Comparative Immunomodulatory Efficacy of Secukinumab and Honokiol in Experimental Asthma and Acute Lung Injury
Abstract
1. Introduction
2. Results
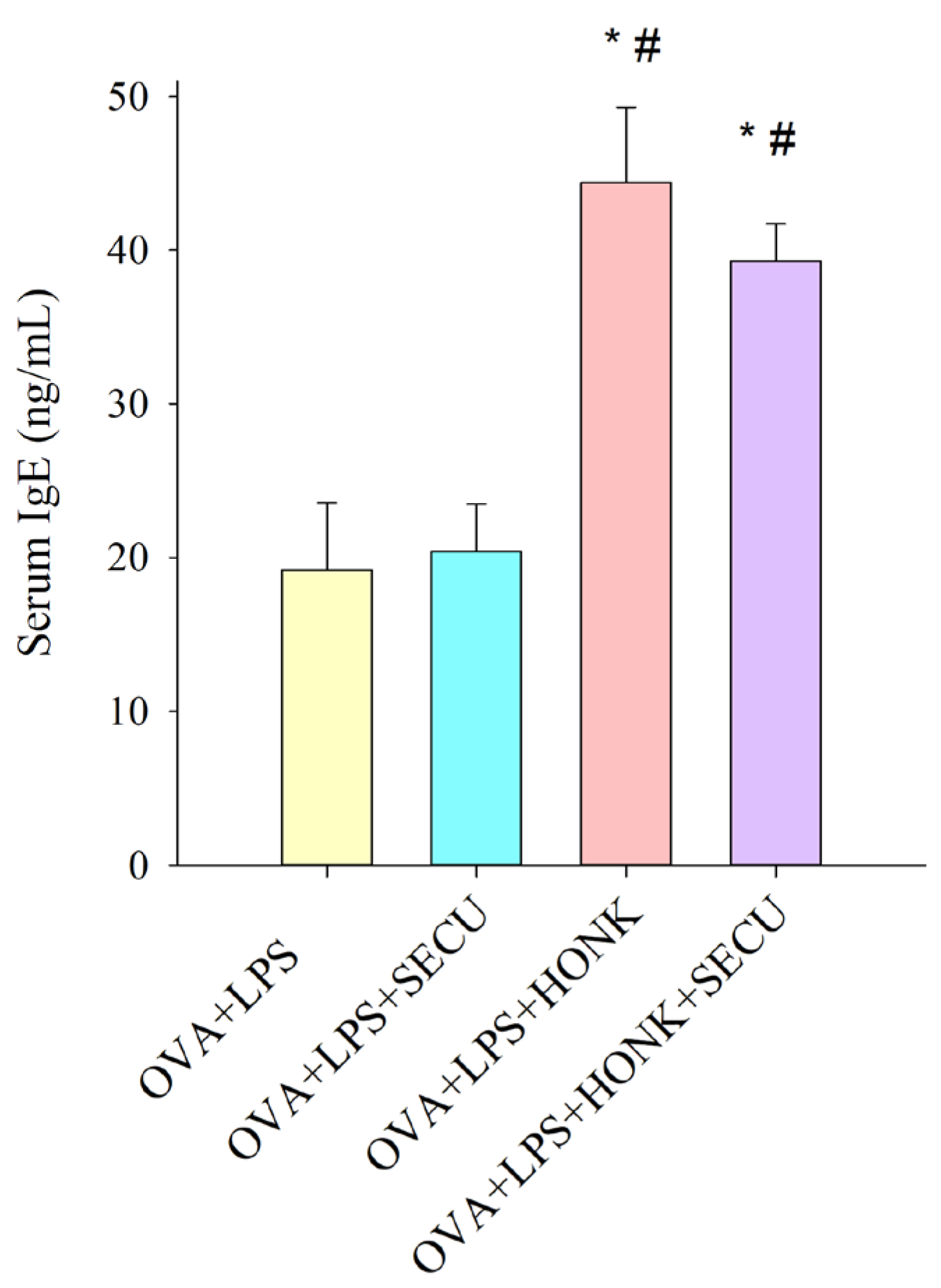
2.1. Effects of SECU, HONK and HONK + SECU Combined Treatment, Respectively, on Serum Levels of Ovalbumin (OVA)-Specific IgE
2.2. Effects of SECU, HONK, and HONK + SECU Combined Treatment, Respectively, on Bronchoalveolar Lavage Fluid (BALF) and on Lung Tissue Homogenate (LTH)
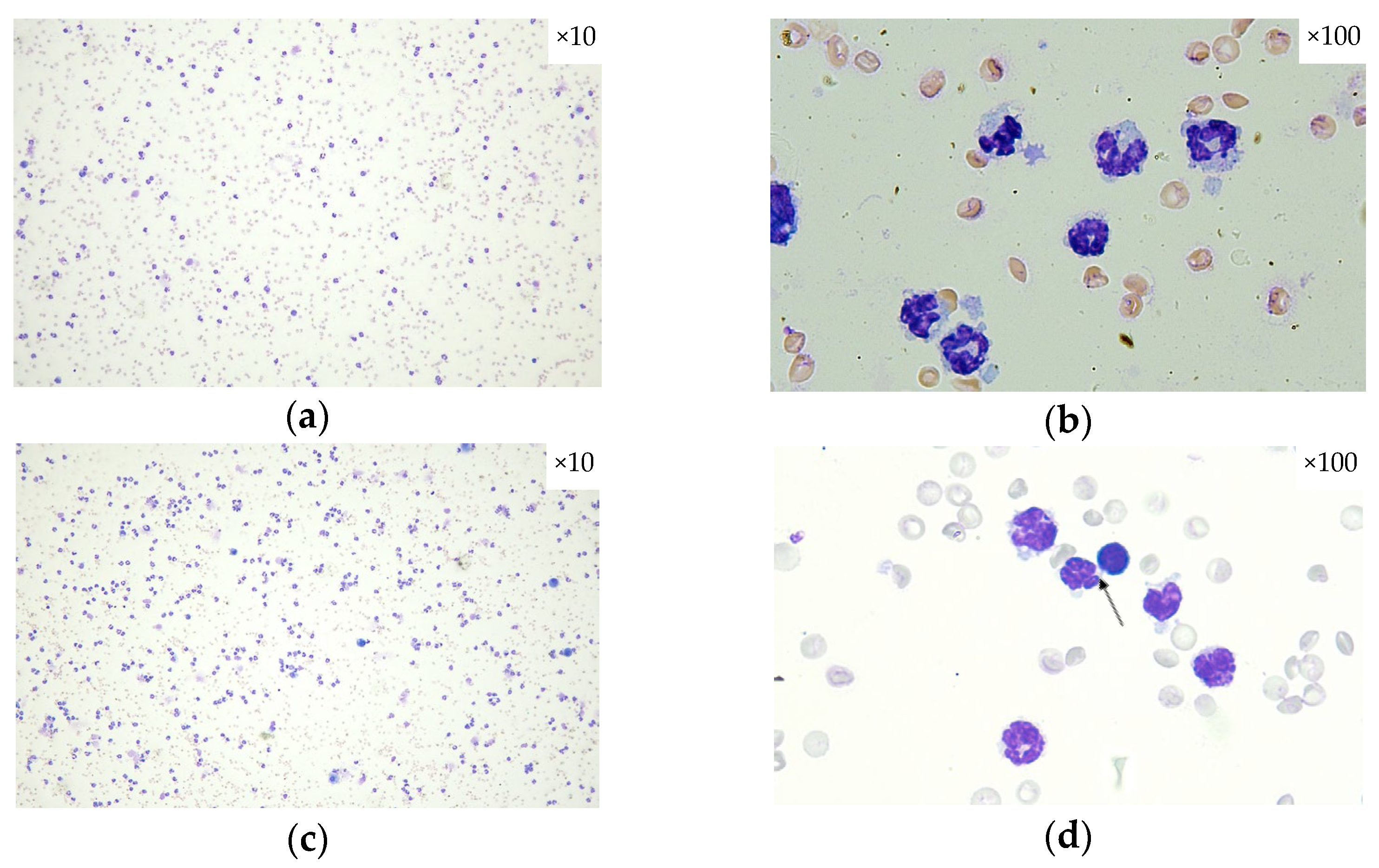
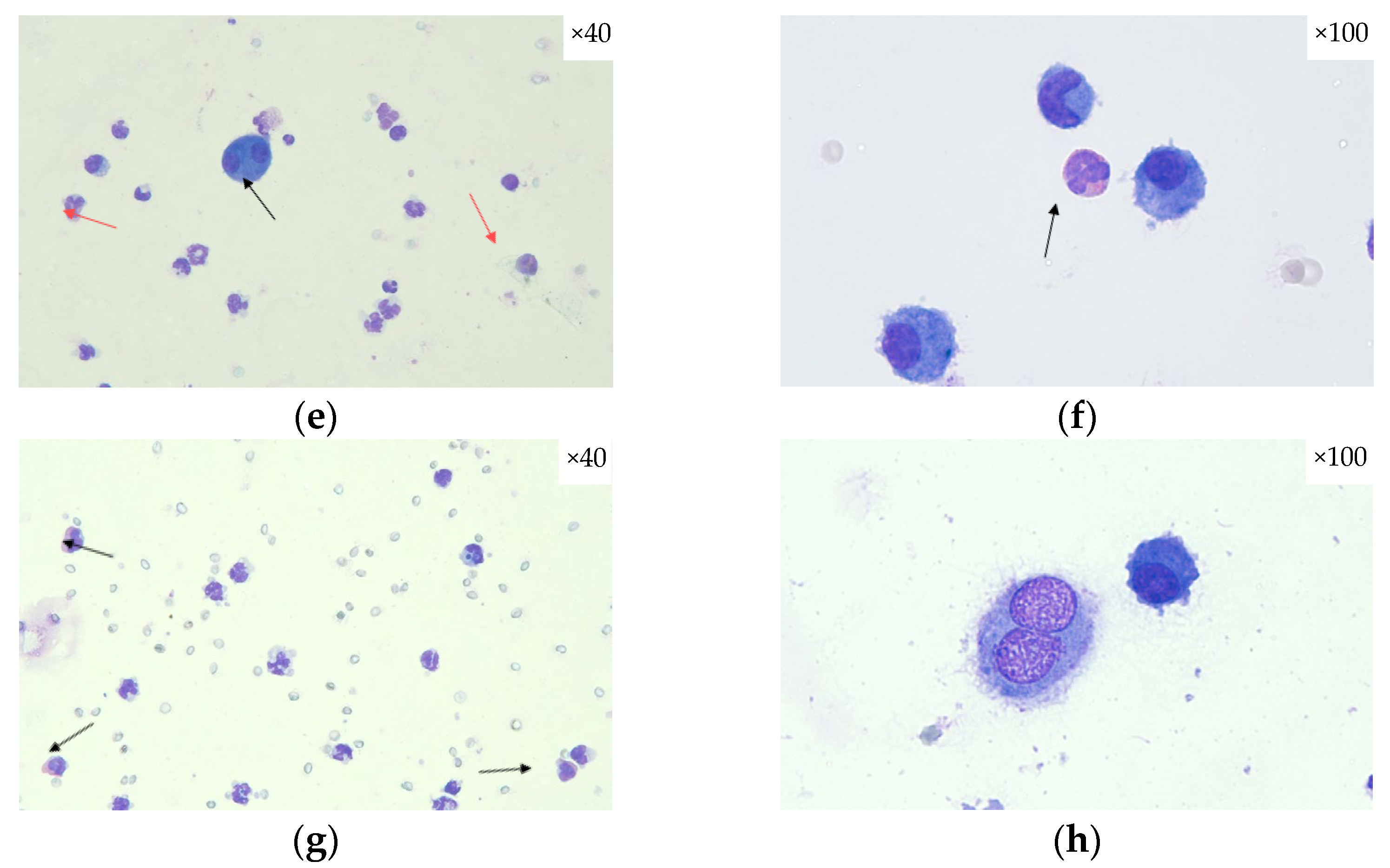
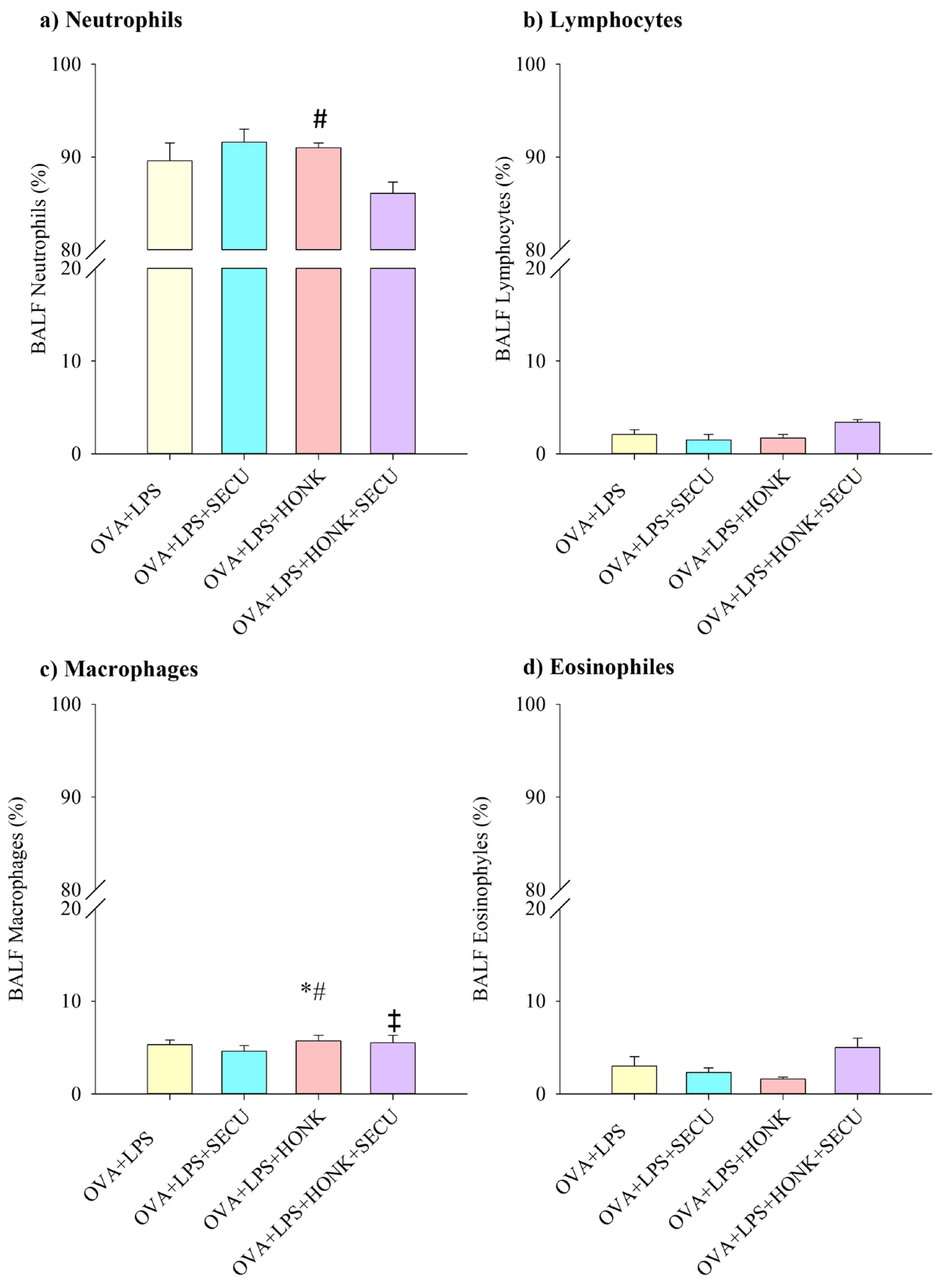
2.2.1. Cell Count in BALF
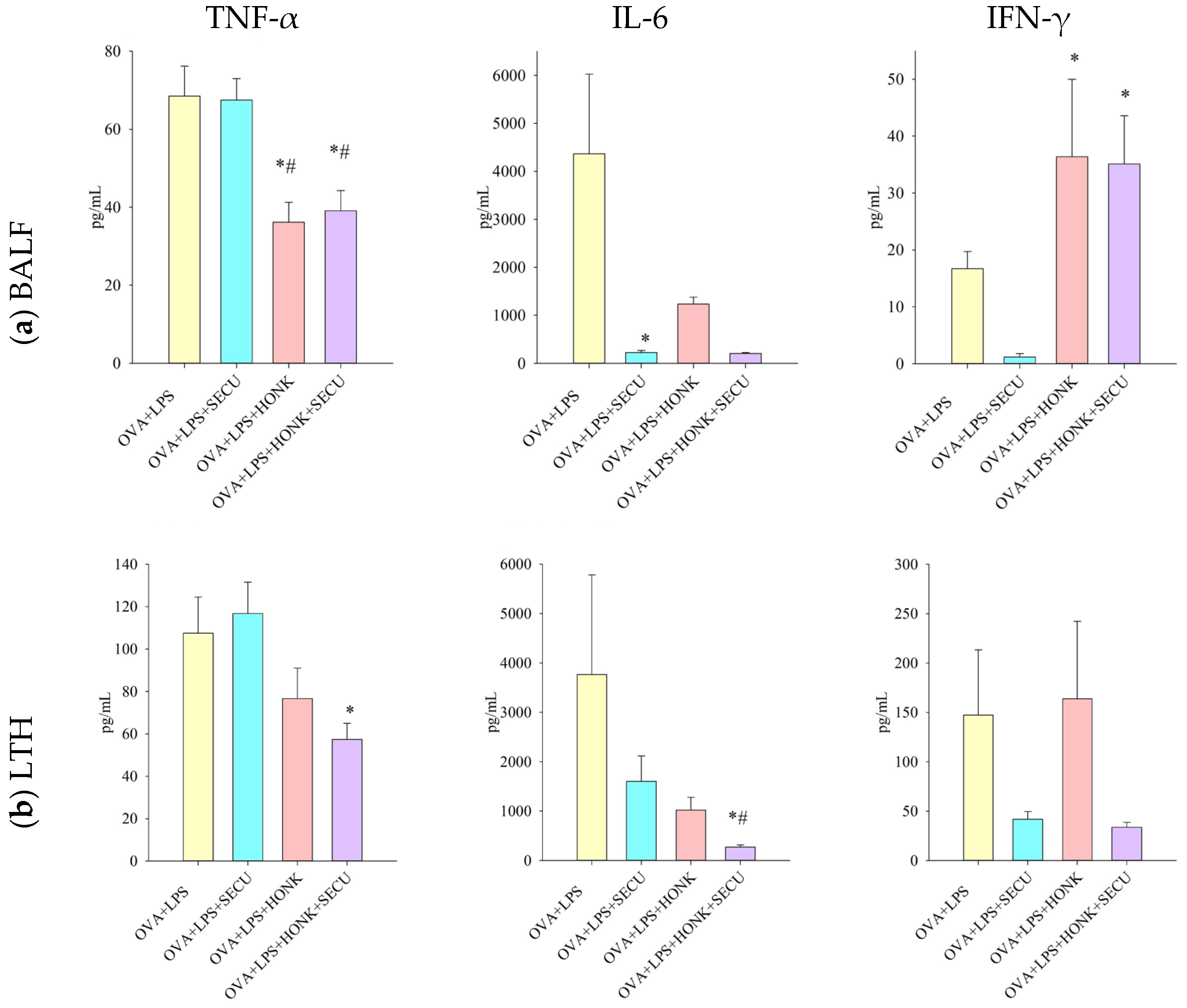
2.2.2. Th 1 Cytokines in BALF vs. In LTH
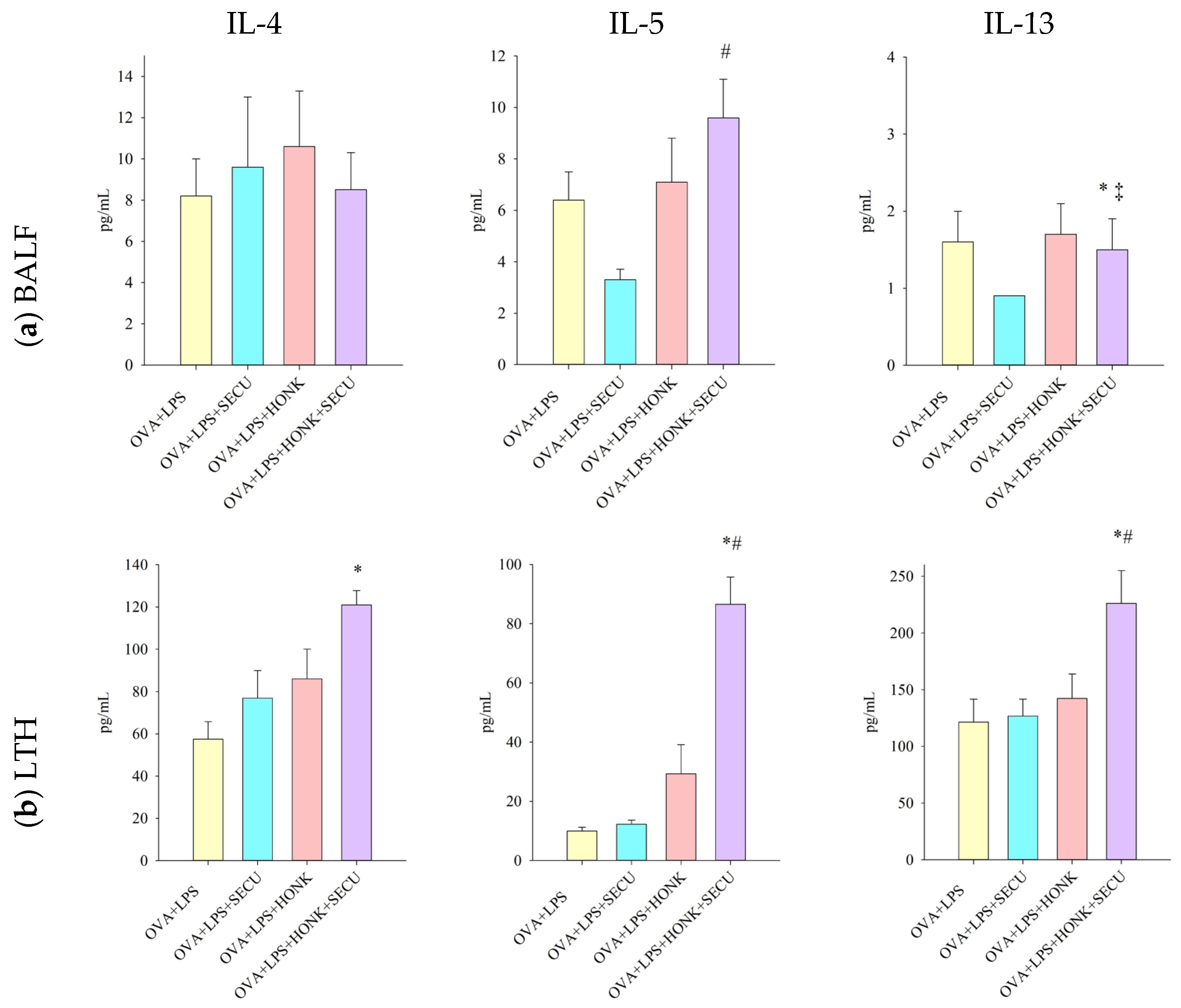
2.2.3. Th 2 Cytokines in BALF vs. In LTH
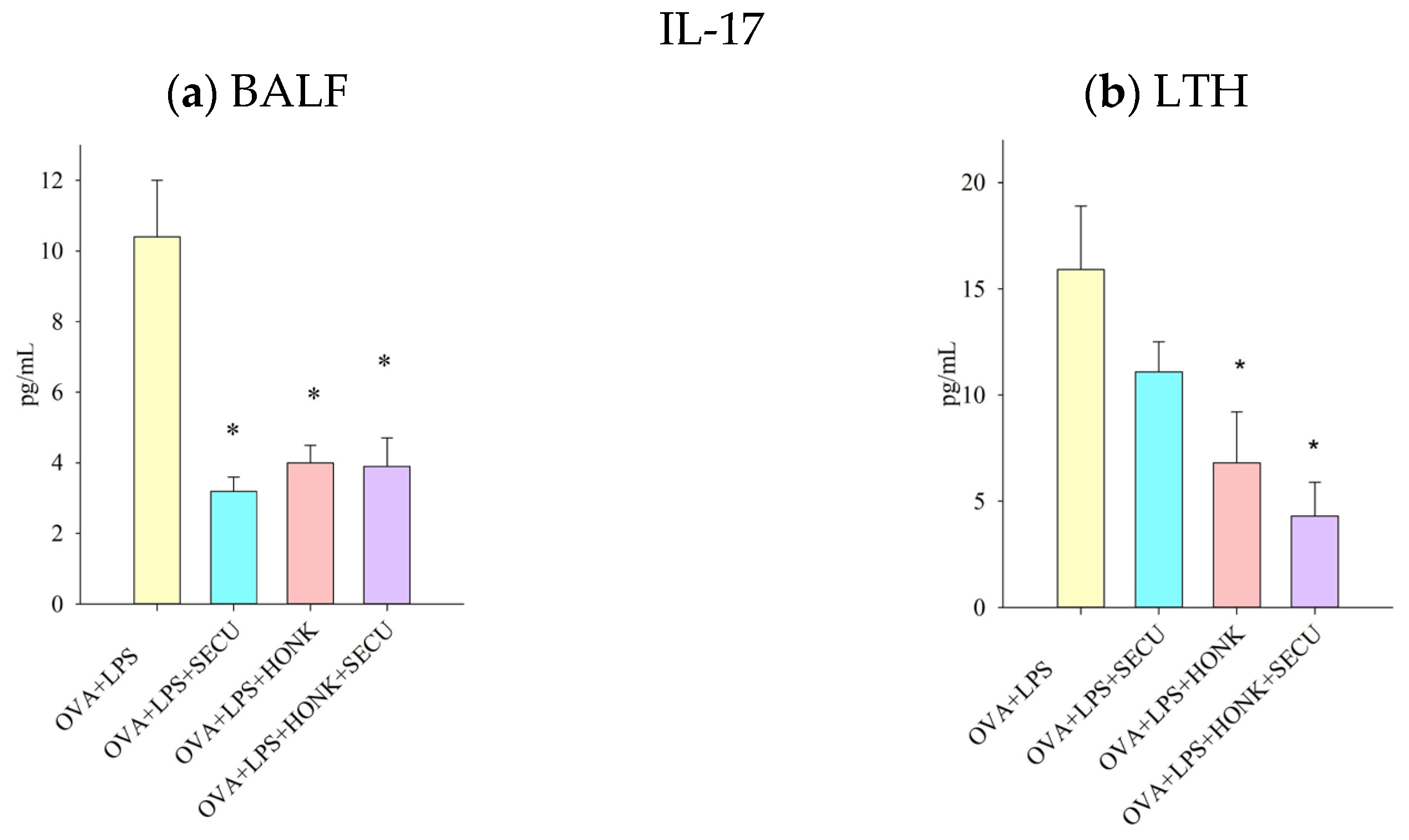
2.2.4. IL-17 Cytokine in BALF vs. In LTH
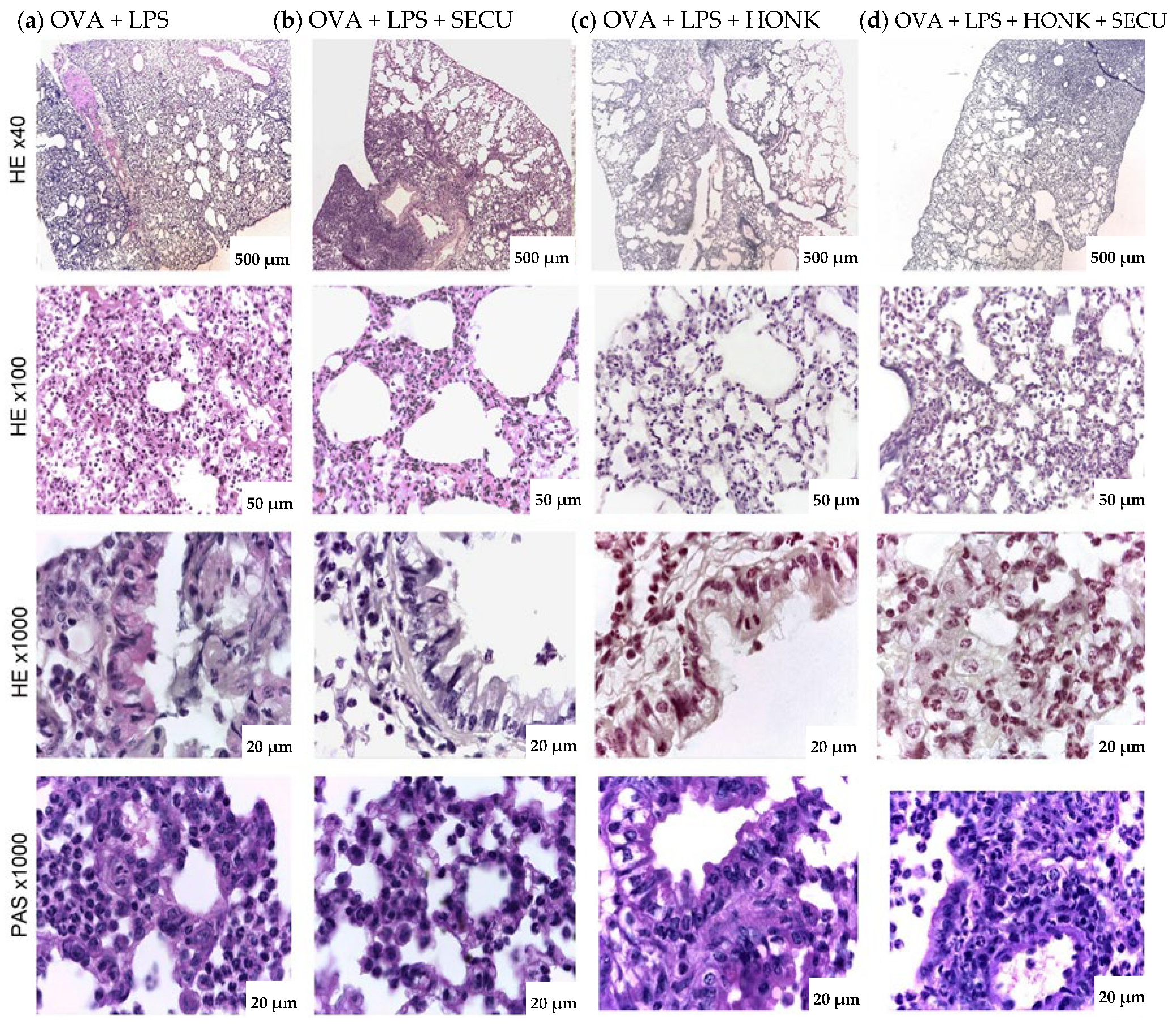
2.3. Histopathological Assessment of SECU, HONK, and HONK + SECU Combined Therapeutic Intervention
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Chemicals, Antibodies, and Reagents
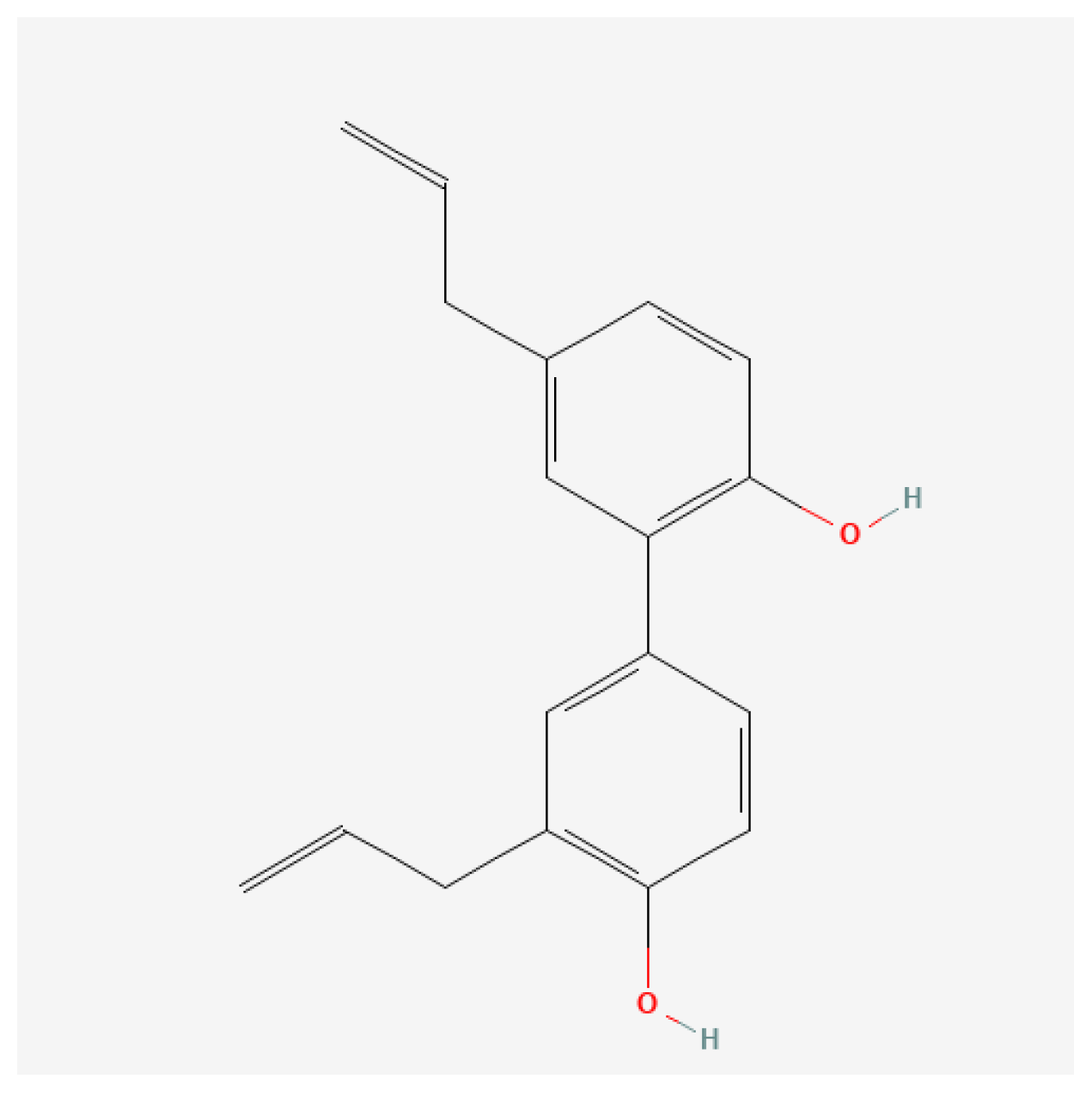
4.2. Preparation of Honokiol Suspension and Ovalbumin Solution
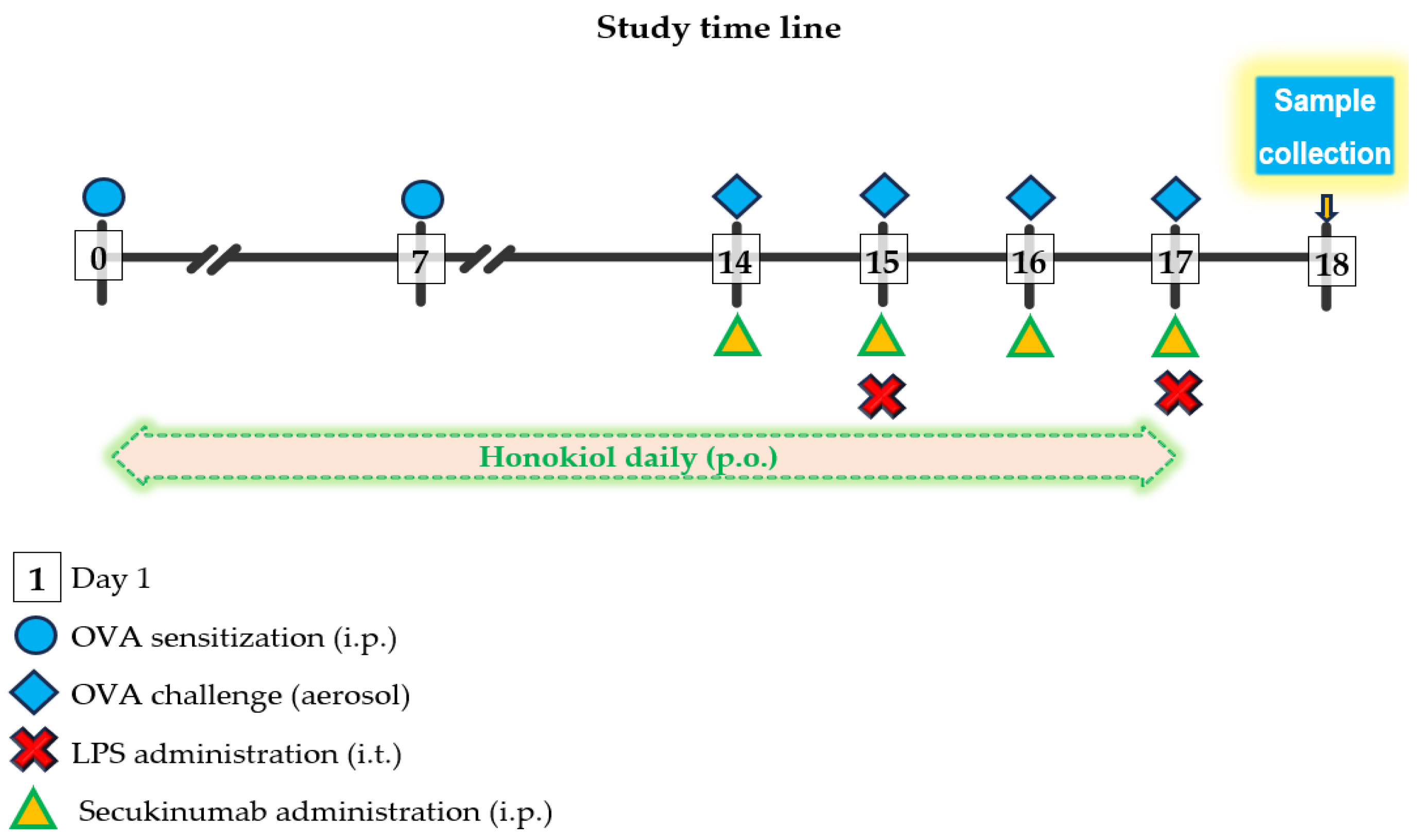
4.3. Experimental Design and Study Protocol
- Group 1 (OVA-LPS) (positive disease control): received inhaled ovalbumin and instilled LPS;
- Group 2 (OVA-LPS + SECU): received inhaled ovalbumin and instilled LPS and subcutaneous administration of secukinumab;
- Group 3 (OVA-LPS + HONK): received inhaled ovalbumin and instilled LPS and oral gavage treatment with honokiol;
- Group 4 (OVA-LPS + HONK + SECU): received inhaled ovalbumin and instilled LPS and both oral gavage treatment with honokiol and subcutaneous administration of secukinumab.
4.4. Assessment of Serum OVA-Specific IgE Levels
4.5. Assessment of Cell Count and Cytokines in Bronchoalveolar Lavage Fluid (BALF)
4.6. Assessment of Cytokines in Lung Homogenates
4.7. Histological Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, N.; Hochhegger, B.; Mukhopadhyay, S.; Teixeira e Silva Torres, P.P.; Mohammed, T.-L. Acute Lung Injury. J. Thorac. Imaging 2025, 40, e0820. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.A.; Erzurum, S.C. Redox Control of Asthma: Molecular Mechanisms and Therapeutic Opportunities. Antioxid. Redox Signal. 2010, 12, 93–124. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative stress in asthma. World Allergy Organ. J. 2011, 4, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Metnitz, P.G.H.; Bartens, C.; Fischer, M.; Fridrich, P.; Steltzer, H.; Druml, W. Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Med. 1999, 25, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; Silva, C.P.E.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants 2023, 12, 548. [Google Scholar] [CrossRef]
- McGovern, T.K.; Chen, M.; Allard, B.; Larsson, K.; Martin, J.G.; Adner, M. Neutrophilic oxidative stress mediates organic dust-induced pulmonary inflammation and airway hyperresponsiveness. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L155–L165. [Google Scholar] [CrossRef]
