Curcumin and Papain-Loaded Liposomal Natural Latex Dressings with Phototherapy: A Synergistic Approach to Diabetic Wound Healing
Abstract
1. Introduction
2. Results
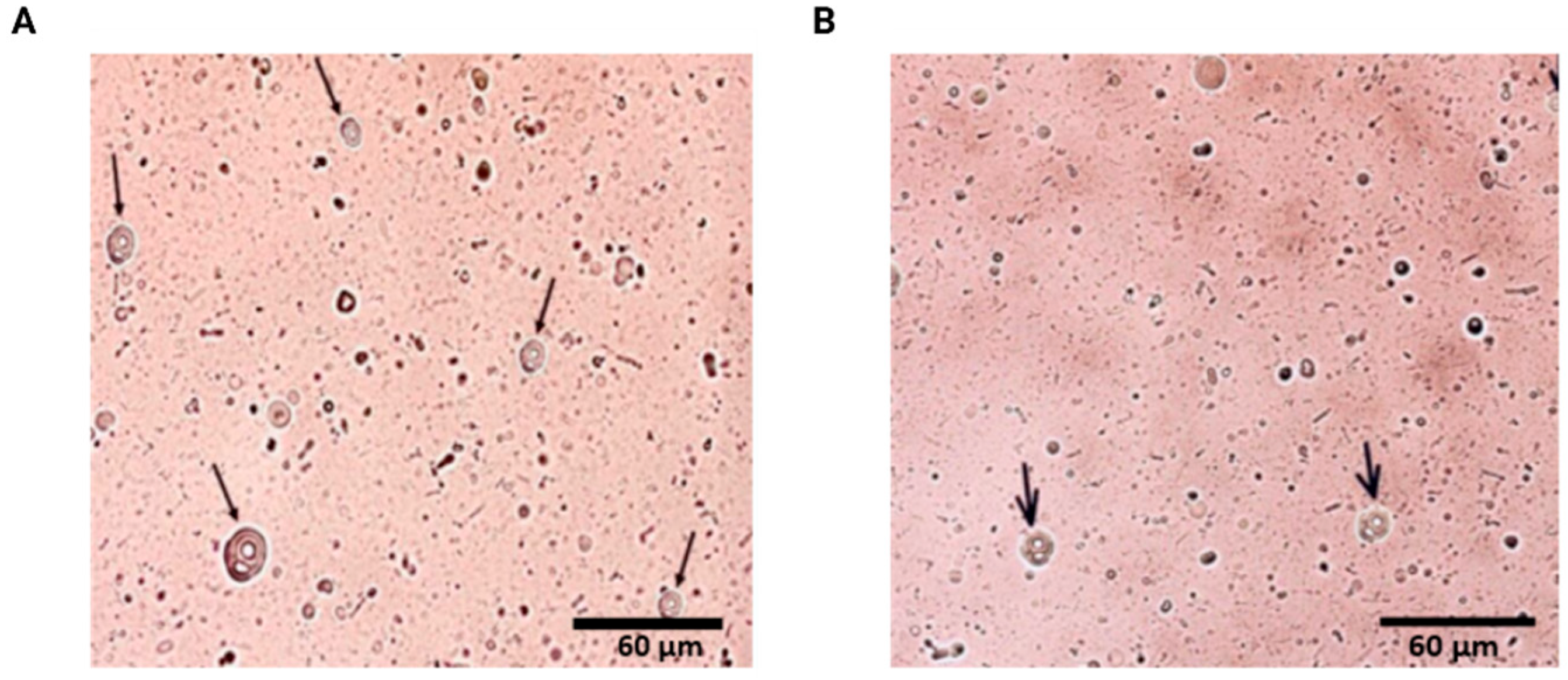
2.1. Characterization of Liposomes
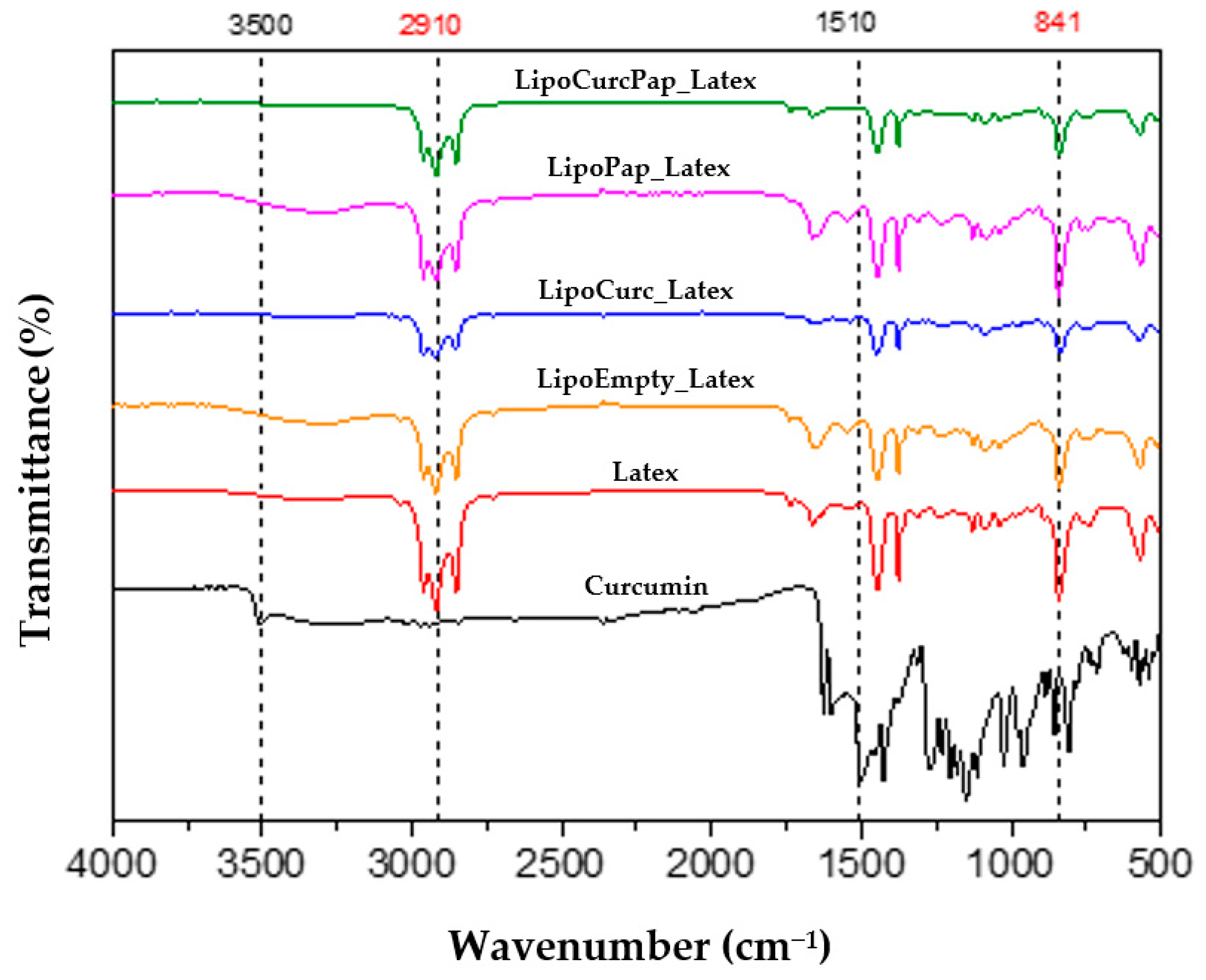
2.2. Characterization of Biomembranes by Fourier Transform Infrared Spectroscopy (FTIR)
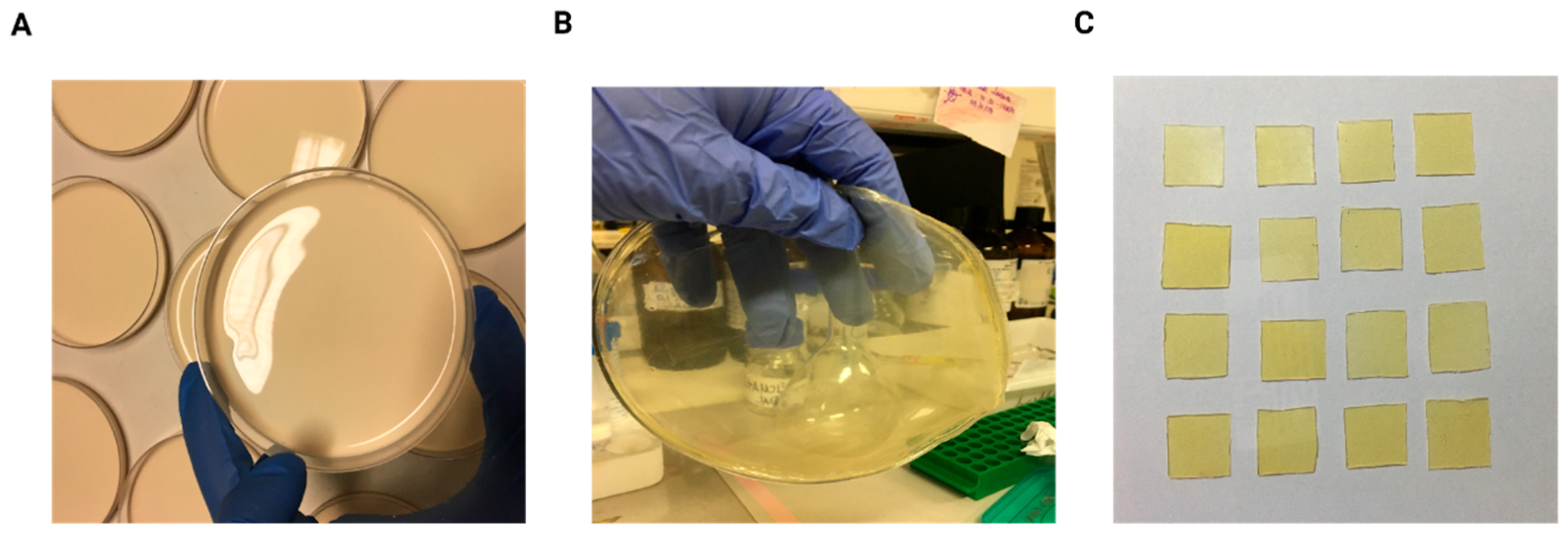
