Association of SLCO1B3 and SLCO1B1 Polymorphisms with Methotrexate Efficacy and Toxicity in Saudi Rheumatoid Arthritis Patients
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Data
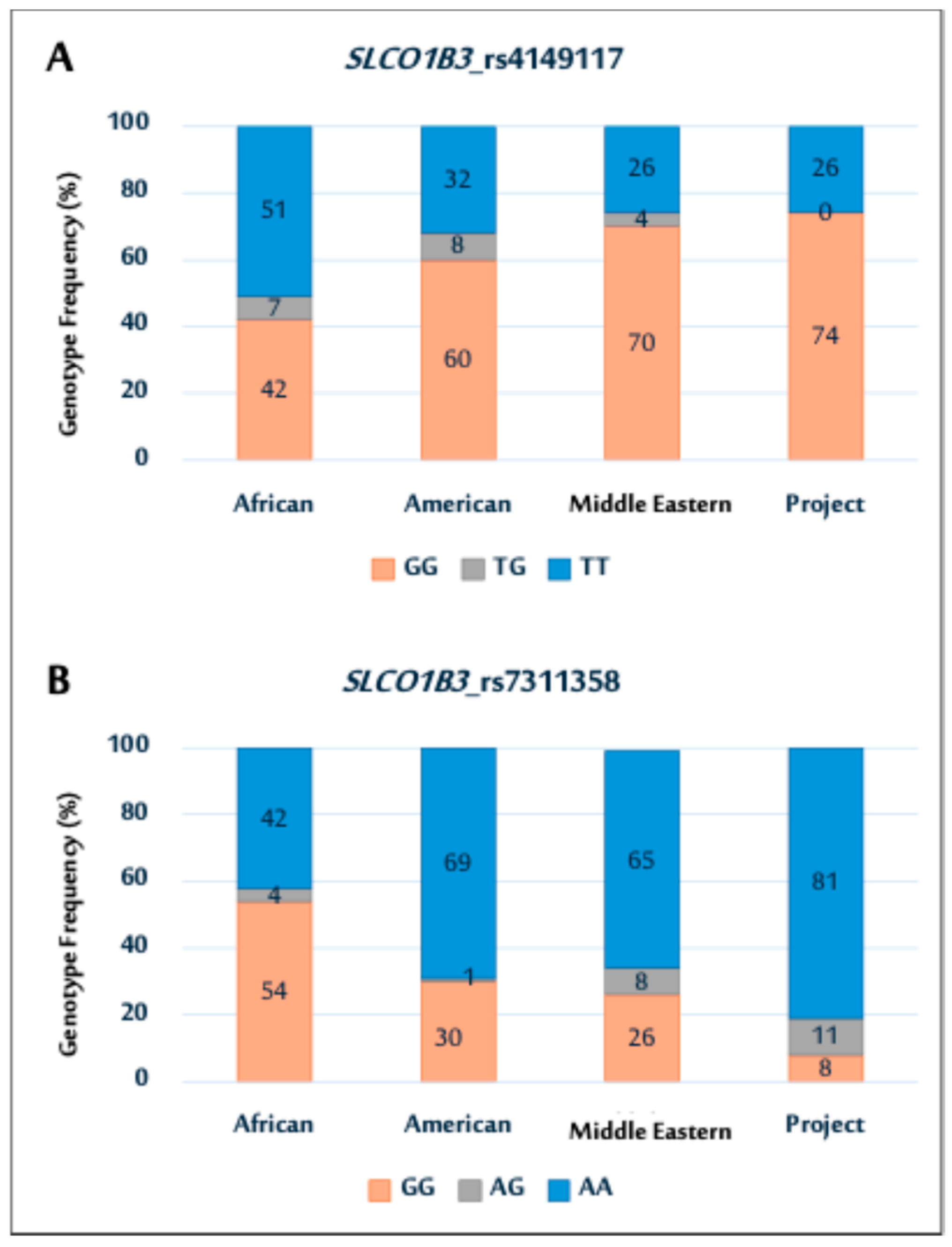
2.2. Genetic Association Data for SLCO1B3 Polymorphisms
Genotype Distributions and Allele Frequencies of SLCO1B3 Polymorphisms
2.3. Association Between SLCO1B3 Gene Polymorphisms and Patient Response to MTX
2.4. Association Between the Haplotypes of SLCO1B3 Polymorphisms and Their Response to MTX
2.5. Associations Between SLCO1B3 Gene Polymorphisms and the Risk of MTX Adverse Reactions
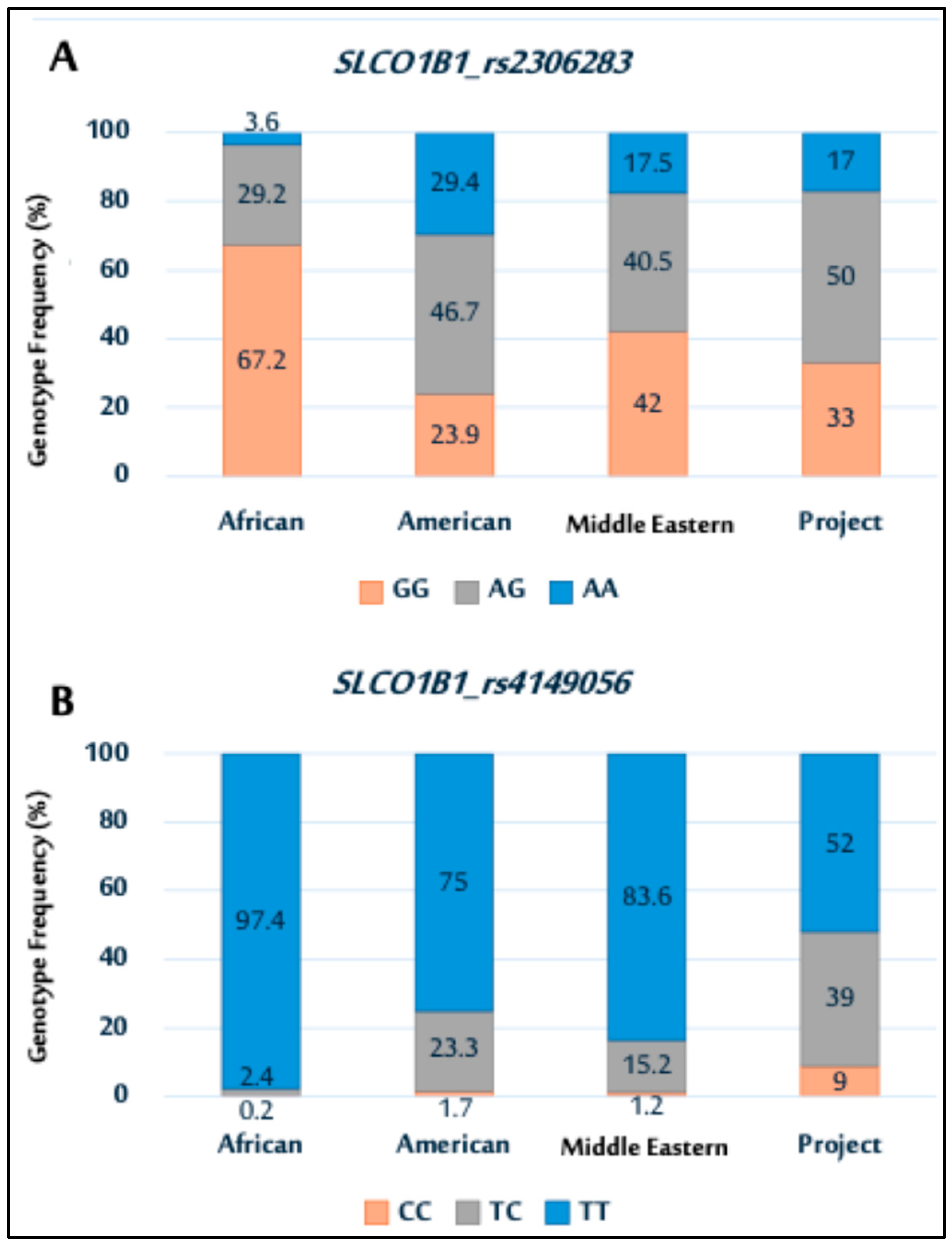
2.6. Genetic Association Data for SLCO1B1 Polymorphisms
Genotype Distributions and Allele Frequencies of SLCO1B1 Polymorphisms
2.7. Association Between SLCO1B1 Gene Polymorphisms and Patient Response to MTX
2.8. Association Between SLCO1B1 Polymorphism Haplotypes and Response to MTX
2.9. Associations Between SLCO1B1 Gene Polymorphisms and the Risk of MTX Adverse Reactions
2.10. Association Between SLCO1B3 and SLCO1B1 Polymorphism Haplotypes and the Response to MTX
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Ethical Approval and Ethical Consideration
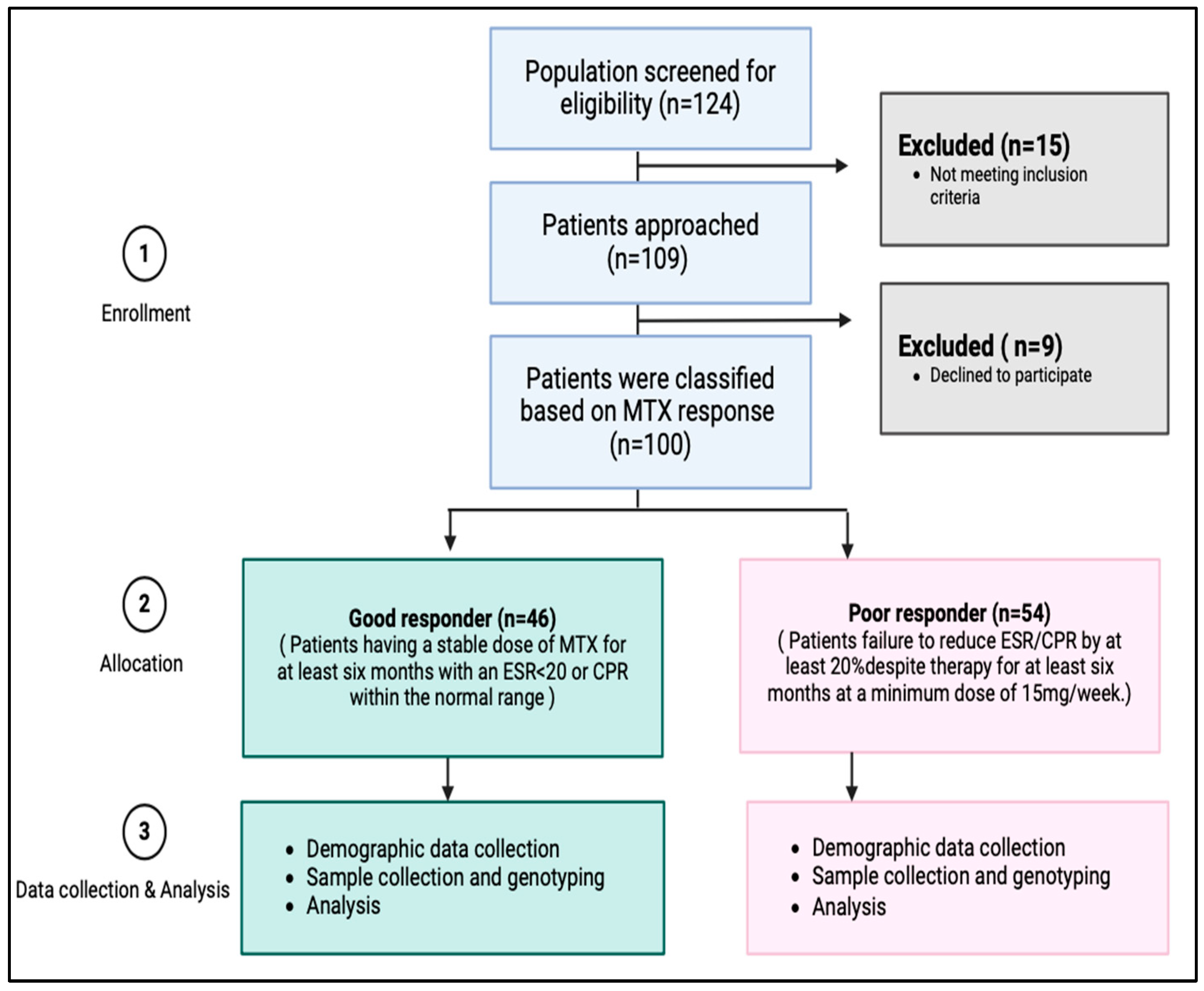
4.3. Subjects and Data Source
4.3.1. Participant Recruitment
4.3.2. Inclusion and Exclusion Criteria
4.4. Outcomes Measure
4.5. Selection of SNPs
4.5.1. Candidate Genes and SNP Selection
4.5.2. Target-Specific Primer Design
4.6. Genotyping
4.6.1. DNA Extraction and Quantification
4.6.2. Polymerase Chain Reaction (PCR)
4.6.3. Sanger Sequencing
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Hadi, S.J.; Naji, H.H.; Ali, S.H.; Radhi, Z.A. A review of some the physiological effects of methotrexate on rheumatoid arthritis. South Asian Res. J. Pharm. Sci. 2024, 6, 22–28. [Google Scholar] [CrossRef]
- Hassen, N.; Lacaille, D.; Xu, A.; Alandejani, A.; Sidi, S.; Mansourian, M.; Butt, Z.A.; Cahill, L.E.; Iyamu, I.O.; Lang, J.J.; et al. National burden of rheumatoid arthritis in Canada, 1990–2019: Findings from the Global Burden of Disease Study 2019—A GBD collaborator-led study. RMD Open 2024, 10, e003533. [Google Scholar] [CrossRef] [PubMed]
- Almoallim, H.M.; Alharbi, L.A. Rheumatoid arthritis in Saudi Arabia. Saudi Med. J. 2014, 35, 1442–1454. [Google Scholar] [PubMed]
- Meade, T.; Joyce, C.; Perich, T.; Manolios, N.; Conaghan, P.G.; Katz, P. Prevalence, severity, and measures of anxiety in rheumatoid arthritis: A systematic review. Arthritis Care Res. 2024, 76, 171–180. [Google Scholar] [CrossRef]
- Sen, R.; Riofrio, M.; Singh, J.A. A narrative review of the comparative safety of disease-modifying anti-rheumatic drugs used for the treatment of rheumatoid arthritis. Expert Opin. Drug Saf. 2024, 23, 687–714. [Google Scholar] [CrossRef]
