Evaluation and DFT Analysis of In Vitro Anticancer Activity of Consolida orientalis, Smyrnium rotundifolium, and Euphorbia virgata Plant Extracts in Colorectal Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Extract Yields
2.2. Cytotoxicity Assay
2.3. Biotic Compound and Target Proteins Identifications
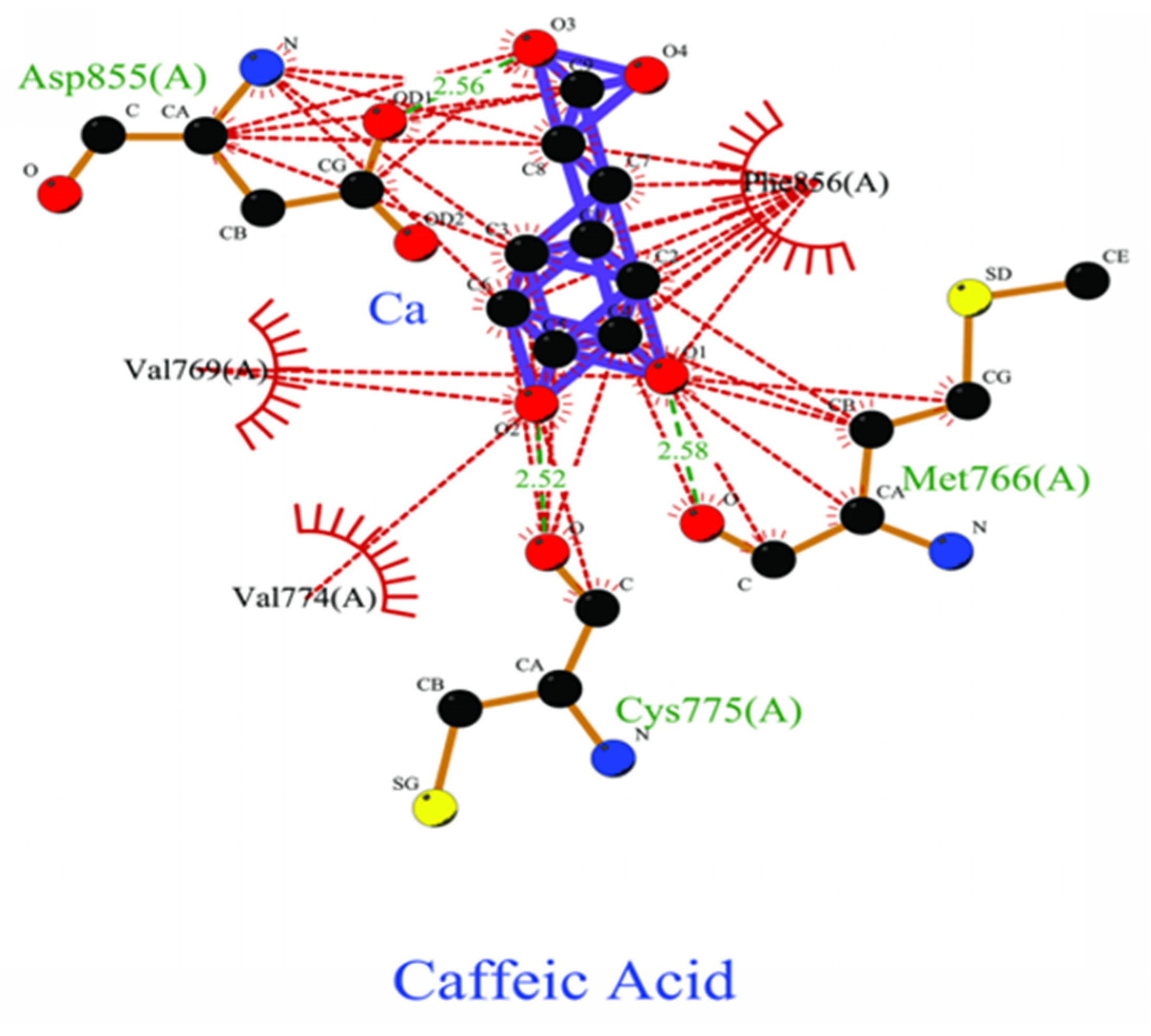
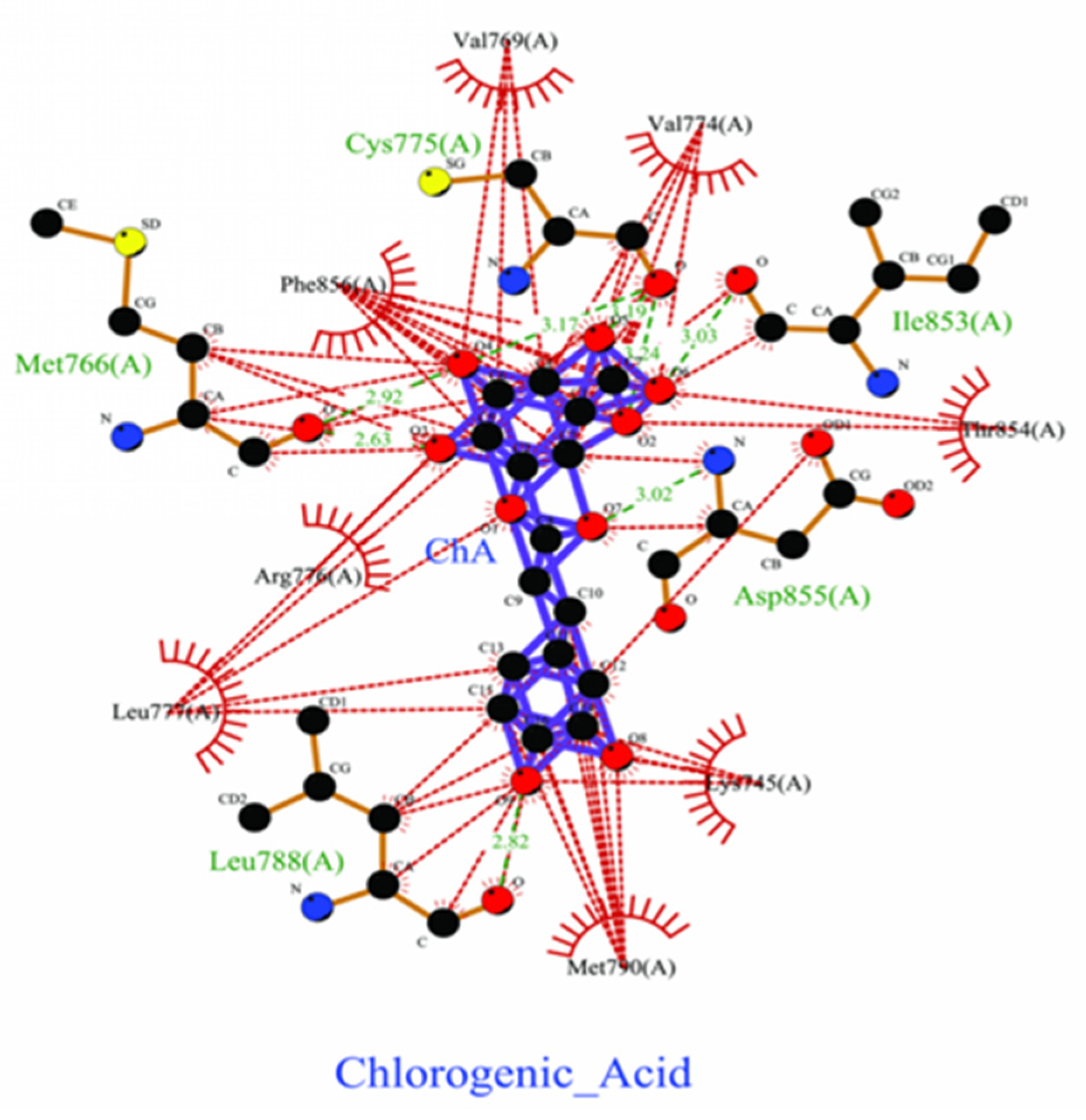
2.4. Bonding Energy Analysis Between Ligands and Proteins
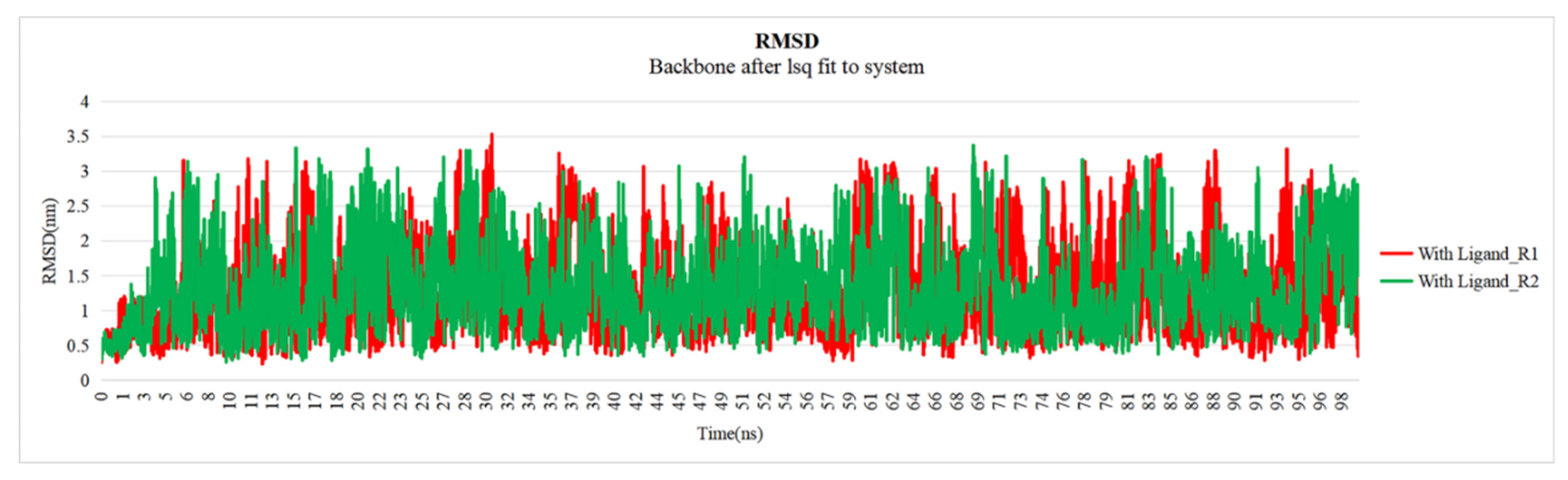
2.5. Evaluation of Docked Complex Proteins Stability Through MD Simulation
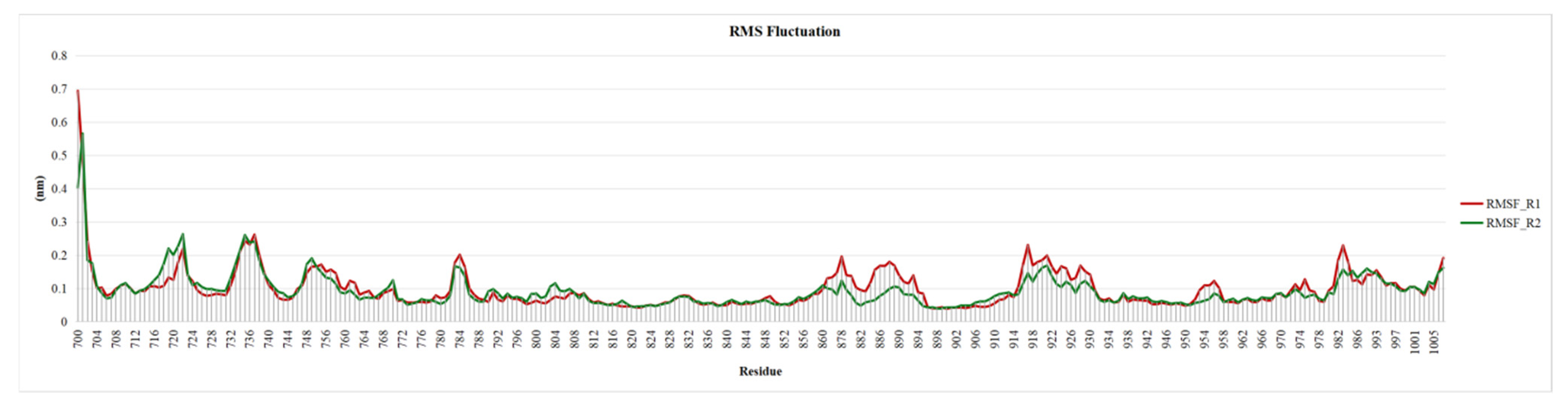
2.6. Root Mean Square Fluctuation of EGRF Proteins
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Plant Extract
3.3. Cell Culture
3.4. In Vitro Cytotoxicity Assay
3.5. Molecular Docking Analysis
3.5.1. Bioactive Compound Identifications
3.5.2. Target Protein Identifications Linked to Bioactive or Hypertensive
3.5.3. Preparation of Ligand, Target Protein, and Stability Check
3.5.4. MD Simulation of EGFR Kinase Docked Complexes with Top Ligand
3.6. Details of DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nesaragi, A.R.; Algethami, J.S.; Alsaiari, M.; Alsareii, S.A.; Mathada, B.S.; Ningaiah, S.; Sasidhar, B.S.; Harraz, F.A.; Patil, S.A. A Comprehensive Overview of Coumarinyl-Triazole Hybrids as Anticancer Agents. J. Mol. Struct. 2024, 1302, 137478. [Google Scholar] [CrossRef]