2.3. Production of Natural Latex Biomembranes (NLBs)
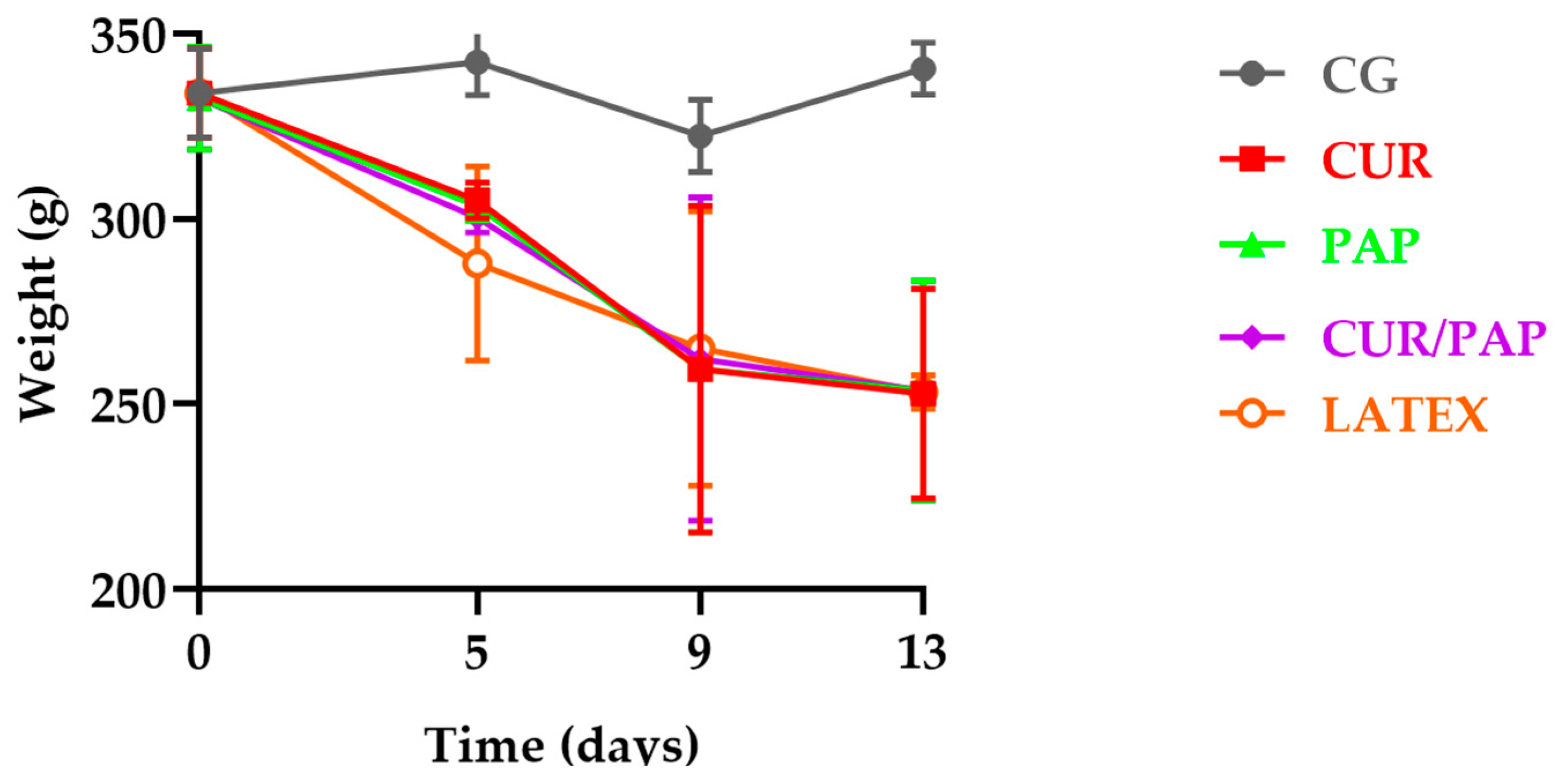
2.4. In Vivo Tests
Clinical Aspects
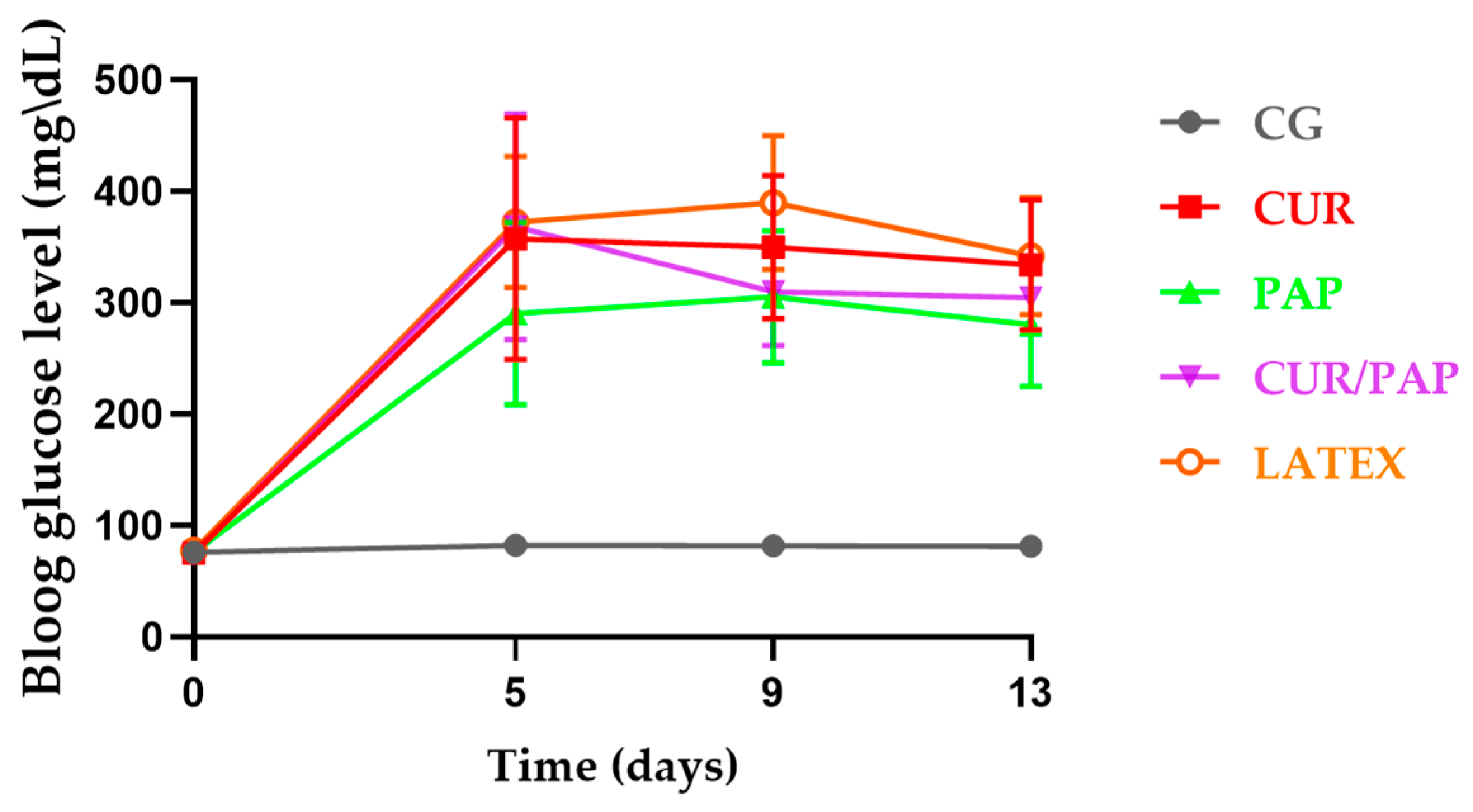
2.5. Biochemical Assessment: Plasma Fructosamine
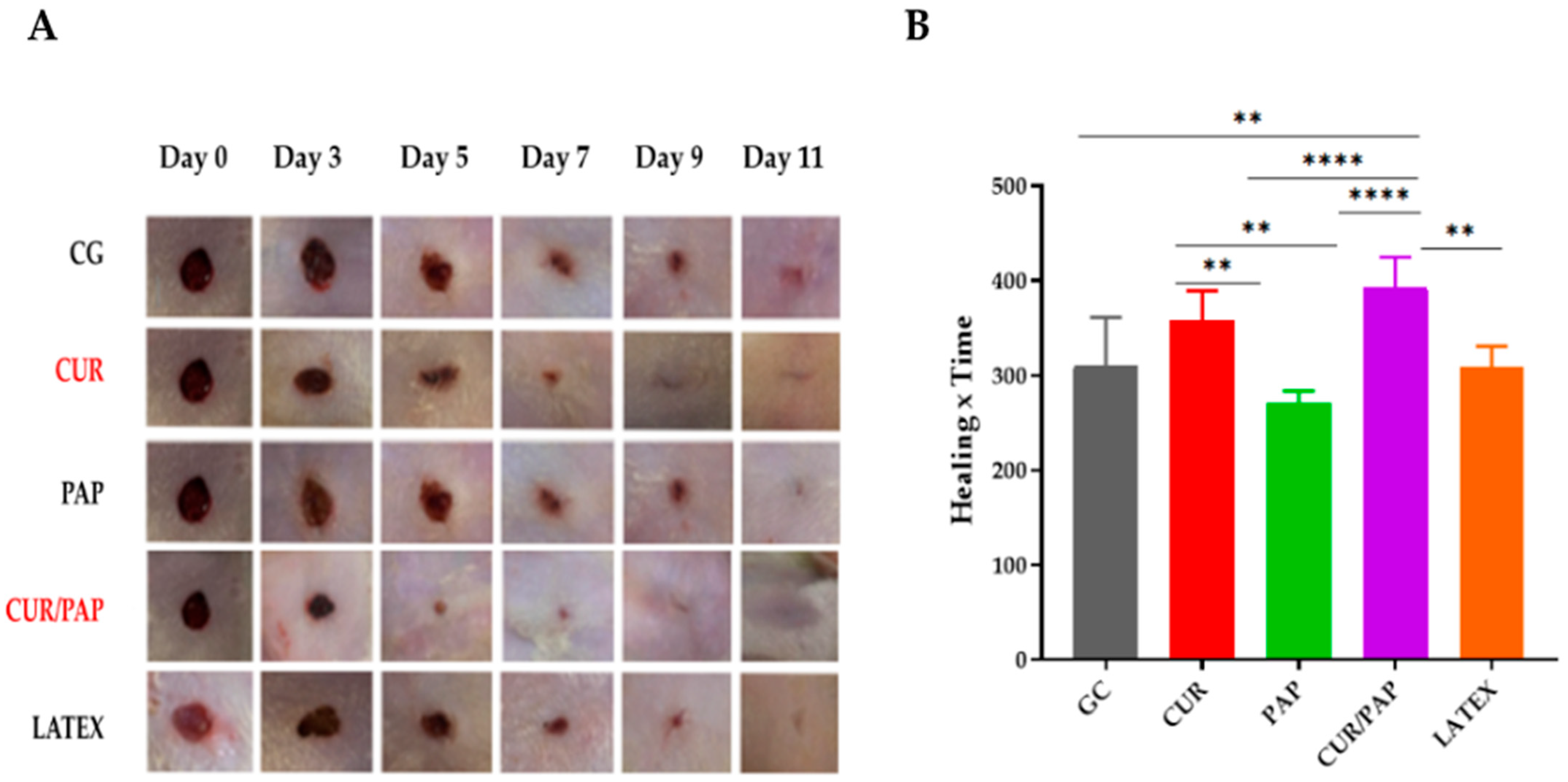
2.6. Evaluation of Wound Healing
3. Discussion
Study Limitations and Perspectives
4. Materials and Methods
4.1. Preparation of Liposomes
4.2. Characterization of Liposomes
