Bisphosphonates in the Management of Patients with Postmenopausal Osteoporosis; Back to the Future
Abstract
1. Introduction
2. Treatment Rationale
3. Approved Treatments
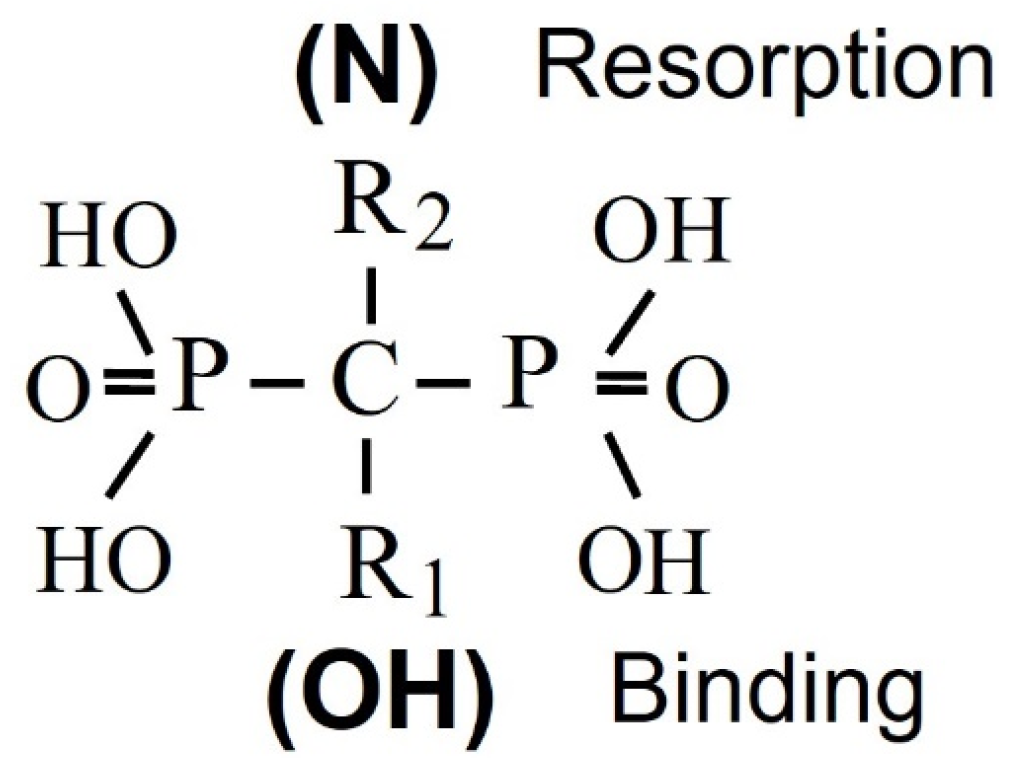
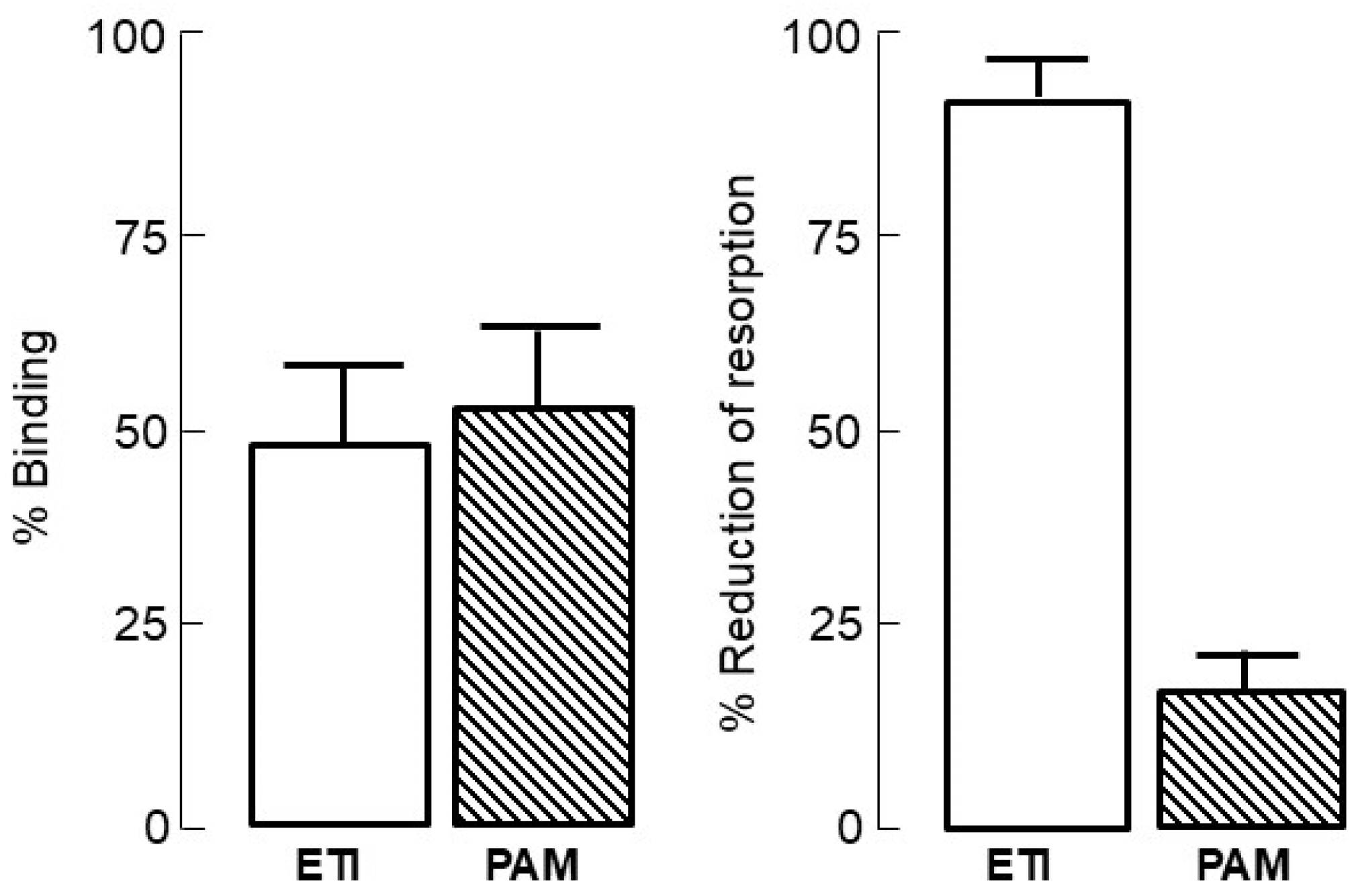
4. Bisphosphonate Mechanism of Action
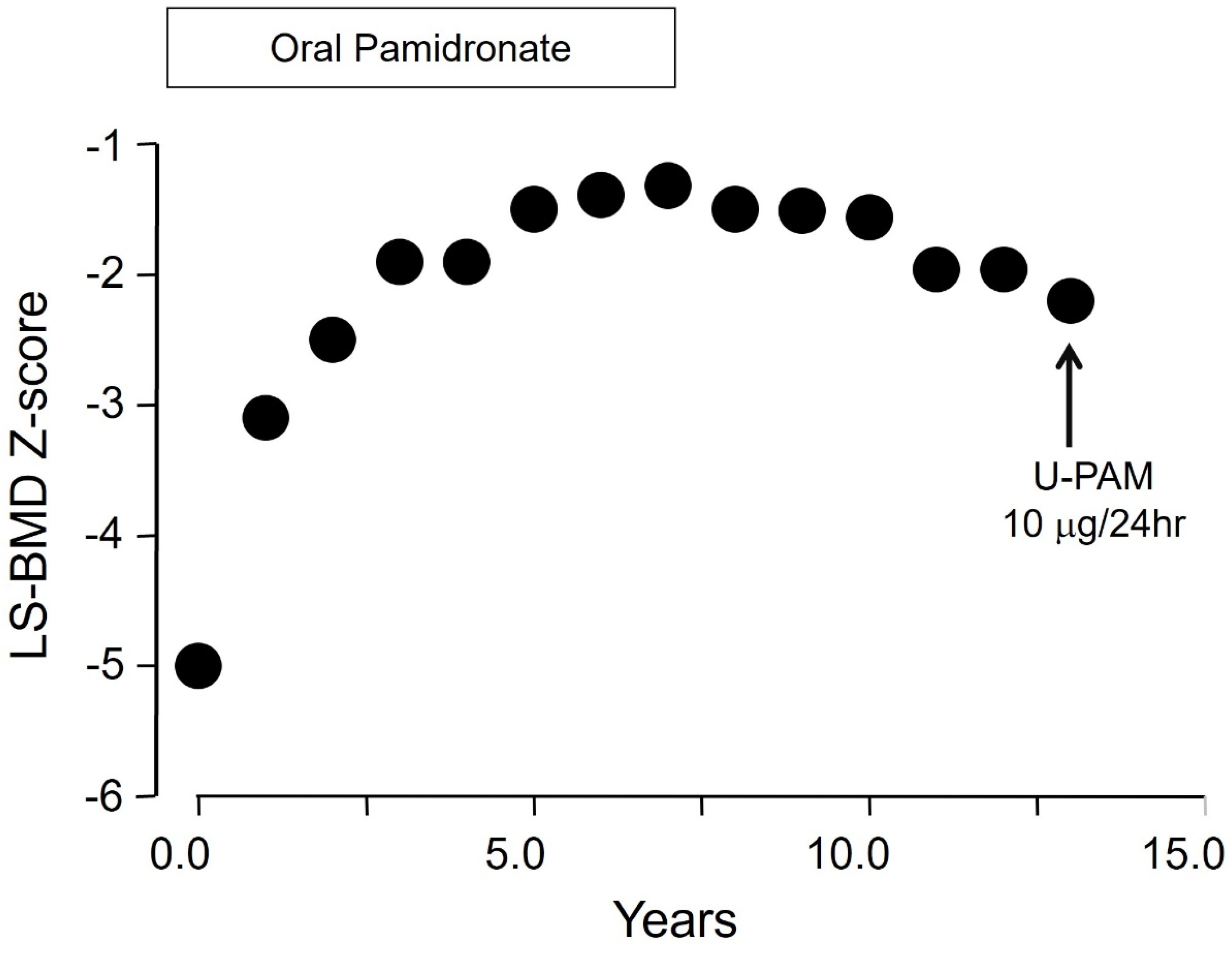
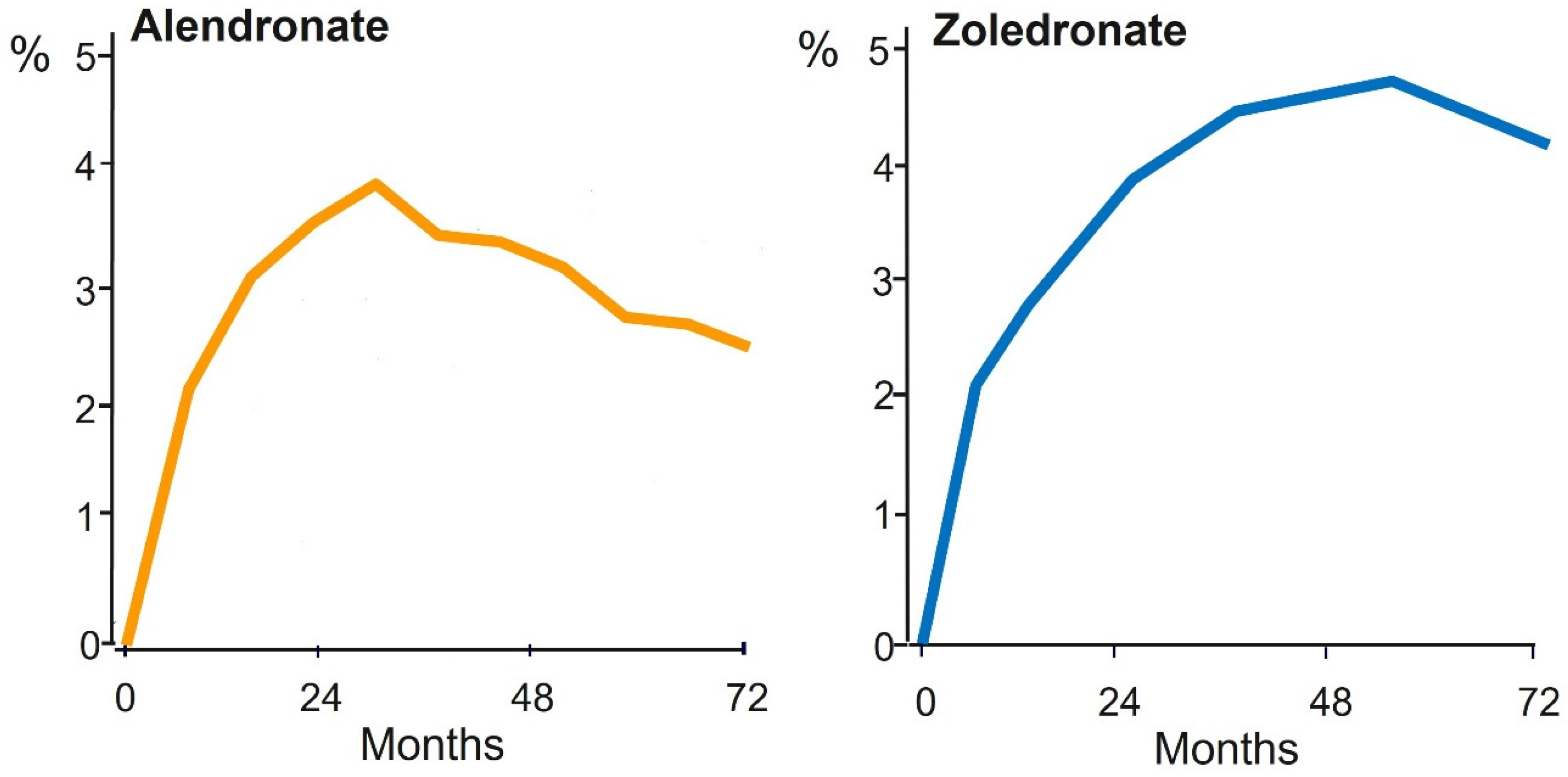
5. Bisphosphonate PK/PD Relevant to Long-Term Use
6. Bisphosphonate and Atypical Femur Fractures
7. The Drug Holiday
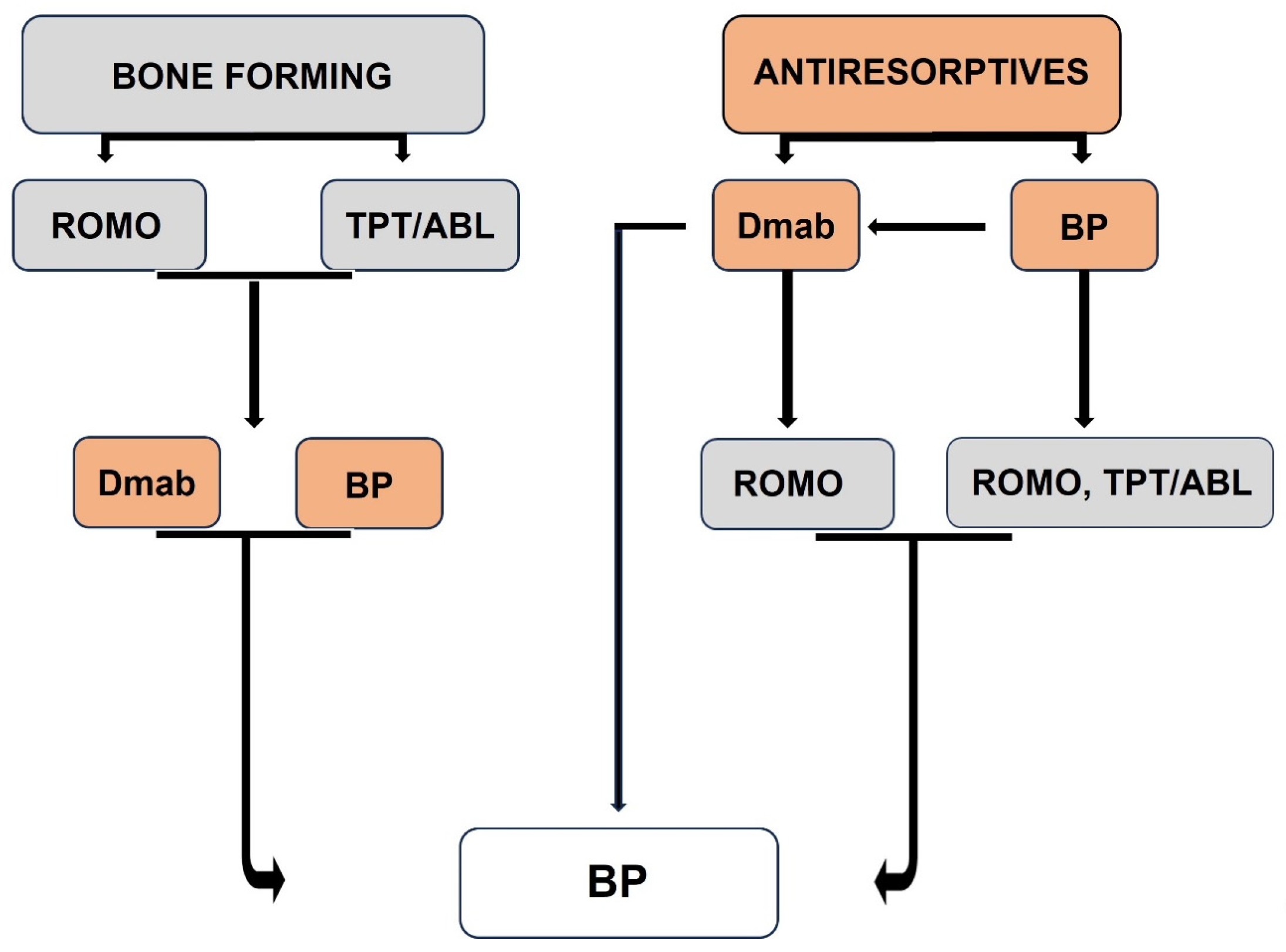
8. Positioning Bisphosphonates in Long-Term Treatment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 39, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef] [PubMed]
- Delgado-Calle, J.; Bellido, T. The osteocyte as a signaling cell. Physiol. Rev. 2021, 102, 379–410. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Recker, R.; Lappe, J.; Davies, K.M.; Heaney, R. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J. Bone Miner. Res. 2004, 19, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Delmas, P.D. Bone quality—The material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Appelman-Dijkstra, N.M.; Papapoulos, S.E. Modulating bone resorption and bone formation in opposite directions in the treatment of postmenopausal osteoporosis. Drugs 2015, 75, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Papapoulos, S.E. Long-term efficacy and safety of treatments for osteoporosis. In The Duration and Safety of Osteoporosis Treatment; Silverman, S.L., Abrahamsen, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 203–232. [Google Scholar]
- Black, D.M.; Schwartz, A.V.; Ensrud, K.E.; Cauley, J.A.; Levis, S.; Quandt, S.A.; Satterfield, S.; Wallace, R.B.; Bauer, D.C.; Palermo, L.; et al. Effects of continuing or stopping alendronate after 5 years of treatment: The Fracture Intervention Trial Long-term Extension (FLEX): A randomized trial. JAMA 2006, 296, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Mellstrom, D.D.; Sorensen, O.H.; Goemaere, S.; Roux, C.; Johnson, T.D.; Chines, A.A. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif. Tissue Int. 2004, 75, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Reid, I.R.; Cauley, J.A.; Cosman, F.; Leung, P.C.; Lakatos, P.; Lippuner, K.; Cummings, S.R.; Hue, T.F.; Mukhopadhyay, A.; et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: A randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J. Bone Miner. Res. 2015, 30, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Hosking, D.; Devogelaer, J.P.; Tucci, J.R.; Emkey, R.D.; Tonino, R.P.; Rodriguez-Portales, J.A.; Downs, R.W.; Gupta, J.; Santora, A.C.; et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N. Engl. J. Med. 2004, 350, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwiński, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J.; Sims, N.A.; Seeman, E. Physiological and Pharmacological Roles of PTH and PTHrP in Bone Using Their Shared Receptor, PTH1R. Endocrine Rev. 2021, 42, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Wein, M.N.; Foretz, M.; Fisher, D.E.; Xavier, R.J.; Kronenberg, H.M. Salt inducible kinases: Physiology, regulation by cAMP, and therapeutic potential. Trends Endocrinol. Metab. 2018, 29, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.-Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Hattersley, G.; Riis, B.J.; Williams, G.C.; Lau, E.; Russo, L.A.; Alexandersen, P.; Zerbini, C.A.F.; Hu, M.-Y.; Harris, A.G.; et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: A randomized clinical trial. JAMA 2016, 316, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Appelman-Dijkstra, N.M.; Papapoulos, S.E. Clinical advantages and disadvantages of anabolic bone therapies targeting the WNT pathway. Nat. Rev. Endocrinol. 2018, 14, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Chavassieux, P.; Chapurlat, R.; Portero-Muzy, N.; Roux, J.P.; Garcia, P.; Brown, J.P.; Libanati, C.; Boyce, R.W.; Wang, A.; Grauer, A. Bone-Forming and Antiresorptive Effects of Romosozumab in Postmenopausal Women with Osteoporosis: Bone Histomorphometry and Microcomputed Tomography Analysis After 2 and 12 Months of Treatment. J. Bone Miner. Res. 2019, 34, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F.; Chapurlat, R.; Boyce, R.W.; Shi, Y.; Brown, J.P.; Horlait, S.; Betah, D.; Libanati, C.; Chavassieux, P. Modeling-based bone formation after 2 months of romosozumab treatment: Results from the FRAME clinical trial. J. Bone Miner. Res. 2022, 37, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.D.O.