The Antidiabetic Activity of Wild-Growing and Cultivated Medicinal Plants Used in Romania for Diabetes Mellitus Management: A Phytochemical and Pharmacological Review
Abstract
1. Introduction
2. Pathogenesis of Diabetes Mellitus
3. Causes and Risk Factors
4. Prevalence of Diabetes Mellitus
5. Classification of Diabetes Mellitus
| I. Type 1 Diabetes Mellitus (β-cell destruction, usually resulting in absolute insulin deficiency) A. Autoimmune—The autoimmune process is marked by the presence of specific autoantibodies, including islet cell antibodies, insulin autoantibodies, anti-glutamic acid decarboxylase antibodies (GAD65), and antibodies against tyrosine phosphatases IA-2 and IA-2β. B. Idiopathic—The mechanisms responsible for β-cell destruction are unknown. Patients exhibit permanent insulinopenia and are prone to ketoacidosis, without any evidence of autoimmune involvement. This form of diabetes is more commonly observed in individuals of African or Asian descent [56]. |
| II. Type 2 Diabetes Mellitus. This form may range from predominant insulin resistance with a relative insulin deficiency to a predominant insulin secretory defect accompanied by insulin resistance [57] |
| III. Other Specific Types of Diabetes Mellitus A. Genetic defects of β-cell function (MODY—Maturity Onset Diabetes of the Young):
Type A insulin resistance, leprechaunism, Rabson–Mendenhall syndrome, lipoatrophic diabetes C. Diseases of the exocrine pancreas: Pancreatitis, pancreatectomy, neoplasia, cystic fibrosis, hemochromatosis, fibrocalculous pancreatopathy D. Endocrinopathies: Acromegaly, Cushing syndrome, glucagonoma, pheochromocytoma, hyperthyroidism, somatostatinoma, aldosteronoma E. Drug- or chemical-induced diabetes: Vacor (a rodenticide), intravenous pentamidine (irreversibly destroys pancreatic β-cells), nicotinic acid, glucocorticoids, thyroid hormones, diazoxide, β-adrenergic agonists, thiazides, phenytoin (Dilantin), α-interferon F. Infections: Congenital rubella, cytomegalovirus G. Uncommon forms of immune-mediated diabetes: Stiff-man syndrome, insulin receptor autoantibodies H. Other genetic syndromes associated with diabetes: Down syndrome, Klinefelter syndrome, Turner syndrome, Wolfram syndrome, Friedreich ataxia, Huntington chorea, Laurence–Moon–Biedl syndrome, myotonic dystrophy, porphyria, Prader–Willi syndrome [58] |
| IV. Gestational Diabetes Mellitus (GDM) Carbohydrate intolerance of variable severity with onset or first recognition during pregnancy. In some cases, this may reflect previously undiagnosed pre-existing diabetes rather than true gestational diabetes. Women diagnosed with GDM require long-term follow-up after delivery [59] |
| V. Prediabetes This category refers to blood glucose abnormalities that are less severe than overt diabetes, but still associated with increased risk of progression and cardiovascular complications. A. Impaired Fasting Glucose (IFG): Fasting plasma glucose levels between 110 and 125 mg/dL. B. Impaired Glucose Tolerance (IGT): Two-hour plasma glucose levels during an oral glucose tolerance test between 140 and 199 mg/dL. Individuals with IFG and/or IGT are at high risk of developing type 2 diabetes, and their cardiovascular risk is elevated—comparable to that of individuals with overt diabetes [60] |
| Tests Used | Reference Ranges for Prediabetes | Interpretation |
|---|---|---|
| FPG (fasting plasma glucose)—no carbohydrate intake 8 h prior | 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L) | Impaired fasting glucose |
| Two-hour plasma glucose after 75 g oral glucose (OGTT—oral glucose tolerance test) | 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L) | Impaired glucose tolerance |
| HbA1C | 5.7–6.4% | Prediabetes |
6. Traditionally Used Herbal Products in the Treatment of Diabetes Mellitus and Its Complications
Key Representatives and Mechanisms of Action
7. Materials and Methods
8. Study Limitations
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 Diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef]
- Matheus, A.S.; Tannus, L.R.; Cobas, R.A.; Palma, C.C.; Negrato, C.A.; Gomes, M.B. Impact of Diabetes on Cardiovascular Disease: An Update. Int. J. Hypertens. 2013, 2013, 653789. [Google Scholar] [CrossRef]
- Ştefan, A.G.; Clenciu, D.; Mitrea, A.; Vladu, I.M.; Protasiewicz-Timofticiuc, D.C.; Roşu, M.M.; Maria, D.T.; Dinu, I.R.; Gheonea, T.C.; Vladu, B.E.; et al. Metabolic Syndrome and Insulin Resistance in Romania. Int. J. Mol. Sci. 2025, 26, 2389. [Google Scholar] [CrossRef]
- Chiles, N.S.; Phillips, C.L.; Volpato, S.; Bandinelli, S.; Ferrucci, L.; Guralnik, J.M.; Patel, K.V. Diabetes, Peripheral Neuropathy, and Lower-Extremity Function. J. Diabetes Complicat. 2014, 28, 91–95. [Google Scholar] [CrossRef]
- Mota, M.; Popa, S.G.; Mota, E.; Mitrea, A.; Catrinoiu, D.; Cheta, D.M.; Guja, C.; Hancu, N.; Ionescu-Tirgoviste, C.; Lichiardopol, R.; et al. Prevalence of Diabetes Mellitus and Prediabetes in the Adult Romanian Population: PREDATORR Study. J. Diabetes 2016, 8, 336–344. [Google Scholar] [CrossRef]
- Gómez-Peralta, F.; Pinés-Corrales, P.J.; Santos, E.; Cuesta, M.; González-Albarrán, O.; Azriel, S.; Castaño, L.; Mathieu, C.; on behalf of the AGORA Diabetes Collaborative Group. Autoimmune Type 1 Diabetes: An Early Approach Appraisal for Spain by the AGORA Diabetes Collaborative Group. J. Clin. Med. 2025, 14, 418. [Google Scholar] [CrossRef]
- Mirea, O.; Oghli, M.G.; Neagoe, O.; Berceanu, M.; Țieranu, E.; Moraru, L.; Raicea, V.; Donoiu, I. All-Cause Mortality Prediction in Subjects with Diabetes Mellitus Using a Machine Learning Model and Shapley Values. Diabetology 2025, 6, 5. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the Endocrine Control of Energy Balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef]
- Akbari, M.; Hassan-Zadeh, V. IL-6 Signalling Pathways and the Development of Type 2 Diabetes. Inflammopharmacology 2018, 26, 685–698. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Fotiadis, G.; Kapelouzou, A.; Kostakis, A.; Liapis, C.D.; Vrabas, I.S. The Differential Anti-Inflammatory Effects of Exercise Modalities and Their Association with Early Carotid Atherosclerosis Progression in Patients with Type 2 Diabetes. Diabet. Med. 2013, 30, e41–e50. [Google Scholar] [CrossRef]
- Freitas Lima, L.C.; de Braga, V.A.; do Socorro de França Silva, M.; de Cruz, J.C.; Sousa Santos, S.H.; de Oliveira Monteiro, M.M.; de Moura Balarini, C. Adipokines, Diabetes and Atherosclerosis: An Inflammatory Association. Front. Physiol. 2015, 6, 304. [Google Scholar] [CrossRef]
- Guerre-Millo, M. Adipose Tissue and Adipokines: For Better or Worse. Diabetes Metab. 2004, 30, 13–19. [Google Scholar] [CrossRef]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity Is a Fibroblast Growth Factor 21 (FGF21)-Resistant State. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef]
- Zajec, A.; Trebušak Podkrajšek, K.; Tesovnik, T.; Šket, R.; Čugalj Kern, B.; Jenko Bizjan, B.; Šmigoc Schweiger, D.; Battelino, T.; Kovač, J. Pathogenesis of Type 1 Diabetes: Established Facts and New Insights. Genes 2022, 13, 706. [Google Scholar] [CrossRef]
- Keshavarzi, E.; Noveiry, B.B.; Rezaei, N. The Relationship Between GAD65 Autoantibody and the Risk of T1DM Onset. J. Diabetes Metab. Disord. 2022, 21, 1935–1942. [Google Scholar] [CrossRef]
- Minniakhmetov, I.; Yalaev, B.; Khusainova, R.; Bondarenko, E.; Melnichenko, G.; Dedov, I.; Mokrysheva, N. Genetic and Epigenetic Aspects of Type 1 Diabetes Mellitus: Modern View on the Problem. Biomedicines 2024, 12, 399. [Google Scholar] [CrossRef]
- Noble, J.A.; Valdes, A.M. Genetics of the HLA Region in the Prediction of Type 1 Diabetes. Curr. Diab. Rep. 2011, 11, 533–542. [Google Scholar] [CrossRef]
- Stene, L.C.; Norris, J.M.; Rewers, M.J. Risk Factors for Type 1 Diabetes. In Diabetes in America [Internet]; Lawrence, J.M., Casagrande, S.S., Herman, W.H., Wexler, D.J., Cefalu, W.T., Eds.; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): Bethesda, MD, USA, 2023. [Google Scholar]
- Pugliese, A.; Boulware, D.; Yu, L.; Babu, S.; Steck, A.K.; Becker, D.; Rodriguez, H.; DiMeglio, L.; Evans-Molina, C.; Harrison, L.C.; et al. HLA-DRB115:01-DQA101:02-DQB1*06:02 Haplotype Protects Autoantibody-Positive Relatives From Type 1 Diabetes Throughout the Stages of Disease Progression. Diabetes 2016, 65, 1109–1119. [Google Scholar] [CrossRef]
- Milicic, T.; Jotic, A.; Lalic, K.; Lukic, L.; Macesic, M.; Stanarcic Gajovic, J.; Stoiljkovic, M.; Milovancevic, M.; Rafailovic, D.; Bozovic, A.; et al. Insulin Secretion and Insulin Sensitivity Change in Different Stages of Adult-Onset Type 1 Diabetes: A Cross-Sectional Study. J. Clin. Med. 2025, 14, 1109. [Google Scholar] [CrossRef]
