Stem Cells and Organoids: A Paradigm Shift in Preclinical Models Toward Personalized Medicine
Abstract
1. Introduction
2. Human Pluripotent Stem Cells
3. Organoids: Innovative 3D Models
4. Applications in Precision Medicine
4.1. Personalized Drug Screening and Therapeutic Stratification
4.2. Modeling Disease Heterogeneity and Genotype–Phenotype Relationships
4.3. Towards Clinical Integration
5. Reducing and Replacing Animal Models: Ethical and Scientific Perspectives
5.1. Scientific Limitations of Animal Models
5.2. Regulatory Trends and Emerging Guidelines
5.3. Ethical Advantages and Public Acceptance
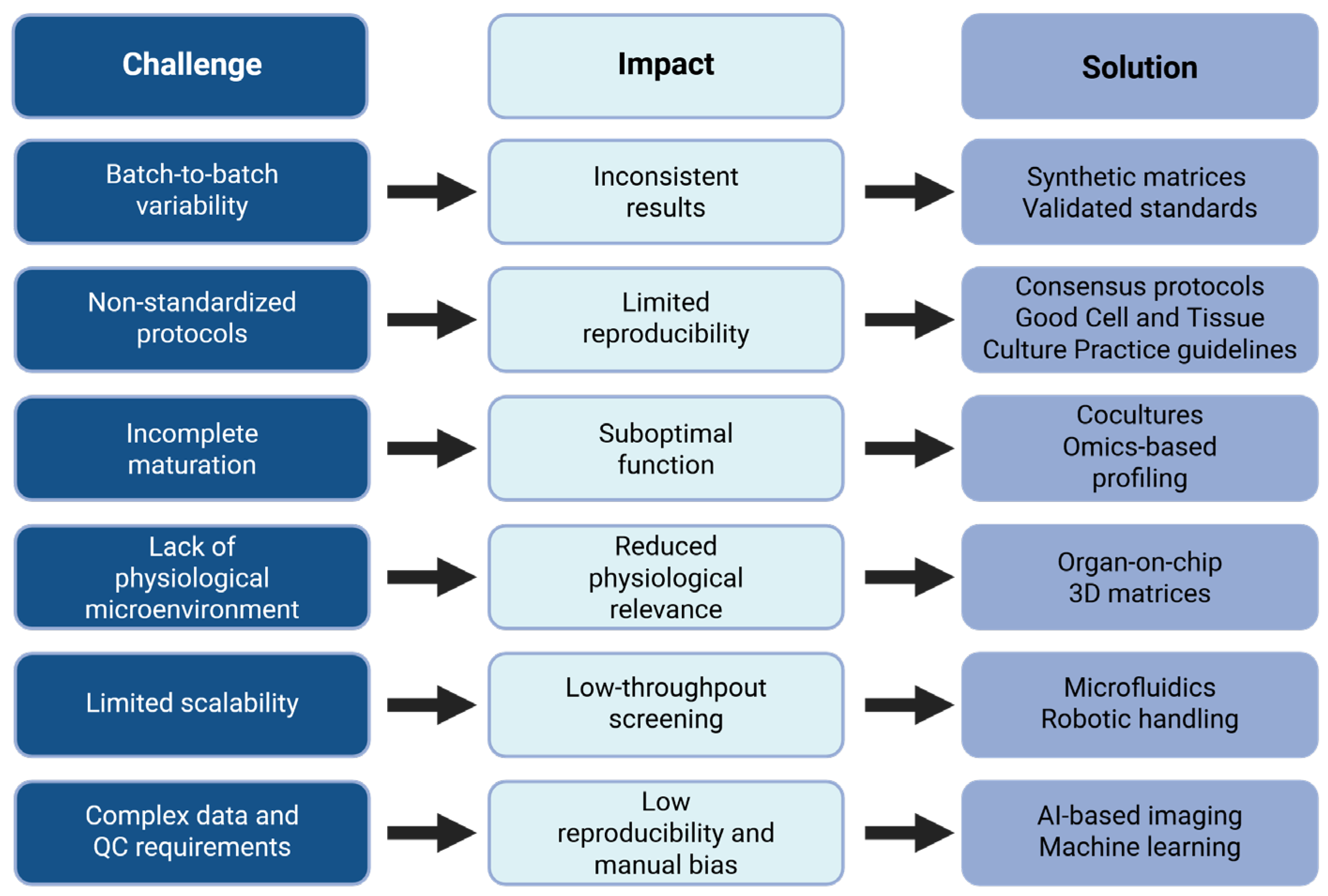
6. Current Challenges and Future Perspectives
6.1. Standardization and Reproducibility Issues
6.2. Incomplete Maturation and Limited Functional Performance
6.3. Lack of Physiological Microenvironment
6.4. Integration of Omics Technologies and Artificial Intelligence
6.5. Toward High-Throughput, Automated Screening Platforms
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSC | Pluripotent stem cell |
| PDO | Patient-derived organoid |
| PDTO | Patient-derived tumor organoid |
| ESC | Embryonic stem cell |
| iPSC | Induced pluripotent stem cell |
References
- Van Der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can Animal Models of Disease Reliably Inform Human Studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in Translation: Animal Models and Clinical Trials in Cancer Treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar] [PubMed]
- Qin, Y.; Cui, Q.; Sun, G.; Chao, J.; Wang, C.; Chen, X.; Ye, P.; Zhou, T.; Jeyachandran, A.V.; Sun, O.; et al. Developing Enhanced Immunotherapy Using NKG2A Knockout Human Pluripotent Stem Cell-Derived NK Cells. Cell Rep. 2024, 43, 114867. [Google Scholar] [CrossRef] [PubMed]
- Verma, I.; Seshagiri, P.B. Current Applications of Human Pluripotent Stem Cells in Neuroscience Research and Cell Transplantation Therapy for Neurological Disorders. Stem Cell Rev. Rep. 2025, 21, 964–987. [Google Scholar] [CrossRef]
- Lee, C.-J.; Nam, Y.; Rim, Y.A.; Ju, J.H. Advanced Animal Replacement Testing Strategies Using Stem Cell and Organoids. Int. J. Stem Cells 2025, 18, 107–125. [Google Scholar] [CrossRef]
- Kumar, D.; Gupta, S.; Gupta, V.; Tanwar, R.; Chandel, A. Engineering the Future of Regenerative Medicines in Gut Health with Stem Cell-Derived Intestinal Organoids. Stem Cell Rev. Rep. 2025, 1–22. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-Derived Organoids Model Treatment Response of Metastatic Gastrointestinal Cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Böttinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G.D.; et al. Long-term Expanding Human Airway Organoids for Disease Modeling. EMBO J. 2019, 38, e100300. [Google Scholar] [CrossRef]
- Esposito, A.; Ferraresi, A.; Vallino, L.; Garavaglia, B.; Dhanasekaran, D.N.; Isidoro, C. Three-Dimensional In Vitro Cell Cultures as a Feasible and Promising Alternative to Two-Dimensional and Animal Models in Cancer Research. Int. J. Biol. Sci. 2024, 20, 5293–5311. [Google Scholar] [CrossRef]
