2.2. Molecular Docking
The molecular docking study of the inhibitors of interest was carried out systematically. First, the possibility of finding binding sites throughout the protein surface was explored by combining three descriptors: the pocket volume, receptor hydrophobicity, and binding energy. Next, localized molecular docking was performed, where the specific affinity of the ligands for the binding sites was estimated. In addition, flexible docking was performed to implicitly allow a certain degree of interpenetration between the receptor protein and the docked ligand. Furthermore, the consequences of solvent participation during ligand binding (by flexible/hydrated docking) were evaluated by considering the presence of charged sites within the ligand. Furthermore, the effect of the enantiomers on the racemic mixture was evaluated by simultaneous coupling. Finally, we present the validation of the method by comparing the relative efficiency of HCQ and MLN-4760.
Initially, a blind coupling evaluation was performed to identify the regions in ACE2, with a greater affinity towards (
R)-CQ, (
S)-CQ, (
R)-HCQ, and (
S)-HCQ. At this stage, plausible protonation states were considered and contrasted. Each ligand docking to ACE2 afforded multiple conformations of the coupled ligand and the corresponding binding scores. Root mean square deviation (RMSD) for the coupled conformations was calculated, and a relatively large RMSD limit value of 8.0 Å was selected upon the consideration of the large binding site determined above and the significant number of rotating bonds in the ligands. It was generally observed that the chosen protonation states of the ligands do favor the three distinct binding sites previously identified in the literature, and labeled as α [
18], β [
15], and γ [
16] (
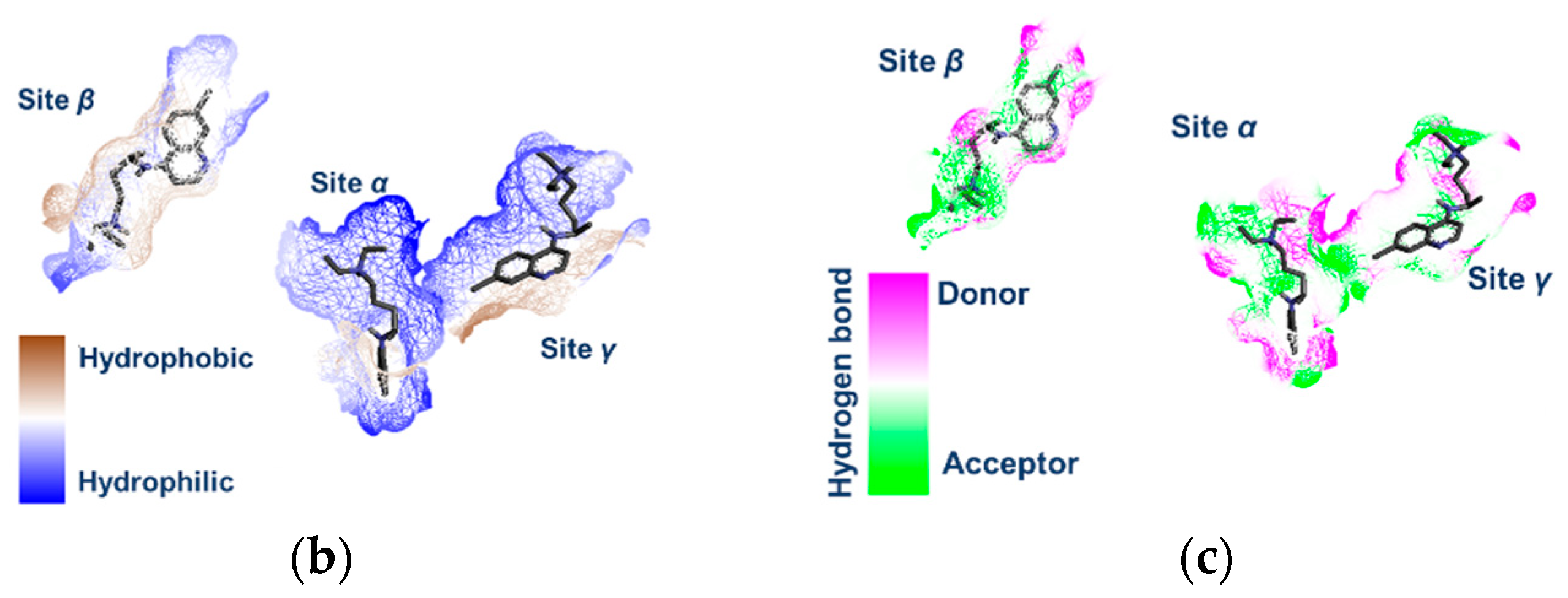
Figure 5a).
