Therapeutic Potential of Bioactive Compounds from Traditional Chinese Medicine in Modulating Macrophage Cholesterol Metabolism for Atherosclerosis Treatment
Abstract
1. Introduction
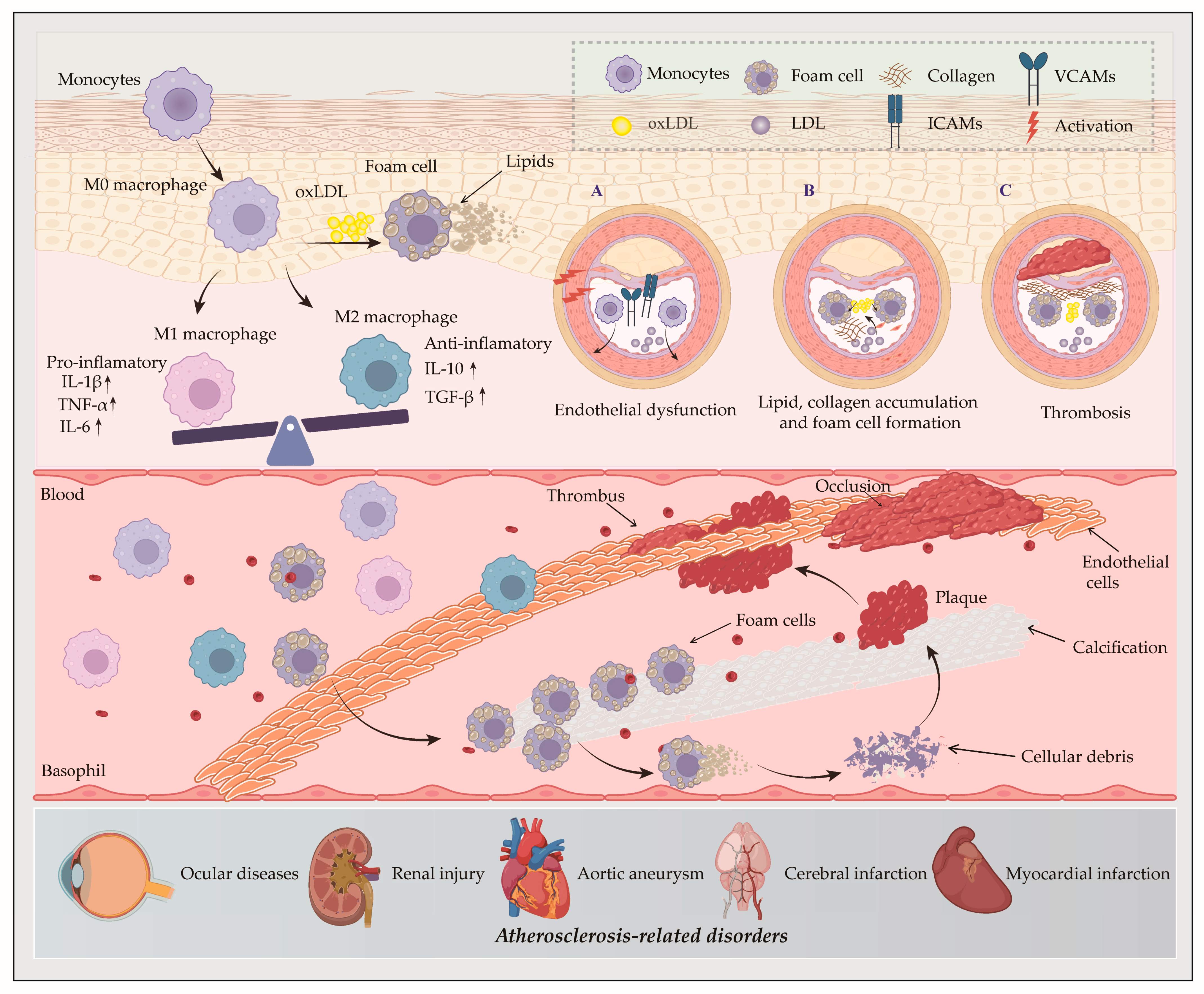
2. The Role of Macrophages in AS
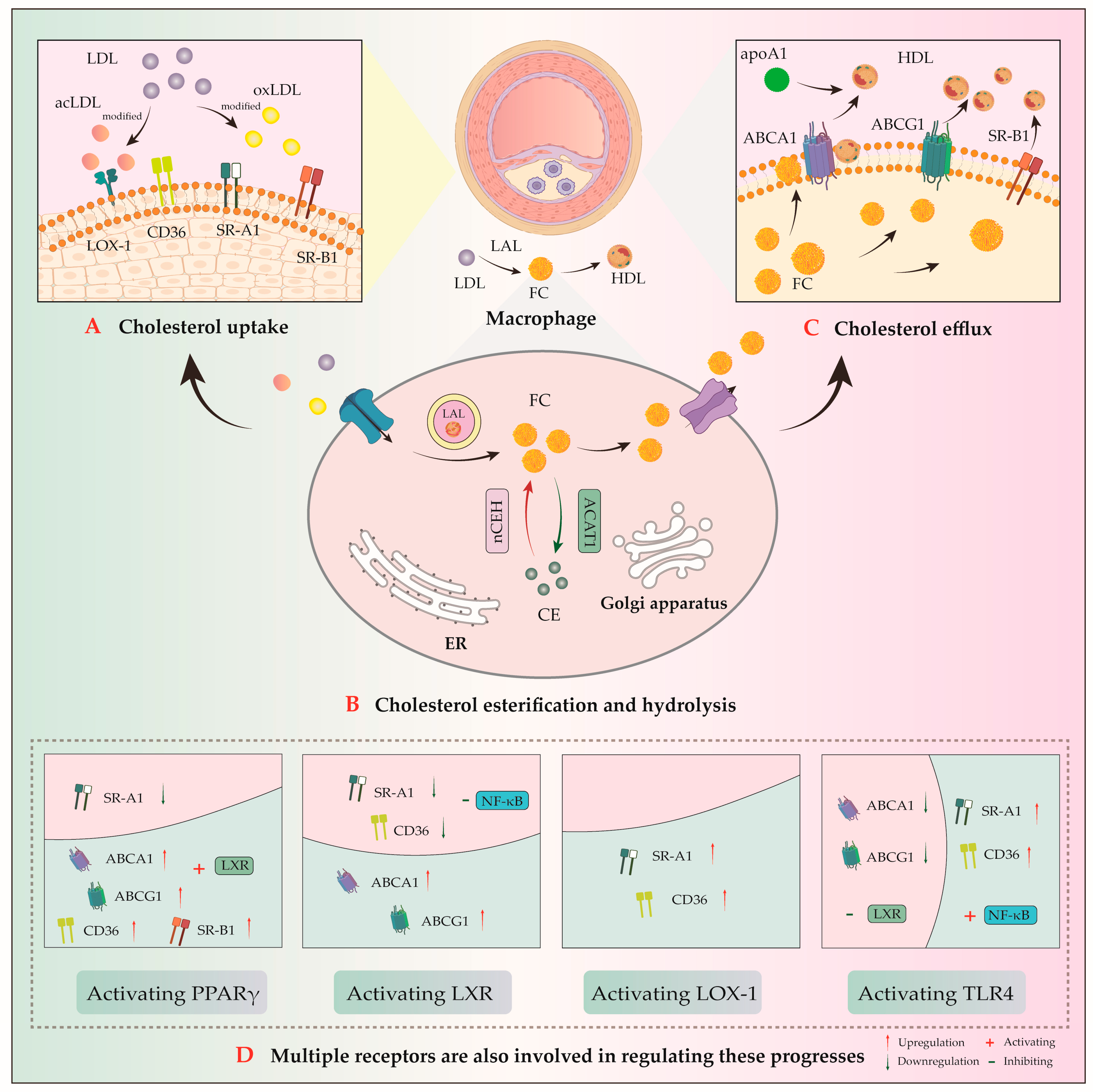
3. Key Regulators of Macrophage in Cholesterol Metabolism
- (1)
- Uptake receptors: scavenger receptor class A1 (SR-A1) and cluster of differentiation 36 (CD36);
- (2)
- Efflux transporters: ATP-binding cassette transporter A1/G1 (ABCA1/ABCG1) and scavenger receptor class B type 1 (SR-B1) [33]. ABCA1-mediated cholesterol efflux serves as the rate-limiting step in reverse cholesterol transport (RCT) process. This process facilitates the transfer of intracellular cholesterol and phospholipids to apolipoprotein A-1 (apoA-1) to generate nascent pre-β high-density lipoprotein (HDL) particles. The RCT pathway ultimately promotes hepatic excretion of excess cholesterol via bile and feces, thereby attenuating AS development [34];
- (3)
- Cholesteryl esterase and hydrolase, cholesterol acyltransferase 1 (ACAT1), and neutral cholesteryl ester hydrolase (nCEH).
3.1. Cholesterol Uptake
3.1.1. Fluid-Phase Pinocytosis
3.1.2. SR-Mediated Cytophagy
SR-A1
CD36
3.2. Cholesterol Efflux
3.2.1. ABCA1
3.2.2. ABCG1
3.2.3. SR-B1
3.3. Cholesterol Esterification and Hydrolysis
3.3.1. ACAT1
3.3.2. nCEH
3.4. Cholesterol Metabolism-Related Receptors
3.4.1. PPAR
3.4.2. LXR
3.4.3. LOX-1
3.4.4. TLR4
4. Modulation of Cholesterol Metabolism in Macrophages by TCM Components
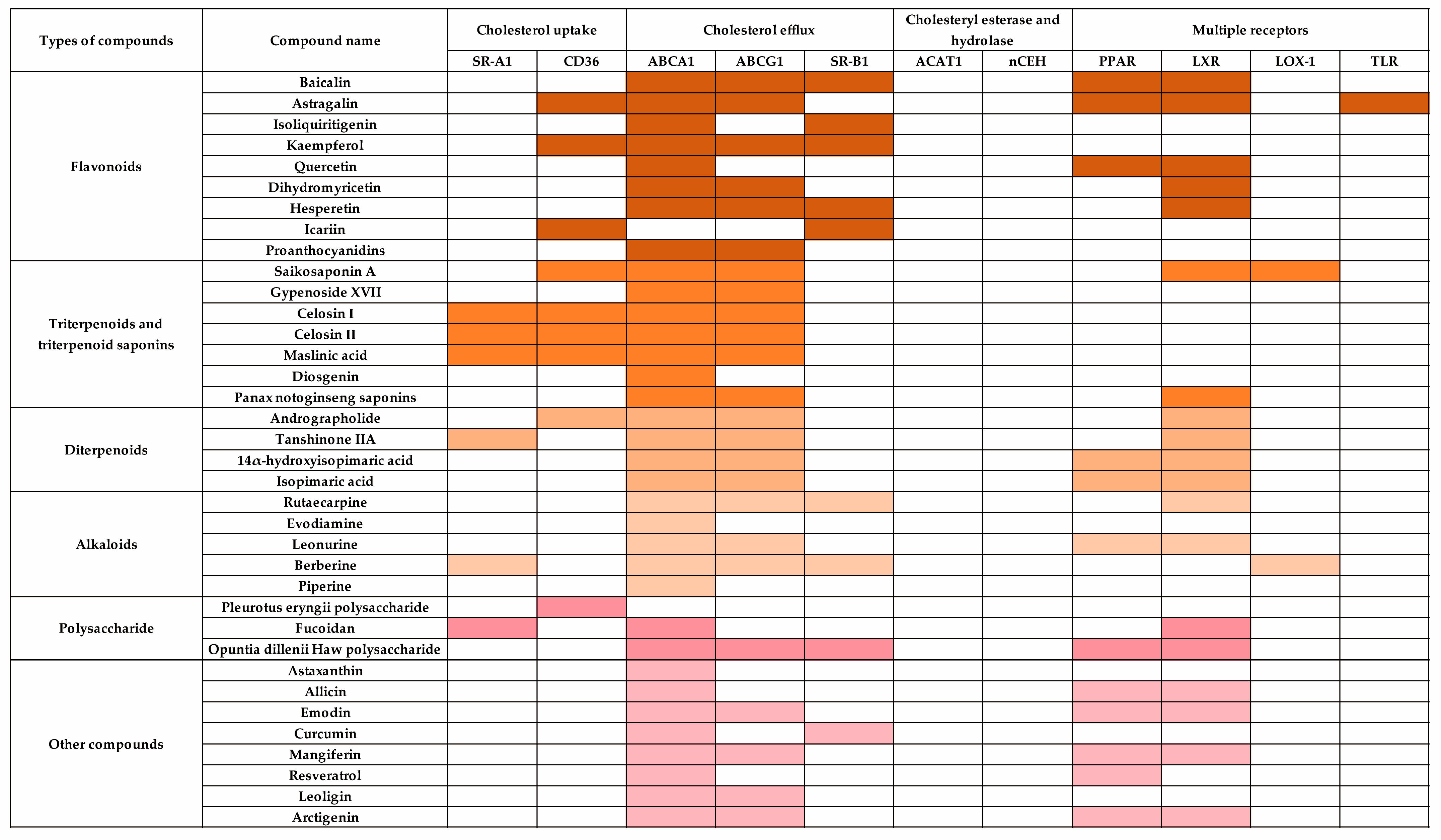
4.1. Flavonoids
| Compound No. | Components | Source TCM | Experimental Model | Dosage | Pathway | Mechanism | Pharmacological Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
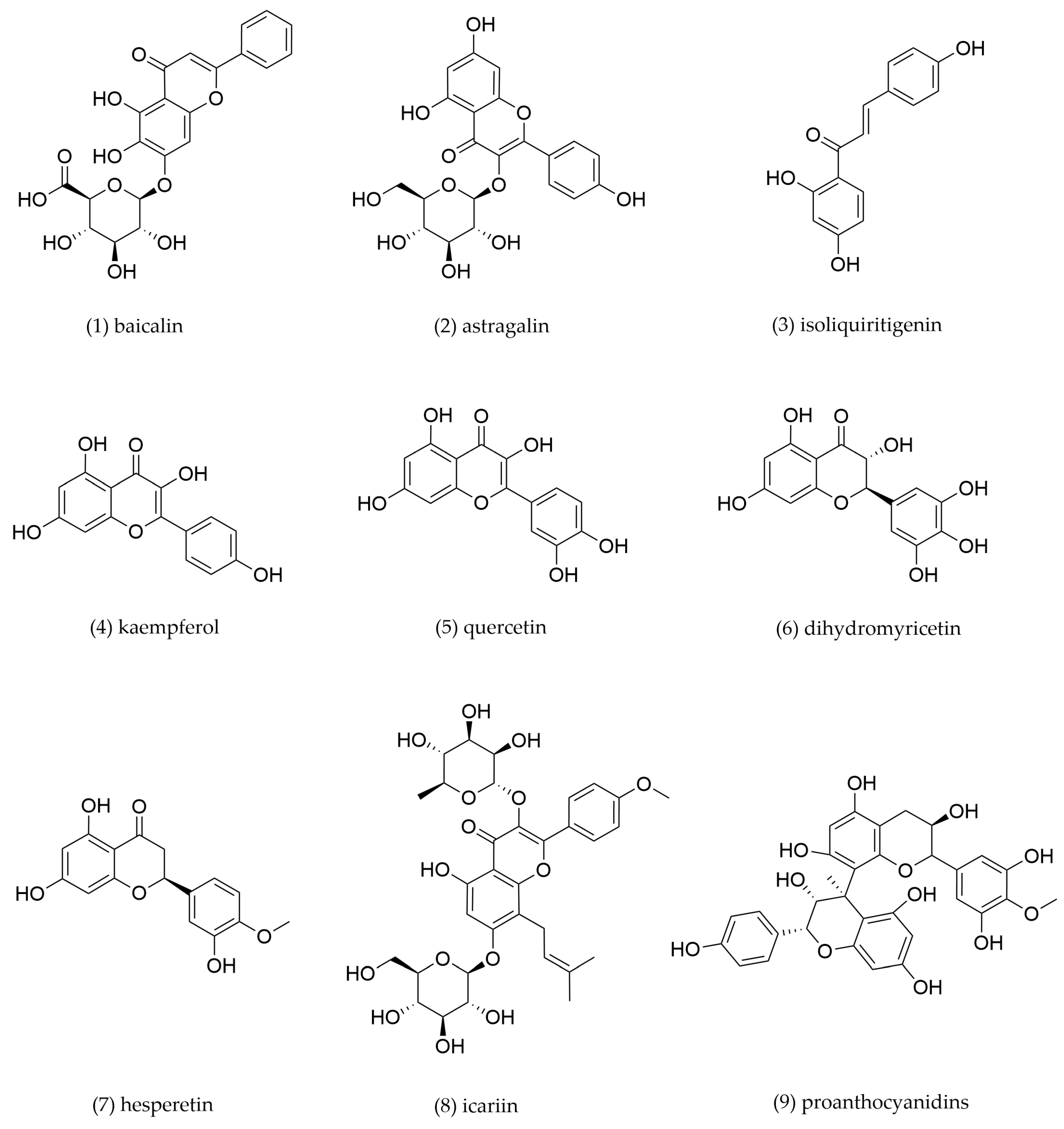
| 1 | Baicalin | Scutellaria baicalensis | THP-1-derived foam cells | 2, 10, 50 μM incubation for 48 h | PPARγ/LXRα | SR-B1↑, PPAR-γ↑, LXRα↑ | Promote cholesterol efflux | [104] |
| THP-1-derived foam cells | 25, 50, 100 μM incubation for 24 h | PPARγ/LXRα- ABCA1/ABCG1 | PPARγ↑, LXRα↑, ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [105] | |||
| 2 | Astragalin | Astragalus membranaceus | THP-1-derived foam cells | 5, 10, 20, 40 μg/mL incubation for 48 h | PPARγ/LXRα | ABCA1↑, ABCG1↑, TLR4↓ | Promote cholesterol efflux | [107] |
| 3 | Isoliquiritigenin | Glycyrrhiza uralensis | Peritoneal macrophage-derived foam cells | 0.5 μg/mL incubation for 12 h | PPARγ | PPARγ↑, ABCA1↑, CD36↓ | Promote cholesterol efflux, inhibit cholesterol intake | [109] |
| Glycyrrhiza uralensis | ApoE−/− mice | 0, 20, 100 mg/kg/day for 12 weeks | / | VLDL/LDL↓, SOD↑, PON1↑, IL-6↓, TNF-α↓, MCP-1↓, SR-B1↑, ABCA1↑, ABCG8↑, CYP7A1↑ and CYP27A1↑ | Promote cholesterol efflux, inhibit cholesterol intake | [109] | ||
| 4 | Kaempferol | Kaempferia galanga | THP-1-derived foam cells | 2.5, 5, 10 μg/mL incubation for 24 h | c-Jun-AP-1/ HO-1 | ABCA1↑, ABCG1↑, SR-B1↑, CD36↓ | Promote cholesterol efflux, inhibit cholesterol intake | [110] |
| 5 | Quercetin | Sophora japonica, Forsythia suspensa | THP-1-derived foam cells | 25, 50, 100, 200 μM incubation for 24 h | PPARγ/LXRα | PPARγ↑, ABCA1↑ | Promote cholesterol efflux | [113] |
| 6 | Dihydromyricetin | Ampelopsis megalophylla | THP-1-derived foam cells | 1, 10, 100 μM incubation for 24 h | LXRα/ABCA1/ ABCG1 | LXRα↑, ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [116] |
| 7 | Hesperetin | Citri reticulatae | THP-1-derived foam cells | 10, 50, 100 μM incubation for 24 h | LXRα/AMPK | LXRα↑, ABCA1↑, ABCG1↑, SR-B1↑, phosphorylated- AMPK↑ | Promote cholesterol efflux | [118] |
| 8 | Icariin | Epimedium brevicornu | THP-1-derived foam cells | 0.8, 4, 20 μM incubation for 12 h | p38MAPK | SR-B1↑, CD36↓, p38 MAPK↓ | Promote cholesterol efflux, inhibit cholesterol intake | [119] |
| 9 | Proanthocyanidins | Crataegus altaica | THP-1-derived foam cells | 100 μg/mL incubation for 72 h | Class III PI3K/Beclin1 | ABCA1↑, ABCG1↑ | Activate autophagy, promote cholesterol efflux | [120] |
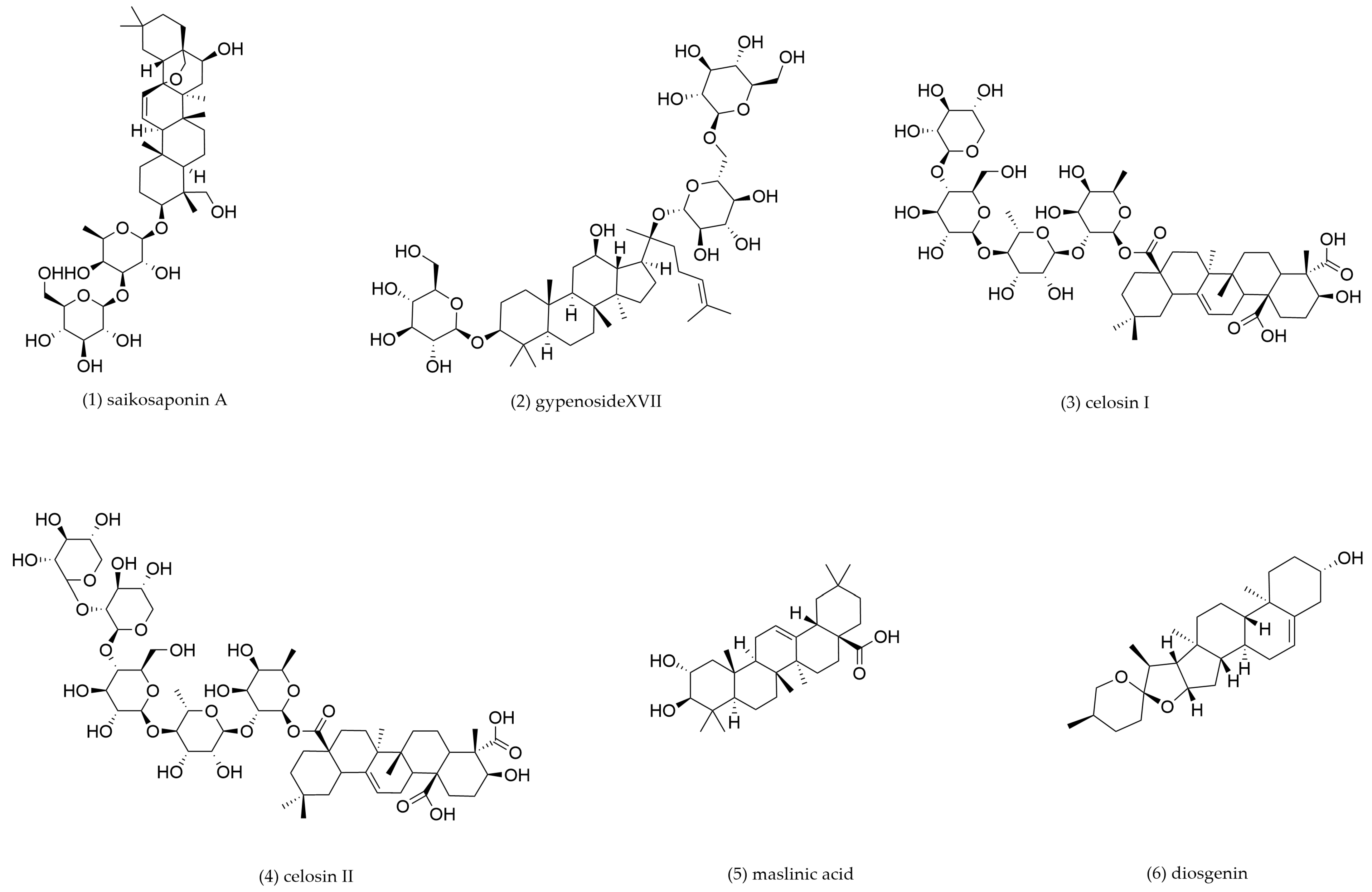
4.2. Triterpenoids and Triterpenoid Saponins
| Compound No. | Components | Source TCM | Experimental Model | Dosage | Pathway | Mechanism | Pharmacological Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Saikosaponin A | Bupleurum chinense, Bupeurum scorzonerifolium | C57/BL6J mouse peritoneal macrophage-derived foam cells | 3, 6, 12 μM incubation for 12 h | LXRα | LXRα↑, ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [123] |
| THP-1-derived foam cells | 6.25, 12.5, 25, 50 μM incubation for 24 h | PPARγ/LOX-1 | PPARγ↑, ABCA1↑, CD36↓, LOX-1↓ | Promote cholesterol efflux, inhibit cholesterol intake | [124] | |||
| 2 | Gypenoside XVII | Gynostemma pentaphyllum | THP-1-derived foam cells | 100 μg/mL incubation for 24 h | miR-182-5p/HDAC9 | ABCA1↑, ABCG1↑, miR-182–5p↑, HDAC9↓ | Promote cholesterol efflux | [126] |
| 3 | Celosin I | Celosia argentea | C57/BL6J mouse peritoneal macrophage-derived foam cells | 12.5, 25, 50 μg/mL incubation for 24 h | / | ABCA1↑, ABCG1↑, CD36↓, SR-A1↓ | Promote cholesterol efflux | [127] |
| 4 | Celosin II | |||||||
| 5 | Maslinic acid | Crataegus pinnatifida | THP-1-derived foam cells | 5, 10 μM incubation for 24 h | / | ABCA1↑, ABCG1↑, SR-A1↓, CD36↓ | Promote cholesterol efflux, inhibit cholesterol intake | [130] |
| 6 | Diosgenin | Dioscorea polystachya | THP-1 and C57/BL6J mouse peritoneal macrophage-derived foam cells | 10, 20, 40, 80 μM incubation for 24 h | miR-19b | ABCA1↑, miR-19b↓ | Promote cholesterol efflux | [132] |
| 7 | Panax notoginseng saponins | Panax notoginseng | THP-1-derived foam cells | 25, 50, 100 mg/L incubation for 12 h | / | LXRα↑, ABCA1↑, ABCG1↑, NF-κB↓ | Promote cholesterol efflux | [135] |
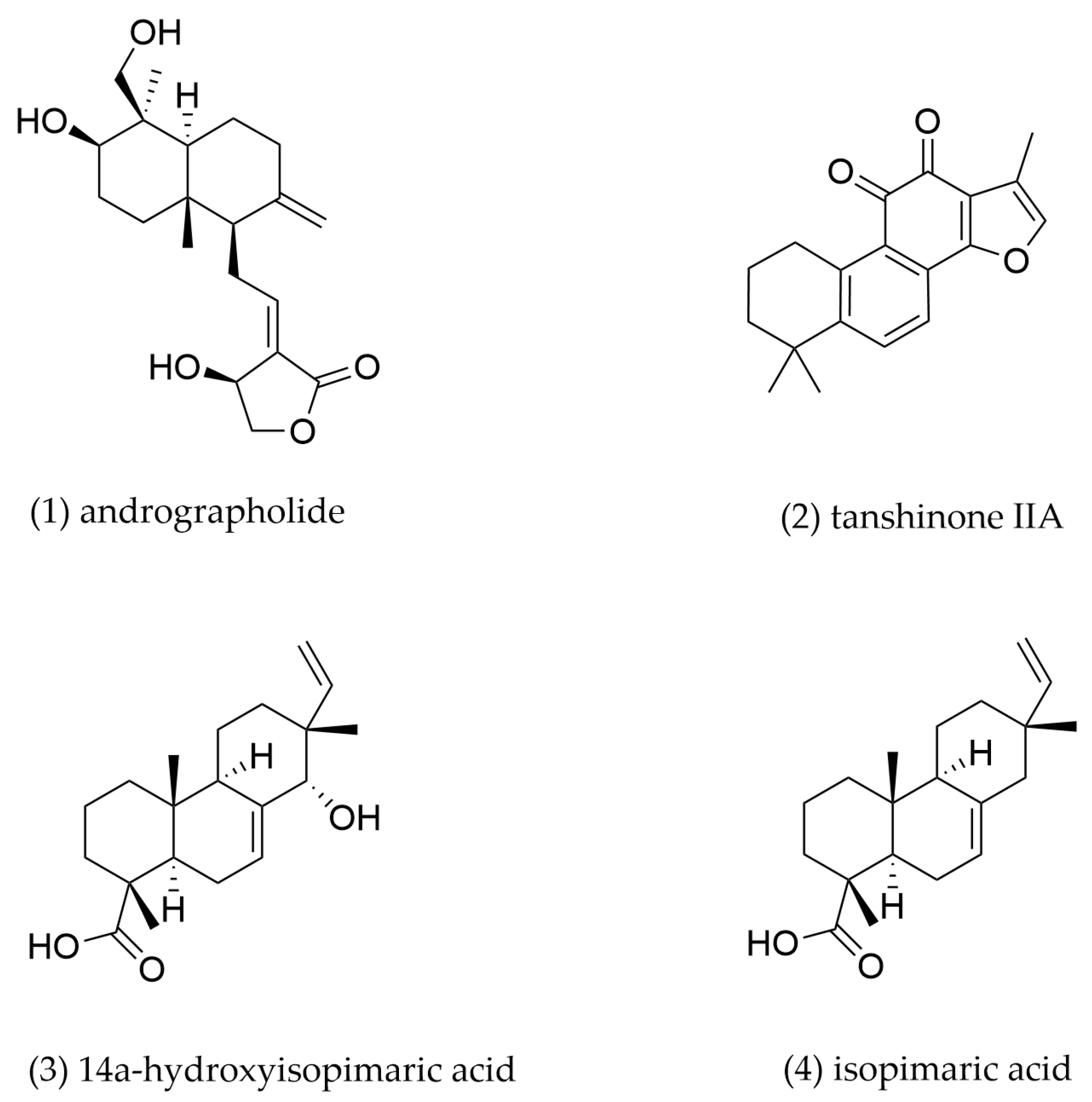
4.3. Diterpenoids
| Compound No. | Components | Source TCM | Experimental Model | Dosage | Pathway | Mechanism | Pharmacological Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Andrographolide | Andrographis paniculata | J774A.1-derived foam cells | 0.5, 1 μM incubation for 24 h | LXRα | LXRα↑, ABCA1↑, ABCG1↑, CD36↓ | Promote cholesterol efflux, inhibit cholesterol intake | [139] |
| 2 | Tanshinone IIA | Salvia miltiorrhiza | THP-1-derived foam cells | 1, 3, 10 μM incubation for 24 h | LXRα/Nrf2/HO-1 | SR-A1↓, ABCA1↑, ABCG1↑ | Promote cholesterol efflux, inhibit cholesterol intake | [141] |
| THP-1-derived foam cells | 20, 40, 80 mg/L incubation for 24 h | Omentin-1/ABCA1 | ABCA1↑, Omentin-1↑ | Promote cholesterol efflux | [142] | |||
| 3 | 14α-hydroxyisopimaric acid | Callicarpa rubella | RAW264.7-derived foam cells | 15 μM incubation for 24 h | PPARγ/LXRα | PPARγ↑, LXRα↑, ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [144] |
| 4 | Isopimaric acid | Callicarpa rubella | RAW264.7-derived foam cells | 15 μM incubation for 24 h | PPARγ/LXRα | PPARγ↑, LXRα↑, ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [144] |
4.4. Alkaloids
| Compound No. | Components | Source TCM | Experimental Model | Dosage | Pathway | Mechanism | Pharmacological Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Rutaecarpine | Evodia rutaecarpa | RAW264.7-derived foam cells | 0.035, 0.35, 3.48, 34.80 μM incubation for 24 h | LXRα/LXRβ | ABCA1↑, ABCG1↑, SR-B1↑ | Promote cholesterol efflux | [145] |
| 2 | Evodiamine | THP-1-derived foam cells | 1, 3, 10, 20 μM incubation for 24 h | / | ABCA1↑ | Promote cholesterol efflux | [146] | |
| 3 | Leonurine | Leonurus japonicu | THP-1-derived foam cells | 5, 10, 20, 40, 80 μM incubation for 24 h | PPARγ/LXRα | PPARγ↑, LXRα↑, ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [64] |