- Popescu, R.C.; Tocia, C.; Brînzan, C.; Cozaru, G.C.; Deacu, M.; Dumitru, A.; Leopa, N.; Mitroi, A.F.; Nicolau, A.; Dumitru, E. Molecular Profiling of the Colon Cancer in South-Eastern Romania. Medicine 2021, 100, e24062. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Gömeç, M.; Yulak, F.; Gezegen, H.; Özkaraca, M.; Sayin, K.; Ataseven, H. Synthesis of Diaryl Urea Derivatives and Evaluation of Their Antiproliferative Activities in Colon Adenocarcinoma. J. Mol. Struct. 2022, 1254, 132318. [Google Scholar] [CrossRef]
- Abedizadeh, R.; Majidi, F.; Khorasani, H.R.; Abedi, H.; Sabour, D. Colorectal Cancer: A Comprehensive Review of Carcinogenesis, Diagnosis, and Novel Strategies for Classified Treatments. Cancer Metastasis Rev. 2024, 43, 729–753. [Google Scholar] [CrossRef] [PubMed]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-Derived Bioactive Compounds in Colon Cancer Treatment: An Updated Review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Pan, T.; Jia, N. Plant Natural Compounds in the Cancer Treatment: A Systematic Bibliometric Analysis. Heliyon 2024, 10, e34462. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a Source of Anti-Cancer Agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Picot-Allain, C.M.N.; Cakmak, Y.S.; Uysal, S.; Aktumsek, A. In Vitro Tyrosinase Inhibitory and Antioxidant Potential of Consolida Orientalis, Onosma Isauricum and Spartium Junceum from Turkey. South. Afr. J. Bot. 2019, 120, 119–123. [Google Scholar] [CrossRef]