4.3. Production of Natural Latex Biomembrane (NLB)
4.4. Preparation of Latex Biomembrane Containing Multilamellar Liposomes
4.5. Sterilization and Packaging of NLB
4.6. Fourier Transform Infrared Spectroscopy (FTIR)
4.7. Animals
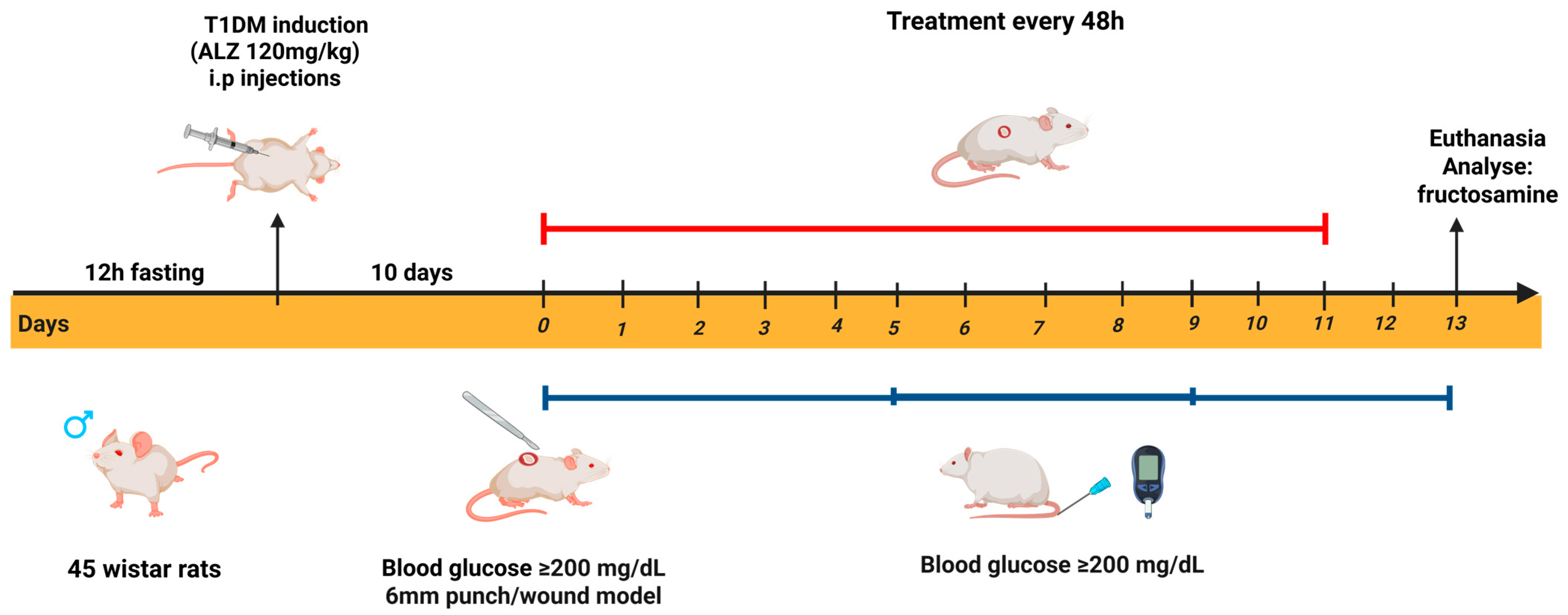
Experimental Design
4.8. Diabetic Model Protocol
4.9. Incisional Wound Model
4.10. Treatment
4.11. LED Irradiation Procedure
4.12. In Vivo Evaluation
4.13. Biochemical Analysis (Fructosamine)
4.14. Measurement of the Wound Area and Photographic Records
4.15. Ethics Statement
4.16. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgess, J.L.; Wyant, W.A.; Abujamra, B.A.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Goyal, R.; Singhal, M.; Jialal, I. Type 2 Diabetes. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513253/ (accessed on 15 March 2025).