- Sha, H.X.; Veerapen, K.; Chow, S.K.; Gun, S.C.; Lau, I.S.; Lim, R.L.H.; Zulkifli, Z.; Yow, Y.Y.; Peh, S.C.; Hwang, J.S. Genetic variations in methotrexate metabolic pathway genes influence methotrexate responses in rheumatoid arthritis patients in Malaysia. Sci. Rep. 2022, 12, 11844. [Google Scholar] [CrossRef]
- Cen, H.; Wen, Q.W.; Zhang, H.Q.; Yu, H.; Zeng, Z.; Jin, T.; Wang, T.H.; Qin, W.; Huang, H.; Wu, X.D. Associations between genetic polymorphisms within transporter genes and clinical response to methotrexate in Chinese rheumatoid arthritis patients: A pilot study. Pharmacogenom. Pers. Med. 2022, 15, 327–339. [Google Scholar] [CrossRef]
- Dashti, M.; Al-Matrouk, A.; Channanath, A.; Al-Mulla, F.; Thanaraj, T.A. Frequency of functional exonic single-nucleotide polymorphisms and haplotype distribution in the SLCO1B1 gene across genetic ancestry groups in the Qatari population. Sci. Rep. 2022, 12, 14858. [Google Scholar] [CrossRef]
- Sun, R.; Ying, Y.; Tang, Z.; Liu, T.; Shi, F.; Li, H.; Guo, T.; Huang, S.; Lai, R. The emerging role of the SLCO1B3 protein in cancer resistance. Protein Pept. Lett. 2020, 27, 17–29. [Google Scholar] [CrossRef]
- Rajanala, S.H.; Plym, A.; Vaselkiv, J.B.; Ebot, E.M.; Matsoukas, K.; Lin, Z.; Chakraborty, G.; Markt, S.C.; Penney, K.L.; Lee, G.M.; et al. SLCO1B3 and SLCO2B1 genotypes, androgen deprivation therapy, and prostate cancer outcomes: A prospective cohort study and meta-analysis. Carcinogenesis 2024, 45, 35–44. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Chen, C.; Chen, S.; Xu, J.; Zhou, Y.; Zhao, X.; Cui, Y. Influence of the OATP polymorphism on the population pharmacokinetics of methotrexate in Chinese patients. Curr. Drug Metab. 2019, 20, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Anabtawi, N.; Drabison, T.; Hu, S.; Sparreboom, A.; Talebi, Z. The Role of OATP1B1 and OATP1B3 transporter polymorphisms in drug disposition and response to anticancer drugs: A review of the recent literature. Expert Opin. Drug Metab. Toxicol. 2022, 18, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, L.F.; Giraldo, R.; Londoño, J.; Pinzón, C.; Cortes, A.; Ballesteros, G.; Santos, A.M. Pharmacogenetics of methotrexate in rheumatoid arthritis: A systematic review. Rev. Colomb. Reumatol. (Engl. Ed.) 2016, 23, 102–114. [Google Scholar] [CrossRef]
- Carmona, L.; Aurrecoechea, E.; García de Yébenes, M.J. Tailoring rheumatoid arthritis treatment through a sex and gender lens. J. Clin. Med. 2023, 13, 55. [Google Scholar] [CrossRef]
- Namas, R.; Joshi, A.; Ali, Z.; Al Saleh, J.; Abuzakouk, M. Demographic and clinical patterns of rheumatoid arthritis in an Emirati cohort from United Arab Emirates. Int. J. Rheumatol. 2019, 2019, 3057578. [Google Scholar] [CrossRef]
