Taurine-Based Hybrid Drugs as Potential Anticancer Therapeutic Agents: In Vitro, In Vivo Evaluations
Abstract
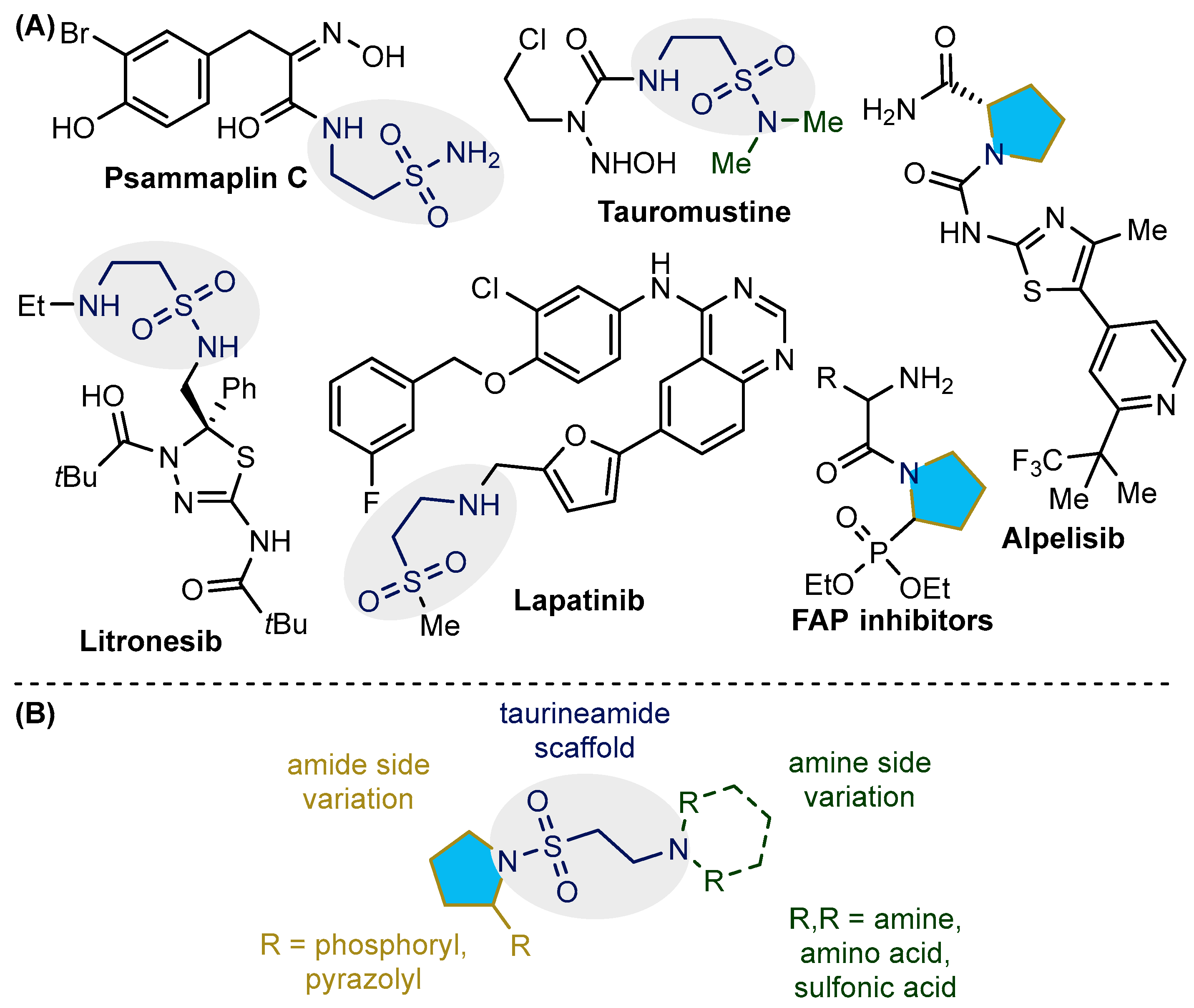
1. Introduction
2. Results
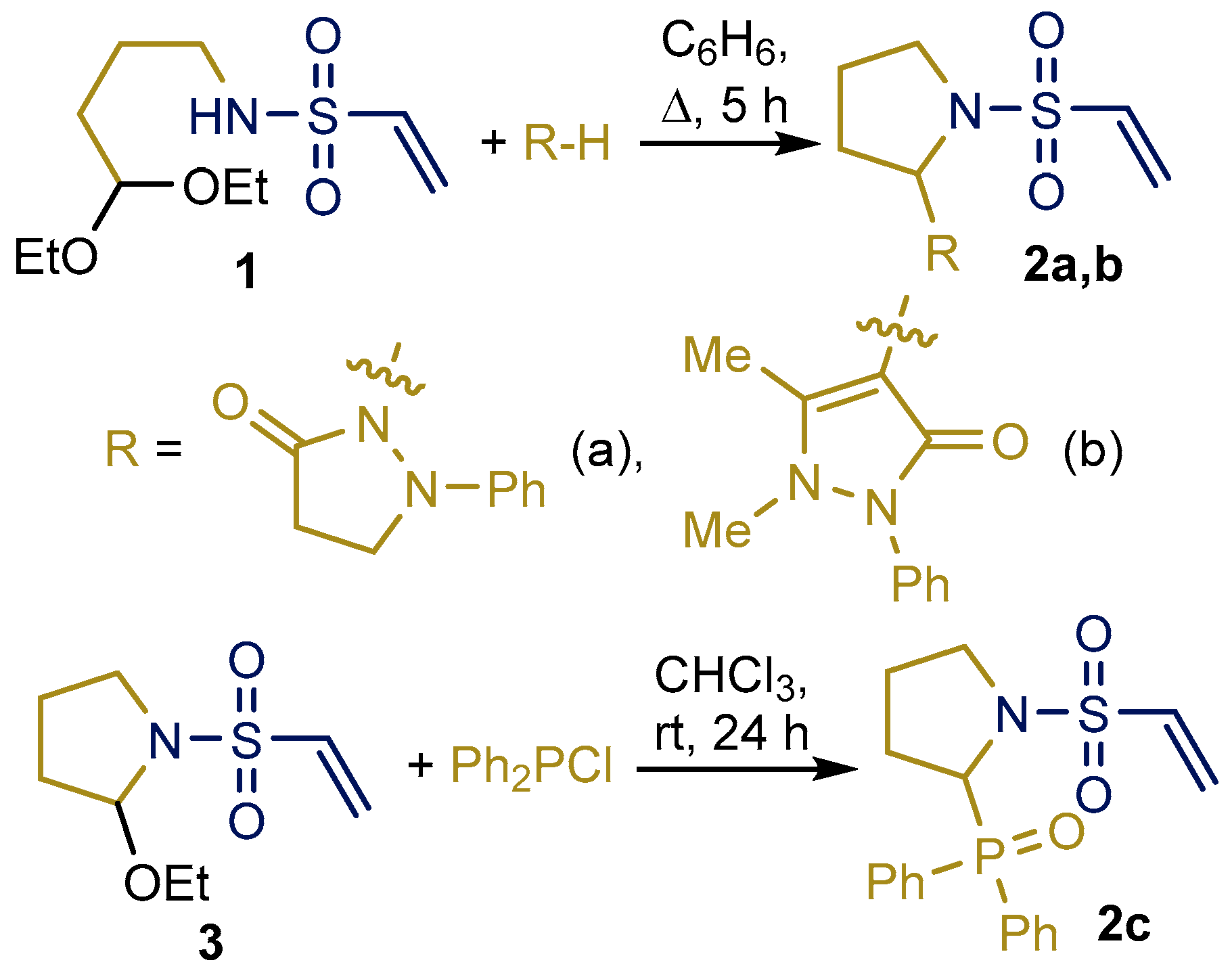
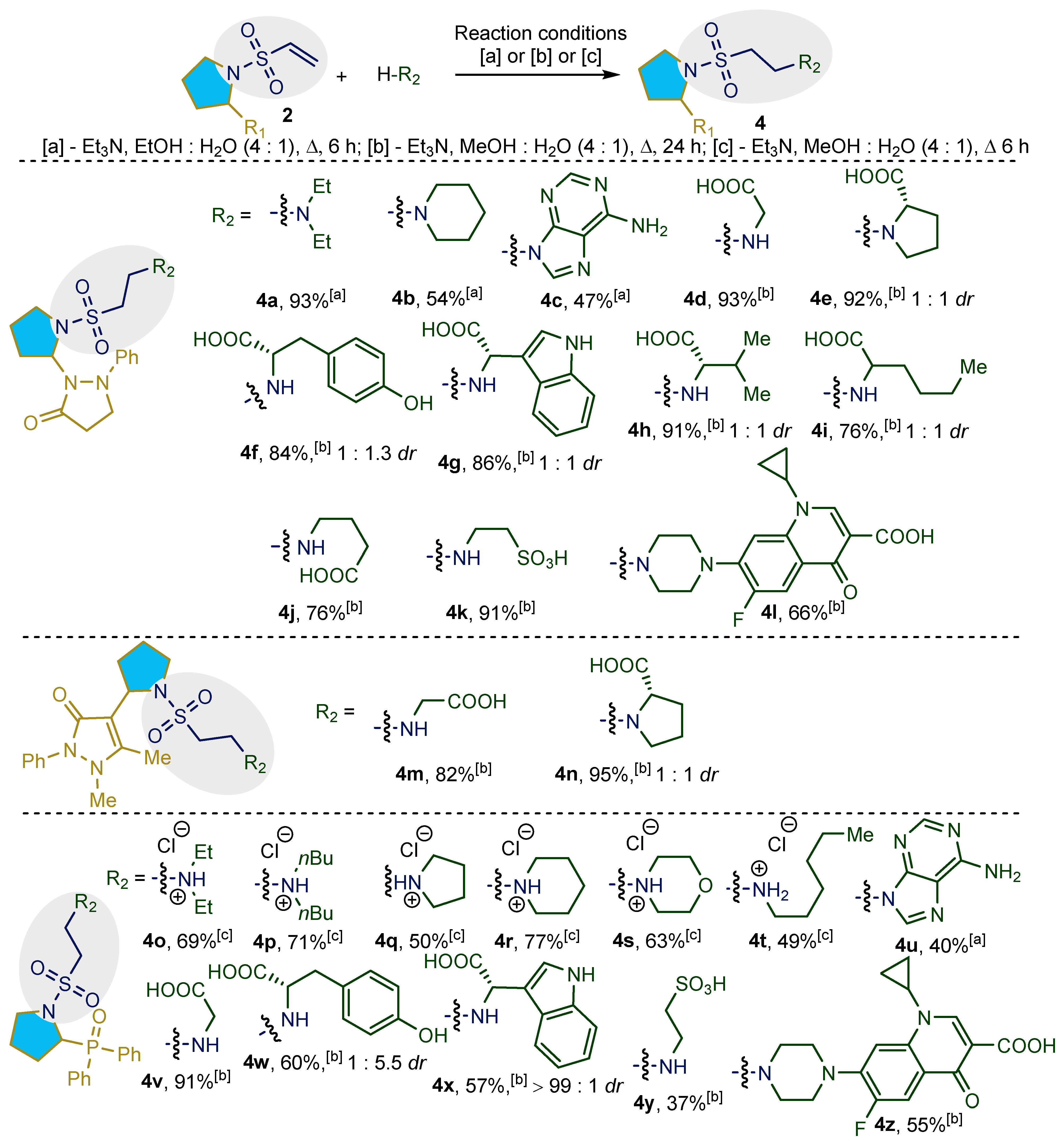
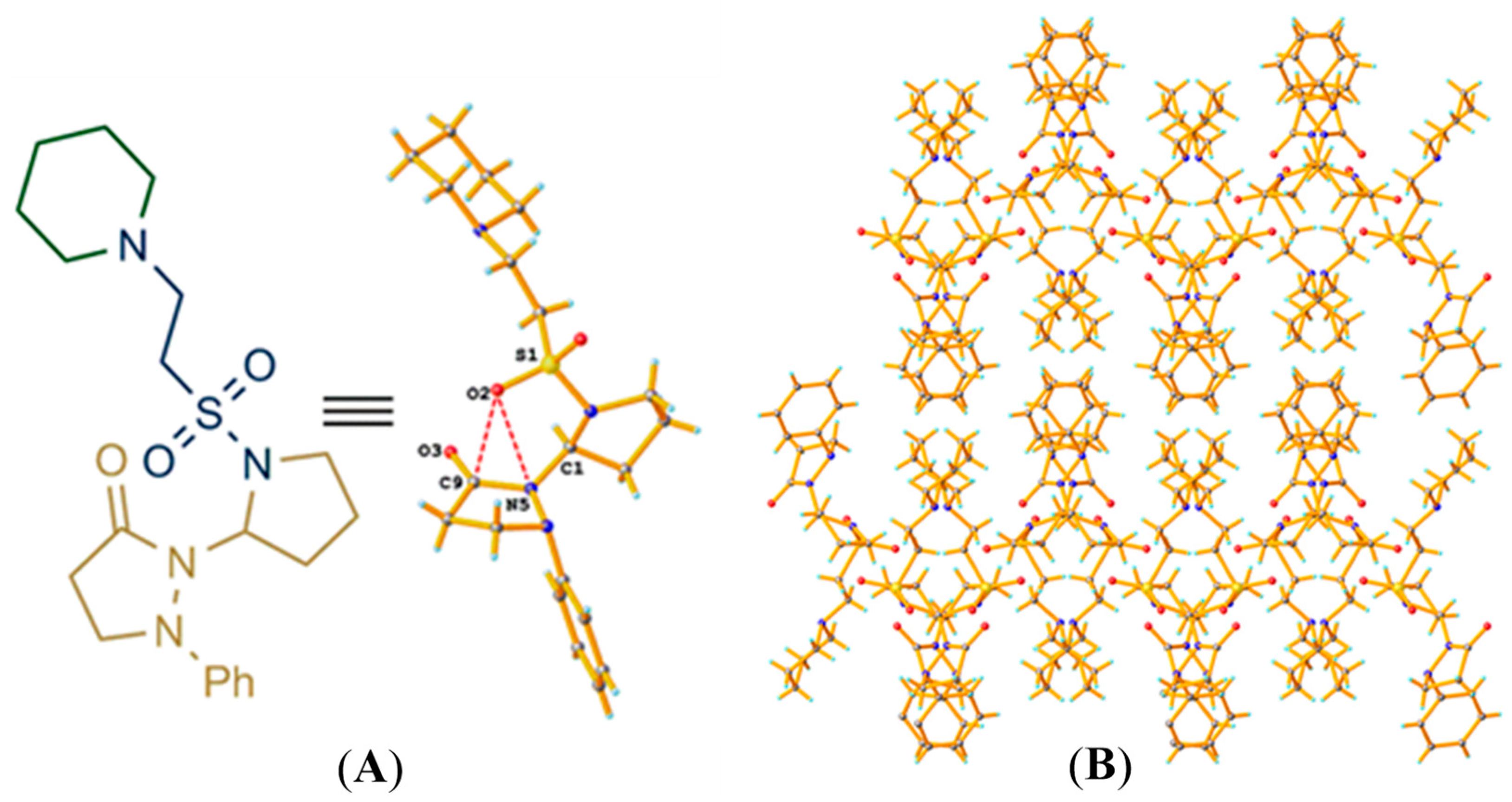
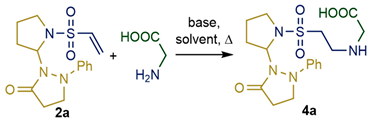
2.1. Synthesis and Characterization of Taurine Amide Derivatives
2.2. In Vitro Studies of Anticancer Activity
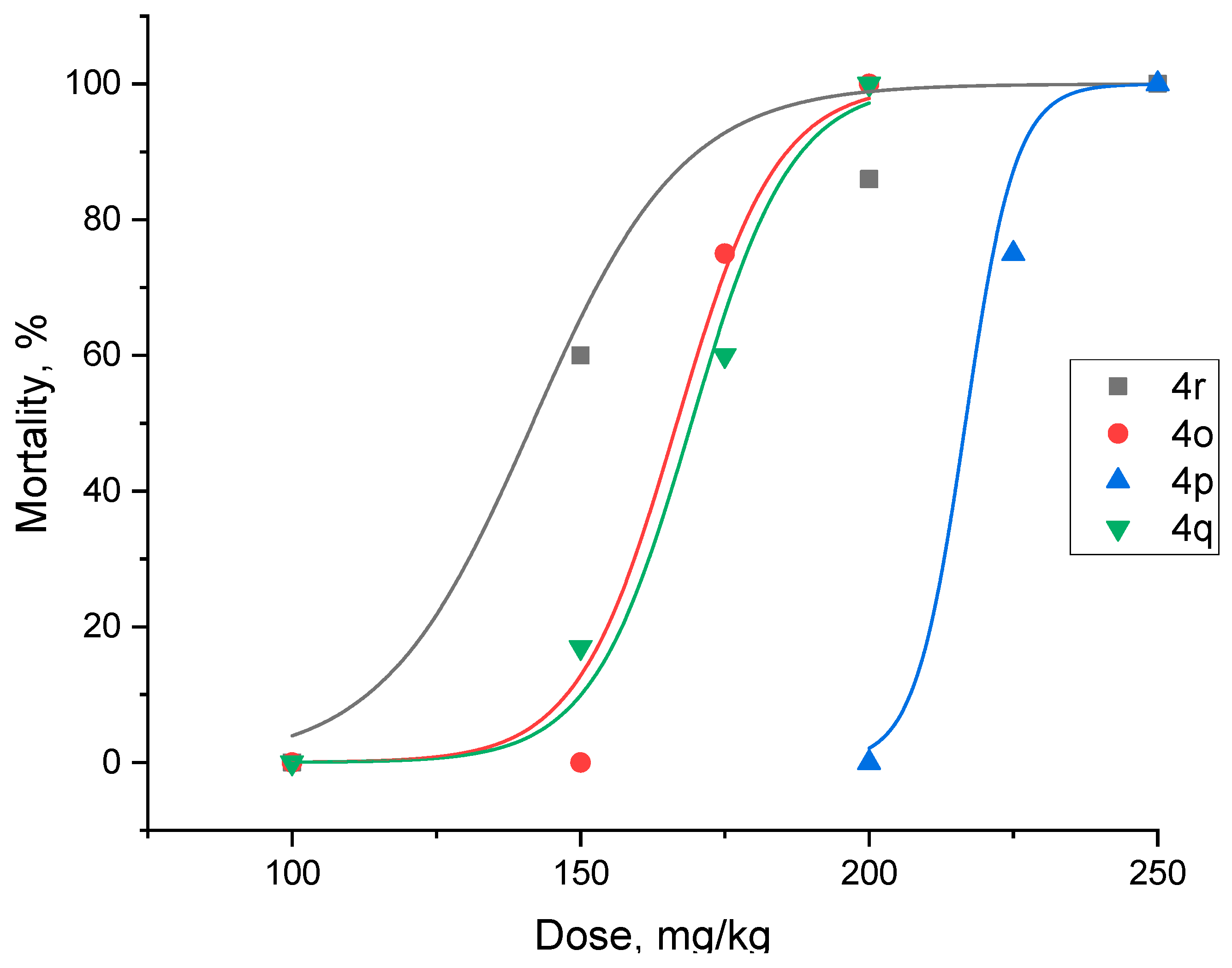
2.3. In Vivo Studies of Anticancer Activity
3. Discussion
4. Materials and Methods
4.1. Diphenyl(1-(vinylsulfonyl)pyrrolidin-2-yl)phosphine Oxide (2c)
4.2. General Experimental Procedure for the Synthesis of 4
4.3. Cytotoxicity Assay
4.4. Evaluation of In Vivo Acute Toxicity
4.5. Antitumor Activity
4.6. Statistical Treatment Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, R.; Win, T.; Bittner, S. Taurine Analogues; A New Class of Therapeutics: Retrospect and Prospects. Curr. Med. Chem. 2005, 12, 2021–2039. [Google Scholar] [CrossRef] [PubMed]
- Schuller-Levis, G.B.; Park, E. Taurine: New Implications for an Old Amino Acid. FEMS Microbiol. Lett. 2003, 226, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. The Sulfur-Containing Amino Acids: An Overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef] [PubMed]
- Baliou, S.; Kyriakopoulos, A.; Spandidos, D.; Zoumpourlis, V. Role of Taurine, Its Haloamines and Its LncRNA TUG1 in Both Inflammation and Cancer Progression. On the Road to Therapeutics? (Review). Int. J. Oncol. 2020, 57, 631–664. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.; Shamsi, A.; Mohammad, T.; Azum, N.; Alfaifi, S.Y.M.; Asiri, A.M.; Mohamed Elasbali, A.; Islam, A.; Hassan, M.I.; Haque, Q.M.R. Inhibiting Cyclin-Dependent Kinase 6 by Taurine: Implications in Anticancer Therapeutics. ACS Omega 2022, 7, 25844–25852. [Google Scholar] [CrossRef] [PubMed]
- Bibby, M.C.; Double, J.A.; Morris, C.M. Anti-Tumour Activity of TCNU in a Panel of Transplantable Murine Colon Tumours. Eur. J. Cancer Clin. Oncol. 1988, 24, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, A.; Khosrawipour, V.; Lau, H.; Li, S.; Migdal, P.; Labbé, M.K.; Kielan, W.; Nicpon, J.; Stieglitz, S.; Khosrawipour, T. Exploring the Potential of Taurolidine in Inducing Mobilization and Detachment of Colon Cancer Cells: A Preliminary in-Vitro Study. BMC Pharmacol. Toxicol. 