| 4 | Berberine | Coptis chinensis | THP-1-derived foam cells, C57/BL6J mouse peritoneal macrophage-derived foam cells | 1, 3, 10 μM incubation for 24 h | Nrf2/HO-1 | ABCA1↑, ABCG1↑, SR-B1↑, LOX-1↓, SR-A1↓ | Promote cholesterol efflux, inhibit cholesterol intake | [152] |
| 5 | Piperine | Piper nigrum | THP-1-derived foam cells | 25, 50, 100 μM incubation for 24 h | / | ABCA1↑ | Promote cholesterol efflux | [155] |
4.5. Polysaccharide
| Compound No. | Components | Source TCM | Experimental Model | Dosage | Pathway | Mechanism | Pharmacological Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Pleurotus eryngii polysaccharide | Pleurotus eryngii | RAW264.7-derived foam cells | 5, 100, 200 μg/mL incubation for 24 h | / | CD36↓ | Inhibit cholesterol intake | [158] |
| 2 | Fucoidan | Laminaria japonica | THP-1-derived foam cells | 50 μg/mL incubation for 24 h | LXRα | LXRα↑, ABCA1↑, SR-A1↓ | Promote cholesterol efflux | [162,163] |
| 3 | Opuntia dillenii Haw polysaccharide | Opuntia dillenii | THP-1-derived foam cells | 5, 10, 20 nM incubation for 24 h | PPARγ/LXRα | PPARγ↑, LXRα↑, ABCA1↑, ABCG1↑, SR-B1↑ | Promote cholesterol efflux | [164] |
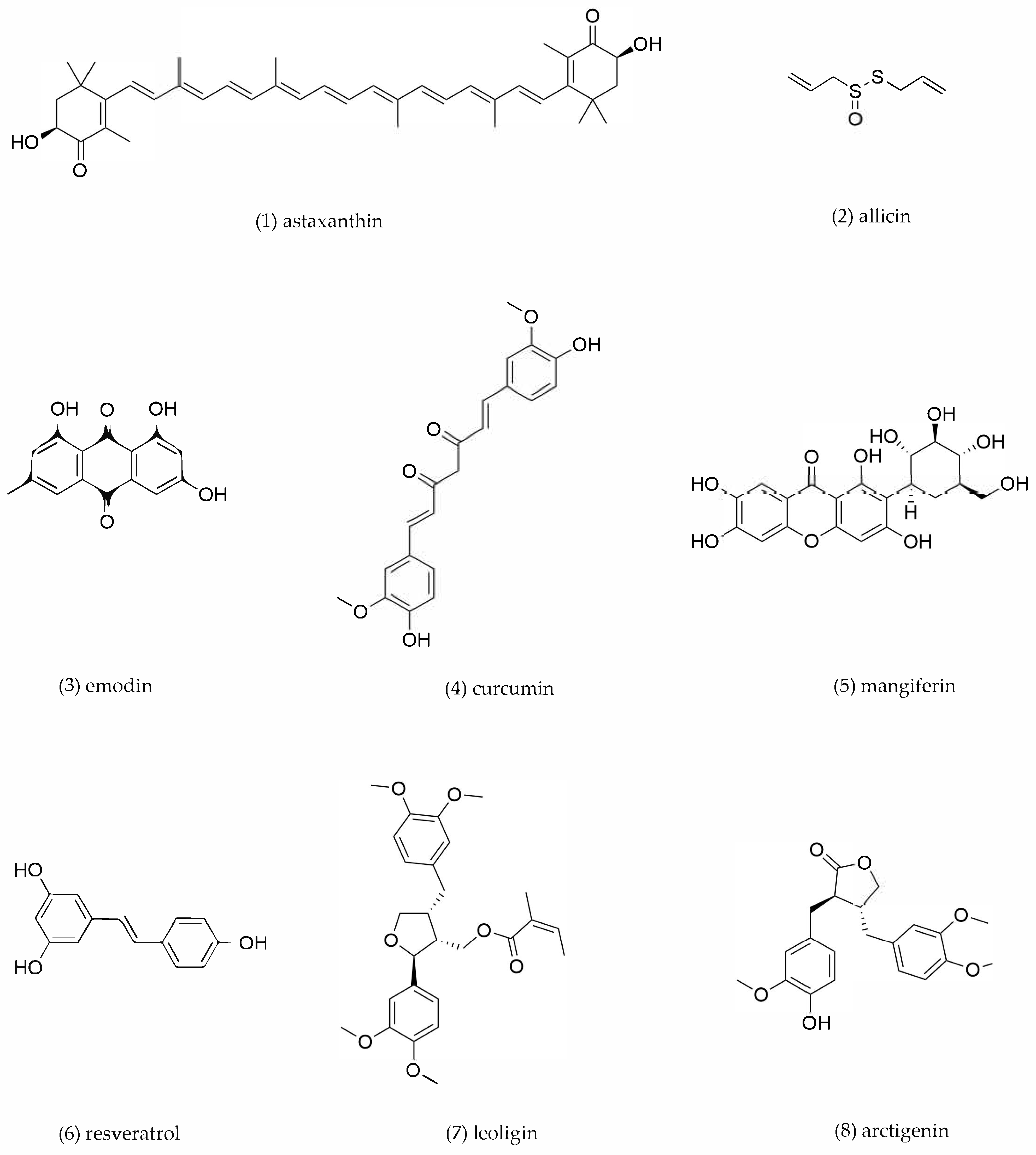
4.6. Other Compounds
| Compound No. | Components | Source TCM | Experimental Model | Dosage | Pathway | Mechanism | Pharmacological Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Astaxanthin | Haematococcus pluvialis | RAW264.7-derived foam cells | 0.5, 5, 50 μM incubation for 48 h | circTPP2/miR-3073b-5p/ABCA1 | ABCA1↑, circTPP2↑, miR-3073b-5p↓ | Promote cholesterol efflux | [166] |
| 2 | Allicin | Allium sativum | THP-1-derived foam cells | 2.5. 5, 10, 20, 40 mg/mL incubation for 24 h | PPARγ/LxRα | PPARγ↑, LXRα↑, ABCA1↑ | Promote cholesterol efflux | [168] |
| 3 | Emodin | Rheum palmatum | THP-1-derived foam cells | 0–10 μM incubation for 18 h | PPARγ/LXRα | PPARγ↑, LXRα↑, ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [169] |
| 4 | Curcumin | Curcuma longa | RAW264.7-derived foam cells | 10, 20, 40 μM incubation for 12 h | Nrf2/ARE | HO-1↑, ABCA1↑, SR-B1↑ | Promote cholesterol efflux | [174] |
| 5 | Mangiferin | Mangifera indica | RAW264.7-derived foam cells | 5, 10, 20 μM incubation for 24 h | PPARγ/LXRα-ABCA1/ABCG1 | PPARγ↑, LXRα↑, ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [179] |
| 6 | Resveratrol | Reynoutria japonica | RAW264.7-derived foam cells | 1.5 μg/mL incubation for 24 h | PPARα/γ | PPARγ↑, PPARα↑, ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [181] |
| 7 | Leoligin | Leontopodium leontopodioides | THP-1-derived foam cells | 10, 20, 40 μM incubation for 24 h | / | ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [182] |
| 8 | Arctigenin | Arctium lappa | THP-1-derived foam cells | 10, 50, 100 μM incubation for12 h | PPARγ/LXRα | PPARγ↑, LXRα↑, ABCA1↑, ABCG1↑ | Promote cholesterol efflux | [184] |
5. Conclusions and Outlook
- (1)
- Identification of potential anti-AS compounds of TCMs and TCM formulas
- (2)
- Structure–activity relationship research
- (3)
- Pharmacokinetics research
- (4)
- Interdisciplinary integration to identify targets
- (5)
- Synergistic effects of TCM bioactive compounds
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette transporter A1 |
| ABCG1 | ATP-binding cassette transporter G1 |
| ACAT1 | cholesterol acyltransferase 1 |
| acLDL | acetylated LDL |
| apoA-1 | apolipoprotein A-1 |
| AS | Atherosclerosis |
| CD36 | cluster of differentiation 36 |
| CEs | cholesteryl esters |
| CVDs | Cardiovascular diseases |
| ECs | endothelial cells |
| ERK | extracellular signal-regulated kinase |
| FASN | fatty acid synthase |
| FC | free cholesterol |
| HDAC9 | histone deacetylase 9 |
| HDL | high-density lipoprotein |
| HMG-CoA | 3-hydroxy-3-methyl glutaryl coenzyme A reductase |
| HO-1 | heme oxygenase-1 |
| ICAMs | intercellular cell adhesion molecules |
| IFN-γ | interferon-γ |
| IL-17A | interleukins 17A |
| IL-33 | interleukins 33 |
| LDLRs | low-density lipoprotein receptors |
| LOX-1 | lectin-like oxidized low-density lipoprotein receptor-1 |
| LXR | Liver X receptor |
| MAPK | mitogen-activated protein kinase |
| M-CSF | macrophage colony-stimulating factor |
| mmLDL | minimally oxidized LDL |
| nCEH | neutral cholesteryl ester hydrolase |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| ODP-Ia | Opuntia dillenii Haw polysaccharide |
| ox-LDL | oxidized low-density lipoprotein |
| PMA | phorbol 12-myristate 13-acetate |
| PON1 | paraoxonase-1 |
| PPAR | peroxisome proliferator-activated receptor |
| RCT | reverse cholesterol transport |
| SOD | superoxide dismutase |
| SR | scavenger receptors |
| SR-A1 | scavenger receptor class A1 |
| SR-B1 | scavenger receptor class B type 1 |
| SYK | spleen tyrosine kinase |
| TCM | traditional Chinese medicine |
| TGF-β | transforming growth factor-β |
| TLR4 | toll-like receptor 4 |
| VCAMs | vascular cell adhesion molecules |
| VLDL | very low-density lipoprotein |
References
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The heart of the world. Glob. Heart 2024, 19, 11–24. [Google Scholar] [CrossRef]
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Varieur Turco, J.; et al. Global burden of cardiovascular diseases and risks collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef]
- Wu, J.; He, S.; Song, Z.; Chen, S.; Lin, X.; Sun, H.; Zhou, P.; Peng, Q.; Du, S.; Zheng, S.; et al. Macrophage polarization states in atherosclerosis. Front. Immunol. 2023, 14, 1185587. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.E.; Gage, A.D.; Joseph, N.T.; Danaei, G.; García-Saisó, S.; Salomon, J.A. Mortality due to low-quality health systems in the universal health coverage era: A systematic analysis of amenable deaths in 137 countries. Lancet 2018, 392, 2203–2212. [Google Scholar] [CrossRef]
- Zhao, F.; Shao, M.; Li, M.; Li, T.; Zheng, Y.; Sun, W.; Ni, C.; Li, L. Sphingolipid metabolites involved in the pathogenesis of atherosclerosis: Perspectives on sphingolipids in atherosclerosis. Cell. Mol. Biol. Lett. 2025, 30, 18–42. [Google Scholar] [CrossRef]
- Liao, Y.; Dong, Z.; Liao, H.; Chen, Y.; Hu, L.; Yu, Z.; Xia, Y.; Zhao, Y.; Fan, K.; Ding, J.; et al. Lipid metabolism patterns and relevant clinical and molecular features of coronary artery disease patients: An integrated bioinformatic analysis. Lipids Health Dis. 2022, 21, 87–99. [Google Scholar] [CrossRef]
- Linton, M.R.F.; Moslehi, J.J.; Babaev, V.R. Akt signaling in macrophage polarization, survival, and atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef]
- Hou, P.; Fang, J.; Liu, Z.; Shi, Y.; Agostini, M.; Bernassola, F.; Bove, P.; Candi, E.; Rovella, V.; Sica, G.; et al. Macrophage polarization and metabolism in atherosclerosis. Cell Death Dis. 2023, 14, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Ye, S. Putative targeting of matrix metalloproteinase-8 in atherosclerosis. Pharmacol. Ther. 2015, 147, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Blagov, A.V.; Markin, A.M.; Bogatyreva, A.I.; Tolstik, T.V.; Sukhorukov, V.N.; Orekhov, A.N. The role of macrophages in the pathogenesis of atherosclerosis. Cells 2023, 12, 522. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.F.; Chen, W.J.; Tang, S.L.; Mo, Z.C.; Tang, Y.Y.; Li, Y.; Wang, J.L.; Liu, X.Y.; Peng, J.; et al. MicroRNA-27a/b regulates cellular cholesterol efflux, influx and esterification/hydrolysis in THP-1 macrophages. Atherosclerosis 2014, 234, 54–64. [Google Scholar] [CrossRef]