- Duong, S.Q.; Crowson, C.S.; Athreya, A.; Atkinson, E.J.; Davis, J.M., III; Warrington, K.J.; Matteson, E.L.; Weinshilboum, R.; Wang, L.; Myasoedova, E. Clinical predictors of response to methotrexate in patients with rheumatoid arthritis: A machine learning approach using clinical trial data. Arthritis Res. Ther. 2022, 24, 162. [Google Scholar] [CrossRef]
- Majorczyk, E.; Mazurek-Mochol, M.; Pawlik, A.; Kuśnierczyk, P. Clinical factors and the outcome of treatment with methotrexate in rheumatoid arthritis: Role of rheumatoid factor, erosive disease, and high level of erythrocyte sedimentation rate. J. Clin. Med. 2022, 11, 6078. [Google Scholar] [CrossRef]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an old drug with new tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef]
- Duquesne, J.; Bouget, V.; Cournede, P.H.; Fautrel, B.; Guillemin, F.; de Jong, P.H.; Heutz, J.W.; Verstappen, M.; van der Helm-van Mil, A.H.; Mariette, X.; et al. Machine learning identifies a profile of inadequate responder to methotrexate in rheumatoid arthritis. Rheumatology 2023, 62, 2402–2409. [Google Scholar] [CrossRef]
- Waki, D.; Tamai, H.; Yokochi, R.; Kido, T.; Yagyu, Y.; Yanai, R.; Sada, K.E. Effects of anti-SSA antibodies on the response to methotrexate in rheumatoid arthritis: A retrospective multicenter observational study. PLoS ONE 2022, 17, e0271921. [Google Scholar] [CrossRef]
- Lima, A.; Bernardes, M.; Azevedo, R.; Monteiro, J.; Sousa, H.; Medeiros, R.; Seabra, V. SLC19A1, SLC46A1, and SLCO1B1 polymorphisms as predictors of methotrexate-related toxicity in Portuguese rheumatoid arthritis patients. Toxicol. Sci. 2014, 142, 196–209. [Google Scholar] [CrossRef]
- Pasanen, M.K.; Neuvonen, P.J.; Niemi, M. Global analysis of genetic variation in SLCO1B1. Pharmacogenomics 2008, 9, 19–33. [Google Scholar] [CrossRef]
- Suarez-Kurtz, G.; Pena, S.D.J.; Struchiner, C.J.; Hutz, M.H. Pharmacogenomic diversity among Brazilians: Influence of ancestry, self-reported color, and geographical origin. Front. Pharmacol. 2012, 3, 191. [Google Scholar] [CrossRef]
- Banach, B.; Modrzejewski, A.; Juzyszyn, Z.; Kurzawski, M.; Sroczynski, T.; Pawlik, A. Association study of SLCO1B3 and ABCC3 genetic variants in gallstone disease. Genes 2022, 13, 512. [Google Scholar] [CrossRef]
