Polymeric Nanoparticle-Mediated Photodynamic Therapy: A Synergistic Approach for Glioblastoma Treatment
Abstract
1. Introduction
| Type of Therapy | Examples | Advantages | Drawbacks | References |
|---|---|---|---|---|
| Surgical resection | Craniotomy | Removal of the bulk of the tumour | Impossible to remove all glioblastoma cells in a tumour; nearly all glioblastoma tumours locally recur; risk of surgical wound complications and direct cortical as well as vascular injury | [12] |
| Chemotherapy | Fotemustine, temozolomide, lomustine, carmustine | Slows tumour growth and reduces tumour size | Some chemotherapeutic agents cannot effectively penetrate blood–brain barrier which limits their use; fortified tumour location hinders the delivery of therapeutics; therapy resistance | [13] |
| Radiation therapy | Brachytherapy, 2D conventional radiotherapy, particle radiation therapy, intensity modulated radiotherapy | Usually combined with chemotherapy to treat high-grade gliomas | Radiation necrosis; normal tissues are inevitably irradiated; toxicity; cognitive dysfunction; some glioblastomas are radioresistant | [13,14] |
2. Principle and Mechanism of Photodynamic Therapy

3. Polymeric Nanoparticles (PNPs) in the Treatment of Brain Tumours
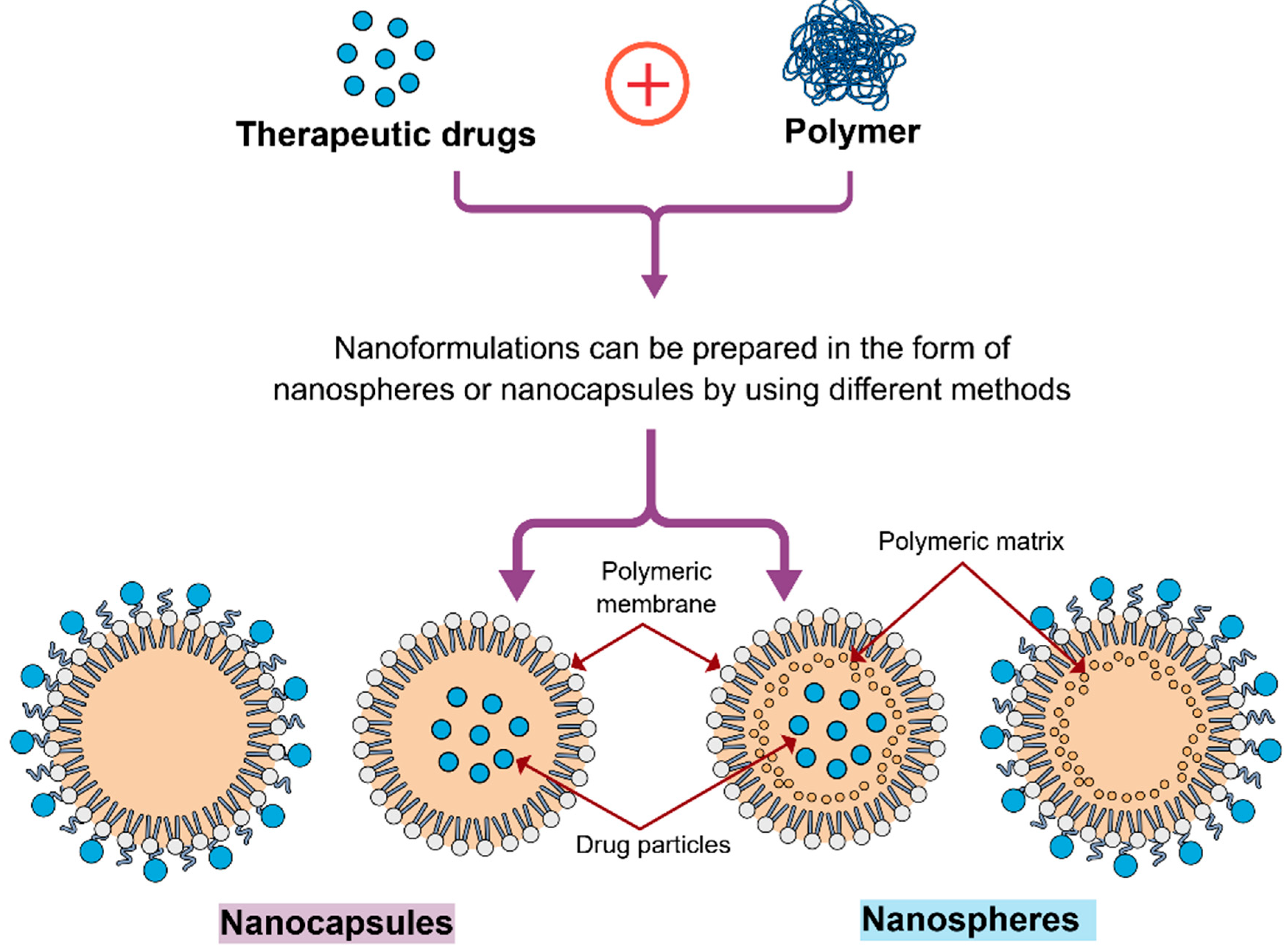
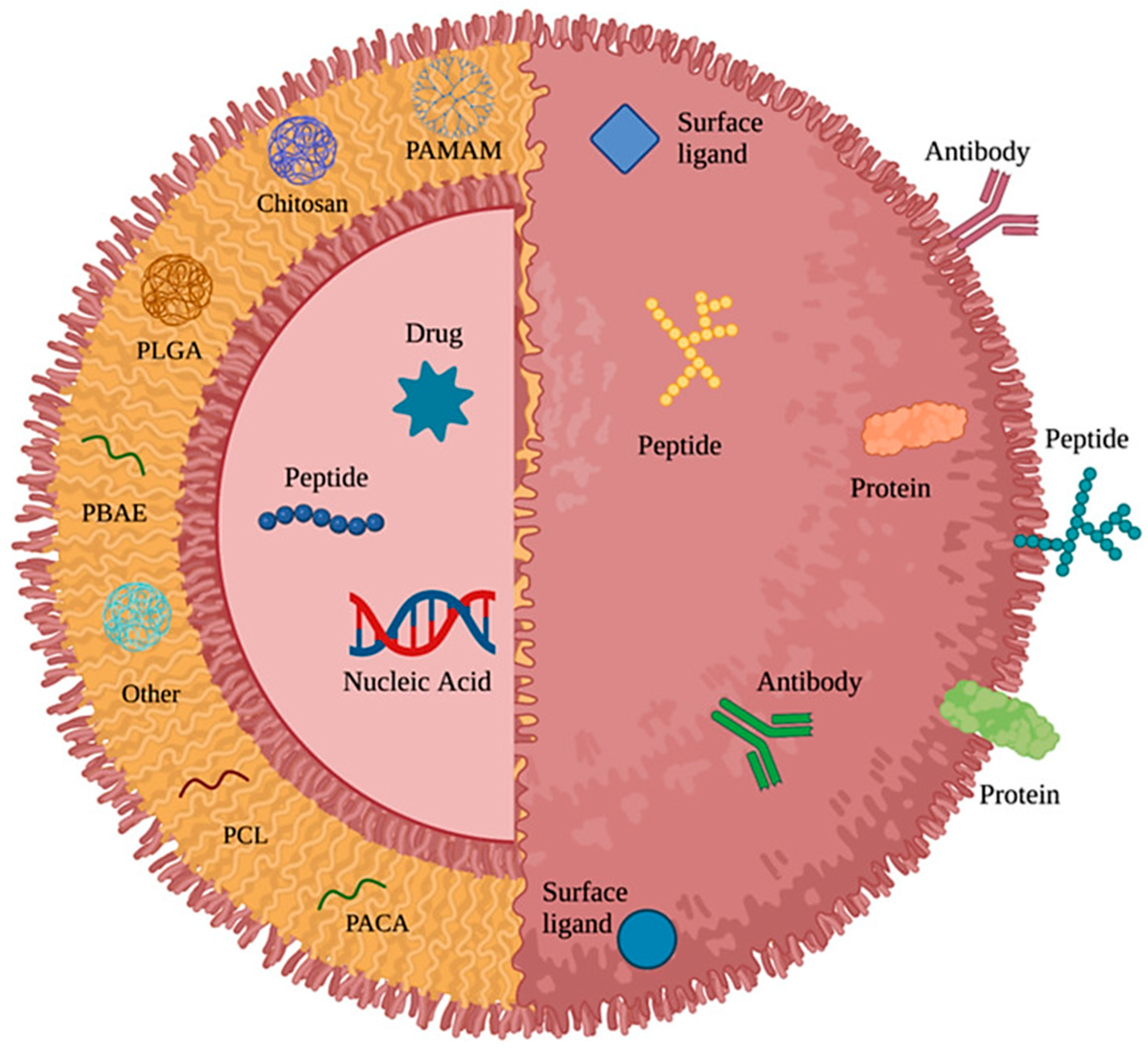
| Polymeric Nanoparticles (NPs) | Particle Size | Drug/Active Molecule | Targeting Strategy | Study Model | Study Outcome | References |
|---|---|---|---|---|---|---|
| Transferrin-functionalised NPs | 137 nm | Temozolomide and the bromodomain inhibitor JQ1 | - | Human U87MG and murine GL261 cells | Transferrin-functionalised NPs elevated DNA damage as well as apoptosis that associates with a 1.5- to 2-fold reduction in tumour burden and corresponding improvement in survival | [123] |
| Chitosan NPs | 184.33 ± 4.4 nm | Superparamagnetic iron oxide and doxorubicin (DOX) | - | Rat glioma C6 cells | Chitosan NPs showed potential as an effective theragnostic formulation for both the treatment and diagnosis of glioblastoma | [124] |
| Hyaluronan (HA)-grafted lipid-based nanoparticles (LNPs) | 100 nm | RNA interference (RNAi) | Active | Human glioblastoma U87MG orthotopic xenograft model | In an orthotopic model, mice treated with RNAi-loaded LNPs coated with HA showed markedly improved longevity | [125] |
| Human serum albumin (HSA)-based NPs | 90.5 ± 3.1 nm | Paclitaxel (PTX) | Active | Orthotopic glioma-bearing mice | Improved anti-glioma efficacy was observed with the dual-enhanced system of dual cationic absorptive transcytosis and glucose-transport by the combined usage of c- and m-HSAs | [126] |
| Albumin NPs | Less than 150 nm | PTX and fenretinide | Active | Human glioma U87, U251 cells, mouse glioma C6, GL261 cells | Albumin NPs showed enhanced blood–brain barrier penetration, intratumoral infiltration, and cellular uptake along with reduced toxic side effects | [127] |
| Activatable low molecular weight protamine (ALMWP) conjugated with polyethylene glycol (PEG)-polycaprolactone (PCL) NPs | 121 nm | PTX | Active | C6 cells implanted into the right striatum of male BALB/c nude mice | Enhanced tumour penetration and glioma-targeting resulted in an anticipated improvement in the in vivo anti-glioblastoma effect; mice treated with ALMWP-NP-PTX showed significantly higher survival | [128] |
| cRGD-directed, NIR-responsive gold nanorod/PEG-PCL hybrid NPs (cRGD-HNs) | 90 nm | DOX | Active | Human glioblastoma U87MG cells | The combined therapy with NIR irradiation and cRGD-HN-DOX completely suppressed tumour growth and showed much lower side effects as compared to free DOX | [129] |
| PCL NPs | 202.1 ± 2.0 nm | Irinotecan hydrochloride trihydrate (IRH) | Active | Primary high-grade glioma (HGG) cells | IRH-loaded nanoparticles exhibited higher encapsulation efficiency and showed cellular toxicity against primary glioma cells | [130] |
| Polysorbate 80 (PS 80)-coated [14C]-Poly(butyl cyanoacrylate) NPs | 252–257 nm | DOX | - | Glioblastoma 101/8-bearing rats | Improved penetration characteristics were seen as a result of nanoparticles that were localised in close proximity to the tumour | [131] |
| Transferrin-modified PEG-poly lactic acid NPs | 153.3 ± 28.2 nm | Resveratrol | - | C6 and U87 glioma cells | As compared to free resveratrol, resveratrol conjugates markedly reduced tumour volume and buildup in brain tumours, which eventually led to prolonged survival of C6 glioma-bearing rats | [132] |
| PLGA NPs | 74 ± 18 nm | PTX | - | Intracranial tumours in immunocompromised rats by injection of U87MG cells | PTX-loaded NPs enhanced survival in tumour-bearing rats | [133] |
| Synthetic protein NP (SPNP) based on polymerised HSA | 115 ± 23.4 nm | Small interfering RNA | - | GL26 syngeneic mouse glioma model | SPNPs resulted in long-term survival in 87.5% of glioblastoma-bearing mice and primed the immune system to develop immunological memory against glioblastoma | [134] |
4. The Importance of Polymeric Nanoparticles in Photodynamic Therapy
5. Applications of Polymeric Nanoparticle-Based Photodynamic Therapy in the Treatment of Glioblastoma
5.1. Photodynamic Therapy with Conjugated Polymer Nanoparticles
5.2. Photodynamic Therapy with Poly(Lactic-Co-Glycolic Acid)-Based Nanoparticles
5.3. Photodynamic Therapy with Lipid–Polymer Hybrid Nanoparticles
5.4. Metronomic Photodynamic Therapy with Conjugated Polymer Nanoparticles
5.5. Metronomic Photodynamic Therapy with Polyethylene-Glycolated (Pegylated) Polymeric Nanoparticles