2022, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Neijenhuis, L.K.A.; Naumann, L.L.; Ferkel, S.A.M.; Rubin, S.J.S.; Rogalla, S. Exploring the Effects of Taurolidine on Tumor Weight and Microvessel Density in a Murine Model of Osteosarcoma. Oncol. Res. 2024, 32, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, C.; Guo, Z.; Li, Y.; Zhao, S.; Yang, S.; Yang, Y.; Zhu, J.; Lin, D. Discovery of a Potent Dual EGFR/HER-2 Inhibitor L-2 (Selatinib) for the Treatment of Cancer. Eur. J. Med. Chem. 2013, 69, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, X.; Cai, Y.; Li, W. Lapatinib and Lapatinib plus Trastuzumab Therapy versus Trastuzumab Therapy for HER2 Positive Breast Cancer Patients: An Updated Systematic Review and Meta-Analysis. Syst. Rev. 2022, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.S.; Fan, L.; Van Horn, R.D.; Nakai, R.; Ohta, Y.; Akinaga, S.; Murakata, C.; Yamashita, Y.; Yin, T.; Credille, K.M.; et al. A Novel Eg5 Inhibitor (LY2523355) Causes Mitotic Arrest and Apoptosis in Cancer Cells and Shows Potent Antitumor Activity in Xenograft Tumor Models. Mol. Cancer Ther. 2015, 14, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Möhler, H.; Pfirman, R.W.; Frei, K. Redox-Directed Cancer Therapeutics: Taurolidine and Piperlongumine as Broadly Effective Antineoplastic Agents (Review). Int. J. Oncol. 2014, 45, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Akgül, Ö.; Erdoğan, M.A.; Birim, D.; Kayabaşi, Ç.; Gündüz, C.; Armağan, G. Design, Synthesis, Cytotoxic Activity, and Apoptosis Inducing Effects of 4- and N-Substituted Benzoyltaurinamide Derivatives. Turk. J. Chem. 2020, 44, 1674–1693. [Google Scholar] [CrossRef] [PubMed]
- Mujumdar, P.; Teruya, K.; Tonissen, K.F.; Vullo, D.; Supuran, C.T.; Peat, T.S.; Poulsen, S.-A. An Unusual Natural Product Primary Sulfonamide: Synthesis, Carbonic Anhydrase Inhibition, and Protein X-Ray Structures of Psammaplin C. J. Med. Chem. 2016, 59, 5462–5470. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, G. An Overview of Molecular Hybrids in Drug Discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Lazar, C.; Kluczyk, A.; Kiyota, T.; Konishi, Y. Drug Evolution Concept in Drug Design: 1. Hybridization Method. J. Med. Chem. 2004, 47, 6973–6982. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.A.; Adami, M.; Istyastono, E.P.; Zuiderveld, O.P.; van Dam, C.M.E.; de Kanter, F.J.J.; Jongejan, A.; Coruzzi, G.; Leurs, R.; de Esch, I.J.P. Synthesis and QSAR of Quinazoline Sulfonamides As Highly Potent Human Histamine H 4 Receptor Inverse Agonists. J. Med. Chem. 2010, 53, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Hollstein, U. Actinomycin. Chemistry and Mechanism of Action. Chem. Rev. 1974, 74, 625–652. [Google Scholar] [CrossRef]
- Cummings, S.R.; Ensrud, K.; Delmas, P.D.; LaCroix, A.Z.; Vukicevic, S.; Reid, D.M.; Goldstein, S.; Sriram, U.; Lee, A.; Thompson, J.; et al. Lasofoxifene in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2010, 362, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Komrokji, R.S.; Seymour, J.F.; Roberts, A.W.; Wadleigh, M.; To, L.B.; Scherber, R.; Turba, E.; Dorr, A.; Zhu, J.; Wang, L.; et al. Results of a Phase 2 Study of Pacritinib (SB1518), a JAK2/JAK2(V617F) Inhibitor, in Patients with Myelofibrosis. Blood 2015, 125, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.; William, A.; Blanchard, S.; Lee, A.; Nagaraj, H.; Wang, H.; Teo, E.; Tan, E.; Goh, K.C.; Dymock, B. Structure-Based Design of Oxygen-Linked Macrocyclic Kinase Inhibitors: Discovery of SB1518 and SB1578, Potent Inhibitors of Janus Kinase 2 (JAK2) and Fms-like Tyrosine Kinase-3 (FLT3). J. Comput.-Aided Mol. Des. 2012, 26, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Burness, C.B.; Duggan, S.T. Trifluridine/Tipiracil: A Review in Metastatic Colorectal Cancer. Drugs 2016, 76, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Cervantes, A.; Moreno Vera, S.; Taieb, J. Trifluridine/Tipiracil: An Emerging Strategy for the Management of Gastrointestinal Cancers. Future Oncol. 2018, 14, 1629–1645. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA -Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Juric, D.; Rodon, J.; Tabernero, J.; Janku, F.; Burris, H.A.; Schellens, J.H.M.; Middleton, M.R.; Berlin, J.; Schuler, M.; Gil-Martin, M.; et al. Phosphatidylinositol 3-Kinase α–Selective Inhibition with Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results From the First-in-Human Study. J. Clin. Oncol. 