Zebrafish Models of Induced Lymphangiogenesis: Current Advancements and Therapeutic Discovery
Abstract
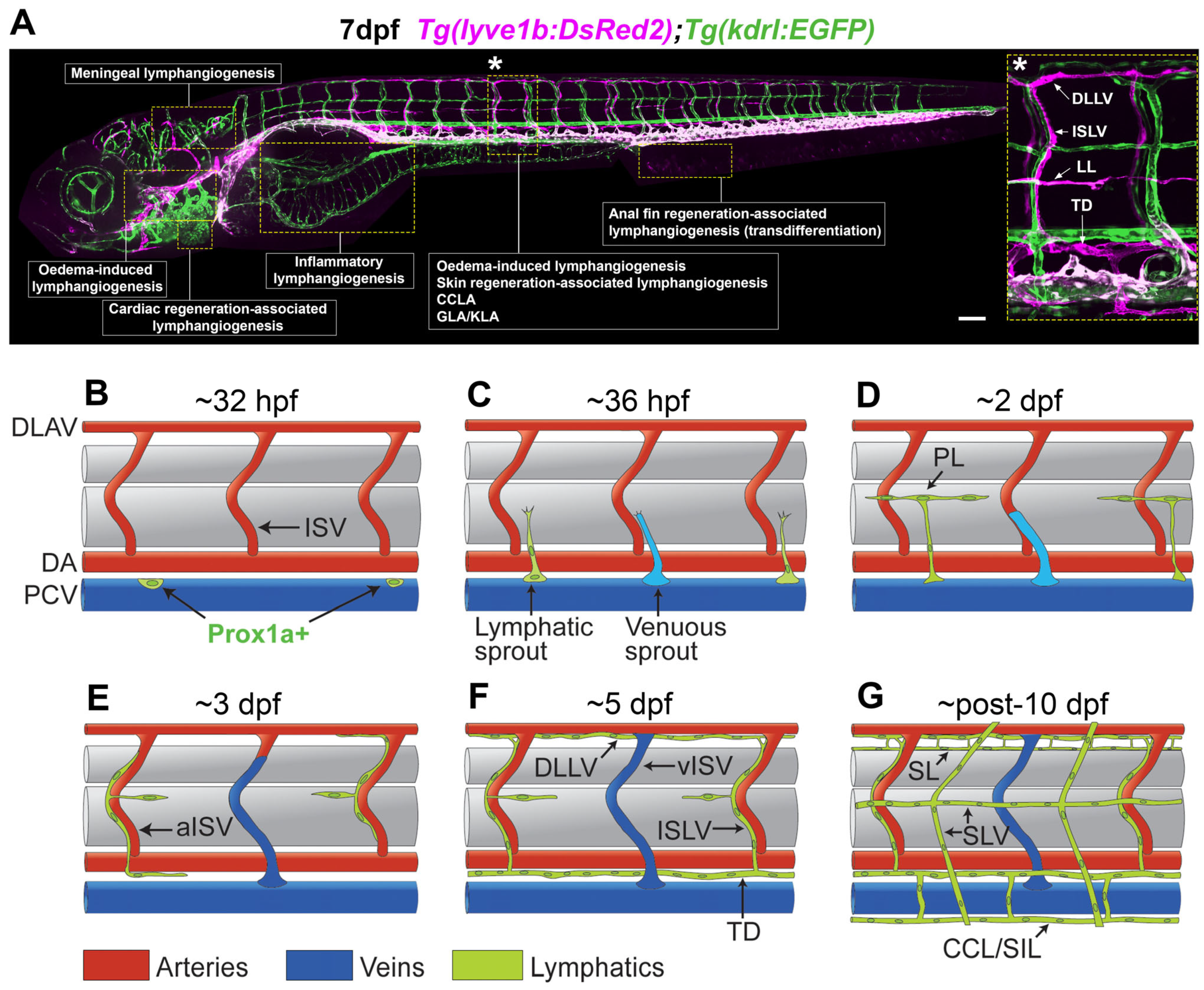
1. Introduction
2. Zebrafish as a Model for Lymphatic Research
3. Cancer, Inflammation, Meningeal, and Oedema-Associated Lymphangiogenesis
4. Tissue Regeneration-Associated Lymphangiogenesis
5. Lymphatic Disease Modelling in Zebrafish
| Drug | Mechanism of Action | Zebrafish Phenotype | Model | Mammalian Translation | Reference |
|---|---|---|---|---|---|
| Cobimetinib | MEK inhibitor | Rescue of ephb4 morpholino-induced vascular phenotypes | ephb4 morpholino-induced CCLA | Not tested | [128] |
| Reduction in oedema | KRAS p.G12D/p.G13D-induced CCLA | Note tested | [30] | ||
| Rescue of TD morphology | ARAF p.S214P-induced CCLA | Not tested | [31] | ||
| BEZ235 | PI3K and mTOR inhibitor | Rescue of epnh4 morpholino-induced vascular phenotypes | ephb4 morpholino induced CCLA | Not tested | [128] |
| Binimetinib | MEK inhibitor | Reduction in oedema | KRAS p.G13D induced CCLA model | Rescues the phenotypes in HDLECs expressing KRAS p.G12D/p.G13D and reduces sprout length in organoid model | [30] |
| AZD8330 | MEK inhibitor | Reduction in oedema | KRAS p.G12D induced CCLA model | Not tested | [30] |
| Pimasertib | MEK inhibitor | Reduction in oedema | KRAS p.G12D induced CCLA model | Not tested | [30] |
| TAK-733 | MEK inhibitor | Reduction in oedema | KRAS p.G12D induced CCLA model | Not tested | [30] |
| Trametinib | MEK inhibitor | Reduction in body swelling, pericardial oedema, and TD dilation | NRAS p.Q61R induced GLA/KLA model | Reduces sprouting of isolated NRAS p.Q61R patient LECs in spheroid-based assay Rescues the phenotypes in ARAF p.S214P expressing HDLECs and was used to treat an ARAF p.S214P-induced CCLA patient. Rescues the phenotypes in HDLECs expressing KRAS p.G12D/p.G13D and reduces sprout length in organoid model | [30,31,129] |
| GSK690693 | AKT inhibitor | Reduces embryo swelling and TD dilation | NRAS p.Q61R induced GLA/KLA model | Reduces sprouting of isolated NRAS p.Q61R patient LECs in spheroid-based assay | [129] |
| Verapamil | Calcium channel blocker | Reduces embryo swelling and TD dilation | NRAS p.Q61R induced GLA/KLA model | No effect on isolated NRAS p.Q61R patient LECs in spheroid-based assay | [129] |
| Cabozantinib | Receptor tyrosine kinase inhibitor (MET, VEGFR2, FLT3, c-KIT) | Reduces embryo swelling and TD dilation | NRAS p.Q61R induced GLA/KLA model | Reduces sprouting of isolated NRAS p.Q61R patient LECs in spheroid-based assay | [129] |
| Kaempferol | VEGFR2/VEGFR3 kinase activity inhibition | Inhibits lymphatic sprouting, resulting in impaired TD formation | Developmental lymphangiogenesis | Inhibits VEGF-C-induced lymphatic growth in a mouse Matrigel plug assay, and reduces tumour-induced lymphangiogenesis and lymph node metastasis in mice | [153] |
| Leflunomide | Dihydroorotate dehydrogenase inhibitor | Inhibits lymphatic sprout migration and morphology, resulting in impaired TD formation | Developmental lymphangiogenesis | Inhibits VEGF-C-induced lymphatic growth in a mouse Matrigel plug assay | [153] |
| A77 1726 (Leflunomide active metabolite) | Dihydroorotate dehydrogenase inhibitor | Reduces number of secondary sprouts, resulting in impaired TD formation | Developmental lymphangiogenesis | Not tested | [153] |
| Cinnarizine | Calcium channel blocker | Impairs TD formation by inhibition of lymphangiogenesis after secondary sprouting | Developmental lymphangiogenesis | Not tested | [153] |
| Flunarizine | Calcium channel blocker | Apoptosis of parachordal lymphatic endothelial cells | Developmental lymphangiogenesis | Inhibits VEGF-C-induced lymphatic growth in a mouse Matrigel plug assay. Ineffective at suppressing tumour-induced lymphangiogenesis and lymph node metastasis in mice | [153] |
| CDF | Inhibition of VEGF-C-induced ERK phosphorylation | Inhibits lymphatic sprouting and migration, leading to impaired lymphatic development. Blocks endothelial cell proliferation in a vegfc-induced model | Developmental lymphangiogenesis | Reduces VEGF-C-induced phospho-ERK levels in HMVEC-dLy-Ad | [154] |
| Toluquinol | Inhibition of VEGF-C-induced phosphorylation of VEGFR3, AKT and ERK | Inhibits TD formation | Developmental lymphangiogenesis | Reduces viability, migration, tube formation, and sprouting of HMVEC-dLy-Ad. Reduces lymphangiogenesis in mouse explanted lymphatic ring, ear sponge, and corneal neovascularization assays | [155] |
6. Anti-Lymphangiogenic Drug Discovery Using Zebrafish
7. Limitations of Using Zebrafish to Model Induced Lymphangiogenesis
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breslin, J.W.; Yang, Y.; Scallan, J.P.; Sweat, R.S.; Adderley, S.P.; Murfee, W.L. Lymphatic Vessel Network Structure and Physiology. Compr. Physiol. 2018, 9, 207–299. [Google Scholar] [CrossRef] [PubMed]
- Bazigou, E.; Wilson, J.T.; Moore, J.E. Primary and Secondary Lymphatic Valve Development: Molecular, Functional and Mechanical Insights. Microvasc. Res. 2014, 96, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef] [PubMed]
- Petrova, T.V.; Koh, G.Y. Biological Functions of Lymphatic Vessels. Science 2020, 369, eaax4063. [Google Scholar] [CrossRef] [PubMed]