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; Belgium International Diabetes Federation: Brussels, Belgium, 2025. [Google Scholar]
- Seedevi, P.; Ramu Ganesan, A.; Moovendhan, M.; Mohan, K.; Sivasankar, P.; Loganathan, S.; Vairamani, S.; Shanmugam, A. Anti-Diabetic Activity of Crude Polysaccharide and Rhamnose-Enriched Polysaccharide from G. Lithophila on Streptozotocin (STZ)-Induced in Wistar Rats. Sci. Rep. 2020, 10, 556. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, A. Type 1 Diabetes Mellitus. Nat. Rev. Dis. Prim. 2017, 3, 17016. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; Gluud, C.; Nicola, S.; Simancas-Racines, D.; Reveiz, L.; Oliva, P.; Cedeño-Taborda, J. Growth Factors for Treating Diabetic Foot Ulcers. Cochrane Database Syst. Rev. 2015, 2015, CD008548. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Murray, R.Z.; West, Z.E.; Cowin, A.J.; Farrugia, B.L. Development and Use of Biomaterials as Wound Healing Therapies. Burn. Trauma 2019, 7, s41038-018-0139-7. [Google Scholar] [CrossRef] [PubMed]
- Pegorin Brasil, G.S.; de Barros, P.P.; Miranda, M.C.R.; de Barros, N.R.; Junqueira, J.C.; Gomez, A.; Herculano, R.D.; de Mendonça, R.J. Natural Latex Serum: Characterization and Biocompatibility Assessment Using Galleria Mellonella as an Alternative In Vivo Model. J. Biomater. Sci. Polym. Ed. 2022, 33, 705–726. [Google Scholar] [CrossRef]
- Asami, J.; Quevedo, B.V.; Santos, A.R.; Giorno, L.P.; Komatsu, D.; de Rezende Duek, E.A. The Impact of Non-Deproteinization on Physicochemical and Biological Properties of Natural Rubber Latex for Biomedical Applications. Int. J. Biol. Macromol. 2023, 253, 126782. [Google Scholar] [CrossRef]
- de Siqueira Rodrigues Fleury Rosa, S.; Rosa, M.F.F.; Fonseca, M.A.M.; da Silva Luz, G.V.; Avila, C.F.D.; Domínguez, A.G.D.; Dantas, A.G.D.; Richter, V.B. Evidence in Practice of Tissue Healing with Latex Biomembrane: Integrative Review. J. Diabetes Res. 2019, 2019, 7457295. [Google Scholar] [CrossRef]
- Frade, M.A.C.; Brum Cursi, I.; Fortes Andrade, F.; Coutinho Netto, J.; Magalhães Barbetta, F.; Foss, N.T. Management of Diabetic Skin Wounds with a Natural Latex Biomembrane Manejo de Úlceras Cutâneas Diabéticas Com a Biomembrana Natural de Látex; Medigraphic: Mexico City, Mexico, 2004; Volume 32. [Google Scholar]
- Araujo, M.M.; Massuda, E.T.; Hyppolito, M.A. Anatomical and Functional Evaluation of Tympanoplasty Using a Transitory Natural Latex Biomembrane Implant from the Rubber Tree Hevea Brasiliensis. Acta Cir. Bras. 2012, 27, 566–571. [Google Scholar] [CrossRef]
- Minatel, D.G.; Frade, M.A.C.; França, S.C.; Enwemeka, C.S. Phototherapy Promotes Healing of Chronic Diabetic Leg Ulcers That Failed to Respond to Other Therapies. Lasers Surg. Med. 2009, 41, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Minatel, D.G.; Enwemeka, C.S.; França, S.C.; Frade, M.A.C. Phototherapy (LEDs 660/890nm) in the Treatment of Leg Ulcers in Diabetic Patients: Case Study. An. Bras. Dermatol. 2009, 84, 279–283. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Nunes, G.A.M.; dos Reis, M.D.C.; Rosa, M.F.F.; Peixoto, L.R.T.; da Rocha, A.F.; de Siqueira Rodrigues Fleury Rosa, S. A System for Treatment of Diabetic Foot Ulcers Using Led Irradiation and Natural Latex. Res. Biomed. Eng. 2016, 32, 3–13. [Google Scholar] [CrossRef]
- Guerra, N.B.; Sant’Ana Pegorin, G.; Boratto, M.H.; de Barros, N.R.; de Oliveira Graeff, C.F.; Herculano, R.D. Biomedical Applications of Natural Rubber Latex from the Rubber Tree Hevea Brasiliensis. Mater. Sci. Eng. C 2021, 126, 112126. [Google Scholar] [CrossRef]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int. J. Nanomed. 2020, 15, 9717–9743. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Ng, S.F.; Khan, S.; Katas, H. Nanoencapsulation, an Efficient and Promising Approach to Maximize Wound Healing Efficacy of Curcumin: A Review of New Trends and State-of-the-Art. Colloids Surf. B Biointerfaces 2017, 150, 223–241. [Google Scholar] [CrossRef]
- Vij, T.; Prashar, Y. A Review on Medicinal Properties of Carica Papaya Linn. Asian Pac. J. Trop. Dis. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Kong, Y.R.; Jong, Y.X.; Balakrishnan, M.; Bok, Z.K.; Weng, J.K.K.; Tay, K.C.; Goh, B.H.; Ong, Y.S.; Chan, K.G.; Lee, L.H.; et al. Beneficial Role of Carica Papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology 2021, 10, 287. [Google Scholar] [CrossRef]
- Karunamoorthi, K.; Kim, H.-M.; Jegajeevanram, K.; Xavier, J.; Vijayalakshmi, J. Papaya: A Gifted Nutraceutical Plant—A Critical Review of Recent Human Health Research. Tang Humanit. Med. 2014, 4, 2. [Google Scholar] [CrossRef][Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Ali Zaidi, S.A.S.; Fatima, F.; Ali Zaidi, S.A.S.; Zhou, D.; Deng, W.; Liu, S. Engineering SiRNA Therapeutics: Challenges and Strategies. J. Nanobiotechnol. 2023, 21, 381. [Google Scholar] [CrossRef] [PubMed]