6. Current Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballard, C.A.P.; Wang, Y.; Kruchko, C.; Barnholtz-Sloan, J.S.; Li, Y.; Ostrom, Q.T. Characteristics of long-term glioblastoma survivors diagnosed from 2010 to 2016 in the United States. Cancer Epidemiol. 2025, 97, 102810. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro-Oncology 2024, 26, vi1–vi85. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, G.S.; Lyutfi, E.; Georgieva, R.; Georgiev, R.; Dzhenkov, D.L.; Petkova, L.; Ivanov, B.D.; Kaprelyan, A.; Ghenev, P. Reclassification of Glioblastoma Multiforme According to the 2021 World Health Organization Classification of Central Nervous System Tumors: A Single Institution Report and Practical Significance. Cureus 2022, 14, e21822. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S.; Green, S.; Ramanathan, L.; Rosenzweig, K.; Labombardi, V. No Consistent Relationship of Glioblastoma Incidence and Cytomegalovirus Seropositivity in Whites, Blacks, and Hispanics. Anticancer Res. 2012, 32, 1113–1115. [Google Scholar] [PubMed]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma survival in the United States before and during the temozolomide era. J. Neuro-Oncol. 2012, 107, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Ali, S.; Qadir, M.G.; De La Fuente, M.I.; Ivan, M.E.; Komotar, R.J. The role of bevacizumab in the treatment of glioblastoma. J. Neuro-Oncol. 2017, 133, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhou, Z.; Huang, X.; Chen, Z.; Zhang, L.; Zhang, J.; Hua, W.; Mao, Y. Use of Bevacizumab in recurrent glioblastoma: A scoping review and evidence map. BMC Cancer 2023, 23, 544. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, U.K.; Bose, R.J.C.; Malhotra, M.; Babikir, H.A.; Afjei, R.; Robinson, E.; Zeng, Y.; Chang, E.; Habte, F.; Sinclair, R.; et al. Intranasal delivery of targeted polyfunctional gold–iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials 2019, 218, 119342. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Shastri, D.H.; Shah, J.; Nair, A.B.; Jacob, S. Nasal Delivery to the Brain: Harnessing Nanoparticles for Effective Drug Transport. Pharmaceutics 2024, 16, 481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tian, S.; Liu, Y.; Zheng, M.; Yang, X.; Zou, Y.; Shi, B.; Luo, L. Near infrared-activatable biomimetic nanogels enabling deep tumor drug penetration inhibit orthotopic glioblastoma. Nat. Commun. 2022, 13, 6835. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Westphal, M.; Quiñones-Hinojosa, A. Complications of glioma surgery. Handb. Clin. Neurol. 2016, 134, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Oliva, C.R.; Noman, A.S.M.; Allen, B.G.; Goswami, P.C.; Zakharia, Y.; Monga, V.; Spitz, D.R.; Buatti, J.M.; Griguer, C.E. Radioresistance in Glioblastoma and the Development of Radiosensitizers. Cancers 2020, 12, 2511. [Google Scholar] [CrossRef] [PubMed]
- Oluwajembola, A.M.; Cleanclay, W.D.; Onyia, A.F.; Chikere, B.N.; Zakari, S.; Ndifreke, E.; De Campos, O.C. Photosensitizers in photodynamic therapy: An advancement in cancer treatment. Results Chem. 2024, 10, 101715. [Google Scholar] [CrossRef]
- Itoo, A.M.; Paul, M.; Padaga, S.G.; Ghosh, B.; Biswas, S. Nanotherapeutic Intervention in Photodynamic Therapy for Cancer. ACS Omega 2022, 7, 45882–45909. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, P.; Jiang, D.; Yang, G.; Xue, Y.; Tang, Z.; Zhang, M.; Wang, H.; Jiang, X.; Wu, Y.; et al. In Situ Catalytic Reaction for Solving the Aggregation of Hydrophobic Photosensitizers in Tumor. ACS Appl. Mater. Interfaces 2020, 12, 5624–5632. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, A.; Zhao, L.; Dong, Q.; Wang, M.; Xu, H.; Yan, X.; Bai, S. Self-Assembly of Monomeric Hydrophobic Photosensitizers with Short Peptides Forming Photodynamic Nanoparticles with Real-Time Tracking Property and without the Need of Release in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 28420–28427. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, C.; Jin, B.; Sun, T.; Sun, K.; Wang, S.; Fan, Z. Advances in smart nanotechnology-supported photodynamic therapy for cancer. Cell Death Discov. 2024, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.E.; Lamberti, M.J.; Rivarola, V.A.; Rumie Vittar, N.B. Developing strategies to predict photodynamic therapy outcome: The role of melanoma microenvironment. Tumor Biol. 2015, 36, 9127–9136. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.E.; Arévalo, D.E.; Sanabria, L.M.; Carrión, F.D.C.; Fanelli, M.A.; Rivarola, V.A. Heat shock protein 27 modulates autophagy and promotes cell survival after photodynamic therapy. Photochem. Photobiol. Sci. 2019, 18, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Girotti, A.W. Upregulation of nitric oxide in tumor cells as a negative adaptation to photodynamic therapy. Lasers Surg. Med. 2018, 50, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Le, T.; Harley, B.A.C.; Imoukhuede, P.I. Characterizing Glioblastoma Heterogeneity via Single-Cell Receptor Quantification. Front. Bioeng. Biotechnol. 2018, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.M.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavaré, S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 4009–4014. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.A.; Vora, P.; Venugopal, C.; Sidhu, S.S.; Moffat, J.; Swanton, C.; Singh, S.K. Intratumoral heterogeneity: Pathways to treatment resistance and relapse in human glioblastoma. Ann. Oncol. 2017, 28, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Skaga, E.; Kulesskiy, E.; Fayzullin, A.; Sandberg, C.J.; Potdar, S.; Kyttälä, A.; Langmoen, I.A.; Laakso, A.; Gaál-Paavola, E.; Perola, M.; et al. Intertumoral heterogeneity in patient-specific drug sensitivities in treatment-naïve glioblastoma. BMC Cancer 2019, 19, 628. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Tran, K.; Chen, Y.; Di Donato, A.T.; Yu, L.; Hu, Y.; Linskey, M.E.; Wang, P.H.; Limoli, C.L.; Zhou, Y.H. Linking differential radiation responses to glioma heterogeneity. Oncotarget 2014, 5, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Motaln, H.; Koren, A.; Gruden, K.; Ramšak, Ž.; Schichor, C.; Lah, T.T. Heterogeneous glioblastoma cell cross-talk promotes phenotype alterations and enhanced drug resistance. Oncotarget 2015, 6, 40998–41017. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Ramiro, A.; Ramírez-Ortega, D.; Pérez de la Cruz, V.; Hérnandez-Pedro, N.Y.; González-Esquivel, D.F.; Sotelo, J.; Pineda, B. Role of Redox Status in Development of Glioblastoma. Front. Immunol. 2016, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Eskilsson, E.; Rosland, G.V.; Talasila, K.M.; Knappskog, S.; Keunen, O.; Sottoriva, A.; Foerster, S.; Solecki, G.; Taxt, T.; Jirik, R.; et al. EGFRvIII mutations can emerge as late and heterogenous events in glioblastoma development and promote angiogenesis through Src activation. Neuro-Oncology 2016, 18, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yu, S.; Xu, H.; Zheng, Y.; Lin, J.; Wu, M.; Wang, J.; Wang, A.; Lan, Q.; Furnari, F.; et al. FHL2 interacts with EGFR to promote glioblastoma growth. Oncogene 2018, 37, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.-W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.J.; Bronson, R.T.; Charest, A. Inhibition of EGFR Induces a c-MET-Driven Stem Cell Population in Glioblastoma. Stem Cells 2014, 32, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Puliyappadamba, V.T.; Chakraborty, S.; Rehman, A.; Vemireddy, V.; Saha, D.; Souza, R.F.; Hatanpaa, K.J.; Koduru, P.; Burma, S.; et al. EGFR wild type antagonizes EGFRvIII-mediated activation of Met in glioblastoma. Oncogene 2015, 34, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.K.; Sulman, E.P.; Wen, P.Y.; Kurz, S.C. Novel Therapies for Glioblastoma. Curr. Neurol. Neurosci. Rep. 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Z.; Yuan, H.; Wang, L.; Gao, L.-H. Poly(p-phenylenevinylene) nanoparticles modified with antiEGFRvIII for specific glioblastoma therapy. Sci. Rep. 2021, 11, 4449. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.K.; Da Silva, C.G.; Kralisch, D.; Chan, A.; Ossendorp, F.; Cruz, L.J. Combinatory therapy adopting nanoparticle-based cancer vaccination with immune checkpoint blockade for treatment of post-surgical tumor recurrences. J. Control. Release 2018, 285, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Chen, Z.; Wang, C.; Liu, Z. Toward Biomaterials for Enhancing Immune Checkpoint Blockade Therapy. Adv. Funct. Mater. 2018, 28, 1802540. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.D.; Kumthekar, P.U. Immune Checkpoint Inhibitors for the Treatment of Central Nervous System (CNS) Metastatic Disease. Front. Oncol. 2018, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, Y.; Shi, Y.; Li, B.; Liu, W.; Yin, W.; Dang, Y.; Chu, Y.; Fan, J.; He, R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3–CCL2 Signaling. Cancer Res. 2016, 76, 4124–4135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, Y.; Liao, X.; Tang, Y.; Ni, Q.; Sun, J.; Zhao, Y.; Zhang, J.; Teng, Z.; Lu, G. Enhancing selective photosensitizer accumulation and oxygen supply for high-efficacy photodynamic therapy toward glioma by 5-aminolevulinic acid loaded nanoplatform. J. Colloid Interface Sci. 2020, 565, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.; Boddu, S.; Abuhijjleh, R.; Selvaraju, K.; Babu, T.; Gorain, B.; Shah, J.; Morsy, M. The emerging role of lipid nanosystems and nanomicelles in liver diseases. Eur. Rev. Med. Pharmacol. Sci. 2023, 27. [Google Scholar]
- Gorain, B.; Al-Dhubiab, B.E.; Nair, A.; Kesharwani, P.; Pandey, M.; Choudhury, H. Multivesicular liposome: A lipid-based drug delivery system for efficient drug delivery. Curr. Pharm. Des. 2021, 27, 4404–4415. [Google Scholar] [CrossRef] [PubMed]
- Shinu, P.; Nair, A.B.; Kumari, B.; Jacob, S.; Kumar, M.; Tiwari, A.; Tiwari, V.; Venugopala, K.N.; Attimarad, M.; Nagaraja, S. Recent Advances and Appropriate use of Niosomes for the Treatment of Skin Cancer. Indian J. Pharm. Educ. Res. 2022, 56, 1–14. [Google Scholar] [CrossRef]
- Shehata, T.M.; Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Jacob, S.; Alhaider, I.A.; Attimarad, M.; Elsewedy, H.S.; Ibrahim, M.M. Vesicular emulgel based system for transdermal delivery of insulin: Factorial design and in vivo evaluation. Appl. Sci. 2020, 10, 5341. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Attimarad, M.; Nair, A.B. Nanosuspension Innovations: Expanding Horizons in Drug Delivery Techniques. Pharmaceutics 2025, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Crintea, A.; Motofelea, A.C.; Șovrea, A.S.; Constantin, A.M.; Crivii, C.B.; Carpa, R.; Duțu, A.G. Dendrimers: Advancements and Potential Applications in Cancer Diagnosis and Treatment-An Overview. Pharmaceutics 2023, 15, 1406. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical applications of metallic nanoparticles in cancer: Current status and future perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Mohammadnejad, J.; Najafi-Taher, R.; Zadeh, Z.B.; Tanhaei, M.; Ramakrishna, S. Multifunctional Carbon-Based Nanoparticles: Theranostic Applications in Cancer Therapy and Diagnosis. ACS Appl. Bio Mater. 2023, 6, 1323–1338. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Md, S.; Nair, A.B.; Deb, P.K. Exploring the potential of carbon dots to combat COVID-19. Front. Mol. Biosci. 2020, 7, 616575. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.P.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small (Weinh. Der Bergstr. Ger.) 2024, 20, e2304848. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Ramteke, S.; Jain, N.K. Pharmaceutical Applications of Quantum Dots. AAPS PharmSciTech 2021, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica nanoparticles: Biomedical applications and toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Shah, J.; Nair, A.B. Innovations in Nanoemulsion Technology: Enhancing Drug Delivery for Oral, Parenteral, and Ophthalmic Applications. Pharmaceutics 2024, 16, 1333. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, T.; Gupta, S.; Nair, A.; Chauhan, S.; Saini, V. Wound healing potential of insulin-loaded nanoemulsion with Aloe vera gel in diabetic rats. J. Drug Deliv. Sci. Technol. 2021, 64, 102601. [Google Scholar] [CrossRef]