- Pham, D.L.; Ban, G.-Y.; Kim, S.-H.; Shin, Y.S.; Ye, Y.-M.; Chwae, Y.-J.; Park, H.-S. Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma. Clin. Exp. Allergy 2017, 47, 57–70. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Camargo, L.D.N.; Righetti, R.F.; Aristóteles, L.R.D.C.R.B.; Dos Santos, T.M.; De Souza, F.C.R.; Fukuzaki, S.; Cruz, M.M.; Alonso-Vale, M.I.C.; Saraiva-Romanholo, B.M.; Prado, C.M.; et al. Effects of Anti-IL-17 on Inflammation, Remodeling, and Oxidative Stress in an Experimental Model of Asthma Exacerbated by LPS. Front. Immunol. 2018, 8, 1835. [Google Scholar] [CrossRef]
- Camargo, L.D.N.; Santos, T.M.D.; Andrade, F.C.P.D.; Fukuzaki, S.; Dos Santos Lopes, F.D.T.Q.; De Arruda Martins, M.; Prado, C.M.; Leick, E.A.; Righetti, R.F.; Tibério, I.D.F.L.C. Bronchial Vascular Remodeling Is Attenuated by Anti-IL-17 in Asthmatic Responses Exacerbated by LPS. Front. Pharmacol. 2020, 11, 1269. [Google Scholar] [CrossRef]
- Dos Santos, T.M.; Righetti, R.F.; Rezende, B.G.; Campos, E.C.; Camargo, L.D.N.; Saraiva-Romanholo, B.M.; Fukuzaki, S.; Prado, C.M.; Leick, E.A.; Martins, M.A.; et al. Effect of anti-IL17 and/or Rho-kinase inhibitor treatments on vascular remodeling induced by chronic allergic pulmonary inflammation. Ther. Adv. Respir. Dis. 2020, 14, 175346662096266. [Google Scholar] [CrossRef]
- Santos, T.M.D.; Righetti, R.F.; Camargo, L.D.N.; Saraiva-Romanholo, B.M.; Aristoteles, L.R.C.R.B.; De Souza, F.C.R.; Fukuzaki, S.; Alonso-Vale, M.I.C.; Cruz, M.M.; Prado, C.M.; et al. Effect of Anti-IL17 Antibody Treatment Alone and in Combination With Rho-Kinase Inhibitor in a Murine Model of Asthma. Front. Physiol. 2018, 9, 1183. [Google Scholar] [CrossRef]
- Righetti, R.F.; dos Santos, T.M.; do Nascimento Camargo, L.; Aristóteles, L.R.C.R.B.; Fukuzaki, S.; de Souza, F.C.R.; Santana, F.P.R.; de Agrela, M.V.R.; Cruz, M.M.; Alonso-Vale, M.I.C.; et al. Protective Effects of Anti-IL17 on Acute Lung Injury Induced by LPS in Mice. Front. Pharmacol. 2018, 9, 1021. [Google Scholar] [CrossRef]
- Shaikh, S.B.; Bhat, S.G.; Bhandary, Y.P. Curcumin attenuates IL-17A mediated pulmonary SMAD dependent and non-dependent mechanism during acute lung injury in vivo. Mol. Biol. Rep. 2020, 47, 5643–5649. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Gao, H.; Hoesel, L.M.; Nadeau, B.A.; Day, D.E.; Zetoune, F.S.; Sarma, J.V.; Huber-Lang, M.S.; Ferrara, J.L.M.; et al. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008, 22, 2198–2205. [Google Scholar] [CrossRef]
- Wiche Salinas, T.R.; Zheng, B.; Routy, J.-P.; Ancuta, P. Targeting the interleukin-17 pathway to prevent acute respiratory distress syndrome associated with SARS-CoV-2 infection. Respirology 2020, 25, 797–799. [Google Scholar] [CrossRef]
- Zijlstra, G.J.; Ten Hacken, N.H.T.; Hoffmann, R.F.; Van Oosterhout, A.J.M.; Heijink, I.H. Interleukin-17A induces glucocorticoid insensitivity in human bronchial epithelial cells. Eur. Respir. J. 2012, 39, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Melton, A.C.; Chen, C.; Engler, M.B.; Huang, K.E.; Ren, X.; Wang, Y.; Bernstein, X.; Li, J.T.; Atabai, K.; et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 2012, 18, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Tong, L.; Wang, J.; Dou, M.; Ji, S.; Bi, J.; Chen, C.; Yang, D.; He, H.; et al. Recovery from acute lung injury can be regulated via modulation of regulatory T cells and Th17 cells. Scand. J. Immunol. 2018, 88, e12715. [Google Scholar] [CrossRef]