- Nemati, F.; Dehpouri, A.A.; Eslami, B.; Mahdavi, V.; Mirzanejad, S. Cytotoxic Properties of Some Medicinal Plant Extracts from Mazandaran, Iran. Iran. Red. Crescent Med. J. 2013, 15, e8871. [Google Scholar] [CrossRef]
- Özpınar, N. Consolida Orientalis (Gay.) Schröd. Ekstraktlarının Acanthamoeba Castellanii Trofozoit ve Kist Formları Üzerine Ameboisidal Etkisi ve Sitotoksik Potansiyeli. Int. J. Acad. Med. Pharm. 2020, EK-1, 34–39. [Google Scholar] [CrossRef]
- Mungan, F.; Yıldız, K.; Kılıç, M.; Kuh, M. A Morphological Study of Smyrnium (Apiaceae) from Turkey. Biol. Divers. Conserv. 2015, 8, 54–59. [Google Scholar]
- Kaçar, D.; Bayraktar, O.; Erdem, C.; Alamri, A.S.; Galanakis, C.M. Antioxidant and Antimicrobial Activities of Plants Grown in the Mediterranean Region. JSFA Rep. 2022, 2, 452–461. [Google Scholar] [CrossRef]
- Ulubelen, A.; Abdolmaleky, H.; Mabry, T.J. Flavonoid Glycosides from Smyrnium Perfoliatum, Smyrniumcreticum and Smyrnium Rotundifolium. J. Nat. Prod. 1982, 45, 507. [Google Scholar] [CrossRef]
- Özbilgin, S.; Küpeli Akkol, E.; Süntar, İ.; Tekin, M.; Saltan İşcan, G. Wound-Healing Activity of Some Species of Euphorbia L. Rec. Nat. Prod. 2018, 13, 104–113. [Google Scholar] [CrossRef]