- Van Den Berg, C.W.; Dumas, S.J.; Little, M.H.; Rabelink, T.J. Challenges in Maturation and Integration of Kidney Organoids for Stem Cell–Based Renal Replacement Therapy. Kidney Int. 2025, 107, 262–270. [Google Scholar] [CrossRef]
- Ge, J.-Y.; Wang, Y.; Li, Q.-L.; Liu, F.-K.; Lei, Q.-K.; Zheng, Y.-W. Trends and Challenges in Organoid Modeling and Expansion with Pluripotent Stem Cells and Somatic Tissue. PeerJ 2024, 12, e18422. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yu, T.; Liu, J.; Wang, T.; Higuchi, A. Introduction to Stem Cells. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2023; Volume 199, pp. 3–32. ISBN 978-0-443-13411-1. [Google Scholar]
- Zhigalina, D.I.; Denisov, E.V.; Lebedev, I.N.; Skryabin, N.A. Embryoid Bodies as a Model System for Exploring Early Human Embryonic Development. J. Assist. Reprod. Genet. 2025, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Luce, E.; Hussein, M.; Dubart-Kupperschmitt, A. Pluripotent-Stem-Cell-Derived Hepatic Cells: Hepatocytes and Organoids for Liver Therapy and Regeneration. Cells 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Kirkeby, A.; Main, H.; Carpenter, M. Pluripotent Stem-Cell-Derived Therapies in Clinical Trial: A 2025 Update. Cell Stem Cell 2025, 32, 10–37. [Google Scholar] [CrossRef]
- Kumar, D.; Baligar, P.; Srivastav, R.; Narad, P.; Raj, S.; Tandon, C.; Tandon, S. Stem Cell Based Preclinical Drug Development and Toxicity Prediction. Curr. Pharm. Des. 2021, 27, 2237–2251. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Aboul-Soud, M.A.M.; Alzahrani, A.J.; Mahmoud, A. Induced Pluripotent Stem Cells (iPSCs)-Roles in Regenerative Therapies, Disease Modelling and Drug Screening. Cells 2021, 10, 2319. [Google Scholar] [CrossRef]
- Luce, E.; Steichen, C.; Allouche, M.; Messina, A.; Heslan, J.; Lambert, T.; Weber, A.; Nguyen, T.H.; Christophe, O.; Dubart-Kupperschmitt, A. In Vitro Recovery of FIX Clotting Activity as a Marker of Highly Functional Hepatocytes in a Hemophilia B iPSC Model. Hepatology 2022, 75, 866–880. [Google Scholar] [CrossRef]
- Wang, L.; Koui, Y.; Kanegae, K.; Kido, T.; Tamura-Nakano, M.; Yabe, S.; Tai, K.; Nakajima, Y.; Kusuhara, H.; Sakai, Y.; et al. Establishment of Human Induced Pluripotent Stem Cell-Derived Hepatobiliary Organoid with Bile Duct for Pharmaceutical Research Use. Biomaterials 2024, 310, 122621. [Google Scholar] [CrossRef]
- Magdy, T.; Schuldt, A.J.T.; Wu, J.C.; Bernstein, D.; Burridge, P.W. Human Induced Pluripotent Stem Cell (hiPSC)-Derived Cells to Assess Drug Cardiotoxicity: Opportunities and Problems. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Police, S.; Rao, N.; Carpenter, M.K. Characterization and Enrichment of Cardiomyocytes Derived from Human Embryonic Stem Cells. Circ. Res. 2002, 91, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Lyra-Leite, D.M.; Gutiérrez-Gutiérrez, Ó.; Wang, M.; Zhou, Y.; Cyganek, L.; Burridge, P.W. A Review of Protocols for Human iPSC Culture, Cardiac Differentiation, Subtype-Specification, Maturation, and Direct Reprogramming. STAR Protoc. 2022, 3, 101560. [Google Scholar] [CrossRef] [PubMed]
- Dimos, J.T.; Rodolfa, K.T.; Niakan, K.K.; Weisenthal, L.M.; Mitsumoto, H.; Chung, W.; Croft, G.F.; Saphier, G.; Leibel, R.; Goland, R.; et al. Induced Pluripotent Stem Cells Generated from Patients with ALS Can Be Differentiated into Motor Neurons. Science 2008, 321, 1218–1221. [Google Scholar] [CrossRef]
