In Vivo Evaluation of the Analgesic and Anti-Inflammatory Activity of Thymus numidicus Essential Oil
Abstract
1. Introduction
2. Results and Discussion
2.1. GC-MS Analysis of Isolated EOs
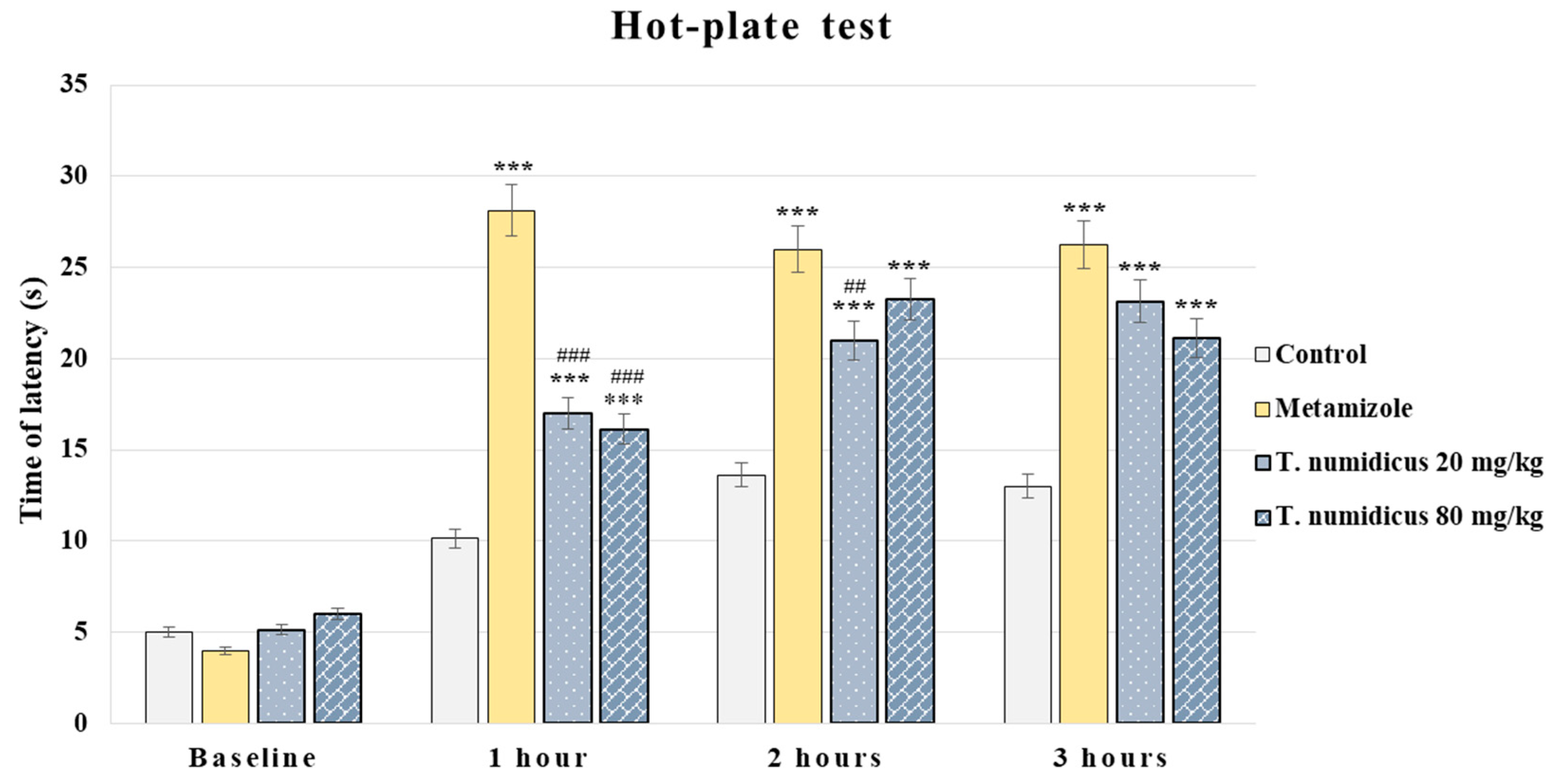
2.2. Analgesic Effect of T. numidicus EO 20 mg/kg and 80 mg/kg After per os Treatment in the Hot-Plate Test in Naïve Animals
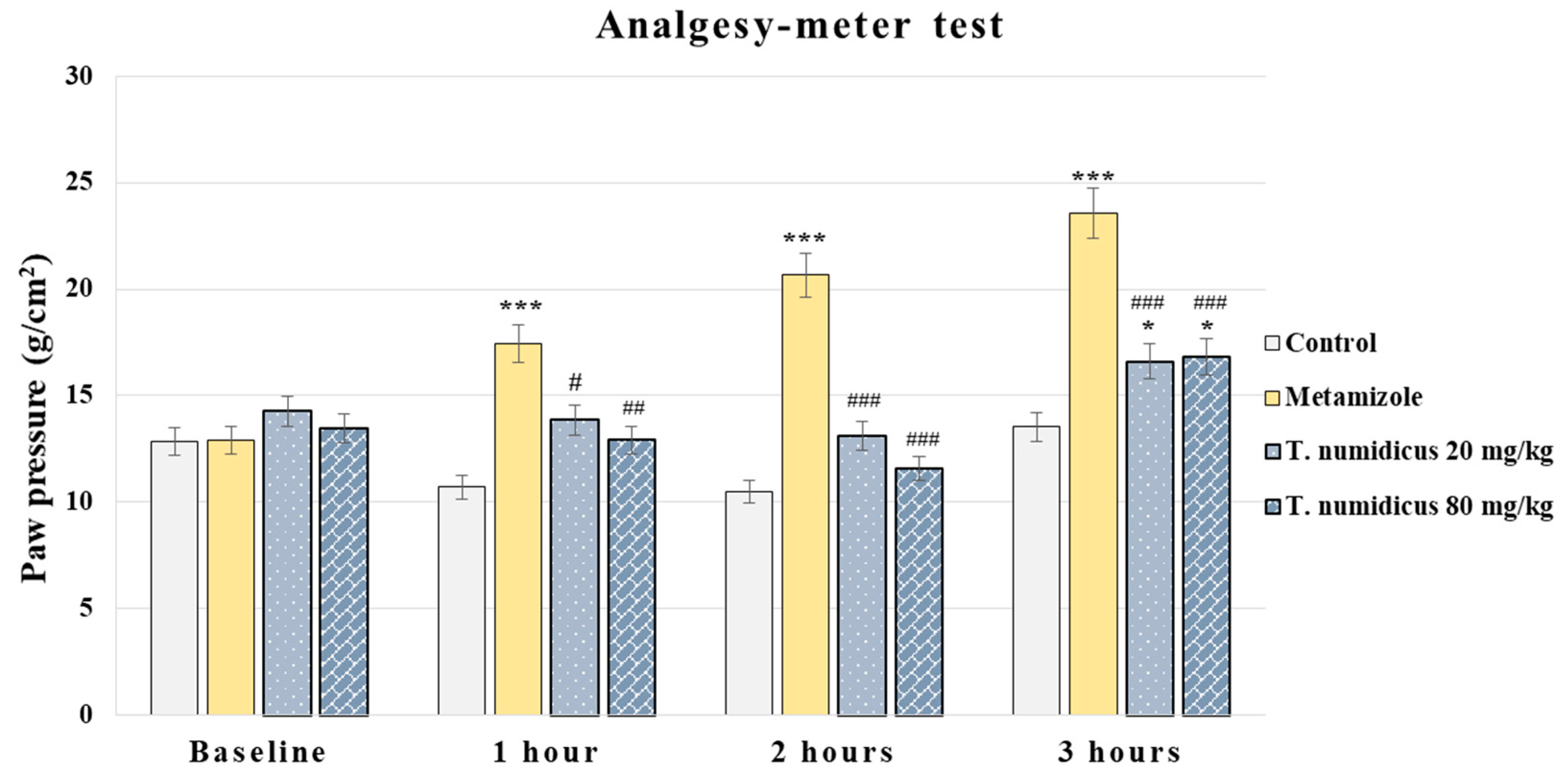
