Nanoparticles for Cancer Immunotherapy: Innovations and Challenges
Abstract
1. Introduction
2. Overcoming Drug Resistance Mechanisms
2.1. Targeted Drug Delivery to Tumor Hypoxia
2.2. Targeted Delivery to Efflux Transporters
3. Harnessing Nanoparticles for Immune Cell Activation
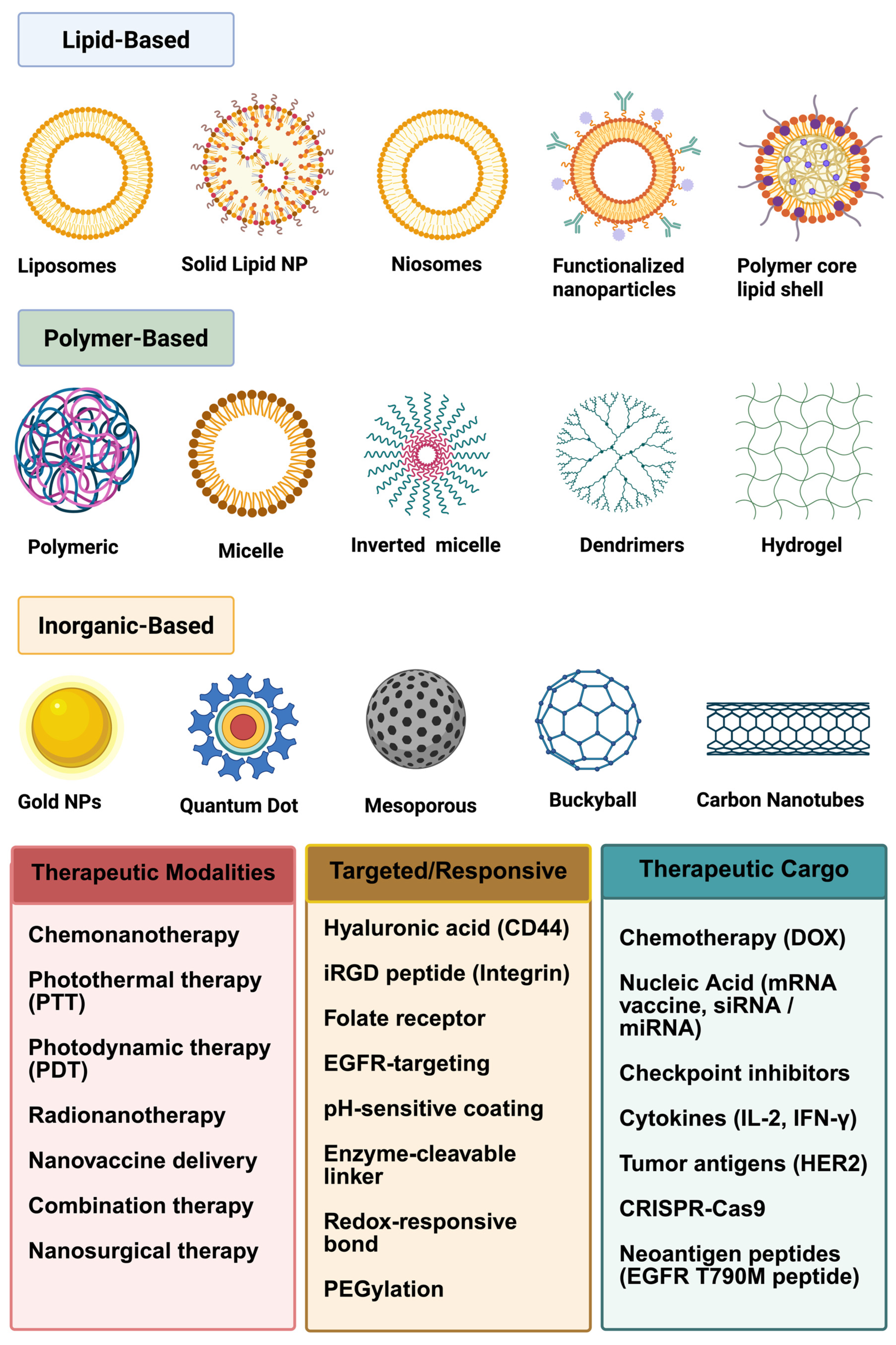
3.1. Innovations in Non-Lipid-Based Nanoparticle Drug Delivery
3.2. Engineering Considerations for Optimized Drug Targeting
3.2.1. Polymeric Nanoparticles: Versatile and Biocompatible Carriers
Poly(ε-caprolactone) (PCL)
Chitosan
3.2.2. Poly(alkyl cyanoacrylate) (PACA)
Poly(glutamic acid)
| Polymeric Nanoparticle | Encapsulated Agent | Cancer Cell Line (s) | Observed Effect | References |
|---|---|---|---|---|
| PLGA | mSTEAP peptide | Adenocarcinoma mouse prostate (TRAMP)-C2 cells, melanoma cell lines (624 and 1351), and human tumor-infiltrating lymphocytes | Synergistic tumor regression (~50% increase over monotherapy) | [4,42] |
| PLGA (folate-functionalized) | Doxorubicin | OVCAR-3 (ovarian) | 5-fold increase in uptake due to folate receptor targeting | [45] |
| PCL (Poly(ε-caprolactone)) | Paclitaxel, antibodies | MCF-7 (breast), A549 (lung) | Sustained drug release, enhanced cytotoxicity, tumor suppression | [57,58] |
| Chitosan | Docetaxel | MCF-7 (breast) | 57% greater tumor inhibition; improved drug delivery | [66,67] |
| PACA (Polyalkyl cyanoacrylate) | Doxorubicin, tumor peptides | Patient-derived basal-like xenograft (MAS98.12) | Increased ratio of M1/M2 (antitumorigenic/protumorigenic) macrophages in the tumors | [6,72,73] |
| PGA (Poly(γ-glutamic acid)) | Cisplatin, Imiquimod | B1F10 (melanoma) and bone marrow (BM)-derived DCs (BMDCs) | 2.5-fold tumor accumulation, reduced nephrotoxicity, enhanced DC activation | [76,77,78] |
3.2.3. Inorganic Nanoparticles: Precision and Multifunctionality
3.2.4. Gold Nanoparticles (AuNPs)
Silver Nanoparticles (AgNPs)
3.2.5. Mesoporous Silica Nanoparticles (MSNs)
3.2.6. Quantum Dots (QDs)
3.2.7. Carbon-Based Nanoparticles: Emerging Platforms for Drug Delivery
3.2.8. Graphene Oxide (GO) Nanoparticles
3.2.9. Carbon Nanotubes (CNTs)
3.2.10. Fullerenes: Antioxidant and Immunomodulatory Properties
3.2.11. Magnetic Nanoparticles: Controlled Targeting and Imaging
3.2.12. Potential In Vivo Toxicities from Non-Lipid-Based Nanoparticle Treatments
3.2.13. FDA-Approved Treatments
3.2.14. Lipid-Based Nanoparticle Delivery Systems
3.2.15. Liposomes
3.2.16. Lipid Nanoparticles (LNPs)
3.2.17. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
3.2.18. Extracellular Vesicles (EVs)
3.2.19. Other Lipid-Based Systems
3.3. Protein-Based Nanoparticles
4. Applications in Cancer Immunotherapy
5. Biocompatibility and Safety Concerns
6. Regulations and Manufacturing Barriers
7. Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AuNPs | Gold Nanoparticles |
| AFP | Alpha-Fetoprotein |
| AML | Acute Myeloid Leukemia |
| APCs | Antigen-Presenting Cells |
| ArgNPs | Arginine-coated Gold Nanoparticles |
| ASO | Antisense Oligonucleotide |
| A-MnO2 NPs | Albumin -Manganese oxide Nanoparticles |
| A549 | Lung carcinoma epithelial cells |
| AI | Artificial intelligence |
| AgNPs | Silver nanoparticles |
| BBB | Blood–Brain Barrier |
| BCLM | Breast Cancer Liver Metastases |
| BCRP | Breast Cancer Resistance Protein |
| Bcl-2 | B-cell lymphoma 2 |
| CD | Cluster of Differentiation (e.g., CD8+ T cells, CD86) |
| CNTs | Carbon Nanotubes |
| CARPA | Complement Activation-Related Pseudoallergy |
| Cdk5 | Cyclin-Dependent Kinase 5 |
| CT | Computed Tomography |
| CTLA-4 | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| CTLs | Cytotoxic T Lymphocytes |
| CD73 | Cluster of Differentiation 73 |
| COX-2 | Cyclooxygenase-2 |
| CD206 | Cluster of Differentiation 206 |
| CRISPR/Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-associated protein 9 |
| ctDNA | Circulating tumor DNA |
| CMC | chemistry, manufacturing, and controls |
| CMO | Contract Manufacturing Organization |
| CRO | Contract Research Organization |
| CAR | chimeric antigen receptor |
| DAMPs | Danger-Associated Molecular Patterns |
| DCs | Dendritic Cells |
| DOX | Doxorubicin |
| DSPE-PEG2000 | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] |
| DSTAP | 1,2-Dioleoyl-3-Trimethylammonium-Propane |
| DTX | Docetaxel |
| EPR | Enhanced Permeability and Retention |
| EGFR | Epidermal Growth Factor Receptor |
| EVs | Extracellular Vesicles |
| EMA | European Medicines Agency |
| FDA | U.S. Food and Drug Administration |
| FH | Factor H |
| GSH | Glutathione |
| GO | Graphene Oxide |
| GEM | Gemcitabine |
| GMP | Good Manufacturing Practice |
| GLP | Good Laboratory Practice |
| HCuSNPs | Hollow Copper Sulfide Nanoparticles |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HO1 | Heme Oxygenase 1 |
| HPV | Human Papillomavirus |
| HSA | Human Serum Albumin |
| H2O2 | Hydrogen peroxide |
| HA | Hyaluronic acid |
| HIF-1α | Hypoxia-inducible factor 1α |
| HepG2 | hepatoblastoma cell line |
| ICD | Immunogenic Cell Death |
| IFN-γ | Interferon-gamma |
| IL-2 | Interleukin-2 |
| IND | Investigational New Drug |
| iNOS | Inducible Nitric Oxide Synthase |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| ICIs | Immune Checkpoint Inhibitors |
| IFNα | Interferon Alpha |
| IL-4R | Interleukin-4 Receptor |
| IFP | interstitial fluid pressure |
| IL-12 | Interleukin 12 |
| IL-10 | Interleukin-10 |
| IL-12@P1 NPs | IL-12-loaded poly (beta-amino ester) nanoparticles |
| IgE | Immunoglobulin E |
| LAG-3 | Lymphocyte-Activation Gene 3 |
| LFA-1 | Lymphocyte Function-Associated Antigen 1 |
| LPH | Liposome-Polycation-Hyaluronic Acid |
| LPHNPs | Lipid-Polymer Hybrid Nanoparticles |
| MDSCs | Myeloid-Derived Suppressor Cells |
| MHC | Major Histocompatibility Complex |
| MMPs | Matrix Metalloproteinases |
| MPa | Megapascal |
| MSNs | Mesoporous Silica Nanoparticles |
| MWCNTs | Multiwalled Carbon Nanotubes |
| mAbs | Monoclonal Antibodies |
| MBP | MnO2 Nanoparticles in PFOB Nanoemulsion |
| miRNA | MicroRNA |
| MRI | Magnetic Resonance Imaging |
| MUC4 | Mucin 4 |
| MDR | multidrug resistance |
| ML | Machine learning |
| MOFs | Metal–organic frameworks |
| MRPs | Multidrug resistance-associated proteins |
| MRX34 | a liposome-encapsulated miR-34a mimic |
| NIR | Near-Infrared |
| NE | Nanoemulsion |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of Activated B cells |
| NK | Natural Killer |
| NLCs | Nanostructured Lipid Carriers |
| NSCLC | Non-Small Cell Lung Cancer |
| NSCs | Neural Stem Cells |
| Ntn1 | Netrin-1 |
| NPs | nanoparticles |
| NO | Nitric oxide |
| NNM | nanoparticulate nanomedicines |
| ODN | Oligodeoxynucleotides |
| O2 | Oxygen |
| PACA | Poly(Alkyl Cyanoacrylate) |
| PBCA | Poly(Butyl Cyanoacrylate) |
| PCL | Poly(ε-caprolactone) |
| PDT | Photodynamic Therapy |
| PEG | Polyethylene Glycol |
| PGA | Poly(γ-glutamic acid) |
| PLA | Polylactic Acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| PTT | Photothermal Therapy |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PEI | Polyethylenimine |
| PFOB | Perfluorooctyl Bromide |
| PLK1 | Polo-like Kinase 1 |
| pM330 | Plasmid encoding Cas9 for Ntn1 targeting |
| poly(I:C) | Polyinosinic-Polycytidylic Acid |
| PTX | Paclitaxel |
| P-gp | P-glycoprotein |
| QDs | Quantum Dots |
| RGD | Arginine–Glycine–Aspartic Acid (Peptide) |