- Rojas-Jiménez, S.; Valladares-Cisneros, M.G.; Salinas-Sánchez, D.O.; Pérez-Ramos, J.; Sánchez-Pérez, L.; Pérez-Gutiérrez, S.; Campos-Xolalpa, N. Anti-Inflammatory and Cytotoxic Compounds Isolated from Plants of Euphorbia Genus. Molecules 2024, 29, 1083. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R. Chemistry and Biological Activity of Secondary Metabolites in Euphorbia from Iran. Phytochemistry 2006, 67, 1977–1984. [Google Scholar] [CrossRef]
- Alsanie, W.F.; Alhomrani, M.; Alamri, A.S.; Alyami, H.; Shakya, S.; Habeeballah, H.; Alkhatabi, H.A.; Felimban, R.I.; Alamri, A.; Alhabeeb, A.A.; et al. Attempting to Increase the Effectiveness of the Antidepressant Trazodone Hydrochloride Drug Using π-Acceptors. Int. J. Env. Res. Public. Health 2022, 19, 11281. [Google Scholar] [CrossRef]
- Alhomrani, M.; Alsanie, W.F.; Alamri, A.S.; Alyami, H.; Habeeballah, H.; Alkhatabi, H.A.; Felimban, R.I.; Haynes, J.M.; Shakya, S.; Raafat, B.M.; et al. Enhancing the Antipsychotic Effect of Risperidone by Increasing Its Binding Affinity to Serotonin Receptor via Picric Acid: A Molecular Dynamics Simulation. Pharmaceuticals 2022, 15, 285. [Google Scholar] [CrossRef]
- Kaya, S.; Kaya, C. A New Equation for Calculation of Chemical Hardness of Groups and Molecules. Mol. Phys. 2015, 113, 1311–1319. [Google Scholar] [CrossRef]
- von Szentpály, L.; Kaya, S.; Karakuş, N. Why and When Is Electrophilicity Minimized? New Theorems and Guiding Rules. J. Phys. Chem. A 2020, 124, 10897–10908. [Google Scholar] [CrossRef]