2.2.1. Analgesic Effect of T. numidicus EO 20 mg/kg and 80 mg/kg After per os Treatment in the Paw Pressure Test (Randall–Selitto Method) in Naïve Animals
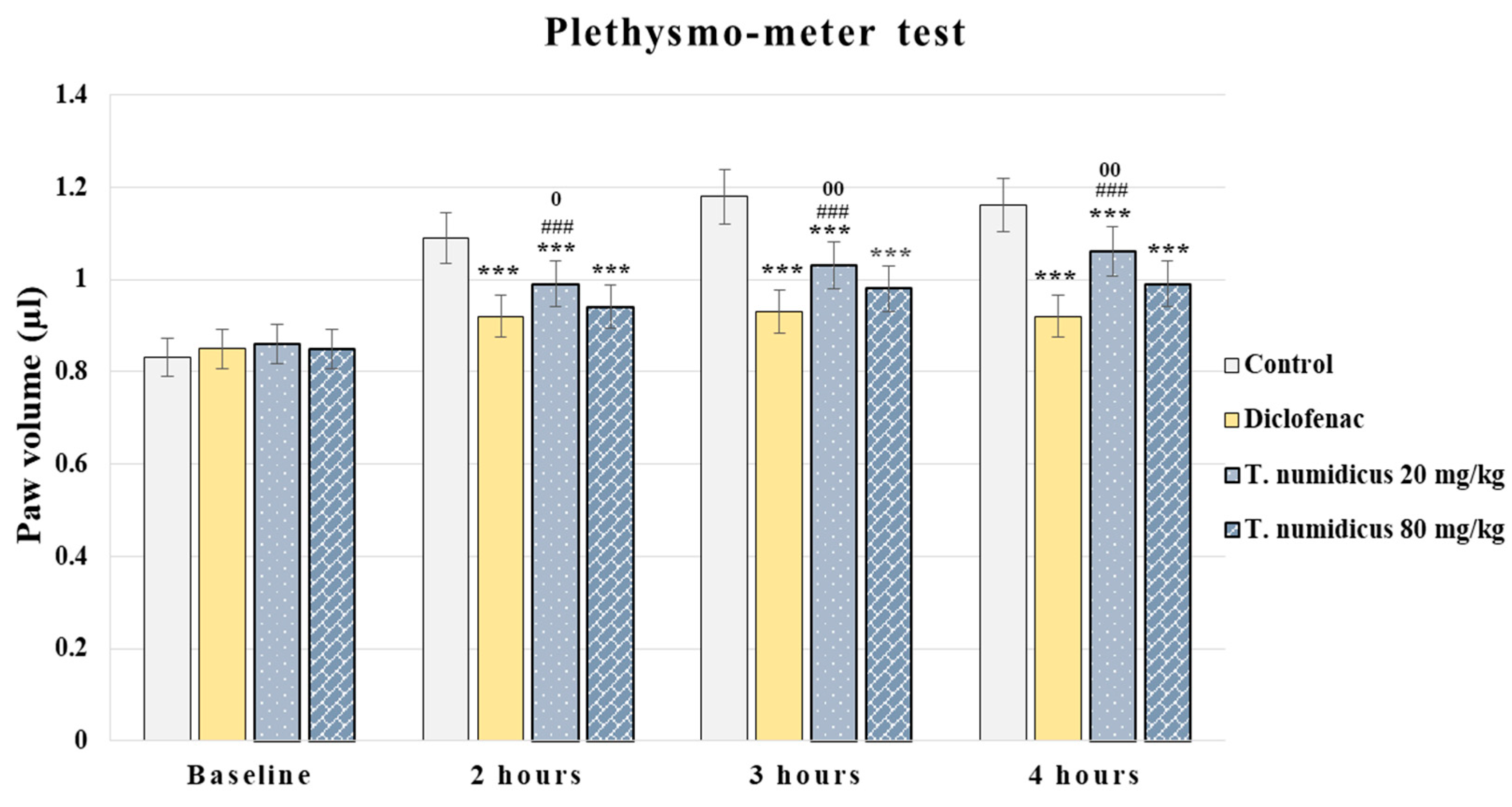
2.2.2. Anti-Inflammatory Effect of T. numidicus EO 20 mg/kg and 80 mg/kg after per os Treatment in the Plethysmometer Test in Naïve Animals