2018, 36, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, B.F.; Lynas, J.F.; Scott, C.J.; McGoohan, C.; Martin, L.; Walker, B. Dipeptide Proline Diphenyl Phosphonates Are Potent, Irreversible Inhibitors of Seprase (FAPα). Biochem. Biophys. Res. Commun. 2006, 346, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Puré, E.; Blomberg, R. Pro-Tumorigenic Roles of Fibroblast Activation Protein in Cancer: Back to the Basics. Oncogene 2018, 37, 4343–4357. [Google Scholar] [CrossRef] [PubMed]
- Busek, P.; Mateu, R.; Zubal, M.; Kotackova, L.; Sedo, A. Targeting Fibroblast Activation Protein in Cancer Ndash Prospects and Caveats. Front. Biosci. 2018, 23, 1933–1968. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Dai, Y.; Qiang, H.; Shi, W.; Liao, C.; Zhao, F.; Huang, W.; Qian, H. Discovery of Novel 2-Substituted-4-(2-Fluorophenoxy) Pyridine Derivatives Possessing Pyrazolone and Triazole Moieties as Dual c-Met/VEGFR-2 Receptor Tyrosine Kinase Inhibitors. Bioorg. Chem. 2017, 72, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kandhasamy, S.; Ramanathan, G.; Muthukumar, T.; Thyagarajan, S.; Umamaheshwari, N.; Santhanakrishnan, V.P.; Sivagnanam, U.T.; Perumal, P.T. Nanofibrous Matrixes with Biologically Active Hydroxybenzophenazine Pyrazolone Compound for Cancer Theranostics. Mater. Sci. Eng. C 2017, 74, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Grauer, A.; König, B. Peptidomimetics—A Versatile Route to Biologically Active Compounds. Eur. J. Org. Chem. 2009, 2009, 5099–5111. [Google Scholar] [CrossRef]
- Albericio, F.; Kruger, H.G. Therapeutic Peptides. Future Med. Chem. 2012, 4, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, A.A.; Reichert, J.M. Future Directions for Peptide Therapeutics Development. Drug Discov. Today 2013, 18, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Rulev, A.Y. Aza-Michael Reaction: Achievements and Prospects. Russ. Chem. Rev. 2011, 80, 197–218. [Google Scholar] [CrossRef]
- Smolobochkin, A.V.; Muravyeva, E.A.; Vagapova, L.I.; Knyazeva, I.R.; Voronina, J.K.; Burilov, A.R.; Pudovik, M.A.; Gildebrant, A.V.; Sazykin, I.S.; Sazykina, M.A.; et al. Synthesis and Evaluation of Water-Soluble 2-Aryl-1-Sulfonylpyrrolidine Derivatives as Bacterial Biofilm Formation Inhibitors. Chem. Biodivers. 2019, 16, e1800490. [Google Scholar] [CrossRef] [PubMed]