- Peters, K.; Ernst, S.; Peters, M. Interaction of Interleukin-17A with a Th2 Response in a Mouse Model of Allergic Airway Inflammation. Cells 2023, 12, 1774. [Google Scholar] [CrossRef]
- Vicovan, A.G.; Petrescu, D.C.; Cretu, A.; Ghiciuc, C.M.; Constantinescu, D.; Iftimi, E.; Strugariu, G.; Ancuta, C.M.; Caratașu, C.-C.; Solcan, C.; et al. Targeting Common Inflammatory Mediators in Experimental Severe Asthma and Acute Lung Injury. Pharmaceuticals 2024, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Chesné, J.; Braza, F.; Mahay, G.; Brouard, S.; Aronica, M.; Magnan, A. IL-17 in severe asthma. Where do we stand? Am. J. Respir. Crit. Care Med. 2014, 190, 1094–1101. [Google Scholar] [CrossRef]
- Menson, K.E.; Mank, M.M.; Reed, L.F.; Walton, C.J.; Van Der Vliet, K.E.; Ather, J.L.; Chapman, D.G.; Smith, B.J.; Rincon, M.; Poynter, M.E. Therapeutic efficacy of IL-17A neutralization with corticosteroid treatment in a model of antigen-driven mixed-granulocytic asthma. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L693–L709. [Google Scholar] [CrossRef]
- Granda, P.; Villamañán, E.; Carpio, C.; Laorden, D.; Quirce, S.; Álvarez-Sala, R. Anti-IL-5 and anti-IL-5R biologics for severe asthma. Are there any differences in their effects? J. Asthma 2024, 61, 857–866. [Google Scholar] [CrossRef]
- Pan, C.; Li, Q.; Xiong, S.; Yang, Y.; Yang, Y.; Huang, C.; Wang, Z.-P. Delivery Strategies, Structural Modification, and Pharmacological Mechanisms of Honokiol: A Comprehensive Review. Chem. Biodivers. 2024, 21, e202302032. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Y.; Luo, Z.; Hu, Y.; Wang, S.; Hu, S.; Yao, Y.; Pan, L.; Shen, C.; Xu, T. Honokiol Inhibits the Inflammatory Response and Lipid Metabolism Disorder by Inhibiting p38α in Alcoholic Liver Disease. Planta Med. 2023, 89, 273–285. [Google Scholar] [CrossRef]
- Chao, L.K.; Liao, P.-C.; Ho, C.-L.; Wang, E.I.-C.; Chuang, C.-C.; Chiu, H.-W.; Hung, L.-B.; Hua, K.-F. Anti-inflammatory bioactivities of honokiol through inhibition of protein kinase C, mitogen-activated protein kinase, and the NF-κB pathway to reduce LPS-induced TNFα and NO expression. J. Agric. Food Chem. 2010, 58, 3472–3478. [Google Scholar] [CrossRef]
- Munroe, M.E.; Businga, T.R.; Kline, J.N.; Bishop, G.A. Anti-Inflammatory Effects of the Neurotransmitter Agonist Honokiol in a Mouse Model of Allergic Asthma. J. Immunol. 2010, 185, 5586–5597. [Google Scholar] [CrossRef]
- Xu, J.; Lu, X.; Han, F. Effects of honokiol on particulate matter 2.5-induced lung injury in asthmatic mice and its mechanisms. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2018, 43, 718–724. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Luo, Y.; Li, J.; Shang, L.; Zhou, F.; Yang, S. Honokiol alleviates LPS-induced acute lung injury by inhibiting NLRP3 inflammasome-mediated pyroptosis via Nrf2 activation in vitro and in vivo. Chin. Med. 2021, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Mac Sharry, J.; Shalaby, K.H.; Marchica, C.; Farahnak, S.; Chieh-Li, T.; Lapthorne, S.; Qureshi, S.T.; Shanahan, F.; Martin, J.G. Concomitant exposure to ovalbumin and endotoxin augments airway inflammation but not airway hyperresponsiveness in a murine model of asthma. PLoS ONE 2014, 9, e98648. [Google Scholar] [CrossRef] [PubMed]