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.L.; Libanati, C.; Crittenden, D.B.; Bolognese, M.A.; Brown, J.P.; Daizadeh, N.S.; Dokoupilova, E.; Engelke, K.; Finkelstein, J.S.; Genant, H.K.; et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: A randomized, open-label, phase 3 trial. Lancet 2017, 390, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Papapoulos, S.E. Bisphosphonates: How do they work? Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Cremers, S.; Papapoulos, S. Pharmacology of bisphosphonates. Bone 2011, 49, 42–49. [Google Scholar] [CrossRef] [PubMed]
- van Beek, E.; Hoekstra, M.; van de Ruit, M.; Löwik, C.; Papapoulos, S. Structural requirements for bisphosphonate actions in vitro. J. Bone Miner. Res. 1994, 9, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Leu, C.-T.; Luegmayr, E.; Freedman, L.P.; Rodan, G.A.; Reszka, A.A. Relative binding affinities of bisphosphonates for human bone and relationship to antiresorptive efficacy. Bone 2006, 38, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J.; Crockett, J.C.; Coxon, F.P.; Monkkonen, J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 2011, 49, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J.; Monkkonen, J.; Munoz, M.A. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J.; Russell, R.G.G.; Blackburn, G.M.; Williamson, M.P.; Watts, D.J. Metabolism of halogenated bisphosphonates by the cellular slime mold Dictyostelium discoideum. Biochem. Biophys. Res. Commun. 1992, 189, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J.; Brown, R.J.; Hodkin, V.; Blackburn, G.M.; Russell, R.G.G.; Watts, D.J. Bisphosphonates are incorporated into adenine nucleotides by human aminoacyltRNA synthetase enzymes. Biochem. Biophys. Res. Commun. 1996, 224, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.C.; Monkkonen, J.; Blackburn, G.M.; Russell, R.G.G.; Rogers, M.J. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J. Bone Miner. Res. 1997, 12, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- van Beek, E.; Pieterman, E.; Cohen, L.; Lowik, C.; Papapoulos, S. Farnesyl Pyrophosphate Synthase Is the Molecular Target of Nitrogen-Containing Bisphosphonates. Biochem. Biophys. Res. Commun. 1999, 264, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Luckman, S.P.; Hughes, D.E.; Coxon, F.P.; Russell, R.G.G.; Rogers, M.J. Nitrogen containing bisphosphonates inhibit the mevalonate pathway and prevent posttranslational prenylation of GTP-binding proteins, including Ras. J. Bone Miner. Res. 1998, 13, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.J. From molds and macrophages to mevalonate: A decade of progress in understanding the molecular mode of action of bisphosphonates. Calcif. Tissue Int. 2004, 75, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Dunford, J.E.; Thompson, K.; Coxon, F.P.; Luckman, S.P.; Hahn, F.M.; Poulter, C.D.; Ebetino, F.H.; Rogers, M.J. Structure-activity relationships for inhibition of pharnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 2001, 296, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences M.J and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Monkkonen, H.; Auriola, S.; Lehenkari, P.; Kellinsalmi, M.; Hassinen, I.E.; Vepsäläinen, J.; Mönkkönen, J. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br. J. Pharmacol. 2006, 147, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Bauer, E.; Wilhelm, M. Gamma/delta T-cell stimulation by pamidronate. N. Engl. J. Med. 1999, 340, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, A.J.; Jauhiainen, M.; Mönkkönen, H.; Rogers, M.J.; Mönkkönen, J.; Thompson, K. Peripheral blood monocytes are responsible for gamma delta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br. J. Haematol. 2009, 144, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Roelofs, A.J.; Jauhiainen, M.; Monkkonen, H.; Monkkonen, J.; Rogers, M.J. Activation of gamma delta T cells by bisphosphonates. Adv. Exp. Med. Biol. 2010, 658, 11–20. [Google Scholar] [PubMed]