- Smolobochkin, A.V.; Rizbayeva, T.S.; Gazizov, A.S.; Voronina, J.K.; Dobrynin, A.B.; Gildebrant, A.V.; Strelnik, A.G.; Sazykin, I.S.; Burilov, A.R.; Pudovik, M.A.; et al. Acid-Catalyzed Intramolecular Imination/Nucleophilic Trapping of 4-Aminobutanal Derivatives: One-Pot Access to 2-(Pyrazolyl)pyrrolidines. Eur. J. Org. Chem. 2019, 2019, 5709–5719. [Google Scholar] [CrossRef]
- Smolobochkin, A.V.; Turmanov, R.A.; Gazizov, A.S.; Voloshina, A.D.; Voronina, J.K.; Sapunova, A.S.; Burilov, A.R.; Pudovik, M.A. One-Pot Imination/Arbuzov Reaction of 4-Aminobutanal Derivatives: Synthesis of 2-Phosphorylpyrrolidines and Evaluation of Anticancer Activity. Tetrahedron 2020, 76, 131369. [Google Scholar] [CrossRef]
- Aranha, O.; Grignon, R.; Fernandes, N.; McDonnell, T.; Wood, D.; Sarkar, F. Suppression of Human Prostate Cancer Cell Growth by Ciprofloxacin Is Associated with Cell Cycle Arrest and Apoptosis. Int. J. Oncol. 2003, 22, 787–794. [Google Scholar] [CrossRef] [PubMed]
- El-Rayes, B.; Grignon, R.; Aslam, N.; Aranha, O.; Sarkar, F. Ciprofloxacin Inhibits Cell Growth and Synergises the Effect of Etoposide in Hormone Resistant Prostate Cancer Cells. Int. J. Oncol. 2002, 21, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Kloskowski, T.; Gurtowska, N.; Olkowska, J.; Nowak, J.M.; Adamowicz, J.; Tworkiewicz, J.; Debski, R.; Grzanka, A.; Drewa, T. Ciprofloxacin Is a Potential Topoisomerase II Inhibitor for the Treatment of NSCLC. Int. J. Oncol. 2012, 41, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Lodochnikova, O.A.; Latypova, L.Z.; Madzhidov, T.I.; Chmutova, G.A.; Voronina, J.K.; Gubaidullin, A.T.; Kurbangalieva, A.R. “Lp···synthon” Interaction as a Reason for the Strong Amplification of Synthon-Forming Hydrogen Bonds. CrystEngComm 2019, 21, 1499–1511. [Google Scholar] [CrossRef]
- Lodochnikova, O.A.; Krivolapov, D.B.; Startseva, V.A.; Nikitina, L.E.; Bodrov, A.V.; Artemova, N.P.; Klochkov, V.V.; Madzhidov, T.I.; Chmutova, G.A.; Litvinov, I.A. S=o…s=o Interactions as a Driving Force for Low-Temperature Conformational Rearrangement of Stable H-Bonding {S(O)-Ch2-Ch2-OH···}2 Synthon in Two Modifications of Diastereomeric Pinanyl Sulfoxides Co-Crystal. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 2222–2231. [Google Scholar] [CrossRef]