- Martinet, W.; Coornaert, I.; Puylaert, P.; De Meyer, G.R. Macrophage death as a pharmacological target in atherosclerosis. Front. Pharmacol. 2019, 10, 306–324. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.J.; Heo, J.H.; Hur, S.H.; Choi, H.H.; Kim, K.J.; Kim, J.H.; Park, K.H.; Lee, J.H.; Choi, Y.J.; et al. Combination moderate-Intensity statin and ezetimibe therapy for elderly patients with atherosclerosis. J. Am. Coll. Cardiol. 2023, 81, 1339–1349. [Google Scholar] [CrossRef]
- Loh, W.J.; Watts, G.F. The management of hypercholesterolemia in patients with neuromuscular disorder. Curr. Atheroscler. Rep. 2023, 25, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, L.; Zhu, W.; Wu, F.; Wu, B. Statin-related neurocognitive disorder: A real-world pharmacovigilance study based on the FDA adverse event reporting system. Expert. Rev. Clin. Pharmacol. 2024, 17, 255–261. [Google Scholar] [CrossRef]
- Wang, C.; Niimi, M.; Watanabe, T.; Wang, Y.; Liang, J.; Fan, J. Treatment of atherosclerosis by traditional chinese medicine: Questions and quandaries. Atherosclerosis 2018, 277, 136–144. [Google Scholar] [CrossRef]
- Xuan, X.; Zhang, J.; Fan, J.; Zhang, S. Research progress of traditional chinese medicine (TCM) in targeting inflammation and lipid metabolism disorder for arteriosclerosis intervention: A review. Medicine 2023, 102, e33748. [Google Scholar] [CrossRef]
- Dai, J.; Qiu, L.; Lu, Y.; Li, M. Recent advances of traditional chinese medicine against cardiovascular disease: Overview and potential mechanisms. Front. Endocrinol. 2024, 15, 1366285. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Han, Y.; Wang, Y. Traditional chinese medicine for cardiovascular disease: Efficacy and safety. Front. Cardiovasc. Med. 2024, 11, 1419169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.Y.; Shi, S.R.; Ma, C.N.; Lin, Y.P.; Song, W.G.; Guo, S.D. Natural products in atherosclerosis therapy by targeting PPARs: A review focusing on lipid metabolism and inflammation. Front. Cardiovasc. Med. 2024, 11, 1372055. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Ren, P.; Yang, Y.; Li, S.; Qin, X.; Zhang, M.; Zhou, M.; Liu, W. Qing-Xue-Xiao-Zhi formula attenuates atherosclerosis by inhibiting macrophage lipid accumulation and inflammatory response via TLR4/MyD88/NF-κB pathway regulation. Phytomedicine 2021, 93, 153812. [Google Scholar] [CrossRef]
- Xue, X.; Chen, T.; Wei, W.; Zhou, X.; Lin, Z.; Chen, L. Effects of alisma decoction on lipid metabolism and inflammatory response are mediated through the activation of the LXRα pathway in macrophage-derived foam cells. Int. J. Mol. Med. 2014, 33, 971–977. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, Y.; Lin, X.; Deng, S.; Sun, B.; Zheng, J.; Zeng, F.; Xue, Y. Relationship between fetal-type posterior cerebral artery and basilar artery atherosclerosis. Front. Neurol. 2025, 16, 1533281. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Guo, L.; Sakamoto, A.; Torii, S.; Sato, Y.; Cornelissen, A.; Kuntz, S.; Paek, K.H.; Fernandez, R.; Fuller, D.; et al. Diversity of macrophage phenotypes and responses in atherosclerosis. Cell. Mol. Life Sci. 2020, 77, 1919–1932. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, H.Q.; Hao, Y.M.; Ren, Z.; Qu, S.L.; Liu, L.S.; Wei, D.H.; Tang, Z.H.; Zhang, J.F.; Jiang, Z.S. Macrophage polarization in atherosclerosis. Clin. Chim. Acta 2020, 501, 142–146. [Google Scholar] [CrossRef]
- Eshghjoo, S.; Kim, D.M.; Jayaraman, A.; Sun, Y.; Alaniz, R.C. Macrophage polarization in atherosclerosis. Genes 2022, 13, 756. [Google Scholar] [CrossRef]
- Tabas, I.; Bornfeldt, K.E. Intracellular and intercellular aspects of macrophage immunometabolism in atherosclerosis. Circ. Res. 2020, 126, 1209–1227. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, H.; Chen, Y.; Liu, X.; Tian, J.; Shen, W. The role of macrophage iron overload and ferroptosis in atherosclerosis. Biomolecules 2022, 12, 1702. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, H.; Tang, B.; Luo, Y.; Yang, Y.; Zhong, X.; Chen, S.; Xu, X.; Huang, S.; Liu, C. Macrophages in cardiovascular diseases: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 130–174. [Google Scholar] [CrossRef]
- Mehta, A.; Shapiro, M.D. Apolipoproteins in vascular biology and atherosclerotic disease. Nat. Rev. Cardiol. 2022, 19, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar] [CrossRef]
- Wang, W.Z.; Liu, C.; Luo, J.Q.; Lei, L.J.; Chen, M.H.; Zhang, Y.Y.; Sheng, R.; Li, Y.N.; Wang, L.; Jiang, X.H.; et al. A novel small-molecule PCSK9 inhibitor E28362 ameliorates hyperlipidemia and atherosclerosis. Acta Pharmacol. Sin. 2024, 45, 2119–2133. [Google Scholar] [CrossRef] [PubMed]
- Doodnauth, S.A.; Grinstein, S.; Maxson, M.E. Constitutive and stimulated macropinocytosis in macrophages: Roles in immunity and in the pathogenesis of atherosclerosis. Phil. Trans. R. Soc. B 2019, 374, 20180147. [Google Scholar] [CrossRef]
- Pacitto, R.; Gaeta, I.; Swanson, J.A.; Yoshida, S. CXCL12-induced macropinocytosis modulates two distinct pathways to activate mTORC1 in macrophages. J. Leukoc. Biol. 2017, 101, 683–692. [Google Scholar] [CrossRef]
- Michael, D.R.; Ashlin, T.G.; Davies, C.S.; Gallagher, H.; Stoneman, T.W.; Buckley, M.L.; Ramji, D.P. Differential regulation of macropinocytosis in macrophages by cytokines: Implications for foam cell formation and atherosclerosis. Cytokine 2013, 64, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, A.; Ramji, D.P. Monitoring modified lipoprotein uptake and macropinocytosis associated with macrophage foam cell formation. Methods Mol. Biol. 2022, 2419, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Mielcarska, M.B.; Bossowska-Nowicka, M.; Gregorczyk-Zboroch, K.P.; Wyżewski, Z.; Szulc-Dąbrowska, L.; Gieryńska, M.; Toka, F.N. Syk and hrs regulate TLR3-mediated antiviral response in murine astrocytes. Oxid. Med. Cell. Longev. 2019, 2019, 6927380. [Google Scholar] [CrossRef]
- Palm, W. Metabolic functions of macropinocytosis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, X.; Zhang, M.; Tian, J.; Liu, M.; Jin, T.; Pan, J.; Gao, M.; An, F. WISP1 alleviates lipid deposition in macrophages via the PPARγ/CD36 pathway in the plaque formation of atherosclerosis. J. Cell. Mol. Med. 2020, 24, 11729–11741. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.; Takahashi, T.; Ueno, K.; Takahashi, K.; Ishitobi, M.; Kikuchi, M.; Higashima, M.; Wada, Y. Changes in EEG complexity with electroconvulsive therapy in a patient with autism spectrum disorders: A multiscale entropy approach. Front. Hum. Neurosci. 2015, 9, 106–113. [Google Scholar] [CrossRef]
- Shen, W.; Anwaier, G.; Cao, Y.; Lian, G.; Chen, C.; Liu, S.; Tuerdi, N.; Qi, R. Atheroprotective mechanisms of tilianin by inhibiting inflammation through down-regulating NF-κB pathway and foam cells formation. Front. Physiol. 2019, 10, 825–837. [Google Scholar] [CrossRef]
- Wang, H.; Franco, F.; Tsui, Y.C.; Xie, X.; Trefny, M.P.; Zappasodi, R.; Mohmood, S.R.; Fernández-García, J.; Tsai, C.H.; Schulze, I.; et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 2020, 21, 298–308. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Nabben, M.; Luiken, J. CD36 (SR-B2) as master regulator of cellular fatty acid homeostasis. Curr. Opin. Lipidol. 2022, 33, 103–111. [Google Scholar] [CrossRef]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The role of CD36 in cardiovascular disease. Cardiovasc. Res. 2022, 118, 115–129. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Ding, Q.; Li, Y.; Chen, Y.; Ruan, X.Z. CD36 senses dietary lipids and regulates lipids homeostasis in the intestine. Front. Physiol. 2021, 12, 115–129. [Google Scholar] [CrossRef]
- Bergquist, J. Proteomics to understand the degenerative matter. Free Radic. Biol. Med. 2014, 75, S10. [Google Scholar] [CrossRef]