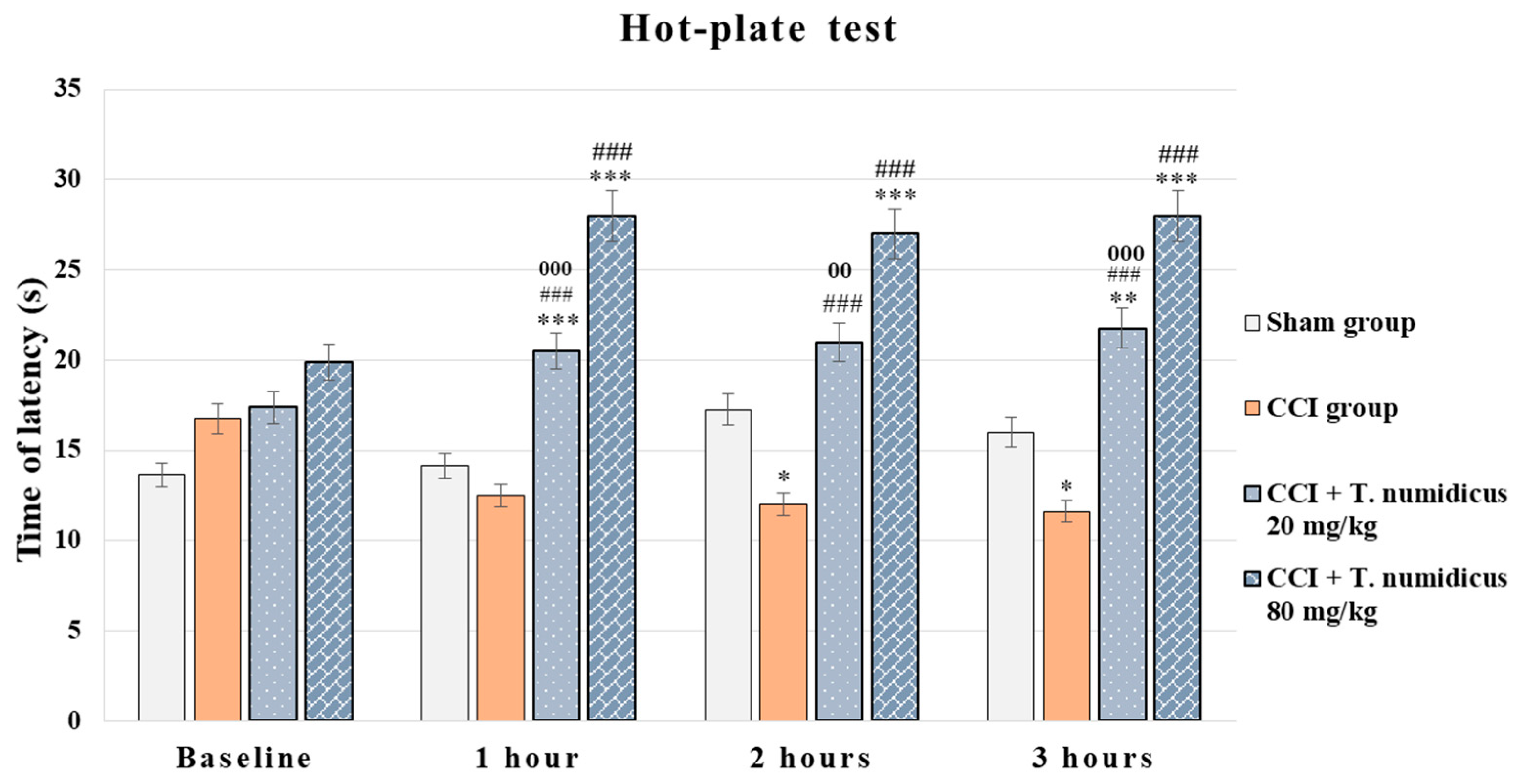
2.2.3. Neuropathic Pain Model Effect of T. numidicus EO 20 mg/kg and 80 mg/kg After per os Treatment on CCI-Induced Thermal Hyperalgesia in Rats
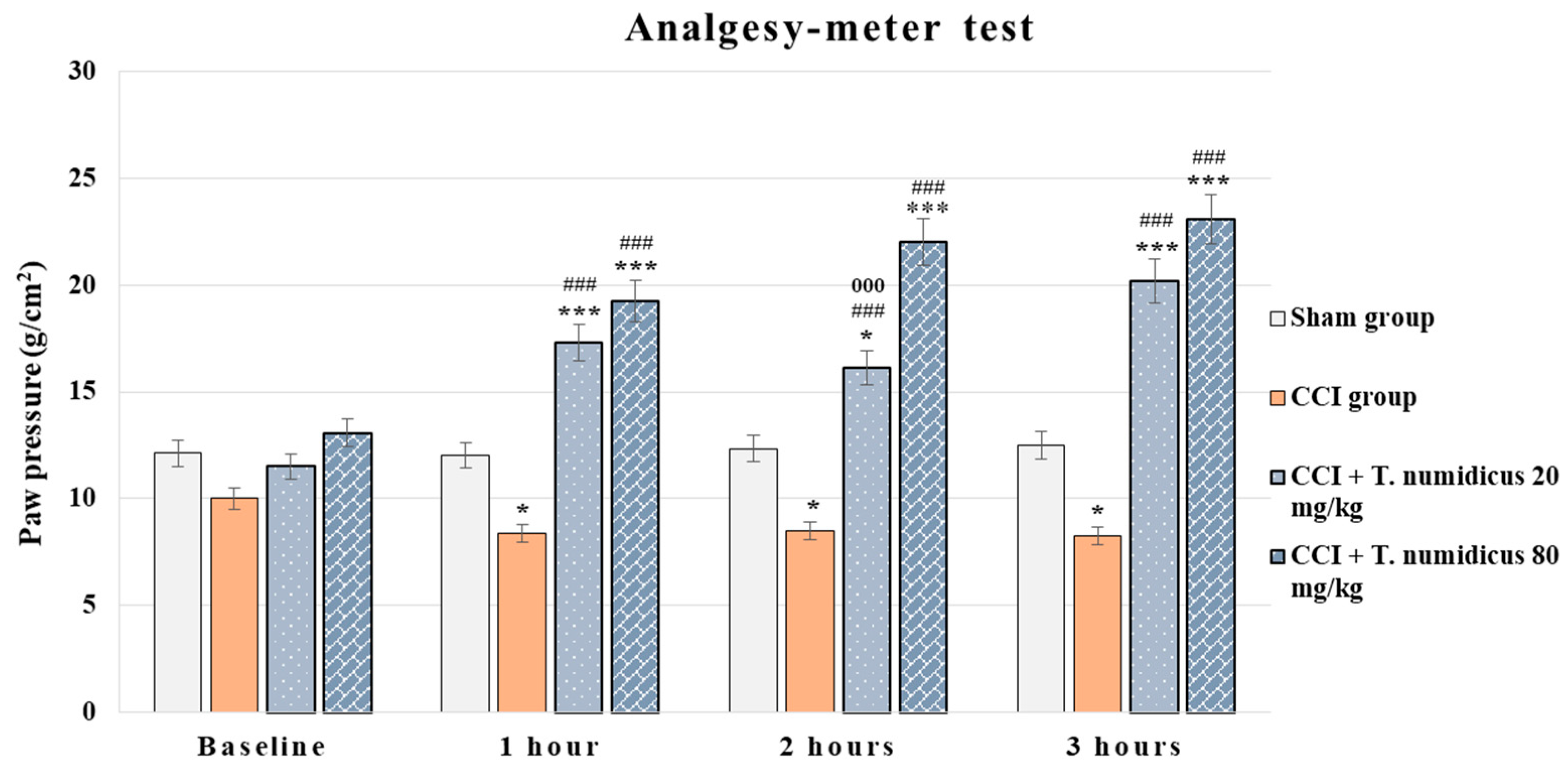
2.2.4. Effect of T. numidicus EO 20 mg/kg and 80 mg/kg after per os Treatment on CCI-Induced Mechanical Allodynia in Rats
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and Oil Extraction
3.3. Chromatographic Conditions
3.4. In Vivo Experiments
3.4.1. Animals
3.4.2. Experimental Groups
- ○
- 1st group Control-Saline 1 mL/kg b.w. per os;
- ○
- 2nd group-metamizole, 150 mg/kg b.w. i. p.—standard for a drug with analgesic effect;
- ○
- 3rd group-T. numidicus EO 20 mg/kg b.w. per os;
- ○
- 4th group-T. numidicus EO 80 mg/kg b.w. per os.