- Wigle, J.T.; Oliver, G. Prox1 Function Is Required for the Development of the Murine Lymphatic System. Cell 1999, 98, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Küchler, A.M.; Gjini, E.; Peterson-Maduro, J.; Cancilla, B.; Wolburg, H.; Schulte-Merker, S. Development of the Zebrafish Lymphatic System Requires VEGFC Signaling. Curr. Biol. 2006, 16, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Koltowska, K.; Lagendijk, A.K.; Pichol-Thievend, C.; Fischer, J.C.; Francois, M.; Ober, E.A.; Yap, A.S.; Hogan, B.M. Vegfc Regulates Bipotential Precursor Division and Prox1 Expression to Promote Lymphatic Identity in Zebrafish. Cell Rep. 2015, 13, 1828–1841. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.S.; Escobedo, N.; Yang, Y.; Interiano, A.; Dillard, M.E.; Finkelstein, D.; Mukatira, S.; Gil, H.J.; Nurmi, H.; Alitalo, K.; et al. The Prox1-Vegfr3 Feedback Loop Maintains the Identity and the Number of Lymphatic Endothelial Cell Progenitors. Genes Dev. 2014, 28, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Atri, D.; Eichmann, A.; Simons, M. Endothelial ERK Signaling Controls Lymphatic Fate Specification. J. Clin. Investig. 2013, 123, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, X.; Simons, M. Molecular Controls of Lymphatic VEGFR3 Signaling. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Male, I.; Beane, T.J.; Villefranc, J.A.; Kok, F.O.; Zhu, L.J.; Lawson, N.D. Vegfc Acts through ERK to Induce Sprouting and Differentiation of Trunk Lymphatic Progenitors. Development 2016, 143, 3785–3795. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular Endothelial Growth Factor C Is Required for Sprouting of the First Lymphatic Vessels from Embryonic Veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, K.; Isogai, S.; Castranova, D.; Dye, L.; Hitomi, J.; Weinstein, B.M. Live Imaging of Lymphatic Development in the Zebrafish. Nat. Med. 2006, 12, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.; Schulte, D.; Brice, G.; Simpson, M.A.; Roukens, M.G.; van Impel, A.; Connell, F.; Kalidas, K.; Jeffery, S.; Mortimer, P.S.; et al. Mutation in Vascular Endothelial Growth Factor-C, a Ligand for Vascular Endothelial Growth Factor Receptor-3, Is Associated With Autosomal Dominant Milroy-Like Primary Lymphedema. Circ. Res. 2013, 112, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Allam, O.; Park, K.E.; Chandler, L.; Mozaffari, M.A.; Ahmad, M.; Lu, X.; Alperovich, M. The Impact of Radiation on Lymphedema: A Review of the Literature. Gland Surg. 2020, 9, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Ferrell, R.E.; Lawrence, E.C.; Kimak, M.A.; Levinson, K.L.; McTigue, M.A.; Alitalo, K.; Finegold, D.N. Missense Mutations Interfere with VEGFR-3 Signalling in Primary Lymphoedema. Nat. Genet. 2000, 25, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.A.; Phillips, T.J. Lymphedema: Pathophysiology and Clinical Manifestations. J. Am. Acad. Dermatol. 2017, 77, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G. Advances in Lymphedema. Circ. Res. 2021, 128, 2003–2016. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, T.; Boon, L.M.; Vikkula, M.; Alitalo, K. Lymphatic Malformations: Genetics, Mechanisms and Therapeutic Strategies. Circ. Res. 2021, 129, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Loll, E.G.; Hassan, A.-E.S.; Cheng, M.; Wang, A.; Farmer, D.L. Genetic and Molecular Determinants of Lymphatic Malformations: Potential Targets for Therapy. J. Dev. Biol. 2022, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Wassef, M.; Blei, F.; Adams, D.; Alomari, A.; Baselga, E.; Berenstein, A.; Burrows, P.; Frieden, I.J.; Garzon, M.C.; Lopez-Gutierrez, J.-C.; et al. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 2015, 136, e203–e214. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, M.; Fukao, T. Generalized Lymphatic Anomaly and Gorham–Stout Disease: Overview and Recent Insights. Adv. Wound Care 2019, 8, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.J.; Sarma, A.; Borst, A.J.; Tekes, A. Lymphatic Anomalies in Children: Update on Imaging Diagnosis, Genetics, and Treatment. Am. J. Roentgenol. 2022, 218, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Luks, V.L.; Kamitaki, N.; Vivero, M.P.; Uller, W.; Rab, R.; Bovée, J.V.M.G.; Rialon, K.L.; Guevara, C.J.; Alomari, A.I.; Greene, A.K.; et al. Lymphatic and Other Vascular Malformative/Overgrowth Disorders Are Caused by Somatic Mutations in PIK3CA. J. Pediatr. 2015, 166, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Osborn, A.J.; Dickie, P.; Neilson, D.E.; Glaser, K.; Lynch, K.A.; Gupta, A.; Dickie, B.H. Activating PIK3CA Alleles and Lymphangiogenic Phenotype of Lymphatic Endothelial Cells Isolated from Lymphatic Malformations. Hum. Mol. Genet. 2015, 24, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Corral, I.; Zhang, Y.; Petkova, M.; Ortsäter, H.; Sjöberg, S.; Castillo, S.D.; Brouillard, P.; Libbrecht, L.; Saur, D.; Graupera, M.; et al. Blockade of VEGF-C Signaling Inhibits Lymphatic Malformations Driven by Oncogenic PIK3CA Mutation. Nat. Commun. 2020, 11, 2869. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, M.; Aoki, Y.; Nozawa, A.; Yasue, S.; Endo, S.; Hori, Y.; Matsuoka, K.; Niihori, T.; Funayama, R.; Shirota, M.; et al. Detection of NRAS Mutation in Cell-Free DNA Biological Fluids from Patients with Kaposiform Lymphangiomatosis. Orphanet J. Rare Dis. 2019, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Manevitz-Mendelson, E.; Leichner, G.S.; Barel, O.; Davidi-Avrahami, I.; Ziv-Strasser, L.; Eyal, E.; Pessach, I.; Rimon, U.; Barzilai, A.; Hirshberg, A.; et al. Somatic NRAS Mutation in Patient with Generalized Lymphatic Anomaly. Angiogenesis 2018, 21, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, A.; Ozeki, M.; Niihori, T.; Suzui, N.; Miyazaki, T.; Aoki, Y. A Somatic Activating KRAS Variant Identified in an Affected Lesion of a Patient with Gorham-Stout Disease. J. Hum. Genet. 2020, 65, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.E.; March, M.; Seiler, C.; Matsuoka, L.S.; Kim, S.; Kao, C.; Rubin, A.I.; Battig, M.R.; Khalek, N.; Schindewolf, E.; et al. Lymphatic Disorders Caused by Mosaic, Activating KRAS Variants Respond to MEK Inhibition. JCI Insight 2023, 8, e155888. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; March, M.E.; Gutierrez-Uzquiza, A.; Kao, C.; Seiler, C.; Pinto, E.; Matsuoka, L.S.; Battig, M.R.; Bhoj, E.J.; Wenger, T.L.; et al. ARAF Recurrent Mutation Causes Central Conducting Lymphatic Anomaly Treatable with a MEK Inhibitor. Nat. Med. 2019, 25, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Clahsen, T.; Hadrian, K.; Notara, M.; Schlereth, S.L.; Howaldt, A.; Prokosch, V.; Volatier, T.; Hos, D.; Schroedl, F.; Kaser-Eichberger, A.; et al. The Novel Role of Lymphatic Vessels in the Pathogenesis of Ocular Diseases. Prog. Retin. Eye Res. 2023, 96, 101157. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D.; Regele, H.M.; Moosberger, I.; Nagy-Bojarski, K.; Watschinger, B.; Soleiman, A.; Birner, P.; Krieger, S.; Hovorka, A.; Silberhumer, G.; et al. Lymphatic Neoangiogenesis in Human Kidney Transplants Is Associated with Immunologically Active Lymphocytic Infiltrates. J. Am. Soc. Nephrol. 2004, 15, 603. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, S.; Dana, M.R.; Tsuru, T. Draining Lymph Nodes Play an Essential Role in Alloimmunity Generated in Response to High-Risk Corneal Transplantation. Cornea 2002, 21, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhao, X.; Wu, Z.; Qu, B.; Yuan, M.; Xing, Y.; Song, Y.; Wang, Z. Lymphatic Vessel: Origin, Heterogeneity, Biological Functions and Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Alitalo, K. The Lymphatic Vasculature in Disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Jung, K.; Jang, C.; Yang, H.; Schwendener, R.A.; Baik, J.E.; Han, S.H.; Alitalo, K.; Koh, G.Y. Critical Role of CD11b+ Macrophages and VEGF in Inflammatory Lymphangiogenesis, Antigen Clearance, and Inflammation Resolution. Blood 2009, 113, 5650–5659. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; Tammela, T.; Ator, E.; Lyubynska, N.; Achen, M.G.; Hicklin, D.J.; Jeltsch, M.; Petrova, T.V.; Pytowski, B.; Stacker, S.A.; et al. Pathogenesis of Persistent Lymphatic Vessel Hyperplasia in Chronic Airway Inflammation. J. Clin. Investig. 2005, 115, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kholová, I.; Dragneva, G.; Cermáková, P.; Laidinen, S.; Kaskenpää, N.; Hazes, T.; Cermáková, E.; Steiner, I.; Ylä-Herttuala, S. Lymphatic Vasculature Is Increased in Heart Valves, Ischaemic and Inflamed Hearts and in Cholesterol-Rich and Calcified Atherosclerotic Lesions. Eur. J. Clin. Investig. 2011, 41, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Norman, S.; Vieira, J.M.; Masters, M.; Rohling, M.; Dubé, K.N.; Bollini, S.; Matsuzaki, F.; Carr, C.A.; Riley, P.R. Cardiac Lymphatics Are Heterogeneous in Origin and Respond to Injury. Nature 2015, 522, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Maruyama, K.; Jackson, D.G.; Streilein, J.W.; Kruse, F.E. Time Course of Angiogenesis and Lymphangiogenesis after Brief Corneal Inflammation. Cornea 2006, 25, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Corral, I.; Olmeda, D.; Diéguez-Hurtado, R.; Tammela, T.; Alitalo, K.; Ortega, S. In Vivo Imaging of Lymphatic Vessels in Development, Wound Healing, Inflammation, and Tumor Metastasis. Proc. Natl. Acad. Sci. USA 2012, 109, 6223–6228. [Google Scholar] [CrossRef] [PubMed]
- Güç, E.; Briquez, P.S.; Foretay, D.; Fankhauser, M.A.; Hubbell, J.A.; Kilarski, W.W.; Swartz, M.A. Local Induction of Lymphangiogenesis with Engineered Fibrin-Binding VEGF-C Promotes Wound Healing by Increasing Immune Cell Trafficking and Matrix Remodeling. Biomaterials 2017, 131, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Abouelkheir, G.R.; Upchurch, B.D.; Rutkowski, J.M. Lymphangiogenesis: Fuel, Smoke, or Extinguisher of Inflammation’s Fire? Exp. Biol. Med. 2017, 242, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Hokari, R.; Tomioka, A. The Role of Lymphatics in Intestinal Inflammation. Inflamm. Regener. 2021, 41, 25. [Google Scholar] [CrossRef] [PubMed]
- Yeo, K.P.; Lim, H.Y.; Angeli, V. Leukocyte Trafficking via Lymphatic Vessels in Atherosclerosis. Cells 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Mandriota, S.J.; Jussila, L.; Jeltsch, M.; Compagni, A.; Baetens, D.; Prevo, R.; Banerji, S.; Huarte, J.; Montesano, R.; Jackson, D.G.; et al. Vascular Endothelial Growth Factor-C-Mediated Lymphangiogenesis Promotes Tumour Metastasis. EMBO J. 2001, 20, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Skobe, M.; Hawighorst, T.; Jackson, D.G.; Prevo, R.; Janes, L.; Velasco, P.; Riccardi, L.; Alitalo, K.; Claffey, K.; Detmar, M. Induction of Tumor Lymphangiogenesis by VEGF-C Promotes Breast Cancer Metastasis. Nat. Med. 2001, 7, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Laguna, L.; Agra, N.; Ibañez, K.; Oliva-Molina, G.; Gordo, G.; Khurana, N.; Hominick, D.; Beato, M.; Colmenero, I.; Herranz, G.; et al. Somatic Activating Mutations in PIK3CA Cause Generalized Lymphatic Anomaly. J. Exp. Med. 2019, 216, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Homayun-Sepehr, N.; McCarter, A.L.; Helaers, R.; Galant, C.; Boon, L.M.; Brouillard, P.; Vikkula, M.; Dellinger, M.T. KRAS-Driven Model of Gorham-Stout Disease Effectively Treated with Trametinib. JCI Insight 2021, 6, e149831. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, A.; Abe, T.; Niihori, T.; Ozeki, M.; Aoki, Y.; Ohnishi, H. Lymphatic Endothelial Cell-Specific NRAS p.Q61R Mutant Embryos Show Abnormal Lymphatic Vessel Morphogenesis. Hum. Mol. Genet. 2024, 33, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, C.G.; Fox, D.; Pastura, P.; Alharbi, S.; Huppert, S.S.; Cras, T.D.L. Lyve1-Driven NrasQ61R Causes Edema, Enlarged Lymphatic Vessels, and Hepatic Vascular Defects in Embryonic Mice. Pediatr. Blood Cancer 2025, 72, e31492. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Nüsslein-Volhard, C. The Zebrafish Issue of Development. Development 2012, 139, 4099–4103. [Google Scholar] [CrossRef] [PubMed]
- Driever, W.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.; Malicki, J.; Stemple, D.L.; Stainier, D.Y.; Zwartkruis, F.; Abdelilah, S.; Rangini, Z.; et al. A Genetic Screen for Mutations Affecting Embryogenesis in Zebrafish. Development 1996, 123, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.M.; Schulte-Merker, S. How to Plumb a Pisces: Understanding Vascular Development and Disease Using Zebrafish Embryos. Dev. Cell 2017, 42, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, R.; Ferrer, T.; Huisken, J.; Spitzer, K.; Stainier, D.Y.R.; Tristani-Firouzi, M.; Chi, N.C. Zebrafish Model for Human Long QT Syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 11316–11321. [Google Scholar] [CrossRef] [PubMed]