- Tian, K.; Xu, Y.; Sahebkar, A.; Xu, S. CD36 in atherosclerosis: Pathophysiological mechanisms and therapeutic implications. Curr. Atheroscler. Rep. 2020, 22, 59–69. [Google Scholar] [CrossRef]
- Feng, L.; Gu, C.; Li, Y.; Huang, J. High glucose promotes CD36 expression by upregulating peroxisome proliferator-activated receptor γ levels to exacerbate lipid deposition in renal tubular cells. Biomed. Res. Int. 2017, 2017, 12–22. [Google Scholar] [CrossRef]
- Liu, Q.; Fan, J.; Bai, J.; Peng, L.; Zhang, T.; Deng, L.; Wang, G.; Zhao, Y.; Nong, J.; Zhang, M.; et al. IL-34 promotes foam cell formation by enhancing CD36 expression through p38 MAPK pathway. Sci. Rep. 2018, 8, 17347–17357. [Google Scholar] [CrossRef]
- Yazgan, B.; Sozen, E.; Karademir, B.; Ustunsoy, S.; Ince, U.; Zarkovic, N.; Ozer, N.K. CD36 expression in peripheral blood mononuclear cells reflects the onset of atherosclerosis. Biofactors 2018, 44, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Kashirskikh, D.A.; Sukhorukov, V.N.; Kalmykov, V.; Omelchenko, A.V.; Orekhov, A.N. Cholesterol transport dysfunction and its involvement in atherogenesis. Int. J. Mol. Sci. 2022, 23, 1332. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Apoprotein e and reverse cholesterol transport. Int. J. Mol. Sci. 2018, 19, 3479. [Google Scholar] [CrossRef]
- Yu, X.H.; Tang, C.K. ABCA1, ABCG1, and cholesterol homeostasis. Adv. Exp. Med. Biol. 2022, 1377, 95–107. [Google Scholar] [CrossRef]
- Mineo, C. Lipoprotein receptor signalling in atherosclerosis. Cardiovasc. Res. 2020, 116, 1254–1274. [Google Scholar] [CrossRef] [PubMed]
- Frambach, S.; de Haas, R.; Smeitink, J.A.M.; Rongen, G.A.; Russel, F.G.M.; Schirris, T.J.J. Brothers in arms: ABCA1- and ABCG1-mediated cholesterol efflux as promising targets in cardiovascular disease treatment. Pharmacol. Rev. 2020, 72, 152–190. [Google Scholar] [CrossRef] [PubMed]
- Lake, N.J.; Taylor, R.L.; Trahair, H.; Harikrishnan, K.N.; Curran, J.E.; Almeida, M.; Kulkarni, H.; Mukhamedova, N.; Hoang, A.; Low, H.; et al. TRAK2, a novel regulator of ABCA1 expression, cholesterol efflux and HDL biogenesis. Eur. Heart J. 2017, 38, 3579–3587. [Google Scholar] [CrossRef]
- Bi, X.; Vitali, C.; Cuchel, M.J.A.T.; Biology, V. ABCA1 and inflammation: From animal models to humans. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1551–1553. [Google Scholar] [CrossRef]
- Liu, P.; Peng, L.; Zhang, H.; Tang, P.M.; Zhao, T.; Yan, M.; Zhao, H.; Huang, X.; Lan, H.; Li, P. Tangshen formula attenuates diabetic nephropathy by promoting ABCA1-mediated renal cholesterol efflux in db/db mice. Front. Physiol. 2018, 9, 343–353. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhai, L.; Lu, J.; Sun, S.; Wang, D.; Zhao, D.; Sun, L.; Zhao, W.; Li, X.; Chen, Y. Shen-hong-tong-luo formula attenuates macrophage inflammation and lipid accumulation through the activation of the PPAR-γ/LXR-α/ABCA1 pathway. Oxid. Med. Cell. Longev. 2020, 2020, 3426925. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ren, K.; Chen, Q.; Li, H.; Yao, R.; Hu, H.; Lv, Y.C.; Zhao, G.J. Leonurine prevents atherosclerosis via promoting the expression of ABCA1 and ABCG1 in a Pparγ/Lxrα signaling pathway-dependent manner. Cell. Physiol. Biochem. 2017, 43, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, X.; Xu, X.; Liu, J.; Zhang, Z.; Wang, M.; Li, X.; Chen, H.; Zhao, D.; Wang, J.; et al. Active polypeptides from Hirudo inhibit endothelial cell inflammation and macrophage foam cell formation by regulating the LOX-1/LXR-α/ABCA1 pathway. Biomed. Pharmacother. 2019, 115, 108840–108850. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, B.; Liang, P.; Tong, Z.; Liu, M.; Lv, Q.; Liu, Y.; Liu, X.; Tang, Y.; Xiao, X. Nucleolin protects macrophages from oxLDL-induced foam cell formation through up-regulating ABCA1 expression. Biochem. Biophys. Res. Commun. 2017, 486, 364–371. [Google Scholar] [CrossRef]
- Liimatta, J.; Curschellas, E.; Altinkilic, E.M.; Naamneh Elzenaty, R.; Augsburger, P.; du Toit, T.; Voegel, C.D.; Breault, D.T.; Flück, C.E.; Pignatti, E. Adrenal abcg1 controls cholesterol flux and steroidogenesis. Endocrinology 2024, 165, bqae014. [Google Scholar] [CrossRef]
- Helal, O.; Berrougui, H.; Loued, S.; Khalil, A. Extra-virgin olive oil consumption improves the capacity of HDL to mediate cholesterol efflux and increases ABCA1 and ABCG1 expression in human macrophages. Br. J. Nutr. 2013, 109, 1844–1855. [Google Scholar] [CrossRef]
- Rafiei, A.; Ferns, G.A.; Ahmadi, R.; Khaledifar, A.; Rahimzadeh-Fallah, T.; Mohmmad-Rezaei, M.; Emami, S.; Bagheri, N. Expression levels of miR-27a, miR-329, ABCA1, and ABCG1 genes in peripheral blood mononuclear cells and their correlation with serum levels of oxidative stress and hs-CRP in the patients with coronary artery disease. IUBMB Life 2021, 73, 223–237. [Google Scholar] [CrossRef]
- Rozhkova, A.V.; Dmitrieva, V.G.; Nosova, E.V.; Dergunov, A.D.; Limborska, S.A.; Dergunova, L.V. Genomic variants and multilevel regulation of ABCA1, ABCG1, and SCARB1 expression in atherogenesis. J. Cardiovasc. Dev. Dis. 2021, 8, 170. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Zhang, M.; Liao, L.X.; Zou, J.; Wang, G.; Wan, X.J.; Zhou, L.; Li, H.; Qin, Y.S.; Yu, X.H.; et al. Long non-coding RNA PCA3 inhibits lipid accumulation and atherosclerosis through the miR-140-5p/RFX7/ABCA1 axis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158904. [Google Scholar] [CrossRef]
- Ramírez, C.M.; Zhang, X.; Bandyopadhyay, C.; Rotllan, N.; Sugiyama, M.G.; Aryal, B.; Liu, X.; He, S.; Kraehling, J.R.; Ulrich, V.; et al. Caveolin-1 regulates atherogenesis by attenuating low-density lipoprotein transcytosis and vascular inflammation independently of endothelial nitric oxide synthase activation. Circulation 2019, 140, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Durham, K.K.; Kluck, G.; Mak, K.C.; Deng, Y.D.; Trigatti, B.L. Treatment with apolipoprotein A1 protects mice against doxorubicin-induced cardiotoxicity in a scavenger receptor class B, type I-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, 1447–1457. [Google Scholar] [CrossRef]
- Huang, L.; Chambliss, K.L.; Gao, X.; Yuhanna, I.S.; Behling-Kelly, E.; Bergaya, S.; Ahmed, M.; Michaely, P.; Luby-Phelps, K.; Darehshouri, A.; et al. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature 2019, 569, 565–569. [Google Scholar] [CrossRef]
- Tao, H.; Yancey, P.G.; Babaev, V.R.; Blakemore, J.L.; Zhang, Y.; Ding, L.; Fazio, S.; Linton, M.F. Macrophage SR-BI mediates efferocytosis via Src/PI3K/Rac1 signaling and reduces atherosclerotic lesion necrosis. J. Lipid Res. 2015, 56, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Gharib, M.; Tao, H.; Fungwe, T.V.; Hajri, T. Cluster differentiating 36 (CD36) deficiency attenuates obesity-associated oxidative stress in the heart. PLoS ONE 2016, 11, e0155611. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Takahashi, M.; Wakabayashi, T.; Sakai, K.; Yamamuro, D.; Takei, A.; Takei, S.; Nagashima, S.; Yagyu, H.; Sekiya, M.; et al. Loss of ACAT1 attenuates atherosclerosis aggravated by loss of NCEH1 in bone marrow-derived cells. J. Atheroscler. Thromb. 2019, 26, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, P.P.; Zeng, G.F.; Zhang, T.; Ou Yang, X.P. miR-467b regulates the cholesterol ester formation via targeting ACAT1 gene in RAW 264.7 macrophages. Biochimie 2017, 132, 38–44. [Google Scholar] [CrossRef]
- Terasaki, M.; Yashima, H.; Mori, Y.; Saito, T.; Matsui, T.; Hiromura, M.; Kushima, H.; Osaka, N.; Ohara, M.; Fukui, T.; et al. A dipeptidyl peptidase-4 inhibitor inhibits foam cell formation of macrophages in type 1 diabetes via suppression of CD36 and ACAT-1 expression. Int. J. Mol. Sci. 2020, 21, 4811. [Google Scholar] [CrossRef]