- Silva, M.C.; Nandi, G.; Haggarty, S.J. Differentiation of Human Induced Pluripotent Stem Cells into Cortical Neurons to Advance Precision Medicine. In Stem Cell Assays; Kannan, N., Beer, P., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; Volume 2429, pp. 143–174. ISBN 978-1-07-161978-0. [Google Scholar]
- Si-Tayeb, K.; Noto, F.K.; Nagaoka, M.; Li, J.; Battle, M.A.; Duris, C.; North, P.E.; Dalton, S.; Duncan, S.A. Highly Efficient Generation of Human Hepatocyte–like Cells from Induced Pluripotent Stem Cells. Hepatology 2010, 51, 297–305. [Google Scholar] [CrossRef]
- Messina, A.; Luce, E.; Benzoubir, N.; Pasqua, M.; Pereira, U.; Humbert, L.; Eguether, T.; Rainteau, D.; Duclos-Vallée, J.-C.; Legallais, C.; et al. Evidence of Adult Features and Functions of Hepatocytes Differentiated from Human Induced Pluripotent Stem Cells and Self-Organized as Organoids. Cells 2022, 11, 537. [Google Scholar] [CrossRef]
- Shim, J.H.; Kim, S.E.; Woo, D.H.; Kim, S.K.; Oh, C.H.; McKay, R.; Kim, J.H. Directed Differentiation of Human Embryonic Stem Cells towards a Pancreatic Cell Fate. Diabetologia 2007, 50, 1228–1238. [Google Scholar] [CrossRef]
- Hogrebe, N.J.; Maxwell, K.G.; Augsornworawat, P.; Millman, J.R. Generation of Insulin-Producing Pancreatic β Cells from Multiple Human Stem Cell Lines. Nat. Protoc. 2021, 16, 4109–4143. [Google Scholar] [CrossRef]
- Zhang, H.; Thai, P.N.; Shivnaraine, R.V.; Ren, L.; Wu, X.; Siepe, D.H.; Liu, Y.; Tu, C.; Shin, H.S.; Caudal, A.; et al. Multiscale Drug Screening for Cardiac Fibrosis Identifies MD2 as a Therapeutic Target. Cell 2024, 187, 7143–7163.e22. [Google Scholar] [CrossRef]
- Schinke, C.; Fernandez Vallone, V.; Ivanov, A.; Peng, Y.; Körtvelyessy, P.; Nolte, L.; Huehnchen, P.; Beule, D.; Stachelscheid, H.; Boehmerle, W.; et al. Modeling Chemotherapy Induced Neurotoxicity with Human Induced Pluripotent Stem Cell (iPSC)—Derived Sensory Neurons. Neurobiol. Dis. 2021, 155, 105391. [Google Scholar] [CrossRef]
- Chaudhari, U.; Ellis, J.K.; Wagh, V.; Nemade, H.; Hescheler, J.; Keun, H.C.; Sachinidis, A. Metabolite Signatures of Doxorubicin Induced Toxicity in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Amino Acids 2017, 49, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Ito, D.; Okada, Y.; Akamatsu, W.; Nihei, Y.; Yoshizaki, T.; Yamanaka, S.; Okano, H.; Suzuki, N. Modeling Familial Alzheimer’s Disease with Induced Pluripotent Stem Cells. Human. Mol. Genet. 2011, 20, 4530–4539. [Google Scholar] [CrossRef] [PubMed]
- Isaja, L.; Rodríguez-Varela, M.S.; Marazita, M.; Mucci, S.; Itzcovich, T.; Chrem-Méndez, P.; Niikado, M.; Ferriol-Laffouillere, S.L.; Allegri, R.; Martinetto, H.; et al. Generation of a Human Induced Pluripotent Stem Cell Line from a Familial Alzheimer’s Disease PSEN1 T119I Patient. Stem Cell Res. 2021, 53, 102325. [Google Scholar] [CrossRef] [PubMed]
- Stepniewski, J.; Kachamakova-Trojanowska, N.; Ogrocki, D.; Szopa, M.; Matlok, M.; Beilharz, M.; Dyduch, G.; Malecki, M.T.; Jozkowicz, A.; Dulak, J. Induced Pluripotent Stem Cells as a Model for Diabetes Investigation. Sci. Rep. 2015, 5, 8597. [Google Scholar] [CrossRef]