- Li, L.; Huh, K.M. Polymeric nanocarrier systems for photodynamic therapy. Biomater Res. 2014, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Bartusik-Aebisher, D.; Żołyniak, A.; Barnaś, E.; Machorowska-Pieniążek, A.; Oleś, P.; Kawczyk-Krupka, A.; Aebisher, D. The Use of Photodynamic Therapy in the Treatment of Brain Tumors-A Review of the Literature. Molecules 2022, 27, 6847. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Zheng, Y.-R.; Gadde, S.; Pfirschke, C.; Zope, H.; Engblom, C.; Kohler, R.H.; Iwamoto, Y.; Yang, K.S.; Askevold, B.; et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun. 2015, 6, 8692. [Google Scholar] [CrossRef] [PubMed]
- Vagena, I.-A.; Malapani, C.; Gatou, M.-A.; Lagopati, N.; Pavlatou, E.A. Enhancement of EPR Effect for Passive Tumor Targeting: Current Status and Future Perspectives. Appl. Sci. 2025, 15, 3189. [Google Scholar] [CrossRef]
- Quaglia, F.; Sortino, S. Polymer Nanoparticles for Cancer Photodynamic Therapy Combined with Nitric Oxide Photorelease and Chemotherapy. In Applied Photochemistry: When Light Meets Molecules; Bergamini, G., Silvi, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 397–426. [Google Scholar]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef] [PubMed]
- Indoria, S.; Singh, V.; Hsieh, M.-F. Recent advances in theranostic polymeric nanoparticles for cancer treatment: A review. Int. J. Pharm. 2020, 582, 119314. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Patel, S.S.; Morsy, M.A.; Patel, V.; Chavda, V.; Jacob, S.; Sreeharsha, N.; Shinu, P.; et al. Development of Asialoglycoprotein Receptor-Targeted Nanoparticles for Selective Delivery of Gemcitabine to Hepatocellular Carcinoma. Molecules 2019, 24, 4566. [Google Scholar] [CrossRef] [PubMed]
- Borzęcka, W.; Domiński, A.; Kowalczuk, M. Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy. Nanomaterials 2021, 11, 2426. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Desai, V.M.; Jain, R.; Agrawal, M.; Dubey, S.K.; Singhvi, G. Unveiling the potential of photodynamic therapy with nanocarriers as a compelling therapeutic approach for skin cancer treatment: Current explorations and insights. RSC Adv. 2024, 14, 21915–21937. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Choi, G.; Choy, J.-H. Recent Developments on Semiconducting Polymer Nanoparticles as Smart Photo-Therapeutic Agents for Cancer Treatments—A Review. Polymers 2021, 13, 981. [Google Scholar] [CrossRef] [PubMed]
- Sadat Tabatabaei Mirakabad, F.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Maliyakkal, N.; Appadath Beeran, A.; Udupa, N. Nanoparticles of cisplatin augment drug accumulations and inhibit multidrug resistance transporters in human glioblastoma cells. Saudi Pharm. J. 2021, 29, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Taghizadehghalehjoughi, A.; Ahmet, H.; Meltem, C.; Busra, U.A.; Bianca, G.; Yaroslav, M.; Ufuk, O.; Numan, T.; Mehmet, T.; Abdullah, U.; et al. Effect of Metformin/Irinotecan-Loaded Poly-Lactic-Co-Glycolic Acid Nanoparticles on Glioblastoma: In Vitro and In Vivo Studies. Nanomedicine 2018, 13, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, Q.; Zhao, L.; Ye, C.; Hua, L.; Liang, J.; Yu, R.; Liu, H. Angiopep-2 Modified Cationic Lipid-Poly-Lactic-Co-Glycolic Acid Delivery Temozolomide and DNA Repair Inhibitor Dbait to Achieve Synergetic Chemo-Radiotherapy Against Glioma. J. Nanosci. Nanotechnol. 2019, 19, 7539–7545. [Google Scholar] [CrossRef] [PubMed]
- Pereverzeva, E.; Treschalin, I.; Treschalin, M.; Arantseva, D.; Ermolenko, Y.; Kumskova, N.; Maksimenko, O.; Balabanyan, V.; Kreuter, J.; Gelperina, S. Toxicological study of doxorubicin-loaded PLGA nanoparticles for the treatment of glioblastoma. Int. J. Pharm. 2019, 554, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.; Esnaashari, S.S.; Bergonzi, M.C.; Webster, T.J.; Younes, H.M.; Khosravani, M.; Adabi, M. Paclitaxel/methotrexate co-loaded PLGA nanoparticles in glioblastoma treatment: Formulation development and in vitro antitumor activity evaluation. Life Sci. 2020, 256, 117943. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Wojtunik-Kulesza, K.A.; Oniszczuk, T.; Kasprzak, K. The potential of photodynamic therapy (PDT)—Experimental investigations and clinical use. Biomed. Pharmacother. 2016, 83, 912–929. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Amin Doustvandi, M.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiagn. Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer-A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef] [PubMed]
- Domka, W.; Bartusik-Aebisher, D.; Rudy, I.; Dynarowicz, K.; Pięta, K.; Aebisher, D. Photodynamic therapy in brain cancer: Mechanisms, clinical and preclinical studies and therapeutic challenges. Front. Chem. 2023, 11, 1250621. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.-A.; Guérin, L.; Lecomte, F.; Baert, G.; Vignion, A.-S.; Mordon, S.; Reyns, N. Is interstitial photodynamic therapy for brain tumors ready for clinical practice? A systematic review. Photodiagn. Photodyn. Ther. 2021, 36, 102492. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, I.O.; Tsubone, T.M.; Pavani, C.; Baptista, M.S. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Sun, W.; Fan, J.; Du, J.; Peng, X. An estrogen receptor targeted ruthenium complex as a two-photon photodynamic therapy agent for breast cancer cells. Chem. Commun. 2018, 54, 7038–7041. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, Y.; He, Y.; Xiong, M.; Huang, H.; Pei, S.; Liao, J.; Wang, Y.; Shao, D. Green synthesis of carrier-free curcumin nanodrugs for light-activated breast cancer photodynamic therapy. Colloids Surf. B Biointerfaces 2019, 180, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Colombeau, L.; Gries, M.; Peterlini, T.; Mathieu, C.; Thomas, N.; Boura, C.; Frochot, C.; Vanderesse, R.; Lux, F.; et al. Ultrasmall AGuIX theranostic nanoparticles for vascular-targeted interstitial photodynamic therapy of glioblastoma. Int. J. Nanomed. 2017, 12, 7075–7088. [Google Scholar] [CrossRef] [PubMed]