- Jafari, F.; Arasteh, O.; Hosseinjani, H.; Allahyari, A.; Ataei Azimi, S.; Askari, V.R. A critical review of methotrexate clinical interactions: Role of transporters. Expert Opin. Drug Metab. Toxicol. 2023, 19, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Chung, J.E.; Yee, J.; Lee, K.E.; Park, K.; Gwak, H.S. Effects of SLCO1B1 and SLCO1B3 genetic polymorphisms on valsartan pharmacokinetics in healthy Korean volunteers. J. Pers. Med. 2021, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Jenko, B.; Tomšič, M.; Jekić, B.; Milić, V.; Dolžan, V.; Praprotnik, S. Clinical pharmacogenetic models of treatment response to methotrexate monotherapy in Slovenian and Serbian rheumatoid arthritis patients: Differences in patient’s management may preclude generalization of the models. Front. Pharmacol. 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.G.; Gao, C.; Zhang, R.D.; Zhao, X.X.; Cui, L.; Li, W.J.; Chen, Z.P.; Yue, Z.X.; Zhang, Y.Y.; Wu, M.Y.; et al. Polymorphisms in methotrexate transporters and their relationship to plasma methotrexate levels, toxicity of high-dose methotrexate, and outcome of pediatric acute lymphoblastic leukemia. Oncotarget 2017, 8, 37761–37772. [Google Scholar] [CrossRef]
- Lui, G.; Treluyer, J.M.; Fresneau, B.; Piperno-Neumann, S.; Gaspar, N.; Corradini, N.; Gentet, J.C.; Marec Berard, P.; Laurence, V.; Schneider, P.; et al. A pharmacokinetic and pharmacogenetic analysis of osteosarcoma patients treated with high-dose methotrexate: Data from the OS2006/Sarcoma-09. Trial. J. Clin. Pharmacol. 2018, 58, 1541–1549. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, P.; Yang, Z.; Miao, R.R. Relationship between the efficacy and adverse effects of methotrexate and gene polymorphism. Egypt. J. Med. Hum. Genet. 2024, 25, 89. [Google Scholar] [CrossRef]
- Strand, V.; Sharp, V.; Koenig, A.S.; Park, G.; Shi, Y.; Wang, B.; Zack, D.J.; Fiorentino, D. Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis and psoriasis and effects of etanercept treatment. Ann. Rheum. Dis. 2012, 71, 1143–1150. [Google Scholar] [CrossRef]
- Jansen, M.E.; Rigter, T.; Fleur, T.M.C.; Souverein, P.C.; Verschuren, W.M.M.; Vijverberg, S.J.; Swen, J.J.; Rodenburg, W.; Cornel, M.C. Predictive value of SLCO1B1 c.521T> C polymorphism on observed changes in the treatment of 1136 statin-users. Genes 2023, 14, 456. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, D.C.; Wanderley, A.V.; Dos Santos, A.M.R.; Fernandes, M.R.; Cohen Lima de Castro, A.N.; Leitão, L.P.C.; de Carvalho, J.A.N.; de Souza, T.P.; Khayat, A.S.; Dos Santos, S.E.B.; et al. Pharmacogenomics and variations in the risk of toxicity during the consolidation/maintenance phases of the treatment of pediatric B-cell leukemia patients from an admixed population in the Brazilian Amazon. Leuk. Res. 2018, 74, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, M.H.; Zhuang, Q.; Lin, B.J.; Chen, Y.Y.; Yang, L.; Liu, M.B.; Que, W.C.; Qiu, H.Q. Genetic factors involved in delayed methotrexate elimination in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2021, 68, e28858. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jin, L.; Yang, J.; Duan, L.Y.; Zhang, M.; Zhou, J.C.; Zhang, H.Y. Study on relationship of tumor status and gene polymorphism with blood concentration of MTX and toxicities in 63 Pediatric Mature B cell lymphomas in a Chinese population. Technol. Cancer Res. Treat. 2021, 20, 1533033821995288. [Google Scholar] [CrossRef]