- Joshi, K.; Cameron, F.; Tiwari, S.; Mannering, S.I.; Elefanty, A.G.; Stanley, E.G. Modeling Type 1 Diabetes Using Pluripotent Stem Cell Technology. Front. Endocrinol. 2021, 12, 635662. [Google Scholar] [CrossRef]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson’s Disease Patient-Derived Induced Pluripotent Stem Cells Free of Viral Reprogramming Factors. Cell 2009, 136, 964–977. [Google Scholar] [CrossRef]
- Bose, A.; Petsko, G.A.; Studer, L. Induced Pluripotent Stem Cells: A Tool for Modeling Parkinson’s Disease. Trends Neurosci. 2022, 45, 608–620. [Google Scholar] [CrossRef]
- Rowe, R.G.; Daley, G.Q. Induced Pluripotent Stem Cells in Disease Modelling and Drug Discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef]
- Volpato, V.; Webber, C. Addressing Variability in iPSC-Derived Models of Human Disease: Guidelines to Promote Reproducibility. Dis. Models Mech. 2020, 13, dmm042317. [Google Scholar] [CrossRef]
- Pleguezuelos-Manzano, C.; Puschhof, J.; van den Brink, S.; Geurts, V.; Beumer, J.; Clevers, H. Establishment and Culture of Human Intestinal Organoids Derived from Adult Stem Cells. Curr. Protoc. Immunol. 2020, 130, e106. [Google Scholar] [CrossRef]
- Xiang, Y.; Tanaka, Y.; Cakir, B.; Patterson, B.; Kim, K.-Y.; Sun, P.; Kang, Y.-J.; Zhong, M.; Liu, X.; Patra, P.; et al. hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell 2019, 24, 487–497.e7. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, O.; Nikolaev, M.; Xu, Q.; Broguiere, N.; Cubela, I.; Camp, J.G.; Bscheider, M.; Lutolf, M.P. Bioengineered Human Colon Organoids with In Vivo-like Cellular Complexity and Function. Cell Stem Cell 2024, 31, 1175–1186.e7. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.X.d.S.d.; Liberali, P. From Single Cells to Tissue Self-Organization. FEBS J. 2019, 286, 1495–1513. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures In Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Velasco, S.; Kedaigle, A.J.; Simmons, S.K.; Nash, A.; Rocha, M.; Quadrato, G.; Paulsen, B.; Nguyen, L.; Adiconis, X.; Regev, A.; et al. Individual Brain Organoids Reproducibly Form Cell Diversity of the Human Cerebral Cortex. Nature 2019, 570, 523–527. [Google Scholar] [CrossRef]
- Al Reza, H.; Santangelo, C.; Al Reza, A.; Iwasawa, K.; Sachiko, S.; Glaser, K.; Bondoc, A.; Merola, J.; Takebe, T. Self-Assembled Generation of Multi-Zonal Liver Organoids from Human Pluripotent Stem Cells. bioRxiv 2024. [Google Scholar]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.-K.; Huch, M. Culture and Establishment of Self-Renewing Human and Mouse Adult Liver and Pancreas 3D Organoids and Their Genetic Manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef]
- Nunez-Nescolarde, A.B.; Santos, L.L.; Kong, L.; Ekinci, E.; Moore, P.; Dimitriadis, E.; Nikolic-Paterson, D.J.; Combes, A.N. Comparative Analysis of Human Kidney Organoid and Tubuloid Models. Kidney360 2025. [Google Scholar] [CrossRef]