- Mokwena, M.G.; Kruger, C.A.; Ivan, M.-T.; Heidi, A. A review of nanoparticle photosensitizer drug delivery uptake systems for photodynamic treatment of lung cancer. Photodiagn. Photodyn. Ther. 2018, 22, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [PubMed]
- Matlou, G.G.; Abrahamse, H. Nanoscale metal–organic frameworks as photosensitizers and nanocarriers in photodynamic therapy. Front. Chem. 2022, 10, 971747. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Yasinjan, F.; Yang, M.; Du, Y.; Geng, H.; He, M.; Wang, Y.; Sun, J.; Jiang, W.; Zhang, L.; et al. A scientometric analysis and up-to-date review of nano-based drug delivery systems in glioblastoma treatment. Nano Today 2023, 52, 101961. [Google Scholar] [CrossRef]
- Prabhu, R.H.; Patravale, V.B.; Joshi, M.D. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomed. 2015, 10, 1001–1018. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; Canney, M.; Carpentier, A.; Idbaih, A. Overcoming the blood brain barrier in glioblastoma: Status and future perspective. Rev. Neurol. 2023, 179, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Chatterji, A.; Dash, B.P.; Raveen Nelson, B.; Sarkar, T.; Shahimi, S.; Atan Edinur, H.; Binti Abd Manan, T.S.; Jena, P.; Mohanta, Y.K.; et al. Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab. Polymers 2020, 12, 2361. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Sarkar, T.; Sheikh, H.I.; Bharadwaj, K.K.; Mohapatra, P.K.; Chatterji, A.; Dash, B.P.; Edinur, H.A.; Nelson, B.R. γ-Irradiated Chitosan From Carcinoscorpius rotundicauda (Latreille, 1802) Improves the Shelf Life of Refrigerated Aquatic Products. Front. Mar. Sci. 2021, 8, 664961. [Google Scholar] [CrossRef]
- Rabha, B.; Bharadwaj, K.K.; Baishya, D.; Sarkar, T.; Edinur, H.A.; Pati, S. Synthesis and Characterization of Diosgenin Encapsulated Poly-ε-Caprolactone-Pluronic Nanoparticles and Its Effect on Brain Cancer Cells. Polymers 2021, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Rabha, B.; Bharadwaj, K.K.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Kari, Z.A.; Edinur, H.A.; Baishya, D.; Atanase, L.I. Development of Polymer-Based Nanoformulations for Glioblastoma Brain Cancer Therapy and Diagnosis: An Update. Polymers 2021, 13, 4114. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-L.; Lin, H.-C.; Hong, S.-T.; Chang, C.-H.; Wang, C.-S.; Lin, A.M.-Y. Lipid polymeric nanoparticles modified with tight junction-modulating peptides promote afatinib delivery across a blood–brain barrier model. Cancer Nanotechnol. 2021, 12, 13. [Google Scholar] [CrossRef]
- Wen, L.; Tan, Y.; Dai, S.; Zhu, Y.; Meng, T.; Yang, X.; Liu, Y.; Liu, X.; Yuan, H.; Hu, F. VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy. Drug Deliv. 2017, 24, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Caraway, C.A.; Gaitsch, H.; Wicks, E.E.; Kalluri, A.; Kunadi, N.; Tyler, B.M. Polymeric Nanoparticles in Brain Cancer Therapy: A Review of Current Approaches. Polymers 2022, 14, 2963. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-Layer polyelectrolyte assemblies for encapsulation and release of active compounds. Adv. Colloid Interface Sci. 2017, 249, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Maroto, A.; Abelenda-Núñez, I.; Ortega, F.; Rubio, R.G.; Guzmán, E. Polyelectrolyte Multilayers on Soft Colloidal Nanosurfaces: A New Life for the Layer-By-Layer Method. Polymers 2021, 13, 1221. [Google Scholar] [CrossRef] [PubMed]
- Soh, W.W.M.; Teoh, R.Y.P.; Zhu, J.; Xun, Y.; Wee, C.Y.; Ding, J.; Thian, E.S.; Li, J. Facile Construction of a Two-in-One Injectable Micelleplex-Loaded Thermogel System for the Prolonged Delivery of Plasmid DNA. Biomacromolecules 2022, 23, 3477–3492. [Google Scholar] [CrossRef] [PubMed]
- Doty, A.C.; Jarvis, C.M.; Munsell, E.V. Formulation Strategies to Enable Delivery of Therapeutic Peptides across Cell Membranes. In Approaching the Next Inflection in Peptide Therapeutics: Attaining Cell Permeability and Oral Bioavailability; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1417, pp. 223–254. [Google Scholar]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Korpusik, A.B.; Tan, Y.; Garrison, J.B.; Tan, W.; Sumerlin, B.S. Aptamer-Conjugated Micelles for Targeted Photodynamic Therapy Via Photoinitiated Polymerization-Induced Self-Assembly. Macromolecules 2021, 54, 7354–7363. [Google Scholar] [CrossRef]
- Tian, H.; Huang, Y.; He, J.; Zhang, M.; Ni, P. CD147 Monoclonal Antibody Targeted Reduction-Responsive Camptothecin Polyphosphoester Nanomedicine for Drug Delivery in Hepatocellular Carcinoma Cells. ACS Appl. Bio Mater. 2021, 4, 4422–4431. [Google Scholar] [CrossRef] [PubMed]
- Ximendes, E.; Benayas, A.; Jaque, D.; Marin, R. Quo Vadis, Nanoparticle-Enabled In Vivo Fluorescence Imaging? ACS Nano 2021, 15, 1917–1941. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Dimri, S.; Prasad, R.; Ravichandran, G.; Naidu, V.; De, A.; Srivastava, R. Characteristics of Molecularly Engineered Anticancer Drug Conjugated Organic Nanomicelles for Site-Selective Cancer Cell Rupture and Growth Inhibition of Tumor Spheroids. ACS Appl. Bio Mater. 2020, 3, 7067–7079. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, M.R.; Kosuru, R.; Singh, S.K.; Prasad, C.B.; Narayan, G.; Muthu, M.S.; Singh, S. Resveratrol loaded PLGA:d-α-tocopheryl polyethylene glycol 1000 succinate blend nanoparticles for brain cancer therapy. RSC Adv. 2016, 6, 74254–74268. [Google Scholar] [CrossRef]
