Chelerythrine Inhibits TGF-β-Induced Epithelial–Mesenchymal Transition in A549 Cells via RRM2
Abstract
1. Introduction
2. Results
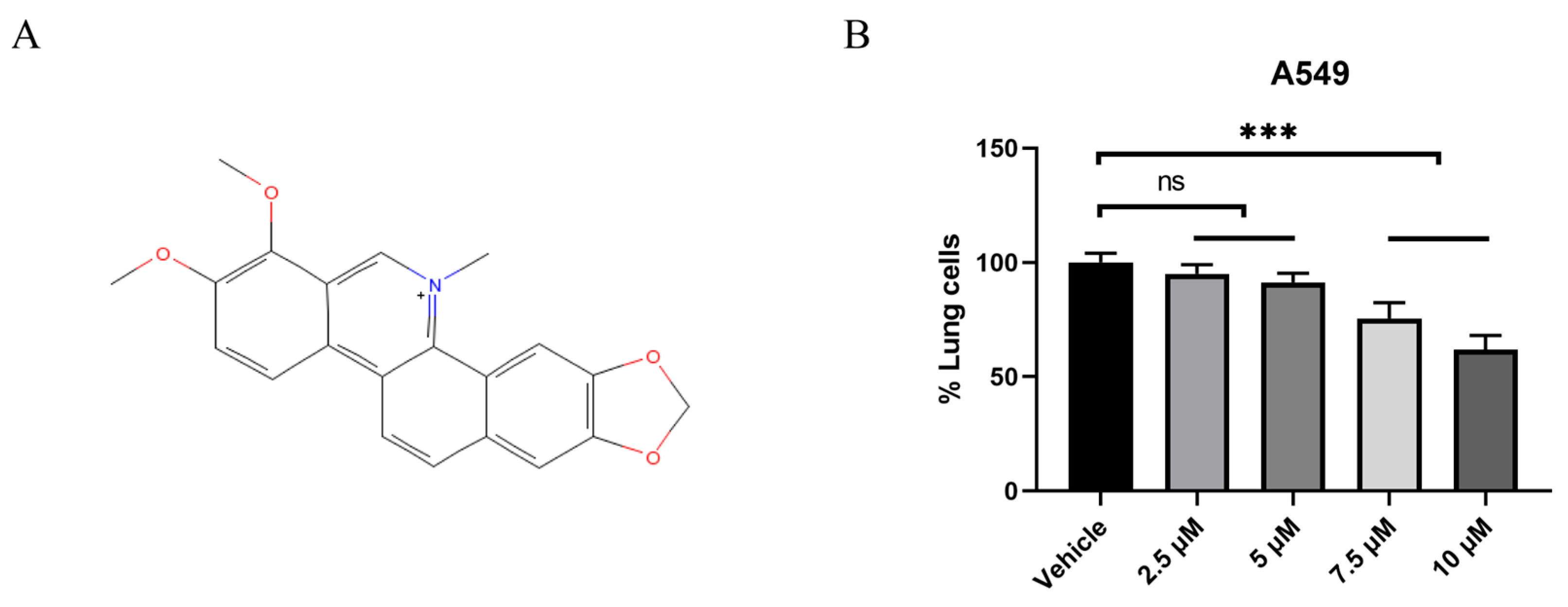
2.1. CHE Inhibited the Viability of A549 Cells
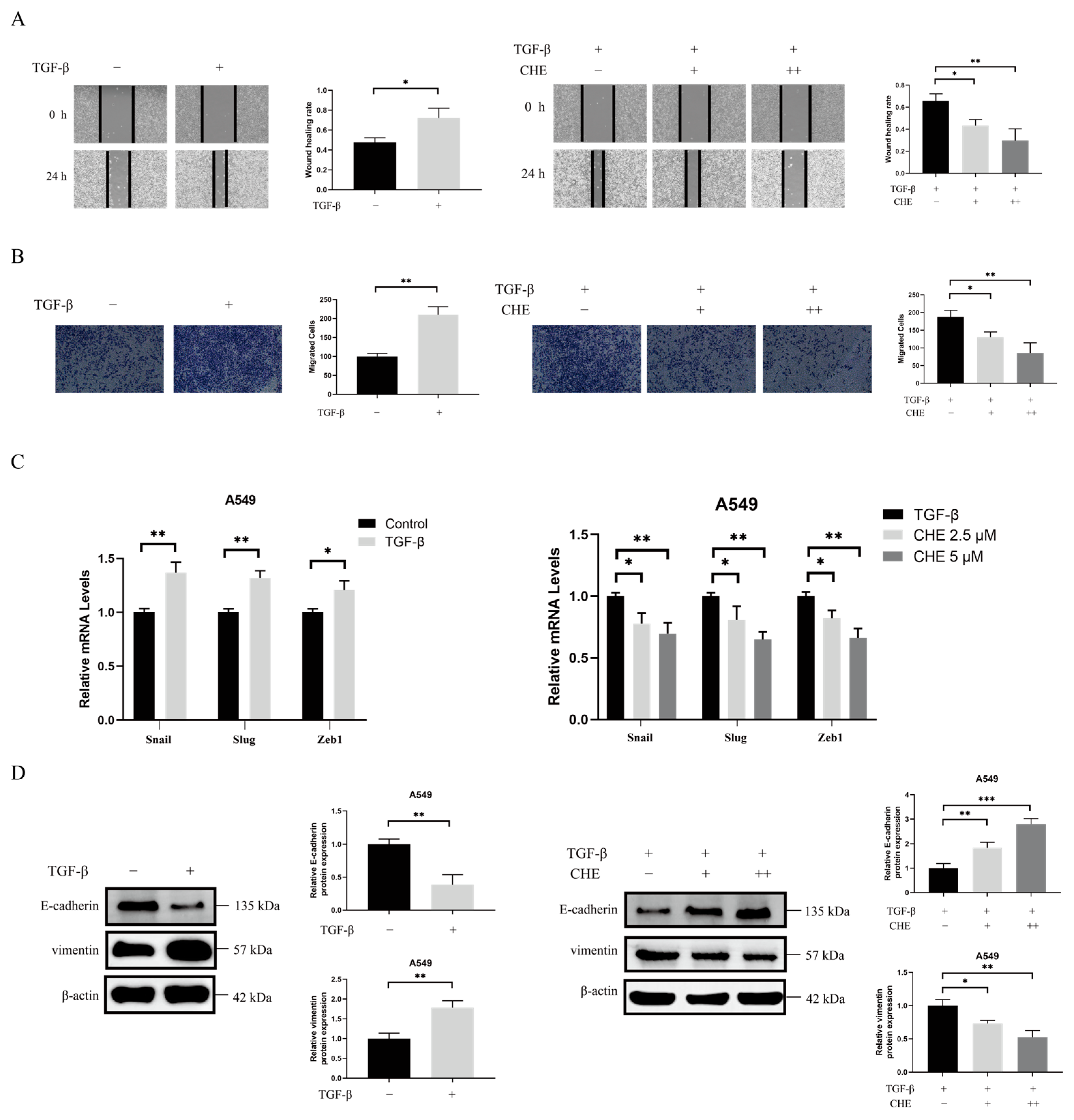
2.2. CHE Inhibits TGF-β-Induced EMT
2.3. A Therapeutic Target of LUAD Cells, RRM2, Was Suppressed by CHE
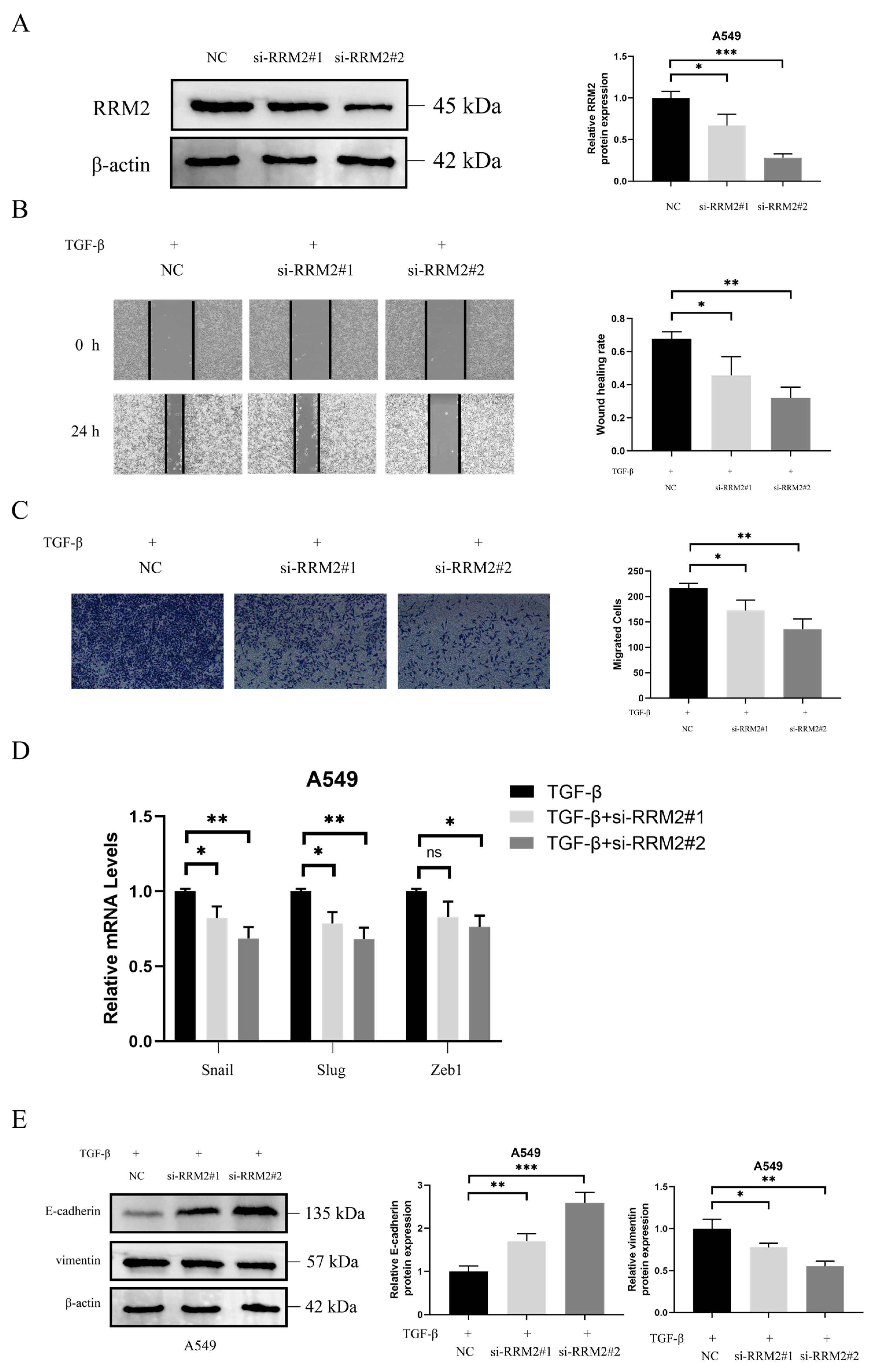
2.4. RRM2 Is Involved in TGF-β-Induced EMT
2.5. CHE Inhibits Cancer Metastasis In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Bioinformatics Analysis
4.3. Molecular Docking
4.4. Cell Counting Kit-8 (CCK8) Assay
4.5. Cell Culture, siRNA, and Plasmids
4.6. Wound Healing Assay
4.7. Transwell Assay
4.8. Quantitative Real-Time PCR
4.9. Immunofluorescence Staining
4.10. Histology
4.11. Western Blot
4.12. Mouse Models of Metastasis
4.13. Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NSCLC | Non-small-cell lung cancer |
| CHE | Chelerythrine |
| TGF-β | Transforming growth factor-beta |
| RRM2 | Ribonucleotide reductase subunit M2 |
| EMT | Epithelial–mesenchymal transition |
| FBS | Fetal bovine serum |
| DMSO | Dimethyl sulfoxide |
| GEPIA | Gene expression profiling interactive analysis |
| PCAS | ProteoCancer analysis suite |
| DAPI | 4′,6-Diamidino-2′-phenylindole |
| BCA | Bicinchoninic acid |
| LUAD | Adenocarcinoma |
| BRCA | Breast invasive carcinoma |
| CCRCC | Clear cell renal cell carcinoma |
| OV | Ovarian serous cystadenocarcinoma |
References
- McMillan, E.A.; Ryu, M.-J.; Diep, C.H.; Mendiratta, S.; Clemenceau, J.R.; Vaden, R.M.; Kim, J.-H.; Motoyaji, T.; Covington, K.R.; Peyton, M.; et al. Chemistry-First Approach for Nomination of Personalized Treatment in Lung Cancer. Cell 2018, 173, 864–878.e29. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Siewert, B.; Csuk, R.; Rauter, A.P. New Antitumor 6-Chloropurine Nucleosides Inducing Apoptosis and G2/M Cell Cycle Arrest. Eur. J. Med. Chem. 2015, 90, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Ibodeng, G.