Water-Soluble Formulations of Curcumin and Eugenol Produced by Spray Drying
Abstract
1. Introduction
2. Results
2.1. Effect of Solvent on Polymer Aggregation
2.2. Preparation and Optimization of Curcumin-Loaded Nanoparticles and Spray-Dried Powders
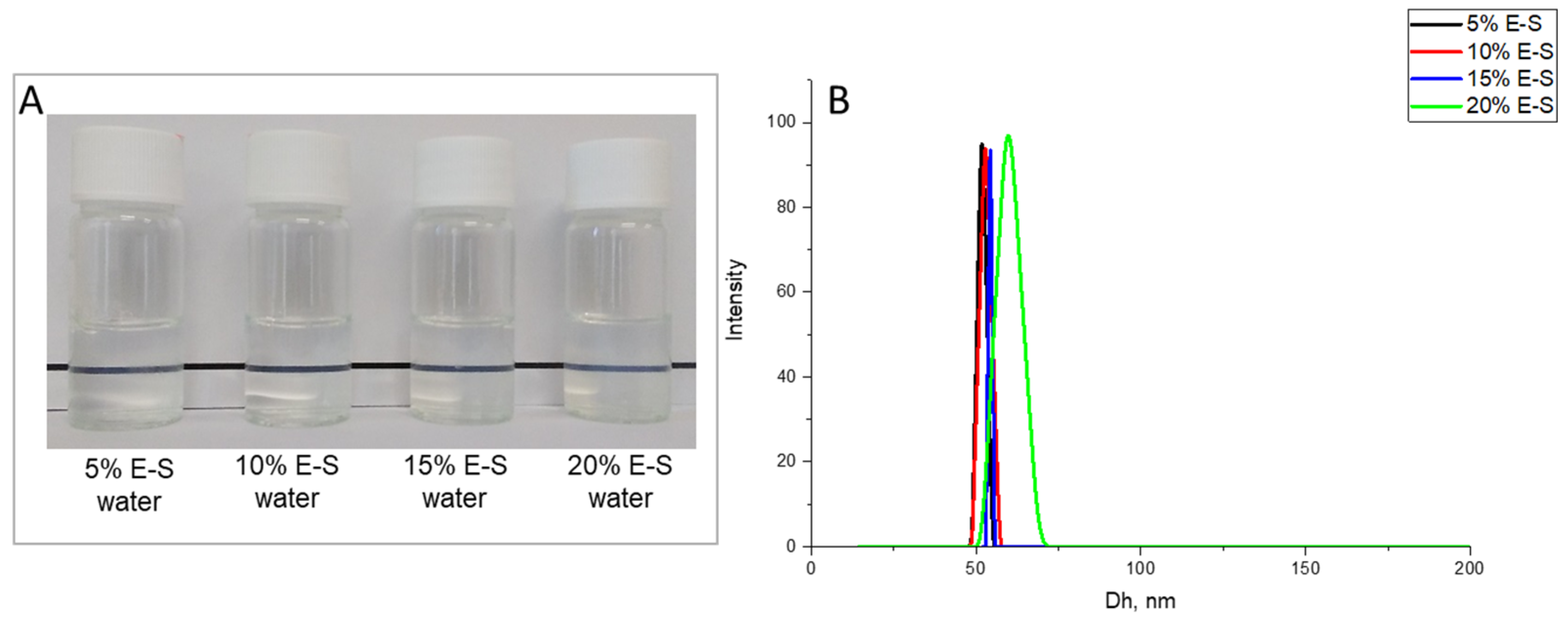
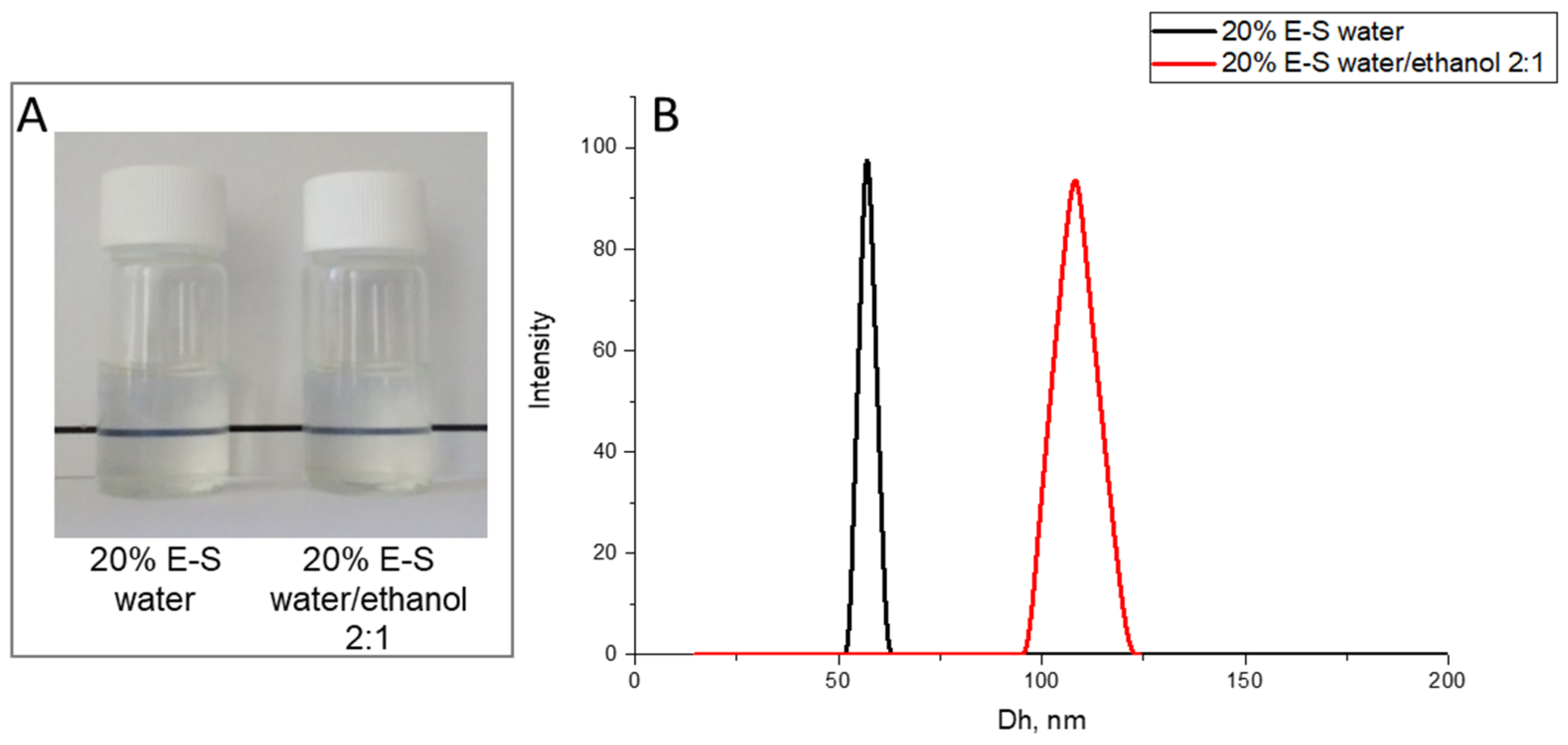
2.3. Preparation and Optimization of Eugenol-Loaded Nanoparticles and Spray-Dried Powders
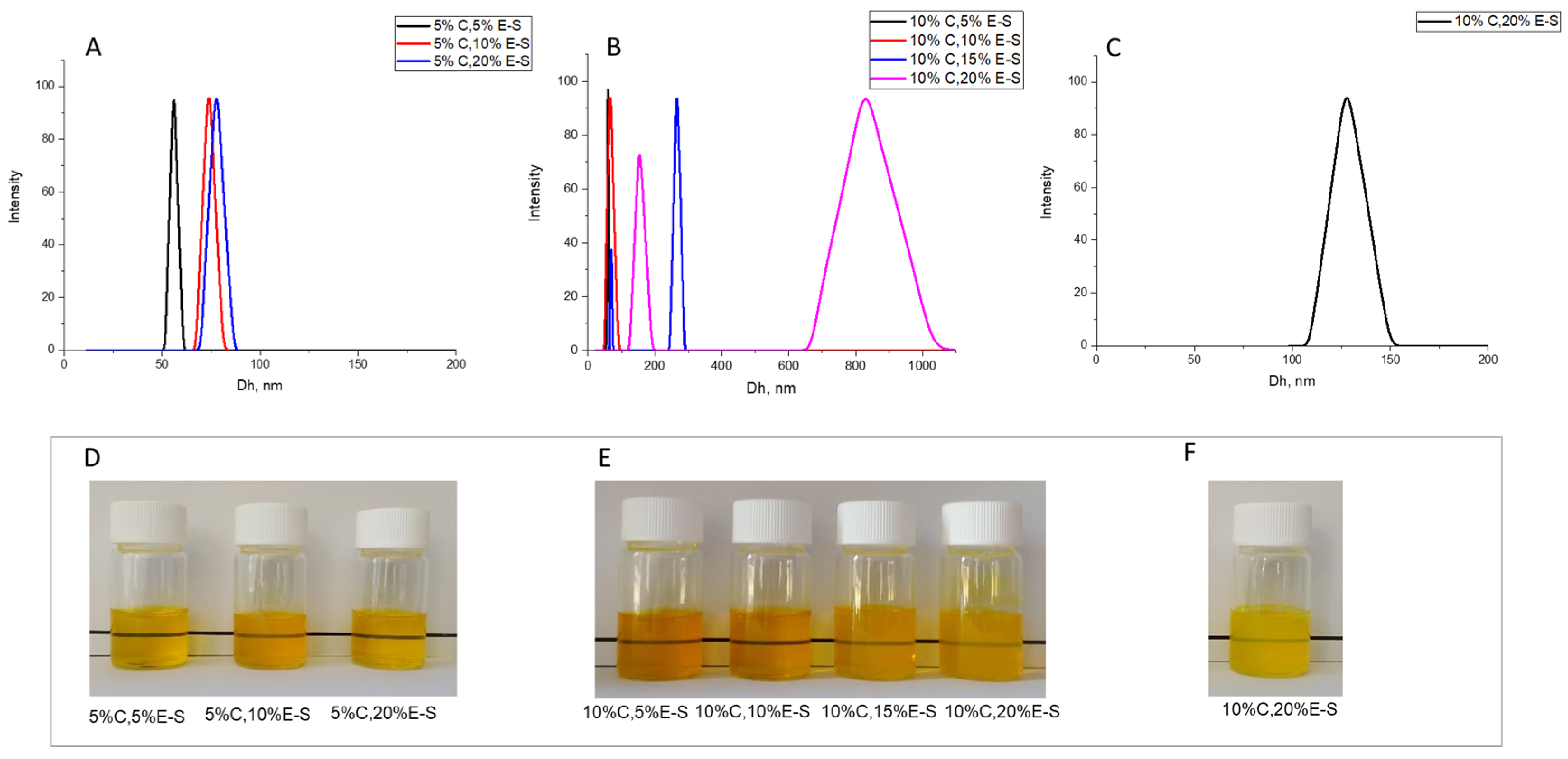
2.4. Preparation and Optimization of Curcumin/Eugenol Double-Loaded Nanoparticles and Spray-Dried Powders
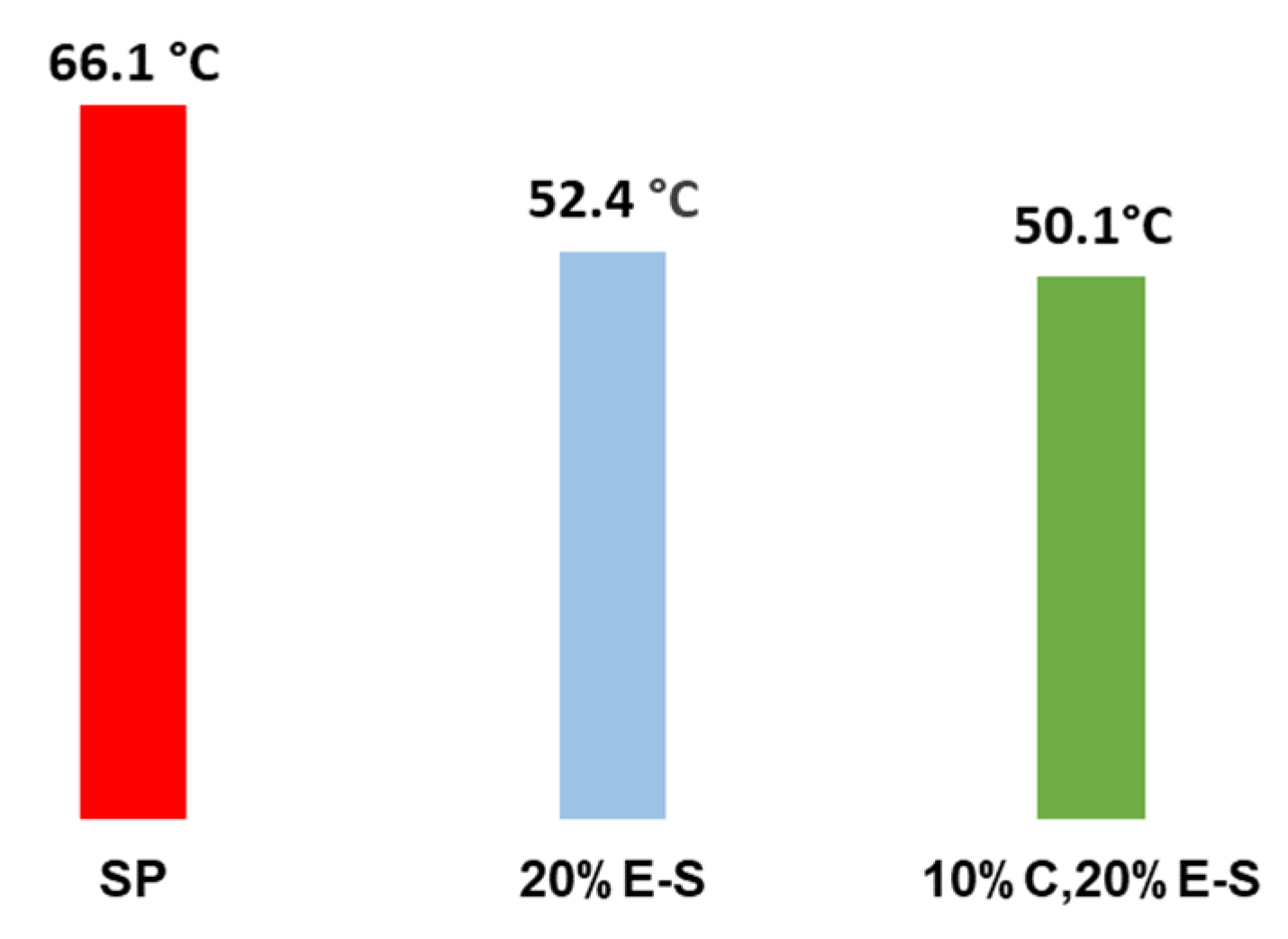
2.5. Appearance and Morphology of the Spray-Dried Powders
2.6. Encapsulation Efficiency and Loading Capacity
2.7. FTIR Analysis
2.8. Differential Scanning Calorimetry Analysis
2.9. X-Ray Analysis
2.10. Dissolving Properties of the Spray-Dried Powders
3. Discussion
3.1. Experimental Results
3.2. Green Technology and Process Optimization
3.3. Implications for Sustainable Manufacturing
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Feed Solutions for Spray Drying
4.2.1. Preparation of Curcumin-Loaded Dispersions for Spray Drying (Summarized in Supplementary Materials, Table S1)
- 2:1 water/ethanol ratio
- 5:1 water/ethanol ratio
4.2.2. Preparation of Eugenol-Loaded Dispersions for Spray Drying
4.2.3. Preparation of Double-Loaded Curcumin/Eugenol Dispersions for Spray Drying
4.3. Spray Drying Conditions
4.4. Particle Size in the Water Solution
4.5. Scanning Electron Microscopy
4.6. Assay of the Encapsulated Curcumin and Eugenol (Encapsulation Efficiency and Loading Capacity)
4.7. Fourier-Transform Infrared Spectroscopy
4.8. Differential Scanning Calorimetry
4.9. X-Ray Diffraction
4.10. Dissolution of Curcumin and Eugenol from the Spray-Dried Powders
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khopde, S.M.; Priyadarsini, K.I.; Venkatesan, P.; Rao, M.N. Free radical scavenging ability and antioxidant efficiency of curcumin and its substituted analogue. Bhiophys. Chem. 1999, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Maekawa, T.; Hidaka, K.; Bando, H.; Takeda, Y.; Yamaguchi, H. Chemical studies on antioxidant mechanism of curcumin: Analysis of oxidative coupling products from curcumin and linoleate. J. Agric. Food Chem. 2001, 49, 2539–2547. [Google Scholar] [CrossRef]