- Darney, K.; Turco, L.; Buratti, F.M.; Di Consiglio, E.; Vichi, S.; Roudot, A.C.; Béchaux, C.; Testai, E.; Dorne, J.L.C.M.; Lautz, L.S. Human variability in influx and efflux transporters in relation to uncertainty factors for chemical risk assessment. Food Chem. Toxicol. 2020, 140, 111305. [Google Scholar] [CrossRef]
- Alam, K.; Farasyn, T.; Ding, K.; Yue, W. Characterization of liver- and cancer-type organic anion transporting polypeptide (OATP) 1B3 messenger RNA expression in normal and cancerous human tissues. Drug Metab. Lett. 2018, 12, 24–32. [Google Scholar] [CrossRef]
- Fransen, J.; Van Riel, P.L.C.M. The Disease Activity Score and the EULAR response criteria. Clin. Exp. Rheumatol. 2005, 23, S93. [Google Scholar] [CrossRef]
- Sortica, V.D.A.; Ojopi, E.B.; Genro, J.P.; Callegari-Jacques, S.; Ribeiro-dos-Santos, Â.; de Moraes, M.O.; Romano-Silva, M.A.; Pena, S.D.; Suarez-Kurtz, G.; Hutz, M.H. Influence of genomic ancestry on the distribution of SLCO1B1, SLCO1B3 and ABCB1 gene polymorphisms among Brazilians. Basic Clin. Pharmacol. Toxicol. 2012, 110, 460–468. [Google Scholar] [CrossRef]
- Magadmi, R.; Alyoubi, R.; Moshrif, T.; Bakhshwin, D.; Suliman, B.A.; Kamel, F.; Jamal, M.; Burzangi, A.S.; Basit, S. Polymorphisms in the Drug Transporter Gene ABCB1 Are Associated with Drug Response in Saudi Epileptic Pediatric Patients. Biomedicines 2023, 11, 2505. [Google Scholar] [CrossRef]
- Solé, X.; Guinó, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A web tool for the analysis of association studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef]
| Feature | Mean | SD | |
|---|---|---|---|
| Age (years) | 53.02 | 12.78 | |
| Age of onset (years) | 46.26 | 12.35 | |
| Frequency | Percentage | ||
| Disease duration | 6 months–1 year | 13 | 13% |
| >1 year | 87 | 87% | |
| Sex | Male | 20 | 20% |
| Female | 80 | 80% | |
| MTX | Mean | SD | |
| MTX dose | 14.98 | 3.7 | |
| MTX | Frequency | Percentage | |
| Duration of MTX treatment | 6 months–1 year | 15 | 15% |
| >1 year | 85 | 85% | |
| Regimen type | Monotherapy | 44 | 44% |
| Combined therapy | 56 | 56% | |
| Markers of disease activity | Mean | SD | |
| DAS-28 | 2.94 | 0.96 | |
| CRP | 10.56 | 7.4 | |
| ESR | 27.44 | 17.33 | |
| Markers of disease activity | Frequency | Percentage | |
| RF | +ve | 68 | 68% |
| −ve | 28 | 28% | |
| Not known | 4 | 4% | |