- ○
- 1st group Control-Saline 1 mL/kg b.w. per os;
- ○
- 2nd group-diclofenac, 25 mg/kg b.w. i. p., positive control—standard for a drug with anti-inflammatory effect;
- ○
- 3d group-T. numidicus EO, 20 mg/kg b.w. per os;
- ○
- 4th group-T. numidicus EO, 80 mg/kg b.w. per os.
- ○
- 1st group sham control-operated animals without ligation of the sciatic nerve, which were administered saline solution 1 mL/kg b.w. per os;
- ○
- 2nd group CCI group-operated animals with ligation of sciatic nerve, which were administered saline solution 1 mL/kg b.w. per os;
- ○
- 3rd group-operated animals with ligation of sciatic nerve, which were administered T. numidicus EO in a dose of 20 mg/kg b.w. per os;
- ○
- 4th group-operated animals with ligation of sciatic nerve, which were administered T. numidicus EO in a dose of 80 mg/kg b.w. per os.
3.4.3. Experimental Methods
Hot-Plate Test for Analgesic Activity
Nociceptive Test Using Mechanical Pressure to Assess the Analgesic Effect
Neuropathic Pain Test to Investigate the Analgesic Effect
Method for Testing Anti-Inflammatory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | Essential oil |
| MH | Monoterpene hydrocarbons |
| MO | Oxygenated monoterpene |
| SH | Sesquiterpene hydrocarbons |
| SO | Oxygenated sesquiterpene |
| O | Others |
| tr | traces |
| RIc | retention index calculated |
| RIl | retention index literature |
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Oubihi, A.; Ballaoui, F.Z.; Imtara, H.; Jaber, H.; Ettouil, A.; Haida, S.; Ouhssine, M.; Noman, O.M.; Mothana, R.A.; Tarayrah, M.; et al. Phytochemical Compounds, Acute Toxicity, Anti-Inflammatory and Antioxidant Activities of Thymus Leptobotrys Murb Essential Oil. Molecules 2023, 28, 1355. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. Biological Activities of Three Essential Oils of the Lamiaceae Family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, M.É.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential Oils from Neotropical Myrtaceae: Chemical Diversity and Biological Properties. Chem. Biodivers. 2011, 8, 73–94. [Google Scholar] [CrossRef]
- Xavier, J.K.A.M.; Alves, N.S.F.; Setzer, W.N.; da Silva, J.K.R. Chemical Diversity and Biological Activities of Essential Oils from Licaria, Nectrandra and Ocotea Species (Lauraceae) with Occurrence in Brazilian Biomes. Biomolecules 2020, 10, 869. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Barral, M.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Otero, P.; Prieto, M.A.; Simal-Gandara, J. Traditional Plants from Asteraceae Family as Potential Candidates for Functional Food Industry. Food Funct. 2021, 12, 2850–2873. [Google Scholar] [CrossRef]
- Hélène, I. Le Marché des Plantes Aromatiques et Médicinales: Analyse Des Tendances Du Marché Mondial et Des Stratégies Économiques En Albanie et En Algérie; Serie B: Etude et rechrche. 2016. Available online: https://hal.science/hal-02153288 (accessed on 27 May 2025).
- da Silva, L.R.R.; Ferreira, O.O.; Cruz, J.N.; Franco, C.d.J.P.; dos Anjos, O.T.; Cascaes, M.M.; da Costa, W.A.; Andrade, E.H.d.A.; Oliveira, M.S. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. Evid.-Based Complement. Altern. Med 2021, 2021, 6748052. [Google Scholar] [CrossRef]
- Kaki, F.A.; Benkiniouar, R.; Touil, A.; Demirtas, I.; Merzoug, A.; Khattabi, L. Thymus Numidicus: Phenolic Constituents, Antibacterial, and Antioxidant Activities of Butanolic Extract. Environ. Exp. Biol. 2021, 19, 67–72. [Google Scholar] [CrossRef]