- Ruby, A.J.; Kuttan, G.; Babu, K.D.; Rajasekharan, K.N.; Kuttan, R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef]
- Sharma, O. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol. 1976, 5, 1811–1812. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food. 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Rifkind, J.M.; Boindala, S.; Nakka, L. Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol. Biol. 2010, 610, 165–180. [Google Scholar] [CrossRef]
- Satoh, K.; Ida, Y.; Sakagami, H.; Tanaka, T.; Fusisawa, S. Effect of antioxidants on radical intensity and cytotoxic activity of eugenol. Anticancer Res. 1998, 18, 1549–1552. [Google Scholar]
- Ito, M.; Murakami, K.; Yoshino, M. Antioxidant action of eugenol: Role of metal ion in the inhibition of lipid peroxidation. Food Chem. Toxicol. 2005, 43, 461–466. [Google Scholar] [CrossRef]
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A Natural Compound with Versatile Pharmacological Actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Srimal, R.C.; Dhawan, B.N. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef]
- Barboza, J.N.; Da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-inflammatory Potential and Antioxidant Profile of Eugenol. Oxidative Med. Cell. Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Khan, S.; Imran, M.; Butt, T.T.; Shah, S.W.A.; Sohail, M.; Malik, A.; Das, S.; Thu, H.E.; Adam, A.; Hussain, Z. Curcumin based nanomedicines as efficient nanoplatform for treatment of cancer: New developments in reversing cancer drug resistance, rapid internalization, and improved anticancer efficacy. Trends Food Sci. Technol. 2018, 80, 8–22. [Google Scholar] [CrossRef]