- Asimaki, A.; Kapoor, S.; Plovie, E.; Karin Arndt, A.; Adams, E.; Liu, Z.; James, C.A.; Judge, D.P.; Calkins, H.; Churko, J.; et al. Identification of a New Modulator of the Intercalated Disc in a Zebrafish Model of Arrhythmogenic Cardiomyopathy. Sci. Transl. Med. 2014, 6, ra74–ra240. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Zhang, Y.; Ding, Y.; Ross, C.A.; Li, H.; Olson, T.M.; Xu, X. Cardiac Transcriptome and Dilated Cardiomyopathy Genes in Zebrafish. Circ. Cardiovasc. Genet. 2015, 8, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart Regeneration in Zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Koopman, C.D.; De Angelis, J.; Iyer, S.P.; Verkerk, A.O.; Da Silva, J.; Berecki, G.; Jeanes, A.; Baillie, G.J.; Paterson, S.; Uribe, V.; et al. The Zebrafish Grime Mutant Uncovers an Evolutionarily Conserved Role for Tmem161b in the Control of Cardiac Rhythm. Proc. Natl. Acad. Sci. USA 2021, 118, e2018220118. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, S.; Ribatti, D.; Cotelli, F.; Presta, M. Mammalian Tumor Xenografts Induce Neovascularization in Zebrafish Embryos. Cancer Res. 2007, 67, 2927–2931. [Google Scholar] [CrossRef] [PubMed]
- Langenau, D.M.; Traver, D.; Ferrando, A.A.; Kutok, J.L.; Aster, J.C.; Kanki, J.P.; Lin, S.; Prochownik, E.; Trede, N.S.; Zon, L.I.; et al. Myc-Induced T Cell Leukemia in Transgenic Zebrafish. Science 2003, 299, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Widlund, H.R.; Kutok, J.L.; Kopani, K.R.; Amatruda, J.F.; Murphey, R.D.; Berghmans, S.; Mayhall, E.A.; Traver, D.; Fletcher, C.D.M.; et al. BRAF Mutations Are Sufficient to Promote Nevi Formation and Cooperate with P53 in the Genesis of Melanoma. Curr. Biol. 2005, 15, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Brunson, D.C.; Tang, Q.; Do, D.; Iftimia, N.A.; Moore, J.C.; Hayes, M.N.; Welker, A.M.; Garcia, E.G.; Dubash, T.D.; et al. Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish. Cell 2019, 177, 1903–1914.e14. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, A.; Schoonaert, L.; Lemmens, R.; Timmers, M.; Staats, K.A.; Laird, A.S.; Peeters, E.; Philips, T.; Goris, A.; Dubois, B.; et al. EPHA4 Is a Disease Modifier of Amyotrophic Lateral Sclerosis in Animal Models and in Humans. Nat. Med. 2012, 18, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Bretaud, S.; Allen, C.; Ingham, P.W.; Bandmann, O. P53-Dependent Neuronal Cell Death in a DJ-1-Deficient Zebrafish Model of Parkinson’s Disease. J. Neurochem. 2007, 100, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Imlach, W.L.; Saieva, L.; Beck, E.S.; Hao, L.T.; Li, D.K.; Jiao, W.; Mentis, G.Z.; Beattie, C.E.; McCabe, B.D.; et al. An SMN-Dependent U12 Splicing Event Essential for Motor Circuit Function. Cell 2012, 151, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, D.B.; Nguyen, P.D.; Siegel, A.L.; Ehrlich, O.V.; Sonntag, C.; Phan, J.M.N.; Berger, S.; Ratnayake, D.; Hersey, L.; Berger, J.; et al. Asymmetric Division of Clonal Muscle Stem Cells Coordinates Muscle Regeneration In Vivo. Science 2016, 353, aad9969. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, G.; Karpf, J.A.; Myers, J.A.; Alexander, M.S.; Guyon, J.R.; Kunkel, L.M. Drug Screening in a Zebrafish Model of Duchenne Muscular Dystrophy. Proc. Natl. Acad. Sci. USA 2011, 108, 5331–5336. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Ramakrishnan, L. The Role of the Granuloma in Expansion and Dissemination of Early Tuberculous Infection. Cell 2009, 136, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Oehlers, S.H.; Cronan, M.R.; Scott, N.R.; Thomas, M.I.; Okuda, K.S.; Walton, E.M.; Beerman, R.W.; Crosier, P.S.; Tobin, D.M. Interception of Host Angiogenic Signalling Limits Mycobacterial Growth. Nature 2015, 517, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Palha, N.; Guivel-Benhassine, F.; Briolat, V.; Lutfalla, G.; Sourisseau, M.; Ellett, F.; Wang, C.-H.; Lieschke, G.J.; Herbomel, P.; Schwartz, O.; et al. Real-Time Whole-Body Visualization of Chikungunya Virus Infection and Host Interferon Response in Zebrafish. PLoS Pathog. 2013, 9, e1003619. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.J.; Flores, M.V.; Oehlers, S.H.; Sanderson, L.E.; Lam, E.Y.; Crosier, K.E.; Crosier, P.S. Infection-Responsive Expansion of the Hematopoietic Stem and Progenitor Cell Compartment in Zebrafish Is Dependent upon Inducible Nitric Oxide. Cell Stem Cell 2012, 10, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal Models of Human Disease: Zebrafish Swim into View. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish Disease Models in Drug Discovery: From Preclinical Modelling to Clinical Trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- García-Caballero, M.; Quesada, A.R.; Medina, M.A.; Marí-Beffa, M. Fishing Anti(Lymph)Angiogenic Drugs with Zebrafish. Drug Discov. Today 2018, 23, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.S.; Lee, H.M.; Velaithan, V.; Ng, M.F.; Patel, V. Utilizing Zebrafish to Identify Anti-(Lymph)Angiogenic Compounds for Cancer Treatment: Promise and Future Challenges. Microcirculation 2016, 23, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.S.; Astin, J.W.; Misa, J.P.; Flores, M.V.; Crosier, K.E.; Crosier, P.S. Lyve1 Expression Reveals Novel Lymphatic Vessels and New Mechanisms for Lymphatic Vessel Development in Zebrafish. Development 2012, 139, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Nozaki, T.; Idrizi, F.; Isogai, S.; Ogasawara, K.; Ishida, K.; Yuge, S.; Roscoe, B.; Wolfe, S.A.; Fukuhara, S.; et al. Valves Are a Conserved Feature of the Zebrafish Lymphatic System. Dev. Cell 2019, 51, 374–386.e5. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Yin, H.-M.; Shih, Y.-H.; Nozaki, T.; Portman, D.; Toles, B.; Kolb, A.; Luk, K.; Isogai, S.; Ishida, K.; et al. Generation and Application of Endogenously Floxed Alleles for Cell-Specific Knockout in Zebrafish. Dev. Cell 2023, 58, 2614–2626.e7. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lv, T.; Zhang, J.; Shen, W.; Li, L.; Li, Y.; Liu, X.; Lei, X.; Lin, X.; Xu, H.; et al. Temporospatial Inhibition of Erk Signaling Is Required for Lymphatic Valve Formation. Sig. Transduct. Target. Ther. 2023, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.M.; Castranova, D.; Swift, M.R.; Pham, V.N.; Venero Galanternik, M.; Isogai, S.; Butler, M.G.; Mulligan, T.S.; Weinstein, B.M. Development of the Larval Lymphatic System in Zebrafish. Development 2017, 144, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Castranova, D.; Samasa, B.; Venero Galanternik, M.; Jung, H.M.; Pham, V.N.; Weinstein, B.M. Live Imaging of Intracranial Lymphatics in the Zebrafish. Circ. Res. 2021, 128, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Castranova, D.; Samasa, B.; Venero Galanternik, M.; Gore, A.V.; Goldstein, A.E.; Park, J.S.; Weinstein, B.M. Long-Term Imaging of Living Adult Zebrafish. Development 2022, 149, dev199667. [Google Scholar] [CrossRef] [PubMed]