A localized docking strategy with the protonated states of the stereoisomers confirmed these binding sites. The most prominent binding site was identified as β, with binding energy values ranging from −8 to −12 kcal/mol. Once the three binding sites had been characterized, the hydrophilic/hydrophobic character of the most relevant peptide residues in those sites was identified. In particular, for the β-site, the presence of hydrophobic zones that can interact with the aromatic segment of the ligands can be appreciated (
Figure 5b). In addition, at the β binding site, the presence of polar compact zones was established (SASA: 281.40 Å
2), whereas in the α and γ sites, the binding pockets are more spacious, which allows the greater mobility of the bound ligands, which exhibit accordingly weaker adhesion (
Figure 5c). In the CQ/ACE2 and HCQ/ACE2 systems, the smaller β site size compared to α and γ sites forced the ligand to adopt a reduced number of effective conformations that met the structural requirements for an effective interaction with the receptor. Thus, for flexible ligands, the affinity between species is regulated by the degree of complementarity and the energy cost associated with the conformational reorganization [
25,
26] that allows the bioactive conformation of the coupled ligand. This fact can be extrapolated to the idea that the average free energy is lower for α and γ sites than for β-site.
From the data obtained by localized docking, it is concluded that the selectivity of the β-site is preferential for the
R-configured species (see
Table 1 and
Table 2), giving rise to a higher level of binding free energy relative to that exhibited by the (
S) isomer. Likewise, the inhibition constant (k
i) of the ligand–protein complex in the β-site was lower, k
i ≈ 0.150 μM for the (
R) enantiomers of CQ and HCQ, which means that the ligand/ACE2 complex was efficient enough to occupy 50% of the binding site even when present in a lower concentration. Statistically, the percentage of effective poses was 8–10% of 150 conformers associated with an RMSD of less than 2 Å, indicating that many conformers can fit within the binding β-site. In contrast, the larger α and γ binding sites exhibit a higher affinity for the (
S) enantiomer of CQ and HCQ as they exhibit a lower inhibition constant and % pose greater than 19%. These facts make this study enjoyable and lead us to specify the ligand–enzyme complex’s activity further.
Considering the physiological pH (≈7.2) [
27], CQ and HCQ are likely to exist in a specific protonation state, with the monoprotonated state at the tertiary amino nitrogen being the most plausible due to its pKa being compatible with the physiological pH [
28]. This monoprotonated species is probably the predominant form in the extracellular physiological environment and plays an important role in the interaction with its target, so it was selected as the most relevant state for the study of CQ and HCQ binding. In particular, attention was paid to the (
R) and (
S) stereoisomers of the monoprotonated species 2CQ and 2HCQ, since they are the most likely to be present in the extracellular environment at a physiological pH.
Flexible coupling [
29] was then performed to find the best binding conformational poses of the protonated ligands 2CQ and 2HCQ at the β binding site. Special attention was paid to interactions involving the residues of Ser-128, Glu-145, Cys-344, Asp-335, and Asp-367, given their ability to participate in hydrogen bonding as well as in electrostatic interactions via so-called “salt bridges” with protonated ligands [
30,
31]. The optimized conformation of the bound ligands suggested a distinction between the two conformational populations: (1)
Bent conformation due to dominant intermolecular interactions between the tertiary amine nitrogen and the aniline-type amino group with one particular amino acid residue in ACE2. (2)
Extended conformation exhibits intermolecular interactions between the tertiary amino group and the secondary aniline-type amino group and two distinct amino acid residues at the β-site that are distant (
Figure 6b).
Additionally,
Figure 6b includes the data collected from the flexible coupling study of the protonated (
R)- and (
S)-stereoisomers of 2CQ, 5CQ, 2HCQ, and 5HCQ at the β-site. For CQ as the substrate, the (
R)-
configured species, 2RCQ, is found to adopt an
extended conformation so that the quinoline ring fits well within the hydrophobic cavity of the pocket and is stabilized by a non-covalent π–π attractive interaction with the Tyr-127 amino acid residue at the β-site. In addition, this
extended conformation is stabilized by electrostatic attractive interactions with the Asp-335 residue (
Figure 6b). A salient observation is the formation of a hydrogen bond between the N-secondary amino group and the thiol group in Cys-344 (NH–S distance = 2.63 Å). These stabilizing interactions with the (
R) enantiomer provide a binding free-energy score value of −11.32 kcal/mol, k
i = 0.005 µM, and 16% number of poses. In contrast, the (
S)-
configured enantiomer 2SCQ exhibited an affinity of −10.13 kcal/mol, k
i = 0.014 µM, and 6% number of poses, also in an
extended conformation induced by an electrostatic attraction between the Asp-335 carboxylate group and the protonated N-tertiary amino group (O
−–HN
+ distance = 3.21 Å). These data suggest the greater stability of the (
R)-
configured 2RCQ complex because it presents a more negative binding free-energy value and a better inhibition constant. Interestingly, for CQ ligands protonated in the aniline-type nitrogen (5RCQ and 5SCQ), which are less likely to be populated at a physiological pH, no significant difference was observed in their inhibition constant and free-energy score values: −8.32 and −8.28 kcal/mol, respectively.