| ROS | Reactive Oxygen Species |
| R848 | Resiquimod |
| RES | Reticuloendothelial System |
| RGD | Arginine-Glycine-Aspartic Acid |
| RICTOR | Rapamycin-Insensitive Companion of mTOR |
| RNS | Reactive nitrogen species |
| siRNA | Small Interfering RNA |
| SPIONs | Superparamagnetic Iron Oxide Nanoparticles |
| Sfn | Sorafenib |
| sgRNA | Single-Guide RNA |
| SIRP-α | Signal Regulatory Protein Alpha |
| SLNs | Solid Lipid Nanoparticles |
| SnMP | Tin Mesoporphyrin |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STING | Stimulator of Interferon Genes |
| stPEI | Stearyl-Polyethylenimine |
| siRNA | Small Interfering Ribonucleic Acid |
| TAAs | Tumor-Associated Antigens |
| TCR | T-Cell Receptor |
| TERT | Telomerase Reverse Transcriptase |
| TILs | Tumor-Infiltrating Lymphocytes |
| TLR | Toll-Like Receptor |
| TME | Tumor Microenvironment |
| TNBC | Triple-Negative Breast Cancer |
| TAMs | Tumor-Associated Macrophages |
| TIGIT | T-cell immunoreceptor with Ig and ITIM domains |
| TIM-3 | T-cell Immunoglobulin and Mucin-domain containing-3 |
| Tregs | Regulatory T Cells |
| TriMix | A combination of mRNAs encoding CD70, CD40L, and constitutively active TLR4 |
| TAFs | Tumor-associated fibroblasts |
| TAT | Trans-Activator of Transcription peptide |
| TNF-α | Tumor necrosis factor alpha |
| TAMs | Tumor-associate macrophages |
| TGF-β | Transforming growth factor beta |
| TMB | tumor mutational burden |
| ZP | Zeta Potential |
References
- Cancer Statistics—NCI. Available online: https://www.cancer.gov/about-cancer/understanding/statistics (accessed on 13 January 2025).
- Cooper, G.M. The Development and Causes of Cancer. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9963/ (accessed on 13 January 2025).
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef] [PubMed]
- Salahpour Anarjan, F. Active targeting drug delivery nanocarriers: Ligands. Nano-Struct. Nano-Objects 2019, 19, 100370. [Google Scholar] [CrossRef]
- Shams, F.; Golchin, A.; Azari, A.; Mohammadi Amirabad, L.; Zarein, F.; Khosravi, A.; Ardeshirylajimi, A. Nanotechnology-based products for cancer immunotherapy. Mol. Biol. Rep. 2022, 49, 1389–1412. [Google Scholar] [CrossRef] [PubMed]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Chapter 8—Lipid-Based Nanoparticles for Drug-Delivery Systems. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–284. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Velpurisiva, P.; Gad, A.; Piel, B.; Jadia, R.; Rai, P. Nanoparticle Design Strategies for Effective Cancer Immunotherapy. J. Biomed. Syd. NSW 2017, 2, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kibria, G.; Hatakeyama, H.; Harashima, H. Cancer multidrug resistance: Mechanisms involved and strategies for circumvention using a drug delivery system. Arch. Pharm. Res. 2014, 37, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Litman, T.; Brangi, M.; Hudson, E.; Fetsch, P.; Abati, A.; Ross, D.D.; Miyake, K.; Resau, J.H.; Bates, S.E. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2). J. Cell Sci. 2000, 113, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Tang, C.; Tian, C.; Sun, Q.; Su, Z.; Xue, L.; Yin, Y.; Ju, C.; Zhang, C. Co-delivery of paclitaxel and anti-survivin siRNA via redox-sensitive oligopeptide liposomes for the synergistic treatment of breast cancer and metastasis. Int. J. Pharm. 2017, 529, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Gordijo, C.R.; Abbasi, A.Z.; Maeda, A.; Ip, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. Multifunctional Albumin–MnO2 Nanoparticles Modulate Solid Tumor Microenvironment by Attenuating Hypoxia, Acidosis, Vascular Endothelial Growth Factor and Enhance Radiation Response. ACS Nano 2014, 8, 3202–3212. [Google Scholar] [CrossRef] [PubMed]
- Vadde, R.; Vemula, S.; Jinka, R.; Merchant, N.; Bramhachari, P.V.; Nagaraju, G.P. Role of hypoxia-inducible factors (HIF) in the maintenance of stemness and malignancy of colorectal cancer. Crit. Rev. Oncol. Hematol. 2017, 113, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Rey, S.; Schito, L.; Wouters, B.G.; Eliasof, S.; Kerbel, R.S. Targeting Hypoxia-Inducible Factors for Antiangiogenic Cancer Therapy. Trends Cancer 2017, 3, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, F.; Moghadaszadeh Ardebili, S.; Baghi Moornani, M.; Masjedi, A.; Atyabi, F.; Kiani, M.; Namdar, A.; Karpisheh, V.; Izadi, S.; Baradaran, B.; et al. Silencing of HIF-1α/CD73 axis by siRNA-loaded TAT-chitosan-spion nanoparticles robustly blocks cancer cell progression. Eur. J. Pharmacol. 2020, 882, 173235. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Wu, Z.-X.; Yang, Y.; Teng, Q.-X.; Li, Y.-D.; Lei, Z.-N.; Jani, K.A.; Kaushal, N.; Chen, Z.-S. ATP-binding cassette (ABC) transporters in cancer: A review of recent updates. J. Evid. Based Med. 2021, 14, 232–256. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Brinkhuis, R.F.; Deemter, L.v.; Wijnholds, J.; Schinkel, A.H. Extensive Contribution of the Multidrug Transporters P-Glycoprotein and Mrp1 to Basal Drug Resistance1. Cancer Res. 2000, 60, 5761–5766. [Google Scholar] [PubMed]
- Murakami, M.; Cabral, H.; Matsumoto, Y.; Wu, S.; Kano, M.R.; Yamori, T.; Nishiyama, N.; Kataoka, K. Improving Drug Potency and Efficacy by Nanocarrier-Mediated Subcellular Targeting. Sci. Transl. Med. 2011, 3, 64ra2. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P.C. pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J. Adv. Res. 2019, 18, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, L.; Jiang, S.; Yang, D.; He, H.; Zhang, F.; Zhang, P. Multifunctional micelle delivery system for overcoming multidrug resistance of doxorubicin. J. Drug Target. 2018, 26, 289–295. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Gong, C.; Qin, J.; Li, M.; Huang, S. Cancer Cell Membrane Decorated Silica Nanoparticle Loaded with miR495 and Doxorubicin to Overcome Drug Resistance for Effective Lung Cancer Therapy. Nanoscale Res. Lett. 2019, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, N.; Wan, G.; Zhang, T.; Li, C.; Wang, Y.; Wang, Y.; Liu, Y. pH and redox dual-responsive nanoparticles based on disulfide-containing poly(β-amino ester) for combining chemotherapy and COX-2 inhibitor to overcome drug resistance in breast cancer. J. Nanobiotechnol. 2019, 17, 109. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, A.; Schioppa, T.; Tiberio, L.; Stabile, H.; Sozzani, S. Leukocyte trafficking in tumor microenvironment. Curr. Opin. Pharmacol. 2017, 35, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, T.D.; Prieto-Dominguez, N.; Gowda, P.S.; Ubil, E. Mechanisms of Macrophage Plasticity in the Tumor Environment: Manipulating Activation State to Improve Outcomes. Front. Immunol. 2021, 12, 642285. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, F.; Huang, Z.; Li, Y.; Shi, H.; Sun, Q.; Ma, Y.; Wang, Y.; Zhang, Y.; Yang, S.; et al. T cells, NK cells, and tumor-associated macrophages in cancer immunotherapy and the current state of the art of drug delivery systems. Front. Immunol. 2023, 14, 1199173. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Roy, K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor-Associated Macrophages Toward M1-like Phenotype and Attenuating Tumor Hypoxia|ACS Nano. Available online: https://pubs-acs-org.nottingham.idm.oclc.org/doi/full/10.1021/acsnano.5b06779 (accessed on 22 February 2025).