- Echeverri, A.F.; Tobón, G.J. Autoimmune Diabetes Mellitus (Type 1A). In Autoimmunity: From Bench to Bedside [Internet]; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Levy, R.A., Cervera, R., Eds.; El Rosario University Press: Bogotá, Colombia, 2013. [Google Scholar]
- Młynarska, E.; Czarnik, W.; Dzieża, N.; Jędraszak, W.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Type 2 Diabetes Mellitus: New Pathogenetic Mechanisms, Treatment and the Most Important Complications. Int. J. Mol. Sci. 2025, 26, 1094. [Google Scholar] [CrossRef]
- Ferdous, S.-E.; Ferrell, J.M. Pathophysiological Relationship between Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 8731. [Google Scholar] [CrossRef]
- Aljafary, M.A.; Al-Suhaimi, E.A. Adiponectin System (Rescue Hormone): The Missing Link Between Metabolic and Cardiovascular Diseases. Pharmaceutics 2022, 14, 1430. [Google Scholar] [CrossRef]
- Ebrahimi-Mameghani, M.; Mohammadi, S.; Arefhosseini, S.R.; Fallah, P.; Bazi, Z. Adiponectin as a Potential Biomarker of Vascular Disease. Vasc. Health Risk Manag. 2015, 11, 55–70. [Google Scholar]
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in Insulin Resistance: Insights Into Mechanisms and Therapeutic Strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Ustianowski, P.; Malinowski, D.; Kopytko, P.; Czerewaty, M.; Tarnowski, M.; Dziedziejko, V.; Safranow, K.; Pawlik, A. ADCY5, CAPN10 and JAZF1 Gene Polymorphisms and Placental Expression in Women with Gestational Diabetes. Life 2021, 11, 806. [Google Scholar] [CrossRef]
- Mine, K.; Yoshikai, Y.; Takahashi, H.; Mori, H.; Anzai, K.; Nagafuchi, S. Genetic Susceptibility of the Host in Virus-Induced Diabetes. Microorganisms 2020, 8, 1133. [Google Scholar] [CrossRef]
- Gillett, M.; Royle, P.; Snaith, A.; Scotland, G.; Poobalan, A.; Imamura, M.; Black, C.; Boroujerdi, M.; Jick, S.; Wyness, L.; et al. Non-Pharmacological Interventions to Reduce the Risk of Diabetes in People with Impaired Glucose Regulation: A Systematic Review and Economic Evaluation. Health Technol. Assess. 2012, 16, 1–236. [Google Scholar] [CrossRef]
- Spanakis, E.K.; Golden, S.H. Race/ethnic difference in diabetes and diabetic complications. Curr. Diab. Rep. 2013, 13, 814–823. [Google Scholar] [CrossRef]
- Mittal, R.; Prasad, K.; Lemos, J.R.N.; Arevalo, G.; Hirani, K. Unveiling Gestational Diabetes: An Overview of Pathophysiology and Management. Int. J. Mol. Sci. 2025, 26, 2320. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef]
- Ghojazadeh, M.; Mobasseri, M.; Pournaghi Azar, F.; Lotfi, A. Prevalence and Incidence of Type 1 Diabetes in the World. In Type 1 Diabetes—Causes, Symptoms, and Treatments; IntechOpen: New York, NY, USA, 2024. [Google Scholar] [CrossRef]
- Pappachan, J.M.; Fernandez, C.J.; Ashraf, A.P. Rising tide: The global surge of type 2 diabetes in children and adolescents demands action now. World J. Diabetes 2024, 15, 797–809. [Google Scholar] [CrossRef]
- Xu, S.T.; Sun, M.; Xiang, Y. Global, regional, and national trends in type 2 diabetes mellitus burden among adolescents and young adults aged 10–24 years from 1990 to 2021: A trend analysis from the Global Burden of Disease Study 2021. World J. Pediatr. 2025, 21, 73–89. [Google Scholar] [CrossRef]
- Zhang, F.S.; Li, H.J.; Yu, X.; Song, Y.P.; Ren, Y.F.; Qian, X.Z.; Liu, J.L.; Li, W.X.; Huang, Y.R.; Gao, K. Global trends and hotspots of type 2 diabetes in children and adolescents: A bibliometric study and visualization analysis. World J. Diabetes 2025, 16, 96032. [Google Scholar] [CrossRef]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes Metab. Syndr. Obes. 2021, 14, 3567–3602. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. WHO Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 May 2025).
- Bala, C.; Rusu, A.; Ciobanu, D.; Roman, G. Length of Hospital Stay, Hospitalization Costs, and Their Drivers in Adults with Diabetes in the Romanian Public Hospital System. Int. J. Environ. Res. Public Health 2022, 19, 10035. [Google Scholar] [CrossRef] [PubMed]
- Hâncu, A.; Roman, G.; Bala, C.; Timar, B.; Roman, D.; Păun, D.; Mechanick, J.I. Diabetes Care in Romania: A Lesson on the Central Role of Lifestyle Medicine. Am. J. Lifestyle Med. 2023, 15598276231195572. [Google Scholar] [CrossRef]
- Green, A.; Hede, S.M.; Patterson, C.C.; Wild, S.H.; Imperatore, G.; Roglic, G.; Beran, D. Type 1 diabetes in 2017: Global estimates of incident and prevalent cases in children and adults. Diabetologia 2021, 64, 2741–2750. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef]
- Abel, E.D.; Gloyn, A.L.; Evans-Molina, C.; Joseph, J.J.; Misra, S.; Pajvani, U.B.; Simcox, J.; Susztak, K.; Drucker, D.J. Diabetes mellitus-Progress and opportunities in the evolving epidemic. Cell 2024, 187, 3789–3820. [Google Scholar] [CrossRef]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, H.; Zhao, Z.; Li, S.; Zhang, X.; Guo, R.; Liu, H.; Yuan, Y.; Li, W.; Song, Q.; et al. Type 3 diabetes and metabolic reprogramming of brain neurons: Causes and therapeutic strategies. Mol. Med. 2025, 31, 61. [Google Scholar] [CrossRef]
- Albairmani, R.A.; Basheer, B.M.; Macky, M.M.; Al Syouti, T.; AlZubaidy, H.; Elfaki, E.; Kidwai, A.; Basheer, Y.M.; Ahmed, F.; Salaheldin, M. Management of Diabetes in Pregnancy: A Review of Clinical Guidelines and Practices. Cureus 2025, 17, e79334. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E. Anti-Islet Autoantibodies in Type 1 Diabetes. Int. J. Mol. Sci. 2023, 24, 10012. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. 1), S16–S38. [Google Scholar]
- Tamás, G.; Kerényi, Z. Gestational Diabetes: Current Aspects on Pathogenesis and Treatment. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. 2), S400–S411. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Selvin, E. Prediabetes and What It Means: The Epidemiological Evidence. Annu. Rev. Public Health 2021, 42, 59–77. [Google Scholar] [CrossRef]
- Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. Diabetes 2018, 42 (Suppl. 1), S1–S325. [Google Scholar]
- Salehi, B.; Ata, A.; Kumar, V.A.N.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Tsouh Fokou, P.V.; Kobarfard, F.; Zakaria, Z.A.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Governa, P.; Baini, G.; Borgonetti, V.; Cettolin, G.; Giachetti, D.; Magnano, A.R.; Miraldi, E.; Biagi, M. Phytotherapy in the Management of Diabetes: A Review. Molecules 2018, 23, 105. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Noor, F.; Tahir ul Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, P.; Talukdar, N.C.; Banerjee, S.K. Therapeutic Benefit of Dillenia indica in Diabetes and Its Associated Complications. J. Diabetes Res. 2019, 2019, 4632491. [Google Scholar] [CrossRef] [PubMed]
- Song, B.-R.; Alam, M.B.; Lee, S.-H. Terpenoid-Rich Extract of Dillenia indica L. Bark Displays Antidiabetic Action in Insulin-Resistant C2C12 Cells and STZ-Induced Diabetic Mice by Attenuation of Oxidative Stress. Antioxidants 2022, 11, 1227. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Jini, D. Antidiabetic Effects of Momordica charantia (Bitter Melon) and Its Medicinal Potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Kim, B.; Lee, H.S.; Kim, H.J.; Lee, H.; Lee, I.-Y.; Ock, S.; Kwon, S.; Kang, S.-S.; Choi, Y. Momordica charantia (Bitter Melon) Efficacy and Safety on Glucose Metabolism in Korean Prediabetes Participants: A 12-Week, Randomized Clinical Study. Food Sci. Biotechnol. 2023, 32, 697–704. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef]
- Sarker, D.K.; Ray, P.; Dutta, A.K.; Rouf, R.; Uddin, S.J. Antidiabetic Potential of Fenugreek (Trigonella foenum-graecum): A Magic Herb for Diabetes Mellitus. Food Sci. Nutr. 2024, 12, 7108–7136. [Google Scholar] [CrossRef]
- Geberemeskel, G.A.; Debebe, Y.G.; Nguse, N.A. Antidiabetic Effect of Fenugreek Seed Powder Solution (Trigonella foenum-graecum L.) on Hyperlipidemia in Diabetic Patients. J. Diabetes Res. 2019, 2019, 8507453. [Google Scholar] [CrossRef]
- Kishore, T.S.; Babu, J.A.; Priyadarshini, K.A. Evaluation of Antidiabetic Activity of Trigonella foenum-graecum Leaves in Alloxan Induced Type-II Diabetes. Int. J. Res. Pharmacol. Pharmacother. 2025, 14, 1–10. [Google Scholar]
- Lertpatipanpong, P.; Janpaijit, S.; Park, E.Y.; Kim, C.T.; Baek, S.J. Potential Anti-Diabetic Activity of Pueraria lobata Flower (Flos Puerariae) Extracts. Molecules 2020, 25, 3970. [Google Scholar] [CrossRef]
- Zhang, S.; Ge, Q.; Chen, L.; Chen, K. Studies of the Anti-Diabetic Mechanism of Pueraria lobata Based on Metabolomics and Network Pharmacology. Processes 2021, 9, 1245. [Google Scholar] [CrossRef]