- Castaneda, D.C.; Jangra, S.; Yurieva, M.; Martinek, J.; Callender, M.; Coxe, M.; Choi, A.; García-Bernalt Diego, J.; Wu, T.-C.; Marches, F.; et al. Protocol for Establishing Primary Human Lung Organoid-Derived Air-Liquid Interface Cultures from Cryopreserved Human Lung Tissue. STAR Protoc. 2023, 4, 102735. [Google Scholar] [CrossRef]
- Tuveson, D.; Clevers, H. Cancer Modeling Meets Human Organoid Technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Hushka, E.A.; Yavitt, F.M.; Brown, T.E.; Dempsey, P.J.; Anseth, K.S. Relaxation of Extracellular Matrix Forces Directs Crypt Formation and Architecture in Intestinal Organoids. Adv. Healthc. Mater. 2020, 9, 1901214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Meyer, S.R.; O’Meara, M.J.; Huang, S.; Capeling, M.M.; Ferrer-Torres, D.; Childs, C.J.; Spence, J.R.; Fontana, R.J.; Sexton, J.Z. A Human Liver Organoid Screening Platform for DILI Risk Prediction. J. Hepatol. 2023, 78, 998–1006. [Google Scholar] [CrossRef]
- Ramli, M.N.B.; Lim, Y.S.; Koe, C.T.; Demircioglu, D.; Tng, W.; Gonzales, K.A.U.; Tan, C.P.; Szczerbinska, I.; Liang, H.; Soe, E.L.; et al. Human Pluripotent Stem Cell-Derived Organoids as Models of Liver Disease. Gastroenterology 2020, 159, 1471–1486.e12. [Google Scholar] [CrossRef]
- Han, Y.; Yu, Z.; Chen, Y.; Guo, X.; Liu, Y.; Zhang, H.; Li, Z.; Chen, L. PM2.5 Induces Developmental Neurotoxicity in Cortical Organoids. Environ. Pollut. 2024, 361, 124913. [Google Scholar] [CrossRef]
- Xu, H.; Jiao, D.; Liu, A.; Wu, K. Tumor Organoids: Applications in Cancer Modeling and Potentials in Precision Medicine. J. Hematol. Oncol. 2022, 15, 58. [Google Scholar] [CrossRef]
- Duzagac, F.; Saorin, G.; Memeo, L.; Canzonieri, V.; Rizzolio, F. Microfluidic Organoids-on-a-Chip: Quantum Leap in Cancer Research. Cancers 2021, 13, 737. [Google Scholar] [CrossRef]
- Kroll, K.T.; Mata, M.M.; Homan, K.A.; Micallef, V.; Carpy, A.; Hiratsuka, K.; Morizane, R.; Moisan, A.; Gubler, M.; Walz, A.-C.; et al. Immune-Infiltrated Kidney Organoid-on-Chip Model for Assessing T Cell Bispecific Antibodies. Proc. Natl. Acad. Sci. USA 2023, 120, e2305322120. [Google Scholar] [CrossRef]
- Monteduro, A.G.; Rizzato, S.; Caragnano, G.; Trapani, A.; Giannelli, G.; Maruccio, G. Organs-on-Chips Technologies—A Guide from Disease Models to Opportunities for Drug Development. Biosens. Bioelectron. 2023, 231, 115271. [Google Scholar] [CrossRef]
- Lopez-Muñoz, G.A.; Mughal, S.; Ramón-Azcón, J. Sensors and Biosensors in Organs-on-a-Chip Platforms. Adv. Exp. Med. Biol. 2022, 1379, 55–80. [Google Scholar] [CrossRef]
- Bellin, M.; Marchetto, M.C.; Gage, F.H.; Mummery, C.L. Induced Pluripotent Stem Cells: The New Patient? Nat. Rev. Mol. Cell Biol. 2012, 13, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Guillen, K.P.; Fujita, M.; Butterfield, A.J.; Scherer, S.D.; Bailey, M.H.; Chu, Z.; DeRose, Y.S.; Zhao, L.; Cortes-Sanchez, E.; Yang, C.-H.; et al. A Human Breast Cancer-Derived Xenograft and Organoid Platform for Drug Discovery and Precision Oncology. Nat. Cancer 2022, 3, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Weeber, F.; Van De Wetering, M.; Hoogstraat, M.; Dijkstra, K.K.; Krijgsman, O.; Kuilman, T.; Gadellaa-van Hooijdonk, C.G.M.; Van Der Velden, D.L.; Peeper, D.S.; Cuppen, E.P.J.G.; et al. Preserved Genetic Diversity in Organoids Cultured from Biopsies of Human Colorectal Cancer Metastases. Proc. Natl. Acad. Sci. USA 2015, 112, 13308–13311. [Google Scholar] [CrossRef] [PubMed]