- Samarai, A.G.M.A.; Obaidi, A.H.A.A.; Jawad, A.K.Y.; Janabi, J.M.A.; Samarai, A.G.M.A.; Obaidi, A.H.A.A.; Jawad, A.K.Y.; Janabi, J.M.A. Association Between C Reactive Protein and Asthma. Thorac. Res. Pract. 2010, 11, 98–104. [Google Scholar] [CrossRef]
- Qasim, J.; Al-Daami, Q.; Bash, H.; Hamid, G.; Makki, A.-A.; Abdul-Amir, H.; Al-Hindy, M.; Makki, H. High-Sensitivity C -reactive protein Assessment in Bronchial Asthma: Impact of Exhaled Nitric Oxide and Body Mass Index. Syst. Rev. Pharm. 2020, 11, 705. [Google Scholar]
- Shi, B.; Li, W.; Hao, Y.; Dong, H.; Cao, W.; Guo, J.; Gao, P. Characteristics of inflammatory phenotypes among patients with asthma: Relationships of blood count parameters with sputum cellular phenotypes. Allergy Asthma Clin. Immunol. 2021, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Ma, Y.-F.; Zhu, J.; Wang, D.-X.; Liu, Y. A clinical study of multiple trauma combined with acute lung injury. J. Acute Dis. 2016, 5, 450–453. [Google Scholar] [CrossRef]
- Hammer, J. Monitoring and assessment of acute lung injury. Pediatr. Pulmonol. 2004, 37, 123–124. [Google Scholar] [CrossRef]
- Hastie, A.T.; Bishop, A.C.; Khan, M.S.; Bleecker, E.R.; Castro, M.; Denlinger, L.C.; Erzurum, S.C.; Fahy, J.V.; Israel, E.; Levy, B.D.; et al. Protein–Protein interactive networks identified in bronchoalveolar lavage of severe compared to nonsevere asthma. Clin. Exp. Allergy 2024, 54, 265–277. [Google Scholar] [CrossRef]
- Sont, J.K.; de Boer, W.I.; van Schadewijk, W.A.A.M.; Grünberg, K.; van Krieken, J.H.J.M.; Hiemstra, P.S.; Sterk, P.J. Fully Automated Assessment of Inflammatory Cell Counts and Cytokine Expression in Bronchial Tissue. Am. J. Respir. Crit. Care Med. 2003, 167, 1496–1503. [Google Scholar] [CrossRef]
- Mummy, D.; Driehuys, B. Illuminating Lung Inflammation at the Alveolar Capillary Interface. J. Magn. Reson. Imaging 2020, 51, 1677–1678. [Google Scholar] [CrossRef]
- Adler, A.; Bergwik, J.; Padra, M.; Papareddy, P.; Schmidt, T.; Dahlgren, M.; Kahn, R.; Berglund, U.W.; Egesten, A. Pharmacological inhibition of MutT homolog 1 (MTH1) in allergic airway inflammation as a novel treatment strategy. Respir. Res. 2025, 26, 101. [Google Scholar] [CrossRef] [PubMed]
- Haeger, S.; Moore, C.M.; McManus, S.A.; Moore, P.K.; Janssen, W.J.; Mould, K.J. The bronchoalveolar lavage dilution conundrum: An updated view on a long-standing problem. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2024, 327, L807–L813. [Google Scholar] [CrossRef] [PubMed]
- Ishimine, T.; Kawakami, K.; Nakamoto, A.; Saito, A. Analysis of cellular response and gamma interferon synthesis in bronchoalveolar lavage fluid and lung homogenate of mice infected with Pneumocystis carinii. Microbiol. Immunol. 1995, 39, 49–58. [Google Scholar] [CrossRef]
