; Zhou, R.; Yang, L.; Cheng, Y. Dapagliflozin alleviates hepatic steatosis by restoring autophagy via the AMPK-mTOR pathway. Front. Pharmacol. 2021, 12, 589273. [Google Scholar] [CrossRef]
- Lin, X.F.; Cui, X.N.; Yang, J.; Jiang, Y.F.; Wei, T.J.; Xia, L.; Liao, X.-Y.; Li, F.; Wang, D.-D.; Li, J.; et al. SGLT2 inhibitors ameliorate NAFLD in mice via downregulating PFKFB3, suppressing glycolysis and modulating macrophage polarization. Acta Pharmacol. Sin. 2024, 45, 2579–2597. [Google Scholar] [CrossRef]
- Kabil, S.L.; Mahmoud, N.M. Canagliflozin protects against non-alcoholic steatohepatitis in type-2 diabetic rats through zinc alpha-2 glycoprotein up-regulation. Eur. J. Pharmacol. 2018, 828, 135–145. [Google Scholar] [CrossRef]
- Mudaliar, S.; Henry, R.R.; Boden, G.; Smith, S.; Chalamandaris, A.G.; Duchesne, D.; Iqbal, N.; List, J. Changes in insulin sensitivity and insulin secretion with the sodium glucose cotransporter 2 inhibitor dapagliflozin. Diabetes Technol. Ther. 2014, 16, 137–144. [Google Scholar] [CrossRef]
- Aithal, G.P.; Thomas, J.A.; Kaye, P.V.; Lawson, A.; Ryder, S.D.; Spendlove, I.; Austin, A.S.; Freeman, J.G.; Morgan, L.; Webber, J. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008, 135, 1176–1184. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.-S.; Harrison, S.A. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Stapff, M.P. Using real world data to assess cardiovascular outcomes of two antidiabetic treatment classes. World J. Diabetes 2018, 9, 252–257. [Google Scholar] [CrossRef]
| (a) | ||||
| Characteristic | SGLT2i Group | Non-SGLT2i Group | p-Value | St. Diff. |
| (n = 19,922) | (n = 19,922) | |||
| Demographics | ||||
| Age (years), mean ± SD | 55.5 ± 11.8 | 55.6 ± 11.9 | 0.87 | 0.0084 |
| Female, n (%) | 9647 (48.4%) | 9644 (48.4%) | 0.94 | 0.0003 |
| Race (%) | ||||
| - White | 12,044 (60.5%) | 12,051 (60.5%) | 0.91 | 0.0014 |
| - Black | 1709 (8.6%) | 1706 (8.6%) | 0.93 | 0.0009 |
| - Asian | 1926 (9.7%) | 1931 (9.7%) | 0.91 | 0.0013 |
| BMI (kg/m2), mean ± SD | 34.0 ± 6.4 | 34.1 ± 6.5 | 0.76 | 0.0156 |
| Comorbidities (%) | ||||
| - Diabetes mellitus | 18,008 (90.4%) | 18,015 (90.4%) | 0.91 | 0.0011 |
| - Hypertension | 13,291 (66.7%) | 13,297 (66.7%) | 0.93 | 0.0008 |
| - Ischemic heart disease | 2202 (11.1%) | 2198 (11.0%) | 0.91 | 0.0012 |
| - Cerebrovascular disease | 525 (2.6%) | 522 (2.6%) | 0.89 | 0.0019 |
| (b) | ||||
| Laboratory Parameter | SGLT2i Group | Non-SGLT2i Group | p-Value | St. Diff. |
| Liver function tests | ||||
| ALT (U/L), mean ± SD | 46.7 ± 37.9 | 46.9 ± 38.2 | 0.89 | 0.0052 |
| (n = 14,743, 74%) | (n = 14,751, 74%) | |||
| AST (U/L), mean ± SD | 35.1 ± 35.1 | 35.3 ± 35.4 | 0.91 | 0.0057 |
| (n = 14,423, 72%) | (n = 14,418, 72%) | |||
| ALP (U/L), mean ± SD | 84.8 ± 35.9 | 85.1 ± 36.2 | 0.88 | 0.0083 |
| (n = 13,806, 69%) | (n = 13,812, 69%) | |||
| Gamma-Glutamyl Transferase (GGT) (U/L), mean ± SD | 95.5 ± 178.4 | 96.2 ± 180.1 | 0.93 | 0.0039 |
| (n = 981, 4.9%) | (n = 978, 4.9%) | |||
| Total bilirubin (mg/dL), mean ± SD | 0.60 ± 0.30 | 0.61 ± 0.31 | 0.87 | 0.0328 |
| (n = 13,745, 69%) | (n = 13,751, 69%) | |||
