Abstract
Background/Objectives: Neurofibromatosis Type 1 (NF1) plexiform neurofibroma (PN) can cause morbidity, including disfigurement that can negatively impact social functioning. Historically, the mainstay treatment is surgical resection. However, complete resection is often prohibitive due to multiple nerve involvement. Moreover, post-operative recurrence is common. MEK inhibitors, including selumetinib and mirdametinib, have recently changed the treatment paradigm for these tumors. In 2020, selumetinib was FDA-approved for pediatric NF1 patients with inoperable symptomatic PNs, but selumetinib remains under investigation for their adult counterparts. In 2025, mirdametinib was FDA-approved for use in adults with symptomatic incompletely resectable NF1 PNs. Lower partial response rates have been reported with mirdametinib compared to selumetinib, but direct comparative analyses have not been conducted to establish the superiority of one agent over the other. Results: We present a case of a 38-year-old male with a right facial PN successfully treated with selumetinib, resulting in a 16.77% tumor volumetric reduction over 7 months. Selumetinib was well tolerated in our patient, with an asymptomatic Grade 3 CPK elevation that subsequently improved with a dose reduction. Conclusion: Our case adds to the growing body of evidence suggesting that selumetinib is effective and well tolerated in adult patients with NF1-associated PNs.
1. Introduction
The Neurofibromin 1 (MIM# 613113, NF1) gene encodes neurofibromin, a tumor suppressor protein that negatively regulates the Ras signaling pathway [1,2]. Neurofibromin converts active guanosine triphosphate-bound RAS to an inactive GDP-bound RAS [3]. As such, heterozygous loss-of-function and inactivating mutations within NF1 upregulate and constitutively activate the MAPK signaling pathway, leading to tumorigenesis [3,4,5,6,7,8,9].
Pathogenic germline variants occur in approximately half of all NF1 cases, giving rise to Neurofibromatosis type 1 (NF1) syndrome (MIM# 162200, NF1) [10,11]. NF1 is an autosomal-dominant inherited tumor predisposition syndrome that is characterized by a broad spectrum of diagnostic findings, including café-au-lait spots, intertriginous freckling, bone dysplasia, and tumors [10,12,13,14]. This condition is clinically heterogeneous, with varying levels of multisystem involvement–even within the same family [15]. Approximately half of these patients develop plexiform neurofibromas (PNs). PNs are tumors comprising of Schwann cells, fibroblasts, and hematopoietic and mast cells, among others [16]. PNs can develop anywhere from the nerve root to the distal peripheral nerve, and may cause morbidity and disfigurement for NF1 patients [2,9,15,17,18,19]. Mortality may be increased in cases of transformation to malignant peripheral nerve sheath tumor, airway compression, or spinal cord impingement [10,20,21,22]. Associated symptoms depend on the tumor location and may have significant impacts on physical functioning, including pain, motor, vision, bowel, and bladder impairment [10,19,23]. PNs causing disfigurement can significantly impact social functioning and limit activity engagement and participation [24,25]. Limited studies address the negative social impact associated with this syndrome. The stigma associated with the appearance of these tumors can bear emotional weight on the lives of these patients [26], including those with facial deformity.
NF1 syndrome may be diagnosed clinically or genetically, with PN as a diagnostic criterion [27]. PNs are diagnosed on MRI and surgical resection is the mainstay treatment for these tumors [2,12,17,28,29,30]. However, multiple nerve involvement, soft tissue invasion, and vascular injury often prohibit complete tumor resection [2,12,17,28,29,31]. Furthermore, 40–50% of PNs recur following surgery [12,29,32,33]. Accordingly, there has been an unmet need for the treatment of these tumors. Trials investigating therapies for NF1 PNs have been primarily focused on the pediatric patient population, wherein PNs exhibit rapid growth during childhood and in the setting of hormonal changes [34,35,36,37,38,39]. In stark contrast, adult NF1 PNs exhibit an insidious growth pattern [35,37,39]. Agents targeting multiple pathways, including pegylated interferon alfa-2b, pirfenidone, tipifarnib, sirolimus, and imatinib, have shown marginal success with limited reductions in tumor volume [40,41,42,43,44,45]. However, molecular advances have now led to the emergence of targeted therapies.
MEK inhibitors (MEKi)s aimed at the MEK gene hinder the MAPK signaling cascade [2]. Selumetinib is a potent allosteric kinase MEK 1/2 inhibitor that impedes ERK1/2 phosphorylation, consequently reducing cell proliferation, survival, and differentiation [46,47,48]. Selumetinib can induce durable responses with no apparent activation of resistance pathways, including the AKT signaling pathway [37]. The pivotal National Cancer Institute (NCI) Phase 1/2 SPRINT trial (NCT01362803) investigating the use of selumetinib in children with symptomatic inoperable NF1 PNs demonstrated partial response rates (PR = tumor volume decrease of 20% or greater for 4 or more weeks) of 68–75%, many of whom maintained durable responses lasting more than one year, and a median progression-free survival of 7 years (Table 1) [17,49]. Accordingly, selumetinib was granted FDA approval for the treatment of NF1 pediatric patients with inoperable PNs based on the success of this trial [50,51]. In a subsequent Phase II study conducted by Kim et al., 87% (26/30) of adult NF1 PN patients treated with selumetinib 50 mg twice daily (BID) achieved PR [52]. All participants who completed 26 cycles achieved PR [52]. In another Phase 2 study (NCT02407405) investigating the use of selumetinib in adult patients with inoperable, progressive, or symptomatic PNs, the overall response rate (ORR) was 63.6% [37]. The safety profiles amongst these trials are comparable, with reported adverse events (AEs) of rash, elevated creatine phosphokinase (CPK) or alanine transaminase, dry skin, and pruritus [17,49,52]. Selumetinib has yet to gain regulatory approval for use in NF1 PN adult patients, but it remains under investigation. The randomized, placebo-controlled Phase 3 KOMET (NCT04924608) and NCI Phase I (NCT02407405) trials are currently underway to assess the efficacy and safety of selumetinib in adult NF1 patients with inoperable PNs. Interim results for the KOMET study are promising, with an ORR of 20% and a 33.9% reduction in tumor volume in participants who completed 16 cycles of selumetinib 25 mg/m2 BID [53]. Notably, even participants who completed only 4 months of selumetinib demonstrated a response to therapy [53]. More recently, in February 2025, mirdametinib was FDA-approved for use for NF1 adults (and children) with symptomatic PNs who are not amenable to complete resection, based on the success of the ReNeu trial [54]. Interestingly, the PR of 42% associated with mirdametinib is lower than the ORR associated with selumetinib for NF1 PN adults (87%) [9,52]. However, the ReNeu trial was not designed to determine the maximal tolerated dose, and higher dosing may have positively influenced the therapeutic response [9]. Further, mirdametinib provides the additional benefit of a liquid formulation to accommodate NF1 head and facial PN patients with dysphagia [25].

Table 1.
Clinical Trials investigating MEK inhibitors in NF1 patients with PNs. ORR = overall response rate; PR = partial response; BID = twice daily; CPK = creatine phosphokinase; ALT = alanine aminotransferase; PN = plexiform neurofibroma.