- Varan, C.; Bilensoy, E. Cationic PEGylated polycaprolactone nanoparticles carrying post-operation docetaxel for glioma treatment. Beilstein J. Nanotechnol. 2017, 8, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Yanxian, H.; Qing, Y.; Weiling, G.; Gang, W.; Menglin, W.; Qiang, F.; Zhonggui, H.; Vadivel, G.; Sun, J. L-Carnitine-conjugated nanoparticles to promote permeation across blood–brain barrier and to target glioma cells for drug delivery via the novel organic cation/carnitine transporter OCTN2. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lin, Z.; Liu, L.; Lin, W.; Xie, X.; Zhang, X. Enhancing glioma-specific drug delivery through self-assembly of macrophage membrane and targeted polymer assisted by low-frequency ultrasound irradiation. Mater Today Bio 2024, 26, 101067. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Zhang, T.; Zhang, X. Development of bioactive materials for glioblastoma therapy. Bioact. Mater. 2016, 1, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Labak, C.M.; Wang, P.Y.; Arora, R.; Guda, M.R.; Asuthkar, S.; Tsung, A.J.; Velpula, K.K. Glucose transport: Meeting the metabolic demands of cancer, and applications in glioblastoma treatment. Am. J. Cancer Res. 2016, 6, 1599–1608. [Google Scholar] [PubMed]
- Sun, P.; Xiao, Y.; Di, Q.; Ma, W.; Ma, X.; Wang, Q.; Chen, W. Transferrin Receptor-Targeted PEG-PLA Polymeric Micelles for Chemotherapy Against Glioblastoma Multiforme. Int. J. Nanomed. 2020, 15, 6673–6688. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Vu Han, T.L.; Hwang, M.K.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Tafaghodi, M.; Abbasi, B.; Daroudi, M.; Kazemi Oskuee, R. Preparation of superparamagnetic iron oxide/doxorubicin loaded chitosan nanoparticles as a promising glioblastoma theranostic tool. J. Cell Physiol. 2019, 234, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.R.; Ramishetti, S.; Peshes-Yaloz, N.; Goldsmith, M.; Wohl, A.; Zibly, Z.; Peer, D. Localized RNAi Therapeutics of Chemoresistant Grade IV Glioma Using Hyaluronan-Grafted Lipid-Based Nanoparticles. ACS Nano 2015, 9, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H.J.; Thao, L.Q.; Lee, S.; Min, S.Y.; Lee, E.S.; Shin, B.S.; Choi, H.-G.; Youn, Y.S. Doxorubicin-loaded nanoparticles consisted of cationic- and mannose-modified-albumins for dual-targeting in brain tumors. J. Control. Release 2016, 225, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood-Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Xia, H.; Hu, Q.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Tu, Y.; Pang, Z.; Song, Q.; et al. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials 2013, 34, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, C.; Cheng, R.; Cheng, L.; Meng, F.; Liu, Z.; Zhong, Z. cRGD-directed, NIR-responsive and robust AuNR/PEG–PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo. J. Control. Release 2014, 195, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.S.; McConville, C. Development and Optimization of Irinotecan-Loaded PCL Nanoparticles and Their Cytotoxicity against Primary High-Grade Glioma Cells. Pharmaceutics 2021, 13, 541. [Google Scholar] [CrossRef] [PubMed]
- Ambruosi, A.; Khalansky, A.S.; Yamamoto, H.; Gelperina, S.E.; Begley, D.J.; Kreuter, J. Biodistribution of polysorbate 80-coated doxorubicin-loaded [14C]-poly(butyl cyanoacrylate) nanoparticles after intravenous administration to glioblastoma-bearing rats. J. Drug Target. 2006, 14, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, A.; Jia, Z.; Yuan, Y.; Dai, H.; Li, H. Transferrin modified PEG-PLA-resveratrol conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharmacol. 2013, 718, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Patel, T.R.; Sirianni, R.W.; Strohbehn, G.; Zheng, M.-Q.; Duong, N.; Schafbauer, T.; Huttner, A.J.; Huang, Y.; Carson, R.E.; et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc. Natl. Acad. Sci. USA 2013, 110, 11751–11756. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.V.; Kadiyala, P.; Doherty, R.; Cadena, M.; Habeel, S.; Ruoslahti, E.; Lowenstein, P.R.; Castro, M.G.; Lahann, J. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 2020, 11, 5687. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-H.; Qiu, W.-X.; Zhang, Y.-H.; Li, B.; Zhang, C.; Gao, F.; Zhang, L.; Zhang, X.-Z. A Charge Reversible Self-Delivery Chimeric Peptide with Cell Membrane-Targeting Properties for Enhanced Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1700220. [Google Scholar] [CrossRef]
- Li, F.; Du, Y.; Liu, J.; Sun, H.; Wang, J.; Li, R.; Kim, D.; Hyeon, T.; Ling, D. Responsive Assembly of Upconversion Nanoparticles for pH-Activated and Near-Infrared-Triggered Photodynamic Therapy of Deep Tumors. Adv. Mater. 2018, 30, 1802808. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhu, Y.; Zhang, M.; Luo, L.; Wu, J.; Zhou, H.; Guan, L.; Battaglia, G.; Tian, Y. Localization matters: A nuclear targeting two-photon absorption iridium complex in photodynamic therapy. Chem. Commun. 2017, 53, 3303–3306. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, L.; Shi, H.; Du, W.; Qi, Y.; Qiu, C.; Liang, X.; Shi, W.; Liu, J. Endoplasmic reticulum-targeting photosensitizer Hypericin confers chemo-sensitization towards oxaliplatin through inducing pro-death autophagy. Int. J. Biochem. Cell Biol. 2017, 87, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, X.; Deng, J.; Lu, D.; Zhang, X.; Chen, Y.; Zhu, J.; Fan, A.; Ding, D.; Kong, D.; et al. Multifunctional Micelles Dually Responsive to Hypoxia and Singlet Oxygen: Enhanced Photodynamic Therapy via Interactively Triggered Photosensitizer Delivery. ACS Appl. Mater. Interfaces 2018, 10, 17117–17128. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, L. Functional Polymer Nanocarriers for Photodynamic Therapy. Pharmaceuticals 2018, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; He, H.; Chen, D.; Yin, L. Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT). Biomater. Sci. 2017, 5, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Qidwai, A.; Annu; Nabi, B.; Kotta, S.; Narang, J.K.; Baboota, S.; Ali, J. Role of nanocarriers in photodynamic therapy. Photodiagn. Photodyn. Ther. 2020, 30, 101782. [Google Scholar] [CrossRef] [PubMed]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Grimland, J.L.; Wu, C.; Ramoutar, R.R.; Brumaghim, J.L.; McNeill, J. Photosensitizer-doped conjugated polymer nanoparticles with high cross-sections for one- and two-photon excitation. Nanoscale 2011, 3, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Hou, W.; Zhou, H.; Zhou, L.; Chen, H.; Wu, C. Therapeutic Considerations and Conjugated Polymer-Based Photosensitizers for Photodynamic Therapy. Macromol. Rapid Commun. 2018, 39, 1700614. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, R.A.; Ibarra, L.E.; Achilli, E.E.; Odella, E.; Chesta, C.A.; Martínez, S.R.; Palacios, R.E. Sweet light o’ mine: Photothermal and photodynamic inactivation of tenacious pathogens using conjugated polymers. J. Photochem. Photobiol. B Biol. 2022, 234, 112510. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, M.; Li, H.; Jiang, J.; Zhou, S.; Chen, W.; Xie, C.; Zhen, X.; Jiang, X. Enhancing Penetration Ability of Semiconducting Polymer Nanoparticles for Sonodynamic Therapy of Large Solid Tumor. Adv. Sci. 2022, 9, 2104125. [Google Scholar] [CrossRef] [PubMed]
- Cesca, B.A.; Pellicer San Martin, K.; Caverzan, M.D.; Oliveda, P.M.; Ibarra, L.E. State-of-the-art photodynamic therapy for malignant gliomas: Innovations in photosensitizers and combined therapeutic approaches. Explor. Target. Anti-Tumor Ther. 2025, 6, 1002303. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, L.E.; Camorani, S.; Agnello, L.; Pedone, E.; Pirone, L.; Chesta, C.A.; Palacios, R.E.; Fedele, M.; Cerchia, L. Selective Photo-Assisted Eradication of Triple-Negative Breast Cancer Cells through Aptamer Decoration of Doped Conjugated Polymer Nanoparticles. Pharmaceutics 2022, 14, 626. [Google Scholar] [CrossRef] [PubMed]
- Caverzán, M.D.; Beaugé, L.; Chesta, C.A.; Palacios, R.E.; Ibarra, L.E. Photodynamic therapy of Glioblastoma cells using doped conjugated polymer nanoparticles: An in vitro comparative study based on redox status. J. Photochem. Photobiol. B Biol. 2020, 212, 112045. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, L.E.; Beaugé, L.; Arias-Ramos, N.; Rivarola, V.A.; Chesta, C.A.; López-Larrubia, P.; Palacios, R.E. Trojan horse monocyte-mediated delivery of conjugated polymer nanoparticles for improved photodynamic therapy of glioblastoma. Nanomedicine 2020, 15, 1687–1707. [Google Scholar] [CrossRef] [PubMed]
- Arias-Ramos, N.; Ibarra, L.E.; Serrano-Torres, M.; Yagüe, B.; Caverzán, M.D.; Chesta, C.A.; Palacios, R.E.; López-Larrubia, P. Iron Oxide Incorporated Conjugated Polymer Nanoparticles for Simultaneous Use in Magnetic Resonance and Fluorescent Imaging of Brain Tumors. Pharmaceutics 2021, 13, 1258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, X.; Zhang, Q.; Zheng, T.; Mohammadniaei, M.; Wang, W.; Shen, J.; Sun, Y. Biodegradable Polymeric Nanoparticles Containing an Immune Checkpoint Inhibitor (aPDL1) to Locally Induce Immune Responses in the Central Nervous System. Adv. Funct. Mater. 2021, 31, 2102274. [Google Scholar] [CrossRef]
- Foresto, E.; Gilardi, P.; Ibarra, L.E.; Cogno, I.S. Light-activated green drugs: How we can use them in photodynamic therapy and mass-produce them with biotechnological tools. Phytomed. Plus 2021, 1, 100044. [Google Scholar] [CrossRef]
- Lu, Q.; Fu, Y.; Li, H. Berberine and its derivatives represent as the promising therapeutic agents for inflammatory disorders. Pharmacol. Rep. 2022, 74, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed. Pharmacother. 2018, 104, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Kumari, M.; Pahuja, R.; Pant, A.B.; Shukla, Y.; Kumar, P.; Gupta, K.C. Hyaluronic acid-grafted PLGA nanoparticles for the sustained delivery of berberine chloride for an efficient suppression of Ehrlich ascites tumors. Drug Deliv. Transl. Res. 2018, 8, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Comincini, S.; Manai, F.; Sorrenti, M.; Perteghella, S.; D’Amato, C.; Miele, D.; Catenacci, L.; Bonferoni, M.C. Development of Berberine-Loaded Nanoparticles for Astrocytoma Cells Administration and Photodynamic Therapy Stimulation. Pharmaceutics 2023, 15, 1078. [Google Scholar] [CrossRef] [PubMed]
- Chanburee, S.; and Tiyaboonchai, W. Mucoadhesive nanostructured lipid carriers (NLCs) as potential carriers for improving oral delivery of curcumin. Drug Dev. Ind. Pharm. 2017, 43, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mou, H.; Xue, C.; Leung, A.W.; Xu, C.; Tang, Q.-J. Photodynamic effect of curcumin on Vibrio parahaemolyticus. Photodiagn. Photodyn. Ther. 2016, 15, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Khorsandi, K. Methylene blue, curcumin and ion pairing nanoparticles effects on photodynamic therapy of MDA-MB-231 breast cancer cell. Photodiagn. Photodyn. Ther. 2017, 18, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Khoobi, M.; Hejazi, S.M.; Eivazi, N.; Abdolahpour, S.; Imanparast, F.; Moradi-Sardareh, H.; Paknejad, M. Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagn. Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloid Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood–Brain Barrier Transfer—Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Duwa, R.; Emami, F.; Lee, S.; Jeong, J.-H.; Yook, S. Polymeric and lipid-based drug delivery systems for treatment of glioblastoma multiforme. J. Ind. Eng. Chem. 2019, 79, 261–273. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid nanoparticles as a promising drug delivery carrier for topical ocular therapy—An overview on recent advances. Pharmaceutics 2022, 14, 533. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, S.; Bala, S.; Nair, A.B. Solid lipid nanoparticles: An effective lipid based technology for poorly water soluble drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 78–90. [Google Scholar]