- Hardyman, M.A.; Wilkinson, E.; Martin, E.; Jayasekera, N.P.; Blume, C.; Swindle, E.J.; Gozzard, N.; Holgate, S.T.; Howarth, P.H.; Davies, D.E.; et al. TNF-α–mediated bronchial barrier disruption and regulation by src-family kinase activation. J. Allergy Clin. Immunol. 2013, 132, 665–675.e8. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.V.; Wilson, M.R.; O’Dea, K.P.; Takata, M. TNF-Induced Death Signaling Triggers Alveolar Epithelial Dysfunction in Acute Lung Injury. J. Immunol. 2013, 190, 4274–4282. [Google Scholar] [CrossRef]
- Aoyagi, T.; Yamamoto, N.; Hatta, M.; Tanno, D.; Miyazato, A.; Ishii, K.; Suzuki, K.; Nakayama, T.; Taniguchi, M.; Kunishima, H.; et al. Activation of pulmonary invariant NKT cells leads to exacerbation of acute lung injury caused by LPS through local production of IFN-γ and TNF-α by Gr-1+ monocytes. Int. Immunol. 2011, 23, 97–108. [Google Scholar] [CrossRef]
- O’Dea, K.P.; Wilson, M.R.; Dokpesi, J.O.; Wakabayashi, K.; Tatton, L.; van Rooijen, N.; Takata, M. Mobilization and margination of bone marrow Gr-1high monocytes during subclinical endotoxemia predisposes the lungs toward acute injury. J. Immunol. 2009, 182, 1155–1166. [Google Scholar] [CrossRef]
- Abraham, E. Neutrophils and acute lung injury. Crit. Care Med. 2003, 31, S195–S199. [Google Scholar] [CrossRef]
- Han, F.; Li, S.; Yang, Y.; Bai, Z. Interleukin-6 promotes ferroptosis in bronchial epithelial cells by inducing reactive oxygen species-dependent lipid peroxidation and disrupting iron homeostasis. Bioengineered 2021, 12, 5279–5288. [Google Scholar] [CrossRef] [PubMed]
- Gubernatorova, E.O.; Gorshkova, E.A.; Namakanova, O.A.; Zvartsev, R.V.; Hidalgo, J.; Drutskaya, M.S.; Tumanov, A.V.; Nedospasov, S.A. Non-redundant Functions of IL-6 Produced by Macrophages and Dendritic Cells in Allergic Airway Inflammation. Front. Immunol. 2018, 9, 2718. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.M.; Spiller, K.L. Pro-inflammatory polarization primes Macrophages to transition into a distinct M2-like phenotype in response to IL-4. J. Leukoc. Biol. 2022, 111, 989–1000. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Xue, G.; Li, K.; Zheng, Y.-F.; Zhang, Z.; Jiang, Q.; Liu, Y.; Zhou, X.; Cao, C. Requirement of Gαi1 and Gαi3 in interleukin-4-induced signaling, macrophage M2 polarization and allergic asthma response. Theranostics 2021, 11, 4894–4909. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Lu, C.; Zhang, P.; Zhou, C.; Ni, Y.; Niu, W.; Yuan, X.; Li, P.; Zheng, J.; et al. Efficacy of pulmonary transplantation of engineered macrophages secreting IL-4 on acute lung injury in C57BL/6J mice. Cell Death Dis. 2019, 10, 664. [Google Scholar] [CrossRef]
- Harris, A.J.; Mirchandani, A.S.; Lynch, R.W.; Murphy, F.; Delaney, L.; Small, D.; Coelho, P.; Watts, E.R.; Sadiku, P.; Griffith, D.; et al. IL4Rα Signaling Abrogates Hypoxic Neutrophil Survival and Limits Acute Lung Injury Responses In Vivo. Am. J. Respir. Crit. Care Med. 2019, 200, 235–246. [Google Scholar] [CrossRef]
- Liu, A.; Xun, S.; Zhou, G.; Zhang, Y.; Lin, L. Honokiol alleviates sepsis-associated cardiac dysfunction via attenuating inflammation, apoptosis and oxidative stress. J. Pharm. Pharmacol. 2023, 75, 397–406. [Google Scholar] [CrossRef]
- Li, F.; Ye, C.; Wang, X.; Li, X.; Wang, X. Honokiol ameliorates cigarette smoke-induced damage of airway epithelial cells via the SIRT3/SOD2 signalling pathway. J. Cell. Mol. Med. 2023, 27, 4009–4020. [Google Scholar] [CrossRef]