- Thompson, K.; Keech, F.; McLernon, D.J.; Vinod, K.; May, R.J.; Simpson, W.G.; Rogers, M.J.; Reid, D.M. Fluvastatin does not prevent the acute-phase response to intravenous zoledronic acid in post-menopausal women. Bone 2011, 49, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Makras, P.; Anastasilakis, A.D.; Polyzos, S.; Bisbinas, I.; Sakellariou, G.T.; Papapoulos, S.E. No effect of rosuvastatin in the zoledronate-induced acute phase response. Calcif. Tissue Int. 2011, 88, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Papapoulos, S.E. Bisphosphonate actions: Physical chemistry revisited. Bone 2006, 38, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Papapoulos, S.E. Pamidronate: A model compound of the pharmacology of nitrogen containing bisphosphonates; A Leiden historical perspective. Bone 2020, 134, 115244. [Google Scholar] [CrossRef] [PubMed]
- Landman, J.O.; Hamdy, N.A.; Pauwels, E.K.; Papapoulos, S.E. Skeletal metabolism in patients with osteoporosis after discontinuation of long-term treatment with oral pamidronate. J. Clin. Endocrinol. Metab. 1995, 80, 3465–3468. [Google Scholar] [CrossRef] [PubMed]
- Papapoulos, S.E. Cremers SC: Prolonged bisphosphonate release after treatment in children. N. Engl. J. Med. 2007, 356, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.K.; Yang, K.Y.; Koh, J.S.; Wong, M.K.; Chua, S.Y.; Chua, D.T.; Howe, T.S. Subtrochanteric insufficiency fractures in patients on alendronate therapy: A caution. J. Bone Jt. Surg. Br. 2007, 89, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Kwek, E.B.; Goh, S.K.; Koh, J.S.; Png, M.A.; Howe, T.S. An emerging pattern of subtrochanteric stress fractures: A long-term complication of alendronate therapy? Injury 2008, 39, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Lenart, B.A.; Lorich, D.G.; Lane, J.M. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N. Engl. J. Med. 2008, 358, 1304–1305. [Google Scholar] [CrossRef] [PubMed]