| Anti-CCP | +ve | 62 | 62% |
| −ve | 15 | 15% | |
| Not known | 23 | 23% | |
| Adverse drug reaction | Frequency | Percentage | |
| Adverse drug reaction § | GI disturbance | 61 | 61% |
| Increased liver enzymes | 25 | 25% | |
| Anemia | 14 | 14% | |
| Classification of RA based on response to MTX | Frequency | Percentage | |
| Patient response | Good responders | 46 | 46% |
| Poor responders | 54 | 54% | |
| Feature | Good Responders (N = 46) |
Poor Responders (N = 54) | p-Value | |
|---|---|---|---|---|
| Age (years) | 52.50 (12.64) | 53.416 (13.00) | 0.709 t | |
| Age of onset (years) | 47.79 (11.76) | 44.98 (12.80) | 0.264 t | |
| Disease duration | 6 months–1 year | 11 (23.9) | 2 (3.7) | 0.009 * |
| >1 year | 35 (76.1) | 52 (96.3) | ||
| Sex | Male | 8 (17.4) | 12 (22.2) | 0.621 |
| Female | 38 (82.6) | 42 (77.8) | ||
| MTX | ||||
| MTX dose (mean) | 15.05 (3.00) | 14.91 (4.23) | 0.844 t | |
| Duration of MTX treatment | 6 months–1 year | 10 (21.7) | 5 (9.3) | 0.098 F |
| >1 year | 36 (78.3) | 49 (90.7) | ||
| Regimen type | Monotherapy | 30 (65.2) | 14 (25.9) | <0.001 * |
| Combined therapy (with steroid) | 16 (34.6) | 40 (74.1) | ||
| Markers of disease activity | ||||
| DAS-28 (mean (SD)) | 2.36 (0.90) | 3.43 (0.70) | <0.001 *t | |
| CRP (mean (SD)) | 7.74 (4.41) | 12.96 (8.54) | <0.001 *t | |
| ESR (mean (SD)) | 23.70 (15.86) | 30.63 (18.02) | 0.046 *t | |
| RF | +ve | 32 (69.6) | 36 (66.7) | 0.690 |
| −ve | 13 (28.3) | 15 (27.8) | ||
| Not known | 1 (2.2) | 3 (5.6) | ||
| Anti-CCP | +ve | 30 (65.2) | 32 (59.3) | 0.442 |
| −ve | 8 (17.4) | 7 (13.0) | ||
| Not known | 8 (17.4) | 15 (27.8) | ||
| Adverse drug reaction | ||||
| Adverse drug reaction § | GI disturbance | 30 (30) | 31 (31) | 0.642 |
| Increased liver enzymes | 11 (11) | 14 (14) | ||
| Anemia | 5 (5) | 9 (9) | ||
| Genotype/Allele | N (%) |
|---|---|
| SLCO1B3_rs4149117 (N = 100) | |
| GG | 74 (74) |
| TG | 0 (0) |
| TT | 26 (26) |
| G | 148 (74) |
| T | 52 (26) |
| SLCO1B3_rs7311358 (N = 100) | |
| AA | 81(81) |
| AG | 11 (11) |
| GG | 8 (8) |
| A | 173 (87) |
| G | 27 (13) |
| Genotype/Allele | Good Responders N (%) | Poor Responders N (%) | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| N = 46 | N = 54 | |||
| SLCO1B3_rs4149117 (N = 100) | ||||
| GG | 35 (76.1%) | 39 (72.2%) | 0.81 (0.33–2.013) | 0.66 |
| TT | 11 (23.9%) | 15 (27.8%) | 1.2 (0.496–3.01) | |
| G | 70 (76.1%) | 78 (72.2%) | 1.2 (0.64–2.31) | 0.53 |
| T | 22 (23.9%) | 30 (27.8%) | 0.81 (0.43–1.54) | |
| SLCO1B3_rs7311358 (N = 100) | ||||
| AA | 39 (84.8%) | 42 (77.8%) | 1.5 (0.56–4.45) | 0.45 |
| AG | 5 (10.9%) | 6 (11.1%) | 0.97 (0.27–3.4) | |
| GG | 2 (4.3%) | 6 (11.1%) | 0.36 (0.06–1.8) | |
| A | 83 (90%) | 90 (83%) | 1.84 (0.78–4.33) | 0.16 |