- Weng, T.I.; Wu, H.Y.; Chen, B.L.; Liu, S.H. Honokiol attenuates the severity of acute pancreatitis and associated lung injury via acceleration of acinar cell apoptosis. Shock 2012, 37, 478–484. [Google Scholar] [CrossRef]
- Pillar, A.; Ali, M.K. IL-22 Binding Protein/IL-22 Axis in Regulating Acute Lung Injury. Am. J. Pathol. 2024, 194, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Paoletti, G.; Puggioni, F.; Racca, F.; Pelaia, G.; Canonica, G.W.; Heffler, E. Interleukin-5 in the Pathophysiology of Severe Asthma. Front. Physiol. 2019, 10, 1514. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Provost, K.; Niu, N.; Homer, R.; Cohn, L. IFN-γ Acts on the Airway Epithelium to Inhibit Local and Systemic Pathology in Allergic Airway Disease. J. Immunol. 2011, 187, 3815–3820. [Google Scholar] [CrossRef]
- Hachem, P.; Lisbonne, M.; Michel, M.-L.; Diem, S.; Roongapinun, S.; Lefort, J.; Marchal, G.; Herbelin, A.; Askenase, P.W.; Dy, M.; et al. α-Galactosylceramide-induced iNKT cells suppress experimental allergic asthma in sensitized mice: Role of IFN-γ. Eur. J. Immunol. 2005, 35, 2793–2802. [Google Scholar] [CrossRef]
- Kumar, M.; Kong, X.; Behera, A.K.; Hellermann, G.R.; Lockey, R.F.; Mohapatra, S.S. Chitosan IFN-γ-pDNA Nanoparticle (CIN) Therapy for Allergic Asthma. Genet. Vaccines Ther. 2003, 1, 3. [Google Scholar] [CrossRef]
- Chenery, A.L.; Rosini, S.; Parkinson, J.E.; Ajendra, J.; Herrera, J.A.; Lawless, C.; Chan, B.H.; Loke, P.; MacDonald, A.S.; Kadler, K.E.; et al. IL-13 deficiency exacerbates lung damage and impairs epithelial-derived type 2 molecules during nematode infection. Life Sci. Alliance 2021, 4, e202001000. [Google Scholar] [CrossRef]
- Sugita, K.; Steer, C.A.; Martinez-Gonzalez, I.; Altunbulakli, C.; Morita, H.; Castro-Giner, F.; Kubo, T.; Wawrzyniak, P.; Rückert, B.; Sudo, K.; et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J. Allergy Clin. Immunol. 2018, 141, 300–310.e11. [Google Scholar] [CrossRef]
- Ma, Q.; Tong, H.; Jing, J. High throughput virtual screening strategy to develop a potential treatment for bronchial asthma by targeting interleukin 13 cytokine signaling. Allergol. Immunopathol. 2022, 50, 22–31. [Google Scholar] [CrossRef]
- Wu, L.C.; Scheerens, H. Targeting IgE production in mice and humans. Curr. Opin. Immunol. 2014, 31, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pouw Kraan, T.C.T.M.; Van Der Zee, J.S.; Boeije, L.C.M.; DE Groot, E.R.; Stapel, S.O.; Aarden, L.A. The role of IL-13 in IgE synthesis by allergic asthma patients. Clin. Exp. Immunol. 1998, 111, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Badalyan, V.; Thompson, R.; Addo, K.; Borthwick, L.A.; Fisher, A.J.; Ort, T.; Myers, T.G.; Wynn, T.A.; Ramalingam, T.R. TNF-α/IL-17 synergy inhibits IL-13 bioactivity via IL-13Rα2 induction. J. Allergy Clin. Immunol. 2014, 134, 975–978.e5. [Google Scholar] [CrossRef]
- Hynes, G.M.; Hinks, T.S.C. The role of interleukin-17 in asthma: A protective response? ERJ Open Res. 2020, 6, 00364–02019. [Google Scholar] [CrossRef]