- Boj, S.F.; Hwang, C.-I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid Models of Human and Mouse Ductal Pancreatic Cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef]
- Lê, H.; Seitlinger, J.; Lindner, V.; Olland, A.; Falcoz, P.-E.; Benkirane-Jessel, N.; Quéméneur, E. Patient-Derived Lung Tumoroids—An Emerging Technology in Drug Development and Precision Medicine. Biomedicines 2022, 10, 1677. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Wiegerinck, C.L.; de Jonge, H.R.; Bronsveld, I.; Janssens, H.M.; de Winter-de Groot, K.M.; Brandsma, A.M.; de Jong, N.W.M.; Bijvelds, M.J.C.; Scholte, B.J.; et al. A Functional CFTR Assay Using Primary Cystic Fibrosis Intestinal Organoids. Nat. Med. 2013, 19, 939–945. [Google Scholar] [CrossRef]
- Avior, Y.; Sagi, I.; Benvenisty, N. Pluripotent Stem Cells in Disease Modelling and Drug Discovery. Nat. Rev. Mol. Cell Biol. 2016, 17, 170–182. [Google Scholar] [CrossRef]
- Caron, J.; Pène, V.; Tolosa, L.; Villaret, M.; Luce, E.; Fourrier, A.; Heslan, J.-M.; Saheb, S.; Bruckert, E.; Gómez-Lechón, M.J.; et al. Low-Density Lipoprotein Receptor-Deficient Hepatocytes Differentiated from Induced Pluripotent Stem Cells Allow Familial Hypercholesterolemia Modeling, CRISPR/Cas-Mediated Genetic Correction, and Productive Hepatitis C Virus Infection. Stem Cell Res. Ther. 2019, 10, 221. [Google Scholar] [CrossRef]
- Xie, F.; Ye, L.; Chang, J.C.; Beyer, A.I.; Wang, J.; Muench, M.O.; Kan, Y.W. Seamless Gene Correction of β-Thalassemia Mutations in Patient-Specific iPSCs Using CRISPR/Cas9 and piggyBac. Genome Res. 2014, 24, 1526–1533. [Google Scholar] [CrossRef]
- Rehbach, K.; Fernando, M.B.; Brennand, K.J. Integrating CRISPR Engineering and hiPSC-Derived 2D Disease Modeling Systems. J. Neurosci. 2020, 40, 1176–1185. [Google Scholar] [CrossRef]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; Van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; Van De Haar, J.; et al. Patient-Derived Organoids Can Predict Response to Chemotherapy in Metastatic Colorectal Cancer Patients. Sci. Transl. Med. 2019, 11, eaay2574. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Parveen, M.; Bala, A.; Sur, D. The 3C (Cell Culture, Computer Simulation, Clinical Trial) Solution for Optimizing the 3R (Replace, Reduction, Refine) Framework during Preclinical Research Involving Laboratory Animals. ACS Pharmacol. Transl. Sci. 2025, 8, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Martić-Kehl, M.I.; Schibli, R.; Schubiger, P.A. Can Animal Data Predict Human Outcome? Problems and Pitfalls of Translational Animal Research. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Thew, M.; Balls, M. An Analysis of the Use of Animal Models in Predicting Human Toxicology and Drug Safety. Altern. Lab. Anim. 2014, 42, 181–199. [Google Scholar] [CrossRef]
- Haslam, A.; Olivier, T.; Powell, K.; Tuia, J.; Prasad, V. Eventual Success Rate and Predictors of Success for Oncology Drugs Tested in Phase I Trials. Int. J. Cancer 2023, 152, 276–282. [Google Scholar] [CrossRef]