| G | 9 (10%) | 18 (17%) | 0.54 (0.23–1.27) | |
| Haplotype | Haplotype Frequency | Adjusted OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| SLCO1B3 _rs4149117 | SLCO1B3 _rs7311358 | Good Responder (N = 46) | Poor Responder (N = 54) | ||
| G | A | 70 | 78 | 1 | - |
| T | A | 14 | 13 | 0.78 (0.41–1.51) | 0.47 |
| T | G | 6 | 15 | 1.76 (0.78–4.01) | 0.18 |
| G | G | 2 | 2 | 0.95 (0.23–3.86) | 0.94 |
| Genotype | GI Disturbance (N = 61) | p-Value | Increased Liver Enzymes (N = 25) | p-Value | Anemia (N = 14) | p-Value |
|---|---|---|---|---|---|---|
| SLCO1B3_rs4149117 | ||||||
| TT | 17 (27.9) | 0.25 | 6 (24) | 0.549 | 3 (21.4) | 0.0463 * |
| GG | 44 (72.1) | 19 (76) | 11 (78.6) | |||
| SLCO1B3_rs7311358 | ||||||
| AA | 48 (78.7) | 0.084 | 20 (80) | 0.576 | 13 (92.9) | 0.0487 * |
| AG | 10 (16.4) | 1 (4) | 0 | |||
| GG | 3 (4.9) | 4 (16) | 1 (7.1) | |||
| Genotype/Allele | N (%) |
|---|---|
| SLCO1B1_rs2306283 (N = 100) | |
| AA | 17 (17) |
| AG | 50 (50) |
| GG | 33 (33) |
| A | 84 (42) |
| G | 116 (58) |
| SLCO1B1_rs4149056 (N = 100) | |
| TT | 52 (52) |
| TC | 39 (39) |
| CC | 9 (9) |
| T | 143 (72) |
| C | 57 (28) |
| Genotype/Allele | Good Responders N (%) | Poor Responders N (%) | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|---|
| N = 46 | N = 54 | |||
| SLCO1B1_rs2306283 (N = 100) | ||||
| AA | 8 (17.4%) | 9 (16.7%) | 1.05 (0.36–2.9) | 0.18 |
| AG | 27 (58.7%) | 23 (42.6%) | 1.9 (0.89–4.3) | |
| GG | 11 (23.9%) | 22 (40.7%) | 0.46 (0.195–1.08) | |
| A | 43 (47%) | 41(38%) | 1.43 (0.81–2.52) | 0.21 |
| G | 49 (53%) | 67 (62%) | 0.69 (0.39–1.22) | |
| SLCO1B1_rs4149056 (N = 100) | ||||
| TT | 27 (58.7%) | 25 (46.3%) | 1.6 (0.74–3.6) | 0.44 |
| TC | 15 (32.6%) | 24 (44.4%) | 0.6 (0.26–1.3) | |
| CC | 4 (8.7%) | 5 (9.3%) | 0.93 (0.23–3.7) | |
| T | 69 (75%) | 74 (69%) | 1.37 (0.73–2.5) | 0.31 |
| C | 23 (25%) | 34 (31%) | 0.72 (0.38–1.35) | |
| Haplotype | Haplotype Frequency | Adjusted OR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| SLCO1B1 _rs2306283 | SLCO1B1 _rs4149056 | Good Responder (N = 46) | Poor Responder (N = 54) | ||
| A | T | 45 | 36 | 0.46 (0.19–1.09) | 0.01 * |
| G | T | 28 | 37 | 1.24 (0.61–2.50) | 0.55 |
| G | C | 15 | 32 | 1.71 (0.81–3.62) | 0.16 |
| A | C | 4 | 3 | 0.86 (0.20–3.63) | 0.84 |
| Genotype | GI Disturbance (N = 61) | p-Value | Increased Liver Enzymes (N = 25) | p-Value | Anemia (N = 14) | p-Value |
|---|---|---|---|---|---|---|
| SLCO1B1_ rs2306283 | ||||||
| AA | 7 (11.5) | 0.001 * | 5 (20) | 0.111 | 5 (37.7) | 0.009 * |
| AG | 25 (40.9) | 16 (64) | 9 (64.3) | |||
| GG | 29 (47.6) | 4 (16) | 0 | |||
| SLCO1B1_rs4149056 | ||||||
| TT | 24 (39.3) | 0.007 * | 19 (76) | 0.021 * | 9 (64.3) | 0.61 |
| TC | 30 (49.2) | 5 (20) | 4 (28.6) | |||