- Sutedja, A.M.; Yanase, E.; Batubara, I.; Fardiaz, D.; Lioe, H.N. Antidiabetic Components from the Hexane Extract of Red Kidney Beans (Phaseolus vulgaris L.): Isolation and Structure Determination. Biosci. Biotechnol. Biochem. 2020, 84, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.S.P.; Banegas-Luna, A.J.; Peña-García, J.; Pérez-Sánchez, H.; Apostolides, Z. Evaluation of the Anti-Diabetic Activity of Some Common Herbs and Spices: Providing New Insights with Inverse Virtual Screening. Molecules 2019, 24, 4030. [Google Scholar] [CrossRef] [PubMed]
- AlBalawi, A.N.; Elmetwalli, A.; Baraka, D.M.; Alnagar, H.A.; Alamri, E.S.; Hassan, M.G. Chemical Constituents, Antioxidant Potential, and Antimicrobial Efficacy of Pimpinella anisum Extracts Against Multidrug-Resistant Bacteria. Microorganisms 2023, 11, 1024. [Google Scholar] [CrossRef]
- Assaggaf, H.M. Investigating the Antidiabetic Properties of Apium graveolens Extract and Its Inhibition of Enzymes Associated with Hyperglycemia. Int. J. Biol. Macromol. 2025, 290, 138873. [Google Scholar] [CrossRef]
- Meutia, R.; Sembiring, N.B.; Nababan, O.A.; Simanjuntak, N.; Novriani, E.; Nurasni, N. Antidiabetic Activity Test of the Ethyl Acetate Fraction of Celery Leaves (Apium graveolens L.) Against Wistar Male Rats (Rattus norvegicus). J. Pharm. Sci. 2022, 6, 160–166. [Google Scholar]
- Karkute, S.G.; Koley, T.K.; Yengkhom, B.K.; Tripathi, A.; Srivastava, S.; Maurya, A.; Singh, B. Anti-Diabetic Phenolic Compounds of Black Carrot (Daucus carota Subsp. sativus var. atrorubens Alef.) Inhibit Enzymes of Glucose Metabolism: An in silico and in vitro Validation. Med. Chem. 2018, 14, 641–649. [Google Scholar]
- Esatbeyoglu, T.; Rodríguez-Werner, M.; Schlösser, A.; Liehr, M.; Ipharraguerre, I.; Winterhalter, P.; Rimbach, G. Fractionation of Plant Bioactives from Black Carrots (Daucus carota subsp. sativus var. atrorubens Alef.) by Adsorptive Membrane Chromatography and Analysis of Their Potential Anti-Diabetic Activity. J. Agric. Food Chem. 2016, 64, 5901–5908. [Google Scholar]
- Ali, A. Chemical Composition, α-Glucosidase Inhibitory and Anticancer Activity of Essential Oil of Thymus vulgaris Leaves. J. Essent. Oil Bear. Plants 2021, 24, 695–703. [Google Scholar] [CrossRef]
- Aljelehawy, Q.H.A.; Mohammadi, S.; Mohamadian, E.; Mal Allah, O.R.; Mirzaei, A.; Ghahremanlou, M. Antimicrobial, Anticancer, Antidiabetic, Antineurodegenerative, and Antirheumatic Activities of Thymol: Clarification of Mechanisms. Micro Nano Bio Asp. 2023, 2, 1–7. [Google Scholar]
- Tundis, R.; Grande, F.; Occhiuzzi, M.A.; Sicari, V.; Loizzo, M.R.; Cappello, A.R. Lavandula angustifolia Mill. (Lamiaceae) Ethanol Extract and Its Main Constituents as Promising Agents for the Treatment of Metabolic Disorders: Chemical Profile, in vitro Biological Studies, and Molecular Docking. J. Enzym. Inhib. Med. Chem. 2023, 38, 2269481. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodnia, L.; Forouzandeh Shahrakei, Z.; Hojatyar, S.; Setorki, M. Effects of Lavandula angustifolia Hydroalcoholic Extract on the Blood and Urine Biochemical Factors of Diabetic Patients: A Placebo-Controlled Clinical Trial. J. Basic Res. Med. Sci. 2024, 11, 53–61. [Google Scholar]
- Behradmanesh, S.; Derees, F.; Rafieian-Kopaei, M. Effect of Salvia officinalis on Diabetic Patients. J. Ren. Inj. Prev. 2013, 2, 51–54. [Google Scholar] [PubMed]
- Ononamadu, C.J.; Seidel, V. Exploring the Antidiabetic Potential of Salvia officinalis Using Network Pharmacology, Molecular Docking and ADME/Drug-Likeness Predictions. Plants 2024, 13, 2892. [Google Scholar] [CrossRef]
- Huang, S.M.; Lin, C.H.; Chang, W.F.; Shih, C.C. Antidiabetic and Antihyperlipidemic Activities of Phyllanthus emblica L. Extract in vitro and the Regulation of Akt Phosphorylation, Gluconeogenesis, and Peroxisome Proliferator-Activated Receptor α in Streptozotocin-Induced Diabetic Mice. Food Nutr. Res. 2023, 67, 9492. [Google Scholar] [CrossRef]
- Lin, C.H.; Kuo, Y.H.; Shih, C.C. Antidiabetic and Immunoregulatory Activities of Extract of Phyllanthus emblica L. in NOD with Spontaneous and Cyclophosphamide-Accelerated Diabetic Mice. Int. J. Mol. Sci. 2023, 24, 9922. [Google Scholar] [CrossRef]
- Vadivelan, R.; Gopala Krishnan, R.; Kannan, R. Antidiabetic Potential of Asparagus racemosus Willd Leaf Extracts through Inhibition of α-Amylase and α-Glucosidase. J. Tradit. Complement. Med. 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Hannan, J.M.; Ali, L.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H. Antihyperglycaemic Activity of Asparagus racemosus Roots Is Partly Mediated by Inhibition of Carbohydrate Digestion and Absorption, and Enhancement of Cellular Insulin Action. Br. J. Nutr. 2012, 107, 1316–1323. [Google Scholar] [CrossRef]
- Satyanarayana, K.; Sravanthi, K.; Shaker, I.A.; Ponnulakshmi, R. Molecular Approach to Identify Antidiabetic Potential of Azadirachta indica. J. Ayurveda Integr. Med. 2015, 6, 165–174. [Google Scholar] [CrossRef]
- Mohammed, A.; Kumar, R.; Ashfaq, F.; Alsayegh, A.A.; Al Areefy, A.A.E.H.; Khan, M.I.; Rizvi, S.I. Young and Mature Leaves of Azadirachta indica (Neem) Display Different Antidiabetic and Antioxidative Effects. Egypt. J. Basic Appl. Sci. 2023, 10, 316–328. [Google Scholar] [CrossRef]
- Shetty, P.; Rai, M.; Ravindran, A.; Gopalakrishna, H.N.; Pai, V.R.; Kalal, B.S. Hypoglycemic and Hypolipidemic Effects of Garcinia cambogia Extracts in Streptozotocin-Nicotinamide Induced Diabetic Rat Model. Int. J. Clin. Exp. Pathol. 2022, 15, 380–387. [Google Scholar] [PubMed]
- Chen, T.-H.; Tsai, M.-J.; Fu, Y.-S.; Weng, C.-F. The Exploration of Natural Compounds for Anti-Diabetes from Distinctive Species Garcinia linii with Comprehensive Review of the Garcinia Family. Biomolecules 2019, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Issac, A.; Nm, J.; Ninan, E.; Maliakel, B.; Kuttan, R. Effects of the Polyphenol Content on the Anti-Diabetic Activity of Cinnamomum zeylanicum Extracts. Food Funct. 2014, 5, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Ali, T.; Naeem, M.; Hussain, F.; Li, Z.; Nasir, A. Biochemical, Structural Characterization and In vitro Evaluation of Antioxidant, Antibacterial, Cytotoxic, and Antidiabetic Activities of Nanosuspensions of Cinnamomum zeylanicum Bark Extract. Front. Chem. 2023, 11, 1194389. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Review of Ginseng Anti-Diabetic Studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef]
- Liu, Z.; Qu, C.Y.; Li, J.X.; Wang, Y.F.; Li, W.; Wang, C.Z.; Wang, D.S.; Song, J.; Sun, G.Z.; Yuan, C.S. Hypoglycemic and Hypolipidemic Effects of Malonyl Ginsenosides from American Ginseng (Panax quinquefolius L.) on Type 2 Diabetic Mice. ACS Omega 2021, 6, 33652–33664. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, B.; Li, Y. Antihyperglycemic Effect of Ginkgo biloba Extract in Streptozotocin-Induced Diabetes in Rats. BioMed. Res. Int. 2013, 2013, 162724. [Google Scholar] [CrossRef]
- Aziz, T.A.; Hussain, S.A.; Mahwi, T.O.; Ahmed, Z.A.; Rahman, H.S.; Rasedee, A. The Efficacy and Safety of Ginkgo biloba Extract as an Adjuvant in Type 2 Diabetes Mellitus Patients Ineffectively Managed with Metformin: A Double-Blind, Randomized, Placebo-Controlled Trial. Drug Des. Devel. Ther. 2018, 12, 735–742. [Google Scholar] [CrossRef]
- Jurčević Šangut, I.; Šola, I.; Šamec, D. Neuroprotective, Anti-Hyperpigmentation, and Anti-Diabetic Effects and Bioaccessibility of Flavonoids in Ginkgo Leaf Infusions from Green and Yellow Leaves. Appl. Sci. 2024, 14, 10231. [Google Scholar] [CrossRef]
- Kazazis, C.E.; Evangelopoulos, A.A.; Kollas, A.; Vallianou, N.G. The Therapeutic Potential of Milk Thistle in Diabetes. Rev. Diabet. Stud. 2014, 11, 167–174. [Google Scholar] [CrossRef]
- Lekmine, S.; Benslama, O.; Ola, M.S.; Touzout, N.; Moussa, H.; Tahraoui, H.; Hafsa, H.; Zhang, J.; Amrane, A. Preliminary Data on Silybum marianum Metabolites: Comprehensive Characterization, Antioxidant, Antidiabetic, Antimicrobial Activities, LC-MS/MS Profiling, and Predicted ADMET Analysis. Metabolites 2025, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhang, Y.; Cao, Y.; Sun, Y.; Wang, Y.; Zhang, C.; Liang, D.; Liu, Y.; Feng, W. Chemical Constituents from Acorus calamus with Potent Anti-Diabetic and Hepatoprotective Activities. Fitoterapia 2023, 169, 105591. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Silva, F.; Cerón-Romero, L.; Arias-Durán, L.; Navarrete-Vázquez, G.; Almanza-Pérez, J.; Román-Ramos, R.; Ramírez-Ávila, G.; Perea-Arango, I.; Villalobos-Molina, R.; Estrada-Soto, S. Antidiabetic Effect of Achillea millefollium through Multitarget Interactions: α-Glucosidases Inhibition, Insulin Sensitization and Insulin Secretagogue Activities. J. Ethnopharmacol. 2018, 212, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Ashkar, F.; Koohpeyma, F.; Mahmoodi, M.; Gholamalizadeh, M.; Mazloom, Z.; Doaei, S. Hydroalcoholic Extract of Achillea millefolium Improved Blood Glucose, Liver Enzymes and Lipid Profile Compared to Metformin in Streptozotocin-Induced Diabetic Rats. Lipids Health Dis. 2020, 19, 81. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Heidari, H.; Oroojan, A.A.; Mirzavandi, F.; Nasr Esfehani, K.; Dehghan Mohammadi, Z. Antidiabetic, Hypolipidemic and Hepatoprotective Effects of Arctium lappa Root’s Hydro-Alcoholic Extract on Nicotinamide-Streptozotocin Induced Type 2 Model of Diabetes in Male Mice. Avicenna J. Phytomed. 2017, 7, 169–179. [Google Scholar]
