Toosendanin Induces Hepatotoxicity by Facilitating ALOX5-Mediated Lipid Peroxidation and Sensitizing Cells to Ferroptosis
Abstract
1. Introduction
2. Results
2.1. TSN Induces Hepatotoxicity via Ferroptosis In Vitro
2.2. TSN Induces Ferroptosis In Vivo
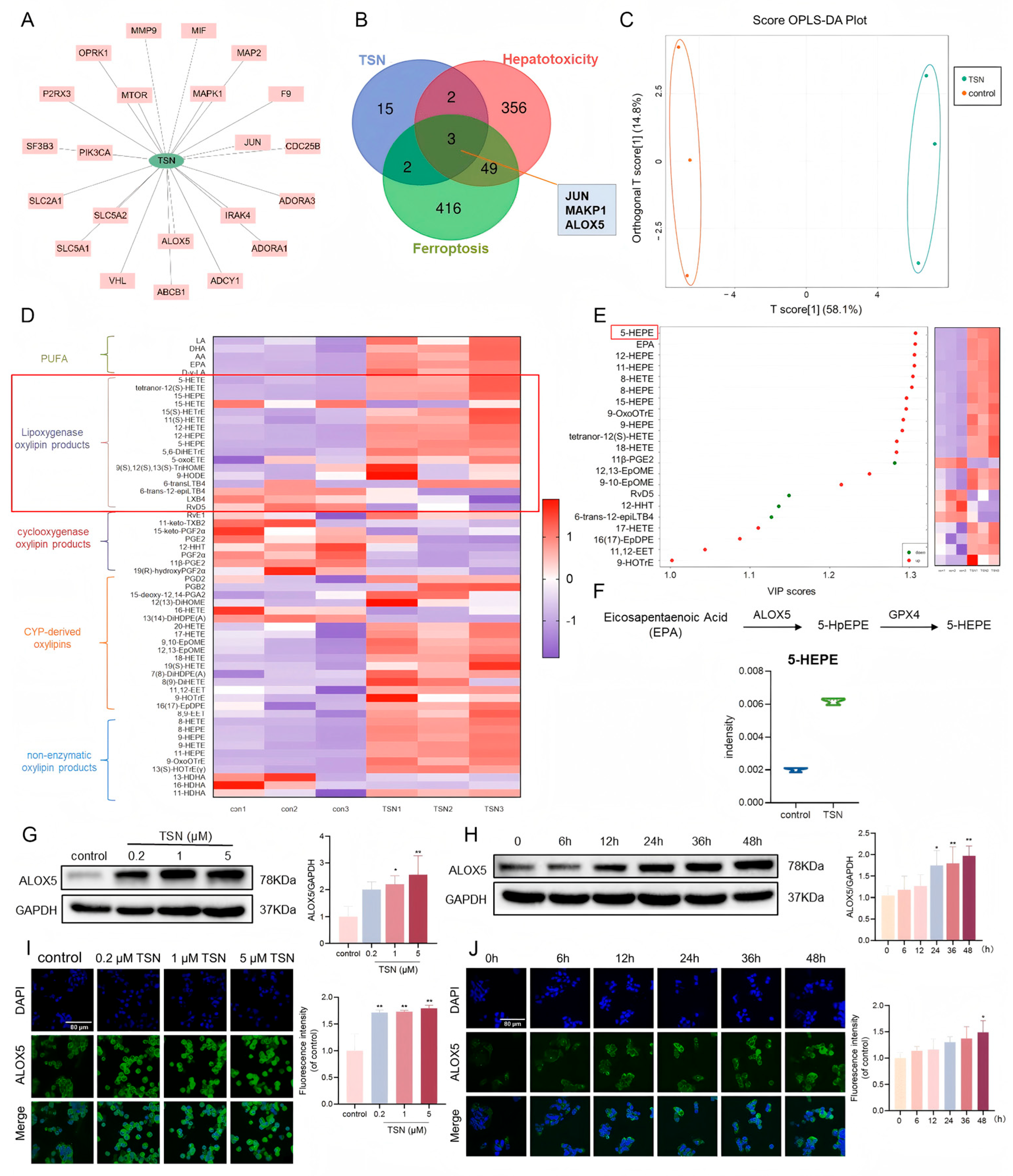
2.3. TSN Promotes the Expression of ALOX5 and Its Downstream Metabolites In Vitro
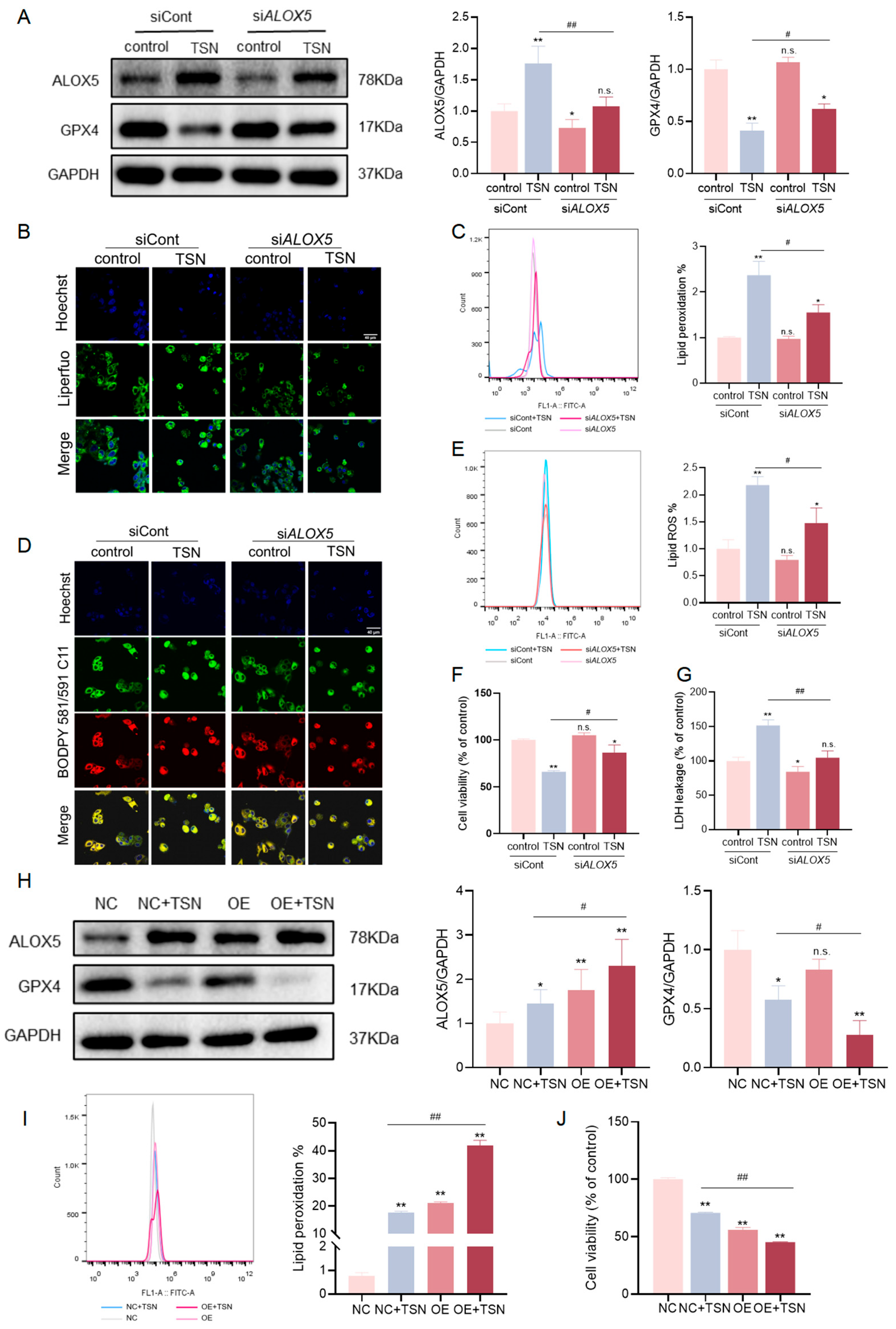
2.4. The Level of ALOX5 Affects the Sensitivity of TSN-Induced Cell Damage Involving Ferroptosis
2.5. ALOX5 Downregulation Attenuates TSN-Induced Hepatic Injury In Vivo
2.6. ALOX5 Knockdown Protects Against TSN-Induced Ferroptosis In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. HepG2 Cell Culture and Transfection
4.3. Cell Viability and Cytotoxicity Assay
4.4. Western Blotting
4.5. Quantitative Real-Time PCR (RT-qPCR)
4.6. Measurement of Glutathione (GSH)
4.7. Fluorescence Staining
4.8. Animals and Treatment
4.9. Serum Biochemical Assay
4.10. Histopathological Examination
4.11. IHC
4.12. Malondialdehyde (MDA) Assay
4.13. Acquisition of Related Targets
4.14. Component–Target Visualization Network Construction
4.15. Oxidative Lipidomics Analysis
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HEPE | 5-hydroxyeicosapentaenoic acid |
| 5-HPETE | 5-hydroperoxyeicosatetraenoic acid |
| ALOX5 | 5-lipoxygenase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| CCK-8 | Cell Counting Kit-8 |
| DILI | Drug-induced liver injury |
| Era | erastin |
| FMT | Fructus Meliae Toosendan |
| Fer-1 | ferrostatin-1 |
| GPX4 | glutathione peroxidase 4 |
| GSH | glutathione |
| IF | immunofluorescence |
| LDH | lactate dehydrogenase |
| Lip-1 | liproxstatin-1 |
| MDA | malondialdehyde |
| Nec-1 | necrostatin-1 |
| TSN | toosendanin |
| TFRC | transferrin receptor protein 1 |
| VIP | variable importance in projection |
References