| Albumin (g/dL), mean ± SD | 4.30 ± 0.40 | 4.29 ± 0.41 | 0.91 | 0.0248 |
| (n = 13,847, 70%) | (n = 13,843, 69%) | |||
| Metabolic parameters | ||||
| HbA1c (%), mean ± SD | 8.00 ± 1.70 | 8.02 ± 1.72 | 0.88 | 0.0117 |
| (n = 13,934, 70%) | (n = 13,928, 70%) | |||
| Total cholesterol (mg/dL), mean ± SD | 170.0 ± 47.8 | 170.4 ± 48.1 | 0.92 | 0.0083 |
| (n = 12,500, 63%) | (n = 12,495, 63%) | |||
| LDL (mg/dL), mean ± SD | 89.8 ± 36.9 | 90.1 ± 37.2 | 0.9 | 0.0081 |
| (n = 12,312, 62%) | (n = 12,308, 62%) | |||
| HDL (mg/dL), mean ± SD | 41.5 ± 14.5 | 41.4 ± 14.6 | 0.94 | 0.0069 |
| (n = 12,670, 64%) | (n = 12,665, 64%) | |||
| Triglycerides (mg/dL), mean ± SD | 210.3 ± 207.0 | 211.5 ± 208.8 | 0.88 | 0.0058 |
| (n = 12,567, 63%) | (n = 12,562, 63%) | |||
| Hematologic parameters | ||||
| Platelet count (×103/μL), mean ± SD | 256.9 ± 71.0 | 257.4 ± 71.5 | 0.91 | 0.007 |
| (n = 12,087, 61%) | (n = 12,092, 61%) | |||
| INR, mean ± SD | 1.00 ± 0.10 | 1.01 ± 0.11 | 0.89 | 0.0953 |
| (n = 1867, 9.4%) | (n = 1863, 9.3%) | |||
| Other markers | ||||
| AFP (ng/mL), mean ± SD | 3.20 ± 1.50 | 3.22 ± 1.52 | 0.93 | 0.0133 |
| (n = 95, 0.5%) | (n = 93, 0.5%) | |||
| Creatinine (mg/dL), mean ± SD | 0.90 ± 1.90 | 0.91 ± 1.92 | 0.9 | 0.0052 |
| (n = 15,129, 76%) | (n = 15,124, 76%) | |||
| (a) | ||||||
| Clinical Outcomes | SGLT2i Status | 1 Year | 5 Years | 10 Years | HR (95% CI) | p-Value |
| Mortality | ||||||
| Exposed | 0.30% | 0.87% | 1.06% | 0.28 (0.24–0.33) | <0.001 | |
| Unexposed | 1.63% | 3.21% | 3.75% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Survival | ||||||
| Exposed | 99.64% | 98.11% | 95.00% | - | - | |
| Unexposed | 98.11% | 94.24% | 88.69% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Cardiovascular events | ||||||
| Exposed | 5.99% | 9.50% | 10.19% | 0.86 (0.82–0.90) | <0.001 | |
| Unexposed | 6.19% | 10.28% | 11.80% | |||
| p-value | 0.390 | 0.009 | <0.001 | |||
| Advanced liver disease | ||||||
| Exposed | 2.67% | 6.27% | 6.90% | 0.48 (0.45–0.51) | <0.001 | |
| Unexposed | 8.70% | 12.81% | 14.15% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| ALD (clinical) | ||||||
| Exposed | 0.87% | 2.00% | 2.20% | 0.61 (0.54–0.68) | <0.001 | |
| Unexposed | 2.30% | 3.30% | 3.60% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| ALD (laboratory) | ||||||
| Exposed | 1.20% | 2.80% | 3.10% | 0.38 (0.35–0.42) | <0.001 | |
| Unexposed | 5.90% | 7.60% | 8.10% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| ALD (medications) | ||||||
| Exposed | 0.90% | 2.70% | 3.10% | 0.49 (0.44–0.54) | <0.001 | |
| Unexposed | 2.90% | 5.30% | 6.30% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Liver transplantation | ||||||
| Exposed | 0.01% | 0.06% | 0.08% | 1.00 (0.50–2.00) | 1.000 | |
| Unexposed | 0.05% | 0.06% | 0.08% | |||
| p-value | 0.002 | 0.670 | 0.414 | |||
| Hepatocellular carcinoma | ||||||
| Exposed | 0.13% | 0.32% | 0.36% | 0.78 (0.60–1.02) | 0.070 | |
| Unexposed | 0.17% | 0.41% | 0.46% | |||
| p-value | 0.018 | 0.035 | 0.051 | |||
| Ascites | ||||||
| Exposed | 0.15% | 0.35% | 0.39% | 0.50 (0.40–0.63) | <0.001 | |
| Unexposed | 0.43% | 0.69% | 0.78% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Encephalopathy | ||||||