- Mooibroek, T.J.; Gamez, P.; Reedijk, J. Lone Pair–π Interactions: A New Supramolecular Bond? CrystEngComm 2008, 10, 1501. [Google Scholar] [CrossRef]
- Voronina, Y.K.; Litvinov, I.A.; Lyssenko, K.A. The Lp(O)…π-Interactions in 1-[1-(Methoxycarbonylmethylthio)methyl]-3,5-dimethylisocyanurate: Topological Analysis of the Electron Density Distribution from X-Ray Diffraction Data and Quantum Chemical Calculations. Russ. Chem. Bull. 2013, 62, 1699–1706. [Google Scholar] [CrossRef]
- Thakur, A.K.; Fezio, W.L. A Computer Program for Estimating Ld50 and its Confidence Limits using Modified Behrens-Reed-Muench Cumulant Method. Drug Chem. Toxicol. 1981, 4, 297–305. [Google Scholar] [CrossRef] [PubMed]
- GOST 12.1.007-76; Occupational Safety Standards System. Noxious Substances. Classification and General Safety Requirements. Belgiss: Minsk, Belarus, 1977.
- Teicher, B.A. Tumor Models for Efficacy Determination. Mol. Cancer Ther. 2006, 5, 2435–2443. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.N.; Bunyatyan, N.D. (Eds.) Handbook for Preclinical Drug Trials; Ministry of Public Health and Social Development of the RF, FGBU Scientific Centre for the Expert Evaluation of Medicinal Products: Moscow, Russia, 2012.
- Smolobochkin, A.V.; Gazizov, A.S.; Yakhshilikova, L.J.; Bekrenev, D.D.; Burilov, A.R.; Pudovik, M.A.; Lyubina, A.P.; Amerhanova, S.K.; Voloshina, A.D. Synthesis and Biological Evaluation of Taurine-Derived Diarylmethane and Dibenzoxanthene Derivatives as Possible Cytotoxic and Antimicrobial Agents. Chem. Biodivers. 2022, 19, e202100970. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; He, F.; Kawanokuchi, J.; Wang, G.; Yamashita, T. Taurine and Its Anticancer Functions: In Vivo and In Vitro Study. Adv. Exp. Med. Biol. 2022, 1370, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Stary, D.; Bajda, M. Taurine and Creatine Transporters as Potential Drug Targets in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 3788. [Google Scholar] [CrossRef] [PubMed]