- Ayyagari, V.N.; Wang, X.; Diaz-Sylvester, P.L.; Groesch, K.; Brard, L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS ONE 2020, 15, e0228024. [Google Scholar] [CrossRef]
- Chiwata, T.; Aragane, K.; Fujinami, K.; Kojima, K.; Ishibashi, S.; Yamada, N.; Kusunoki, J. Direct effect of an acyl-CoA:cholesterol acyltransferase inhibitor, F-1394, on atherosclerosis in apolipoprotein E and low density lipoprotein receptor double knockout mice. Br. J. Pharmacol. 2001, 133, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cheng, B.; Guo, X.; Wu, Q.; Sun, S.; He, P. PPARα/γ signaling pathways are involved in Chlamydia pneumoniae-induced foam cell formation via upregulation of SR-A1 and ACAT1 and downregulation of ABCA1/G1. Microb. Pathog. 2021, 161, 105284. [Google Scholar] [CrossRef]
- Sakai, K.; Igarashi, M.; Yamamuro, D.; Ohshiro, T.; Nagashima, S.; Takahashi, M.; Enkhtuvshin, B.; Sekiya, M.; Okazaki, H.; Osuga, J.; et al. Critical role of neutral cholesteryl ester hydrolase 1 in cholesteryl ester hydrolysis in murine macrophages. J. Lipid Res. 2014, 55, 2033–2040. [Google Scholar] [CrossRef]
- Sekiya, M.; Osuga, J.; Igarashi, M.; Okazaki, H.; Ishibashi, S. The role of neutral cholesterol ester hydrolysis in macrophage foam cells. J. Atheroscler. Thromb. 2011, 18, 359–364. [Google Scholar] [CrossRef]
- Gu, H.F.; Li, N.; Xu, Z.Q.; Hu, L.; Li, H.; Zhang, R.J.; Chen, R.M.; Zheng, X.L.; Tang, Y.L.; Liao, D.F. Chronic unpredictable mild stress promotes atherosclerosis via HMGB1/TLR4-mediated downregulation of PPARγ/LXRα/ABCA1 in ApoE-/- mice. Front. Physiol. 2019, 10, 165–178. [Google Scholar] [CrossRef]
- Luo, P.; Yang, J.; Jian, L.; Dong, J.; Yin, S.; Luo, C.; Zhou, S. Knockdown of PGBD5 inhibits the malignant progression of glioma through upregulation of the PPAR pathway. Int. J. Oncol. 2024, 64, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Changizi, Z.; Kajbaf, F.; Moslehi, A. An overview of the role of peroxisome proliferator-activated receptors in liver diseases. J. Clin. Transl. Hepatol. 2023, 11, 1542–1552. [Google Scholar] [CrossRef]
- Zheng, Y.; Shao, M.; Zheng, Y.; Sun, W.; Qin, S.; Sun, Z.; Zhu, L.; Guan, Y.; Wang, Q.; Wang, Y.; et al. PPARs in atherosclerosis: The spatial and temporal features from mechanism to druggable targets. J. Adv. Res. 2025, 69, 225–244. [Google Scholar] [CrossRef]
- Deng, H.; Wu, D.; Guo, M.; Sun, C.; Lu, B.; Yang, L.; Sun, Y.; Fan, G.; Chen, Y.; Gao, Q.; et al. Ethanol extracts of danlou tablet attenuate atherosclerosis via inhibiting inflammation and promoting lipid effluent. Pharmacol. Res. 2019, 146, 104306. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Chang, X.; Wang, J.; Zou, X.; Zhao, Z.; Huang, Z.; Wang, Y.; Yu, B. Sini decoction intervention on atherosclerosis via PPARγ-LXRα-ABCA1 pathway in rabbits. Open Life Sci. 2018, 13, 446–455. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, H.; Li, Y.; Wang, Y.; Zheng, Y.; Liang, J.; Zhang, S.; Liu, M.; Fang, Z. Yin-xing-tong-mai decoction attenuates atherosclerosis via activating PPARγ-LXRα-ABCA1/ABCG1 pathway. Pharmacol. Res. 2021, 169, 105639. [Google Scholar] [CrossRef]
- Liang, P.L.; Chen, X.L.; Gong, M.J.; Xu, Y.; Tu, H.S.; Zhang, L.; Liao, B.S.; Qiu, X.H.; Zhang, J.; Huang, Z.H.; et al. Guang chen pi (the pericarp of citrus reticulata blanco’s cultivars ‘chachi’) inhibits macrophage-derived foam cell formation. J. Ethnopharmacol. 2022, 293, 115328. [Google Scholar] [CrossRef]
- Kaseda, R.; Tsuchida, Y.; Yang, H.C.; Yancey, P.G.; Zhong, J.; Tao, H.; Bian, A.; Fogo, A.B.; Linton, M.R.F.; Fazio, S.; et al. Chronic kidney disease alters lipid trafficking and inflammatory responses in macrophages: Effects of liver X receptor agonism. BMC Nephrol. 2018, 19, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, X. Activation of PPARγ does not contribute to macrophage ABCA1 expression and ABCA1-mediated cholesterol efflux to apoAI. Biochem. Biophys. Res. Commun. 2017, 482, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Bellora, F.; Ricciarelli, R.; De Ciucis, C.; Furfaro, A.; Leardi, R.; Colla, R.; Pacini, D.; Traverso, N.; Moretta, A.; et al. Oxysterol mixture and, in particular, 27-hydroxycholesterol drive M2 polarization of human macrophages. Biofactors 2016, 42, 80–92. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, Y.; Wu, Q.; Gao, G.; Liu, Y.; Xiong, Y.; Huang, C.; Wu, S. Activation of LXRα improves cardiac remodeling induced by pulmonary artery hypertension in rats. Sci. Rep. 2017, 7, 6169. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, signaling and its role in atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, oxLDL, and atherosclerosis. Mediators Inflamm. 2013, 2013, 52786. [Google Scholar] [CrossRef]
- Singh, R.K.; Haka, A.S.; Asmal, A.; Barbosa-Lorenzi, V.C.; Grosheva, I.; Chin, H.F.; Xiong, Y.; Hla, T.; Maxfield, F.R. TLR4 (toll-like receptor 4)-dependent signaling drives extracellular catabolism of LDL (low-density lipoprotein) aggregates. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 86–102. [Google Scholar] [CrossRef]
- Li, Y.; Shen, S.; Ding, S.; Wang, L. Toll-like receptor 2 downregulates the cholesterol efflux by activating the nuclear factor-κB pathway in macrophages and may be a potential therapeutic target for the prevention of atherosclerosis. Exp. Ther. Med. 2018, 15, 198–204. [Google Scholar] [CrossRef]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Byeon, H.E.; Kim, J.W.; Kim, H.A.; Suh, C.H.; Choi, S.; Linton, M.F.; Jung, J.Y. Inhibition of toll-like receptors alters macrophage cholesterol efflux and foam cell formation. Int. J. Mol. Sci. 2024, 25, 6808. [Google Scholar] [CrossRef]
- Taront, S.; Dieudonné, A.; Blanchard, S.; Jeannin, P.; Lassalle, P.; Delneste, Y.; Gosset, P. Implication of scavenger receptors in the interactions between diesel exhaust particles and immature or mature dendritic cells. Part. Fibre Toxicol. 2009, 6, 9–23. [Google Scholar] [CrossRef]
- Yu, R.; Lv, Y.; Wang, J.; Pan, N.; Zhang, R.; Wang, X.; Yu, H.; Tan, L.; Zhao, Y.; Li, B. Baicalin promotes cholesterol efflux by regulating the expression of SR-BI in macrophages. Exp. Ther. Med. 2016, 12, 4113–4120. [Google Scholar] [CrossRef]
- He, X.W.; Yu, D.; Li, W.L.; Zheng, Z.; Lv, C.L.; Li, C.; Liu, P.; Xu, C.Q.; Hu, X.F.; Jin, X.P. Anti-atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARγ-LXRα-ABCA1/ABCG1 pathway. Biomed. Pharmacother. 2016, 83, 257–264. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, K.; Qin, S.; Jing, Y.; Liu, S.; Li, D.; Peng, C. Astragalin: A food-origin flavonoid with therapeutic effect for multiple diseases. Front. Pharmacol. 2023, 14, 1265960. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Zhang, M.; Wang, G.; Zou, J.; Gao, J.H.; Zhou, L.; Wan, X.J.; Zhang, D.W.; Yu, X.H.; Tang, C.K. Astragalin retards atherosclerosis by promoting cholesterol efflux and inhibiting the inflammatory response via upregulating ABCA1 and ABCG1 expression in macrophages. J. Cardiovasc. Pharmacol. 2021, 77, 217–227. [Google Scholar] [CrossRef]
- Qi, J.; Cui, J.; Mi, B.; Yan, X.; Xu, W.; Ma, H.; Zhang, Q.; Xu, F. Isoliquiritigenin inhibits atherosclerosis by blocking TRPC5 channel expression. Cardiovasc. Ther. 2020, 2020, 1926249. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Gesang, Q.; Cao, J.; Qian, M.; Ma, L.; Wu, D.; Yu, H. Isoliquiritigenin attenuates atherogenesis in apolipoprotein e-deficient mice. Int. J. Mol. Sci. 2016, 17, 1932. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Kong, L.X.; Li, J.; He, H.X.; Zhou, Y.D. Kaempferol suppresses lipid accumulation in macrophages through the downregulation of cluster of differentiation 36 and the upregulation of scavenger receptor class B type I and ATP-binding cassette transporters A1 and G1. Int. J. Mol. Med. 2013, 31, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, L.; Guo, X.; Zhang, S.; Wang, J.; Zhou, F.; Liu, L.; Tang, Y.; Yao, P. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein e knockout mice: A critical role of NADPH oxidase. Food Chem. Toxicol. 2017, 105, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, E.; Wang, F.; Wang, T.; Qin, Z.; Niu, S.; Qiu, C. Quercetin increases macrophage cholesterol efflux to inhibit foam cell formation through activating PPARγ-ABCA1 pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 10854–10860. [Google Scholar]