- Venero Galanternik, M.; Castranova, D.; Gober, R.D.; Nguyen, T.; Kenton, M.; Margolin, G.; Kraus, A.; Sur, A.; Dye, L.E.; Pham, V.; et al. Anatomical and Molecular Characterization of the Zebrafish Meninges. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Castranova, D.; Kenton, M.I.; Kraus, A.; Dell, C.W.; Park, J.S.; Venero Galanternik, M.; Park, G.; Lumbantobing, D.N.; Dye, L.; Marvel, M.; et al. The Axillary Lymphoid Organ Is an External, Experimentally Accessible Immune Organ in the Zebrafish. J. Exp. Med. 2025, 222, e20241435. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.; Hogan, B.M. Network Patterning, Morphogenesis and Growth in Lymphatic Vascular Development. Curr. Top. Dev. Biol. 2021, 143, 151–204. [Google Scholar] [CrossRef] [PubMed]
- Koltowska, K.; Betterman, K.L.; Harvey, N.L.; Hogan, B.M. Getting out and about: The Emergence and Morphogenesis of the Vertebrate Lymphatic Vasculature. Development 2013, 140, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.; Padberg, Y.; van de Pavert, S.A.; Dierkes, C.; Morooka, N.; Peterson-Maduro, J.; van de Hoek, G.; Adrian, M.; Mochizuki, N.; Sekiguchi, K.; et al. An Evolutionarily Conserved Role for Polydom/Svep1 During Lymphatic Vessel Formation. Circ. Res. 2017, 120, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Gauvrit, S.; Villasenor, A.; Strilic, B.; Kitchen, P.; Collins, M.M.; Marín-Juez, R.; Guenther, S.; Maischein, H.-M.; Fukuda, N.; Canham, M.A.; et al. HHEX Is a Transcriptional Regulator of the VEGFC/FLT4/PROX1 Signaling Axis during Vascular Development. Nat. Commun. 2018, 9, 2704. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Hrupka, B.; van Bebber, F.; Sunil Kumar, S.; Feng, X.; Tschirner, S.K.; Aßfalg, M.; Müller, S.A.; Hilger, L.S.; Hofmann, L.I.; et al. The Alzheimer’s Disease-Linked Protease BACE2 Cleaves VEGFR3 and Modulates Its Signaling. J. Clin. Investig. 2024, 134, e170550. [Google Scholar] [CrossRef] [PubMed]
- Coxam, B.; Sabine, A.; Bower, N.I.; Smith, K.A.; Pichol-Thievend, C.; Skoczylas, R.; Astin, J.W.; Frampton, E.; Jaquet, M.; Crosier, P.S.; et al. Pkd1 Regulates Lymphatic Vascular Morphogenesis during Development. Cell Rep. 2014, 7, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Alders, M.; Hogan, B.M.; Gjini, E.; Salehi, F.; Al-Gazali, L.; Hennekam, E.A.; Holmberg, E.E.; Mannens, M.M.A.M.; Mulder, M.F.; Offerhaus, G.J.A.; et al. Mutations in CCBE1 Cause Generalized Lymph Vessel Dysplasia in Humans. Nat. Genet. 2009, 41, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.M.; Bos, F.L.; Bussmann, J.; Witte, M.; Chi, N.C.; Duckers, H.J.; Schulte-Merker, S. Ccbe1 Is Required for Embryonic Lymphangiogenesis and Venous Sprouting. Nat. Genet. 2009, 41, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Murtomaki, A.; Uh, M.K.; Choi, Y.K.; Kitajewski, C.; Borisenko, V.; Kitajewski, J.; Shawber, C.J. Notch1 Functions as a Negative Regulator of Lymphatic Endothelial Cell Differentiation in the Venous Endothelium. Development 2013, 140, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Geudens, I.; Herpers, R.; Hermans, K.; Segura, I.; de Almodovar, C.R.; Bussmann, J.; De Smet, F.; Vandevelde, W.; Hogan, B.M.; Siekmann, A.; et al. Role of Dll4 / Notch in the Formation and Wiring of the Lymphatic Network in Zebrafish. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, L.C.; Klein, S.; Mathelier, A.; Sliwa-Primorac, A.; Ma, Q.; Hong, Y.-K.; Shin, J.W.; Hamada, M.; Lizio, M.; Itoh, M.; et al. DeepCAGE Transcriptomics Reveal an Important Role of the Transcription Factor MAFB in the Lymphatic Endothelium. Cell Rep. 2015, 13, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Rondon-Galeano, M.; Skoczylas, R.; Bower, N.I.; Simons, C.; Gordon, E.; Francois, M.; Koltowska, K.; Hogan, B.M. MAFB Modulates the Maturation of Lymphatic Vascular Networks in Mice. Dev. Dyn. 2020, 249, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Koltowska, K.; Paterson, S.; Bower, N.I.; Baillie, G.J.; Lagendijk, A.K.; Astin, J.W.; Chen, H.; Francois, M.; Crosier, P.S.; Taft, R.J.; et al. Mafba Is a Downstream Transcriptional Effector of Vegfc Signaling Essential for Embryonic Lymphangiogenesis in Zebrafish. Genes Dev. 2015, 29, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Nicenboim, J.; Malkinson, G.; Lupo, T.; Asaf, L.; Sela, Y.; Mayseless, O.; Gibbs-Bar, L.; Senderovich, N.; Hashimshony, T.; Shin, M.; et al. Lymphatic Vessels Arise from Specialized Angioblasts within a Venous Niche. Nature 2015, 522, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hogan, B.M.; Herpers, R.; Witte, M.; Heloterä, H.; Alitalo, K.; Duckers, H.J.; Schulte-Merker, S. Vegfc/Flt4 Signalling Is Suppressed by Dll4 in Developing Zebrafish Intersegmental Arteries. Development 2009, 136, 4001–4009. [Google Scholar] [CrossRef] [PubMed]
- Villefranc, J.A.; Nicoli, S.; Bentley, K.; Jeltsch, M.; Zarkada, G.; Moore, J.C.; Gerhardt, H.; Alitalo, K.; Lawson, N.D. A Truncation Allele in Vascular Endothelial Growth Factor c Reveals Distinct Modes of Signaling during Lymphatic and Vascular Development. Development 2013, 140, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Astin, J.W.; Haggerty, M.J.L.; Okuda, K.S.; Le Guen, L.; Misa, J.P.; Tromp, A.; Hogan, B.M.; Crosier, K.E.; Crosier, P.S. Vegfd Can Compensate for Loss of Vegfc in Zebrafish Facial Lymphatic Sprouting. Development 2014, 141, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Koltowska, K.; Okuda, K.S.; Gloger, M.; Rondon-Galeano, M.; Mason, E.; Xuan, J.; Dudczig, S.; Chen, H.; Arnold, H.; Skoczylas, R.; et al. The RNA Helicase Ddx21 Controls Vegfc-Driven Developmental Lymphangiogenesis by Balancing Endothelial Cell Ribosome Biogenesis and P53 Function. Nat. Cell Biol. 2021, 23, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Das, R.N.; Tevet, Y.; Safriel, S.R.; Han, Y.; Moshe, N.; Lambiase, G.; Bassi, I.; Nicenboim, J.; Brückner, M.; Hirsch, D.; et al. Generation of Specialized Blood Vessels via Lymphatic Transdifferentiation. Nature 2022, 606, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Suster, M.L.; Kikuta, H.; Urasaki, A.; Asakawa, K.; Kawakami, K. Transgenesis in Zebrafish with the Tol2 Transposon System. Methods Mol. Biol. 2009, 561, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.-R.J.; Joung, J.K. Efficient Genome Editing in Zebrafish Using a CRISPR-Cas System. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef] [PubMed]