- Noble Metal Nanoparticle-Induced Oxidative Stress Modulates Tumor Associated Macrophages (TAMs) from an M2 to M1 Phenotype: An In Vitro Approach—ScienceDirect. Available online: https://www-sciencedirect-com.nottingham.idm.oclc.org/science/article/pii/S1567576916302314 (accessed on 22 February 2025).
- Wang, Y.; Lin, Y.-X.; Qiao, S.-L.; An, H.-W.; Ma, Y.; Qiao, Z.-Y.; Rajapaksha, R.P.Y.J.; Wang, H. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials 2017, 112, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 707319. [Google Scholar] [CrossRef] [PubMed]
- Cancer Nanomedicine: Progress, Challenges and Opportunities|Nature Reviews Cancer. Available online: https://www.nature.com/articles/nrc.2016.108 (accessed on 5 May 2025).
- Bruneau, M.; Bennici, S.; Brendle, J.; Dutournie, P.; Limousy, L.; Pluchon, S. Systems for stimuli-controlled release: Materials and applications. J. Control. Release 2019, 294, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Panigaj, M.; Johnson, M.B.; Ke, W.; McMillan, J.; Goncharova, E.A.; Chandler, M.; Afonin, K.A. Aptamers as Modular Components of Therapeutic Nucleic Acid Nanotechnology. ACS Nano 2019, 13, 12301–12321. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Luiz, M.T.; Abriata, J.P.; Raspantini, G.L.; Tofani, L.B.; Fumagalli, F.; de Melo, S.M.G.; Emery, F.d.S.; Swiech, K.; Marcato, P.D.; Lee, R.; et al. In vitro evaluation of folate-modified PLGA nanoparticles containing paclitaxel for ovarian cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110038. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up polymeric-based nanoparticles drug delivery systems: Development and challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Egwu, C.O.; Aloke, C.; Onwe, K.T.; Umoke, C.I.; Nwafor, J.; Eyo, R.A.; Chukwu, J.A.; Ufebe, G.O.; Ladokun, J.; Audu, D.T.; et al. Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine. Molecules 2024, 29, 2584. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Biocompatibility, Biodegradation and Biomedical Applications of Poly(lactic acid)/poly(lactic-co-glycolic acid) Micro and Nanoparticles|Journal of Pharmaceutical Investigation. Available online: https://link.springer.com/article/10.1007/s40005-019-00439-x (accessed on 5 May 2025).
- Polymer-Protein Conjugates. 2019. Available online: https://shop.elsevier.com/books/polymer-protein-conjugates/pasut/978-0-444-64081-9 (accessed on 5 May 2025).
- Yoo, J.; Won, Y.-Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Batty, C.J.; Bachelder, E.M.; Ainslie, K.M. Historical Perspective of Clinical Nano and Microparticle Formulations for Delivery of Therapeutics. Trends Mol. Med. 2021, 27, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Waterkotte, T.; He, X.; Wanasathop, A.; Li, S.K.; Park, Y.C. Long-Term Antibody Release Polycaprolactone Capsule and the Release Kinetics in Natural and Accelerated Degradation. ACS Biomater. Sci. Eng. 2022, 8, 4428–4438. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, E.; Notopoulou, M.; Nakiou, E.; Kostoglou, M.; Barmpalexis, P.; Bikiaris, D.N. Branched Poly(ε-caprolactone)-Based Copolyesters of Different Architectures and Their Use in the Preparation of Anticancer Drug-Loaded Nanoparticles. Int. J. Mol. Sci. 2022, 23, 15393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, W.; Kong, G.; Zhen, Y.; Zeng, Q.; Wang, S.; Chen, S.; Gu, J.; Li, C.; Guo, K. Paclitaxel-incorporated nanoparticles improve functional recovery after spinal cord injury. Front. Pharmacol. 2022, 13, 957433. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Helguera, G.; Legaspi, M.J.; Gonzalez, L.; Hocht, C.; Taira, C.; Chiappetta, D.A. Paclitaxel-loaded PCL-TPGS nanoparticles: In vitro and in vivo performance compared with Abraxane®. Colloids Surf. B Biointerfaces 2014, 113, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-F.; Woodrow, K.A. Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. J. Mech. Behav. Biomed. Mater. 2017, 65, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Y.; Blázquez, J.P.F.; Yin, G.-Z.; Wang, D.-Y.; Llorca, J.; Echeverry-Rendón, M. A strategy to tailor the mechanical and degradation properties of PCL-PEG-PCL based copolymers for biomedical application. arXiv 2023, arXiv:2308.15503. [Google Scholar] [CrossRef]
- Ramírez-Ruiz, F.; Núñez-Tapia, I.; Piña-Barba, M.C.; Alvarez-Pérez, M.A.; Guarino, V.; Serrano-Bello, J. Polycaprolactone for Hard Tissue Regeneration: Scaffold Design and In Vivo Implications. Bioengineering 2025, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Emadi, H.; Karevan, M.; Masoudi Rad, M.; Sadeghzade, S.; Pahlevanzadeh, F.; Khodaei, M.; Khayatzadeh, S.; Lotfian, S. Bioactive and Biodegradable Polycaprolactone-Based Nanocomposite for Bone Repair Applications. Polymers 2023, 15, 3617. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, X.; Su, C.; Shi, Y.; Zhao, L. Chitosan nanoparticles triggered the induction of ROS-mediated cytoprotective autophagy in cancer cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Al-Shadidi, J.R.M.H.; Al-Shammari, S.; Al-Mutairi, D.; Alkhudhair, D.; Thu, H.E.; Hussain, Z. Chitosan Nanoparticles for Targeted Cancer Therapy: A Review of Stimuli-Responsive, Passive, and Active Targeting Strategies. Int. J. Nanomed. 2024, 19, 8373–8400. [Google Scholar] [CrossRef] [PubMed]
- Chitosan Coated Solid Lipid Nanoparticles as Promising Carriers for Docetaxel. Available online: http://ouci.dntb.gov.ua/en/works/7noODG64/ (accessed on 5 May 2025).
- Frontiers|Recent Advances in Chitosan and Its Derivatives in Cancer Treatment. Available online: https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2022.888740/full (accessed on 5 May 2025).