- Ma, L.; Zeng, J.; Mo, B.; Wang, C.; Huang, J.; Sun, Y.; Yu, Y.; Liu, S. High mobility group box 1: A novel mediator of Th2-type response-induced airway inflammation of acute allergic asthma. J. Thorac. Dis. 2015, 7, 1732–1741. [Google Scholar] [CrossRef]
- Vicovan, A.G.; Petrescu, D.C.; Constantinescu, D.; Iftimi, E.; Cernescu, I.T.; Ancuta, C.M.; Caratașu, C.-C.; Șorodoc, L.; Ceasovschih, A.; Solcan, C.; et al. Experimental Insights on the Use of Secukinumab and Magnolol in Acute Respiratory Diseases in Mice. Biomedicines 2024, 12, 1538. [Google Scholar] [CrossRef]
- Liu, D.; Tan, Y.; Bajinka, O.; Wang, L.; Tang, Z. Th17/IL-17 Axis Regulated by Airway Microbes Get Involved in the Development of Asthma. Curr. Allergy Asthma Rep. 2020, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.H.; Kim, W.K.; Park, J.S.; Park, C.S.; Jin, G.Y. A Quantitative Study of Airway Changes on Micro-CT in a Mouse Asthma Model: Comparison with Histopathological Findings. Allergy Asthma Immunol Res. 2014, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Oh, J.S.; Bae, B.; Ahn, Y.H.; Kim, L.W.; Choi, J.; Kim, H.Y.; Kang, H.R.; Lee, C.H. Ultra-high-resolution computed tomography shows changes in the lungs related with airway hyperresponsiveness in a murine asthma model. Sci. Rep. 2021, 11, 17584. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, C.M.; Wang, C.; Chou, H.C. Microcomputed tomography assessment of lipopolysaccharide-induced acute lung injury in rat. Exp. Lung Res. 2016, 42, 103–109. [Google Scholar] [CrossRef]
- Lamb, T.; Kaur, G.; Rahman, I. Tobacco-derived and tobacco-free nicotine cause differential inflammatory cell influx and MMP-9 in mouse lung. Respir. Res. 2024, 25, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhong, H.; Zhang, X.; Huang, X.; Wang, J.; Li, Z.; Chen, M.; Xiao, Z. EGCG promotes PRKCA expression to alleviate LPS-induced acute lung injury and inflammatory response. Sci. Rep. 2021, 11, 11014. [Google Scholar] [CrossRef]
- Debeuf, N.; Haspeslagh, E.; Van Helden, M.; Hammad, H.; Lambrecht, B.N. Mouse Models of Asthma. Curr. Protoc. Mouse Biol. 2016, 6, 169–184. [Google Scholar] [CrossRef]
- Melgert, B.N.; Postma, D.S.; Kuipers, I.; Geerlings, M.; Luinge, M.A.; van der Strate, B.W.A.; Kerstjens, H.A.M.; Timens, W.; Hylkema, M.N. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin. Exp. Allergy J. 2005, 35, 1496–1503. [Google Scholar] [CrossRef]
- Liu, B.; Lee, J.-B.; Chen, C.-Y.; Burwinkel, K.; Wang, Y.-H. Type-2 innate lymphoid cells facilitate antigen-induced CD4+ TH2 cell immune response that exacerbates chronic allergic asthma (HYP7P.291). J. Immunol. 2014, 192, 119.14. [Google Scholar] [CrossRef]
- Liu, J.; Li, W. Role of Th1 and Th17 imbalance in acute lung injury mice. Crit. Care 2014, 18, P336. [Google Scholar] [CrossRef]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced Cytokine Production in Human Monocytes and Macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef]
- Zhu, C.; Weng, Q.-Y.; Zhou, L.-R.; Cao, C.; Li, F.; Wu, Y.-F.; Wu, Y.-P.; Li, M.; Hu, Y.; Shen, J.-X.; et al. Homeostatic and early-recruited CD101− eosinophils suppress endotoxin-induced acute lung injury. Eur. Respir. J. 2020, 56, 1902354. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Han, L.; Lv, R.; Ling, L. Magnolol exerts anti-asthmatic effects by regulating Janus kinase-signal transduction and activation of transcription and Notch signaling pathways and modulating Th1/Th2/Th17 cytokines in ovalbumin-sensitized asthmatic mice. Korean J. Physiol. Pharmacol. 2019, 23, 251. [Google Scholar] [CrossRef]