- Zolotova, D.; Teterovska, R.; Bandere, D.; Lauberte, L.; Niedra, S. Antidiabetic Properties of the Root Extracts of Dandelion (Taraxacum officinale) and Burdock (Arctium lappa). Plants 2024, 13, 1021. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Semwal, P.; Painuli, S.; Badoni, H.; Ezzat, S.M.; Farid, M.M.; Merghany, R.M.; Aborehab, N.M.; Salem, M.A.; et al. Artemisia spp.: An Update on Its Chemical Composition, Pharmacological and Toxicological Profiles. Oxid. Med. Cell. Longev. 2022, 2022, 5628601. [Google Scholar] [CrossRef]
- Neagu, E.; Paun, G.; Albu, C.; Apreutesei, O.T.; Radu, G.L. In vitro Assessment of the Antidiabetic and Anti-Inflammatory Potential of Artemisia absinthium, Artemisia vulgaris and Trigonella foenum-graecum Extracts Processed Using Membrane Technologies. Molecules 2023, 28, 7156. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Rafiullah, M.; Hossain, M.A.; Ali, M. Antidiabetic Activity of Cichorium intybus L. Water Extract Against Streptozotocin-Induced Diabetic Rats. J. Umm Al Qura Univ. Appl. Sci. 2023, 9, 565–571. [Google Scholar] [CrossRef]
- Ferrare, K.; Bidel, L.P.R.; Awwad, A.; Poucheret, P.; Cazals, G.; Lazennec, F.; Azay-Milhau, J.; Tournier, M.; Lajoix, A.D.; Tousch, D. Increase in Insulin Sensitivity by the Association of Chicoric Acid and Chlorogenic Acid Contained in a Natural Chicoric Acid Extract (NCRAE) of Chicory (Cichorium intybus L.) for an Antidiabetic Effect. J. Ethnopharmacol. 2018, 215, 241–248. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Abdel Fattah, A.A.; Abdul-Hamid, M.; Abdel-Aziz, A.M.; Sakr, H.I.; Damanhory, A.A.; Abdel-Kawi, S.H.; Ghaboura, N.; Awad, M.M.Y. Antidiabetic and Liver Histological and Ultrastructural Effects of Cynara scolymus Leaf and Flower Head Hydroethanolic Extracts in Nicotinamide/Streptozotocin-Induced Diabetic Rats. Evid. Based Complement. Alternat. Med. 2023, 2023, 4223026. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.; Benameur, T.; Cianciulli, A.; Vacca, M.; Chiarini, M.; De Angelis, M.; Panaro, M.A. Functional and Therapeutic Potential of Cynara scolymus in Health Benefits. Nutrients 2024, 16, 872. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, I.; Laila, O.; Drabu, I.; Ahmad, A.; Charifi, W.; Popescu, S.M.; Mansoor, S. Nutritional Profiling, Phytochemical Composition and Antidiabetic Potential of Taraxacum officinale, an Underutilized Herb. Molecules 2022, 27, 5380. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M. In vitro Antioxidant Activity, Total Phenolic and Total Flavonoid Contents of Taraxacum officinale Leaves. Int. J. Innov. Pharm. Sci. Res. 2015, 3, 697–707. [Google Scholar]
- Studzińska-Sroka, E.; Paczkowska-Walendowska, M.; Kledzik, J.; Galanty, A.; Gościniak, A.; Szulc, P.; Korybalska, K.; Cielecka-Piontek, J. Antidiabetic Potential of Black Elderberry Cultivars Flower Extracts: Phytochemical Profile and Enzyme Inhibition. Molecules 2024, 29, 5775. [Google Scholar] [CrossRef]
- Krejpcio, Z.; Król, E.; Staniek, H.; Oluwatobi, J.M.; Rocha, S.M.; Salvador, A.; Kurek, J. Evaluation of the Effect of Elderberry (Sambucus nigra) Fruit Extracts on Calcium and Magnesium Status in STZ-Induced Diabetic Rats. Proceedings 2023, 91, 228. [Google Scholar]
- Mehmood, A.; Zeb, A.; Ateeq, M.K. In Vivo Antidiabetic Effects of Phenolic Compounds of Spinach, Mustard, and Cabbage Leaves in Mice. Heliyon 2023, 9, e16616. [Google Scholar] [CrossRef]
- Subhash, G.P.; Virbhadrappa, S.R.; Vasant, O.K.; Otari, K.V. Spinacia oleracea Linn, A Pharmacognostic and Pharmacological Overview. Int. J. Res. Ayurveda Pharm. 2010, 1, 78–84. [Google Scholar]
- Dzhafarova, R.E.; Garaev, G.S.; Dzhafarkulieva, Z.S. Antidiabetic Action of Extract of Juglans regia L. Georgian Med. News 2009, 170, 110–114. [Google Scholar]
- Bourais, I.; Elmarrkechy, S.; Taha, D.; Badaoui, B.; Mourabit, Y.; Salhi, N.; Alshahrani, M.M.; Al Awadh, A.A.; Bouyahya, A.; Goh, K.W.; et al. Comparative Investigation of Chemical Constituents of Kernels, Leaves, Husk, and Bark of Juglans regia L., Using HPLC-DAD-ESI-MS/MS Analysis and Evaluation of Their Antioxidant, Antidiabetic, and Anti-Inflammatory Activities. Molecules 2022, 27, 8989. [Google Scholar] [CrossRef]
- Muñiz-Ramirez, A.; Perez, R.M.; Garcia, E.; Garcia, F.E. Antidiabetic Activity of Aloe vera Leaves. Evid. Based Complement. Alternat. Med. 2020, 2020, 6371201. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Iqbal, S.; Biswas, J.; Riaz, U.; Datta, S. Antidiabetic Property of Aloe vera (Aloe barbadensis) and Bitter Melon (Momordica charantia). In Medicinal and Aromatic Plants; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Hafizur, R.M.; Kabir, N.; Chishti, S. Asparagus officinalis Extract Controls Blood Glucose by Improving Insulin Secretion and β-Cell Function in Streptozotocin-Induced Type 2 Diabetic Rats. Br. J. Nutr. 2012, 108, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. A Review of the Pro-Health Activity of Asparagus officinalis L. and Its Components. Foods 2024, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Esakki, A.; Ramadoss, R.; Ananthapadmanabhan, L.; Sundar, S.; Panneerselvam, S.; Ramani, P. Quantification of the Anti-Diabetic Effect of Allium cepa. Cureus 2024, 16, e59174. [Google Scholar] [CrossRef]
- Vu, N.K.; Kim, C.S.; Ha, M.T.; Ngo, Q.-M.T.; Park, S.E.; Kwon, H.; Lee, D.; Choi, J.S.; Kim, J.A.; Min, B.S. Isolation and Identification of Antidiabetic Compounds from Allium cepa and Evaluation of Their Activity in Diabetic Mice. J. Agric. Food Chem. 2020, 68, 8797–8811. [Google Scholar] [CrossRef]
- Ashraf, R.; Khan, R.A.; Ashraf, I. Garlic (Allium sativum) Supplementation with Standard Antidiabetic Agent Provides Better Diabetic Control in Type 2 Diabetes Patients. Pak. J. Pharm. Sci. 2011, 24, 565–570. [Google Scholar]
- Aminabee, S.; Shankar, K.R.; Lakshmi, K.N.V.C.; Saritha, K.; Kavya, R.; Babu, K.C.; Dasari, S.K. Influence of Allium sativum on the Hypoglycaemic Activity of Gliclazide in Normal Rats: A Possible Approach to Herb-Drug Interaction. Biomed. Pharmacol. J. 2024, 17, 377–382. [Google Scholar] [CrossRef]
- Turkkan, A.; Savas, H.B.; Yavuz, B.; Yigit, A.; Uz, E.; Bayram, N.A.; Kale, B. The Prophylactic Effect of Viscum album in Streptozotocin-Induced Diabetic Rats. North Clin. Istanb. 2016, 3, 83–89. [Google Scholar]
- Szurpnicka, A.; Kowalczuk, A.; Szterk, A. Biological Activity of Mistletoe: In vitro and In Vivo Studies and Mechanisms of Action. Arch. Pharm. Res. 2020, 43, 593–629. [Google Scholar] [CrossRef]
- Morales Ramos, J.G.; Esteves Pairazamán, A.T.; Mocarro Willis, M.E.S.; Collantes Santisteban, S.; Caldas Herrera, E. Medicinal Properties of Morus alba for the Control of Type 2 Diabetes Mellitus: A Systematic Review. F1000Research 2021, 10, 1022. [Google Scholar] [CrossRef]
- Özgür, M.; Uçar, A.; Yılmaz, S. The Multifaceted Benefits of Morus nigra L.: A Pharmacological Powerhouse. Phytochem. Rev. 2025. [Google Scholar] [CrossRef]
- Jelača, S.; Dajić-Stevanović, Z.; Vuković, N.; Kolašinac, S.; Trendafilova, A.; Nedialkov, P.; Stanković, M.; Tanić, N.; Tanić, N.T.; Acović, A.; et al. Beyond Traditional Use of Alchemilla vulgaris: Genoprotective and Antitumor Activity In vitro. Molecules 2022, 27, 8113. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.G.; Ganai, B.A.; Akbar, S.; Dar, M.Y.; Masood, A. β-Cell Protective Efficacy, Hypoglycemic and Hypolipidemic Effects of Extracts of Achillea millefolium in Diabetic Rats. Chin. J. Nat. Med. 2012, 10, 185–189. [Google Scholar]
- Mallhi, I.Y.; Sohaib, M.; Khan, A.U.; Rabbani, I. Antidiabetic, Antioxidative and Antihyperlipidemic Effects of Strawberry Fruit Extract in Alloxan-Induced Diabetic Rats. Foods 2023, 12, 2911. [Google Scholar] [CrossRef]
- Ibrahim, D.S.; Abd El-Maksoud, M.A. Effect of Strawberry (Fragaria × ananassa) Leaf Extract on Diabetic Nephropathy in Rats. Int. J. Exp. Pathol. 2015, 96, 87–93. [Google Scholar] [CrossRef]
- Fattahi, A.; Niyazi, F.; Shahbazi, B.; Farzaei, M.H.; Bahrami, G. Antidiabetic Mechanisms of Rosa canina Fruits: An In vitro Evaluation. J. Evid. Based Complement. Altern. Med. 2017, 22, 127–133. [Google Scholar] [CrossRef]
- Vasić, D.; Katanić Stanković, J.S.; Urošević, T.; Kozarski, M.; Naumovski, N.; Khan, H.; Popović-Djordjević, J. Insight into Bioactive Compounds, Antioxidant and Anti-Diabetic Properties of Rosehip (Rosa canina L.)-Based Tisanes with Addition of Hibiscus sabdariffa L. and Crocus sativus L. Beverages 2024, 10, 1. [Google Scholar] [CrossRef]