- Guo, L.; Yan, D.D.; Yang, D.; Li, Y.; Wang, X.; Zalewski, O.; Yan, B.; Lu, W. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano 2014, 8, 5670–5681. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.B.M.; Douka, S.; Mertins, O.; Mastrobattista, E.; Han, S.W. Efficacy of Chitosan-N-Arginine Chitosomes in mRNA Delivery and Cell Viability Enhancement. ACS Appl. Bio Mater. 2024, 7, 8261–8271. [Google Scholar] [CrossRef] [PubMed]
- Caraway, C.A.; Gaitsch, H.; Wicks, E.E.; Kalluri, A.; Kunadi, N.; Tyler, B.M. Polymeric Nanoparticles in Brain Cancer Therapy: A Review of Current Approaches. Polymers 2022, 14, 2963. [Google Scholar] [CrossRef] [PubMed]
- Kante, B.; Couvreur, P.; Dubois-Krack, G.; De Meester, C.; Guiot, P.; Roland, M.; Mercier, M.; Speiseru, P. Toxicity of Polyalkylcyanoacrylate Nanoparticles I: Free Nanoparticles. J. Pharm. Sci. 1982, 71, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Gulyaev, A.E.; Gelperina, S.E.; Skidan, I.N.; Antropov, A.S.; Kivman, G.Y.; Kreuter, J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm. Res. 1999, 16, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B. Brain targeting PBCA nanoparticles and the blood-brain barrier. Nanomedicine 2009, 4, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, M.T.; Fattal, E.; Desmaële, D.; Besnard, M.; Noël, J.P.; Gomis, J.M.; Appel, M.; d’Angelo, J.; Couvreur, P. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J. Control. Release 1999, 60, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, V.; Rahiman, N.; Cabral, H.; Quader, S.; Zirak, M.R.; Taghavizadeh Yazdi, M.E.; Jaafari, M.R.; Alavizadeh, S.H. Poly-γ-glutamic acid nanoparticles as adjuvant and antigen carrier system for cancer vaccination. J. Control. Release 2023, 362, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Feng, X.; Zou, H.; Xu, W.; Zhuang, X. Poly(l-glutamic acid)-cisplatin nanoformulations with detachable PEGylation for prolonged circulation half-life and enhanced cell internalization. Bioact. Mater. 2021, 6, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent Advances in Poly(α-L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Toyama, M.; Nishi, Y.; Akagi, T.; Shima, F.; Akashi, M.; Baba, M. Uptake of biodegradable poly(γ-glutamic acid) nanoparticles and antigen presentation by dendritic cells in vivo. Results Immunol. 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Purohit, A.; Mishra, S.; Sahu, A.; Singhai, N.; Verma, A. Inorganic Nanoparticles-Based Strategies for Cancer Immunotherapy. In Nanotechnology Based Strategies for Cancer Immunotherapy: Concepts, Design, and Clinical Applications; Sharma, R., Pandey, V., Mishra, N., Eds.; Springer Nature: Singapore, 2024; pp. 327–353. [Google Scholar] [CrossRef]
- Badir, A.; Refki, S.; Sekkat, Z. Utilizing gold nanoparticles in plasmonic photothermal therapy for cancer treatment. Heliyon 2025, 11, e42738. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarlou, M.; Rashidzadeh, H.; Mohammadi, A.; Mousazadeh, N.; Barsbay, M.; Sharafi, A.; Gharbavi, M.; Danafar, H.; Javani, S. Photothermal and radiotherapy with alginate-coated gold nanoparticles for breast cancer treatment. Sci. Rep. 2024, 14, 13299. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.S.; Park, J.-H.; Lim, D.-K.; Ahn, C.-H.; Sun, I.-C.; Kim, K. How Did Conventional Nanoparticle-Mediated Photothermal Therapy Become “Hot” in Combination with Cancer Immunotherapy? Cancers 2022, 14, 2044. [Google Scholar] [CrossRef] [PubMed]
- Hiep Tran, T.; Thu Phuong Tran, T. Current status of nanoparticle-mediated immunogenic cell death in cancer immunotherapy. Int. Immunopharmacol. 2024, 142, 113085. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Kou, D.; Lou, S.; Ashrafizadeh, M.; Aref, A.R.; Canadas, I.; Tian, Y.; Niu, X.; Wang, Y.; Torabian, P.; et al. Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.P.P.; Lee, M.; Kim, T. Unlocking the Potential of Gold as Nanomedicine in Cancer Immunotherapy. J. Nanotheranostics 2024, 5, 29–59. [Google Scholar] [CrossRef]
- Different-Sized Gold Nanoparticle Activator/Antigen Increases Dendritic Cells Accumulation in Liver-Draining Lymph Nodes and CD8+ T Cell Responses—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26771692/ (accessed on 5 May 2025).
- Huang, H.; Liu, R.; Yang, J.; Dai, J.; Fan, S.; Pi, J.; Wei, Y.; Guo, X. Gold Nanoparticles: Construction for Drug Delivery and Application in Cancer Immunotherapy. Pharmaceutics 2023, 15, 1868. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.B.R.; Choi, J.-H.; Kim, U.K.; Hwang, D.S.; Kim, G.C. Gold nanoparticles conjugated with programmed death-ligand 1 antibodies induce apoptosis of SCC-25 oral squamous cell carcinoma cells via programmed death-ligand 1/signal transducer and transcription 3 pathway. Arch. Oral Biol. 2021, 125, 105085. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Banstola, A.; Vatanara, A.; Lee, S.; Kim, J.O.; Jeong, J.-H.; Yook, S. Doxorubicin and Anti-PD-L1 Antibody Conjugated Gold Nanoparticles for Colorectal Cancer Photochemotherapy. Mol. Pharm. 2019, 16, 1184–1199. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, R.; Chan, C.; Lu, Y.; Winnik, M.A.; Cescon, D.W.; Reilly, R.M. 90Y-Labeled Gold Nanoparticle Depot (NPD) Combined with Anti-PD-L1 Antibodies Strongly Inhibits the Growth of 4T1 Tumors in Immunocompetent Mice and Induces an Abscopal Effect on a Distant Non-Irradiated Tumor. Mol. Pharm. 2022, 19, 4199–4211. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Azharuddin, M.; Al-Otaibi, N.; Hinkula, J. Efficacy and Immune Response Elicited by Gold Nanoparticle- Based Nanovaccines against Infectious Diseases. Vaccines 2022, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Pitirollo, O.; Micoli, F.; Necchi, F.; Mancini, F.; Carducci, M.; Adamo, R.; Evangelisti, C.; Morelli, L.; Polito, L.; Lay, L. Gold nanoparticles morphology does not affect the multivalent presentation and antibody recognition of Group A Streptococcus synthetic oligorhamnans. Bioorganic Chem. 2020, 99, 103815. [Google Scholar] [CrossRef] [PubMed]
- Budhadev, D.; Poole, E.; Nehlmeier, I.; Liu, Y.; Hooper, J.; Kalverda, E.; Akshath, U.S.; Hondow, N.; Turnbull, W.B.; Pöhlmann, S.; et al. Glycan-Gold Nanoparticles as Multifunctional Probes for Multivalent Lectin-Carbohydrate Binding: Implications for Blocking Virus Infection and Nanoparticle Assembly. J. Am. Chem. Soc. 2020, 142, 18022–18034. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.Y.; McGee, J.K.; Killius, M.G.; Suarez, D.A.; Blackman, C.F.; DeMarini, D.M.; Simmons, S.O. Investigating oxidative stress and inflammatory responses elicited by silver nanoparticles using high-throughput reporter genes in HepG2 cells: Effect of size, surface coating, and intracellular uptake. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2013, 27, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Sargsian, A.; Koutsoumpou, X.; Girmatsion, H.; Egil, C.; Buttiens, K.; Luci, C.R.; Soenen, S.J.; Manshian, B.B. Silver nanoparticle induced immunogenic cell death can improve immunotherapy. J. Nanobiotechnol. 2024, 22, 691. [Google Scholar] [CrossRef] [PubMed]
- Habiba, K.; Aziz, K.; Sanders, K.; Santiago, C.M.; Mahadevan, L.S.K.; Makarov, V.; Weiner, B.R.; Morell, G.; Krishnan, S. Enhancing Colorectal Cancer Radiation Therapy Efficacy using Silver Nanoprisms Decorated with Graphene as Radiosensitizers. Sci. Rep. 2019, 9, 17120. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Liu, M.; Wang, Z.; Zhou, R.; Ai, K. Enhancing radiotherapy-induced anti-tumor immunity via nanoparticle-mediated STING agonist synergy. Mol. Cancer 2025, 24, 176. [Google Scholar] [CrossRef] [PubMed]
- Theivendran, S.; Lazarev, S.; Yu, C. Mesoporous silica/organosilica nanoparticles for cancer immunotherapy. Exploration 2023, 3, 20220086. [Google Scholar] [CrossRef] [PubMed]
- Godakhindi, V.; Tarannum, M.; Dam, S.K.; Vivero-Escoto, J.L. Mesoporous Silica Nanoparticles as an Ideal Platform for Cancer Immunotherapy: Recent Advances and Future Directions. Adv. Healthc. Mater. 2024, 13, e2400323. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Tang, J.; Qiao, Q.; Wu, T.; Qi, Y.; Tan, S.; Gao, X.; Zhang, Z. Biodegradable Hollow Mesoporous Silica Nanoparticles for Regulating Tumor Microenvironment and Enhancing Antitumor Efficiency. Theranostics 2017, 7, 3276–3292. [Google Scholar] [CrossRef] [PubMed]
- Nairi, V.; Magnolia, S.; Piludu, M.; Nieddu, M.; Caria, C.A.; Sogos, V.; Vallet-Regì, M.; Monduzzi, M.; Salis, A. Mesoporous silica nanoparticles functionalized with hyaluronic acid. Effect of the biopolymer chain length on cell internalization. Colloids Surf. B Biointerfaces 2018, 168, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Extra-Large Pore Mesoporous Silica Nanoparticles Enabling Co-Delivery of High Amounts of Protein Antigen and Toll-like Receptor 9 Agonist for Enhanced Cancer Vaccine Efficacy—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29721531/ (accessed on 5 May 2025).
- Escriche-Navarro, B.; Escudero, A.; Lucena-Sánchez, E.; Sancenón, F.; García-Fernández, A.; Martínez-Máñez, R. Mesoporous Silica Materials as an Emerging Tool for Cancer Immunotherapy. Adv. Sci. 2022, 9, e2200756. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Ito, A.; Sogo, Y.; Ohno, T. Synergistic anti-tumor efficacy of a hollow mesoporous silica-based cancer vaccine and an immune checkpoint inhibitor at the local site. Acta Biomater. 2022, 145, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Sobhanan, J.; Rival, J.V.; Anas, A.; Sidharth Shibu, E.; Takano, Y.; Biju, V. Luminescent quantum dots: Synthesis, optical properties, bioimaging and toxicity. Adv. Drug Deliv. Rev. 2023, 197, 114830. [Google Scholar] [CrossRef] [PubMed]
- In Vivo Cation Exchange in Quantum Dots for Tumor-Specific Imaging|Nature Communications. Available online: https://www.nature.com/articles/s41467-017-00153-y (accessed on 5 May 2025).