| Exposed | 0.15% | 0.35% | 0.45% | 0.42 (0.34–0.52) | <0.001 | |
| Unexposed | 0.60% | 0.95% | 1.15% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Varices | ||||||
| Exposed | 0.08% | 0.18% | 0.25% | 0.73 (0.52–1.02) | 0.064 | |
| Unexposed | 0.16% | 0.25% | 0.35% | |||
| p-value | <0.001 | 0.017 | 0.189 | |||
| Ammonia lowering agents | ||||||
| Exposed | 1.15% | 2.95% | 3.40% | 0.52 (0.47–0.57) | <0.001 | |
| Unexposed | 2.90% | 5.75% | 6.65% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Diuretics | ||||||
| Exposed | 3.65% | 8.00% | 8.85% | 0.68 (0.64–0.72) | <0.001 | |
| Unexposed | 6.20% | 11.90% | 13.20% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| NSBB | ||||||
| Exposed | 0.70% | 2.05% | 2.35% | 0.55 (0.49–0.62) | <0.001 | |
| Unexposed | 1.65% | 3.75% | 4.35% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| (b) | ||||||
| Laboratory Parameter | SGLT2i Status | 1 Year | 5 Years | 10 Years | p-Value (10 Years) | |
| ALT (U/L) | ||||||
| Exposed | 40.3 ± 31.5 | 38.8 ± 29.6 | 38.6 ± 29.4 | <0.001 | ||
| Unexposed | 48.3 ± 41.2 | 45.4 ± 38.5 | 45.1 ± 38.2 | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| AST (U/L) | ||||||
| Exposed | 30.6 ± 24.8 | 29.9 ± 22.7 | 29.8 ± 22.6 | <0.001 | ||
| Unexposed | 36.4 ± 35.1 | 34.7 ± 31.2 | 34.5 ± 31.0 | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| ALP (U/L) | ||||||
| Exposed | 81.4 ± 33.2 | 80.8 ± 32.5 | 80.7 ± 32.4 | <0.001 | ||
| Unexposed | 86.9 ± 38.7 | 85.6 ± 37.1 | 85.4 ± 36.9 | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Total bilirubin (mg/dL) | ||||||
| Exposed | 0.56 ± 0.29 | 0.57 ± 0.30 | 0.57 ± 0.30 | <0.001 | ||
| Unexposed | 0.63 ± 0.40 | 0.65 ± 0.43 | 0.66 ± 0.44 | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Albumin (g/dL) | ||||||
| Exposed | 4.27 ± 0.41 | 4.26 ± 0.41 | 4.26 ± 0.41 | <0.001 | ||
| Unexposed | 4.20 ± 0.46 | 4.18 ± 0.47 | 4.17 ± 0.47 | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Platelet count (×103/μL) | ||||||
| Exposed | 255.8 ± 68.2 | 253.4 ± 66.5 | 251.9 ± 65.8 | <0.001 | ||
| Unexposed | 249.3 ± 72.8 | 244.8 ± 70.5 | 241.2 ± 69.3 | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| HbA1c (%) | ||||||
| Exposed | 7.35 ± 1.51 | 7.28 ± 1.47 | 7.26 ± 1.46 | <0.001 | ||
| Unexposed | 7.93 ± 1.72 | 7.88 ± 1.69 | 7.86 ± 1.68 | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| BMI (kg/m2) | ||||||
| Exposed | 33.2 ± 6.2 | 32.8 ± 6.1 | 32.7 ± 6.1 | <0.001 | ||
| Unexposed | 34.1 ± 6.5 | 33.9 ± 6.4 | 33.8 ± 6.4 | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Creatinine (mg/dL) | ||||||
| Exposed | 0.91 ± 0.82 | 0.93 ± 0.85 | 0.94 ± 0.86 | 0.026 | ||
| Unexposed | 0.96 ± 1.24 | 0.99 ± 1.31 | 1.01 ± 1.35 | |||
| p-value | <0.001 | <0.001 | 0.026 | |||
| (c) | ||||||
| Categorical Outcome | SGLT2i Status | 1 Year | 5 Years | 10 Years | p-Value (10 Years) | |
| ALT > 50 U/L | ||||||
| Exposed | 21.70% | 25.00% | 25.15% | <0.001 | ||
| Unexposed | 29.10% | 34.80% | 35.60% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| AST > 40 U/L | ||||||
| Exposed | 13.85% | 15.70% | 15.85% | <0.001 | ||