- Padhy, I.; Paul, P.; Sharma, T.; Banerjee, S.; Mondal, A. Molecular Mechanisms of Action of Eugenol in Cancer: Recent Trends and Advancement. Life 2022, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.N.; Ray, A.S.; Rahaman, C.H. A comprehensive and systematic review on potential anticancer activities of eugenol: From pre-clinical evidence to molecular mechanisms of action. Phytomedicine 2022, 107, 154456. [Google Scholar] [CrossRef]
- Fangjun, L.; Zhijia, Y. Tumor suppressive roles of eugenol in human lung cancer cells. Thorac. Cancer 2018, 9, 25–29. [Google Scholar] [CrossRef]
- Lestari, M.L.; Indrayanto, G. Chapter Three—Curcumin. In Profiles of Drug Substances, Excipients, and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 39, pp. 113–204. [Google Scholar] [CrossRef]
- Dable-Tupas, G.; Tulika, V.; Jain, V.; Maheshwari, K.; Brakad, D.D.; Naresh, P.N.; Suruthimeenakshi, S. 11—Bioactive compounds of nutrigenomic importance. In Role of Nutrigenomics in Modern-Day Healthcare and Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 301–342. [Google Scholar] [CrossRef]
- Cortés Rojas, D.F.; de Souza, C.R.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Abdelsalam, M.M. Solitary and Synergistic Influences of Eugenol and Curcumin against Asthma in Experimental Model. Int. J. Clin. Exp. Physiol. 2021, 8, 75–80. [Google Scholar] [CrossRef]
- Setiawan, P.Y.B.; Kertia, N.; Nurrochmad, A.; Wahyuono, S. Curcumin in combination: Review of synergistic effects and mechanisms in the treatment of inflammation. J. Appl. Pharm. Sci. 2021, 11, 001–011. [Google Scholar] [CrossRef]
- Farhoudi, L.; Kesharwani, P.; Majeed, M.; Johnston, T.P.; Sahebkar, A. Polymeric nanomicelles of curcumin: Potential applications in cancer. Int. J. Pharm. 2022, 617, 121622. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Kamenova, K.; Perperieva, T.; Hadjimitova, V.; Donchev, P.; Kaloyanov, K.; Konstantinov, S.; Kondeva-Burdina, M.; Tzankova, V.; Petrov, P. Cationic triblock copolymer micelles enhance antioxidant activity, intracellular uptake and cytotoxicity of curcumin. Int. J. Pharm. 2015, 490, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Koleva, I.Z.; Tzachev, C.T. Efficient Improvement of Eugenol Water Solubility by Spray Drying Encapsulation in Soluplus® and Lutrol F 127. Pharmaceuticals 2024, 17, 1156. [Google Scholar] [CrossRef]