-O.; Uche, I.N.; Mokua, R.; Galo, M.; Odigwe, B.; Galeas, J.N.; Dasgupta, S. A Snapshot of Lung Cancer: Where Are We Now?-A Narrative Review. Ann. Transl. Med. 2023, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The Emerging Treatment Landscape of Targeted Therapy in Non-Small-Cell Lung Cancer. Signal Transduct. Target Ther. 2019, 4, 61. [Google Scholar] [CrossRef]
- Islami, F.; Marlow, E.C.; Thomson, B.; McCullough, M.L.; Rumgay, H.; Gapstur, S.M.; Patel, A.V.; Soerjomataram, I.; Jemal, A. Proportion and Number of Cancer Cases and Deaths Attributable to Potentially Modifiable Risk Factors in the United States, 2019. CA Cancer J. Clin. 2024, 74, 405–432. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial-Mesenchymal Transitions in Tumour Progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Kang, Y.; Massagué, J. Epithelial-Mesenchymal Transitions: Twist in Development and Metastasis. Cell 2004, 118, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH Factors in Tumour Progression: An Alliance against the Epithelial Phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Hoot, K.E.; Lighthall, J.; Han, G.; Lu, S.-L.; Li, A.; Ju, W.; Kulesz-Martin, M.; Bottinger, E.; Wang, X.-J. Keratinocyte-Specific Smad2 Ablation Results in Increased Epithelial-Mesenchymal Transition during Skin Cancer Formation and Progression. J. Clin. Invest. 2008, 118, 2722–2732. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Katsuno, Y.; Lamouille, S.; Derynck, R. TGF-β Signaling and Epithelial-Mesenchymal Transition in Cancer Progression. Curr. Opin. Oncol. 2013, 25, 76–84. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Morikawa, T.; Maeda, D.; Kume, H.; Homma, Y.; Fukayama, M. Ribonucleotide Reductase M2 Subunit Is a Novel Diagnostic Marker and a Potential Therapeutic Target in Bladder Cancer. Histopathology 2010, 57, 885–892. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, B.; Xue, L.; Yen, F.; Chu, P.; Un, F.; Yen, Y. Ribonucleotide Reductase Subunits M2 and p53R2 Are Potential Biomarkers for Metastasis of Colon Cancer. Clin. Colorectal. Cancer 2007, 6, 374–381. [Google Scholar] [CrossRef]