- Kuczmannová, A.; Balažová, A.; Račanská, E.; Kameníková, M.; Fialová, S.; Majerník, J.; Nagy, M.; Gál, P.; Mučaji, P. Agrimonia eupatoria L. and Cynara cardunculus L. Water Infusions: Comparison of Anti-Diabetic Activities. Molecules 2016, 21, 564. [Google Scholar] [CrossRef]
- Jung, C.H.; Zhou, S.; Ding, G.X.; Kim, J.H.; Hong, M.H.; Shin, Y.-C.; Kim, G.J.; Ko, S.-G. Antihyperglycemic Activity of Herb Extracts on Streptozotocin-Induced Diabetic Rats. Biosci. Biotechnol. Biochem. 2006, 70, 2556–2559. [Google Scholar] [CrossRef]
- Ammar, A.H.; Bouajila, J.; Lebrihi, A.; Mathieu, F.; Romdhane, M.; Zagrouba, F. Chemical Composition and In vitro Antimicrobial and Antioxidant Activities of Citrus aurantium L. Flowers Essential Oil (Neroli Oil). Pak. J. Biol. Sci. 2012, 15, 1034–1040. [Google Scholar] [CrossRef]
- Osfor, M.M.H.; Hegazy, A.; Mohammad, A.; Elmadbouly, M.A.; Afify, R.A.M.; Elbahnasawy, A.S. Hypo-Cholesterolemic and Hypoglycemic Effects of Orange Albedo Powder (Citrus aurantium L.) on Male Albino Rats. Int. J. Nutr. Food Sci. 2013, 2, 70–76. [Google Scholar] [CrossRef][Green Version]
- Akinnuga, A.M.; Bamidele, O.; Ebunlomo, O.A.; Adeniyi, O.S.; Adeleyea, G.S.; Ebomuche, L.C. Hypoglycaemic Effects of Dietary Intake of Ripe and Unripe Lycopersicon esculentum (Tomatoes) on Streptozotocin-Induced Diabetes Mellitus in Rats. OnLine J. Biol. Sci. 2010, 10, 50–53. [Google Scholar] [CrossRef][Green Version]
- Aisami, A.; James, J.I.; Maigari, F.U.; Atiku, M.K. Hypoglycemic Effect of Lycopersicon esculentum (Tomato) on Alloxan-Induced Diabetic Rats. J. Biochem. Microbiol. Biotechnol. 2021, 9, 15–18. [Google Scholar] [CrossRef]
- Chehri, A.; Yarani, R.; Yousefi, Z.; Novin Bahador, T.; Shakouri, S.K.; Ostadrahimi, A.; Mobasseri, M.; Pociot, F.; Araj-Khodaei, M. Anti-Diabetic Potential of Urtica dioica: Current Knowledge and Future Direction. J. Diabetes Metab. Disord. 2022, 21, 931–940. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Orhan, D.D.; Ergun, F.; Yeşilada, E. In-Vivo Assessment of Antidiabetic and Antioxidant Activities of Grapevine Leaves (Vitis vinifera) in Diabetic Rats. J. Ethnopharmacol. 2006, 108, 280–286. [Google Scholar] [CrossRef]
- Karageçili, H.; İzol, E.; Kireçci, E.; Gülçin, İ. Antioxidant, Antidiabetic, Antiglaucoma, and Anticholinergic Effects of Tayfi Grape (Vitis vinifera): A Phytochemical Screening by LC-MS/MS Analysis. Open Chem. 2023, 21, 20230120. [Google Scholar] [CrossRef]
- Soltani, R.; Hakimi, M.; Asgary, S.; Ghanadian, S.M.; Keshvari, M.; Sarrafzadegan, N. Evaluation of the Effects of Vaccinium arctostaphylos L. Fruit Extract on Serum Lipids and hs-CRP Levels and Oxidative Stress in Adult Patients with Hyperlipidemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Evid. Based Complement. Alternat. Med. 2014, 2014, 217451. [Google Scholar] [CrossRef]
- Ozkan, G.; Ercisli, S.; Zeb, A.; Agar, G.; Sagbas, H.I.; Ilhan, G. Some Morphological and Biochemical Characteristics of Wild Grown Caucasian Whortleberry (Vaccinium arctostaphylos L.) Genotypes from Northeastern Turkey. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 47, 378–383. [Google Scholar] [CrossRef]
- Dupak, R.; Hrnkova, J.; Simonova, N.; Kovac, J.; Ivanisova, E.; Kalafova, A.; Schneidgenova, M.; Prnova, M.S.; Brindza, J.; Tokarova, K.; et al. The Consumption of Sea Buckthorn (Hippophae rhamnoides L.) Effectively Alleviates Type 2 Diabetes Symptoms in Spontaneous Diabetic Rats. Res. Vet. Sci. 2022, 152, 261–269. [Google Scholar] [CrossRef]
- Jaszcza, K.; Grzegorzewska, A.K.; Sachman, A.; Kalafova, A.; Massanyi, P.; Kovacik, A.; Dupak, R.; Capcarova, M. The Effect of Metformin and Sea Buckthorn (Hippophae rhamnoides L.) in Alleviating the Symptoms of Type 2 Diabetes Mellitus in the Liver of Zucker Diabetic Fatty Rats. J. Physiol. Pharmacol. 2025, 76, 7. [Google Scholar]

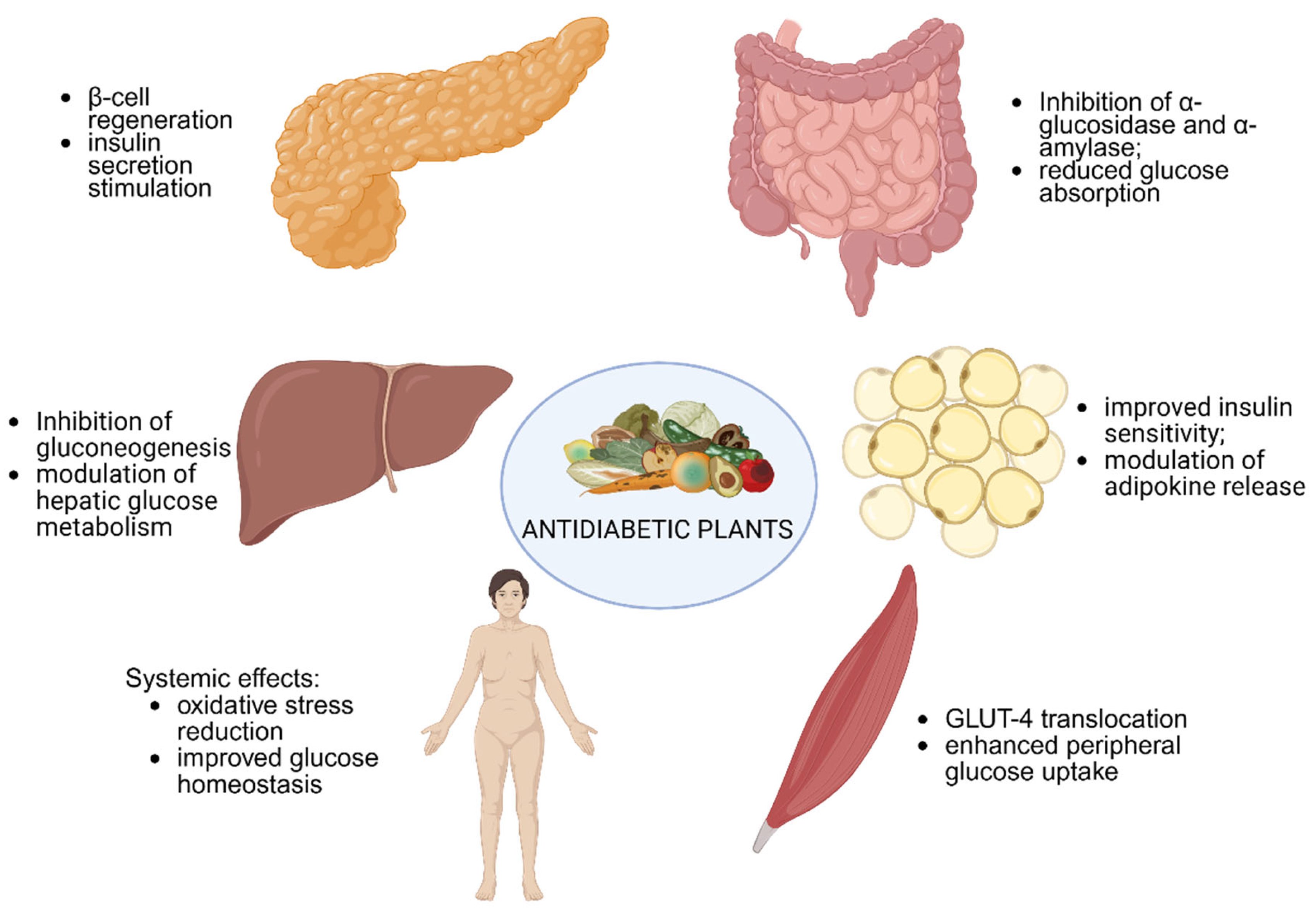
| Factor/Gene (Variant) | Description | Population (s)/Effect | Reference |
|---|---|---|---|
| South Asian origin populations | Heightened sensitivity to diabetes and increased insulin resistance; protective Pro12Ala PPARγ polymorphism in Caucasians shows no protection in Indians | Indian descent vs. Caucasians | [27] |
| Candidate genes (overall) | >50 genes associated with T2DM have been studied; results often inconsistent due to small samples, ethnic differences, environment, gene–environment interplay | Various worldwide populations | – |
| PPARγ Pro allele | Variant reduces insulin sensitivity and markedly increases T2DM risk; carries significant burden of cases | ~98% of Europeans carry ≥1 Pro allele (~25% of Caucasian T2DM cases) | [28] |
| KCNJ11 (Lys allele) and ABCC8 (Ala variant) | Both encode subunits of the ATP-sensitive K+ channel (Kir6.2/SUR1) critical for insulin secretion; associated with T2DM | Pancreatic β-cells | [28] |
| CAPN10 | Encodes calpain-10, a Ca2+-dependent protease; altered activity impairs insulin secretion; higher genetic risk in Mexican-Americans, lower in Caucasians | Mexican-American (↑ risk); Caucasian (↓ risk) | [29] |
| Viral infections (EBV, Coxsackie, mumps, CMV) | Proposed triggers for diabetes via direct β-cell destruction or autoimmune activation | – | – |
| Plant Species (Family) | Active Compounds | Effective Concentration (In Vitro In Vivo) | IC50/EC50 (Target/Assay) | Mechanism of Action |
|---|---|---|---|---|
| Dillenia indica (Dilleniaceae), elephant apple | Tannins, pentacyclic triterpenic alcohols (betulin, betulinic acid, betulinic aldehyde), sterols (β-sitosterol), flavonosides, phenolic compounds | Betulinic acid (approx. 0.3% DW; tested as part of methanolic fruit extract at 100–400 μg/mL, in vitro) | α-glucosidase IC50: 30.75 μg/mL (methanolic fruit extract) | Similar to glibenclamide; has beneficial effects on the histopathological changes in the pancreas, liver, and kidneys [66,67] |
| Momordica charantia (Cucurbitaceae), bitter melon | Polypeptide-P, gurmarin (“plant insulin”), bitter principles (charantin) | mcIRBP peptide (natural content in fresh fruit: approx. 0.5–1.2 mg/g; tested in vitro/in vivo); cucurbitane triterpenoids (content not specified; tested at 10 μM, in vitro) | Charantin α-glucosidase IC50: 10.8 μg/mL (isolated compound); mcIRBP EC50: 0.96 μg/mL | Action begins 30–60 min after oral administration with peak activity at 4 h; mimics bovine insulin, has antioxidant properties, regenerates β-cells, stimulates insulin secretion (sulfonylurea-like mechanism), increases glycogenogenesis, insulin-like activity [68,69] |
| Glycyrrhiza glabra (Fabaceae), licorice | Sterols, flavones, tannins, enzymes, saponins, volatile oils | Glycyrrhizin (natural content in root: approx. 2–9% DW; tested as part of ethanolic root extract at 10–100 μM, in vitro) | α-glucosidase IC50: 12.8 μg/mL (ethanolic root extract) | Acts as a PPAR agonist, improving hyperinsulinemia [70] |
| Trigonella foenum-graecum (Fabaceae), fenugreek | Sterols (lecithin, phytic acid, other phytosterols), bitter substances, volatile oil, tannins, saponins, coumarins | Diosgenin (natural content in seeds: approx. 0.1–0.3%; tested at 8–19 mM, in vitro); ethanolic seed extract (galactomannan: 30–50%; tested at 3–11 μg/mL, in vitro) | Diosgenin EC50 (GLUT4): 8 mM (pure compound); α-glucosidase IC50: 30.15 μg/mL (ethanolic seed extract) | Stimulates glucose transport; regulates glycolysis, gluconeogenesis, and fatty acid synthesis; reduces oxidative stress associated with hyperglycemia; and delays the progression of diabetic retinopathy and neuropathy [71,72,73] |
| Pueraria lobata (Fabaceae), kudzu | Sterols, coumarins, saponins, isoflavones | Puerarin (natural content in root: approx. 3–9%; tested at 100 μM, in vitro; as part of ethanolic root extract) | α-glucosidase IC50: 45.4 μg/mL (ethanolic root extract) | Restores secretory function of β-cells, enhances insulin secretion, inhibits glucose absorption; isoflavones act as PPAR agonists, block IL-12 synthesis, inhibit TH-1 differentiation; prevent and delay T2DM and cardiovascular complications [74,75] |
| Phaseolus vulgaris (Fabaceae), common bean | Sulfur-containing amino acids, alkaloids, anthocyanins, flavonoids, saponins, tannins, terpenoids | Phaseolamin (natural content in extract: approx. 0.5–2%; tested as part of aqueous seed extract at 100 μg/mL, in vitro | α-amylase IC50: 44.2 μg/mL (aqueous seed extract) | Stimulates insulin secretion, increases glucose tolerance; potency similar to tolbutamide [76] |