In the case of HCQ ligands, the (R) enantiomer 2RHCQ adopts a bent conformation as a consequence of two convergent stabilizing interactions: one hydrogen bond between the carboxylate group of Glu-145 and the secondary nitrogen in HCQ (O−–H-N distance = 1.98 Å) and one electrostatic interaction between the same residue Glu-145 and the protonated tertiary nitrogen in the HCQ ligand (O−–HN+ distance = 3.1 Å). These stabilizing interactions provide a free bond energy value of −10.43 kcal/mol, ki = 0.022 µM, and 19% number of poses.
Although the (S) enantiomer 2SHCQ also adopts a bent conformation converging on the amino acid residue Glu-145, the responsible attractive interactions seem to be weaker, with one of the type π–anion involving the quinoline ring and the carboxylate group on the amino acid residue (π–O− distance = 2.91 Å), reflected in a binding energy score value of −9.23 kcal/mol, ki = 0.171 μM, and 12% number of poses.
Furthermore,
Figure 6b shows the superposition of the enantiomers, revealing the distribution of the ligand atoms within the β-site, and highlights the spatial differences in the calculated conformations for each enantiomer, providing insight into the spatial constraint of the ligands within the binding site (SASA = 281.40 Å
2).
The molecular docking study features a rigorous statistical analysis to support the results, including F and Mann–Whitney tests, as well as confidence intervals to estimate the accuracy of the binding free energies. The F test (with an alpha parameter equal to 0.05) suggests that the variation in the data distribution between the enantiomer pairs is equal, although caution should be exercised due to unequal sample sizes and the potential non-normality of the data (see
Tables S2–S7 in the Supporting Information). The 95% confidence intervals for each enantiomer show no overlap between the binding free energies for the ligand pairs 2CQ and 2HCQ, indicating significant differences: 2CQ [2RCQ (−10.44 to −9.93 kcal/mol) and 2SCQ (−9.24 to −8.71 kcal/mol)] and 2HCQ [2RHCQ (−9.66 to −9.31 kcal/mol) and 2SHCQ (−8.76 to −8.21 kcal/mol)]. Furthermore, the Mann–Whitney [
32,
33] test confirms with 95% confidence that there is a difference in the binding energy distributions (
Table S8, Supplementary Information). Taken together, the evidence suggests a slight preference of the β-site for the configured (
R) enantiomers.
The attractive electrostatic interactions between the ligand and the β-site are crucial in determining the lowest-energy conformations the ligand adopts within the receptor pocket. Furthermore, the susceptibility of enantiomers varies depending on the ionic strength of the solution, suggesting that ionic strength significantly influences ligand binding [
11]. Indeed, ionic strength can affect the molecule in hydrated docking by modulating electrostatic interactions, molecular conformation, solvation, and the stability of the complex. By including ions in the simulation, the accuracy of the results can be improved and specific effects, such as the influence of salinity on ligand binding, can be studied.
In this regard, it was considered that since these binding processes take place in an aqueous solution, they should be modulated by solvating water molecules. To evaluate these potential interactions, the solvation entropy at the enzymic pocket with the bound ligand must be considered [
34]; thus, a flexible molecular docking analysis with the explicit inclusion of water molecules was performed. In particular, the protocol developed by A. Olson, which calculates water molecules at specific positions on the surface of ligands prior to coupling, was implemented [
35,
36]. The derived binding score values showed an improvement due to the increase in the solvation potential. In addition, exploring hydration patterns for the protein as a function of the coupled ligand allowed for optimizing the ligand/receptor fit at the binding site.
The results of the flexible and hydrated docking models with protonated species of 2RCQ, 2SCQ, 2RHCQ, and 2SHCQ are summarized in
Figure 7. During the flexible docking simulation, the ligand experienced rotational motions of its single bonds and significant conformational changes due to the flexibility allowed in the enzyme and the presence of pre-calculated water molecules.
Figure S1 of the Supporting Information shows the conformational difference before and after docking, illustrating the adaptability of the ligand in the binding site. As expected, water binding occurred mainly in polar areas near the protonated N tertiary amino group and the protonated aniline-type secondary amino group on the ligands.
The resulting hydrated species did participate in hydrogen-bonding interactions with hydrophilic residues within the β-site. For instance, species 2RCQ is involved in hydrogen bond formation that incorporates bridging water between Ser-128 and Asp-335 residues, affording an extended conformation of the ligand. An energy stabilization value of 12.62 kcal/mol, ki = 500 pM, and 12% of the number of poses was estimated. On the other hand, 2SCQ presented two water bridges, one between Tyr-127 and the protonated secondary nitrogen and another between Glu-145 and the protonated tertiary ammonium nitrogen, giving rise to an
extended conformation and an energy stabilization value of −11.48 kcal/mol, k
i = 3000 pM, and 2% number of poses (see
Figure 7b, where the blue spheres correspond to water molecules). These results indicate that (
R)
configured species bind more efficiently to the β-site.