- Liu, S.; Ai, Q.; Feng, K.; Li, Y.; Liu, X. The cardioprotective effect of dihydromyricetin prevents ischemia-reperfusion-induced apoptosis in vivo and in vitro via the PI3K/Akt and HIF-1α signaling pathways. Apoptosis 2016, 21, 1366–1385. [Google Scholar] [CrossRef]
- Zhou, Q.; Gu, Y.; Lang, H.; Wang, X.; Chen, K.; Gong, X.; Zhou, M.; Ran, L.; Zhu, J.; Mi, M. Dihydromyricetin prevents obesity-induced slow-twitch-fiber reduction partially via FLCN/FNIP1/AMPK pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1282–1291. [Google Scholar] [CrossRef]
- Zeng, Y.; Peng, Y.; Tang, K.; Wang, Y.Q.; Zhao, Z.Y.; Wei, X.Y.; Xu, X.L. Dihydromyricetin ameliorates foam cell formation via LXRα-ABCA1/ABCG1-dependent cholesterol efflux in macrophages. Biomed. Pharmacother. 2018, 101, 543–552. [Google Scholar] [CrossRef]
- Sugasawa, N.; Katagi, A.; Kurobe, H.; Nakayama, T.; Nishio, C.; Takumi, H.; Higashiguchi, F.; Aihara, K.I.; Shimabukuro, M.; Sata, M.; et al. Inhibition of atherosclerotic plaque development by oral Administration of α-glucosyl hesperidin and water-dispersible hesperetin in apolipoprotein E knockout mice. J. Am. Coll. Nutr. 2019, 38, 15–22. [Google Scholar] [CrossRef]
- Chen, X.; Zou, D.; Chen, X.; Wu, H.; Xu, D. Hesperetin inhibits foam cell formation and promotes cholesterol efflux in THP-1-derived macrophages by activating LXRα signal in an AMPK-dependent manner. J. Physiol. Biochem. 2021, 77, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, L.; Qian, P.; Duan, H.; Wu, J.; Li, B.; Wang, S. Icariin inhibits foam cell formation by down-regulating the expression of CD36 and up-regulating the expression of SR-BI. J. Cell. Biochem. 2015, 116, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Jamuna, S.; Ashokkumar, R.; Sakeena Sadullah, M.S.; Devaraj, S.N. Oligomeric proanthocyanidins and epigallocatechin gallate aggravate autophagy of foam cells through the activation of Class III PI3K/Beclin1-complex mediated cholesterol efflux. Biofactors 2019, 45, 763–773. [Google Scholar] [CrossRef]
- Lu, C.N.; Yuan, Z.G.; Zhang, X.L.; Yan, R.; Zhao, Y.Q.; Liao, M.; Chen, J.X. Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of NF-κB signaling pathway. Int. Immunopharmacol. 2012, 14, 121–126. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Qi, G. Mechanism of the effect of saikosaponin on atherosclerosis in vitro is based on the MAPK signaling pathway. Mol. Med. Report 2017, 16, 8868–8874. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Shi, M.; Liu, W.; Yang, Z.; Fu, Y. Saikosaponin a inhibits LPS-induced inflammatory response by inducing liver X receptor alpha activation in primary mouse macrophages. Oncotarget 2016, 7, 48995–49007. [Google Scholar] [CrossRef]
- He, D.; Wang, H.; Xu, L.; Wang, X.; Peng, K.; Wang, L.; Liu, P.; Qu, P. Saikosaponin-a attenuates oxidized LDL uptake and prompts cholesterol efflux in THP-1 cells. J. Cardiovasc. Pharmacol. 2016, 67, 510–518. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, H.; Luo, Y.; Zhang, J.; Wang, M.; Liao, P.; Cao, L.; Guo, P.; Sun, G.; Sun, X. Gypenoside XVII prevents atherosclerosis by attenuating endothelial apoptosis and oxidative stress: Insight into the eRα-mediated PI3K/Akt pathway. Int. J. Mol. Sci. 2017, 18, 77. [Google Scholar] [CrossRef]
- Deng, W.Y.; Zhou, C.L.; Zeng, M.Y. Gypenoside XVII inhibits ox-LDL-induced macrophage inflammatory responses and promotes cholesterol efflux through activating the miR-182-5p/HDAC9 signaling pathway. J. Ethnopharmacol. 2024, 319, 117070. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, H.; Shao, B.; Wang, Y.; Liu, C.; Guo, M. Celosins inhibit atherosclerosis in apoE-/- mice and promote autophagy flow. J. Ethnopharmacol. 2018, 215, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.H.; Ooi, B.K.; Ahmed, N.; Lim, Y.M. Maslinic acid modulates secreted phospholipase A2-IIA (sPLA2-IIA)-mediated inflammatory effects in macrophage foam cells formation. J. Biosci. 2018, 43, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.K.; Phang, S.W.; Yong, P.V.C.; Chellappan, D.K.; Dua, K.; Khaw, K.Y.; Goh, B.H.; Pusparajah, P.; Yap, W.H. In vitro evaluation of the involvement of Nrf2 in maslinic acid-mediated anti-inflammatory effects in atheroma pathogenesis. Life Sci. 2021, 278, 119658. [Google Scholar] [CrossRef]
- Phang, S.W.; Ooi, B.K.; Ahemad, N.; Yap, W.H. Maslinic acid suppresses macrophage foam cells formation: Regulation of monocyte recruitment and macrophage lipids homeostasis. Vascul. Pharmacol. 2020, 128–129, 106675. [Google Scholar] [CrossRef]
- Yu, L.; Lu, H.; Yang, X.; Li, R.; Shi, J.; Yu, Y.; Ma, C.; Sun, F.; Zhang, S.; Zhang, F. Diosgenin alleviates hypercholesterolemia via SRB1/CES-1/CYP7A1/FXR pathway in high-fat diet-fed rats. Toxicol. Appl. Pharmacol. 2021, 412, 115388. [Google Scholar] [CrossRef]
- Lv, Y.C.; Yang, J.; Yao, F.; Xie, W.; Tang, Y.Y.; Ouyang, X.P.; He, P.P.; Tan, Y.L.; Li, L.; Zhang, M.; et al. Diosgenin inhibits atherosclerosis via suppressing the MiR-19b-induced downregulation of ATP-binding cassette transporter A1. Atherosclerosis 2015, 240, 80–114. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Z.; Hu, X.; Liu, X.; Gui, L.; Cai, Z.; Dai, C. Protective effect of panax notoginseng saponins on apolipoprotein-E-deficient atherosclerosis-prone mice. Curr. Pharm. Des. 2022, 28, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, J.; Shi, K.; Lu, Y. Panax notoginseng saponins improves lipid metabolism and prevents atherosclerosis in mice with steroid-resistant lupus nephritis via the SIRT1/PPARγ signaling pathway. J. Steroid Biochem. Mol. Biol. 2025, 245, 106631. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.S.; Liu, D.N.; Huang, G.; Xu, Z.Z.; Jia, Y.; Zhang, H.G.; Li, X.H.; He, F.T. Panax notoginseng saponins attenuate atherosclerosis via reciprocal regulation of lipid metabolism and inflammation by inducing liver X receptor alpha expression. J. Ethnopharmacol. 2012, 142, 732–738. [Google Scholar] [CrossRef]
- Gonde, D.P.; Bhole, B.K.; Kakad, K.S. Andrographolide, diterpenoid constituent of andrographis paniculata: Review on botany, phytochemistry, molecular docking analysis, and pharmacology. Ann. Pharm. Fr. 2024, 82, 15–43. [Google Scholar] [CrossRef]
- Wu, T.; Peng, Y.; Yan, S.; Li, N.; Chen, Y.; Lan, T. Andrographolide ameliorates atherosclerosis by suppressing pro-inflammation and ROS generation-mediated foam cell formation. Inflammation 2018, 41, 1681–1689. [Google Scholar] [CrossRef]
- Hamidy, M.; Oenzil, F.; Yanwirasti, Y.; Aldi, Y.J.K.E. Effect of Andrographolide on Foam Cell Formation at the Initiation Stage of Atherosclerosis. KnE Eng. 2019, 2019, 329–336. [Google Scholar] [CrossRef]
- Lin, H.C.; Lii, C.K.; Chen, H.C.; Lin, A.H.; Yang, Y.C.; Chen, H.W. Andrographolide inhibits oxidized LDL-induced cholesterol accumulation and foam cell formation in macrophages. Am. J. Chin. Med. 2018, 46, 87–106. [Google Scholar] [CrossRef]