- van Impel, A.; Zhao, Z.; Hermkens, D.M.A.; Roukens, M.G.; Fischer, J.C.; Peterson-Maduro, J.; Duckers, H.; Ober, E.A.; Ingham, P.W.; Schulte-Merker, S. Divergence of Zebrafish and Mouse Lymphatic Cell Fate Specification Pathways. Development 2014, 141, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Grimm, L.; Mason, E.; Yu, H.; Dudczig, S.; Panara, V.; Chen, T.; Bower, N.I.; Paterson, S.; Rondon Galeano, M.; Kobayashi, S.; et al. Single-cell Analysis of Lymphatic Endothelial Cell Fate Specification and Differentiation during Zebrafish Development. EMBO J. 2023, 42, e112590. [Google Scholar] [CrossRef] [PubMed]
- Beis, D.; Bartman, T.; Jin, S.-W.; Scott, I.C.; D’Amico, L.A.; Ober, E.A.; Verkade, H.; Frantsve, J.; Field, H.A.; Wehman, A.; et al. Genetic and Cellular Analyses of Zebrafish Atrioventricular Cushion and Valve Development. Development 2005, 132, 4193–4204. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, J.; Bos, F.L.; Urasaki, A.; Kawakami, K.; Duckers, H.J.; Schulte-Merker, S. Arteries Provide Essential Guidance Cues for Lymphatic Endothelial Cells in the Zebrafish Trunk. Development 2010, 137, 2653–2657. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.R.; Fujita, M.; Butler, M.; Isogai, S.; Kochhan, E.; Siekmann, A.F.; Weinstein, B.M. Chemokine Signaling Directs Trunk Lymphatic Network Formation along the Preexisting Blood Vasculature. Dev. Cell 2012, 22, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Muhl, L.; Padberg, Y.; Dupont, L.; Peterson-Maduro, J.; Stehling, M.; le Noble, F.; Colige, A.; Betsholtz, C.; Schulte-Merker, S.; et al. Specific Fibroblast Subpopulations and Neuronal Structures Provide Local Sources of Vegfc-Processing Components during Zebrafish Lymphangiogenesis. Nat. Commun. 2020, 11, 2724. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Ando, K.; Hußmann, M.; Gloger, M.; Skoczylas, R.; Mochizuki, N.; Betsholtz, C.; Fukuhara, S.; Schulte-Merker, S.; Lawson, N.D.; et al. Proper Migration of Lymphatic Endothelial Cells Requires Survival and Guidance Cues from Arterial Mural Cells. Elife 2022, 11, e74094. [Google Scholar] [CrossRef] [PubMed]
- Eng, T.C.; Chen, W.; Okuda, K.S.; Misa, J.P.; Padberg, Y.; Crosier, K.E.; Crosier, P.S.; Hall, C.J.; Schulte-Merker, S.; Hogan, B.M.; et al. Zebrafish Facial Lymphatics Develop through Sequential Addition of Venous and Non-venous Progenitors. EMBO Rep. 2019, 20, e47079. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.R.; Feng, X.; Mo, G.; Aguayo, A.; Villafuerte, J.; Yoshida, T.; Pearson, C.A.; Schulte-Merker, S.; Lien, C.-L. Late Developing Cardiac Lymphatic Vasculature Supports Adult Zebrafish Heart Function and Regeneration. Elife 2019, 8, e42762. [Google Scholar] [CrossRef] [PubMed]
- Gancz, D.; Perlmoter, G.; Yaniv, K. Formation and Growth of Cardiac Lymphatics during Embryonic Development, Heart Regeneration, and Disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a037176. [Google Scholar] [CrossRef] [PubMed]
- Vivien, C.J.; Pichol-Thievend, C.; Sim, C.B.; Smith, J.B.; Bower, N.I.; Hogan, B.M.; Hudson, J.E.; Francois, M.; Porrello, E.R. Vegfc/d-Dependent Regulation of the Lymphatic Vasculature during Cardiac Regeneration Is Influenced by Injury Context. Npj Regen. Med. 2019, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Coffindaffer-Wilson, M.; Criag, M.P.; Hove, J.R. Determination of Lymphatic Vascular Identity and Developmental Timecourse in Zebrafish (Danio rerio). Lymphology 2011, 44, 1–12. [Google Scholar] [PubMed]
- Hoffman, S.J.; Psaltis, P.J.; Clark, K.J.; Spoon, D.B.; Chue, C.D.; Ekker, S.C.; Simari, R.D. An In Vivo Method to Quantify Lymphangiogenesis in Zebrafish. PLoS ONE 2012, 7, e45240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okuda, K.S.; Misa, J.P.; Oehlers, S.H.; Hall, C.J.; Ellett, F.; Alasmari, S.; Lieschke, G.J.; Crosier, K.E.; Crosier, P.S.; Astin, J.W. A Zebrafish Model of Inflammatory Lymphangiogenesis. Biol. Open 2015, 4, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Olayinka, O.; Ryu, H.; Wang, X.; Malik, A.B.; Jung, H.M. Compensatory Lymphangiogenesis Is Required for Edema Resolution in Zebrafish. Sci. Rep. 2025, 15, 8177. [Google Scholar] [CrossRef] [PubMed]
- Gancz, D.; Raftrey, B.C.; Perlmoter, G.; Marín-Juez, R.; Semo, J.; Matsuoka, R.L.; Karra, R.; Raviv, H.; Moshe, N.; Addadi, Y.; et al. Distinct Origins and Molecular Mechanisms Contribute to Lymphatic Formation during Cardiac Growth and Regeneration. Elife 2019, 8, e44153. [Google Scholar] [CrossRef] [PubMed]
- Duca, S.; Xia, Y.; Elmagid, L.A.; Bakis, I.; Qiu, M.; Cao, Y.; Guo, Y.; Eichenbaum, J.V.; McCain, M.L.; Kang, J.; et al. Differential Vegfc Expression Dictates Lymphatic Response during Zebrafish Heart Development and Regeneration. Development 2024, 151, dev202947. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wenger, T.L.; Seiler, C.; March, M.E.; Gutierrez-Uzquiza, A.; Kao, C.; Bhoj, E.; Tian, L.; Rosenbach, M.; Liu, Y.; et al. Pathogenic Variant in EPHB4 Results in Central Conducting Lymphatic Anomaly. Hum. Mol. Genet. 2018, 27, 3233–3245. [Google Scholar] [CrossRef] [PubMed]
- Bassi, I.; Jabali, A.; Farag, N.; Egozi, S.; Moshe, N.; Leichner, G.S.; Geva, P.; Levin, L.; Barzilai, A.; Avivi, C.; et al. A High-Throughput Zebrafish Screen Identifies Novel Candidate Treatments for Kaposiform Lymphangiomatosis (KLA). bioRxiv 2024. [Google Scholar] [CrossRef]