In the case of the enantiomeric pair of 2HCQ, the hydrogen bonding interactions between the residues of Glu-145 and Asp-367 and the protonated secondary and tertiary amino groups are reflected in a stronger and more negative binding energy (greater stability) relative to the CQ ligands.
Figure 7b highlights the water bridges between 2RHCQ and 2SHCQ (hydroxyl and N-tertiary protonated group) and the amino acid residue Glu-145. The calculated binding energy value of −14.1 kcal/mol and −13.3 kcal/mol for 2RHCQ and 2SHCQ, respectively, indicates a more negative (stronger) binding free energy for the (
R)-
configured enantiomer (k
i = 54 pM and 5% number of poses). The F-tests were unreliable because of insufficient binding free energy data available within each cluster at RMSD = 2 Å.
Likewise,
Figure 7b includes the superposition of the enantiomers, revealing significant spatial differences relative to the calculated highest-energy conformations. This allowed us to better understand the changes in the β-site and the enantiomer specificity, which is crucial for understanding the interaction between the ligands and the protein.
Furthermore, we performed simultaneous docking on the whole ACE2 protein using Autodock Vina 1.2.0 software and the multiligand simultaneous docking (MLSD) protocol [
37] to evaluate the racemate effect of the ligands (
R)-2CQ, (
S)-2CQ, (
R)-2HCQ, and (
S)-2HCQ. This approach allowed us to assess the affinity and selectivity of the binding sites on ACE2. Our results reveal that both enantiomers preferentially bind to the α-site, even when ligand pairs with the same configuration (
R,
R and
S,
S) were tested. This is consistent with the larger size of the α-site (SASA = 304.45 Å
2). However, we observed a notable difference in the orientation of the (
R)-enantiomers located closer to the β-site, suggesting higher compatibility and selectivity for this site. The selectivity of binding sites is crucial for understanding protein–ligand interactions. Our results indicate that the α site has a higher affinity for both enantiomers. In contrast, the β-site shows greater selectivity for the (
R) enantiomer (see
Figures S2 and S3 in the Supporting Information).
Finally, the natural ACE2 inhibitor (MLN-4760) redocking was performed to validate our molecular docking studies. As it turns out, the MLN-4760 inhibitor shares similar structural characteristics with HCQ and CQ; for instance, its structure presents several degrees of freedom due to the single bonds within the structure. A RMSD of 0.74 Å was obtained with an affinity energy of −7.15 kcal/mol, k
i = 5.76 µM, and 11% number of poses (see
Figure S4), and these data demonstrate that the method employed has the capacity to perform accurate molecular docking, with precision similar to that achieved from crystallized structures. Furthermore,
Figure 8 shows the overlay of the experimental MLN-4760 structure of the 1R4L protein adjacent to the (
R)-hydroxychloroquine. Both ligands are very close to the assigned β-site in this work, which leads to the conclusion that the β binding site shares characteristics with the receptor site of the crystallized ligand, which presents similar residues: Arg-273 and His-345. It is also observed that (
R)-HCQ should be a more efficient inhibitor than (
S)-HCQ as a result of better binding free energy (−9.65 kcal/mol), a lower inhibition constant (0.150 µM), and a higher percentage of available good poses (10%).
Our molecular docking studies identify three major interaction sites (α, β, and γ) on ACE2 that interact with the enantiomers of CQ and HCQ. We observe that the β-site shows preferential selectivity for species with the (R) configuration, whereas the α and γ sites exhibit higher affinity for the (S) enantiomer of CQ and HCQ. Ligand stereochemistry, protonation states, and cooperativity between binding sites significantly influence binding. These findings offer detailed insight into the molecular interplay underlying the binding of CQ and HCQ enantiomers to ACE2, which may be critical for understanding the efficacy and selectivity.
2.3. Molecular Dynamics
The next step in our study is to investigate the β-site selectivity for CQ and HCQ: (R)-2CQ, (S)-2CQ, (R)-2HCQ, and (S)-2HCQ enantiomers. To achieve this, we performed molecular dynamics (MD) simulations to evaluate the conformational processes when the ligand and receptor bind and the conformational perturbations that occur during ligand binding to the β-site.
These MD simulations allowed us to predict the relative stability of the protein–ligand complexes and analyze the binding dynamics. In particular, we determined the RMSD of the docked system and the root mean square fluctuation (RMSF) during binding, which provided us with valuable information on the stability and flexibility of the complexes. Furthermore, we studied the influence of hydrogen bonds in the various complexes, which allowed us to better understand the factors contributing to the β-site selectivity for the CQ and HCQ enantiomers.