- Advances in Quantum Dot-Mediated siRNA Delivery. Available online: https://html.rhhz.net/zghxkb/20170909.htm (accessed on 5 May 2025).
- Lin, G.; Chen, T.; Zou, J.; Wang, Y.; Wang, X.; Li, J.; Huang, Q.; Fu, Z.; Zhao, Y.; Lin, M.C.-M.; et al. Quantum Dots-siRNA Nanoplexes for Gene Silencing in Central Nervous System Tumor Cells. Front. Pharmacol. 2017, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Derfus, M.; Chen, A.A.; Min, D.-H.; Ruoslahti, E.; Bhatia, S.N. Targeted quantum dot conjugates for siRNA delivery. Bioconjug. Chem. 2007, 18, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Getz, T.; Qin, J.; Medintz, I.L.; Delehanty, J.B.; Susumu, K.; Dawson, P.E.; Dawson, G. Quantum dot-mediated delivery of siRNA to inhibit sphingomyelinase activities in brain-derived cells. J. Neurochem. 2016, 139, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Raut, J.; Sahoo, P. Gene Silencing and Gene Delivery in Therapeutics: Insights Using Quantum Dots. Front. Biosci. 2023, 28, 364. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Wilson, R.L.; Cubeddu, L.X. Quantum Dot Research in Breast Cancer: Challenges and Prospects. Materials 2024, 17, 2152. [Google Scholar] [CrossRef] [PubMed]
- Mohkam, M.; Sadraeian, M.; Lauto, A.; Gholami, A.; Nabavizadeh, S.H.; Esmaeilzadeh, H.; Alyasin, S. Exploring the potential and safety of quantum dots in allergy diagnostics. Microsyst. Nanoeng. 2023, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lv, Y.; Wu, R.; Li, J.; Shen, H.; Yang, H.; Zhang, H.; Li, L.S. Sensitive Immunoassay Based on Biocompatible and Robust Silica-Coated Cd-Free InP-Based Quantum Dots. Inorg. Chem. 2021, 60, 6503–6513. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Zhang, M.; Kim, K. Quantum Dots and Their Interaction with Biological Systems. Int. J. Mol. Sci. 2022, 23, 10763. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, T. A Review of in vivo Toxicity of Quantum Dots in Animal Models. Int. J. Nanomed. 2023, 18, 8143–8168. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.A.; Ramli, M.M.; Osman, N.H.; Mohamed, R. Stimulation of Innate and Adaptive Immune Cells with Graphene Oxide and Reduced Graphene Oxide Affect Cancer Progression. Arch. Immunol. Ther. Exp. 2021, 69, 20. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Ghorbani, S.H.; Mahdavian, L.; Aghamohammadi, M. Graphene-based hybrid composites for cancer diagnostic and therapy. J. Transl. Med. 2024, 22, 611. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-J.; Vayalakkara, R.K.; Dash, B.S.; Hu, S.-H.; Premji, T.P.; Wu, C.-Y.; Shen, Y.-J.; Chen, J.-P. Immunomodulatory R848-Loaded Anti-PD-L1-Conjugated Reduced Graphene Oxide Quantum Dots for Photothermal Immunotherapy of Glioblastoma. Pharmaceutics 2024, 16, 1064. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Biswas, K.; Rauta, P.R.; Mishra, A.K.; De, D.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; Abd-Allah, E.F.; Mahanta, S.; et al. Development of Graphene Oxide Nanosheets as Potential Biomaterials in Cancer Therapeutics: An In-Vitro Study Against Breast Cancer Cell Line. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4236–4249. [Google Scholar] [CrossRef]
- Li, Z.; Bu, J.; Zhu, X.; Zhou, H.; Ren, K.; Chu, P.K.; Li, L.; Hu, X.; Ding, X. Anti-tumor immunity and ferroptosis of hepatocellular carcinoma are enhanced by combined therapy of sorafenib and delivering modified GO-based PD-L1 siRNAs. Biomater. Adv. 2022, 136, 212761. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, J.; Ya, N.; Deng, L.; Gan, Q.; Liu, J.; Zeng, Y. Golgi Apparatus Targeted Graphene Oxide Nanocomposites for Synergistic Chemotherapy, Photothermal Therapy, and Photodynamic Therapy of Metastatic Breast Cancer. ACS Appl. Nano Mater. 2024, 7, 7520–7532. [Google Scholar] [CrossRef]
- Báez, D.F. Graphene-Based Nanomaterials for Photothermal Therapy in Cancer Treatment. Pharmaceutics 2023, 15, 2286. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.B.; Sweeney, E.E.; Ramanujam, A.S.; Fernandes, R. Photothermal therapies to improve immune checkpoint blockade for cancer. Int. J. Hyperth. 2020, 37, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xu, B.; Sun, J.; Zhang, Z. Nanotechnological advances in cancer: Therapy a comprehensive review of carbon nanotube applications. Front. Bioeng. Biotechnol. 2024, 12, 1351787. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnol. 2021, 19, 423. [Google Scholar] [CrossRef] [PubMed]
- Naief, M.F.; Mohammed, S.N.; Mayouf, H.J.; Mohammed, A.M. A review of the role of carbon nanotubes for cancer treatment based on photothermal and photodynamic therapy techniques. J. Organomet. Chem. 2023, 999, 122819. [Google Scholar] [CrossRef]
- Thakur, C.K.; Karthikeyan, C.; Ashby, C.R.; Neupane, R.; Singh, V.; Babu, R.J.; Narayana Moorthy, N.S.H.; Tiwari, A.K. Ligand-conjugated multiwalled carbon nanotubes for cancer targeted drug delivery. Front. Pharmacol. 2024, 15, 1417399. [Google Scholar] [CrossRef] [PubMed]
- Faria, G.N.F.; Karch, C.G.; Chakraborty, S.; Gu, T.; Woodward, A.; Aissanou, A.; Lageshetty, S.; Silvy, R.P.; Resasco, D.; Ballon, J.A.; et al. Immunogenic Treatment of Metastatic Breast Cancer Using Targeted Carbon Nanotube Mediated Photothermal Therapy in Combination with Anti-Programmed Cell Death Protein-1. J. Pharmacol. Exp. Ther. 2024, 390, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wu, S.; Song, S.; Chen, W.R.; Resasco, D.E.; Xing, D. Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials 2012, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, M.; Florek, E.; Mrówczyński, R. Assessment of Pristine Carbon Nanotubes Toxicity in Rodent Models. Int. J. Mol. Sci. 2022, 23, 15343. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Pietroiusti, A.; Fadeel, B.; Kagan, V.E. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol. Appl. Pharmacol. 2012, 261, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xu, Y.; Zhou, C.; Huo, J.; Su, S.; Liu, L.; Zhu, Z.; Li, L.; Jia, W.; Wang, C.; et al. Oral Immunotherapy Reshapes Intestinal Immunosuppression via Metabolic Reprogramming to Enhance Systemic Anti-Tumor Immunity. Adv. Sci. 2023, 10, e2302910. [Google Scholar] [CrossRef] [PubMed]
- Fullerene-Based Immunoregulatory Nanomaterials for Immunotherapy of Tumor and Immune-Related Inflammatory Diseases—Zhen—2024—Advanced Functional Materials—Wiley Online Library. Available online: https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/adfm.202409319 (accessed on 5 May 2025).
- Dias, A.M.M.; Courteau, A.; Bellaye, P.-S.; Kohli, E.; Oudot, A.; Doulain, P.-E.; Petitot, C.; Walker, P.-M.; Decréau, R.; Collin, B. Superparamagnetic Iron Oxide Nanoparticles for Immunotherapy of Cancers through Macrophages and Magnetic Hyperthermia. Pharmaceutics 2022, 14, 2388. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Enhancing Organ and Vascular Contrast in Preclinical Ultra-Low Field MRI Using Superparamagnetic Iron Oxide Nanoparticles—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/39342051/ (accessed on 5 May 2025).