| Pimpinella anisum (Apiaceae), anise | Volatile oil (80–90% anethole, methyl chavicol or isomethylchavicol, small amounts of anisic ketones and aldehydes) | Anethole (main component, essential oil; tested as methanolic seed extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: ~67 μg/mL (methanolic seed extract) | Increases glutathione-S-transferase activity, has antioxidant effect, reduces cholesterol and triglyceride levels [77,78] |
| Apium graveolens (Apiaceae), celery | Sedanenolides, neocnidilide, neophytadiene, essential oil (limonene, β-selinene, nerolidol, α-selinene, β-pinene, carvone, β-myrcene) | Phthalides and apigenin (main actives; natural content not specified; tested as ethanolic seed extract at 100–400 μg/mL, in vitro) | Not available (no IC50 for glucose uptake); α-glucosidase IC50: ~80 μg/mL (ethanolic seed extract) | Induces insulin receptor phosphorylation Promotes GLUT-4 translocation (in muscle and adipose cells) Inhibits gene expression involved in adipogenesis Enhances peripheral glucose utilization [79,80] |
| Daucus carota (Apiaceae), carrot | Mineral salts, carotenoids, vitamins (C, B-complex, folic acid), fibers (cellulose and lignin), acids (glutamic, succinic, lactic, glycolic), polyphenolcarboxylic acids (caffeic acid), anthocyanins | Carotenoids, caffeic acid derivatives (natural content: β-carotene ~7–14 mg/100g; extract tested at 100–400 μg/mL, in vitro) | α-glucosidase IC50: 75 μg/mL (aqueous root extract) | Improves glucose tolerance Inhibit enzymes of glucose metabolism [81,82] |
| Thymus vulgaris (Lamiaceae), thyme | Volatile oils (thymol, p-cymene, borneol, geraniol, carvacrol, linalool, bornyl acetate, α-pinene), saponins, ursolic acid, oleanolic acid, caffeic acid, flavonoids (luteolin, luteolin-7-glycoside), sterols, waxes, triterpenes, bitter principles | Thymol, carvacrol (essential oil; tested as ethanolic aerial part extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 49.8 μg/mL (ethanolic aerial part extract) | Reduces hyperinsulinemia, increases SOD (superoxide dismutase) concentration, counteracts oxidative effects, preventing diabetic complications [83,84] |
| Lavandula angustifolia (Lamiaceae), lavender | Volatile oil (linalyl acetate, linalyl butyrate, geraniol, free linalool, linalyl valerate, borneol, α-pinene), coumarins, caryophyllene, tannins, bitter principles | Linalool (main in oil; natural content not specified; hydroalcoholic flower extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 72.8 μg/mL (hydroalcoholic flower extract) | Stimulates glucose uptake in muscle cell cultures, increases insulin secretion via a sulfonylurea-like mechanism [85,86] |
| Salvia officinalis (Lamiaceae), sage | Essential oil, thujone, α- and β-pinene, camphor, borneol, cineole, tannins, sitosterols, estrogen-like substances, bitter principles (picrosalvin), nicotinic acid, caffeic acid, fumaric acid, resins, vitamins B1 and C, mineral salts | Thujone, rosmarinic acid (thujone ~30–50% oil; extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 59.4 μg/mL (ethanolic leaf extract) | Stimulates insulin production and secretion; increases glucose utilization in tissues similarly to metformin; inhibits glucose absorption [87,88] |
| Phyllanthus emblica (Phyllanthaceae), Indian gooseberry | Tannins (gallic acid, ellagic acid), norsesquiterpenoids, flavonosides | Gallic/ellagic acid (main actives; total phenolics ~50–100 mg GAE/g; extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 46.2 μg/mL (ethanolic fruit extract) | Inhibits neuropathic pain by modulating oxidative stress, nitrite levels, cytokines (IL-1β, TGF-β1); used as a strong antioxidant and immunomodulator; inhibits α-amylase and α-glucosidase; reduces severity of acute pancreatitis and promotes pancreas repair; lowers cholesterol and triglycerides, improves liver function via ALT normalization [89,90] |
| Asparagus racemosus (Asparagaceae), shatavari | Tannins, saponins (shatavarins A and B, filiasparoside C, asparanin A), isoflavones, satavarin (a glycoside of glucose, rhamnose, and sarsapogenin), vanillin, coniferin, sarsaponin | Shatavarin IV (main saponin; content ~0.1–0.2% root; extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 85 μg/mL (ethanolic root extract) | Tannic acid induces insulin receptor phosphorylation, mediates GLUT-4 translocation; inhibits adipogenesis-related gene expression; prevents diabetic nephropathy; used for polydipsia in diabetes insipidus [91,92] |
| Azadirachta indica (Meliaceae), Neem | Bitter compounds: nimbin, nimbinin, and nimbidin. Leaves contain quercetin, beta-sitosterol, the diterpenoids mahogany and nimbogone, vitamins (A, E, C, riboflavin, and niacin), and minerals (Se, Zn, Cu, Mg, Cr) | Nimbin, nimbidin (main actives; extract tested at 50–200 μg/mL, in vitro) | α-amylase IC50: 52.4 μg/mL (aqueous leaf extract) | Decreases glucose absorption Antioxidant properties, inhibits α-amylase and α-glucosidase Inhibits SGLUT-1 activity Increases activity of glucose-6-phosphate dehydrogenase Exerts insulin-like effect Increases levels of superoxide dismutase, catalase, glutathione peroxidase, and glutathione transferase Hypoglycemic effect comparable to glibenclamide [93,94] |
| Garcinia cambogia (Clusiaceae), garcinia | Tartaric, citric, and phosphoric acids; two poly-isoprenylated benzophenones; mangostin derivatives of camboginol and cambogin | Hydroxycitric acid (HCA; content in fruit rind ~16–20%; extract tested at 100–500 μg/mL, in vitro) | α-amylase IC50: 60.5 μg/mL (aqueous fruit extract) | The mentioned acids suppress lipogenesis by inhibiting citrate lyase, which assists in converting excess carbohydrates into fat Reduces triglycerides and cholesterol; resin confers satiety Main constituent, hydroxycitric acid, reduces appetite and increases fat oxidation [95,96] |
| Cinnamomum zeylanicum (Lauraceae), cinnamon tree | Diterpenes, essential oil rich in eugenol, cinnamaldehyde, cinnamyl acetate, cinnamic alcohol, and 2-hydroxycinnamaldehyde, coumarins, terpenes, tannins, proanthocyanidins | Cinnamaldehyde, polyphenols (content in bark oil: cinnamaldehyde 65–80%; extract tested at 10–100 μg/mL, in vitro) | α-glucosidase IC50: 16.3 μg/mL (aqueous bark extract) | Mimics insulin action (via tannic acid) Stimulates glycogen synthesis Contains glutathione and flavonoids (MHCP—methylhydroxychalcone polymer), increasing adipose tissue sensitivity to insulin [97,98] |
| Panax quinquefolius (Araliaceae), American ginseng | Saponozide, saponins, tannins, bitter principles, vitamins A, B1, B2, B5, B6, B12, D3, E, folic acid, nicotinamide, mucilage, waxes, Zn, K, Fe, Si | Ginsenosides (content in root: 3–5%; extract tested at 10–100 μg/mL, in vitro) | α-glucosidase IC50: 38.2 μg/mL (ethanolic root extract) | Stabilizes blood glucose levels by increasing tissue insulin sensitivity Polypeptides exert hypoglycemic action and stimulate hepatic glycogen synthesis Strong antioxidant activity Increases HDL-cholesterol plasma fraction [99,100] |
| Ginkgo biloba (Ginkgoaceae), maidenhair tree | Diterpenes (ginkgolides A, B, C and ginkgolide J), sesquiterpenes (bilobalide); leaves contain flavonols (kaempferol, quercetin, isorhamnetin), biflavones (bilobetin, ginkgetin, isoginkgetin), catechins, proanthocyanidins, sterols, 6-hydroxymurenic acid | Ginkgolide/flavone (leaf extract; content: ginkgolide 0.5–1% DW; tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 41.3 μg/mL (ethanolic leaf extract) | Improves blood circulation and prevents complications Prevents insulin resistance [101,102,103] |
| Silybum marianum (Asteraceae), milk thistle | Saponins, essential oil; fruits contain silymarin (silibinin, silidianin, silicristin), betaine hydrochloride, amino acids (L-cysteine, glycine, glutamic acid, D-2-aminobutyric acid, D-leucine, tyramine), lipids (3–4%), polyhydroxyphenylchromones, fumaric acid | Silymarin (fruit/seed extract; content: silymarin 1.5–3% seeds; tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 32.4 μg/mL (methanolic seed extract) | Stimulates glucose transport Regulates glycolysis, gluconeogenesis, and fatty acid synthesis Reduces oxidative stress related to hyperglycemia Delays diabetic retinopathy and neuropathy Increases glucose tolerance Reduces AGE and ALE formation Silibinin improves β-cell viability and may serve as a therapeutic agent for type 2 diabetes [104,105] |
| Acorus calamus (Araceae), sweet flag | Volatile oil (1.5–3.5%) containing asarone, azaril aldehyde, methyl isoeugenol, linalool, sesquiterpenes, α-pinene, camphene, camphor, eugenol, tannins, bitter substances, resins, mineral salts | β-asarone (rhizome oil; content: 1–3% in oil; extract tested at 25–100 μg/mL, in vitro) | α-glucosidase IC50: 28.1 μg/mL (ethanolic rhizome extract) | Increases insulin release and secretion similarly to gliclazide; inhibits α-glucosidase and improves insulin resistance; inhibits preadipocyte differentiation into adipocytes; β-asarone attenuates ERK1/2 phosphorylation involved in early adipogenesis [106] |