- Oehlers, S.H.; Flores, M.V.; Okuda, K.S.; Hall, C.J.; Crosier, K.E.; Crosier, P.S. A Chemical Enterocolitis Model in Zebrafish Larvae That Is Dependent on Microbiota and Responsive to Pharmacological Agents. Dev. Dyn. 2011, 240, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Jerkic, M.; Peter, M.; Ardelean, D.; Fine, M.; Konerding, M.A.; Letarte, M. Dextran Sulfate Sodium Leads to Chronic Colitis and Pathological Angiogenesis in Endoglin Heterozygous Mice. Inflamm. Bowel Dis. 2010, 16, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.-L.; Iwasaki, A. VEGF-C-Driven Lymphatic Drainage Enables Immunosurveillance of Brain Tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional Aspects of Meningeal Lymphatics in Ageing and Alzheimer’s Disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-R.; Yang, J.-H.; Wang, X.; Yao, Z.-B. Induced Dural Lymphangiogenesis Facilities Soluble Amyloid-Beta Clearance from Brain in a Transgenic Mouse Model of Alzheimer’s Disease. Neural Regen. Res. 2018, 13, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Boisen, A.M.Z.; Amstrup, J.; Novak, I.; Grosell, M. Sodium and Chloride Transport in Soft Water and Hard Water Acclimated Zebrafish (Danio rerio). Biochim. Biophys. Acta BBA–Biomembr. 2003, 1618, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Planas-Paz, L.; Strilić, B.; Goedecke, A.; Breier, G.; Fässler, R.; Lammert, E. Mechanoinduction of Lymph Vessel Expansion. EMBO J. 2012, 31, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Morejón, A.; Mercader, N. Recent Insights into Zebrafish Cardiac Regeneration. Curr. Opin. Genet. Dev. 2020, 64, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Chablais, F.; Veit, J.; Rainer, G.; Jaźwińska, A. The Zebrafish Heart Regenerates after Cryoinjury-Induced Myocardial Infarction. BMC Dev. Biol. 2011, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Panáková, D.; Kikuchi, K.; Holdway, J.E.; Gemberling, M.; Burris, J.S.; Singh, S.P.; Dickson, A.L.; Lin, Y.-F.; Sabeh, M.K.; et al. The Regenerative Capacity of Zebrafish Reverses Cardiac Failure Caused by Genetic Cardiomyocyte Depletion. Development 2011, 138, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Marín-Juez, R.; Marass, M.; Gauvrit, S.; Rossi, A.; Lai, S.-L.; Materna, S.C.; Black, B.L.; Stainier, D.Y.R. Fast Revascularization of the Injured Area Is Essential to Support Zebrafish Heart Regeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 11237–11242. [Google Scholar] [CrossRef] [PubMed]
- Gladka, M.M.; Kohela, A.; Molenaar, B.; Versteeg, D.; Kooijman, L.; Monshouwer-Kloots, J.; Kremer, V.; Vos, H.R.; Huibers, M.M.H.; Haigh, J.J.; et al. Cardiomyocytes Stimulate Angiogenesis after Ischemic Injury in a ZEB2-Dependent Manner. Nat. Commun. 2021, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, X.; Kanisicak, O.; Li, Y.; Wang, Y.; Li, Y.; Pu, W.; Liu, Q.; Zhang, H.; Tian, X.; et al. Preexisting Endothelial Cells Mediate Cardiac Neovascularization after Injury. J. Clin. Investig. 2017, 127, 2968–2981. [Google Scholar] [CrossRef] [PubMed]
- Henri, O.; Pouehe, C.; Houssari, M.; Galas, L.; Nicol, L.; Edwards-Lévy, F.; Henry, J.-P.; Dumesnil, A.; Boukhalfa, I.; Banquet, S.; et al. Selective Stimulation of Cardiac Lymphangiogenesis Reduces Myocardial Edema and Fibrosis Leading to Improved Cardiac Function Following Myocardial Infarction. Circulation 2016, 133, 1484–1497. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.M.; Norman, S.; Villa Del Campo, C.; Cahill, T.J.; Barnette, D.N.; Gunadasa-Rohling, M.; Johnson, L.A.; Greaves, D.R.; Carr, C.A.; Jackson, D.G.; et al. The Cardiac Lymphatic System Stimulates Resolution of Inflammation Following Myocardial Infarction. J. Clin. Investig. 2018, 128, 3402–3412. [Google Scholar] [CrossRef] [PubMed]
- Dahl Ejby Jensen, L.; Cao, R.; Hedlund, E.-M.; Söll, I.; Lundberg, J.O.; Hauptmann, G.; Steffensen, J.F.; Cao, Y. Nitric Oxide Permits Hypoxia-Induced Lymphatic Perfusion by Controlling Arterial-Lymphatic Conduits in Zebrafish and Glass Catfish. Proc. Natl. Acad. Sci. USA 2009, 106, 18408–18413. [Google Scholar] [CrossRef] [PubMed]
- Panara, V.; Varaliová, Z.; Wilting, J.; Koltowska, K.; Jeltsch, M. The Relationship between the Secondary Vascular System and the Lymphatic Vascular System in Fish. Biol. Rev. 2024, 99, 2108–2133. [Google Scholar] [CrossRef] [PubMed]
- Kazenwadel, J.; Venugopal, P.; Oszmiana, A.; Toubia, J.; Arriola-Martinez, L.; Panara, V.; Piltz, S.G.; Brown, C.; Ma, W.; Schreiber, A.W.; et al. A Prox1 Enhancer Represses Haematopoiesis in the Lymphatic Vasculature. Nature 2023, 614, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Dillard, M.E.; Baluk, P.; McDonald, D.M.; Harvey, N.L.; Frase, S.L.; Oliver, G. Lymphatic Endothelial Cell Identity Is Reversible and Its Maintenance Requires Prox1 Activity. Genes Dev. 2008, 22, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Bertozzi, C.; Zou, Z.; Yuan, L.; Lee, J.S.; Lu, M.; Stachelek, S.J.; Srinivasan, S.; Guo, L.; Vicente, A.; et al. Blood Flow Reprograms Lymphatic Vessels to Blood Vessels. J. Clin. Investig. 2012, 122, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.S.; Baek, S.; Hogan, B.M. Visualization and Tools for Analysis of Zebrafish Lymphatic Development. In Lymphangiogenesis: Methods and Protocols; Oliver, G., Kahn, M.L., Eds.; Springer: New York, NY, USA, 2018; pp. 55–70. ISBN 978-1-4939-8712-2. [Google Scholar]
- Sevick-Muraca, E.M.; King, P.D. Lymphatic Vessel Abnormalities Arising from Disorders of Ras Signal Transduction. Trends Cardiovasc. Med. 2014, 24, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Barclay, S.F.; Inman, K.W.; Luks, V.L.; McIntyre, J.B.; Al-Ibraheemi, A.; Church, A.J.; Perez-Atayde, A.R.; Mangray, S.; Jeng, M.; Kreimer, S.R.; et al. A Somatic Activating NRAS Variant Associated with Kaposiform Lymphangiomatosis. Genet. Med. 2019, 21, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Astin, J.W.; Jamieson, S.M.F.; Eng, T.C.Y.; Flores, M.V.; Misa, J.P.; Chien, A.; Crosier, K.E.; Crosier, P.S. An In Vivo Antilymphatic Screen in Zebrafish Identifies Novel Inhibitors of Mammalian Lymphangiogenesis and Lymphatic-Mediated Metastasis. Mol. Cancer Ther. 2014, 13, 2450–2462. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.S.; Ng, M.F.; Ruslan, N.F.; Bower, N.I.; Song, D.S.S.; Chen, H.; Baek, S.; Crosier, P.S.; Koltowska, K.; Astin, J.W.; et al. 3,4-Difluorobenzocurcumin Inhibits Vegfc-Vegfr3-Erk Signalling to Block Developmental Lymphangiogenesis in Zebrafish. Pharmaceuticals 2021, 14, 614. [Google Scholar] [CrossRef] [PubMed]