- Zhang, Y.; Li, Z.; Huang, Y.; Zou, B.; Xu, Y. Amplifying cancer treatment: Advances in tumor immunotherapy and nanoparticle-based hyperthermia. Front. Immunol. 2023, 14, 1258786. [Google Scholar] [CrossRef] [PubMed]
- Janko, C.; Ratschker, T.; Nguyen, K.; Zschiesche, L.; Tietze, R.; Lyer, S.; Alexiou, C. Functionalized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as Platform for the Targeted Multimodal Tumor Therapy. Front. Oncol. 2019, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Muthana, M.; Kennerley, A.J.; Hughes, R.; Fagnano, E.; Richardson, J.; Paul, M.; Murdoch, C.; Wright, F.; Payne, C.; Lythgoe, M.F.; et al. Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nat. Commun. 2015, 6, 8009. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.R.; Port, J.D.; Pandey, M.K. Use of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) via Multiple Imaging Modalities and Modifications to Reduce Cytotoxicity: An Educational Review. J. Nanotheranostics 2020, 1, 105–135. [Google Scholar] [CrossRef]
- Saleh, D.M.; Luo, S.; Ahmed, O.H.M.; Alexander, D.B.; Alexander, W.T.; Gunasekaran, S.; El-Gazzar, A.M.; Abdelgied, M.; Numano, T.; Takase, H.; et al. Assessment of the toxicity and carcinogenicity of double-walled carbon nanotubes in the rat lung after intratracheal instillation: A two-year study. Part. Fibre Toxicol. 2022, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Sulheim, E.; Iversen, T.-G.; To Nakstad, V.; Klinkenberg, G.; Sletta, H.; Schmid, R.; Hatletveit, A.R.; Wågbø, A.M.; Sundan, A.; Skotland, T.; et al. Cytotoxicity of Poly(Alkyl Cyanoacrylate) Nanoparticles. Int. J. Mol. Sci. 2017, 18, 2454. [Google Scholar] [CrossRef] [PubMed]
- Vakili-Ghartavol, R.; Momtazi-Borojeni, A.A.; Vakili-Ghartavol, Z.; Aiyelabegan, H.T.; Jaafari, M.R.; Rezayat, S.M.; Arbabi Bidgoli, S. Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues. Artif. Cells Nanomed. Biotechnol. 2020, 48, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Fojtu, M.; Gumulec, J.; Stracina, T.; Raudenska, M.; Skotakova, A.; Vaculovicova, M.; Adam, V.; Babula, P.; Novakova, M.; Masarik, M. Reduction of Doxorubicin-Induced Cardiotoxicity Using Nanocarriers: A Review. Curr. Drug Metab. 2017, 18, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Bawab, A.A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Patel, H.M. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system—The concept of tissue specificity. Adv. Drug Deliv. Rev. 1998, 32, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, L. Nanoparticles Escaping RES and Endosome: Challenges for siRNA Delivery for Cancer Therapy. J. Nanomater. 2011, 2011, 742895. [Google Scholar] [CrossRef]
- Hong, R.-L.; Huang, C.-J.; Tseng, Y.-L.; Pang, V.F.; Chen, S.-T.; Liu, J.-J.; Chang, F.-H. Direct Comparison of Liposomal Doxorubicin with or without Polyethylene Glycol Coating in C-26 Tumor-bearing Mice: Is Surface Coating with Polyethylene Glycol Beneficial? Clin. Cancer Res. 1999, 5, 3645–3652. [Google Scholar] [PubMed]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, T.; Csincsi, Á.I.; Uzonyi, B.; Hebecker, M.; Fülöp, T.G.; Erdei, A.; Szebeni, J.; Józsi, M. Factor H inhibits complement activation induced by liposomal and micellar drugs and the therapeutic antibody rituximab in vitro. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Kwong, B.; Gai, S.A.; Elkhader, J.; Wittrup, K.D.; Irvine, D.J. Localized Immunotherapy via Liposome-Anchored Anti-CD137 + IL-2 Prevents Lethal Toxicity and Elicits Local and Systemic Antitumor Immunity. Cancer Res. 2013, 73, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, N.; Suh, H.; Irvine, D.J. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Bayyurt, B.; Tincer, G.; Almacioglu, K.; Alpdundar, E.; Gursel, M.; Gursel, I. Encapsulation of two different TLR ligands into liposomes confer protective immunity and prevent tumor development. J. Control. Release 2017, 247, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Abumanhal-Masarweh, H.; da Silva, D.; Poley, M.; Zinger, A.; Goldman, E.; Krinsky, N.; Kleiner, R.; Shenbach, G.; Schroeder, J.E.; Shklover, J.; et al. Tailoring the lipid composition of nanoparticles modulates their cellular uptake and affects the viability of triple negative breast cancer cells. J. Control. Release 2019, 307, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.; Chao, P.-H.; Qin, Z.; Böttger, R.; Lee, S.E.; Li, S.-D. Liposomal Resiquimod for Enhanced Immunotherapy of Peritoneal Metastases of Colorectal Cancer. Pharmaceutics 2021, 13, 1696. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.-H.; Chan, V.; Wu, J.; Andrew, L.J.; Li, S.-D. Resiquimod-loaded cationic liposomes cure mice with peritoneal carcinomatosis and induce specific anti-tumor immunity. J. Control. Release 2024, 372, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of immune checkpoints—PD-1, PD-L1, CTLA-4—New opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, A.; Kostine, M.; Barnetche, T.; Truchetet, M.-E.; Schaeverbeke, T. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Weber, J. Review: Anti–CTLA-4 Antibody Ipilimumab: Case Studies of Clinical Response and Immune-Related Adverse Events. Oncologist 2007, 12, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Nikpoor, A.R.; Tavakkol-Afshari, J.; Sadri, K.; Jalali, S.A.; Jaafari, M.R. Improved tumor accumulation and therapeutic efficacy of CTLA-4-blocking antibody using liposome-encapsulated antibody: In vitro and in vivo studies. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2671–2682. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Jiang, Q.; Xiao, X.; Xu, X.; Xu, Y.; Kong, Y.; Zhang, W.; Zeng, Y.; Liu, X.; Luo, B. Assisting anti-PD-1 antibody treatment with a liposomal system capable of recruiting immune cells. Nanoscale 2019, 11, 7996–8011. [Google Scholar] [CrossRef] [PubMed]
- Hei, Y.; Teng, B.; Zeng, Z.; Zhang, S.; Li, Q.; Pan, J.; Luo, Z.; Xiong, C.; Wei, S. Multifunctional Immunoliposomes Combining Catalase and PD-L1 Antibodies Overcome Tumor Hypoxia and Enhance Immunotherapeutic Effects Against Melanoma. Int. J. Nanomed. 2020, 15, 1677–1691. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Wang, Q.; Shi, Y.; Huang, Y.; Zhang, J.; Zhang, X.; Lin, G. Nanotechnology-mediated immunochemotherapy combined with docetaxel and PD-L1 antibody increase therapeutic effects and decrease systemic toxicity. J. Control. Release 2018, 286, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in Regulatory T Cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ngiow, S.F.; von Scheidt, B.; Akiba, H.; Yagita, H.; Teng, M.W.L.; Smyth, M.J. Anti-TIM3 Antibody Promotes T Cell IFN-γ–Mediated Antitumor Immunity and Suppresses Established Tumors. Cancer Res. 2011, 71, 3540–3551. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Monnard, P.-A.; Oberholzer, T.; Luisi, P. Entrapment of nucleic acids in liposomes. Biochim. Biophys. Acta BBA Biomembr. 1997, 1329, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Masuda, T.; Katayama, K.; Yoshikawa, T.; Tsutsumi, Y.; Akashi, M.; Mayumi, T.; Nakagawa, S. Fusogenic liposome delivers encapsulated nanoparticles for cytosolic controlled gene release. J. Control. Release 2005, 105, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Stremersch, S.; Vandenbroucke, R.E.; Van Wonterghem, E.; Hendrix, A.; De Smedt, S.C.; Raemdonck, K. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J. Control. Release 2016, 232, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Salomon, N.; Vascotto, F.; Selmi, A.; Vormehr, M.; Quinkhardt, J.; Bukur, T.; Schrörs, B.; Löewer, M.; Diken, M.; Türeci, Ö.; et al. A liposomal RNA vaccine inducing neoantigen-specific CD4+ T cells augments the antitumor activity of local radiotherapy in mice. OncoImmunology 2020, 9, 1771925. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Geetha, K.M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; Sahly, H.M.E.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Kon, E.; Sharma, P.; Peer, D. Endosomal escape: A bottleneck for LNP-mediated therapeutics. Proc. Natl. Acad. Sci. USA 2024, 121, e2307800120. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tam, Y.Y.C.; Lin, P.J.C.; Sung, M.M.H.; Tam, Y.K.; Cullis, P.R. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Control. Release 2016, 235, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; You, X.; Wang, X.; Cui, L.; Wang, Z.; Xu, F.; Li, M.; Yang, Z.; Liu, J.; Huang, P.; et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2005191118. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Q.; Zhang, C.; Zhang, X.; Yan, J.; Zeng, C.; Talebian, F.; Lynch, K.; Zhao, W.; Hou, X.; Du, S.; et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control. Release 2022, 345, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Rybakova, Y.; Kowalski, P.S.; Huang, Y.; Gonzalez, J.T.; Heartlein, M.W.; DeRosa, F.; Delcassian, D.; Anderson, D.G. mRNA Delivery for Therapeutic Anti-HER2 Antibody Expression In Vivo. Mol. Ther. 2019, 27, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Hobo, W.; Novobrantseva, T.I.; Fredrix, H.; Wong, J.; Milstein, S.; Epstein-Barash, H.; Liu, J.; Schaap, N.; van der Voort, R.; Dolstra, H. Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation. Cancer Immunol. Immunother. CII 2012, 62, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef] [PubMed]
- Uner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; De, M.; Dey, G.; Bharti, R.; Chattopadhyay, S.; Ali, N.; Chakrabarti, P.; Reis, R.L.; Kundu, S.C.; Mandal, M. A peptide-modified solid lipid nanoparticle formulation of paclitaxel modulates immunity and outperforms dacarbazine in a murine melanoma model. Biomater. Sci. 2019, 7, 1161–1178. [Google Scholar] [CrossRef] [PubMed]
- Bondì, M.L.; Botto, C.; Amore, E.; Emma, M.R.; Augello, G.; Craparo, E.F.; Cervello, M. Lipid nanocarriers containing sorafenib inhibit colonies formation in human hepatocarcinoma cells. Int. J. Pharm. 2015, 493, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Extracellular Vesicles as Tools and Targets in Therapy for Diseases|Signal Transduction and Targeted Therapy. Available online: https://www.nature.com/articles/s41392-024-01735-1 (accessed on 15 January 2025).