Herein, we present the case of a 38-year-old male with NF1 and a right facial PN who was successfully treated with selumetinib, with a tumor volumetric response of −16.77% following 7 months of therapy. Our case adds to the growing body of evidence supporting the use of selumetinib in adults with NF1 PNs.
2. Case Presentation
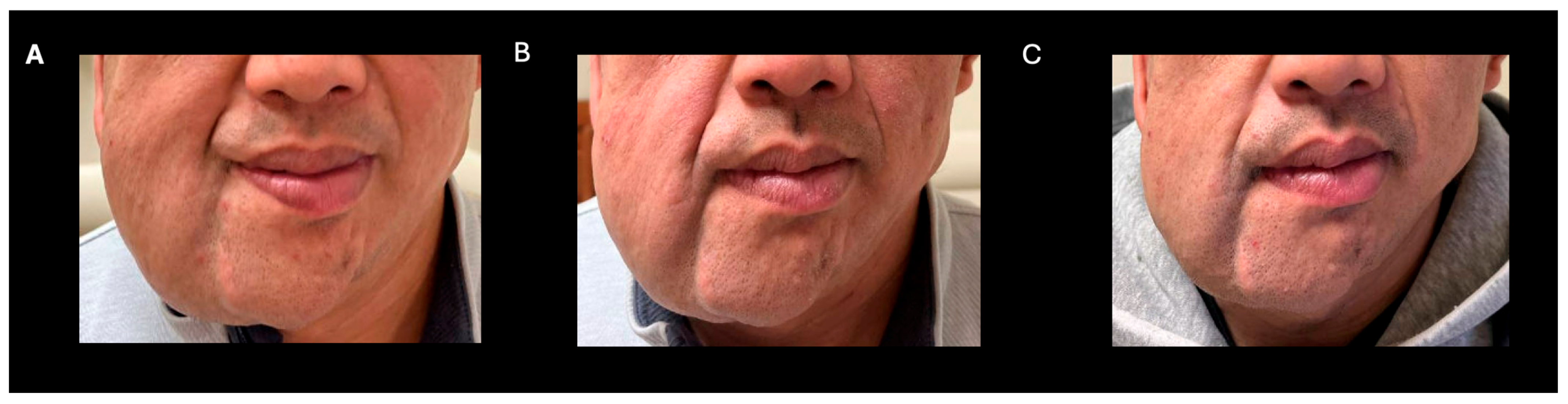
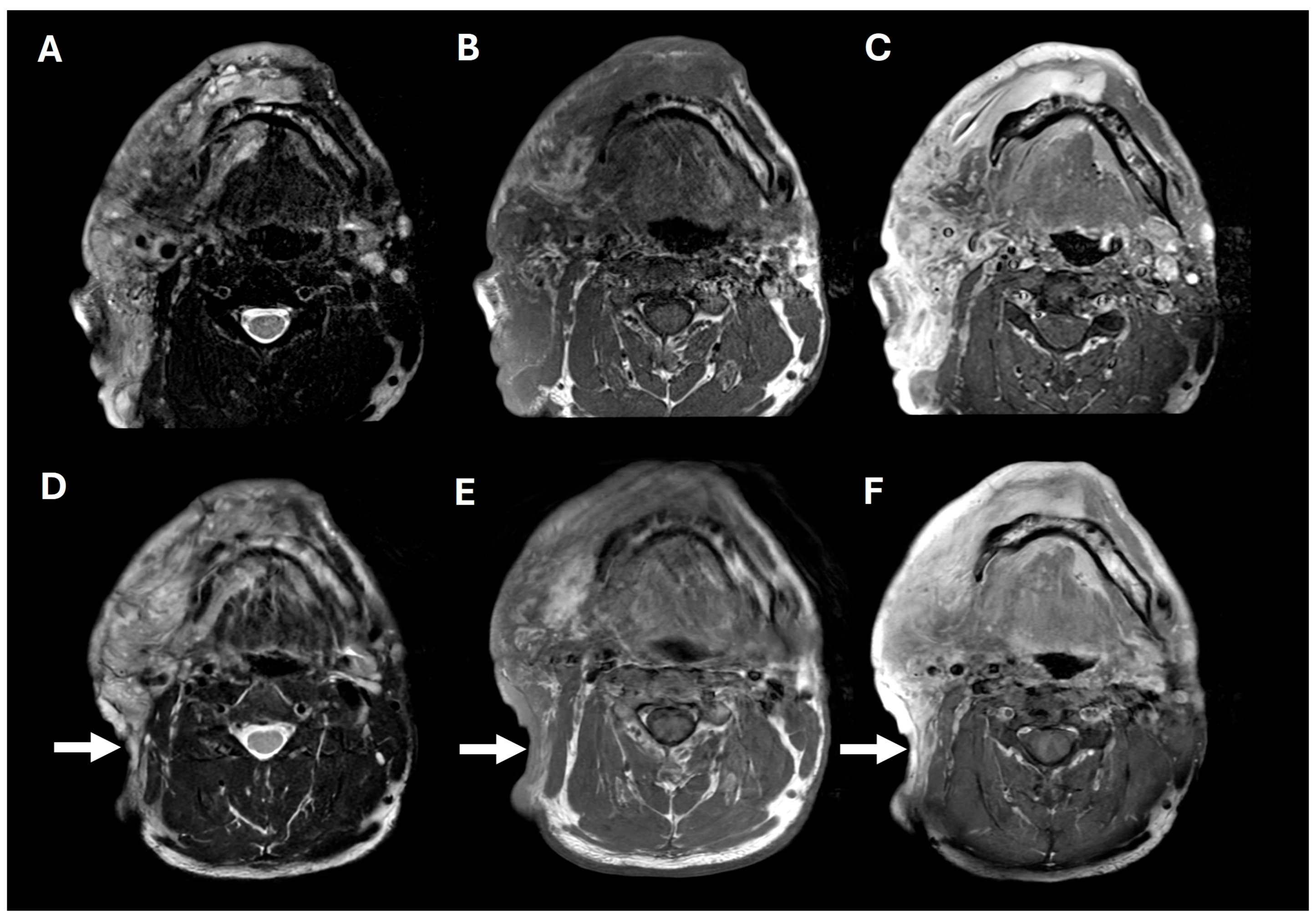
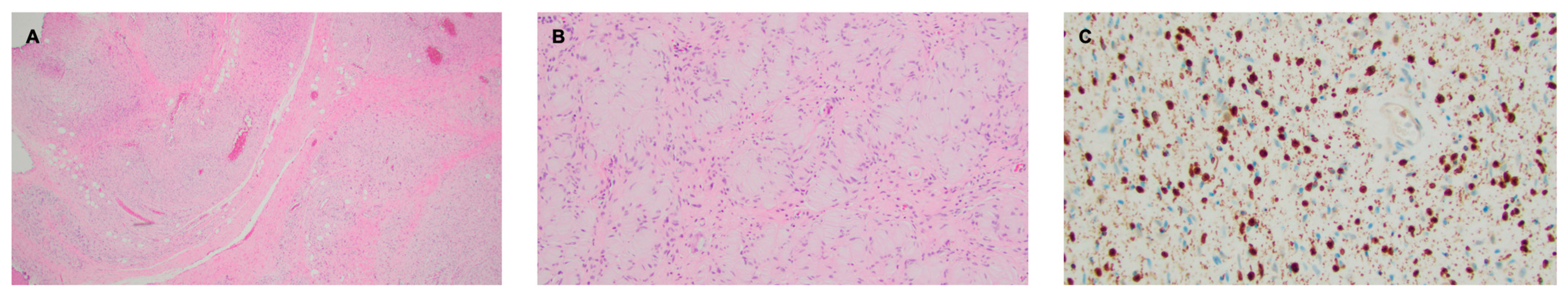
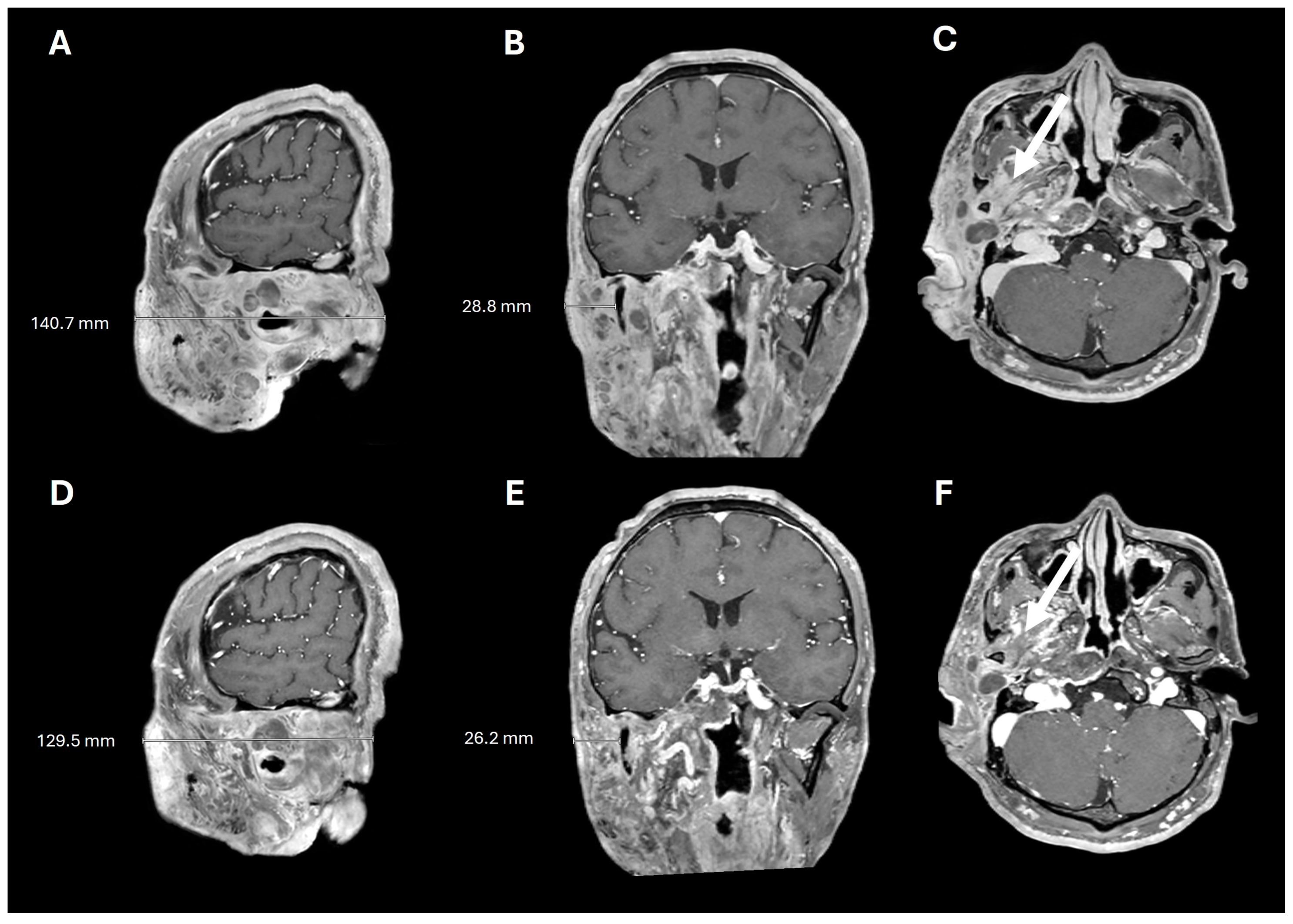
A 38-year-old man with genetically confirmed NF1 and a large conglomerate R facial PN, with status post debulking at age 8, presented to our institution for further management of his disfiguring PN. His family history was positive for NF1 in his mother and daughter. Genetic testing revealed a heterozygous pathogenic truncating variant in exon 38 of NF1: c.5438C>A and p.Sesr1813Ter. No reportable variants were found in SPRED1. Neurological examination showed a large right facial mass (Figure 1A), >6 café au lait macules—all >15 mm in size, axillary freckling, and numerous cutaneous neurofibromas. A brain MRI demonstrated a right predominantly facial PN (Figure 2A–C). Following posterior auricular tumor debulking, a brain MRI showed an interval reduction in the tumor size posterior to his ear (Figure 2D–F). Pathological examination showed positive S100, SOX-10, and CD34, and negative SMA, consistent with