- Rahman, M.A.; Amin, A.R.M.R.; Wang, D.; Koenig, L.; Nannapaneni, S.; Chen, Z.; Wang, Z.; Sica, G.; Deng, X.; Chen, Z.G.; et al. RRM2 Regulates Bcl-2 in Head and Neck and Lung Cancers: A Potential Target for Cancer Therapy. Clin. Cancer Res. 2013, 19, 3416–3428. [Google Scholar] [CrossRef]
- Yoshida, Y.; Tsunoda, T.; Doi, K.; Tanaka, Y.; Fujimoto, T.; Machida, T.; Ota, T.; Koyanagi, M.; Takashima, Y.; Sasazuki, T.; et al. KRAS-Mediated up-Regulation of RRM2 Expression Is Essential for the Proliferation of Colorectal Cancer Cell Lines. Anticancer. Res. 2011, 31, 2535–2539. [Google Scholar]
- He, J.; Wei, Q.; Jiang, R.; Luan, T.; He, S.; Lu, R.; Xu, H.; Ran, J.; Li, J.; Chen, D. The Core-Targeted RRM2 Gene of Berberine Hydrochloride Promotes Breast Cancer Cell Migration and Invasion via the Epithelial-Mesenchymal Transition. Pharmaceuticals 2022, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, L.; Wang, X.; Ma, G.; Chen, J. Expression of RRM1 and RRM2 as a Novel Prognostic Marker in Advanced Non-Small Cell Lung Cancer Receiving Chemotherapy. Tumour. Biol. 2014, 35, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yang, Z.; Zhang, L.; Song, C.; Li, Y.; Zhang, X. Additive Effects of Cherlerythrine Chloride Combination with Erlotinib in Human Non-Small Cell Lung Cancer Cells. PLoS ONE 2017, 12, e0175466. [Google Scholar] [CrossRef]
- Lu, N.; Jiang, Q.; Xu, T.; Gao, Q.; Wang, Y.; Huang, Z.; Huang, Z.; Xu, X. LncOCMRL1 Promotes Oral Squamous Cell Carcinoma Growth and Metastasis via the RRM2/EMT Pathway. J. Exp. Clin. Cancer Res. 2024, 43, 267. [Google Scholar] [CrossRef] [PubMed]
- Valipour, M.; Zarghi, A.; Ebrahimzadeh, M.A.; Irannejad, H. Therapeutic Potential of Chelerythrine as a Multi-Purpose Adjuvant for the Treatment of COVID-19. Cell Cycle 2021, 20, 2321–2336. [Google Scholar] [CrossRef]