- Ge, F.L.; Niu, M.; Han, Z.X.; Cao, J.L.; Wang, J.B.; Bai, Z.F.; Song, H.B.; Guo, Y.M.; Xiao, X.H. Landscape of Hepatobiliary Adverse Drug Reactions Related to Preparations Containing Psoraleae Fructus and Its Application in Pharmacovigilance. Chin. J. Integr. Med. 2021, 27, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ge, F.L.; Tang, J.F.; Qin, S.L.; Zeng, R.; Yao, M.L.; Xiao, X.H.; Bai, Z.F.; Tang, C.L. A review of herb-induced liver injury in mainland china. Front. Pharmacol. 2022, 13, 813073. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, C.; Gong, L.L.; Zhang, Y.H.; Liang, C.L.; Liu, H.Y. An overview of Fructus Meliae Toosendan: Botany, traditional uses, phytochemistry, pharmacology and toxicology. Biomed. Pharmacother. 2023, 157, 113795. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, S.; Shi, D.; Wang, Z.; Yao, X.; Yu, Y.; Xiao, W. Research progress on chemical constituents, pharmacological action and toxicity of Melia toosendan. Chin. Tradit. Herb. Drugs 2020, 51, 4059–4074. [Google Scholar] [CrossRef]
- Zhao, X.P.; Ge, Z.W.; Zhang, Y.F.; Lan, X.H.; Zhang, B.L. Study on rapid screening and identifying hepatotoxic compounds in Toosendan Fructus. Zhongguo Zhongyao Zazhi 2013, 38, 1820–1822. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Lu, J.; Chen, X.; Wang, Y. The pharmacological effect and its underlying mcchanisms of toosendanin: A review. Pharmacol. Clin. Chin. Mater. Medica 2016, 32, 161–164. [Google Scholar] [CrossRef]
- Jin, Y.H.; Kwon, S.; Choi, J.G.; Cho, W.K.; Lee, B.; Ma, J.Y. Toosendanin From Melia Fructus Suppresses Influenza A Virus Infection by Altering Nuclear Localization of Viral Polymerase PA Protein. Front. Pharmacol. 2019, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, L.; Wang, Z.R.; Li, Z.; Ma, J. Anti-cancer effect of toosendanin and its underlying mechanisms. J. Asian Nat. Prod. Res. 2019, 21, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Huang, Z.-L.; Li, L.; Yang, Y.; Wang, C.-H.; Wang, Z.-T.; Ji, L.-L. Quercetin attenuates toosendanin-induced hepatotoxicity through inducing the Nrf2/GCL/GSH antioxidant signaling pathway. Acta Pharmacol. Sin. 2018, 40, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liang, Y.; Fu, Y.; Liang, Z.; Zheng, J.; Lan, J.; Shen, F.; Huang, Z. Toosendanin Induces Hepatocyte Damage by Inhibiting Autophagic Flux via TFEB-Mediated Lysosomal Dysfunction. Pharmaceuticals 2022, 15, 1509. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ni, J.; Luo, L.; Lin, J.; Peng, H.; Shen, F.; Huang, Z. Toosendanin induces hepatotoxicity via disrupting LXRα/Lipin1/SREBP1 mediated lipid metabolism. Food Chem. Toxicol. 2024, 187, 114631. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Jin, X.; Xiao, Q.A.; Jiang, W.; Han, S.; Chao, J.; Zhang, D.; Xia, X.; Wang, D. Ferroptosis: Action and mechanism of chemical/drug-induced liver injury. Drug Chem. Toxicol. 2024, 47, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, S.; Han, S.; Luo, L.; Shen, F.; Huang, Z. Toosendanin induced hepatotoxicity via triggering PERK-eIF2α-ATF4 mediated ferroptosis. Toxicol. Lett. 2023, 377, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, B.J. EPR Spectroscopic Studies of Lipoxygenases. Chem. Asian J. 2020, 15, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; Barros, P.d.L.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef] [PubMed]
- Haeggström, J.Z.; Funk, C.D. Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem. Rev. 2011, 111, 5866–5898. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, M.; Huang, Z.H.; Wang, M.; Sun, W.Y.; Kurihara, H.; Huang, R.T.; Wang, R.; Huang, F.; Liang, L.; et al. ALOX5 inhibition protects against dopaminergic neurons undergoing ferroptosis. Pharmacol. Res. 2023, 193, 106779. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Ren, L.; Liu, Q.; Kuang, J.; Shen, J.; Cheng, S.; Zhang, Y.; Jiang, W.; Zhang, Z.; Jiang, C.; et al. Loss of 5-lipoxygenase activity protects mice against paracetamol-induced liver toxicity. Br. J. Pharmacol. 2016, 173, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiao, Q.; Zhao, H.; Zhang, J.; Yang, C.; Zou, Y.; Zhang, B.; Liu, J.; Sun, H.; Liu, H. Schisanhenol ameliorates non-alcoholic fatty liver disease via inhibiting miR-802 activation of AMPK-mediated modulation of hepatic lipid metabolism. Acta Pharm. Sin. B 2024, 14, 3949–3963. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Seiler, A.; Schneider, M.; Förster, H.; Roth, S.; Wirth, E.K.; Culmsee, C.; Plesnila, N.; Kremmer, E.; Rådmark, O.; Wurst, W.; et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Fukuhara, A.; Shin, J.; Hayakawa, T.; Otsuki, M.; Shimomura, I. Eicosapentaenoic acid and 5-HEPE enhance macrophage-mediated Treg induction in mice. Sci. Rep. 2017, 7, 4560. [Google Scholar] [CrossRef] [PubMed]