- Chen, S.-W.; Zhu, S.-Q.; Pei, X.; Qiu, B.-Q.; Xiong, D.; Long, X.; Lin, K.; Lu, F.; Xu, J.-J.; Wu, Y.-B. Cancer cell-derived exosomal circUSP7 induces CD8+ T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol. Cancer 2021, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Huang, Z.; Shi, C.; Pu, X.; Xu, X.; Wu, Z.; Ding, G.; Cao, L. Pancreatic cancer-derived exosomes induce apoptosis of T lymphocytes through the p38 MAPK-mediated endoplasmic reticulum stress. FASEB J. 2020, 34, 8442–8458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhong, W.; Wang, B.; Yang, J.; Yang, J.; Yu, Z.; Qin, Z.; Shi, A.; Xu, W.; Zheng, C.; et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev. Cell 2022, 57, 329–343.e7. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Hu, S.; Sun, H.; Tuo, B.; Jia, B.; Chen, C.; Wang, W.; Liu, J.; Liu, Y.; Sun, Z.; et al. Extracellular vesicles remodel tumor environment for cancer immunotherapy. Mol. Cancer 2023, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zuo, B.; Jing, R.; Gao, X.; Rao, Q.; Liu, Z.; Qi, H.; Guo, H.; Yin, H. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J. Hepatol. 2017, 67, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Gunassekaran, G.R.; Poongkavithai Vadevoo, S.M.; Baek, M.-C.; Lee, B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials 2021, 278, 121137. [Google Scholar] [CrossRef] [PubMed]
- Adamus, T.; Hung, C.-Y.; Yu, C.; Kang, E.; Hammad, M.; Flores, L.; Nechaev, S.; Zhang, Q.; Gonzaga, J.M.; Muthaiyah, K.; et al. Glioma-targeted delivery of exosome-encapsulated antisense oligonucleotides using neural stem cells. Mol. Ther. Nucleic Acids 2021, 27, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Jung, I.; Jung, D.; Kim, C.S.; Kang, S.-M.; Ryu, S.; Choi, S.-J.; Noh, S.; Jeong, J.; Lee, B.Y.; et al. Novel antitumor therapeutic strategy using CD4+ T cell-derived extracellular vesicles. Biomaterials 2022, 289, 121765. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Shin, S.; Kang, S.; Jung, I.; Ryu, S.; Noh, S.; Choi, S.; Jeong, J.; Lee, B.Y.; Kim, K.; et al. Reprogramming of T cell-derived small extracellular vesicles using IL2 surface engineering induces potent anti-cancer effects through miRNA delivery. J. Extracell. Vesicles 2022, 11, 12287. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xie, Y.; Wang, J.; Bao, C.; Duan, J.; Liu, Y.; Luo, Q.; Xu, J.; Ren, Y.; Jiang, M.; et al. Gene engineered exosome reverses T cell exhaustion in cancer immunotherapy. Bioact. Mater. 2024, 34, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control. Release 2016, 223, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Kuai, X.; Zhu, Y.; Yuan, Z.; Wang, S.; Lin, L.; Ye, X.; Lu, Y.; Luo, Y.; Pang, Z.; Geng, D.; et al. Perfluorooctyl bromide nanoemulsions holding MnO2 nanoparticles with dual-modality imaging and glutathione depletion enhanced HIFU-eliciting tumor immunogenic cell death. Acta Pharm. Sin. B 2022, 12, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Middelberg, A.P.J.; Gemiarto, A.; MacDonald, K.; Baxter, A.G.; Talekar, M.; Moi, D.; Tullett, K.M.; Caminschi, I.; Lahoud, M.H.; et al. Self-adjuvanting nanoemulsion targeting dendritic cell receptor Clec9A enables antigen-specific immunotherapy. J. Clin. Investig. 2018, 128, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, M.; Kumar, P.; Verma, J.; Rosenholm, J.M.; Bansal, K.K.; Vaidya, A. Lipid–Polymer Hybrid Nanosystems: A Rational Fusion for Advanced Therapeutic Delivery. J. Funct. Biomater. 2023, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ji, Z.; Zhou, H.; Wu, D.; Gu, Z.; Wang, D.; ten Dijke, P. Lipid-based nanoparticles as drug delivery systems for cancer immunotherapy. MedComm 2023, 4, e339. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.; Kim, J.; Chung, J.Y.; Ra, S.; kim, S.S.; Kim, Y. Heme Oxygenase 1-Targeted Hybrid Nanoparticle for Chemo- and Immuno-Combination Therapy in Acute Myelogenous Leukemia. Adv. Sci. 2020, 7, 2000487. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Qin, Z.; Gao, L.; Zhou, M.; Xue, Y.; Li, Y.; Xie, J. Protein nanoparticles as drug delivery systems for cancer theranostics. J. Control. Release 2024, 371, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. BioMed Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Park, I.-K. Protein-Caged Nanoparticles: A Promising Nanomedicine Against Cancer. Chonnam Med. J. 2023, 59, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sandra, F.; Khaliq, N.U.; Sunna, A.; Care, A. Developing Protein-Based Nanoparticles as Versatile Delivery Systems for Cancer Therapy and Imaging. Nanomaterials 2019, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, H. Immune gene therapy of cancer. Turk. J. Med. Sci. 2020, 50, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Lykhopiy, V.; Malviya, V.; Humblet-Baron, S.; Schlenner, S.M. IL-2 immunotherapy for targeting regulatory T cells in autoimmunity. Genes Immun. 2023, 24, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Halima, A.; Chan, T.A. Antigen presentation in cancer—Mechanisms and clinical implications for immunotherapy. Nat. Rev. Clin. Oncol. 2023, 20, 604–623. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Zhang, Y.; Zhou, S.; Lin, S.; Zhang, X.-B.; Zhu, G. Nucleic Acid Immunotherapeutics for Cancer. ACS Appl. Bio Mater. 2020, 3, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Collotta, D.; Bertocchi, I.; Chiapello, E.; Collino, M. Antisense oligonucleotides: A novel Frontier in pharmacological strategy. Front. Pharmacol. 2023, 14, 1304342. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.S.; Thelemann, T.; Klar, R.; Kallert, S.M.; Festag, J.; Buchi, M.; Hinterwimmer, L.; Schell, M.; Michel, S.; Jaschinski, F.; et al. Antisense oligonucleotide targeting CD39 improves anti-tumor T cell immunity. J. Immunother. Cancer 2019, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Seok, S.H.; Yoon, H.Y.; Ryu, J.H.; Kwon, I.C. Advancing cancer immunotherapy through siRNA-based gene silencing for immune checkpoint blockade. Adv. Drug Deliv. Rev. 2024, 209, 115306. [Google Scholar] [CrossRef] [PubMed]
- Warashina, S.; Nakamura, T.; Sato, Y.; Fujiwara, Y.; Hyodo, M.; Hatakeyama, H.; Harashima, H. A lipid nanoparticle for the efficient delivery of siRNA to dendritic cells. J. Control. Release 2016, 225, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, X.; Zhang, X.; Liu, B.; Huang, L. Nanoparticles Modified with Tumor-targeting scFv Deliver siRNA and miRNA for Cancer Therapy. Mol. Ther. 2010, 18, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Swaminathan, S.K.; Kirtane, A.; Srivastava, S.K.; Bhardwaj, A.; Singh, S.; Panyam, J.; Singh, A.P. Synthesis, characterization, and evaluation of poly (D,L-lactide-co-glycolide)-based nanoformulation of miRNA-150: Potential implications for pancreatic cancer therapy. Int. J. Nanomed. 2014, 9, 2933–2942. [Google Scholar] [CrossRef]
- Devulapally, R.; Foygel, K.; Sekar, T.V.; Willmann, J.K.; Paulmurugan, R. Gemcitabine and Antisense-microRNA Co-encapsulated PLGA–PEG Polymer Nanoparticles for Hepatocellular Carcinoma Therapy. ACS Appl. Mater. Interfaces 2016, 8, 33412–33422. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Parikh, A.; Amiji, M.M. Repolarization of Tumor-Associated Macrophages in a Genetically Engineered Nonsmall Cell Lung Cancer Model by Intraperitoneal Administration of Hyaluronic Acid-Based Nanoparticles Encapsulating MicroRNA-125b. Nano Lett. 2018, 18, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Vishweshwaraiah, Y.L.; Dokholyan, N.V. mRNA vaccines for cancer immunotherapy. Front. Immunol. 2022, 13, 1029069. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. Recent advances in mRNA cancer vaccines: Meeting challenges and embracing opportunities. Front. Immunol. 2023, 14, 1246682. [Google Scholar] [CrossRef] [PubMed]
- Al Fayez, N.; Nassar, M.S.; Alshehri, A.A.; Alnefaie, M.K.; Almughem, F.A.; Alshehri, B.Y.; Alawad, A.O.; Tawfik, E.A. Recent Advancement in mRNA Vaccine Development and Applications. Pharmaceutics 2023, 15, 1972. [Google Scholar] [CrossRef] [PubMed]
- Thran, M.; Mukherjee, J.; Pönisch, M.; Fiedler, K.; Thess, A.; Mui, B.L.; Hope, M.J.; Tam, Y.K.; Horscroft, N.; Heidenreich, R.; et al. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol. Med. 2017, 9, 1434–1447. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Bong, Y.S.; Chen, R.; Zhang, M.; Long, S.; Shen, D. Abstract 1368: Novel mRNA encoding anti-PD-L1 monoclonal antibodies for cancer immunotherapy. Cancer Res. 2024, 84, 1368. [Google Scholar] [CrossRef]