- Attia, M.S.; Elshahat, A.; Hamdy, A.; Fathi, A.M.; Emad-Eldin, M.; Ghazy, F.-E.S.; Chopra, H.; Ibrahim, T.M. Soluplus® as a solubilizing excipient for poorly water-soluble drugs: Recent advances in formulation strategies and pharmaceutical product features. J. Drug Deliv. Sci. Technol. 2023, 84, 104519. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, J.; Shu, L.; Tan, X.; An, Y.; Yang, X.; Wang, D.; Gao, Q. Optimization of spray drying conditions for the green manufacture of γ-aminobutyric acid-rich powder from Lactobacillus brevis fermentation broth. Biochem. Eng. J. 2020, 156, 107499. [Google Scholar] [CrossRef]
- Broadhead, J.; Edmond Rouan, S.K.; Rhodes, C.T. The Spray Drying of Pharmaceuticals. Drug Dev. Ind. Pharm. 1992, 18, 1169–1206. [Google Scholar] [CrossRef]
- Vehringa, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle formation in spray drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Tran, P.; Pyo, Y.C.; Kim, D.H.; Lee, S.E.; Kim, J.K.; Park, J.S. Overview of the Manufacturing Methods of Solid Dispersion Technology for Improving the Solubility of Poorly Water-Soluble Drugs and Application to Anticancer Drugs. Pharmaceutics 2019, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Ziaeea, A.; Albadarina, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1021. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid. Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Klojdová, I.; Milota, T.; Smetanová, J.; Stathopoulos, C. Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds? Pharmaceuticals 2023, 16, 362. [Google Scholar] [CrossRef]
- Kamal, M.M.; Salawi, A.; Lam, M.; Nokhodchi, A.; Abu-Fayyad, A.; El Sayed, K.A.; Nazzal, S. Development and characterization of curcumin-loaded solid self-emulsifying drug delivery system (SEDDS) by spray drying using Soluplus® as solid carrier. Powder Technol. 2020, 369, 137–145. [Google Scholar] [CrossRef]
- Guo, J.; Li, P.; Kong, L.; Xu, B. Microencapsulation of curcumin by spray drying and freeze drying. LWT 2020, 132, 109892. [Google Scholar] [CrossRef]