| CC | 7 (11.5) | 1 (4) | 1 (7.1) | |||
| Haplotype | Haplotype Frequency | p-Value | ||||
|---|---|---|---|---|---|---|
| SLCO1B3 rs4149117 | SLCO1B3 rs7311358 | SLCO1B1 rs2306283 | SLCO1B1 rs4149056 | Good Responder (N = 46) | Poor Responder (N = 54) | |
| G | A | A | T | 33 | 28 | 0.01 * |
| G | A | G | T | 18 | 20 | 0.2 |
| G | A | G | C | 16 | 19 | 0.2 |
| T | A | G | T | 7 | 7 | 0.18 |
| T | G | G | T | 4 | 7 | 0.01 * |
| T | A | A | T | 7 | 1 | 0.2 |
| T | G | G | C | 1 | 4 | 0.2 |
| G | A | A | C | 4 | 2 | 0.18 |
| T | A | G | C | 1 | 2 | 0.2 |
| T | G | A | T | 1 | 1 | 0.2 |
| G | G | A | T | 0 | 1 | 0.18 |
| G | G | G | T | 2 | 0 | 0.01 * |
| T | G | A | C | 0 | 1 | 0.2 |
| SNP ID | Exon Location | Amino Acid Change Location | Protein Change Location | Forward Primer | Reverse Primer |
|---|---|---|---|---|---|
| rs4149117 | Exon 4 | c.334T>G | p.Ser112Ala | CTACACAGACCGAAGTTA | ATGGGAACTGGAAGTATT |
| rs7311358 | Exon 7 | 699G>A | p.Met235Ile | GTAGTTTGAATGCAATAG | GCACTGGGATCTCTGTTT |
| rs2306283 | Exon 5 | 388A>G | p.Asn130Asp | GCATCACCTGAGATAGTA | ACAGGTATTCTAAAGAAT |
| rs4149056 | Exon 5 | 521T>C | p.V174A | TCGTGGAATAGGGGAGAC | ACATGTGGATATATGTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magadmi, R.; Alharthi, A.M.; Alqurashi, L.A.; Jali, I.M.; Sharawi, Z.W.; Jamal, M.H.; Bawazir, Y.; Mustafa, M.; Bahlas, S.M.; Jamal, B.T.; et al. Association of SLCO1B3 and SLCO1B1 Polymorphisms with Methotrexate Efficacy and Toxicity in Saudi Rheumatoid Arthritis Patients. Pharmaceuticals 2025, 18, 1069. https://doi.org/10.3390/ph18071069
Magadmi R, Alharthi AM, Alqurashi LA, Jali IM, Sharawi ZW, Jamal MH, Bawazir Y, Mustafa M, Bahlas SM, Jamal BT, et al. Association of SLCO1B3 and SLCO1B1 Polymorphisms with Methotrexate Efficacy and Toxicity in Saudi Rheumatoid Arthritis Patients. Pharmaceuticals. 2025; 18(7):1069. https://doi.org/10.3390/ph18071069
Chicago/Turabian StyleMagadmi, Rania, Ahlam M. Alharthi, Lina A. Alqurashi, Ibtisam M. Jali, Zeina W. Sharawi, Maha H. Jamal, Yasser Bawazir, Mohammad Mustafa, Sami M. Bahlas, Basma T. Jamal, and et al. 2025. "Association of SLCO1B3 and SLCO1B1 Polymorphisms with Methotrexate Efficacy and Toxicity in Saudi Rheumatoid Arthritis Patients" Pharmaceuticals 18, no. 7: 1069. https://doi.org/10.3390/ph18071069
APA StyleMagadmi, R., Alharthi, A. M., Alqurashi, L. A., Jali, I. M., Sharawi, Z. W., Jamal, M. H., Bawazir, Y., Mustafa, M., Bahlas, S. M., Jamal, B. T., Daghasi, H., Altowairqi, A. S., & Al Shaer, D. S. (2025). Association of SLCO1B3 and SLCO1B1 Polymorphisms with Methotrexate Efficacy and Toxicity in Saudi Rheumatoid Arthritis Patients. Pharmaceuticals, 18(7), 1069. https://doi.org/10.3390/ph18071069