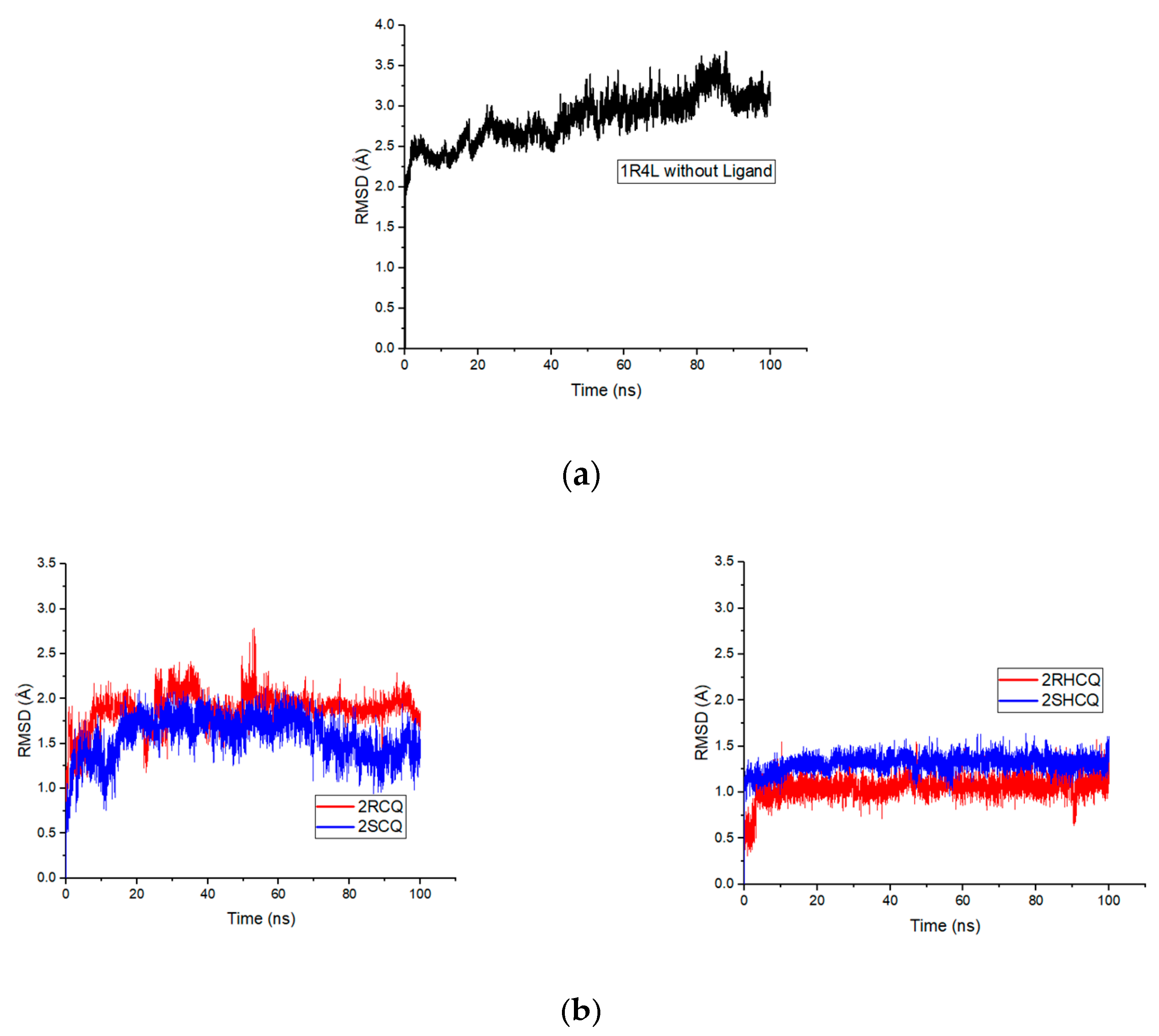
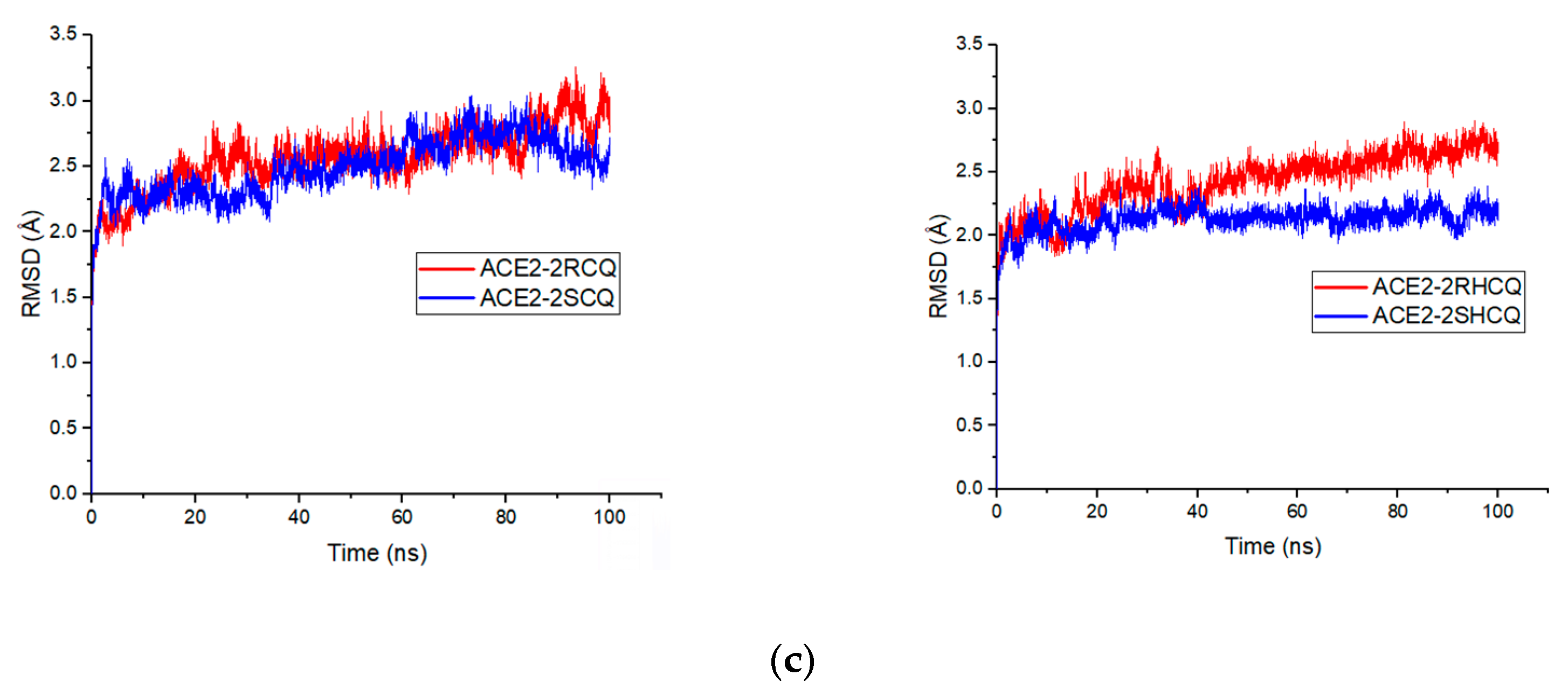
In this regard, RMSD refers to measuring structural deviations of the molecular framework (ligand and protein) from its free form to its final conformation after binding. In particular, the structural modifications produced throughout the simulation determine the stability of the protein in terms of its conformation. A stable protein structure shows smaller deviations in the protein’s backbone relative to the free enzyme. In this sense, it is shown that the free protein 1R4L without a ligand shows a positive trend with maximum values of 3.6 Å at 87.8 ns, indicating the destabilization of the protein owing to the absence of the ligand, something that was already anticipated from the control experiment (see
Figure 9a). On the other hand, RMSD analysis revealed that with the 2RCQ ligand, the entire coupling ligand trajectory stabilizes after 50 ns, while 2SCQ stabilizes after 75 ns. This indicates that chloroquine’s (
R) enantiomer adapts more rapidly to the active pocket in ACE2. On the other hand, in the case of the enantiomeric pair in hydroxychloroquine 2RHCQ/2SHCQ, the process reached stabilization after only 20 ns, suggesting that hydrogen bonding involving the hydroxyl group lowers the activation energy for binding. Furthermore, HCQ ligands display fewer structural alterations during binding than their original conformation (
Figure 9b). On the other hand, the (
R) enantiomer of chloroquine 2RCQ presented a positive slope in the RMSD values during the binding process, which implies greater molecular deformation after 50 ns, reaching a maximum deformation value of 2.83 Å at 95 ns. The (
S) enantiomer 2SCQ exhibited similar behavior with a deviation value of 2.72 Å at 77 ns, which fell to 2.31 Å in the more stable arrangement. A positive slope was observed for 2RHCQ with a maximum deviation of 2.7 Å at 78.6 ns, while 2SHCQ remained stable throughout the simulation (
Figure 9c).