- Touhami, A.; Chefrour, A.; Khellaf, N.; Bukhari, A.; Fadel, I. Phytochemical Characterization of the Essential Oils Obtained from Mediterranean Thymus spp. (Lamiacea) Harvested at Different Stages of Growth. J. Pharm. Pharmacol. 2017, 5, 37–45. [Google Scholar] [CrossRef]
- Azaz, A.D.; Irtem, H.A.; Kurkcuoǧlu, M.; Can Baser, K.H. Composition and the in Vitro Antimicrobial Activities of the Essential Oils of Some Thymus Species. Z. Naturforsch. C 2004, 59, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the Essential Oils of Thymus and Origanum Species from Algeria and Their Antioxidant and Antimicrobial Activities. J. Agric. Food Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef]
- Benayache, F.; Chalard, P.; Figueredo, G.; Benayache, F.; Benayache, S. Chemical Composition of the Essential of Thymus Numidicus Poiret. Pharm. Lett. 2014, 6, 182–185. [Google Scholar]
- Ghorab, H.; Kabouche, A.; Kabouche, Z. Comparative Compositions of Essential Oils of Thymus Growing in Various Soils and Climates of North Africa. Sahara 2014, 355, 13. [Google Scholar]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, Thyme, and Other Plant Sources: Health and Potential Uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Ardasheva, R.; Popov, V.; Yotov, V.; Prissadova, N.; Pencheva, M.; Slavova, I.; Turiyski, V.; Krastev, A. Accelerated Electron Ionization-Induced Changes in the Myenteric Plexus of the Rat Stomach. Int. J. Mol. Sci. 2024, 25, 6807. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, P.; Zaytseva, E.; Ardasheva, R.; Turiyski, V.I. Clostridium Difficile Toxins Impact on Rat Colon Smooth Muscle Reactivity. Folia Medica 2023, 65, 116–123. [Google Scholar] [CrossRef]
- Kabouche, Z.; Boutaghane, N.; Laggoune, S.; Kabouche, A.; Ait-Kaki, Z.; Benlabed, K. Comparative Antibacterial Activity of Five Lamiaceae Essential Oils from Algeria. Int. J. Aromather. 2005, 15, 129–133. [Google Scholar] [CrossRef]
- Hadef, Y.; Kaloustian, J.; Chefrour, A.; Mikail, C.; Abou, L.; Giodani, R.; Nicolay, A.; Portugal, H. Chemical Composition and Variability of the Essential Oil of Thymus Numidicus Poir. from Algeria. Acta Bot. Gall. 2007, 154, 265–274. [Google Scholar] [CrossRef]
- Messara, Y.; Fernane, F.; Meddour, R. Chemical Composition, Antibacterial, and Antifungal Activities of the Essential Oil of Thymus Numidicus Poiret from Algeria. Phytothérapie 2018, 16, 163–168. [Google Scholar] [CrossRef]
- Can Baser, K.H. Biological and Pharmacological Activities of Carvacrol and Carvacrol Bearing Essential Oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and Human Health: A Comprehensive Review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Sá, R.D.C.d.S.e.; Andrade, L.N.; Oliveira, R.D.R.B.d.; De Sousa, D. A Review on Anti-Inflammatory Activity of Phenylpropanoids Found in Essential Oils. Molecules 2014, 19, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Gvozdeva, Y.; Staynova, R. pH-Dependent Drug Delivery Systems for Ulcerative Colitis Treatment. Pharmaceutics 2025, 17, 226. [Google Scholar] [CrossRef]
- Kokova, V.; Apostolova, E. Effect of Etifoxine on Locomotor Activity and Passive Learning in Rats with Diazepam-Induced Cognitive Deficit. Sci. Pharm. 2023, 91, 25. [Google Scholar] [CrossRef]
- Pérez, G.S.; Zavala, S.M.; Arias, G.L.; Ramos, L.M. Anti-Inflammatory Activity of Some Essential Oils. J. Essent. Oil Res. 2011, 23, 38–44. [Google Scholar] [CrossRef]
- Stavrakeva, K.; Popova, M.; Esad, M.; Kokova, V.; Bacelova, M.; Alakidi, A.; Bivolarska, A.; Apostolova, E. Drug-Induced Liver Toxicity. Acta Medica Bulg. 2024, 51, 77–85. [Google Scholar] [CrossRef]