- Mosca, R.C.; Ong, A.A.; Albasha, O.; Bass, K.; Arany, P. Photobiomodulation Therapy for Wound Care: A Potent, Noninvasive, Photoceutical Approach. Adv. Ski. Wound Care 2019, 32, 157–167. [Google Scholar] [CrossRef]
- Karu, T. Primary and Secondary Mechanisms of Action of Visible to Near-IR Radiation on Cells. J. Photochem. Photobiol. B Biol. 1999, 49, 1–17. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Demidova, T.N. Mechanisms of Low Level Light Therapy. Proc. Mech. Low-Light Ther. 2006, 6140, 614001. [Google Scholar]
- Ferraresi, C.; Kaippert, B.; Avci, P.; Huang, Y.Y.; De Sousa, M.V.P.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Low-Level Laser (Light) Therapy Increases Mitochondrial Membrane Potential and ATP Synthesis in C2C12 Myotubes with a Peak Response at 3-6 H. Photochem. Photobiol. 2015, 91, 411–416. [Google Scholar] [CrossRef]
- Gupta, A.; Dai, T.; Hamblin, M.R. Effect of Red and Near-Infrared Wavelengths on Low-Level Laser (Light) Therapy-Induced Healing of Partial-Thickness Dermal Abrasion in Mice. Lasers Med. Sci. 2014, 29, 257–265. [Google Scholar] [CrossRef]
- Almeida-Lopes, L.; Rigau, J.; Zângaro, R.A.; Guidugli-Neto, J.; Jaeger, M.M.M. Comparison of the Low Level Laser Therapy Effects on Cultured Human Gingival Fibroblasts Proliferation Using Different Irradiance and Same Fluence. Lasers Surg. Med. 2001, 29, 179–184. [Google Scholar] [CrossRef]
- Posten, W.; Wrone, D.A.; Dover, J.S.; Arndt, K.A.; Silapunt, S.; Alam, M. Low-Level Laser Therapy for Wound Healing: Mechanism and Efficacy. Dermatol. Surg. 2005, 31, 334–340. [Google Scholar] [CrossRef]
- Gomes, T.F.S.; Guimarães, N.C.; Abreu, L.P.G.C.; Silva, G.d.O.; da Silva, V.R.P.; da Silva, F.d.M.; Veiga-Souza, F.H.; de Souza, P.E.N.; Rosa, M.F.F.; Joanitti, G.A.; et al. A Natural Latex-Based Smart Dressing for Curcumin Delivery Combined with LED Phototherapy in Diabetic Foot Ulcers: A Pilot Clinical Study. Pharmaceutics 2025, 17, 772. [Google Scholar] [CrossRef]
- Rosa, S.S.R.F.; Rosa, M.F.F.; Marques, M.P.; Guimarães, G.A.; Motta, B.C.; Macedo, Y.C.L.; Inazawa, P.; Dominguez, A.; Macedo, F.S.; Lopes, C.A.P.; et al. Regeneration of Diabetic Foot Ulcers Based on Therapy with Red LED Light and a Natural Latex Biomembrane. Ann. Biomed. Eng. 2019, 47, 1153–1164. [Google Scholar] [CrossRef]
- López-Delis, A.; de S. Rodrigues Fleury Rosa, S.; de Souza, P.E.N.; Carneiro, M.L.B.; Rosa, M.F.F.; Macedo, Y.C.L.; Veiga-Souza, F.H.; da Rocha, A.F. Characterization of the Cicatrization Process in Diabetic Foot Ulcers Based on the Production of Reactive Oxygen Species. J. Diabetes Res. 2018, 2018, 4641364. [Google Scholar] [CrossRef]
- Nogueira, V.F.; da Rocha, A.F.; Rosa, S.S.; Nogueira, O.S.; Rosa, M.F. Development and Application of the Rapha® Device for the Treatment of Diabetic Foot Ulcers. Am. J. Transl. Res. 2024, 16, 1044–1061. [Google Scholar] [CrossRef]
- Mrue, F.; Coutinho Netto, J.; Ceneviva, R.; Lachat, J.J.; Thomazini, J.A.; Tambelini, H. Evaluation of the Biocompatibility of a New Biomembrane. Mat. Res. 2004, 7, 277–283. [Google Scholar] [CrossRef]
- Gemeinder, J.L.P.; de Barros, N.R.; Pegorin, G.S.; de Lacorte Singulani, J.; Borges, F.A.; Arco, M.C.G.D.; Giannini, M.J.S.M.; Almeida, A.M.F.; de Souza Salvador, S.L.; Herculano, R.D. Gentamicin Encapsulated within a Biopolymer for the Treatment of Staphylococcus Aureus and Escherichia Coli Infected Skin Ulcers. J. Biomater. Sci. Polym. Ed. 2021, 32, 93–111. [Google Scholar] [CrossRef]
- Ismail, E.H.; Sabry, D.Y.; Mahdy, H.; Khalil, M.M.H. Synthesis and Characterization of Some Ternary Metal Complexes of Curcumin with 1,10-Phenanthroline and Their Anticancer Applications. J. Sci. Res. 2014, 6, 509–519. [Google Scholar] [CrossRef]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-Applied Curcumin for Different Diseases Therapy. Biomed. Res. Int. 2014, 2014, 394264. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 369–400. ISBN 9780128179093. [Google Scholar]
- Raghunath, I.; Koland, M.; Sarathchandran, C.; Saoji, S.; Rarokar, N. Design and Optimization of Chitosan-Coated Solid Lipid Nanoparticles Containing Insulin for Improved Intestinal Permeability Using Piperine. Int. J. Biol. Macromol. 2024, 280, 135849. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Karperien, M.; Johnbosco, C.; Mahmood, A.; Kousar, M. Chitosan and Carboxymethyl Cellulose-Based 3D Multifunctional Bioactive Hydrogels Loaded with Nano-Curcumin for Synergistic Diabetic Wound Repair. Int. J. Biol. Macromol. 2023, 227, 1203–1220. [Google Scholar] [CrossRef]
- Zhai, X.; Hu, H.; Hu, M.; Ji, S.; Lei, T.; Wang, X.; Zhu, Z.; Dong, W.; Teng, C.; Wei, W. A Nano-Composite Hyaluronic Acid-Based Hydrogel Efficiently Antibacterial and Scavenges ROS for Promoting Infected Diabetic Wound Healing. Carbohydr. Polym. 2024, 334, 122064. [Google Scholar] [CrossRef]