Our results suggest that the protein system maintains its original conformation after ligand binding, indicating that ligand incorporation is less disruptive in HCQ-related complexes. Furthermore, none of the ligands caused a significant conformational change in the ACE2 enzyme (average RMSD of the docked protein: 2.40 ± 0.16 Å), suggesting that the open (holoprotein) conformation is not adopted. This implies that CQ and HCQ ligands can effectively prevent viral infection. During molecular dynamics, the ligands underwent conformational changes due to single-bond rotation, but their stereochemistry remained intact and they remained in the β-site. The stability of the complex appears to be determined by spatial and polar–electrostatic complementarity, as well as by hydrophobic contributions that stabilize the binding between the ligand and the protein binding site. The included molecular dynamics video illustrates the stability of the complex and supports this interpretation (See the link at the end of the
Supplementary Information).
The root-mean-square fluctuation (RMSF) analysis addresses conformational changes during ligand binding in the flexible regions of the protein–ligand complexes. In proteins, a higher RMSF is exhibited by flexible, poorly organized structures such as loops, turns, and coils, while well-structured segments such as those involved in α-helix and β-sheets present slight fluctuations. We have calculated the RMSF value to predict the structural changes induced in the structure of proteins by ligand binding. A small but significant difference in the RMSF was observed between the enantiomeric ligands concerning the fluctuations in the interaction pocket, showing greater fluctuations for the (
R)-
configured species 2RCQ and 2RHCQ (see
Figure 10a; the interacting amino acid residues at the β-site are shown in green). Regarding the protein framework, greater fluctuations were observed in residues located far from the β-site and the DP domain (binding interface for SARS-CoV-2), particularly in residues that are located in the more flexible regions where the loops, turns, and coils are localized: Glu-87, Asp-136, Gly-211, Pro-289, Lys-353, Gln-429, Glu-536, Ser-545, and Gly-561 (
Figure 10a, the participant amino acid residues are highlighted in purple).
Figure 10b shows the specific localization of those participant amino acid residues in the protein framework.
Hydrogen bonding interactions play an essential role in stabilizing protein–ligand complexes. In the present study, different strengths of hydrogen bonding interactions among enantiomeric ligands align with distinct affinities between enantiomers towards the β-site. A statistical analysis was performed according to the number of binding events (% frequency) and their distribution over time, obtaining an average number of hydrogen bonds stabilizing the corresponding complexes. The (R)-configured enantiomer (2RCQ) presented a frequency of 73.6% for these H-bonding interactions after 30 ns. In comparison, the 2SCQ had a higher frequency of 88.6% before 60 ns, which is in line with a higher number of interactions by hydrogen bonding.
According to the structural data [
38], both enantiomers showed a similar frequency in the participation of H-bonding interactions; in particular, a similar behavior is found in hydrogen bond interactions for the donor–hydrogen–acceptor angle and the distance between the acceptor atom–donor atom. Nevertheless, a lower frequency of 24.7% was observed for the (
R)-
configured species relative to the opposite enantiomer (2SCQ, 35.4%), which suggests a higher number of interactions of this type in the latter enantiomer (
Figure 11a).
On the other hand, a screenshot of 2RCQ at 30 ns (
Figure 11b) shows an extended conformation with a hydrogen bond interaction between Cys-344 and N-aromatic (O-H–N distance = 3.07 Å), together with two simultaneous electrostatic attractive interactions between protonated N-tertiary amine and Glu-145 (NH
+–O
− distance = 1.79 Å) and Asp-335 (HN
+–O
− distance = 5.32 Å). In the case of 2SCQ (time = 55 ns), an extended conformation with an electrostatic attraction interaction between Glu-145 and protonated N-tertiary amine (O
−–HN
+ distance = 1.7 Å) is observed, together with weaker hydrogen bonding interactions involving carbon atoms adjacent to the protonated amine as acceptors (
Figure 11c).
Regarding the 2HCQ enantiomeric pair, the analysis that was carried out according to the geometric criteria for the detection of H-bonding interactions in the ligand complex with the receptor highlights the affinity of the (
R) enantiomer with a frequency of 61.8% after 30 ns, against 40.42% for the (
S) enantiomer (
Figure 12a). A screenshot at 30 ns shows that 2RHCQ adopts a
bent conformation with a hydrogen bonding interaction between Asp-367 and the hydroxyl group (O–HO distance = 1.6 Å), along with an electrostatic attractive interaction between Asp-367 and the protonated N-tertiary amino group. An additional non-bonded interaction was observed between Arg-273 and the N-aromatic nitrogen (NH–N
+ distance = 2.9 Å), as well as two electrostatic attractive interactions with the same residue of Glu-145 and the aromatic system of the ligand (cation–π) (
Figure 12b). In contrast, the species 2SHCQ presented two simultaneous hydrogen bonding interactions with the Glu-145 residue and the N-secondary nitrogen (O–HN distance = 2.3 Å), which induced the ligand to adopt a
bent conformation. This arrangement is reinforced by a simultaneous hydrogen bond interaction between the Leu-144 residue and the ligand’s hydroxyl group (at 2.2 Å and 1.9 Å distances, respectively). At the same time, the aromatic system was anchored by attractive π–π interactions between the aromatic rings of Tpr-271 and His-345 (
Figure 12c). These results are consistent with the previous study reported by Machado and [
6].