- Farley, L.L.; Winkler, R.A. Flowing Oxygen Schöniger Combustion for Large Samples. Anal. Chem. 1963, 35, 772–773. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXT 2014/4; Bruker-AXS: Madison, WI, USA, 2014. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Belenok, M.G.; Strobykina, I.Y.; Lyubina, A.P.; Gumerova, S.K.; Kataev, V.E. Antimicrobial and Cytotoxic Effects of Ammonium Derivatives of Diterpenoids Steviol and Isosteviol. Bioorg. Med. Chem. 2021, 32, 115974. [Google Scholar] [CrossRef] [PubMed]
 | ||||
|---|---|---|---|---|
| Entry | Solvent | Base | Time [h] | Yield [%] |
| 1 | EtOH:H2O (4:1) | Et3N | 6 | 15 [b] |
| 2 | EtOH:H2O (4:1) | Et3N | 24 | 20 [b] |
| 3 | H2O | Et3N | 24 | 10 [b] |
| 4 | EtOH:H2O (4:1) | Pyridine | 24 | 34 [b] |
| 5 | EtOH:H2O (4:1) | DMAP | 1 | 24 [b] |
| 6 | EtOH:H2O (4:1) | DMAP | 24 | 35 [b] |
| 7 | MeOH:H2O (4:1) | Et3N | 6 | 75 [b] |
| 8 | MeOH:H2O (4:1) | Et3N | 24 | 93 [c] |
| Test Compounds | Cancer Cell Lines | Normal Cell Lines | ||||||
|---|---|---|---|---|---|---|---|---|
| M-HeLa [b] | HuTu 80 [c] | HL-60 [d] | WI38 [e] | RPMI 1788 [f] | ||||
| IC50 (μM) | SI [g] | IC50 (μM) | SI [g] | IC50 (μM) | SI [g] | IC50 (μM) | IC50 (μM) | |
| 4b | 87 ± 6.8 | 1 | >100 | - | - | - | 88.3 ± 7.1 | - |
| 4c | >100 | - | >100 | - | - | - | 100 | - |
| 4l | 85.4 ± 7.5 | 1.8 | >100 | - | - | - | 154 ± 12 | - |
| 4m | >100 | 1 | 87.0 ± 7.0 | >1.1 | - | - | >100 | - |
| 4o | 56.7 ± 4.8 | 1.5 | >100 | - | >100 | - | 84.3 ± 7.2 | >100 |
| 4p | 57.0 ± 4.6 | 1.1 | 94.4 ± 8.7 | - | 76.7 ± 6.1 | 2.3 | 63.2 ± 5.7 | 176.3 ± 3.4 |
| 4q | >100 | - | >100 | - | >100 | - | >100 | >100 |
| 4r | 96.0 ± 8.6 | >1 | 97.3 ± 8.2 | >1 | >100 | - | >100 | >100 |
| 4s | 78.4 ± 6.6 | 1.2 | >100 | - | >100 | - | 91.8 ± 8.4 | >100 |
| 4u | >100 | - | >100 | - | - | - | >100 | - |
| 4v | 96.2 ± 8.4 | 2.2 | >100 | - | - | - | 211 ± 17 | - |
| 4x | 98.2 ± 8.3 | 1.3 | >100 | - | - | - | 128 ± 10 | - |
| 4y | 62.5 ± 5.3 | 1.9 | 100 ± 9.2 | 1.2 | - | - | 120 ± 9.3 | - |
| 4z | 85.9 ± 8.2 | 1.4 | 64.6 ± 5.1 | 1.9 | - | - | 123 ± 9.6 | - |
| Tamoxifen | 28.0 ± 2.5 | 1.6 | - | - | - | - | 46.2 ± 3.5 | - |
| 5-Fluorouracil | 75.6 ± 6.1 | - | 65.2 ± 5.5 | 1 | - | - | 62.0 ± 4.8 | - |
| Doxorubicin | - | - | - | - | 3.0 ± 4.7 | 1.1 | - | 3.3 ± 6.6 |