| Achillea millefolium (Asteraceae), yarrow | Volatile oil, chamazulene, azulenes, asarone, proazulenes, cineole, borneol, pinene, limonene, caryophyllene, achilleine, achilleic acid, organic acids (formic, valeric), tannins | Chamazulene, apigenin (extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 61.2 μg/mL (methanolic aerial part extract) | Regenerates β-pancreatic cells; hypolipidemic effect [107,108] |
| Arctium lappa (Asteraceae), burdock | Arctiin, essential oil, flavonoids, inulin, palmitic acid, caffeic acid, stigmasterol, sitosterol, bitter substances, carotenoids, mineral salts | Arctiin, inulin (inulin content: 30–50% root DW; extract tested at 100–400 μg/mL, in vitro) | α-glucosidase IC50: 37.4 μg/mL (aqueous root extract) | β-sitosterol-D glucopyranoside inhibits α-glucosidase; inulin improves glucose tolerance [109,110] |
| Artemisia absinthium (Asteraceae), wormwood | Essential oil (0.5%) with myrcene, α-pinene, thujone, nerol, camphor, limonene, phellandrene, β-caryophyllene, sesquiterpene lactones (artabsin, absinthin) | Thujone, absinthin (oil: thujone 30–50%; extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 43.1 μg/mL (methanolic aerial part extract) | Hypoglycemic effects similar to metformin; stimulates glycogenogenesis [111,112] |
| Cichorium intybus (Asteraceae), chicory | Inulin, cichorin, chicoric acid, volatile oil, flavonoids, anthocyanins, bitter triterpenes (lactucopicrin), tannins, mineral salts | Inulin (root content: 15–20% DW; methanolic root extract tested at 100–400 μg/mL, in vitro) | α-glucosidase IC50: 45.5 μg/mL (methanolic root extract) | Improves glucose tolerance; reduces hepatic glucose-6-phosphatase activity [113,114] |
| Cynara scolymus (Asteraceae), artichoke | Polyphenols (caffeic acid, chlorogenic acid, cynarin), flavones, potassium, and magnesium salts | Cynarin, chlorogenic acid (leaf content: chlorogenic acid 1–2% DW; extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 36.5 μg/mL (methanolic leaf extract) | Insulin-mimetic, hypolipidemic, antioxidant effects [115,116] |
| Taraxacum officinale (Asteraceae), dandelion | Flavones (rutoside, hyperoside, quercetol), hydroxycinnamic acid derivatives (caffeic and chlorogenic acid), catechin tannins, sterols, triterpenes, carotenoids, coumarins, mucilage | Caffeic acid, inulin (root: inulin 15–25% DW; extract tested at 100–400 μg/mL, in vitro) | α-glucosidase IC50: 42.7 μg/mL (aqueous root extract) | Tannins reduce amylase activity by chelating calcium; hypolipidemic effects [117,118] |
| Sambucus nigra (Caprifoliaceae), elderberry | Anthocyanins, essential oil, quercetin derivatives, cyanogenic glycoside (sambunigrin), mucilage, flavonosides | Anthocyanins, flavonols (flower content: total anthocyanins ~200–400 mg/100g DW; extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 55.1 μg/mL (aqueous flower extract) | Insulin-like effect; increases insulin secretion and release [119,120] |
| Spinacia oleracea (Chenopodiaceae), spinach | Flavonoids (quercetin, myricetin, kaempferol, apigenin, luteolin, spinacetin), phenolic acids (ferulic, coumaric), carotenoids, vitamins (A, E, C, K, folic acid), minerals | Quercetin, kaempferol (leaf content: quercetin 10–30 mg/100 g FW; extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 62.3 μg/mL (aqueous leaf extract) | Potentiates insulin and protects β-pancreatic cells from oxidative damage similar to glibenclamide [121,122] |
| Juglans regia (Juglandaceae), walnut | Riboflavin, niacin, vitamin C, ellagic tannins, inositol, juglone, essential oil. The pericarp contains juglone (5-hydroxy-1,4-naphthoquinone), tannins, etheric oil, chlorophylls, starch, pectins, organic acids | Juglone, ellagitannins (leaf content: juglone ~20–50 mg/100 g; extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 35.2 μg/mL (methanolic leaf extract) | Increases tissue sensitivity to insulin, induces phosphorylation of insulin receptors, involved in GLUT-4 translocation, and inhibits the expression of certain genes [123,124] |
| Aloe vera, (Liliaceae), aloe | Aloe-emodin, aloin A and B, aloe-emodin, chrysophanol (free or glycosidic), resins (10–20%), essential oil in small quantities, mineral salts | Aloin, aloe-emodin (gel content: aloin 0.1–0.4%; extract tested at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 50.9 μg/mL (aqueous gel extract) | Reduces blood glucose in type 2 diabetes with insulin-like activity. Lowers blood lipids and triglycerides Enhances insulin effects and protects β-pancreatic cells from oxidative damage Hypoglycemic action similar to metformin Increases GLUT-4 mRNA synthesis Lowers TC, LDL, TG, and VLDL; increases hepatic glycogen; inhibits lipogenesis [125,126] |
| Asparagus officinalis (Liliaceae), asparagus | Asparagine, lipids, carbohydrates, phytohormones, enzymes, sterols, cellulose, mineral salts | Asparagine, saponins (root: asparagine ~0.03%; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 69.3 μg/mL (methanolic root extract) | Potentiates insulin and protects β-pancreatic cells from oxidative damage [127,128] |
| Allium cepa (Liliaceae), onion | Cycloalliin, methylalliin, propylalliin, cepaenes, flavonoid derivatives (quercetin and kaempferol glycosides), saponins, amines, enzymes | Quercetin, alliin (bulb: quercetin ~10–30 mg/100 g FW; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 40.1 μg/mL (aqueous bulb extract) | Hypoglycemic effect similar to glibenclamide and insulin [129,130] |
| Allium sativum (Liliaceae), garlic | Sulfur compounds; flavonosides; vitamins (A, B1, B2, C); phytosterol; glycerides of palmitic, stearic, oleic, linoleic, and myristic acids; allicin; steroid derivatives (erubosides) | Allicin, alliin (bulb: allicin ~0.1–0.5%; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 28.9 μg/mL (ethanolic bulb extract) | Hypoglycemic and hypolipidemic effects, similar to glibenclamide and insulin [131,132] |
| Viscum album (Loranthaceae), mistletoe | Triterpenic saponins, oleanolic acid derivatives, viscotoxin, viscol, amines (choline, acetylcholine), β-phenylethylamine, lipids, glycosidic substances | Oleanolic acid glycosides, viscotoxins (leaf: oleanolic acid ~0.2–0.4%; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 58.4 μg/mL (aqueous leaf extract) | Increases insulin secretion and peripheral glucose utilization; hypoglycemic effect comparable to glibenclamide [133,134] |
| Morus alba / nigra (Moraceae), mulberry | Citric, aspartic, folic, and folinic acids, volatile compounds, β-carotene, tannins, phenolic compounds, alkaloids, anthocyanins, minerals, vitamins C, B2, B3 | DNJ (1-deoxynojirimycin, leaf: 0.1–0.2%; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 32.1 μg/mL (methanolic leaf extract) | Stimulates cellular glucose uptake; insulin-mimetic effect [135,136] |
| Alchemilla vulgaris (Rosaceae), lady mantle | Ellagic tannins, polyphenolcarboxylic acids (chlorogenic acid), saponins, flavonoids, ellagic and luteic acid, fatty compounds (stearic and palmitic acids), phytosterols, mineral salts | Ellagitannins, chlorogenic acid (aerial part: ellagitannins ~1%; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 57.6 μg/mL (aqueous aerial part extract) | Improves glucose tolerance; anorexigenic effect [137,138] |
| Fragaria ananassa (Rosaceae), strawberry | Fragarol; oily substances; citric, malic, and ascorbic acids; anthocyanins; citrol; polyphenols; vitamins A, B, C | Anthocyanins, ellagic acid (fruit: anthocyanins 20–50 mg/100 g FW; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 47.9 μg/mL (methanolic fruit extract) | Inhibits α-glucosidase; antioxidant effect [139,140] |
| Rosa canina (Rosaceae), rosehip | Carotenoids, terpenoids, anthocyanins, vitamins C, B1, B2, PP, K | Ascorbic acid, flavonoids (fruit: ascorbic acid 0.3–0.7%; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 53.2 μg/mL (methanolic fruit extract) | Stimulates insulin secretion [141,142] |
| Agrimonia eupatoria (Rosaceae), common agrimony | Catechin-type tannins, gallotannins, ellagitannins, free quercetin, hyperin, rutin, apigenin and luteolin glycosides, bitter substances, traces of essential oil, ursolic acid, organic acids, mucilage, coumarins, vitamins (C, K), triterpenes, fatty acids, flavonoids, saponins | Catechin, ellagitannins (aerial part: ellagitannins ~0.8%; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 50.2 μg/mL (methanolic aerial part extract) | Stimulates glucose uptake in cultured muscle cells Increases insulin secretion via sulfonylurea-like mechanism [143,144] |