- Sherawat, K.; Mehan, S. Tanshinone-IIA mediated neuroprotection by modulating neuronal pathways. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 1647–1667. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Huang, E.; Gao, S.; Li, H.; Lu, J.; Tian, K.; Little, P.J.; Shen, X.; Xu, S.; et al. Tanshinone IIA suppresses cholesterol accumulation in human macrophages: Role of heme oxygenase-1. J. Lipid Res. 2014, 55, 201–213. [Google Scholar] [CrossRef]
- Tan, Y.L.; Ou, H.X.; Zhang, M.; Gong, D.; Zhao, Z.W.; Chen, L.Y.; Xia, X.D.; Mo, Z.C.; Tang, C.K. Tanshinone IIA promotes macrophage cholesterol efflux and attenuates atherosclerosis of apoE-/- mice by omentin-1/ABCA1 pathway. Curr. Pharm. Biotechnol. 2019, 20, 422–432. [Google Scholar] [CrossRef]
- Wu, X.W.; Feng, Q.L.; Xie, Y.F.; Song, S.C.; Cheng, B.; Ma, Y.; Li, Q.; Ji, X.; Li, X.L.; Cao, G.; et al. Diverse diterpenoids from Callicarpa rubella Lindl. as natural inhibitors of macrophage foam cell formation. Phytochemistry 2023, 213, 113748. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, X.; Shi, P.; Ma, H.; Fang, F.; Feng, Q.; Zhao, S.; Zhang, R.; Huang, J.; Xu, X.; et al. Diterpenoids inhibit ox-LDL-induced foam cell formation in RAW264.7 cells by promoting ABCA1 mediated cholesterol efflux. Front. Pharmacol. 2023, 14, 1066758. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Q.; Xu, Y.; Liu, C.; Wang, X.; He, X.; Zhu, N.; Liu, J.; Wu, Y.; Li, Y.; et al. Rutaecarpine suppresses atherosclerosis in ApoE-/- mice through upregulating ABCA1 and SR-BI within RCT. J. Lipid Res. 2014, 55, 1634–1647. [Google Scholar] [CrossRef]
- Wang, L.; Eftekhari, P.; Schachner, D.; Ignatova, I.D.; Palme, V.; Schilcher, N.; Ladurner, A.; Heiss, E.H.; Stangl, H.; Dirsch, V.M.; et al. Novel interactomics approach identifies ABCA1 as direct target of evodiamine, which increases macrophage cholesterol efflux. Sci. Rep. 2018, 8, 11061. [Google Scholar] [CrossRef]
- Yang, D.; Jia, W.; Zhu, Y.Z. Leonurine, a potential agent of traditional chinese medicine: Recent updates and future perspectives. Nat. Prod. Commun. 2016, 11, 1757–1761. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Feng, J.; Dou, Z.; Sun, X.; Hu, Y.; Chen, Z.; Liu, L.; Xu, H.; Du, M.; Tang, P.; et al. Berberine as a novel ACSL4 inhibitor to suppress endothelial ferroptosis and atherosclerosis. Biomed. Pharmacother. 2024, 177, 117081. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, J.; Geng, J.; Hu, T.; Wang, B.; Yan, W.; Jiang, Y.; Li, J.; Liu, S. Berberine treatment reduces atherosclerosis by mediating gut microbiota in apoE-/- mice. Biomed. Pharmacother. 2018, 107, 1556–1563. [Google Scholar] [CrossRef]
- Man, B.; Hu, C.; Yang, G.; Xiang, J.; Yang, S.; Ma, C. Berberine attenuates diabetic atherosclerosis via enhancing the interplay between KLF16 and PPARα in ApoE-/- mice. Biochem. Biophys. Res. Commun. 2022, 624, 59–67. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, Q.; Yu, Y.; Yang, F.; Bai, R.; Fan, X. Efficacy and underlying mechanisms of berberine against lipid metabolic diseases: A review. Front. Pharmacol. 2023, 14, 1283784. [Google Scholar] [CrossRef]
- Yang, X.J.; Liu, F.; Feng, N.; Ding, X.S.; Chen, Y.; Zhu, S.X.; Yang, L.C.; Feng, X.F. Berberine attenuates cholesterol accumulation in macrophage foam cells by suppressing AP-1 activity and activation of the Nrf2/HO-1 pathway. J. Cardiovasc. Pharmacol. 2020, 75, 45–53. [Google Scholar] [CrossRef]
- Han, J.; Zhang, S.; He, J.; Li, T. Piperine: Chemistry and biology. Toxins 2023, 15, 696. [Google Scholar] [CrossRef]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef]
- Wang, L.; Palme, V.; Rotter, S.; Schilcher, N.; Cukaj, M.; Wang, D.; Ladurner, A.; Heiss, E.H.; Stangl, H.; Dirsch, V.M.; et al. Piperine inhibits ABCA1 degradation and promotes cholesterol efflux from THP-1-derived macrophages. Mol. Nutr. Food Res. 2017, 61, 1500960. [Google Scholar] [CrossRef]
- Wei, H.; Yue, S.; Zhang, S.; Lu, L. Lipid-lowering effect of the pleurotus eryngii (king oyster mushroom) polysaccharide from solid-state fermentation on both macrophage-derived foam cells and zebrafish models. Polymers 2018, 10, 492. [Google Scholar] [CrossRef]
- Nakahara, D.; Nan, C.; Mori, K.; Hanayama, M.; Kikuchi, H.; Hirai, S.; Egashira, Y. Effect of mushroom polysaccharides from Pleurotus eryngii on obesity and gut microbiota in mice fed a high-fat diet. Eur. J. Nutr. 2020, 59, 3231–3244. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yong, Y.; Xia, X.; Wang, Z.; Liang, Y.; Zhang, S.; Lu, L. The excreted polysaccharide of pleurotus eryngii inhibits the foam-cell formation via down-regulation of CD36. Carbohydr. Polym. 2014, 112, 16–23. [Google Scholar] [CrossRef]
- Chen, J.; Yong, Y.; Xing, M.; Gu, Y.; Zhang, Z.; Zhang, S.; Lu, L. Characterization of polysaccharides with marked inhibitory effect on lipid accumulation in Pleurotus eryngii. Carbohydr. Polym. 2013, 97, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, Y.; Wang, H.; Tian, X.; Zhang, X.; Zhang, Q.; Wei, Q.; Ji, K. Fucoidan-induced reduction of lipid accumulation in foam cells through overexpression of lysosome genes. Int. J. Biol. Macromol. 2024, 263, 130451. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, W.; Wang, T.; Jin, L.; Liu, T.; Li, X.; Guan, Z.; Jiang, Z.; Meng, X.; Wang, J.; et al. Low molecule weight fucoidan mitigates atherosclerosis in ApoE-/- mouse model through activating multiple signal pathway. Carbohydr. Polym. 2019, 206, 110–120. [Google Scholar] [CrossRef]
- Mirza, Z.; Al-Saedi, D.A.; Saddeek, S.; Almowallad, S.; AlMassabi, R.F.; Huwait, E. Atheroprotective effect of fucoidan in THP-1 macrophages by potential upregulation of ABCA1. Biomedicines 2023, 11, 2929. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, M.; Wang, C.; Guan, S.; Wang, L.; Cong, B.; Zhu, W.; Xu, Y. Low-molecular-weight fucoidan bidirectionally regulates lipid uptake and cholesterol efflux through the p38 MAPK phosphorylation. Int. J. Biol. Macromol. 2022, 220, 371–384. [Google Scholar] [CrossRef]
- Li, H.; Huang, Z.; Zeng, F. Opuntia dillenii Haw. Polysaccharide promotes cholesterol efflux in THP-1-derived foam cells via the PPARγ-LXRα signaling pathway. Molecules 2022, 27, 8639. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Zhu, C. Biological and neurological activities of astaxanthin (review). Mol. Med. Rep. 2022, 26, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiu, Y.; Li, W.; Tang, A.; Huang, H.; Yao, W.; Li, H.; Zou, T. Astaxanthin alleviates foam cell formation and promotes cholesterol efflux in ox-LDL-induced raw264.7 cells via circTPP2/miR-3073b-5p/ABCA1 pathway. Molecules 2023, 28, 1701. [Google Scholar] [CrossRef]
- Sánchez-Gloria, J.L.; Arellano-Buendía, A.S.; Juárez-Rojas, J.G.; García-Arroyo, F.E.; Argüello-García, R.; Sánchez-Muñoz, F.; Sánchez-Lozada, L.G.; Osorio-Alonso, H. Cellular mechanisms underlying the cardioprotective role of allicin on cardiovascular diseases. Int. J. Mol. Sci. 2022, 23, 9082. [Google Scholar] [CrossRef]
- Lin, X.L.; Hu, H.J.; Liu, Y.B.; Hu, X.M.; Fan, X.J.; Zou, W.W.; Pan, Y.Q.; Zhou, W.Q.; Peng, M.W.; Gu, C.H. Allicin induces the upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells. Int. J. Mol. Med. 2017, 39, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Xu, A.G.; Yao, M.Y.; Guo, L.; Zhao, L.S. Emodin enhances cholesterol efflux by activating peroxisome proliferator-activated receptor-γ in oxidized low density lipoprotein-loaded THP1 macrophages. Clin. Exp. Pharmacol. Physiol. 2014, 41, 679–684. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Abdollahi, E.; Nikfar, B.; Chaichian, S.; Ekhlasi-Hundrieser, M. Curcumin as a potential modulator of M1 and M2 macrophages: New insights in atherosclerosis therapy. Heart Fail. Rev. 2019, 24, 399–409. [Google Scholar] [CrossRef]