neurofibroma (Figure 3). Ki-67 was less than 1%. Molecular analysis identified a pathogenic variant (NM_000267.3: c.5438C>A, p.Ser1813Ter) in the heterozygous state in NF1. At the time of diagnosis, there were no FDA-approved agents for adult NF1 PNs. On the basis of the higher reported ORR in selumetinib compared to mirdametinib, selumetinib was the targeted agent selected for our patient. However, no head-to-head clinical trials have directly compared mirametinib and selumetinib. He was administered 25 mg/m2 (40 mg BID) selumetinib, which was complicated by a facial acneiform rash and asymptomatic elevated Grade 3 CPK after 1 month. His rash resolved with topical adapalene 0.1%/benzoyl peroxide 2.5% gel, and his CPK improved to Grade 1 with a dose reduction in selumetinib to 35 mg BID. At 7-month follow-up, a reduction in tumor burden was noted clinically (Figure 1A–C), with a corresponding reduction in tumor volume radiographically (Figure 4A–F). On his brain MRI, the pre-selumetinib tumor volume was 284.65 cm3 and the post-selumetinib tumor volume was 236.92 cm3, showing a 16.77% reduction in tumor volume. Given the infiltrative nature of the tumor, volume was calculated using an ellipsoid volume formula of 0.5 × length × width × height. At time of publication, he remains on selumetinib and endorses improvement in quality of life (QoL).
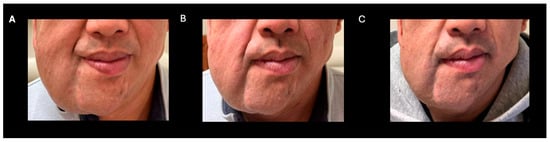
Figure 1.