- Giusti, A.; Hamdy, N.A.T.; Papapoulos, S.E. Atypical fractures of the femur and use of bisphosphonates: A systematic review of cases/case studies. Bone 2010, 47, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Akesson, K.; Bouxsein, M.; Kanis, J.A.; Napoli, N.; Papapoulos, S.; Reginster, J.Y.; Cooper, C. Subtrochanteric fractures after long-term treatment with bisphosphonates: A European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos. Int. 2011, 22, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.A. Update on Rare Adverse Events from Osteoporosis Therapy and Bisphosphonate Drug Holidays. Endocrinol. Metab. Clin. N. Am. 2021, 50, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Ebeling, P.R.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2010, 25, 2267–2294. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Ebeling, P.R.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American society for bone and mineral research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Giusti, A.; Hamdy, N.A.T.; Dekkers, O.M.; Ramautar, S.R.; Dijkstra, S.; Papapoulos, S.E. Atypical fractures and bisphosphonate therapy: A cohort study of patients with femoral fractures with radiographic adjudication of fracture site and features. Bone 2011, 48, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.C.; Black, D.M.; Dell, R.; Fan, B.; Smith, C.D.; Ernst, M.T.; Jurik, A.G.; Frøkjær, J.B.; Boesen, M.; Vittinghoff, E.; et al. Bisphosphonate Use and Risk of Atypical Femoral Fractures: A Danish Case-Cohort Study with Blinded Radiographic Review. J. Clin. Endocrinol. Metab. 2024, 109, e2141–e2150. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Abrahamsen, B.; Bouxsein, M.L.; Einhorn, T.; Napoli, N. Atypical femur fractures: Review of epidemiology, relationship to bisphosphonates, prevention, and clinical management. Endocr. Rev. 2019, 40, 333–368. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Condra, K.; Adams, A.L.; Eastell, R. Bisphosphonates and the risk of atypical femur fractures. Bone 2022, 156, 116297. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Reid, I.R.; Boonen, S.; Bucci-Rechtweg, C.; Cauley, J.A.; Cosman, F.; Cummings, S.R.; Hue, T.F.; Lippuner, K.; Lakatos, P.; et al. The effect of 3 versus 6 years of Zoledronic acid treatment of osteoporosis: A randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J. Bone Miner. Res. 2012, 27, 2612. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Napoli, N.; Palermo, A.; Magopoulos, C.; Khan, A.A.; Zillikens, M.C.; Body, J.