- Halarnekar, D.; Ayyanar, M.; Gangapriya, P.; Kalaskar, M.; Redasani, V.; Gurav, N.; Nadaf, S.; Saoji, S.; Rarokar, N.; Gurav, S. Eco Synthesized Chitosan/Zinc Oxide Nanocomposites as the next Generation of Nano-Delivery for Antibacterial, Antioxidant, Antidiabetic Potential, and Chronic Wound Repair. Int. J. Biol. Macromol. 2023, 242, 124764. [Google Scholar] [CrossRef] [PubMed]
- Blacklow, S.O.; Li, J.; Freedman, B.R.; Zeidi, M.; Chen, C.; Mooney, D.J. Bioinspired Mechanically Active Adhesive Dressings to Accelerate Wound Closure. Sci. Adv. 2019, 5, eaaw3963. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin Improves Wound Healing by Modulating Collagen and Decreasing Reactive Oxygen Species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Fereydouni, N.; Movaffagh, J.; Amiri, N.; Darroudi, S.; Gholoobi, A.; Goodarzi, A.; Hashemzadeh, A.; Darroudi, M. Synthesis of Nano-Fibers Containing Nano-Curcumin in Zein Corn Protein and Its Physicochemical and Biological Characteristics. Sci. Rep. 2021, 11, 1902. [Google Scholar] [CrossRef]
- Salehi, B.; Rodrigues, C.F.; Peron, G.; Dall’Acqua, S.; Sharifi-Rad, J.; Azmi, L.; Shukla, I.; Singh Baghel, U.; Prakash Mishra, A.; Elissawy, A.M.; et al. Curcumin Nanoformulations for Antimicrobial and Wound Healing Purposes. Phyther. Res. 2021, 35, 2487–2499. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Mani, H.; Gaddipati, J.P.; Singh, A.K.; Seth, P.; Banaudha, K.K.; Patnaik, G.K.; Maheshwari, R.K. Curcumin Enhances Wound Healing in Streptozotocin Induced Diabetic Rats and Genetically Diabetic Mice. Wound Repair Regen. 1999, 7, 362–374. [Google Scholar] [CrossRef]
- Islam, M.S.Z.; Akter, J.; Hossain, M.A.; Islam, M.S.Z.; Islam, P.; Goswami, C.; Nguyen, H.T.T.; Miyamoto, A. Anti-Inflammatory, Wound Healing, and Anti-Diabetic Effects of Pure Active Compounds Present in the Ryudai Gold Variety of Curcuma Longa. Molecules 2024, 29, 2795. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Wang, J.; Li, R.; Li, T.; Chang, M.; Yan, F.; Wang, Y. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermos-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.G.; Lee, B.T. Curcumin Incorporation into an Oxidized Cellulose Nanofiber-Polyvinyl Alcohol Hydrogel System Promotes Wound Healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Kamar, S.S.; Abdel-Kader, D.H.; Rashed, L.A. Beneficial Effect of Curcumin Nanoparticles-Hydrogel on Excisional Skin Wound Healing in Type-I Diabetic Rat: Histological and Immunohistochemical Studies. Ann. Anat. 2019, 222, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent Advances in Emulsion-Based Delivery Approaches for Curcumin: From Encapsulation to Bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Sapkota, R.; Munt, D.J.; Kincaid, A.E.; Dash, A.K. Liposomes and Transferosomes in the Delivery of Papain for the Treatment of Keloids and Hypertrophic Scars. PLoS ONE 2023, 18, e0290224. [Google Scholar] [CrossRef] [PubMed]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A.; et al. Curcumin-Encapsulated Nanoparticles as Innovative Antimicrobial and Wound Healing Agent. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 195–206. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Correction: Photobiomodulation: Lasers vs. Light Emitting Diodes? Photochem. Photobiol. Sci. 2019, 18, 259. [Google Scholar] [CrossRef]
- Houreld, N.N. Shedding Light on a New Treatment for Diabetic Wound Healing: A Review on Phototherapy. Sci. World J. 2014, 2014, 398412. [Google Scholar] [CrossRef]
- Dong, J.; Xiong, D. Applications of Light Emitting Diodes in Health Care. Ann. Biomed. Eng. 2017, 45, 2509–2523. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Applications of the Anti-Inflammatory Effects of Photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Houreld, N.N. Photobiomodulation Hastens Diabetic Wound Healing via Modulation of the PI3K/AKT/FoxO1 Pathway in an Adipose Derived Stem Cell-Fibroblast Co-Culture. J. Photochem. Photobiol. 2022, 12, 100157. [Google Scholar] [CrossRef]
- Wu, J.; Deng, L.; Yin, L.; Mao, Z.; Gao, X. Curcumin Promotes Skin Wound Healing by Activating Nrf2 Signaling Pathways and Inducing Apoptosis in Mice. Turk. J. Med. Sci. 2023, 53, 1127–1135. [Google Scholar] [CrossRef]
- Figueiredo Azevedo, F.; Santanna, L.P.; Bóbbo, V.C.; Libert, E.A.; Araújo, E.P.; Abdalla Saad, M.; Lima, M.H.M. Evaluating the Effect of 3% Papain Gel Application in Cutaneous Wound Healing in Mice. Wounds Compend. Clin. Res. Pract. 2017, 29, 96–101. [Google Scholar]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a Wound Healing Agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ramundo, J.; Gray, M. Enzymatic Wound Debridement. J. Wound Ostomy Cont. Nurs. 2008, 35, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Lu, Y.H.; Ma, C.H.; Tao, W.W.; Zhu, J.J.; Zhang, X. A Novel Elastic Liposome for Skin Delivery of Papain and Its Application on Hypertrophic Scar. Biomed. Pharmacother. 2017, 87, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, D.P. Photobiomodulation in Promoting Wound Healing: A Review. Regen. Med. 2016, 11, 107–122. [Google Scholar] [CrossRef]