Clinical improvement after selumetinib (A) baseline (B) 3 months post-selumetinib (C) 7 months post-selumetinib.
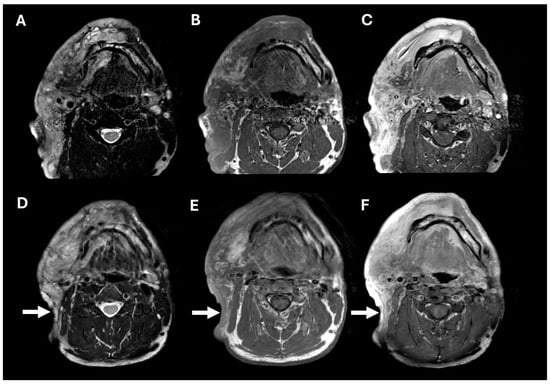
Figure 2.
Pre- and post-operative brain MRIs. Pre-operative T2, T1, and T1+contrast imaging (A–C), showing a large, T2-hyperintense, enhancing, predominantly right facial mass. Post-operative T2, T1, and T1+contrast imaging (D–F), showing posterior tumor debulking (arrows).
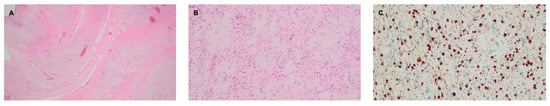
Figure 3.
Right facial plexiform neurofibroma. (A) H&E stain, 2× objective, highlighting a diffusely infiltrative proliferation of spindled to ovoid cells with loose collagenous stroma. A vague bundled and tortuous pattern is visible at this power. (B) H&E stain, 10× objective, demonstrating an area featuring abundant pesudo-Meissner corpuscles, a feature frequently encountered in diffuse and plexiform neurofibromas. (C) S100 stain, 20× objective, showing nuclear and granular cytoplasmic staining, as compared to the diffuse staining seen in schwannomas.
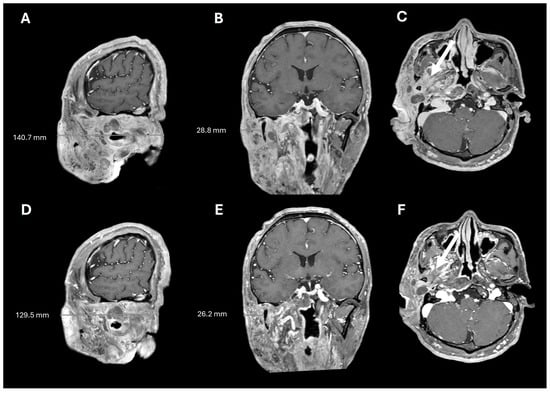
Figure 4.
Pre- and post-selumetinib brain MRI. T1+contrast imaging before (A–C) and after (D–F) 7 months of selumetinib, showing decreased tumor burden and decreased tumor infiltration into the masticator space (arrows).
Ethical guidelines set out by the Declaration of Helsinki were followed in the preparation of this report, and the patient provided written consent.
3. Discussion
Plexiform neurofibromas may cause morbidity and disfigurement in adult NF1 patients [2,9,15,17,18,19]. Studies addressing the negative social impact of facial PNs on these patients are limited. Facial disfigurement can significantly affect social functioning for these patients and hinder participation in activities, leading to a poor QoL [24,25,26,56]. For these reasons, our patient sought further management to reduce his right facial tumor burden. However, NF1 PNs present surgical challenges due to their infiltrative nature, and effective therapy remains a treatment gap in neuro-oncology practice to date [28,38,57]. In particular, facial PNs are arduous due to multiple nerve involvement, soft tissue invasion, and vascular injury, which highlights the need for alternative therapies [28,57,58].
MEKis are a promising therapy to fulfill the treatment gap for adults with NF1 PNs. Recently, mirdametinib was FDA approved for this indication on the basis of the Phase 2 single-arm ReNeu trial [2,54], four months after our patient initiated treatment on selumetinib. However, of the adult NF1 PN participants treated with mirdametinib in this trial, the results were modest compared to results with selumetinib (PR = 42% vs. PR = 87%, respectively) [9,52]. At time of publication, selumetinib was regulatory approved for use in pediatric NF1 patients with symptomatic inoperable PNs, but was not approved for use in their adult counterparts [50]. An inverse correlation between age and NF1 PN growth rate has been proposed, with suggestions that pediatric NF1 PN patients may be more responsive to MEKis, compared to NF1 PN adults [9,34,35,36,37,59]. This increased sensitivity to MEKi has been attributed to higher PN growth rates during childhood compared to the insidious growth rate that is observed in adults with NF1 PN [2,9,35,39]. Despite this, our adult patient achieved significant tumor regression 30 years after his initial diagnosis, with a 16.77% tumor volumetric reduction following 7 months of selumetinib, and he reported improvement in QoL. Limited evidence suggests that neoadjuvant MEKi can provide benefit to permit surgery [60].
To our knowledge, only three other NF1 PN adults with facial PNs treated with MEKis have been reported; two patients were treated with selumetinib 50 mg/m2 BID, and one patient was treated with mirdametinib 2 mg/m2 (4 mg maximum) BID in a 3-week on/1-week off schedule [37,52]. The existing evidence suggests that tumor response can occur later in the treatment trajectory and initial non-responders may be later identified as delayed responders to MEKis [2,17,49]. Nevertheless, our patient was an initial responder and achieved a volumetric response after only 7 months of therapy, corroborating with reported results of some adult patients responding after only 6 cycles of therapy [52]. Kim et al. suggest that selumetinib pharmacokinetics may differ between Asian and Caucasian NF1 patients, with higher drug exposure in Asian individuals [52,61,62,63]. However, it remains unclear whether this factor contributed to our patient’s response to treatment.
This early response was attained on selumetinib 40 mg BID, with a dose reduction to selumetinib 35 mg BID due to asymptomatic Grade 3 elevated CPK. Other NF1 adult studies were designed with higher doses of selumetinib at 50 mg BID and 75 mg BID, and some assert that higher rates of response are associated with higher doses of selumetinib [37,52]. However, elevated Grade 3 CPK, though asymptomatic in our patient, prohibited a higher dose, and his CPK levels were sustained at Grade 1 following the dose reduction. Tumor regrowth was observed in a subset of patients requiring selumetinib dose reduction [17], but dose reduction did not negatively impact our patient’s response to treatment. Elevated CPK is a common adverse event observed in selumetinib and is customarily asymptomatic [17]. Avoidance of vigorous exercise can be recommended to prevent myositis in this scenario; however, our patient continued his regular exercise regimen without complication.
Our patient remains on selumetinib and may not have yet attained maximal volumetric response. The optimal duration of therapy with selumetinib to attain peak and sustained response remains unknown and is an area of future investigation [2,9]. Some assert that peak response is reached at 36–42 months [2]. Long-term use of selumetinib has acceptable rates of toxicity, and tumor recurrences have been reported in cases where selumetinib was discontinued [17,49,52,53,64]. For these reasons, he will continue with prolonged therapy, barring disease progression or any unacceptable treatment-related toxicities in the future. The significance of this study is highlighted by our patient’s early response to selumetinib, which may be due to differences in the pharmacokinetics of individuals of Asian origin and the limited number of reported cases of facial PNs treated with MEKis.