- Ehrentraut, H.; Weisheit, C.K.; Frede, S.; Hilbert, T. Inducing Acute Lung Injury in Mice by Direct Intratracheal Lipopolysaccharide Instillation. J. Vis. Exp. 2019, 149, e59999. [Google Scholar] [CrossRef]
- Karatas, A.; Celik, C.; Oz, B.; Akar, Z.A.; Etem, E.O.; Dagli, A.F.; Koca, S.S. Secukinumab and metformin ameliorate dermal fibrosis by decreasing tissue interleukin-17 levels in bleomycin-induced dermal fibrosis. Int. J. Rheum. Dis. 2021, 24, 795–802. [Google Scholar] [CrossRef]
- Hong, T.; Min, H.; Hui, Z.; Yuejian, L.; Lixing, Y.; Liang, X.Z. Oral administration of honokiol attenuates airway inflammation in asthmatic mouse model. Pak. J. Pharm. Sci. 2018, 31, 1279–1285. [Google Scholar] [PubMed]
- Gottlieb, A.B.; Deodhar, A.; Mcinnes, I.B.; Baraliakos, X.; Reich, K.; Schreiber, S.; Bao, W.; Marfo, K.; Richards, H.B.; Pricop, L.; et al. Long-term Safety of Secukinumab Over Five Years in Patients with Moderate-to-severe Plaque Psoriasis, Psoriatic Arthritis and Ankylosing Spondylitis: Update on Integrated Pooled Clinical Trial and Post-marketing Surveillance Data. Acta Derm. Venereol. 2022, 102, adv00698. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Masuda, T.; Tokuoka, S.; Komai, M.; Nagao, K.; Takahashi, Y.; Nagai, H. The effect of allergen-induced airway inflammation on airway remodeling in a murine model of allergic asthma. Inflamm. Res. 2001, 50, 616–624. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicovan, A.G.; Petrescu, D.C.; Ochiuz, L.; Cianga, P.; Constantinescu, D.; Iftimi, E.; Pavel-Tanasa, M.; Ancuta, C.M.; Caratașu, C.-C.; Glod, M.; et al. Comparative Immunomodulatory Efficacy of Secukinumab and Honokiol in Experimental Asthma and Acute Lung Injury. Pharmaceuticals 2025, 18, 1108. https://doi.org/10.3390/ph18081108
Vicovan AG, Petrescu DC, Ochiuz L, Cianga P, Constantinescu D, Iftimi E, Pavel-Tanasa M, Ancuta CM, Caratașu C-C, Glod M, et al. Comparative Immunomodulatory Efficacy of Secukinumab and Honokiol in Experimental Asthma and Acute Lung Injury. Pharmaceuticals. 2025; 18(8):1108. https://doi.org/10.3390/ph18081108
Chicago/Turabian StyleVicovan, Andrei Gheorghe, Diana Cezarina Petrescu, Lacramioara Ochiuz, Petru Cianga, Daniela Constantinescu, Elena Iftimi, Mariana Pavel-Tanasa, Codrina Mihaela Ancuta, Cezar-Cătălin Caratașu, Mihai Glod, and et al. 2025. "Comparative Immunomodulatory Efficacy of Secukinumab and Honokiol in Experimental Asthma and Acute Lung Injury" Pharmaceuticals 18, no. 8: 1108. https://doi.org/10.3390/ph18081108
APA StyleVicovan, A. G., Petrescu, D. C., Ochiuz, L., Cianga, P., Constantinescu, D., Iftimi, E., Pavel-Tanasa, M., Ancuta, C. M., Caratașu, C.-C., Glod, M., Solcan, C., & Ghiciuc, C. M. (2025). Comparative Immunomodulatory Efficacy of Secukinumab and Honokiol in Experimental Asthma and Acute Lung Injury. Pharmaceuticals, 18(8), 1108. https://doi.org/10.3390/ph18081108