- Kaya, S.; Robles-Navarro, A.; Mejía, E.; Gómez, T.; Cardenas, C. On the Prediction of Lattice Energy with the Fukui Potential: Some Supports on Hardness Maximization in Inorganic Solids. J. Phys. Chem. A 2022, 126, 4507–4516. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, E.; Chattaraj, P.K.; Fuentealba, P. Variation of the Electrophilicity Index along the Reaction Path. J. Phys. Chem. A 2003, 107, 7068–7072. [Google Scholar] [CrossRef]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and Antiacetylcholinesterase Activities of Five Plants Used as Portuguese Food Spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Mohanraj, K.; Karthikeyan, B.S.; Vivek-Ananth, R.P.; Chand, R.P.B.; Aparna, S.R.; Mangalapandi, P.; Samal, A. IMPPAT: A Curated Database of Indian Medicinal Plants, Phytochemistry and Therapeutics. Sci. Rep. 2018, 8, 4329. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.Org: Online Mendelian Inheritance in Man (OMIM®), an Online Catalog of Human Genes and Genetic Disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET Knowledge Platform for Disease Genomics: 2019 Update. Nucleic Acids Res. 2019, 48, D845–D855. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the Worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: A Package for Molecular Simulation and Trajectory Analysis. J. Mol. Model. 