- Lin, W.; Huang, J.; Yuan, Z.; Feng, S.; Xie, Y.; Ma, W. Protein Kinase C Inhibitor Chelerythrine Selectively Inhibits Proliferation of Triple-Negative Breast Cancer Cells. Sci. Rep. 2017, 7, 2022. [Google Scholar] [CrossRef]
- Malíková, J.; Zdarilová, A.; Hlobilková, A.; Ulrichová, J. The Effect of Chelerythrine on Cell Growth, Apoptosis, and Cell Cycle in Human Normal and Cancer Cells in Comparison with Sanguinarine. Cell Biol. Toxicol. 2006, 22, 439–453. [Google Scholar] [CrossRef]
- Chmura, S.J.; Dolan, M.E.; Cha, A.; Mauceri, H.J.; Kufe, D.W.; Weichselbaum, R.R. In Vitro and in Vivo Activity of Protein Kinase C Inhibitor Chelerythrine Chloride Induces Tumor Cell Toxicity and Growth Delay in Vivo. Clin. Cancer Res. 2000, 6, 737–742. [Google Scholar]
- Zhu, Y.; Pan, Y.; Zhang, G.; Wu, Y.; Zhong, W.; Chu, C.; Qian, Y.; Zhu, G. Chelerythrine Inhibits Human Hepatocellular Carcinoma Metastasis in Vitro. Biol. Pharm. Bull. 2018, 41, 36–46. [Google Scholar] [CrossRef]
- Wan, K.F.; Chan, S.-L.; Sukumaran, S.K.; Lee, M.-C.; Yu, V.C. Chelerythrine Induces Apoptosis through a Bax/Bak-Independent Mitochondrial Mechanism. J. Biol. Chem. 2008, 283, 8423–8433. [Google Scholar] [CrossRef]
- Li, W.-F.; Hao, D.-J.; Fan, T.; Huang, H.-M.; Yao, H.; Niu, X.-F. Protective Effect of Chelerythrine against Ethanol-Induced Gastric Ulcer in Mice. Chem. Biol. Interact. 2014, 208, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.C.; Ryu, H.W.; Kang, M.-G.; Lee, H.; Park, D.; Cho, M.-L.; Oh, S.-R.; Kim, H. Selective Inhibition of Monoamine Oxidase A by Chelerythrine, an Isoquinoline Alkaloid. Bioorg Med. Chem. Lett. 2018, 28, 2403–2407. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Fan, Y.; Liu, L.; Tao, W.; Shan, X.; Dong, Y.; Li, L.; Zhang, S.; Wang, H. Chelerythrine Attenuates the Inflammation of Lipopolysaccharide-Induced Acute Lung Inflammation Through NF-κB Signaling Pathway Mediated by Nrf2. Front. Pharmacol. 2018, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Liu, L.; Shi, Y.; Hong, Y.; Xu, W.; Xu, H.; Feng, J.; Xie, M.; Li, Y.; et al. The Therapeutic Potential of Four Main Compounds of Zanthoxylum Nitidum (Roxb.) DC: A Comprehensive Study on Biological Processes, Anti-Inflammatory Effects, and Myocardial Toxicity. Pharmaceuticals 2024, 17, 524. [Google Scholar] [CrossRef]