- Chen, F.; Liu, L.; Tang, C. Spray-drying microencapsulation of curcumin nanocomplexes with soy protein isolate: Encapsulation, water dispersion, bioaccessibility and bioactivities of curcumin. Food Hydrocoll. 2020, 105, 105821. [Google Scholar] [CrossRef]
- Bucurescu, A.; Blaga, A.C.; Estevinho, B.N.; Rocha, F. Microencapsulation of Curcumin by a Spray-Drying Technique Using Gum Arabic as Encapsulating Agent and Release Studies. Food Bioprocess Technol. 2018, 11, 1795–1806. [Google Scholar] [CrossRef]
- Lucas, J.; Ralaivao, M.; Estevinho, B.N.; Fernando, R. A new approach for the microencapsulation of curcumin by a spray drying method, in order to value food products. Powder Technol. 2020, 362, 428–435. [Google Scholar] [CrossRef]
- Patel, S.S.; Pushpadass, H.A.; Franklin, M.E.E.; Battula, S.N.; Vellingiri, P. Microencapsulation of curcumin by spray drying: Characterization and fortification of milk. J. Food Sci. Technol. 2022, 59, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Al-Akayleh, F.; Al-Naji, I.; Adwan, S.; Al-Remawi, M.; Shubair, M. Enhancement of Curcumin Solubility Using a Novel Solubilizing Polymer Soluplus®. J. Pharm. Innov. 2022, 17, 142–154. [Google Scholar] [CrossRef]
- Homayouni, A.; Amini, M.; Sohrabi, M.; Varshosaz, J.; Nokhodchi, A. Curcumin nanoparticles containing poloxamer or soluplus tailored by high pressure homogenization using antisolvent crystallization. Int. J. Pharm. 2019, 562, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Lin, X.; Yu, E.; Dian, C.; Yan, X.; Li, L.; Zhang, M.; Zhao, W.; Dian, L.J. Curcumin-Loaded Mixed Micelles: Preparation, Characterization, and In Vitro Antitumor Activity. J. Nanotechnol. 2018, 2018, 22. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Manzoor, H.; Khan, I.U.; Asghar, S.; Irfan, M.; Albekairi, N.A.; Alshammari, A.; Alqahtani, A.F.; Alotaibi, S.; Munir, R.; et al. Curcumin-loaded soluplus® based ternary solid dispersions with enhanced solubility, dissolution and antibacterial, antioxidant, antiinflammatory activities. Heliyon 2024, 10, e34636. [Google Scholar] [CrossRef]