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual Density Functional Theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Kaya, S.; Kaya, C.; Islam, N. Maximum Hardness and Minimum Polarizability Principles through Lattice Energies of Ionic Compounds. Phys. B Condens. Matter 2016, 485, 60–66. [Google Scholar] [CrossRef]
- İslam, N.; Kaya, S. Conceptual Density Functional Theory and Its Application in the Chemical Domain; Islam, N., Kaya, S., Eds.; Apple Academic Press: New York, NY, USA, 2018; ISBN 9780203711392. [Google Scholar]
- Koopmans, T. Über Die Zuordnung von Wellenfunktionen Und Eigenwerten Zu Den Einzelnen Elektronen Eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
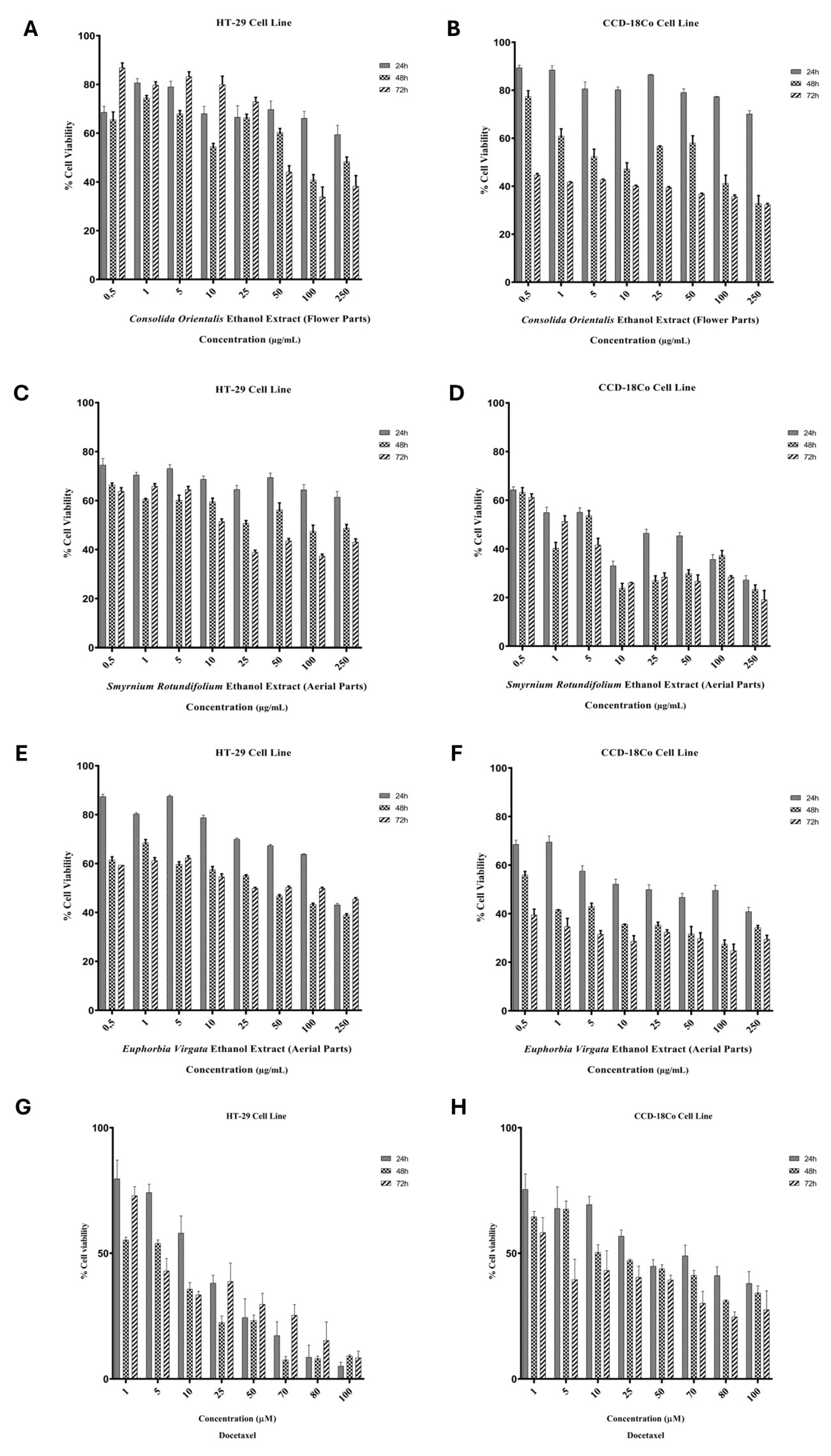
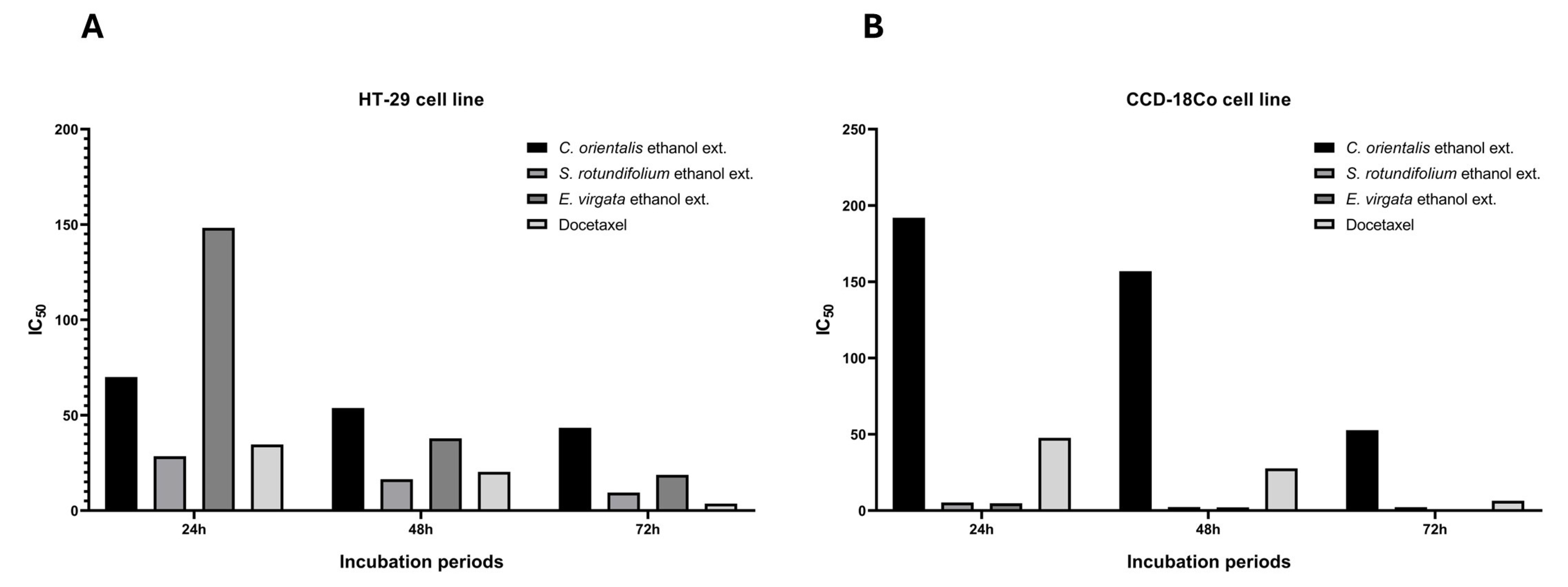



| Plant Extracts | HT-29 | CCD-18Co | ||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| C. orientalis ethanol ext. | 70.00 | 53.82 | 43.43 | 191.90 | 156.90 | 52.67 |
| S. rotundifolium ethanol ext. | 28.48 | 16.47 | 9.44 | 5.34 | 2.35 | 2.25 |
| E. virgata ethanol ext. | 148.30 | 37.85 | 18.75 | 4.80 | 2.13 | 0.32 |
| Docetaxel | 34.68 | 20.31 | 3.70 | 47.67 | 27.69 | 6.44 |
| No | Plant Name | Plant Parts | Phytochemical Name |
|---|---|---|---|
| 1 | C. orientalis | flower | 2,4-Dihydroxy-2H-1,4-benzoxazin-3(4H)-one |
| 2 | C. orientalis | flower | 2-Benzoxazolinone |