| Unexposed | 19.40% | 22.35% | 22.90% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Bilirubin ≥ 2 mg/dL | ||||||
| Exposed | 0.65% | 0.90% | 0.95% | <0.001 | ||
| Unexposed | 1.70% | 2.35% | 2.55% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Albumin ≤ 2.8 g/dL | ||||||
| Exposed | 0.75% | 1.05% | 1.10% | <0.001 | ||
| Unexposed | 2.20% | 2.95% | 3.20% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| INR ≥ 1.7 | ||||||
| Exposed | 0.30% | 0.50% | 0.60% | <0.001 | ||
| Unexposed | 0.75% | 1.20% | 1.45% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Platelets < 150 × 103/μL | ||||||
| Exposed | 7.15% | 8.65% | 9.05% | <0.001 | ||
| Unexposed | 11.45% | 14.05% | 14.95% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Platelets < 100 × 103/μL | ||||||
| Exposed | 1.80% | 2.20% | 2.35% | <0.001 | ||
| Unexposed | 3.75% | 4.65% | 5.00% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| HbA1c > 8.5% | ||||||
| Exposed | 24.50% | 27.30% | 27.60% | <0.001 | ||
| Unexposed | 33.20% | 38.45% | 39.35% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Poor metabolic markers | ||||||
| Exposed | 36.45% | 41.20% | 41.65% | <0.001 | ||
| Unexposed | 43.85% | 50.70% | 51.80% | |||
| p-value | <0.001 | <0.001 | <0.001 | |||
| Outcome | NNT at 1 Year | NNT at 5 Years | NNT at 10 Years |
|---|---|---|---|
| All-cause mortality | 74 | 43 | 36 |
| Advanced liver disease (any) | 17 | 16 | 14 |
| Clinical ALD manifestations | 70 | 77 | 71 |
| Laboratory-defined ALD | 21 | 21 | 20 |
| ALD requiring medications | 50 | 39 | 31 |
| Cardiovascular event | 500 | 125 | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suki, M.; Imam, A.; Amer, J.; Milgrom, Y.; Massarwa, M.; Hazou, W.; Tiram, Y.; Perzon, O.; Sharif, Y.; Sackran, J.; et al. SGLT2 Inhibitors in MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease) Associated with Sustained Hepatic Benefits, Besides the Cardiometabolic. Pharmaceuticals 2025, 18, 1118. https://doi.org/10.3390/ph18081118
Suki M, Imam A, Amer J, Milgrom Y, Massarwa M, Hazou W, Tiram Y, Perzon O, Sharif Y, Sackran J, et al. SGLT2 Inhibitors in MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease) Associated with Sustained Hepatic Benefits, Besides the Cardiometabolic. Pharmaceuticals. 2025; 18(8):1118. https://doi.org/10.3390/ph18081118
Chicago/Turabian StyleSuki, Mohamad, Ashraf Imam, Johnny Amer, Yael Milgrom, Muhammad Massarwa, Wadi Hazou, Yariv Tiram, Ofer Perzon, Yousra Sharif, Joseph Sackran, and et al. 2025. "SGLT2 Inhibitors in MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease) Associated with Sustained Hepatic Benefits, Besides the Cardiometabolic" Pharmaceuticals 18, no. 8: 1118. https://doi.org/10.3390/ph18081118
APA StyleSuki, M., Imam, A., Amer, J., Milgrom, Y., Massarwa, M., Hazou, W., Tiram, Y., Perzon, O., Sharif, Y., Sackran, J., Alon, R., Lourie, N., Hershko Klement, A., Shibli, S., Safadi, T., Raz, I., Khalaileh, A., & Safadi, R. (2025). SGLT2 Inhibitors in MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease) Associated with Sustained Hepatic Benefits, Besides the Cardiometabolic. Pharmaceuticals, 18(8), 1118. https://doi.org/10.3390/ph18081118