- Rani, S.; Mishra, S.; Sharma, M.; Nandy, A.; Mozumdar, S. Solubility and stability enhancement of curcumin in Soluplus® polymeric micelles: A spectroscopic study. J. Disper. Sci. Technol. 2020, 41, 523–536. [Google Scholar] [CrossRef]
- Caballero-Román, A.; Nardi-Ricart, A.; Vila, R.; Cañigueral, S.; Ticó, J.R.; Miñarro, M. Use of Natural Polymers for the Encapsulation of Eugenol by Spray Drying. Pharmaceutics 2024, 16, 1251. [Google Scholar] [CrossRef]
- Talón, E.; Lampi, A.-M.; Vargas, M.; Chiralt, A.; Jouppila, K.; González-Martínez, C. Encapsulation of eugenol by spray-drying using whey protein isolate or lecithin: Release kinetics, antioxidant and antimicrobial properties. Food Chem. 2019, 295, 588–598. [Google Scholar] [CrossRef]
- Bittencourt, P.R.S.; dos Santos da Veiga, R.; da Silva-Buzanello, R.A.; Scremin, F.R.; Becker-Algeri, T.A.; Corso, M.P.; de Moraes Flores, É.L.; Canan, C. Optimization of eugenol-loaded microcapsules obtained by spray drying using a simplex-centroid mixture design. Quim. Nova 2022, 45, 152–158. [Google Scholar] [CrossRef]
- Ridwan, P.R.M.; Rehan, D.; Usama, K.; Saifali, T.; Ansari, H.; Musa, S.M.; Nawaz, M.S.; Aaquil, M. Formulation and evaluation of curcumin and eugenol nanoemulsion in-situ mucoadhesive gel. Biochem. Cell. Arch. 2024, 24, 2639–2646. [Google Scholar] [CrossRef]
- Ridwan, P.R.M.; Rehan, D.; Bhura, M.R.G.; Gomase, P.; Patil, S.G.; Ansari, H.; Saifali, T.; Ahmad, I.; Kalal, V.V.; Band, A.; et al. Preformulation of curcumin and eugenol nanoemulsion in-situ mucoadhesive gel. Biochem. Cell. Arch. 2024, 24, 3123–3131. [Google Scholar] [CrossRef]
- Ferreira, M.P.A.; Martins, J.P.; Hirvonen, J.; Santos, H.A. Chapter 9—Spray-drying for the formulation of oral drug delivery systems. In Nanotechnology for Oral Drug Delivery; Martins, J.P., Hélder, A.S., Eds.; Academic Press: Brookline, MA, USA, 2020; pp. 253–284. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- BASF, Soluplus® Technical Information (03_90801e-06). Available online: https://pharma.basf.com/products/soluplus (accessed on 10 January 2024).