-J. Osteonecrosis of the Jaw and Antiresorptive Agents in Benign and Malignant Diseases: A Critical Review Organized by the ECTS. J. Clin. Endocrinol. Metab. 2022, 107, 1441–1460. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B.; Grbic, J.T.; Binkley, N.; Papapoulos, S.; Butler, P.W.; Yin, X.; Tierney, A.; Wagman, R.B.; McClung, M. Invasive Oral Procedures and Events in Women with Postmenopausal Osteoporosis Treated with Denosumab for up to 10 Years. J. Clin. Endocrinol. Metab. 2019, 104, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.A.; El-Hajj Fuleihan, G.; Bauer, D.C.; Camacho, P.M.; Clarke, B.L.; Clines, G.A.; Compston, J.E.; Drake, M.T.; Edwards, B.J.; Favus, M.J.; et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2016, 31, 16–35. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.R. Sequential and Long-term Therapy for Osteoporosis. Curr. Osteoporos. Rep. 2025, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Roux, C.; Benhamou, C.L.; Brown, J.P.; Lillestol, M.; Siddhanti, S.; Man, H.-S.; Martin, J.S.; Bone, H.G. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J. Bone Miner. Res. 2010, 25, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kendler, D.L.; Marin, F.; Zerbini, C.A.F.; Russo, L.A.; Greenspan, S.L.; Zikan, V.; Bagur, A.; Malouf-Sierra, J.; Lakatos, P.; Fahrleitner-Pammer, A.; et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO); a multicentre, double-blind, double-dummy, randomized controlled trial. Lancet 2018, 391, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Bolognese, M.A.; Yuen, C.K.; Kendler, D.L.; Miller, P.D.; Yang, Y.C.; Grazette, L.; Martin, J.S.; Gallagher, J.C. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J. Clin. Endocrinol. Metab. 2011, 96, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Aubry-Rozier, B.; Kaouri, S.; Lamy, O. Clinical Features of 24 Patients with Rebound-Associated Vertebral Fractures After Denosumab Discontinuation: Systematic Review and Additional Cases. J. Bone Miner. Res. 2017, 32, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Huang, S.; McDermott, M.; Cummings, S.R. Multiple vertebral fractures after denosumab discontinuation: FREEDOM and FREEDOM Extension trials additional post hoc analyses. J. Bone Miner. Res. 2022, 37, 2112–2120. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Zillikens, M.C.; Meier, C.; Body, J.J.; Gonzalez Rodriguez, E.; Anastasilakis, A.D.; Abrahamsen, B.; McCloskey, E.; Hofbauer, L.C.; Guañabens, N.; et al. Fracture risk and management of discontinuation of denosumab therapy: A systematic review and position statement by ECTS. J. Clin. Endocrinol. Metab. 2021, 106, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Lewiecki, E.M.; Eastell, R.; Ebeling, P.R.; Jan De Beur, S.; Langdahl, B.; Rhee, Y.; Fuleihan, G.E.-H.; Kiel, D.P.; Schousboe, J.T.; et al. Goal-directed osteoporosis treatment: ASBMR/BHOF task force position statement 2024. J. Bone Miner. Res. 2024, 39, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Langdahl, B.; Leder, B.Z. Treatment sequence for osteoporosis. Endocr. Pract. 2024, 30, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Makras, P.; Papapoulos, S. Bisphosphonates for postmenopausal osteoporosis. In Primer of the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 10th ed.; Bilezikian, J.P., Ed.; LWW: Philadelphia, PA, USA, 2025; pp. 529–534. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papapoulos, S.E.; Makras, P. Bisphosphonates in the Management of Patients with Postmenopausal Osteoporosis; Back to the Future. Pharmaceuticals 2025, 18, 1068. https://doi.org/10.3390/ph18071068
Papapoulos SE, Makras P. Bisphosphonates in the Management of Patients with Postmenopausal Osteoporosis; Back to the Future. Pharmaceuticals. 2025; 18(7):1068. https://doi.org/10.3390/ph18071068
Chicago/Turabian StylePapapoulos, Socrates E., and Polyzois Makras. 2025. "Bisphosphonates in the Management of Patients with Postmenopausal Osteoporosis; Back to the Future" Pharmaceuticals 18, no. 7: 1068. https://doi.org/10.3390/ph18071068
APA StylePapapoulos, S. E., & Makras, P. (2025). Bisphosphonates in the Management of Patients with Postmenopausal Osteoporosis; Back to the Future. Pharmaceuticals, 18(7), 1068. https://doi.org/10.3390/ph18071068