- Giltnane, J.M.; Balko, J.M. Rationale for Targeting the Ras/MAPK Pathway in Triple-Negative Breast Cancer. Discov. Med. 2014, 17, 275–283. [Google Scholar]
- Gomes, L.R.; Terra, L.F.; Wailemann, R.A.; Labriola, L.; Sogayar, M.C. TGF-Β1 Modulates the Homeostasis between MMPs and MMP Inhibitors through P38 MAPK and ERK1/2 in Highly Invasive Breast Cancer Cells. BMC Cancer 2012, 12, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, S.; Zhao, Z.; Liu, P.; Ke, C.; Xu, S. New Insights into Small-Cell Lung Cancer Development and Therapy. Cell Biol. Int. 2020, 44, 1564–1576. [Google Scholar] [CrossRef]
- Huang, W.-C.; Su, H.-H.; Fang, L.-W.; Wu, S.-J.; Liou, C.-J. Licochalcone A Inhibits Cellular Motility by Suppressing E-Cadherin and MAPK Signaling in Breast Cancer. Cells 2019, 8, 218. [Google Scholar] [CrossRef]
- Liang, D.; Liu, L.; Zheng, Q.; Zhao, M.; Zhang, G.; Tang, S.; Tang, J.; Chen, N. Chelerythrine Chloride Inhibits the Progression of Colorectal Cancer by Targeting Cancer-Associated Fibroblasts through Intervention with WNT10B/β-Catenin and TGFβ2/Smad2/3 Axis. Phytother. Res. 2023, 37, 4674–4689. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Viscardi, S.; Szewczyk, W.; Topola, E. Natural Alkaloids in Cancer Therapy: Berberine, Sanguinarine and Chelerythrine against Colorectal and Gastric Cancer. Int. J. Mol. Sci. 2024, 25, 8375. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Cao, W.-X.; Wang, Z.-Y.; Lu, J.-H.; Liu, B.; Chen, X.; Lu, J.-J. Induction of Reactive Oxygen Species-Stimulated Distinctive Autophagy by Chelerythrine in Non-Small Cell Lung Cancer Cells. Redox Biol. 2017, 12, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.S.; Cheah, S.