| Citrus aurantium (Rutaceae), bitter orange | Citric and malic acids, calcium and potassium citrate, vitamins A, B1, B2, B3, D, E, PP, essential oil (limonene, nerolidol, terpineol, farnesol, pinene, phellandrene), flavonoids | Hesperidin, synephrine (peel: hesperidin ~0.2–0.5%; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 54.5 μg/mL (ethanolic fruit peel extract) | Reduces insulin resistance, lowers LDL-cholesterol and triglycerides, antioxidant activity [145,146] |
| Lycopersicon esculentum (Solanaceae), tomato | Flavonoids, lycopene, organic acids (malic, pectic, citric), vitamins A, B1, B2, B6, C, PP, E, K, β-carotene, minerals | Lycopene, β-carotene (fruit: lycopene 2–5 mg/100g FW; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 60.1 μg/mL (methanolic fruit extract) | Stimulates insulin secretion, improves insulin resistance [147,148] |
| Urtica dioica (Urticaceae), stinging nettle | Polyphenols, amino acids, sterols, essential oil, sitosterols, ursolic acid, vitamins C, B2, K, chlorophylls, protoporphyrin, β-carotene, alkaloids | Phenolic compounds, sterols (leaf: total phenolics 20–40 mg/g DW; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 44.7 μg/mL (methanolic leaf extract) | Insulin-mimetic effect, reduces LDL-cholesterol [149] |
| Vitis vinifera (Vitaceae), grapevine | Polyphenols, resveratrol, anthocyanins, flavonosides, tartaric and malic acid, tannins, minerals, vitamins A, C, E | Resveratrol, proanthocyanidins (seed: proanthocyanidins 5–10%; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 38.6 μg/mL (methanolic seed extract) | Antioxidant, reduces oxidative stress, insulin-like effect, lowers glucose absorption, regenerates β-pancreatic cells [150,151] |
| Vaccinium arctostaphylos (Ericaceae), bearberry | Flavonoids (quercetin), tannins | Quercetin, arbutin (leaf: arbutin 5–7% DW; extract at 50–200 μg/mL, in vitro) | α-amylase IC50: 42.2 μg/mL (aqueous leaf extract) | Inhibits α-amylase [152,153] |
| Hippophae rhamnoides (Elaeagnaceae), sea buckthorn | Carotenoids, flavonoids, proanthocyanidins, catechin tannins, triterpenic acids, vitamin C | Carotenoids, flavonoids, vitamin C (fruit: carotenoids 3–8 mg/100g FW; extract at 50–200 μg/mL, in vitro) | α-glucosidase IC50: 48.3 μg/mL (ethanolic fruit extract) | Inhibits α-glucosidase [154,155] |
| Experiment Duration/Model | Plants Tested in This Model |
|---|---|
| Cell culture (glucose uptake/GLUT4, 24–48 h, insulin secretion) | Momordica charantia, Trigonella foenum-graecum, Pueraria lobata, Glycyrrhiza glabra, Phaseolus vulgaris, Agrimonia eupatoria, Spinacia oleracea, Juglans regia, (unele studii pentru Allium cepa, Allium sativum, Morus alba/nigra, Rosa canina, Apium graveolens, Silybum marianum, Cichorium intybus, Asparagus officinalis, Aloe vera, Urtica dioica, Vitis vinifera) |
| Enzyme inhibition (α-glucosidase/α-amylase, 30–60 min, in vitro enzyme assay) | Dillenia indica, Glycyrrhiza glabra, Phaseolus vulgaris, Trigonella foenum-graecum, Pueraria lobata, Apium graveolens, Daucus carota, Thymus vulgaris, Lavandula angustifolia, Salvia officinalis, Pimpinella anisum, Silybum marianum, Acorus calamus, Achillea millefolium, Arctium lappa, Artemisia absinthium, Cichorium intybus, Cynara scolymus, Taraxacum officinale, Sambucus nigra, Spinacia oleracea, Juglans regia, Urtica dioica, Vitis vinifera, Vaccinium arctostaphylos, Hippophae rhamnoides, Alchemilla vulgaris, Fragaria ananassa, Rosa canina, Citrus aurantium, Lycopersicon esculentum, Morus alba/nigra, Allium cepa, Allium sativum, Viscum album, Asparagus racemosus, Azadirachta indica, Garcinia cambogia, Cinnamomum zeylanicum, Panax quinquefolius, Phyllanthus emblica |
| Animal studies (in vivo, 2–8 weeks, effect on glycemia, β-cell, complications, etc.) | Momordica charantia, Trigonella foenum-graecum, Glycyrrhiza glabra, Silybum marianum, Achillea millefolium, Cynara scolymus, Taraxacum officinale, Sambucus nigra, Juglans regia, Urtica dioica, Rosa canina, Citrus aurantium, Hippophae rhamnoides, Allium cepa, Allium sativum, Viscum album, Azadirachta indica, Panax quinquefolius, Phyllanthus emblica, Cichorium intybus, Pueraria lobata, Spinacia oleracea, Alchemilla vulgaris, Fragaria ananassa, Agrimonia eupatoria, Asparagus racemosus, Morus alba/nigra, Acorus calamus, Daucus carota, Apium graveolens, Arctium lappa, Lavandula angustifolia, Salvia officinalis, Thymus vulgaris, Vaccinium arctostaphylos, Aloe vera, Vitis vinifera, Garcinia cambogia, Dillenia indica, Phyllanthus emblica, Pimpinella anisum, Thymus vulgaris |
| Human/clinical studies (weeks–months, when available) | Momordica charantia, Trigonella foenum-graecum, Silybum marianum, Cinnamomum zeylanicum, Allium sativum, Vitis vinifera, Salvia officinalis, Panax quinquefolius, Morus alba/nigra, Aloe vera, Glycyrrhiza glabra |
| Wild-Growing Plants in Romania (Native Wild Flora) | Not Wild-Growing but Cultivated in Romania: | Neither Wild-Growing nor Commonly Cultivated in Romania: |
|---|---|---|
|
|
|
| Molecular Target/Pathway | Medicinal Plants (Main Bioactive Compounds) |
|---|---|
| AMPK activation | Momordica charantia (charantin), Silybum marianum (silymarin), Vitis vinifera (resveratrol), Viscum album, Arctium lappa (oleanolic acid), Spinacia oleracea (quercetin), Pueraria lobata (puerarin), Glycyrrhiza glabra (possibly via anti-inflam.), Lavandula angustifolia (rosmarinic acid) |
| PPAR-γ modulation | Glycyrrhiza glabra (glycyrrhizin), Pueraria lobata (puerarin), Viscum album, Silybum marianum, Vitis vinifera |
| PI3K/Akt → GLUT4 translocation | Trigonella foenum-graecum (diosgenin), Momordica charantia (mcIRBP), Glycyrrhiza glabra, Pueraria lobata, Allium cepa (quercetin), Spinacia oleracea |
| α-glucosidase inhibition | Morus alba/nigra (DNJ), Phaseolus vulgaris (phaseolamin), Cinnamomum zeylanicum (cinnamaldehyde), Dillenia indica, Arctium lappa (arctiin), Vaccinium arctostaphylos, Salvia officinalis, Apium graveolens, Cichorium intybus, Silybum marianum, Acorus calamus, Achillea millefolium, Cynara scolymus, Taraxacum officinale, Sambucus nigra, Allium cepa, Urtica dioica, Fragaria ananassa, Rosa canina, Hippophae rhamnoides, Allium sativum, Azadirachta indica, Asparagus racemosus, Morus alba, Citrus aurantium, Phyllanthus emblica, Vitis vinifera, Garcinia cambogia, Panax quinquefolius, Aloe vera, Vitis vinifera |
| α-amylase inhibition | Dillenia indica, Phaseolus vulgaris, Taraxacum officinale, Garcinia cambogia, Vaccinium arctostaphylos, Azadirachta indica |
| Insulin receptor agonism/similar effect | Momordica charantia (mcIRBP, polypeptide-p), Allium sativum (allicin), Urtica dioica, Salvia officinalis, Citrus aurantium |
| DPP-IV inhibition | Trigonella foenum-graecum (trigonelline), Glycyrrhiza glabra, Salvia officinalis, Citrus aurantium |
| SGLT inhibition | Trigonella foenum-graecum (trigonelline), Morus alba (DNJ), Citrus aurantium |
| Antioxidant/Anti-inflammatory | Vitis vinifera, Sambucus nigra, Rosa canina, Spinacia oleracea, Lavandula angustifolia, Achillea millefolium, Thymus vulgaris, Fragaria ananassa, Phyllanthus emblica, Asparagus officinalis, Artemisia absinthium |
| β-cell regeneration/insulin secretion | Pueraria lobata, Sambucus nigra, Allium sativum, Salvia officinalis, Citrus aurantium, Agrimonia eupatoria, Artemisia absinthium |
| Miscellaneous (hepatic glucose regulation, lipid metabolism) | Cichorium intybus (hepatic effect), Garcinia cambogia (ATP citrate lyase), Cynara scolymus (lipid/glucose), Aloe vera (GLUT4), Hippophae rhamnoides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trasca, D.M.; Dop, D.; Stoica, G.-A.; Adrian, N.S.; Carmen, N.E.; Văruț, R.M.; Singer, C.E. The Antidiabetic Activity of Wild-Growing and Cultivated Medicinal Plants Used in Romania for Diabetes Mellitus Management: A Phytochemical and Pharmacological Review. Pharmaceuticals 2025, 18, 1035. https://doi.org/10.3390/ph18071035
Trasca DM, Dop D, Stoica G-A, Adrian NS, Carmen NE, Văruț RM, Singer CE. The Antidiabetic Activity of Wild-Growing and Cultivated Medicinal Plants Used in Romania for Diabetes Mellitus Management: A Phytochemical and Pharmacological Review. Pharmaceuticals. 2025; 18(7):1035. https://doi.org/10.3390/ph18071035
Chicago/Turabian StyleTrasca, Diana Maria, Dalia Dop, George-Alin Stoica, Niculescu Stefan Adrian, Niculescu Elena Carmen, Renata Maria Văruț, and Cristina Elena Singer. 2025. "The Antidiabetic Activity of Wild-Growing and Cultivated Medicinal Plants Used in Romania for Diabetes Mellitus Management: A Phytochemical and Pharmacological Review" Pharmaceuticals 18, no. 7: 1035. https://doi.org/10.3390/ph18071035
APA StyleTrasca, D. M., Dop, D., Stoica, G.-A., Adrian, N. S., Carmen, N. E., Văruț, R. M., & Singer, C. E. (2025). The Antidiabetic Activity of Wild-Growing and Cultivated Medicinal Plants Used in Romania for Diabetes Mellitus Management: A Phytochemical and Pharmacological Review. Pharmaceuticals, 18(7), 1035. https://doi.org/10.3390/ph18071035