| 3 | C. orientalis | flower | 4-Hydroxybenzoic acid |
| 4 | C. orientalis | flower | Chlorogenic acid |
| 5 | C. orientalis | flower | Caffeic acid |
| 6 | C. orientalis | flower | 4-Hydroxycinnamic acid |
| 7 | C. orientalis | seed | Ajadinine |
| 8 | C. orientalis | seed | Ajacine |
| 9 | C. orientalis | seed | Unii-DW4U480C61 |
| 10 | C. orientalis | seed | (1S,2R,3R,4S,5R,6S,8R,9S,10S,13S,16S,17R,18S)-11-ethyl-13-(hydroxymethyl)-4,6,18-trimethoxy-11-azahexacyclo [7.7.2.12,5.01,10.03,8.013,17]nonadecane-8,9,16-triol |
| 11 | C. orientalis | seed | Delsoline |
| 12 | C. orientalis | seed | (1S,2R,3R,4S,5S,6S,8R,9S,10S,13S,16S,17R,18S)-11-ethyl-6,18-dimethoxy-13-(methoxymethyl)-11-azahexacyclo [7.7.2.12,5.01,10.03,8.013,17]nonadecane-4,8,9,16-tetrol |
| 13 | C. orientalis | NA | (1S,2R,3R,4S,5R,6S,8R,9S,10S,13S,16S,17R,18S)-11-ethyl-13-(hydroxymethyl)-4,6,18-trimethoxy-11-azahexacyclo [7.7.2.12,5.01,10.03,8.013,17]nonadecane-8,9,16-triol |
| 14 | C. orientalis | NA | 18-hydroxy-14-O-methylgadesine |
| EHOMO (eV) | ELUMO (eV) | χ | η | ω1 | ω2 | Dipole Moment (Debye) | α (a.u) | |
|---|---|---|---|---|---|---|---|---|
| 4-Hydroxycinnamic acid | −6.235 | −2.135 | 4.185 | 4.100 | 2.136 | 3.247 | 5.56 | 202.811 |
| Caffeic acid | −6.112 | −2.159 | 4.136 | 3.953 | 2.164 | 3.338 | 5.07 | 211.760 |