-C. Chelerythrine Chloride Downregulates β-Catenin and Inhibits Stem Cell Properties of Non-Small Cell Lung Carcinoma. Molecules 2020, 25, 224. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Eichhorn, P.J.A.; Thiery, J.P. TGF-β, EMT, and Resistance to Anti-Cancer Treatment. Semin Cancer Biol. 2023, 97, 1–11. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; Zhang, N.; Gao, Y.; Han, L.; Li, S.; Li, J.; Liu, X.; Gong, Y.; Xie, C. RRM2 Silencing Suppresses Malignant Phenotype and Enhances Radiosensitivity via Activating cGAS/STING Signaling Pathway in Lung Adenocarcinoma. Cell BioSci. 2021, 11, 74. [Google Scholar] [CrossRef]
- Jin, C.-Y.; Du, L.; Nuerlan, A.-H.; Wang, X.-L.; Yang, Y.-W.; Guo, R. High Expression of RRM2 as an Independent Predictive Factor of Poor Prognosis in Patients with Lung Adenocarcinoma. Aging 2020, 13, 3518–3535. [Google Scholar] [CrossRef]
- Ma, C.; Luo, H.; Cao, J.; Gao, C.; Fa, X.; Wang, G. Independent Prognostic Implications of RRM2 in Lung Adenocarcinoma. J. Cancer 2020, 11, 7009–7022. [Google Scholar] [CrossRef]
- Guan, X. Cancer Metastases: Challenges and Opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef]
- Fernando, W.; Coyle, K.; Marcato, P.; Vasantha Rupasinghe, H.P.; Hoskin, D.W. Phloridzin Docosahexaenoate, a Novel Fatty Acid Ester of a Plant Polyphenol, Inhibits Mammary Carcinoma Cell Metastasis. Cancer Lett. 2019, 465, 68–81. [Google Scholar] [CrossRef]
- Chen, N.; Qi, Y.; Ma, X.; Xiao, X.; Liu, Q.; Xia, T.; Xiang, J.; Zeng, J.; Tang, J. Rediscovery of Traditional Plant Medicine: An Underestimated Anticancer Drug of Chelerythrine. Front. Pharmacol. 2022, 13, 906301. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-H.; Kao, S.-Y.; Chen, C.-L.; Yuliani, F.S.; Lin, L.-Y.; Lin, C.-H.; Chen, B.-C. Amphiregulin Induces CCN2 and Fibronectin Expression by TGF-β through EGFR-Dependent Pathway in Lung Epithelial Cells. Respir. Res. 2022, 23, 381. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, W.; Wang, X.; Li, X.; Qi, S.; Zhang, Y.; Gao, M.-Q. TGF-Β1 Induces EMT in Bovine Mammary Epithelial Cells Through the TGFβ1/Smad Signaling Pathway. Cell Physiol. BioChem. 2017, 43, 82–93. [Google Scholar] [CrossRef]