We acknowledge the limitations of this study. First, a single case is not generalizable to all NF1 adult patients with PNs. Second, we were unable to determine the durability and maximal volumetric response of selumetinib in our patient given the lack of long-term follow-up. Existing studies on MEKis report higher response rates with longer treatment durations [9,37,52,53,65]. Third, the pharmacokinetics of selumetinib was not analyzed in our patient, and it is unknown if the pharmacokinetics played a role in his tumor’s early response to treatment. Lastly, though our patient reported clinical improvement in his QoL following his tumor reduction, formal QoL assessments were not administered to our patient.
Efforts investigating other MEKis for the treatment of NF1 PNs, including trametinib and binimetinib, are ongoing [2,66]. Though single-agent selumetinib remains effective in our patient, a longer follow-up may uncover delayed resistance to therapy. Some studies suggest a resistance mechanism to selumetinib monotherapy with downstream activation of AKT [67,68,69]. However, data garnered from the KOMET study suggests otherwise [37]. If resistance mechanisms are uncovered, studies aimed at combinatorial therapy with MEKis and other agents should become areas of further investigation, including the addition of immunotherapy or another targeted agent within the MAPK pathway to MEKIs [2]. Alternatively, cabozantinib, a multi-targeted tyrosine kinase inhibitor that is not a direct MEKi, shows early promise for use in NF1 PN patients, with a PR of 42% in a Phase 2 study [55,70]. Cabozantinib is a small-molecule inhibitor of AXL, MET, and VEGFRs [70]. The mechanism by which cabozantinib exerts anti-tumor effects in PNs is not fully understood, but is postulated to be associated with downregulation of AXL, fibroblast regulation, and collagen production within the tumor microenvironment [55]. Lastly, insight into the ideal duration and need for continuous therapy can optimize MEKi treatment for these patients and determine the impact of a protracted course of MEKi therapy. Deepening our understanding of predictors of response, including single-cell RNA sequencing, may better target a subset of patients who can derive greater efficacy from MEKis [71]. Lastly, the PR rates between selumetinib and mirdametinib (87% vs. 42%) vary significantly [9,52]. The Phase II mirdametinib trial was not designed to determine the maximal tolerated dose for mirdametinib, and dosing above 2 mg/m2/dose may have potentially influenced therapeutic response [9]. Though this variance may potentially be attributed differences in trial design, no direct comparative analyses have been conducted [9]. Studies comparing different MEKis may reveal the superiority of one agent over the others.
4. Conclusions
Neurofibromatosis 1 plexiform neurofibromas can cause disfigurement and can negatively impact the social functioning of patients. Most NF1 PN investigations have been aimed at the pediatric population. Selumetinib, a MEK 1/2 inhibitor, achieved FDA approval for use in pediatric patients with symptomatic inoperable NF1 PNs, but it is currently under investigation for use in their adult counterparts. Mirdametinib was recently approved for use in adult patients with NF1 PNs, but it reports lower partial response rates compared to selumetinib. Comparative studies evaluating the superiority of one agent over the others have not been performed. Our case adds to the growing body of evidence suggesting that selumetinib is effective and well tolerated in NF1 PN adult patients. Prospective studies are indicated to evaluate the long-term efficacy, optimal dosing, and safety of selumetinib in adults with NF1-associated plexiform neurofibromas.
Author Contributions
C.A.Y.—study concept, data collection, analysis, interpretation, manuscript drafting, revision, and final approval. E.C.—data collection, analysis, interpretation, revision, and final approval. R.O.—data collection, analysis, interpretation, revision, and final approval. B.K.S.—manuscript revision and final approval. R.V.—manuscript revision and final approval. M.Z.—data collection, drafting, and final approval. E.E.—manuscript revision, and final approval. C.X.— manuscript editing, revision and final approval. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the University of California, Irvine, Institutional Review Board of University of California, Irvine (protocol code IRB# 7460 and date of approval is 18 June 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BID | twice daily |
| CPK | creatine phosphokinase |
| MAPK | mitogen-activated protein kinase |
| MEK | mitogen-activated protein kinase kinase |
| NF1 | Neurofibromatosis 1 |
| ORR | overall response rate |
| PN | plexiform neurofibroma |
| PR | partial response |
References
- Bollag, G.; McCormick, F.; Clark, R. Characterization of full-length neurofibromin: Tubulin inhibits Ras GAP activity. EMBO J. 1993, 12, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Solares, I.; Vinal, D.; Morales-Conejo, M.; Rodriguez-Salas, N.; Feliu, J. Novel molecular targeted therapies for patients with neurofibromatosis type 1 with inoperable plexiform neurofibromas: A comprehensive review. ESMO Open 2021, 6, 100223. [Google Scholar] [CrossRef] [PubMed]
- The, I.; Murthy, A.E.; Hannigan, G.E.; Jacoby, L.B.; Menon, A.G.; Gusella, J.F.; Bernards, A. Neurofibromatosis type 1 gene mutations in neuroblastoma. Nat. Genet. 1993, 3, 62–66. [Google Scholar] [CrossRef]
- Messiaen, L.M.; Callens, T.; Mortier, G.; Beysen, D.; Vandenbroucke, I.; Van Roy, N.; Speleman, F.; Paepe, A.D. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum. Mutat. 2000, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.F.; Yasuda, R. Neurofibromin is the major ras inactivator in dendritic spines. J. Neurosci. 2014, 34, 776–783. [Google Scholar] [CrossRef]
- Xu, G.F.; O’Connell, P.; Viskochil, D.; Cawthon, R.; Robertson, M.; Culver, M.; Dunn, D.; Stevens, J.; Gesteland, R.; White, R.; et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell 1990, 62, 599–608. [Google Scholar] [CrossRef]
- Lawson McLean, A.C.; Rosahl, S.K. Growth Dynamics of Intracranial Tumors in Patients with Neurofibromatosis Type 2. World Neurosurg. 2017, 98, 152–161. [Google Scholar] [CrossRef]