- Santana, T.F.; Joanitti, G.A.; Castro Germano, E.L.; Leite Germano, R.N.; Espindola, T.A.; Ferreira, L.S.; Luz, G.V.d.S.; Fleury Rosa, M.F.; Rosa, S.R.F.; Carneiro, M.L.B. Tecidual. In Interdisciplinaridade no Contexto do pé Diabético: Tratamentos Clínicos, Políticas Públicas e Tecnologia em Saúde; Leite, C.R.M., Parisi, M.C.R., Rosa, M.F.F., Eds.; EDUERN: Mossoró, Brazil, 2021; p. 569. [Google Scholar]
- da Silva, A.K.A.; Nunes, G.A.M.d.A.; Faria, R.M.; Fleury Rosa, M.F.; Costa, L.B.M.d.; de Faria, N.; da Rocha, A.F.; Tatmatsu-Rocha, J.C.; Fleury Rosa, S.D.S.R. Development of Mathematical Model for Understanding Microcirculation in Diabetic Foot Ulcers Based on Ankle–Brachial Index. Bioengineering 2025, 12, 206. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, A.W.; Hua, H.; Rao, X.; Xu, C. Photodynamic Action of LED-Activated Curcumin against Staphylococcus Aureus Involving Intracellular ROS Increase and Membrane Damage. Int. J. Photoenergy 2014, 2014, 637601. [Google Scholar] [CrossRef]
- Kubizna, M.; Dawiec, G.; Wiench, R. Efficacy of Curcumin-Mediated Antimicrobial Photodynamic Therapy on Candida Spp.—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8136. [Google Scholar] [CrossRef]
- Kim, H.M.; Kang, Y.M.; Lee, M.; An, H.J. Papain Suppresses Atopic Skin Inflammation through Anti-Inflammatory Activities Using In Vitro and In Vivo Models. Antioxidants 2024, 13, 928. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Weston, N.; Coombes, A.G.A.; Fitzgerald, M.; Perrie, Y. Liposome Formulation of Poorly Water Soluble Drugs: Optimisation of Drug Loading and ESEM Analysis of Stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef]
- Yin, P.; Wang, Y.; Yang, L.; Sui, J.; Liu, Y. Hypoglycemic Effects in Alloxan-Induced Diabetic Rats of the Phenolic Extract from Mongolian Oak Cups Enriched in Ellagic Acid, Kaempferol and Their Derivatives. Molecules 2018, 23, 1046. [Google Scholar] [CrossRef]
- Banda, M.; Nyirenda, J.; Muzandu, K.; Sijumbila, G.; Mudenda, S. Antihyperglycemic and Antihyperlipidemic Effects of Aqueous Extracts of Lannea Edulis in Alloxan-Induced Diabetic Rats. Front. Pharmacol. 2018, 9, 1099. [Google Scholar] [CrossRef]
- Babakhani, A.; Nobakht, M.; Torodi, H.P.; Dahmardehei, M.; Hashemi, P.; Ansari, J.M.; Ramhormozi, P.; Yari, A.; Heidari, F. Effects of Hair Follicle Stem Cells on Partial-Thickness Burn Wound Healing and Tensile Strength. Iran. Biomed. J. 2020, 24, 99–109. [Google Scholar] [CrossRef]

| Groups | Fructosamine (µmol/L) |
|---|---|
| GC (Control) | 250 ± 22 |
| G1 (Latex + CUR liposomes + LED) | 306 ± 23 |
| G2 (Latex + PAP liposomes + LED) | 305.4 ± 22 |
| G3 (Latex + LED) | 310.1 ± 25 |
| G4 (Latex + CUR + PAP and + LED) | 305.5 ± 22 |
| Groups | Description | Treatment |
|---|---|---|
| CG | Control | No treatment |
| G1 | NLB + CUR liposomes + LED | NLB with curcumin liposomes, associated with LED phototherapy |
| G2 | NLB + PAP liposomes + LED | NLB with papain liposomes, associated with LED phototherapy |
| G3 | NLB + LED | NLB associated with LED phototherapy |
| G4 | NLB + CUR and PAP liposomes + LED | NLB with a combination of curcumin and papain liposomes, associated with LED phototherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.M.; Silva, J.R.; Rodrigues, W.; Sousa, B.A.S.M.; Gomes, T.F.S.; Rosa, M.F.F.; Rosa, S.S.R.F.; Carneiro, M.L.B. Curcumin and Papain-Loaded Liposomal Natural Latex Dressings with Phototherapy: A Synergistic Approach to Diabetic Wound Healing. Pharmaceuticals 2025, 18, 1067. https://doi.org/10.3390/ph18071067
Silva FM, Silva JR, Rodrigues W, Sousa BASM, Gomes TFS, Rosa MFF, Rosa SSRF, Carneiro MLB. Curcumin and Papain-Loaded Liposomal Natural Latex Dressings with Phototherapy: A Synergistic Approach to Diabetic Wound Healing. Pharmaceuticals. 2025; 18(7):1067. https://doi.org/10.3390/ph18071067
Chicago/Turabian StyleSilva, Franciéle M., Jaqueline R. Silva, Wellington Rodrigues, Breno A. S. M. Sousa, Thamis F. S. Gomes, Mario F. F. Rosa, Suélia S. R. F. Rosa, and Marcella L. B. Carneiro. 2025. "Curcumin and Papain-Loaded Liposomal Natural Latex Dressings with Phototherapy: A Synergistic Approach to Diabetic Wound Healing" Pharmaceuticals 18, no. 7: 1067. https://doi.org/10.3390/ph18071067
APA StyleSilva, F. M., Silva, J. R., Rodrigues, W., Sousa, B. A. S. M., Gomes, T. F. S., Rosa, M. F. F., Rosa, S. S. R. F., & Carneiro, M. L. B. (2025). Curcumin and Papain-Loaded Liposomal Natural Latex Dressings with Phototherapy: A Synergistic Approach to Diabetic Wound Healing. Pharmaceuticals, 18(7), 1067. https://doi.org/10.3390/ph18071067