- Senatore, F. Influence of Harvesting Time on Yield and Composition of the Essential Oil of a Thyme (Thymus pulegioides L.) Growing Wild in Campania (Southern Italy). J. Agric. Food Chem. 1996, 44, 1327–1332. [Google Scholar] [CrossRef]
- Saidj, F.; Rezzoug, S.-A.; Bentahar, F.; Boutekedjiret, C. Chemical Composition and Insecticidal Properties of Thymus Numidicus (Poiret) Essential Oil from Algeria. J. Essent. Oil Bear. Plants 2008, 11, 397–405. [Google Scholar] [CrossRef]
- Laouer, H.; Boulaacheb, N.; Akkal, S.; Meierhenrich, U.J.; Baldovini, N.; Prado, S. Composition and In Vitro Antimicrobial Activities of the Essential Oils of Two Populations of Thymus Numidicus Poiret. J. Essent. Oil Res. 2009, 21, 374–377. [Google Scholar] [CrossRef]
- Bendjabeur, S.; Bensouici, C.; Hazzit, M. Study of Chemical Composition, Anticholinesterase and Antioxidant Properties of Essential Oil and Ethanolic Extract from Thymus Numidicus Poiret, an Algerian Endemic Medicinal Plant. Pharm. Chem. J. 2023, 57, 858–868. [Google Scholar] [CrossRef]
- Bekka-Hadji, F.; Bombarda, I.; Touati, A. Antibacterial Activity against Methicillin-Resistant Staphylococcus Aureus of Five Essential Oils from Algerian Medicinal Plants (Lamiaceae). J. Essent. Oil Res. 2016, 28, 518–527. [Google Scholar] [CrossRef]
- Gouyon, P.H.; Vernet, P.; Guillerm, J.L.; Valdeyron, G. Polymorphisms and Environment: The Adaptive Value of the Oil Polymorphisms in Thymus vulgaris L. Heredity 1986, 57, 59–66. [Google Scholar] [CrossRef]
- Elaissi, A.; Elsharkawy, E.; El Mokni, R.; Debbabi, H.; Brighenti, V.; Nardoni, S.; Pellati, F.; Hammami, S. Chemical Composition, Antifungal and Antiproliferative Activities of Essential Oils from Thymus numidicus L. Nat. Prod. Res. 2021, 35, 5888–5893. [Google Scholar] [CrossRef]
- Chaouchi, O.; Fernane, F.; Zerrouki, N.D.; Issad, H.A.; Chaouchi, T.; Zidane, A.; Houali, K. Protective Effects of Lavandula Stoechas and Thymus Numidicus Essential Oils against Deltamethrin-Induced Hematological and Biochemical Toxicity in Female Rabbits. Toxicon 2025, 258, 108309. [Google Scholar] [CrossRef]
- Gonçalves, J.C.R.; De Meneses, D.A.; de Vasconcelos, A.P.; Piauilino, C.A.; Almeida, F.R.D.C.; Napoli, E.M.; Ruberto, G.; de Araujo, D.A.M. Essential Oil Composition and Antinociceptive Activity of Thymus Capitatus. Pharm. Biol. 2017, 55, 782–786. [Google Scholar] [CrossRef]
- Bardoni, R. Role of Presynaptic Glutamate Receptors in Pain Transmission at the Spinal Cord Level. Curr. Neuropharmacol. 2013, 11, 477–483. [Google Scholar] [CrossRef]
- Mohammadifard, F.; Alimohammadi, S. Chemical Composition and Role of Opioidergic System in Antinociceptive Effect of Ziziphora Clinopodioides Essential Oil. Basic Clin. Neurosci. 2018, 9, 357–366. [Google Scholar] [CrossRef]
- Klein, A.H.; Trannyguen, M.; Joe, C.L.; Iodi, C.M.; Carstens, E. Thermosensitive Transient Receptor Potential (TRP) Channel Agonists and Their Role in Mechanical, Thermal and Nociceptive Sensations as Assessed Using Animal Models. Chemosens. Percept. 2015, 8, 96–108. [Google Scholar] [CrossRef]
- Haeseler, G.; Maue, D.; Grosskreutz, J.; Bufler, J.; Nentwig, B.; Piepenbrock, S.; Dengler, R.; Leuwer, M. Voltage-Dependent Block of Neuronal and Skeletal Muscle Sodium Channels by Thymol and Menthol. Eur. J. Anaesthesiol. 2002, 19, 571–579. [Google Scholar] [CrossRef]