- Ratner, N.; Miller, S.J. A RASopathy gene commonly mutated in cancer: The neurofibromatosis type 1 tumour suppressor. Nat. Rev. Cancer 2015, 15, 290–301. [Google Scholar] [CrossRef]
- Weiss, B.D.; Wolters, P.L.; Plotkin, S.R.; Widemann, B.C.; Tonsgard, J.H.; Blakeley, J.; Allen, J.C.; Schorry, E.; Korf, B.; Robison, N.J.; et al. NF106: A Neurofibromatosis Clinical Trials Consortium Phase II Trial of the MEK Inhibitor Mirdametinib (PD-0325901) in Adolescents and Adults With NF1-Related Plexiform Neurofibromas. J. Clin. Oncol. 2021, 39, 797–806. [Google Scholar] [CrossRef]
- Ferner, R.E.; Huson, S.M.; Thomas, N.; Moss, C.; Willshaw, H.; Evans, D.G.; Upadhyaya, M.; Towers, R.; Gleeson, M.; Steiger, C.; et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J. Med. Genet. 2007, 44, 81–88. [Google Scholar] [CrossRef]
- Evans, D.G.; Howard, E.; Giblin, C.; Clancy, T.; Spencer, H.; Huson, S.M.; Lalloo, F. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am. J. Med. Genet. A 2010, 152, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Paria, N.; Cho, T.J.; Choi, I.H.; Kamiya, N.; Kayembe, K.; Mao, R.; Margraf, R.L.; Obermosser, G.; Oxendine, I.; Sant, D.W.; et al. Neurofibromin deficiency-associated transcriptional dysregulation suggests a novel therapy for tibial pseudoarthrosis in NF1. J. Bone Miner. Res. 2014, 29, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Yoon, H.M.; Lee, B.H. Neurofibromatosis type I: Points to be considered by general pediatricians. Clin. Exp. Pediatr. 2021, 64, 149–156. [Google Scholar] [CrossRef]
- DeBella, K.; Szudek, J.; Friedman, J.M. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics 2000, 105, 608–614. [Google Scholar] [CrossRef]
- Blakeley, J.O.; Plotkin, S.R. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol. 2016, 18, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Staser, K.; Yang, F.C.; Clapp, D.W. Pathogenesis of plexiform neurofibroma: Tumor-stromal/hematopoietic interactions in tumor progression. Annu. Rev. Pathol. 2012, 7, 469–495. [Google Scholar] [CrossRef]
- Dombi, E.; Baldwin, A.; Marcus, L.J.; Fisher, M.J.; Weiss, B.; Kim, A.; Whitcomb, P.; Martin, S.; Aschbacher-Smith, L.E.; Rizvi, T.A.; et al. Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas. N. Engl. J. Med. 2016, 375, 2550–2560. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Bredella, M.A.; Cai, W.; Kassarjian, A.; Harris, G.J.; Esparza, S.; Merker, V.L.; Munn, L.L.; Muzikansky, A.; Askenazi, M.; et al. Quantitative assessment of whole-body tumor burden in adult patients with neurofibromatosis. PLoS ONE 2012, 7, e35711. [Google Scholar] [CrossRef]
- Gross, A.M.; Singh, G.; Akshintala, S.; Baldwin, A.; Dombi, E.; Ukwuani, S.; Goodwin, A.; Liewehr, D.J.; Steinberg, S.M.; Widemann, B.C. Association of plexiform neurofibroma volume changes and development of clinical morbidities in neurofibromatosis 1. Neuro Oncol. 2018, 20, 1643–1651. [Google Scholar] [CrossRef]
- Prada, C.E.; Rangwala, F.A.; Martin, L.J.; Lovell, A.M.; Saal, H.M.; Schorry, E.K.; Hopkin, R.J. Pediatric plexiform neurofibromas: Impact on morbidity and mortality in neurofibromatosis type 1. J. Pediatr. 2012, 160, 461–467. [Google Scholar] [CrossRef]
- Dunn, G.P.; Spiliopoulos, K.; Plotkin, S.R.; Hornicek, F.J.; Harmon, D.C.; Delaney, T.F.; Williams, Z. Role of resection of malignant peripheral nerve sheath tumors in patients with neurofibromatosis type 1. J. Neurosurg. 2013, 118, 142–148. [Google Scholar] [CrossRef]
- Uusitalo, E.; Rantanen, M.; Kallionpaa, R.A.; Poyhonen, M.; Leppavirta, J.; Yla-Outinen, H.; Riccardi, V.M.; Pukkala, E.; Pitkaniemi, J.; Peltonen, S.; et al. Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J. Clin. Oncol. 2016, 34, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Baser, M.E.; McGaughran, J.; Sharif, S.; Howard, E.; Moran, A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J. Med. Genet. 2002, 39, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.E.; Patel, Z.S.; Listernick, R.; Charrow, J.; Lai, J.S. Lifespan Development: Symptoms Experienced by Individuals with Neurofibromatosis Type 1 Associated Plexiform Neurofibromas from Childhood into Adulthood. J. Clin. Psychol. Med. Settings 2019, 26, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.K.; Porteous, A.; Ng, A.; Haria, K.; Griffiths, A.; Lloyd, A.; Yang, X.; Kazeem, G.; Barut, V. Impact of neurofibromatosis type 1 with plexiform neurofibromas on the health-related quality of life and work productivity of adult patients and caregivers in the UK: A cross-sectional survey. BMC Neurol. 2023, 23, 419. [Google Scholar] [CrossRef]
- Lai, J.S.; Jensen, S.E.; Patel, Z.S.; Listernick, R.; Charrow, J. Using a qualitative approach to conceptualize concerns of patients with neurofibromatosis type 1 associated plexiform neurofibromas (pNF) across the lifespan. Am. J. Med. Genet. A 2017, 173, 79–87. [Google Scholar] [CrossRef]
- Legius, E.; Messiaen, L.; Wolkenstein, P.; Pancza, P.; Avery, R.A.; Berman, Y.; Blakeley, J.; Babovic-Vuksanovic, D.; Cunha, K.S.; Ferner, R.; et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: An international consensus recommendation. Genet. Med. 2021, 23, 1506–1513. [Google Scholar] [CrossRef]
- Korf, B.R. Plexiform neurofibromas. Am. J. Med. Genet. 1999, 89, 31–37. [Google Scholar] [CrossRef]
- Canavese, F.; Krajbich, J.I. Resection of plexiform neurofibromas in children with neurofibromatosis type 1. J. Pediatr. Orthop. 2011, 31, 303–311. [Google Scholar] [CrossRef]
- Ansari, S.; Dixe de Oliveira Santo, I.; Yazdian Anari, P.; Badawy, M.; Flug, J.A. Plexiform Neurofibroma. Radiographics 2025, 45, e240271. [Google Scholar] [CrossRef]
- Miller, D.T.; Freedenberg, D.; Schorry, E.; Ullrich, N.J.; Viskochil, D.; Korf, B.R.; Council on Genetics; American College of Medical Genetics and Genomics; Chen, E. Health Supervision for Children With Neurofibromatosis Type 1. Pediatrics 2019, 143, e20190660. [Google Scholar] [CrossRef] [PubMed]
- Needle, M.N.; Cnaan, A.; Dattilo, J.; Chatten, J.; Phillips, P.C.; Shochat, S.; Sutton, L.N.; Vaughan, S.N.; Zackai, E.H.; Zhao, H.; et al. Prognostic signs in the surgical management of plexiform neurofibroma: The Children’s Hospital of Philadelphia experience, 1974-1994. J. Pediatr. 1997, 131, 678–682. [Google Scholar] [CrossRef]