ACE2 displays selectivity for (R)-configuration enantiomers at the β-site, resulting in conformational stability of the ligand–ACE2 complexes after 45 ns of simulation. ACE2 has a higher affinity for HCQ than CQ, supporting its potential as a SARS-CoV-2 inhibitor. The (R)-enantiomer of HCQ binds more efficiently to ACE2 through electrostatic hydrogen bonding interactions, making it more active. In contrast, CQ’s (R)- and (S)-enantiomers have similar activity. Ligand binding does not induce significant conformational changes in ACE2.
2.4. Free Energy of Binding According to Molecular Mechanics Poisson–Boltzmann Surface Area Solvation Analysis (MM-PBSA)
Molecular mechanics combined with the Poisson–Boltzmann surface area continuum solvation method allowed the estimation of the free energy of the binding of small ligands to biological molecules and was obtained from molecular dynamics simulations of the receptor–ligand complex. In particular, the binding energy reveals how much energy is released when a ligand and protein interact. In this work, the binding energies for each of the four complexes between (R)-2CQ, (S)-2CQ, (R)-2HCQ, and (S)-2HCQ and the β-site were estimated separately following a molecular dynamics simulation. The energies that contribute to the total interaction-free energy (van der Waals energy, electrostatic energy, polar solvation energy, and solvent-accessible surface energy (SASA)) were calculated to establish which type of non-bonded interaction has the most significant contribution to the stability of the final complex.
MM-PBSA calculations (
Table 3) reveal that both 2CQ and 2HCQ enantiomers exhibit significant affinity for the binding site. For 2CQ, both enantiomers (R and S) show similar total binding free energies (−32.21 ± 6.62 kcal/mol and −33.22 ± 3.91 kcal/mol, respectively), whereas for 2HCQ, the (
R)-enantiomer exhibits a slightly higher affinity (−39.04 ± 6.09 kcal/mol) compared to the (
S)-enantiomer (−36.52 ± 4.46 kcal/mol). In both cases, favorable van der Waals and electrostatic interactions compensate for the unfavorable desolvation, allowing binding. Desolvation is primarily due to the hydrophobic nature of the ligand, specifically the aromatic system and the carbon chain, which results in a loss of interactions with water molecules and a reduction in the system’s entropy.
It is important to emphasize that these results must be interpreted within the context of the limitations of the MM-PBSA method. Although they provide a detailed estimate of the binding free energy, they may not fully capture the complexity of the system’s conformational dynamics and the contribution of the receptor’s conformational entropy. Despite these limitations, the results suggest that the enantiomers of 2CQ and 2HCQ have significant affinity for the binding site, which can be useful in guiding ligand design and binding optimization.
Our study has intrinsic limitations arising from the dependence of docking and molecular dynamics methods on force fields. These methods may not fully capture the complexity of molecular interactions, including dynamical and solvation effects, which may influence the accuracy of the results. Additionally, accurate crystallographic data are lacking and the molecular interactions are complex. Nevertheless, our results, based on in silico simulations, provide detailed insights into the interactions between CQ, HCQ, and ACE2. Our findings reveal the discrepancy associated with the enantioselectivity of the drugs, a topic that has not been clearly defined in the literature due to the multitude of ACE2 interaction sites. Our findings suggest that the (R) enantiomer of HCQ binds more efficiently to ACE2 through electrostatic interactions, positioning it as a potential SARS-CoV-2 inhibitor.
However, the literature suggests that the (S) enantiomer of HCQ may exhibit greater acute toxicity in vivo, an apparent discrepancy between antiviral efficacy and toxicity. This could be due to the complex interaction between HCQ and ACE2, involving multiple binding sites and the allosteric reorganization of the protein. Additionally, the enantioselective pharmacokinetics of HCQ, which are characterized by higher blood concentrations of the (R) enantiomer and higher (S) enantiomer metabolites, may contribute to the discrepancy between antiviral efficacy and toxicity.
Despite these complexities, our results underscore the importance of considering enantioselectivity when developing drugs against SARS-CoV-2. A deeper understanding of the interaction between hydroxychloroquine (HCQ) and angiotensin-converting enzyme 2 (ACE2), as well as the enantioselective pharmacokinetics and pharmacodynamics of HCQ, is essential for developing safer and more effective drugs. Our findings provide a molecular basis for understanding the differences in the binding and activity of the (R) and (S) enantiomers of HCQ. This information could be useful for designing new drugs and optimizing their efficacy and safety.