- Zhang, S.; Zou, J.; Li, P.; Zheng, X.; Feng, D. Curcumin protects against atherosclerosis in apolipoprotein e-knockout mice by inhibiting toll-like receptor 4 expression. J. Agric. Food Chem. 2018, 66, 449–456. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, S.; Li, P.; Zheng, X.; Feng, D. Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice. Nutr. Res. 2018, 56, 32–40. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Zabihi, N.A.; Bagheri, R.K.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Intravenous curcumin mitigates atherosclerosis progression in cholesterol-fed rabbits. Adv. Exp. Med. Biol. 2021, 1308, 45–54. [Google Scholar] [CrossRef]
- Zhong, Y.; Feng, J.; Fan, Z.; Li, J. Curcumin increases cholesterol efflux via heme oxygenase-1-mediated ABCA1 and SR-BI expression in macrophages. Mol. Med. Rep. 2018, 17, 6138–6143. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Fan, X.; Zhang, H.; Xue, D.; Pan, Z. The protective effect of mangiferin on osteoarthritis: An in vitro and in vivo study. Physiol. Res. 2022, 71, 135–145. [Google Scholar] [CrossRef]
- He, Z.; Zhu, H.; Liu, J.; Kwek, E.; Ma, K.Y.; Chen, Z.Y. Mangiferin alleviates trimethylamine-N-oxide (TMAO)-induced atherogenesis and modulates gut microbiota in mice. Food Funct. 2023, 14, 9212–9225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Karmakar, T.; Ghosh, N.; Basak, S.; Gopal Sahoo, N. Targeting mangiferin ioaded n-succinyl chitosan-alginate grafted nanoparticles against atherosclerosis-acase study against diabetes mediated hyperlipidemia in rat. Food Chem. 2022, 370, 131376. [Google Scholar] [CrossRef]
- Ren, K.; Li, H.; Zhou, H.F.; Liang, Y.; Tong, M.; Chen, L.; Zheng, X.L.; Zhao, G.J. Mangiferin promotes macrophage cholesterol efflux and protects against atherosclerosis by augmenting the expression of ABCA1 and ABCG1. Aging 2019, 11, 10992–11009. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, S.; Bao, R.; Wang, D.; Wu, Y.; Zhang, Y.; Liu, M.; Wang, T. Combination of mangiferin and T0901317 targeting autophagy promotes cholesterol efflux from macrophage foam cell in atherosclerosis. Chin. Med. 2024, 19, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhang, L.; Chen, W.; Sun, M.; Liu, W.; Li, X. Resveratrol modulates the redox response and bile acid metabolism to maintain the cholesterol homeostasis in fish megalobrama amblycephala offered a high-carbohydrate diet. Antioxidants 2023, 12, 121. [Google Scholar] [CrossRef]
- Ye, G.; Chen, G.; Gao, H.; Lin, Y.; Liao, X.; Zhang, H.; Liu, X.; Chi, Y.; Huang, Q.; Zhu, H.; et al. Resveratrol inhibits lipid accumulation in the intestine of atherosclerotic mice and macrophages. J. Cell. Mol. Med. 2019, 23, 4313–4325. [Google Scholar] [CrossRef]
- Wang, L.; Ladurner, A.; Latkolik, S.; Schwaiger, S.; Linder, T.; Hošek, J.; Palme, V.; Schilcher, N.; Polanský, O.; Heiss, E.H.; et al. Leoligin, the major lignan from edelweiss (leontopodium nivale subsp. alpinum), promotes cholesterol efflux from THP-1 macrophages. J. Nat. Prod. 2016, 79, 1651–1657. [Google Scholar] [CrossRef]
- Xu, X.; Piao, H.N.; Aosai, F.; Zeng, X.Y.; Cheng, J.H.; Cui, Y.X.; Li, J.; Ma, J.; Piao, H.R.; Jin, X.; et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br. J. Pharmacol. 2020, 177, 5224–5245. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Pang, L.; Huang, G.; Huang, J.; Shi, M.; Sun, X.; Wang, Y. Arctigenin promotes cholesterol efflux from THP-1 macrophages through PPAR-γ/LXR-α signaling pathway. Biochem. Biophys. Res. Commun. 2013, 441, 321–326. [Google Scholar] [CrossRef]
- Men, X.; Shi, X.; Xu, Q.; Liu, M.; Yang, H.; Wang, L.; Men, X.; Xu, H. Exploring the pathogenesis of chronic atrophic gastritis with atherosclerosis via microarray data analysis. Medicine 2024, 103, e37798. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, L.; Cui, C.; Chen, H.; Zeng, W.; Li, X. MicroRNA-19a-3p inhibits endothelial dysfunction in atherosclerosis by targeting JCAD. BMC Cardiovasc. Disord. 2024, 24, 394–407. [Google Scholar] [CrossRef]
- Li, C.; Hao, J.; Zheng, Y.; Wang, C.; Yang, J.; Wang, W.; Zhang, K.; Shao, C.; Hui, W.; Wang, J.; et al. The changing landscape of drug clinical trials on cardiometabolic diseases in China, 2009–2021. Diabetol. Metab. Syndr. 2023, 15, 66–79. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, H.; Wang, Y.; Xiao, W.; Zhang, Y.; Shi, D. Small rodent models of atherosclerosis. Biomed. Pharmacother. 2020, 129, 110426. [Google Scholar] [CrossRef]
- Peng, J.; Ge, C.; Shang, K.; Liu, S.; Jiang, Y. Comprehensive profiling of the chemical constituents in Dayuanyin decoction using UPLC-QTOF-MS combined with molecular networking. Pharm. Biol. 2024, 62, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, Z.; Yang, L.; Fang, Y.; Lu, S.; Akakuru, O.U.; Huang, S.; Li, J.; Ma, S.; Wu, A. HPDA/Zn as a CREB inhibitor for ultrasound imaging and stabilization of atherosclerosis plaque. Chin. J. Chem. 2023, 41, 199–206. [Google Scholar] [CrossRef]
- Bao, M.H.; Li, G.Y.; Huang, X.S.; Tang, L.; Dong, L.P.; Li, J.M. Long noncoding RNA LINC00657 acting as a miR-590-3p sponge to facilitate low concentration oxidized low-density lipoprotein-induced angiogenesis. Mol. Pharmacol. 2018, 93, 368–375. [Google Scholar] [CrossRef]
- Du, F.; Ye, Z.; He, A.; Yuan, J.; Su, M.; Jia, Q.; Wang, H.; Yang, P.; Yang, Z.; Ning, P.; et al. An engineered α1β1 integrin-mediated FcγRI signaling component to control enhanced CAR macrophage activation and phagocytosis. J. Control. Release 2025, 377, 689–703. [Google Scholar] [CrossRef]
- Cheng, M.; Li, T.; Hu, E.; Yan, Q.; Li, H.; Wang, Y.; Luo, J.; Tang, T. A novel strategy of integrating network pharmacology and transcriptome reveals antiapoptotic mechanisms of buyang huanwu decoction in treating intracerebral hemorrhage. J. Ethnopharmacol. 2024, 319, 117123. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; He, X. The mechanism of bisphenol S-induced atherosclerosis elucidated based on network toxicology, molecular docking, and machine learning. J. Appl. Toxicol. 2025, 45, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, Y.; Pang, B.; Jiang, L. Astragaloside IV mediates the PI3K/Akt/mTOR pathway to alleviate injury and modulate the composition of intestinal flora in apoE-/- atherosclerosis model rats. Discov. Med. 2024, 36, 1070–1079. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Guo, J.; Huang, D.; Zhang, F.; Du, Z.; Hou, X.; Deng, J.; Xie, Y.; Hao, E. Therapeutic Potential of Bioactive Compounds from Traditional Chinese Medicine in Modulating Macrophage Cholesterol Metabolism for Atherosclerosis Treatment. Pharmaceuticals 2025, 18, 1113. https://doi.org/10.3390/ph18081113
Yan L, Guo J, Huang D, Zhang F, Du Z, Hou X, Deng J, Xie Y, Hao E. Therapeutic Potential of Bioactive Compounds from Traditional Chinese Medicine in Modulating Macrophage Cholesterol Metabolism for Atherosclerosis Treatment. Pharmaceuticals. 2025; 18(8):1113. https://doi.org/10.3390/ph18081113
Chicago/Turabian StyleYan, Lijiao, Jiageng Guo, Dan Huang, Fan Zhang, Zhengcai Du, Xiaotao Hou, Jiagang Deng, Yan Xie, and Erwei Hao. 2025. "Therapeutic Potential of Bioactive Compounds from Traditional Chinese Medicine in Modulating Macrophage Cholesterol Metabolism for Atherosclerosis Treatment" Pharmaceuticals 18, no. 8: 1113. https://doi.org/10.3390/ph18081113
APA StyleYan, L., Guo, J., Huang, D., Zhang, F., Du, Z., Hou, X., Deng, J., Xie, Y., & Hao, E. (2025). Therapeutic Potential of Bioactive Compounds from Traditional Chinese Medicine in Modulating Macrophage Cholesterol Metabolism for Atherosclerosis Treatment. Pharmaceuticals, 18(8), 1113. https://doi.org/10.3390/ph18081113