- Mazár, J.; Albert, K.; Kovács, Z.; Koris, A.; Nath, A.; Bánvölgyi, S. Advances in Spray-Drying and Freeze-Drying Technologies for the Microencapsulation of Instant Tea and Herbal Powders: The Role of Wall Materials. Foods 2025, 14, 486. [Google Scholar] [CrossRef]
- Solghi, S.; Emam-Djomeh, Z.; Fathi, M.; Farahani, F. The Encapsulation of Curcumin by Whey Protein: Assessment of the Stability and Bioactivity. J. Food Process Eng. 2020, 43, e13403. [Google Scholar] [CrossRef]
- Wijiani, N.; Isadiartuti, D.; Rijal, M.A.S.; Yusuf, H. Characterization and Dissolution Study of Micellar Curcumin-Spray Dried Powder for Oral Delivery. Int. J. Nanomed. 2020, 15, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Fei, Y.; Wang, Y.; Lin, Q.; Ke, Q.; Feng, G.; Xu, L. Solubility Improvement of Curcumin by Crystallization Inhibition from Polymeric Surfactants in Amorphous Solid Dispersions. J. Drug Deliv. Sci. Technol. 2023, 83, 104351. [Google Scholar] [CrossRef]
- Alopaeus, J.F.; Hagesæther, E.; Tho, I. Micellisation Mechanism and Behaviour of Soluplus–Furosemide Micelles: Preformulation Studies of an Oral Nanocarrier-Based System. Pharmaceuticals 2019, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Magid, L. Solvent Effects on Amphiphilic Aggregation. In Solution Chemistry of Surfactants; Mittal, K.L., Ed.; Springer: Boston, MA, USA, 1979. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Majeed, H.; Bian, Y.-Y.; Ali, B.; Jamil, A.; Majeed, U.; Khan, Q.F.; Iqbal, K.J.; Shoemaker, C.F.; Fang, Z. Essential oil encapsulations: Uses, procedures, and trends. RSC Adv. 2015, 5, 58449–58463. [Google Scholar] [CrossRef]
- Homayouni, A.; Sadeghi, F.; Nokhodchi, A.; Varshosaz, J.; Garekani, H.A. Preparation and Characterization of Celecoxib Dispersions in Soluplus®: Comparison of Spray Drying and Conventional Methods. Iran. J. Pharm. Res. 2015, 14, 35–50. [Google Scholar]
- Fang, Z.; Bhandari, B. Spray Drying of Bioactives. In Engineering Foods for Bioactives Stability and Delivery; Roos, Y., Livney, Y., Eds.; Food Engineering Series; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Shah, D.S.; Moravkar, K.K.; Jha, D.K.; Lonkar, V.; Amin, P.D.; Chalik, S.S. A concise summary of powder processing methodologies for flow enhancement. Heliyon 2023, 9, e16498. [Google Scholar] [CrossRef] [PubMed]
- Rowley, G.; Mackin, L.A. The effect of moisture sorption on electrostatic charging of selected pharmaceutical excipient powders. Powder Technol. 2003, 135–136, 50–58. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Chen, W. Trends of spray drying: A critical review on drying of fruit and vegetable juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Huyge, K.; Van Reeth, K.; De Beer, T.; Landman, W.J.M.; van Eck, J.H.H.; Remon, J.P.; Vervaet, C. Suitability of differently formulated dry powder Newcastle disease vaccines for mass vaccination of poultry. Eur. J. Pharm. Biopharm. 2012, 80, 649–656. [Google Scholar] [CrossRef]
- Pinto, J.T.; Faulhammer, E.; Dieplinger, J.; Dekner, M.; Makert, C.; Nieder, M.; Paudel, A. Progress in spray-drying of protein pharmaceuticals: Literature analysis of trends in formulation and process attributes. Dry. Technol. 2021, 39, 1415–1446. [Google Scholar] [CrossRef]













| Formulation | Cur, g | SP, g | H2O:EtOH Ratio | H2O + EtOH, mL | Yield, % | Conc Polymer Solution, % | Dh 1, nm | Zeta Potential 1, mV | DI 1,2 |
|---|---|---|---|---|---|---|---|---|---|
| S | 0.000 | 4 | 1:0 | 60.0 | 27.1 | 6.7 | 54 ± 2 | −3.00 ± 0.8 | 0.06 ± 0.011 |
| 1%C-S | 0.040 | 4 | 1:0 | 60.0 | 26.4 | 6.7 | 53 ± 2 | −3.80 ± 1.1 | 0.09 ± 0.023 |
| 5%C-S | 0.200 | 4 | 1:0 | 60.0 | 22.5 | 6.7 | Precipitation | ||