- Nguyen, R.; Ibrahim, C.; Friedrich, R.E.; Westphal, M.; Schuhmann, M.; Mautner, V.F. Growth behavior of plexiform neurofibromas after surgery. Genet. Med. 2013, 15, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Akshintala, S.; Baldwin, A.; Liewehr, D.J.; Goodwin, A.; Blakeley, J.O.; Gross, A.M.; Steinberg, S.M.; Dombi, E.; Widemann, B.C. Longitudinal evaluation of peripheral nerve sheath tumors in neurofibromatosis type 1: Growth analysis of plexiform neurofibromas and distinct nodular lesions. Neuro Oncol. 2020, 22, 1368–1378. [Google Scholar] [CrossRef]
- Dombi, E.; Solomon, J.; Gillespie, A.J.; Fox, E.; Balis, F.M.; Patronas, N.; Korf, B.R.; Babovic-Vuksanovic, D.; Packer, R.J.; Belasco, J.; et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: Relationship to age and body weight. Neurology 2007, 68, 643–647. [Google Scholar] [CrossRef]
- Kotch, C.; Dombi, E.; Shah, A.C.; Smith, K.; Brown, S.; Li, Y.; Widemann, B.C.; Fisher, M.J. Retrospective Cohort Analysis of the Impact of Puberty on Plexiform Neurofibroma Growth in Patients with Neurofibromatosis Type 1. J. Pediatr. 2023, 260, 113513. [Google Scholar] [CrossRef]
- Gross, A.M.; O’Sullivan Coyne, G.; Dombi, E.; Tibery, C.; Herrick, W.G.; Martin, S.; Angus, S.P.; Shern, J.F.; Rhodes, S.D.; Foster, J.C.; et al. Selumetinib in adults with NF1 and inoperable plexiform neurofibroma: A phase 2 trial. Nat. Med. 2025, 31, 105–115. [Google Scholar] [CrossRef]
- Jange, R.G.; Nisar, S.P. Into the abyss: Facial plexiform neurofibromatosis. Eye 2025, 39, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.I.; Merker, V.L.; Cai, W.; Bredella, M.A.; Muzikansky, A.; Thalheimer, R.D.; Da, J.L.; Orr, C.C.; Herr, H.P.; Morris, M.E.; et al. Ten-Year Follow-up of Internal Neurofibroma Growth Behavior in Adult Patients With Neurofibromatosis Type 1 Using Whole-Body MRI. Neurology 2023, 100, e661–e670. [Google Scholar] [CrossRef]
- Widemann, B.C.; Dombi, E.; Gillespie, A.; Wolters, P.L.; Belasco, J.; Goldman, S.; Korf, B.R.; Solomon, J.; Martin, S.; Salzer, W.; et al. Phase 2 randomized, flexible crossover, double-blinded, placebo-controlled trial of the farnesyltransferase inhibitor tipifarnib in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Neuro Oncol. 2014, 16, 707–718. [Google Scholar] [CrossRef]
- Widemann, B.C.; Babovic-Vuksanovic, D.; Dombi, E.; Wolters, P.L.; Goldman, S.; Martin, S.; Goodwin, A.; Goodspeed, W.; Kieran, M.W.; Cohen, B.; et al. Phase II trial of pirfenidone in children and young adults with neurofibromatosis type 1 and progressive plexiform neurofibromas. Pediatr. Blood Cancer 2014, 61, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.; Widemann, B.C.; Wolters, P.; Dombi, E.; Vinks, A.; Cantor, A.; Perentesis, J.; Schorry, E.; Ullrich, N.; Gutmann, D.H.; et al. Sirolimus for progressive neurofibromatosis type 1-associated plexiform neurofibromas: A neurofibromatosis Clinical Trials Consortium phase II study. Neuro Oncol. 2015, 17, 596–603. [Google Scholar] [CrossRef]
- Weiss, B.; Widemann, B.C.; Wolters, P.; Dombi, E.; Vinks, A.A.; Cantor, A.; Korf, B.; Perentesis, J.; Gutmann, D.H.; Schorry, E.; et al. Sirolimus for non-progressive NF1-associated plexiform neurofibromas: An NF clinical trials consortium phase II study. Pediatr. Blood Cancer 2014, 61, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.A.; Nalepa, G.; Yang, F.C.; Bowers, D.C.; Ho, C.Y.; Hutchins, G.D.; Croop, J.M.; Vik, T.A.; Denne, S.C.; Parada, L.F.; et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: A phase 2 trial. Lancet Oncol. 2012, 13, 1218–1224. [Google Scholar] [CrossRef]
- Jakacki, R.I.; Dombi, E.; Steinberg, S.M.; Goldman, S.; Kieran, M.W.; Ullrich, N.J.; Pollack, I.F.; Goodwin, A.; Manley, P.E.; Fangusaro, J.; et al. Phase II trial of pegylated interferon alfa-2b in young patients with neurofibromatosis type 1 and unresectable plexiform neurofibromas. Neuro Oncol. 2017, 19, 289–297. [Google Scholar] [CrossRef]
- Hedayat, M.; Jafari, R.; Majidi Zolbanin, N. Selumetinib: A selective MEK1 inhibitor for solid tumor treatment. Clin. Exp. Med. 2023, 23, 229–244. [Google Scholar] [CrossRef]
- Bhalla, S.; Evens, A.M.; Dai, B.; Prachand, S.; Gordon, L.I.; Gartenhaus, R.B. The novel anti-MEK small molecule AZD6244 induces BIM-dependent and AKT-independent apoptosis in diffuse large B-cell lymphoma. Blood 2011, 118, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Cichowski, K.; Jacks, T. NF1 tumor suppressor gene function: Narrowing the GAP. Cell 2001, 104, 593–604. [Google Scholar] [CrossRef]
- Gross, A.M.; Dombi, E.; Wolters, P.L.; Baldwin, A.; Dufek, A.; Herrera, K.; Martin, S.; Derdak, J.; Heisey, K.S.; Whitcomb, P.M.; et al. Long-term safety and efficacy of selumetinib in children with neurofibromatosis type 1 on a phase 1/2 trial for inoperable plexiform neurofibromas. Neuro Oncol. 2023, 25, 1883–1894. [Google Scholar] [CrossRef]
- Casey, D.; Demko, S.; Sinha, A.; Mishra-Kalyani, P.S.; Shen, Y.L.; Khasar, S.; Goheer, M.A.; Helms, W.S.; Pan, L.; Xu, Y.; et al. FDA Approval Summary: Selumetinib for Plexiform Neurofibroma. Clin. Cancer Res. 2021, 27, 4142–4146. [Google Scholar] [CrossRef]
- Gross, A.M.; Wolters, P.L.; Dombi, E.; Baldwin, A.; Whitcomb, P.; Fisher, M.J.; Weiss, B.; Kim, A.; Bornhorst, M.; Shah, A.C.; et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N. Engl. J. Med. 2020, 382, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoon, H.M.; Kim, E.K.; Ra, Y.S.; Kim, H.W.; Yum, M.S.; Kim, M.J.; Baek, J.S.; Sung, Y.S.; Lee, S.M.; et al. Safety and efficacy of selumetinib in pediatric and adult patients with neurofibromatosis type 1 and plexiform neurofibroma. Neuro Oncol. 2024, 26, 2352–2363. [Google Scholar] [CrossRef]
- Chen, A.P.; Coyne, G.O.S.; Wolters, P.L.; Martin, S.; Farschtschi, S.; Blanco, I.; Chen, Z.; Darrigo, L.G., Jr.; Eoli, M.; Whittle, J.R.; et al. Efficacy and safety of selumetinib in adults with neurofibromatosis type 1 and symptomatic, inoperable plexiform neurofibromas (KOMET): A multicentre, international, randomised, placebo-controlled, parallel, double-blind, phase 3 study. Lancet 2025, 405, 2217–2230. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves Mirdametinib for Adult and Pediatric Patients with Neurofibromatosis Type 1 Who Have Symptomatic Plexiform Neurofibromas Not Amenable To Complete Resection. Available online: https://www.fda.gov/drugs/resourcesinformation-approved-drugs/fda-approves-mirdametinib-adult-andpediatric-patients-neurofibromatosis-type-1-who-have-symptomatic (accessed on 19 June 2025).