- Schowalter, D.B.; Himeda, C.L.; Winther, B.L.; Wilson, C.B.; Kay, M.A. Implication of interfering antibody formation and apoptosis as two different mechanisms leading to variable duration of adenovirus-mediated transgene expression in immune-competent mice. J. Virol. 1999, 73, 4755–4766. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Yao, W.; Yuan, D.; Zhou, S.; Chen, X.; Zhang, Y.; Li, H.; Xia, Z.; Hei, Z. Brg1-mediated Nrf2/HO-1 pathway activation alleviates hepatic ischemia-reperfusion injury. Cell Death Dis. 2017, 8, e2841. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Hamosh, A. Searching Online Mendelian Inheritance in Man (OMIM): A Knowledgebase of Human Genes and Genetic Phenotypes. Curr. Protoc. Bioinform. 2017, 58, 1.2.1–1.2.12. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, J.; Huang, L.; Tian, Y.; Zhao, C.; Zhou, Z.; Shen, F.; Huang, Z. Toosendanin Induces Hepatotoxicity by Facilitating ALOX5-Mediated Lipid Peroxidation and Sensitizing Cells to Ferroptosis. Pharmaceuticals 2025, 18, 1078. https://doi.org/10.3390/ph18071078
Ni J, Huang L, Tian Y, Zhao C, Zhou Z, Shen F, Huang Z. Toosendanin Induces Hepatotoxicity by Facilitating ALOX5-Mediated Lipid Peroxidation and Sensitizing Cells to Ferroptosis. Pharmaceuticals. 2025; 18(7):1078. https://doi.org/10.3390/ph18071078
Chicago/Turabian StyleNi, Jiajie, Liru Huang, Yifan Tian, Changxin Zhao, Ziyi Zhou, Feihai Shen, and Zhiying Huang. 2025. "Toosendanin Induces Hepatotoxicity by Facilitating ALOX5-Mediated Lipid Peroxidation and Sensitizing Cells to Ferroptosis" Pharmaceuticals 18, no. 7: 1078. https://doi.org/10.3390/ph18071078
APA StyleNi, J., Huang, L., Tian, Y., Zhao, C., Zhou, Z., Shen, F., & Huang, Z. (2025). Toosendanin Induces Hepatotoxicity by Facilitating ALOX5-Mediated Lipid Peroxidation and Sensitizing Cells to Ferroptosis. Pharmaceuticals, 18(7), 1078. https://doi.org/10.3390/ph18071078