- Nordlund, P.; Reichard, P. Ribonucleotide Reductases. Annu. Rev. BioChem. 2006, 75, 681–706. [Google Scholar] [CrossRef] [PubMed]
- Grolmusz, V.K.; Karászi, K.; Micsik, T.; Tóth, E.A.; Mészáros, K.; Karvaly, G.; Barna, G.; Szabó, P.M.; Baghy, K.; Matkó, J.; et al. Cell Cycle Dependent RRM2 May Serve as Proliferation Marker and Pharmaceutical Target in Adrenocortical Cancer. Am. J. Cancer Res. 2016, 6, 2041–2053. [Google Scholar]
- Grossi, F.; Dal Bello, M.G.; Salvi, S.; Puzone, R.; Pfeffer, U.; Fontana, V.; Alama, A.; Rijavec, E.; Barletta, G.; Genova, C.; et al. Expression of Ribonucleotide Reductase Subunit-2 and Thymidylate Synthase Correlates with Poor Prognosis in Patients with Resected Stages I-III Non-Small Cell Lung Cancer. Dis. Markers 2015, 2015, 302649. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xu, M.; Han, L.; Rao, Y.; Han, H.; Zheng, H.; Wu, J.; Sun, X. Chelerythrine Inhibits TGF-β-Induced Epithelial–Mesenchymal Transition in A549 Cells via RRM2. Pharmaceuticals 2025, 18, 1036. https://doi.org/10.3390/ph18071036
Liu J, Xu M, Han L, Rao Y, Han H, Zheng H, Wu J, Sun X. Chelerythrine Inhibits TGF-β-Induced Epithelial–Mesenchymal Transition in A549 Cells via RRM2. Pharmaceuticals. 2025; 18(7):1036. https://doi.org/10.3390/ph18071036
Chicago/Turabian StyleLiu, Jinlong, Mengran Xu, Liu Han, Yuxuan Rao, Haoming Han, Haoran Zheng, Jinying Wu, and Xin Sun. 2025. "Chelerythrine Inhibits TGF-β-Induced Epithelial–Mesenchymal Transition in A549 Cells via RRM2" Pharmaceuticals 18, no. 7: 1036. https://doi.org/10.3390/ph18071036
APA StyleLiu, J., Xu, M., Han, L., Rao, Y., Han, H., Zheng, H., Wu, J., & Sun, X. (2025). Chelerythrine Inhibits TGF-β-Induced Epithelial–Mesenchymal Transition in A549 Cells via RRM2. Pharmaceuticals, 18(7), 1036. https://doi.org/10.3390/ph18071036