- García-Caballero, M.; Blacher, S.; Paupert, J.; Quesada, A.R.; Medina, M.A.; Noël, A. Novel Application Assigned to Toluquinol: Inhibition of Lymphangiogenesis by Interfering with VEGF-C/VEGFR-3 Signalling Pathway. Br. J. Pharmacol. 2016, 173, 1966–1987. [Google Scholar] [CrossRef] [PubMed]
- Willett, C.E.; Cortes, A.; Zuasti, A.; Zapata, A.G. Early Hematopoiesis and Developing Lymphoid Organs in the Zebrafish. Dev. Dyn. 1999, 214, 323–336. [Google Scholar] [CrossRef]
- Langenau, D.M.; Ferrando, A.A.; Traver, D.; Kutok, J.L.; Hezel, J.-P.D.; Kanki, J.P.; Zon, L.I.; Thomas Look, A.; Trede, N.S. In Vivo Tracking of T Cell Development, Ablation, and Engraftment in Transgenic Zebrafish. Proc. Natl. Acad. Sci. USA 2004, 101, 7369–7374. [Google Scholar] [CrossRef] [PubMed]
- Dubey, L.K.; Karempudi, P.; Luther, S.A.; Ludewig, B.; Harris, N.L. Interactions between Fibroblastic Reticular Cells and B Cells Promote Mesenteric Lymph Node Lymphangiogenesis. Nat. Commun. 2017, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Elhadad, S.; Mehrara, B. Helper T Cell Differentiation and Regulation of Inflammatory Lymphangiogenesis (IRC10P.468). J. Immunol. 2014, 192, 192.6. [Google Scholar] [CrossRef]
- García Nores, G.D.; Ly, C.L.; Cuzzone, D.A.; Kataru, R.P.; Hespe, G.E.; Torrisi, J.S.; Huang, J.J.; Gardenier, J.C.; Savetsky, I.L.; Nitti, M.D.; et al. CD4+ T Cells Are Activated in Regional Lymph Nodes and Migrate to Skin to Initiate Lymphedema. Nat. Commun. 2018, 9, 1970. [Google Scholar] [CrossRef] [PubMed]
- Vogrin, A.J.; Bower, N.I.; Gunzburg, M.J.; Roufail, S.; Okuda, K.S.; Paterson, S.; Headey, S.J.; Stacker, S.A.; Hogan, B.M.; Achen, M.G. Evolutionary Differences in the Vegf/Vegfr Code Reveal Organotypic Roles for the Endothelial Cell Receptor Kdr in Developmental Lymphangiogenesis. Cell Rep. 2019, 28, 2023–2036.e4. [Google Scholar] [CrossRef] [PubMed]
- Kugler, E.; Plant, K.; Chico, T.; Armitage, P. Enhancement and Segmentation Workflow for the Developing Zebrafish Vasculature. J. Imaging 2019, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Lubin, A.; Otterstrom, J.; Hoade, Y.; Bjedov, I.; Stead, E.; Whelan, M.; Gestri, G.; Paran, Y.; Payne, E. A Versatile, Automated and High-Throughput Drug Screening Platform for Zebrafish Embryos. Biol. Open 2021, 10, bio058513. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, Q.; Do, D.; Brunson, D.C.; Langenau, D.M. Adult Immune Compromised Zebrafish for Xenograft Cell Transplantation Studies. Ebiomedicine 2019, 47, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Panara, V.; Yu, H.; Peng, D.; Staxäng, K.; Hodik, M.; Filipek-Gorniok, B.; Kazenwadel, J.; Skoczylas, R.; Mason, E.; Allalou, A.; et al. Multiple Cis-Regulatory Elements Control Prox1a Expression in Distinct Lymphatic Vascular Beds. Development 2024, 151, dev202525. [Google Scholar] [CrossRef] [PubMed]
- Auer, T.O.; Duroure, K.; De Cian, A.; Concordet, J.-P.; Del Bene, F. Highly Efficient CRISPR/Cas9-Mediated Knock-in in Zebrafish by Homology-Independent DNA Repair. Genome Res. 2014, 24, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Panara, V.; Arnold, H.; Gloger, M.; Skoczylas, R.; Gutierrez, V.V.; Johansson, A.; Smialowska, A.; Koltowska, K. Distinct Topologically Associated Domains Underlie Regulatory Logic in Lymphatic Endothelial Cells Contributing to Proper Cell Differentiation. bioRxiv 2025. [Google Scholar] [CrossRef]
| Model of Induced Lymphangiogenesis | Induction Method | Developmental Stage | Reference |
|---|---|---|---|
| Cancer-associated lymphangiogenesis | Mouse melanoma cell xenotransplantation | 3 dpf | [123] |
| Inflammatory lymphangiogenesis | Treatment with enterocolitic agents DSS or TNBS | 7 dpf | [124,130] |
| Meningeal lymphangiogenesis | Intracranial human VEGF-C recombinant protein injection | 50 dpf | [86] |
| Compensatory lymphangiogenesis | Osmotic shock by 24 h incubation in hypertonic medium | 4–9 dpf | [125] |
| Cardiac regeneration-associated lymphangiogenesis | Heart injury (resection, cryoinjury, cell ablation) | 3–6 mpf | [119,121,126,127] |
| Anal fin regeneration-associated lymphangiogenesis | Anal fin resection | 3–6 mpf | [108] |
| Skin regeneration-associated lymphangiogenesis | Removal of scales and scraping of skin with a dissecting knife | 6–8 mpf | [87] |
| Central conductive lymphatic anomaly model | Injection of ephb4-targeting morpholino at single-cell stage | 4 dpf | [128] |
| Mosaic expression of mrc1a-driven KRAS p.G12D or p.G13D by construct injection at single-cell stage | 5 dpf | [30] | |
| Mosaic expression of mrc1a-driven ARAF p.S214P by construct injection at single-cell stage | 7 dpf | [31] | |
| Generalised lymphatic anomaly/Kaposiform lymphangiomatosis model | 4-hydroxytamoxifen-inducible lyve1b-driven expression of NRAS p.Q61R at 24 hpf | 5 dpf | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boskovic, S.; Okuda, K.S. Zebrafish Models of Induced Lymphangiogenesis: Current Advancements and Therapeutic Discovery. Pharmaceuticals 2025, 18, 1076. https://doi.org/10.3390/ph18071076
Boskovic S, Okuda KS. Zebrafish Models of Induced Lymphangiogenesis: Current Advancements and Therapeutic Discovery. Pharmaceuticals. 2025; 18(7):1076. https://doi.org/10.3390/ph18071076
Chicago/Turabian StyleBoskovic, Srdjan, and Kazuhide Shaun Okuda. 2025. "Zebrafish Models of Induced Lymphangiogenesis: Current Advancements and Therapeutic Discovery" Pharmaceuticals 18, no. 7: 1076. https://doi.org/10.3390/ph18071076
APA StyleBoskovic, S., & Okuda, K. S. (2025). Zebrafish Models of Induced Lymphangiogenesis: Current Advancements and Therapeutic Discovery. Pharmaceuticals, 18(7), 1076. https://doi.org/10.3390/ph18071076