- Jain, R.; Frederick, J.P.; Huang, E.Y.; Burke, K.E.; Mauger, D.M.; Andrianova, E.A.; Farlow, S.J.; Siddiqui, S.; Pimentel, J.; Cheung-Ong, K.; et al. MicroRNAs Enable mRNA Therapeutics to Selectively Program Cancer Cells to Self-Destruct. Nucleic Acid Ther. 2018, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Bevers, S.; Kooijmans, S.A.A.; Velde, E.V.d.; Evers, M.J.W.; Seghers, S.; Gitz-Francois, J.J.J.M.; van Kronenburg, N.C.H.; Fens, M.H.A.M.; Mastrobattista, E.; Hassler, L.; et al. mRNA-LNP vaccines tuned for systemic immunization induce strong antitumor immunity by engaging splenic immune cells. Mol. Ther. 2022, 30, 3078–3094. [Google Scholar] [CrossRef] [PubMed]
- Vimal, S.; Madar, I.H.; Thirumani, L.; Thangavelu, L.; Sivalingam, A.M. CRISPR/Cas9: Role of genome editing in cancer immunotherapy. Oral Oncol. Rep. 2024, 10, 100251. [Google Scholar] [CrossRef]
- Luo, Y.-L.; Xu, C.-F.; Li, H.-J.; Cao, Z.-T.; Liu, J.; Wang, J.-L.; Du, X.-J.; Yang, X.-Z.; Gu, Z.; Wang, J. Macrophage-Specific in Vivo Gene Editing Using Cationic Lipid-Assisted Polymeric Nanoparticles. ACS Nano 2018, 12, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.; Deng, H.; Kong, L.; Wang, Y.; Yang, T.; Hu, Q.; Hu, M.; Yang, C.; Zhang, Z. Reshaping Tumor Immune Microenvironment through Acidity-Responsive Nanoparticles Featured with CRISPR/Cas9-Mediated Programmed Death-Ligand 1 Attenuation and Chemotherapeutics-Induced Immunogenic Cell Death. ACS Appl. Mater. Interfaces 2020, 12, 16018–16030. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-J.; Chen, L.-C.; Ho, H.-O.; Lin, H.-L.; Sheu, M.-T. Stearyl polyethylenimine complexed with plasmids as the core of human serum albumin nanoparticles noncovalently bound to CRISPR/Cas9 plasmids or siRNA for disrupting or silencing PD-L1 expression for immunotherapy. Int. J. Nanomed. 2018, 13, 7079–7094. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Lee, Y.-W.; Hardie, J.; Mout, R.; Tonga, G.Y.; Farkas, M.E.; Rotello, V.M. CRISPRed Macrophages for Cell-Based Cancer Immunotherapy. Bioconjug. Chem. 2018, 29, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Leonard, F.; Curtis, L.T.; Hamed, A.R.; Zhang, C.; Chau, E.; Sieving, D.; Godin, B.; Frieboes, H.B. Nonlinear response to cancer nanotherapy due to macrophage interactions revealed by mathematical modeling and evaluated in a murine model via CRISPR-modulated macrophage polarization. Cancer Immunol. Immunother. CII 2020, 69, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, J.; Zhou, Y.; Zhang, S.; Zhu, D. CRISPR-Cas9 genome editing for cancer immunotherapy: Opportunities and challenges. Brief. Funct. Genom. 2020, 19, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Valdivieso, J.; Girotti, A.; Schneider, J.; Arias, F.J. Advanced nanomedicine and cancer: Challenges and opportunities in clinical translation. Int. J. Pharm. 2021, 599, 120438. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Meng, H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. VIEW 2021, 2, 20200190. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, Y.; Sun, X.; Lin, X.; Koo, S.; Yaremenko, A.V.; Qin, D.; Kong, N.; Farokhzad, O.C.; Tao, W. Cancer nanomedicine toward clinical translation: Obstacles, opportunities, and future prospects. Med 2023, 4, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Seethy, A.; Singh, S.; Pethusamy, K.; Srivastava, T.; Talukdar, J.; Rath, G.K.; Karmakar, S. Cancer immunotherapy: Recent advances and challenges. J. Cancer Res. Ther. 2021, 17, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Novak, N.; Cabanillas, B.; Jutel, M.; Bousquet, J.; Akdis, C.A. Allergenic components of the mRNA-1273 vaccine for COVID-19: Possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy 2021, 76, 3307–3313. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.N.; Tian, C. Engineered Extracellular Vesicles: Emerging Therapeutic Strategies for Translational Applications. Int. J. Mol. Sci. 2023, 24, 15206. [Google Scholar] [CrossRef] [PubMed]
- Ghodasara, A.; Raza, A.; Wolfram, J.; Salomon, C.; Popat, A. Clinical Translation of Extracellular Vesicles. Adv. Healthc. Mater. 2023, 12, 2301010. [Google Scholar] [CrossRef] [PubMed]
- Csóka, I.; Ismail, R.; Jójárt-Laczkovich, O.; Pallagi, E. Regulatory Considerations, Challenges and Risk-Based Approach in Nanomedicine Development. Available online: https://www.eurekaselect.com/article/115214 (accessed on 1 May 2025).
- Lousa, I.R. Pharmaceutical Regulation of Nanomedicines: Principles and Challenges. Master’s Thesis, Universidade De Lisboa, Lisbon, Portugal, 2024. Available online: https://repositorio.ulisboa.pt/handle/10451/64466 (accessed on 1 May 2025).
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742. [Google Scholar]
- Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Wang, Q.; Wang, X.; Ke, L.; Shi, K. Engineering Nanoparticles for Targeted Remodeling of the Tumor Microenvironment to Improve Cancer Immunotherapy. Theranostics 2019, 9, 126–151. [Google Scholar] [CrossRef] [PubMed]
- Allemailem, K.S.; Alsahli, M.A.; Almatroudi, A.; Alrumaihi, F.; Alkhaleefah, F.K.; Rahmani, A.H.; Khan, A.A. Current updates of CRISPR/Cas9-mediated genome editing and targeting within tumor cells: An innovative strategy of cancer management. Cancer Commun. 2022, 42, 1257–1287. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Aikins, M.E.; Xu, C.; Moon, J.J. Engineered Nanoparticles for Cancer Vaccination and Immunotherapy. Acc. Chem. Res. 2020, 53, 2094. [Google Scholar] [CrossRef] [PubMed]
- Liquid Biopsies Come of Age: Towards Implementation of Circulating Tumour DNA|Nature Reviews Cancer. Available online: https://www-nature-com.nottingham.idm.oclc.org/articles/nrc.2017.7 (accessed on 22 February 2025).
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Heydari, S.; Masoumi, N.; Esmaeeli, E.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorpeh, F.; Ahmadi, M. Artificial intelligence in nanotechnology for treatment of diseases. J. Drug Target. 2024, 32, 1247–1266. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, H.; Khondakar, K.R.; Das, S.; Halder, A.; Kaushik, A. Artificial intelligence for personalized nanomedicine; from material selection to patient outcomes. Expert Opin. Drug Deliv. 2025, 22, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lu, J.; Lin, W. Hybrid nanoparticles for combination therapy of cancer. J. Control. Release 2015, 219, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Hybrid Nanomaterials for Cancer Immunotherapy—Li—2023—Advanced Science—Wiley Online Library. Available online: https://advanced-onlinelibrary-wiley-com.nottingham.idm.oclc.org/doi/full/10.1002/advs.202204932 (accessed on 22 February 2025).
| Delivery System | Advantages | Limitations |
|---|---|---|
| Liposomes |
|
|
| Lipid Nanoparticles (LNPs) |
|
|
| Extracellular Vesicles (EVs) |
|
|
| Solid Lipid Nanoparticles (SLNs) |
|
|
| Nanostructured Lipid Carriers (NLCs) |
|
|
| Nanoemulsions |
|
|
| Lipid–Polymer Hybrid Nanoparticles (LPHNPs) |
|
|
| Cancer Nanoparticles | Challenges |
|---|---|
| Liposomes |
|
| Polymeric micelles |
|
| Nano-biomaterials |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fallatah, M.M.; Alradwan, I.; Alfayez, N.; Aodah, A.H.; Alkhrayef, M.; Majrashi, M.; Jamous, Y.F. Nanoparticles for Cancer Immunotherapy: Innovations and Challenges. Pharmaceuticals 2025, 18, 1086. https://doi.org/10.3390/ph18081086
Fallatah MM, Alradwan I, Alfayez N, Aodah AH, Alkhrayef M, Majrashi M, Jamous YF. Nanoparticles for Cancer Immunotherapy: Innovations and Challenges. Pharmaceuticals. 2025; 18(8):1086. https://doi.org/10.3390/ph18081086
Chicago/Turabian StyleFallatah, Mohannad M., Ibrahim Alradwan, Nojoud Alfayez, Alhassan H. Aodah, Mohammad Alkhrayef, Majed Majrashi, and Yahya F. Jamous. 2025. "Nanoparticles for Cancer Immunotherapy: Innovations and Challenges" Pharmaceuticals 18, no. 8: 1086. https://doi.org/10.3390/ph18081086
APA StyleFallatah, M. M., Alradwan, I., Alfayez, N., Aodah, A. H., Alkhrayef, M., Majrashi, M., & Jamous, Y. F. (2025). Nanoparticles for Cancer Immunotherapy: Innovations and Challenges. Pharmaceuticals, 18(8), 1086. https://doi.org/10.3390/ph18081086