| Chlorogenic acid | −6.054 | −2.088 | 4.071 | 3.966 | 2.089 | 3.187 | 4.38 | 350.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sönmez Gürer, E.; Tunçbilek, Z.; Zontul, C.; Kutlay, A.; Kumar, A.; Jhaa, G. Evaluation and DFT Analysis of In Vitro Anticancer Activity of Consolida orientalis, Smyrnium rotundifolium, and Euphorbia virgata Plant Extracts in Colorectal Cancer. Pharmaceuticals 2025, 18, 943. https://doi.org/10.3390/ph18070943
Sönmez Gürer E, Tunçbilek Z, Zontul C, Kutlay A, Kumar A, Jhaa G. Evaluation and DFT Analysis of In Vitro Anticancer Activity of Consolida orientalis, Smyrnium rotundifolium, and Euphorbia virgata Plant Extracts in Colorectal Cancer. Pharmaceuticals. 2025; 18(7):943. https://doi.org/10.3390/ph18070943
Chicago/Turabian StyleSönmez Gürer, Eda, Zuhal Tunçbilek, Cemile Zontul, Ahu Kutlay, Amrendra Kumar, and Gaurav Jhaa. 2025. "Evaluation and DFT Analysis of In Vitro Anticancer Activity of Consolida orientalis, Smyrnium rotundifolium, and Euphorbia virgata Plant Extracts in Colorectal Cancer" Pharmaceuticals 18, no. 7: 943. https://doi.org/10.3390/ph18070943
APA StyleSönmez Gürer, E., Tunçbilek, Z., Zontul, C., Kutlay, A., Kumar, A., & Jhaa, G. (2025). Evaluation and DFT Analysis of In Vitro Anticancer Activity of Consolida orientalis, Smyrnium rotundifolium, and Euphorbia virgata Plant Extracts in Colorectal Cancer. Pharmaceuticals, 18(7), 943. https://doi.org/10.3390/ph18070943