- Braam, S.R.; Tertoolen, L.; Casini, S.; Matsa, E.; Lu, H.R.; Teisman, A.; Passier, R.; Denning, C.; Gallacher, D.J.; Towart, R.; et al. Repolarization Reserve Determines Drug Responses in Human Pluripotent Stem Cell Derived Cardiomyocytes. Stem Cell Res. 2013, 10, 48–56. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.; Wang, Z.; Liu, Y.; Yu, J.; Wang, W.; Chen, S.; Wu, W.; Wang, J.; Qian, G.; et al. Standardization of Organoid Culture in Cancer Research. Cancer Med. 2023, 12, 14375–14386. [Google Scholar] [CrossRef]
- Phipson, B.; Er, P.X.; Combes, A.N.; Forbes, T.A.; Howden, S.E.; Zappia, L.; Yen, H.-J.; Lawlor, K.T.; Hale, L.J.; Sun, J.; et al. Evaluation of Variability in Human Kidney Organoids. Nat. Methods 2019, 16, 79–87. [Google Scholar] [CrossRef]
- Pamies, D. Good Cell Culture Practice for Stem Cells and Stem-Cell-Derived Models. ALTEX Altern. Anim. Exp. 2016, 34, 95–132. [Google Scholar] [CrossRef]
- Jiang, X.; Lian, X.; Wei, K.; Zhang, J.; Yu, K.; Li, H.; Ma, H.; Cai, Y.; Pang, L. Maturation of Pluripotent Stem Cell-Derived Cardiomyocytes: Limitations and Challenges from Metabolic Aspects. Stem Cell Res. Ther. 2024, 15, 354. [Google Scholar] [CrossRef]
- Budi, N.Y.P.; Lai, W.-Y.; Huang, Y.-H.; Ho, H.-N. 3D Organoid Cultivation Improves the Maturation and Functional Differentiation of Cholangiocytes from Human Pluripotent Stem Cells. Front. Cell Dev. Biol. 2024, 12, 1361084. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-H.; Jeon, T.J.; So, Y.I.; Shin, Y.K.; Lee, H.J. Applications of Bioinspired Platforms for Enhancing Immunomodulatory Function of Mesenchymal Stromal Cells. Int. J. Stem Cells 2023, 16, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Nwokoye, P.N.; Abilez, O.J. Bioengineering Methods for Vascularizing Organoids. Cell Rep. Methods 2024, 4, 100779. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.A.; Gupta, N.; Kroll, K.T.; Kolesky, D.B.; Skylar-Scott, M.; Miyoshi, T.; Mau, D.; Valerius, M.T.; Ferrante, T.; Bonventre, J.V.; et al. Flow-Enhanced Vascularization and Maturation of Kidney Organoids In Vitro. Nat. Methods 2019, 16, 255–262. [Google Scholar] [CrossRef]
- Li, Z.; Yu, D.; Zhou, C.; Wang, F.; Lu, K.; Liu, Y.; Xu, J.; Xuan, L.; Wang, X. Engineering Vascularised Organoid-on-a-Chip: Strategies, Advances and Future Perspectives. Biomater. Transl. 2024, 5, 21–32. [Google Scholar] [CrossRef]
- Tan, S.Y.; Feng, X.; Cheng, L.K.W.; Wu, A.R. Vascularized Human Brain Organoid On-Chip. Lab. Chip 2023, 23, 2693–2709. [Google Scholar] [CrossRef]
- Zhang, S.; Kan, E.L.; Kamm, R.D. Integrating Functional Vasculature into Organoid Culture: A Biomechanical Perspective. APL Bioeng. 2022, 6, 030401. [Google Scholar] [CrossRef]
- Ha, D.; Kong, J.; Kim, D.; Lee, K.; Lee, J.; Park, M.; Ahn, H.; Oh, Y.; Kim, S. Development of Bioinformatics and Multi-Omics Analyses in Organoids. BMB Rep. 2023, 56, 43–48. [Google Scholar] [CrossRef]
- Bai, L.; Wu, Y.; Li, G.; Zhang, W.; Zhang, H.; Su, J. AI-Enabled Organoids: Construction, Analysis, and Application. Bioact. Mater. 2024, 31, 525–548. [Google Scholar] [CrossRef]