- Jain, V.; Kumar, H.; Anod, H.V.; Chand, P.; Gupta, N.V.; Dey, S.; Kesharwani, S.S. A review of nanotechnology-based approaches for breast cancer and triple-negative breast cancer. J. Control. Release 2020, 326, 628–647. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.F.G.; Piva, H.L.; Matsuo, F.S.; de Lima, S.C.G.; de Souza, L.E.B.; Osako, M.K.; Tedesco, A.C. Hybrid lipid-biopolymer nanocarrier as a strategy for GBM photodynamic therapy (PDT). Int. J. Biol. Macromol. 2023, 242, 124647. [Google Scholar] [CrossRef] [PubMed]
- Itazaki, Y.; Sakanoue, K.; Fujita, K.; Kirino, I.; Eguchi, K.; Miyazono, Y.; Yamaguchi, R.; Tsunenari, T.; Sugihara, T.; Kuwada, K.; et al. Metronomic photodynamic therapy for deep organ cancer by implantable wireless OLEDs. APL Bioeng. 2025, 9, 026113. [Google Scholar] [CrossRef] [PubMed]
- Simsek, C.; Esin, E.; Yalcin, S. Metronomic Chemotherapy: A Systematic Review of the Literature and Clinical Experience. J. Oncol. 2019, 2019, 5483791. [Google Scholar] [CrossRef] [PubMed]
- Kirino, I.; Fujita, K.; Sakanoue, K.; Sugita, R.; Yamagishi, K.; Takeoka, S.; Fujie, T.; Uemoto, S.; Morimoto, Y. Metronomic photodynamic therapy using an implantable LED device and orally administered 5-aminolevulinic acid. Sci. Rep. 2020, 10, 22017. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, K.; Kirino, I.; Takahashi, I.; Amano, H.; Takeoka, S.; Morimoto, Y.; Fujie, T. Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy. Nat. Biomed. Eng. 2019, 3, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Caverzán, M.D.; Oliveda, P.M.; Beaugé, L.; Palacios, R.E.; Chesta, C.A.; Ibarra, L.E. Metronomic Photodynamic Therapy with Conjugated Polymer Nanoparticles in Glioblastoma Tumor Microenvironment. Cells 2023, 12, 1541. [Google Scholar] [CrossRef] [PubMed]
- Kydd, J.; Jadia, R.; Rai, P. Co-Administered Polymeric Nano-Antidotes for Improved Photo-Triggered Response in Glioblastoma. Pharmaceutics 2018, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Al-Moujahed, A.; Brodowska, K.; Stryjewski, T.P.; Efstathiou, N.E.; Vasilikos, I.; Cichy, J.; Miller, J.W.; Gragoudas, E.; Vavvas, D.G. Verteporfin inhibits growth of human glioma in vitro without light activation. Sci. Rep. 2017, 7, 7602. [Google Scholar] [CrossRef] [PubMed]
- Momeny, M.; Shamsaiegahkani, S.; Kashani, B.; Hamzehlou, S.; Esmaeili, F.; Yousefi, H.; Irani, S.; Mousavi, S.A.; Ghaffari, S.H. Cediranib, a pan-inhibitor of vascular endothelial growth factor receptors, inhibits proliferation and enhances therapeutic sensitivity in glioblastoma cells. Life Sci. 2021, 287, 120100. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-D.; De-Li, Z.; Meng-Qi, T.; Meng-Ting, L.; Xia-Fang, X.; Xing, T.; Ying-Zheng, Z.; and Xu, H.-L. pH-sensitive polymeric nanoparticles of mPEG-PLGA-PGlu with hybrid core for simultaneous encapsulation of curcumin and doxorubicin to kill the heterogeneous tumour cells in breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neurooncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef] [PubMed]
- Zanders, E.D.; Svensson, F.; Bailey, D.S. Therapy for glioblastoma: Is it working? Drug Discov. Today 2019, 24, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.; Esnaashari, S.S.; Webster, T.J.; Khosravani, M.; Adabi, M. Polymeric nanoparticles for drug delivery in glioblastoma: State of the art and future perspectives. J. Control. Release 2022, 349, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Amir, G.; Omid, G.; Amir, R.; Mahdi, K.; and Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.W.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control. Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Prabhakarpandian, B.; Pant, K.; Mitragotri, S. Clinical and commercial translation of advanced polymeric nanoparticle systems: Opportunities and material challenges. Transl. Mater. Res. 2017, 4, 014001. [Google Scholar] [CrossRef]
- Liu, H.-J.; Xu, P. Strategies to overcome/penetrate the BBB for systemic nanoparticle delivery to the brain/brain tumor. Adv. Drug Deliv. Rev. 2022, 191, 114619. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, L.E.; Vilchez, M.L.; Caverzán, M.D.; Milla Sanabria, L.N. Understanding the glioblastoma tumor biology to optimize photodynamic therapy: From molecular to cellular events. J. Neurosci. Res. 2021, 99, 1024–1047. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-E.K.; Kopelman, R. Polymeric Nanoparticles for Photodynamic Therapy. In Biomedical Nanotechnology: Methods and Protocols; Hurst, S.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 151–178. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldhubiab, B.; Almuqbil, R.M. Polymeric Nanoparticle-Mediated Photodynamic Therapy: A Synergistic Approach for Glioblastoma Treatment. Pharmaceuticals 2025, 18, 1057. https://doi.org/10.3390/ph18071057
Aldhubiab B, Almuqbil RM. Polymeric Nanoparticle-Mediated Photodynamic Therapy: A Synergistic Approach for Glioblastoma Treatment. Pharmaceuticals. 2025; 18(7):1057. https://doi.org/10.3390/ph18071057
Chicago/Turabian StyleAldhubiab, Bandar, and Rashed M. Almuqbil. 2025. "Polymeric Nanoparticle-Mediated Photodynamic Therapy: A Synergistic Approach for Glioblastoma Treatment" Pharmaceuticals 18, no. 7: 1057. https://doi.org/10.3390/ph18071057
APA StyleAldhubiab, B., & Almuqbil, R. M. (2025). Polymeric Nanoparticle-Mediated Photodynamic Therapy: A Synergistic Approach for Glioblastoma Treatment. Pharmaceuticals, 18(7), 1057. https://doi.org/10.3390/ph18071057