| Dose | 4o | Dose | 4p | Dose | 4q | Dose | 4r | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MST ± SD [a] (Days) | ILS [b] (%) | MST ± SD (Days) | ILS (%) | MST ± SD (Days) | ILS (%) | MST ± SD (Days) | ILS (%) | ||||
| 0 | 0 | 0 | 0 | ||||||||
| 12 | 10.7 ± 1.0 | 7 | 16 | 20 ± 3.1 | 100 | 12 | 8.3 ± 0.6 | −9 | 10 | 9.8 ± 0.2 | −9 |
| 17 | 9.5 ± 0.8 | −5 | 22 | 18 ± 3.2 | 80 | 17 | 10.3 ± 0.9 | 13 | 15 | 10.5 ± 0.8 | −2 |
| 26 | 11.7 ± 1.9 | 17 | 34 | 17.5 ± 2.7 | 75 | 27 | 12.2 ± 1.7 | 33 | 22 | 11.2 ± 0.8 | 4 |
| 37 | 10.7 ± 0.9 | 7 | 48 | 15.5 ± 3.4 | 55 | 38 | 15.5 ± 3.9 | 69 | 31 | 10.8 ± 0.7 | 0 |
| 55 | 10.5 ± 1.2 | 5 | 72 | 14.2 ± 2.4 | 42 | 57 | 10.2 ± 0.2 | 11 | 47 | 10.7 ± 0.8 | −1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakypova, S.; Smolobochkin, A.; Rizbayeva, T.; Turmanov, R.; Gazizov, A.; Akylbekov, N.; Zhapparbergenov, R.; Narmanova, R.; Ibadullayeva, S.; Zalaltdinova, A.; et al. Taurine-Based Hybrid Drugs as Potential Anticancer Therapeutic Agents: In Vitro, In Vivo Evaluations. Pharmaceuticals 2025, 18, 1056. https://doi.org/10.3390/ph18071056
Nakypova S, Smolobochkin A, Rizbayeva T, Turmanov R, Gazizov A, Akylbekov N, Zhapparbergenov R, Narmanova R, Ibadullayeva S, Zalaltdinova A, et al. Taurine-Based Hybrid Drugs as Potential Anticancer Therapeutic Agents: In Vitro, In Vivo Evaluations. Pharmaceuticals. 2025; 18(7):1056. https://doi.org/10.3390/ph18071056
Chicago/Turabian StyleNakypova, Saltanat, Andrey Smolobochkin, Tanzilya Rizbayeva, Rakhymzhan Turmanov, Almir Gazizov, Nurgali Akylbekov, Rakhmetulla Zhapparbergenov, Roza Narmanova, Saltanat Ibadullayeva, Alena Zalaltdinova, and et al. 2025. "Taurine-Based Hybrid Drugs as Potential Anticancer Therapeutic Agents: In Vitro, In Vivo Evaluations" Pharmaceuticals 18, no. 7: 1056. https://doi.org/10.3390/ph18071056
APA StyleNakypova, S., Smolobochkin, A., Rizbayeva, T., Turmanov, R., Gazizov, A., Akylbekov, N., Zhapparbergenov, R., Narmanova, R., Ibadullayeva, S., Zalaltdinova, A., Syzdykbayev, M., Voronina, J., Lyubina, A., Voloshina, A., Klimanova, E., Sashenkova, T., Mishchenko, D., & Burilov, A. (2025). Taurine-Based Hybrid Drugs as Potential Anticancer Therapeutic Agents: In Vitro, In Vivo Evaluations. Pharmaceuticals, 18(7), 1056. https://doi.org/10.3390/ph18071056