- Renner, H.; Schöler, H.R.; Bruder, J.M. Combining Automated Organoid Workflows with Artificial Intelligence-Based Analyses: Opportunities to Build a New Generation of Interdisciplinary High-Throughput Screens for Parkinson’s Disease and Beyond. Mov. Disord. 2021, 36, 2745–2762. [Google Scholar] [CrossRef]
- Horvath, P.; Aulner, N.; Bickle, M.; Davies, A.M.; Nery, E.D.; Ebner, D.; Montoya, M.C.; Östling, P.; Pietiäinen, V.; Price, L.S.; et al. Screening out Irrelevant Cell-Based Models of Disease. Nat. Rev. Drug Discov. 2016, 15, 751–769. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, N.; Hoehnel, S.; Kuttler, F.; Homicsko, K.; Ceroni, C.; Ringel, T.; Gjorevski, N.; Schwank, G.; Coukos, G.; Turcatti, G.; et al. High-Throughput Automated Organoid Culture via Stem-Cell Aggregation in Microcavity Arrays. Nat. Biomed. Eng. 2020, 4, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Zhang, Y.; Liu, H.; Han, L. Recent Methods of Droplet Microfluidics and Their Applications in Spheroids and Organoids. Lab. Chip 2023, 23, 1080–1096. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human Organoids: Model Systems for Human Biology and Medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
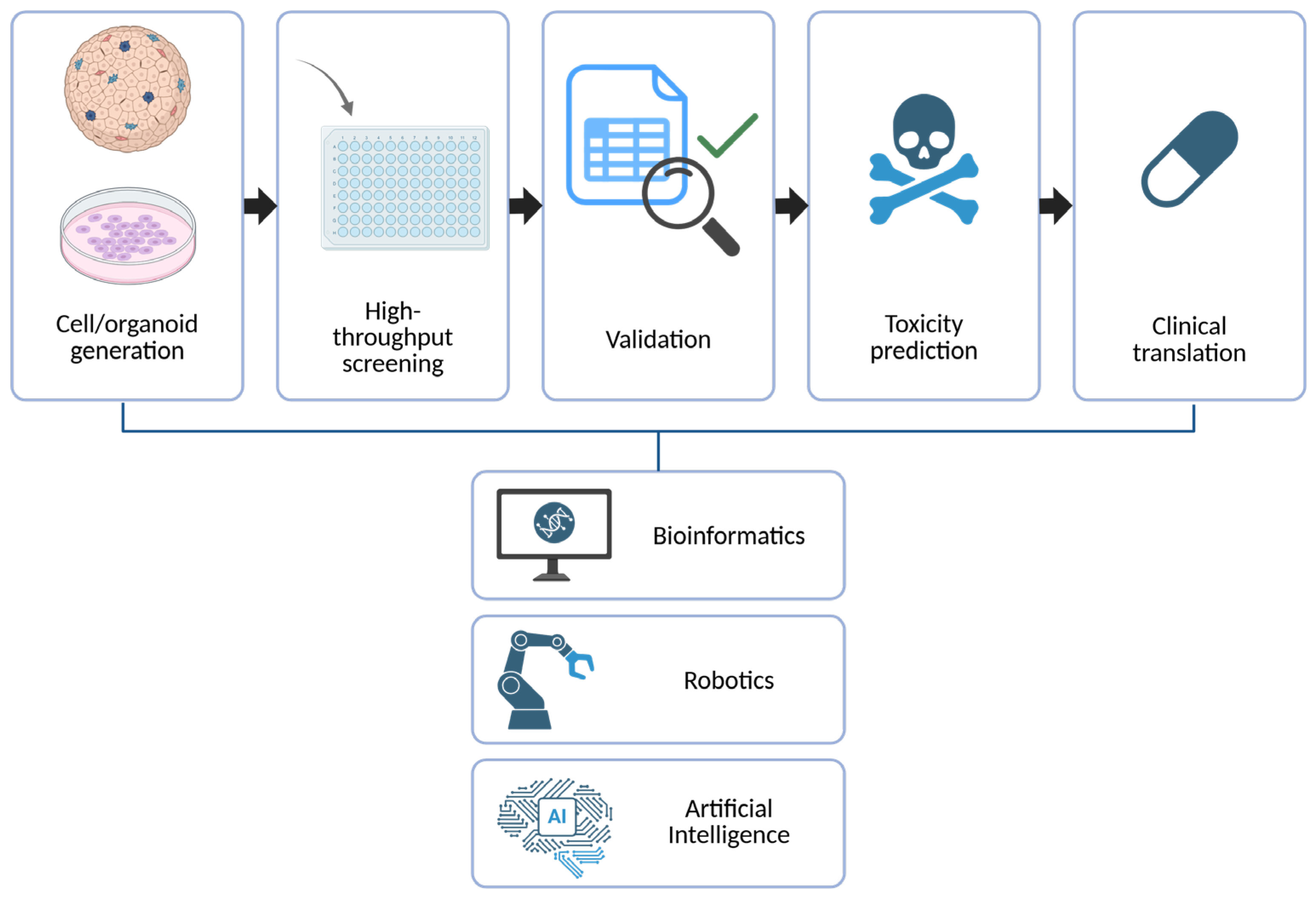
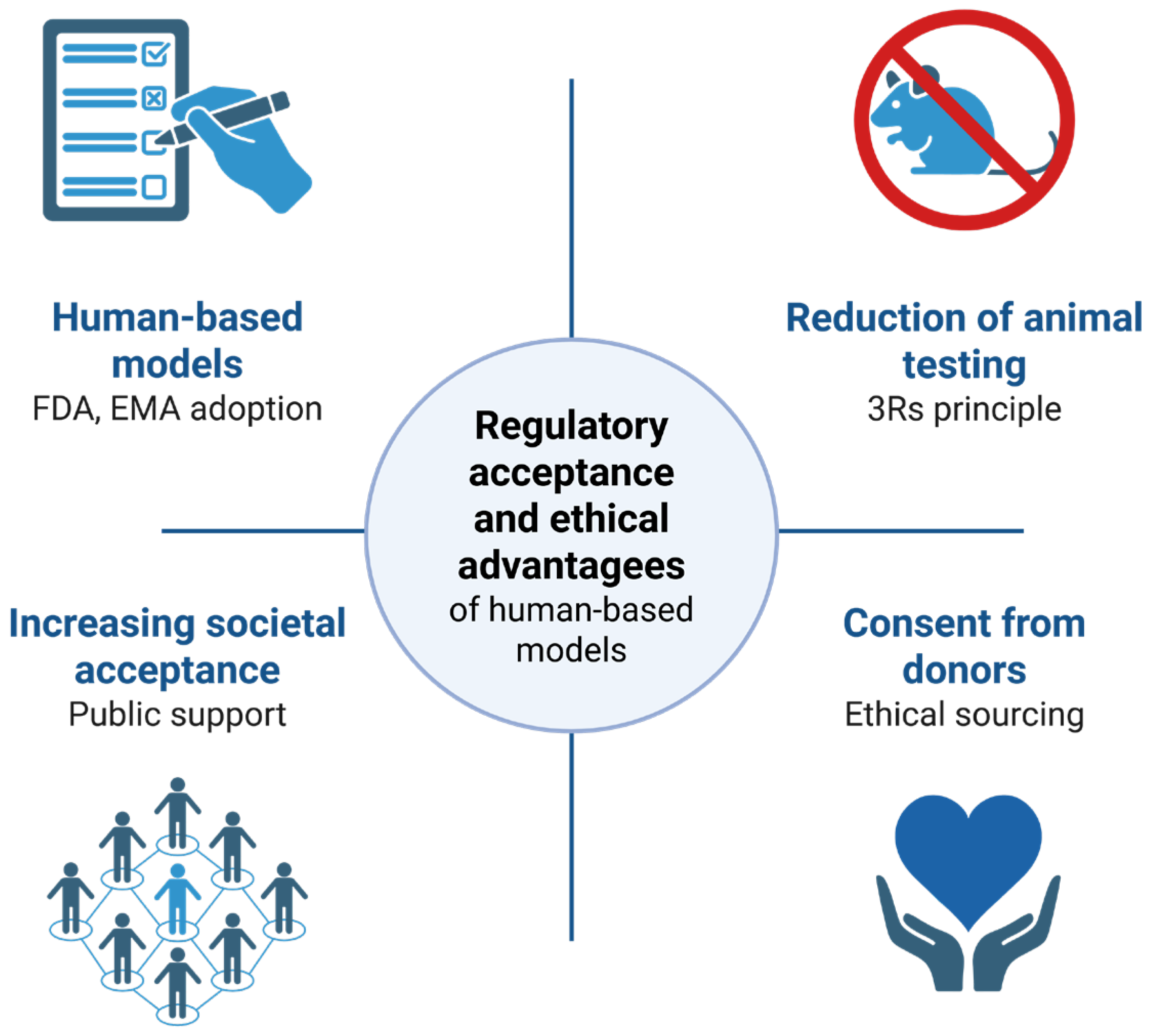
| Application Area | Model Type | Advantages | Limitations |
|---|---|---|---|
| Drug efficacy screening | Organoids hPSC-derived cells [7,31] | Human-specific responses Patient-tailored | Cost Technical complexity |
| Toxicity testing | hPSC-derived hepatocytes/cardiomyocytes [56] | Better prediction of human toxicity | Limited maturity of differentiated cells |
| Disease modeling | iPSC-derived models Organoids [20] | Genetic accuracy Chronic disease modeling | Time-intensive derivation |
| Personalized therapy selection | Patient-derived organoids (PDOs) [64] | Reflects patient heterogeneity Fast screening | Limited scalability Requires biopsy |
| Animal replacement | All hPSC/organoid models [5] | Ethical Human-relevant Scalable | Regulatory acceptance still developing |
| Criteria | 2D Culture | Animal Model | Organoid |
|---|---|---|---|
| Architecture | Flat | Organism level | 3D tissue “organ-like” level |
| Human relevance | Low | Medium | High |
| Cost | Low | High | Medium |
| Clinical predictivity | Low | Medium | High |
| Reproducibility | High | Medium | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luce, E.; Duclos-Vallee, J.-C. Stem Cells and Organoids: A Paradigm Shift in Preclinical Models Toward Personalized Medicine. Pharmaceuticals 2025, 18, 992. https://doi.org/10.3390/ph18070992
Luce E, Duclos-Vallee J-C. Stem Cells and Organoids: A Paradigm Shift in Preclinical Models Toward Personalized Medicine. Pharmaceuticals. 2025; 18(7):992. https://doi.org/10.3390/ph18070992
Chicago/Turabian StyleLuce, Eleanor, and Jean-Charles Duclos-Vallee. 2025. "Stem Cells and Organoids: A Paradigm Shift in Preclinical Models Toward Personalized Medicine" Pharmaceuticals 18, no. 7: 992. https://doi.org/10.3390/ph18070992
APA StyleLuce, E., & Duclos-Vallee, J.-C. (2025). Stem Cells and Organoids: A Paradigm Shift in Preclinical Models Toward Personalized Medicine. Pharmaceuticals, 18(7), 992. https://doi.org/10.3390/ph18070992