- Fisher, M.J.; Shih, C.S.; Rhodes, S.D.; Armstrong, A.E.; Wolters, P.L.; Dombi, E.; Zhang, C.; Angus, S.P.; Johnson, G.L.; Packer, R.J.; et al. Cabozantinib for neurofibromatosis type 1-related plexiform neurofibromas: A phase 2 trial. Nat. Med. 2021, 27, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Darrigo Junior, L.G.; Ferraz, V.E.F.; Cormedi, M.C.V.; Araujo, L.H.H.; Magalhaes, M.P.S.; Carneiro, R.C.; Sales, L.H.N.; Suchmacher, M.; Cunha, K.S.; Filho, A.B.; et al. Epidemiological profile and clinical characteristics of 491 Brazilian patients with neurofibromatosis type 1. Brain Behav. 2022, 12, e2599. [Google Scholar] [CrossRef]
- Abramson, J.S.; Siddiqi, T.; Garcia, J.; Dehner, C.; Kim, Y.; Nguyen, A.; Snyder, S.; McGarvey, N.; Gitlin, M.; Pelletier, C.; et al. Cytokine release syndrome and neurological event costs in lisocabtagene maraleucel-treated patients in the TRANSCEND NHL 001 trial. Blood Adv. 2021, 5, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S. A systematic multiple stage surgical approach for attainment of satisfactory and favourable surgical results in an extremely severe von Recklinghausen’s disease, elephantiasis neurofibromatosa. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 662–674. [Google Scholar] [CrossRef]
- Nguyen, R.; Dombi, E.; Widemann, B.C.; Solomon, J.; Fuensterer, C.; Kluwe, L.; Friedman, J.M.; Mautner, V.F. Growth dynamics of plexiform neurofibromas: A retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J. Rare Dis. 2012, 7, 75. [Google Scholar] [CrossRef]
- Vaassen, P.; Durr, N.; Rohrig, A.; Willing, R.; Rosenbaum, T. Trametinib Induces Neurofibroma Shrinkage and Enables Surgery. Neuropediatrics 2019, 50, 300–303. [Google Scholar] [CrossRef]
- Dymond, A.W.; Elks, C.; Martin, P.; Carlile, D.J.; Mariani, G.; Lovick, S.; Huang, Y.; Lorch, U.; Brown, H.; So, K. Pharmacokinetics and pharmacogenetics of the MEK1/2 inhibitor, selumetinib, in Asian and Western healthy subjects: A pooled analysis. Eur. J. Clin. Pharmacol. 2017, 73, 717–726. [Google Scholar] [CrossRef]
- Schalkwijk, S.; Zhou, L.; Cohen-Rabbie, S.; Jain, L.; Freshwater, T.; So, K.; He, Z.; Gioni, I.; Tomkinson, H.; Vishwanathan, K.; et al. Population pharmacokinetics and exposure-response of selumetinib and its N-desmethyl metabolite in pediatric patients with neurofibromatosis type 1 and inoperable plexiform neurofibromas. Cancer Chemother. Pharmacol. 2021, 88, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Suenobu, S.; Terashima, K.; Akiyama, M.; Oguri, T.; Watanabe, A.; Sugeno, M.; Higashimori, M.; So, K.; Nishida, Y. Selumetinib in Japanese pediatric patients with neurofibromatosis type 1 and symptomatic, inoperable plexiform neurofibromas: An open-label, phase I study. Neurooncol Adv. 2023, 5, vdad054. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Yoon, H.M.; Lee, B.H.; Kim, P.H.; Kim, K.W. Efficacy and Safety of Selumetinib in Pediatric Patients With Neurofibromatosis Type 1: A Systematic Review and Meta-analysis. Neurology 2022, 98, e938–e946. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.J.; Royer, G.L., Jr.; Weiss, R.B. Cytarabine and neurologic toxicity. J. Clin. Oncol. 1991, 9, 679–693. [Google Scholar] [CrossRef]
- McCowage, G.B.; Mueller, S.; Pratilas, C.A.; Hargrave, D.R.; Moertel, C.L.; Whitlock, J.; Fox, E.; Hingorani, P.; Russo, M.W.; Dasgupta, K.; et al. Trametinib in pediatric patients with neurofibromatosis type 1 (NF-1)–associated plexiform neurofibroma: A phase I/IIa study. J. Clin. Oncol. 2018, 36, 10504. [Google Scholar] [CrossRef]
- Chen, C.H.; Hsia, T.C.; Yeh, M.H.; Chen, T.W.; Chen, Y.J.; Chen, J.T.; Wei, Y.L.; Tu, C.Y.; Huang, W.C. MEK inhibitors induce Akt activation and drug resistance by suppressing negative feedback ERK-mediated HER2 phosphorylation at Thr701. Mol. Oncol. 2017, 11, 1273–1287. [Google Scholar] [CrossRef]
- Brown, W.S.; McDonald, P.C.; Nemirovsky, O.; Awrey, S.; Chafe, S.C.; Schaeffer, D.F.; Li, J.; Renouf, D.J.; Stanger, B.Z.; Dedhar, S. Overcoming Adaptive Resistance to KRAS and MEK Inhibitors by Co-targeting mTORC1/2 Complexes in Pancreatic Cancer. Cell Rep. Med. 2020, 1, 100131. [Google Scholar] [CrossRef]
- Butler, D.E.; Marlein, C.; Walker, H.F.; Frame, F.M.; Mann, V.M.; Simms, M.S.; Davies, B.R.; Collins, A.T.; Maitland, N.J. Inhibition of the PI3K/AKT/mTOR pathway activates autophagy and compensatory Ras/Raf/MEK/ERK signalling in prostate cancer. Oncotarget 2017, 8, 56698–56713. [Google Scholar] [CrossRef]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef]
- Bhandarkar, A.R.; Bhandarkar, S.; Babovic-Vuksanovic, D.; Raghunathan, A.; Schwartz, J.; Spinner, R.J. Precision oncology in neurofibromatosis type 1: Quantification of differential sensitivity to selumetinib in plexiform neurofibromas using single-cell RNA sequencing. J. Neurooncol. 2024, 169, 147–153. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).