| 5%C-S | 0.200 | 4 | 2:1 | 60.0 | 24.7 | 6.7 | 57 ± 1 | −4.17 ± 1.3 | 0.07 ± 0.034 |
| 10%C-S | 0.400 | 4 | 1:0 | 60.0 | 25.2 | 6.7 | Precipitation | ||
| 10%C-S | 0.400 | 4 | 0:1 | 60.0 | 52.5 | 6.7 | 74, 204 ± 2 | −5.25 ± 1.8 | 0.24 ± 0.025 |
| 10%C-S | 0.400 | 4 | 2:1 | 60.0 | 31.8 | 6.7 | 90 ± 1 | −6.50 ± 1.5 | 0.10 ± 0.022 |
| 10%C-S | 0.400 | 4 | 2:1 | 120.0 | 56.6 | 3.3 | 100 ± 3 | −2.26 ± 1.4 | 0.18 ± 0.006 |
| 10%C-S | 0.400 | 4 | 5:1 | 120.0 | 52.5 | 3.3 | 62 ± 1 | −5.70 ± 1.9 | 0.08 ± 0.043 |
| Formulation | Eug, g | SP, g | H2O:EtOH Ratio | H2O + EtOH mL | Yield, % | Conc Polymer Solution% | Dh 2, nm | Zeta Potential 2, mV | DI 2,3 |
|---|---|---|---|---|---|---|---|---|---|
| 5%E-S 1 | 0.200 | 4 | 1:0 | 40.0 | 47.1 | 10.0 | 51 ± 3 | −4.6 ± 0.7 | 0.04 ± 0.011 |
| 10%E-S 1 | 0.400 | 4 | 1:0 | 40.0 | 45.9 | 10.0 | 52 ± 3 | −4.5 ± 1.2 | 0.09 ± 0.008 |
| 15%E-S 1 | 0.600 | 4 | 1:0 | 40.0 | 44.1 | 10.0 | 54 ± 2 | −4.3 ± 1.6 | 0.04 ± 0.024 |
| 20%E-S | 0.800 | 4 | 1:0 | 40.0 | 43.4 | 10.0 | 59 ± 3 | −5.7 ± 0.9 | 0.10 ± 0.011 |
| 20%E-S | 0.800 | 4 | 1:0 | 60.0 | 37.0 | 6.7 | 56 ± 2 | −4.9 ± 1.1 | 0.09 ± 0.018 |
| 20%E-S | 0.800 | 4 | 2:1 | 60.0 | 12.2 | 6.7 | 108 ± 6 | −2.9 ± 1.8 | 0.10 ± 0.012 |
| Formulation | Cur, g | Eug, g | SP, g | H2O:EtOH Ratio | H2O + EtOH mL | Yield, % | Conc Polymer Solution% | Dh 1, nm | Zeta Potential 1, mV | DI 1,2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 5%C,5%E-S | 0.200 | 0.200 | 4 | 2:1 | 120.0 | 41.4 | 3.3 | 56 ± 3 | −2.4 ± 1.7 | 0.08 ± 0.008 |
| 5%C,10%E-S | 0.200 | 0.400 | 4 | 2:1 | 120.0 | 37.0 | 3.3 | 73 ± 2 | −3.2 ± 1.4 | 0.12 ± 0.008 |
| 5%C,20%E-S | 0.200 | 0.800 | 4 | 2:1 | 120.0 | 23.4 | 3.3 | 78 ± 1 | −7.2 ± 0.7 | 0.13 ± 0.015 |
| 10%C,5%E-S | 0.400 | 0.200 | 4 | 2:1 | 120.0 | 49.0 | 3.3 | 60 ± 3 | −4.1 ± 1.1 | 0.07 ± 0.017 |
| 10%C,10%E-S | 0.400 | 0.400 | 4 | 2:1 | 120.0 | 46.2 | 3.3 | 66 ± 2 | −5.4 ± 1.3 | 0.14 ± 0.011 |
| 10%C,15%E-S | 0.400 | 0.600 | 4 | 2:1 | 120.0 | 36.7 | 3.3 | 69, 265 ± 4 | −7.7 ± 1.5 | 0.24 ± 0.017 |
| 10%C,20%E-S | 0.400 | 0.800 | 4 | 2:1 | 120.0 | 28.7 | 3.3 | 152,828 ± 3 | −9.6 ± 1.8 | 0.27 ± 0.016 |
| 10%C,20%E-S | 0.400 | 0.800 | 4 | 5:1 | 120.0 | 37.9 | 3.3 | 127 ± 2 | −6.9 ± 1.8 | 0.16 ± 0.029 |
| 5%C,5%E-S-I | 0.200 | 0.200 | 4 | 5:1 | 120.0 | 57.0 | 3.3 | 61 ± 3 | −6.7 ± 1.6 | 0.14 ± 0.018 |
| 10%C,15%E-S-I | 0.400 | 0.600 | 4 | 5:1 | 120.0 | 58.5 | 3.3 | 89 ± 3 | −12.5 ± 2.1 | 0.20 ± 0.010 |
| 10%C,20%E-S-I-A | 0.400 | 0.800 | 4 | 5:1 | 120.0 | 56.3 | 3.3 | 132, 373, 1235 ± 6 | −17.7 ± 2.3 | 0.273 ± 0.009 |
| 10%C,20%E-S-I | 0.400 | 0.800 | 4 | 2:1 | 120.0 | 59.1 | 3.3 | 84, 292 ± 5 | −8.8 ± 2.4 | 0.275 ± 0.013 |
| 10%C,20%E-S-I-A | 0.400 | 0.800 | 4 | 2:1 | 120.0 | 55.7 | 3.3 | 230 ± 2 | −15.4 ± 1.9 | 0.225 ± 0.040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koleva, I.Z.; Kamenova, K.; Petrov, P.D.; Tzachev, C.T. Water-Soluble Formulations of Curcumin and Eugenol Produced by Spray Drying. Pharmaceuticals 2025, 18, 944. https://doi.org/10.3390/ph18070944
Koleva IZ, Kamenova K, Petrov PD, Tzachev CT. Water-Soluble Formulations of Curcumin and Eugenol Produced by Spray Drying. Pharmaceuticals. 2025; 18(7):944. https://doi.org/10.3390/ph18070944
Chicago/Turabian StyleKoleva, Iskra Z., Katya Kamenova, Petar D. Petrov, and Christo T. Tzachev. 2025. "Water-Soluble Formulations of Curcumin and Eugenol Produced by Spray Drying" Pharmaceuticals 18, no. 7: 944. https://doi.org/10.3390/ph18070944
APA StyleKoleva, I. Z., Kamenova, K., Petrov, P. D., & Tzachev, C. T. (2025). Water-Soluble Formulations of Curcumin and Eugenol Produced by Spray Drying. Pharmaceuticals, 18(7), 944. https://doi.org/10.3390/ph18070944