- Sarmento-Neto, J.F.; do Nascimento, L.G.; Felipe, C.F.B.; de Sousa, D.P. Analgesic Potential of Essential Oils. Molecules 2015, 21, 20. [Google Scholar] [CrossRef]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.-Y.; Xie, T.; Bai, R. Natural Terpenoids with Anti-Inflammatory Activities: Potential Leads for Anti-Inflammatory Drug Discovery. Bioorg. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Mrabti, N.N.; Ouassou, H.; Sheikh, R.A.; Assaggaf, H.; Bakrim, S.; Abdallah, E.M.; Alshahrani, M.M.; Al Awadh, A.A.; Lee, L.-H.; et al. Chemical Composition and Antioxidant, Antimicrobial, and Anti-Inflammatory Properties of Origanum Compactum Benth Essential Oils from Two Regions: In Vitro and In Vivo Evidence and In Silico Molecular Investigations. Molecules 2022, 27, 7329. [Google Scholar] [CrossRef] [PubMed]
- Landa, P.; Kokoska, L.; Pribylova, M.; Vanek, T.; Marsik, P. In Vitro Anti-Inflammatory Activity of Carvacrol: Inhibitory Effect on COX-2 Catalyzed Prostaglandin E2 Biosynthesisb. Arch. Pharm. Res. 2009, 32, 75–78. [Google Scholar] [CrossRef]
- Amanlou, M.; Dadkhah, F.; Salehnia, A.; Farsam, H.; Dehpour, A.R. An Anti-Inflammatory and Anti-Nociceptive Effects of Hydroalcoholic Extract of Satureja Khuzistanica Jamzad Extract. J. Pharm. Pharm. Sci. 2005, 8, 102–106. [Google Scholar] [PubMed]
- Chiu, Y.-J.; Huang, T.-H.; Chiu, C.-S.; Lu, T.-C.; Chen, Y.-W.; Peng, W.-H.; Chen, C.-Y. Analgesic and Antiinflammatory Activities of the Aqueous Extract from Plectranthus Amboinicus (Lour.) Spreng. Both In Vitro and In Vivo. Evid.-Based Complement. Alternat. Med. 2012, 2012, 508137. [Google Scholar] [CrossRef]
- Souza, A.C.A.; Abreu, F.F.; Diniz, L.R.L.; Grespan, R.; DeSantana, J.M.; Quintans-Júnior, L.J.; Menezes, P.P.; Araújo, A.A.S.; Correa, C.B.; Teixeira, S.A.; et al. The Inclusion Complex of Carvacrol and β-Cyclodextrin Reduces Acute Skeletal Muscle Inflammation and Nociception in Rats. Pharmacol. Rep. 2018, 70, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Sajjadi, S.E.; Zomorodkia, M. Antinociceptive and Anti-Inflammatory Activities of Bunium Persicum Essential Oil, Hydroalcoholic and Polyphenolic Extracts in Animal Models. Pharm. Biol. 2011, 49, 146–151. [Google Scholar] [CrossRef]
- Abbasi, Z.; Baluchnejadmojarad, T.; Roghani, M.; Susanabadi, A.; Farbin, M.; Mehrabi, S. Acamprosate Effect on Neuropathic Pain in Rats: With Emphasis on the Role of ERK/MAPK Pathway and SCN9A Sodium Channel. J. Chem. Neuroanat. 2023, 131, 102282. [Google Scholar] [CrossRef]
- Arruri, V.K.; Gundu, C.; Kalvala, A.K.; Sherkhane, B.; Khatri, D.K.; Singh, S.B. Carvacrol Abates NLRP3 Inflammasome Activation by Augmenting Keap1/Nrf-2/P62 Directed Autophagy and Mitochondrial Quality Control in Neuropathic Pain. Nutr. Neurosci. 2022, 25, 1731–1746. [Google Scholar] [CrossRef]
- Bilbrey, J.A.; Ortiz, Y.T.; Felix, J.S.; McMahon, L.R.; Wilkerson, J.L. Evaluation of the Terpenes β-Caryophyllene, α-Terpineol, and γ-Terpinene in the Mouse Chronic Constriction Injury Model of Neuropathic Pain: Possible Cannabinoid Receptor Involvement. Psychopharmacology 2022, 239, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Linstrom, P. NIST Chemistry WebBook; NIST Standard Reference Database 69. 1997. Available online: https://webbook.nist.gov/chemistry/ (accessed on 27 May 2025).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass spectrometryADAMS, 4th ed.; Allured Publ.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Bennett, G.J.; Xie, Y.-K. A Peripheral Mononeuropathy in Rat That Produces Disorders of Pain Sensation like Those Seen in Man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.O.; Selitto, J.J. A Method for Measurement of Analgesic Activity on Inflamed Tissue. Arch. Int. Pharmacodyn. Ther. 1957, 111, 409–419. [Google Scholar] [PubMed]
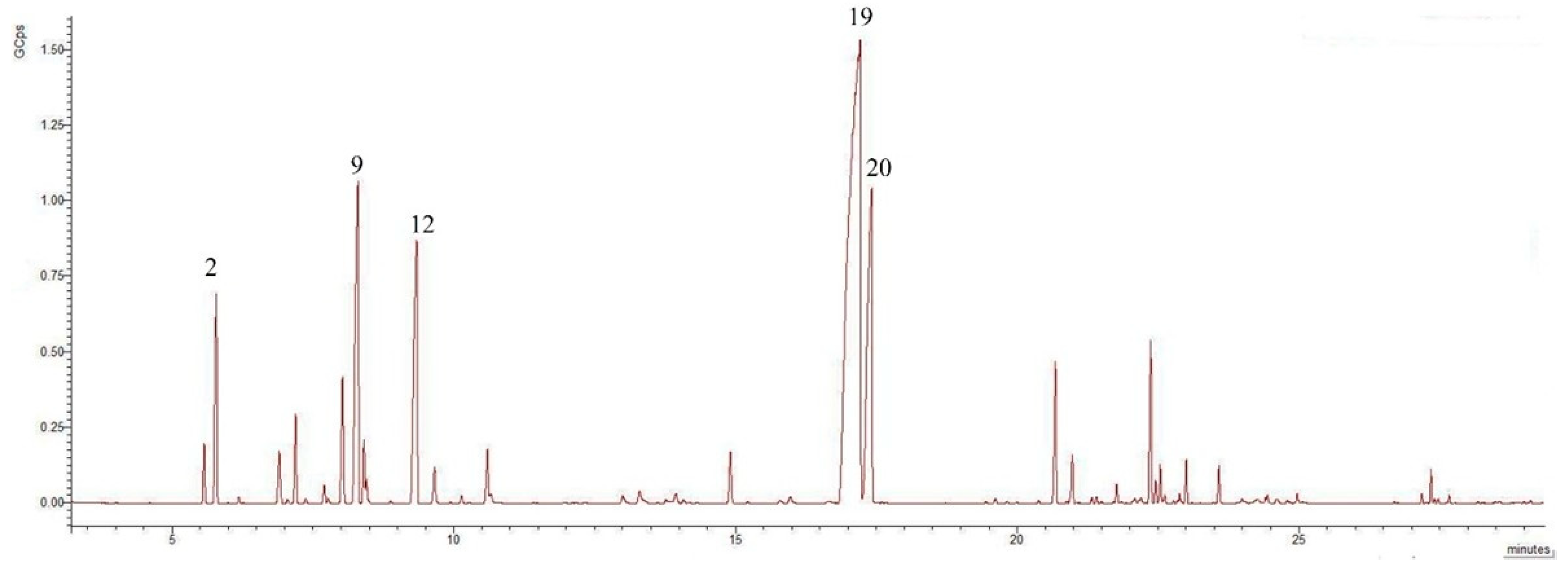
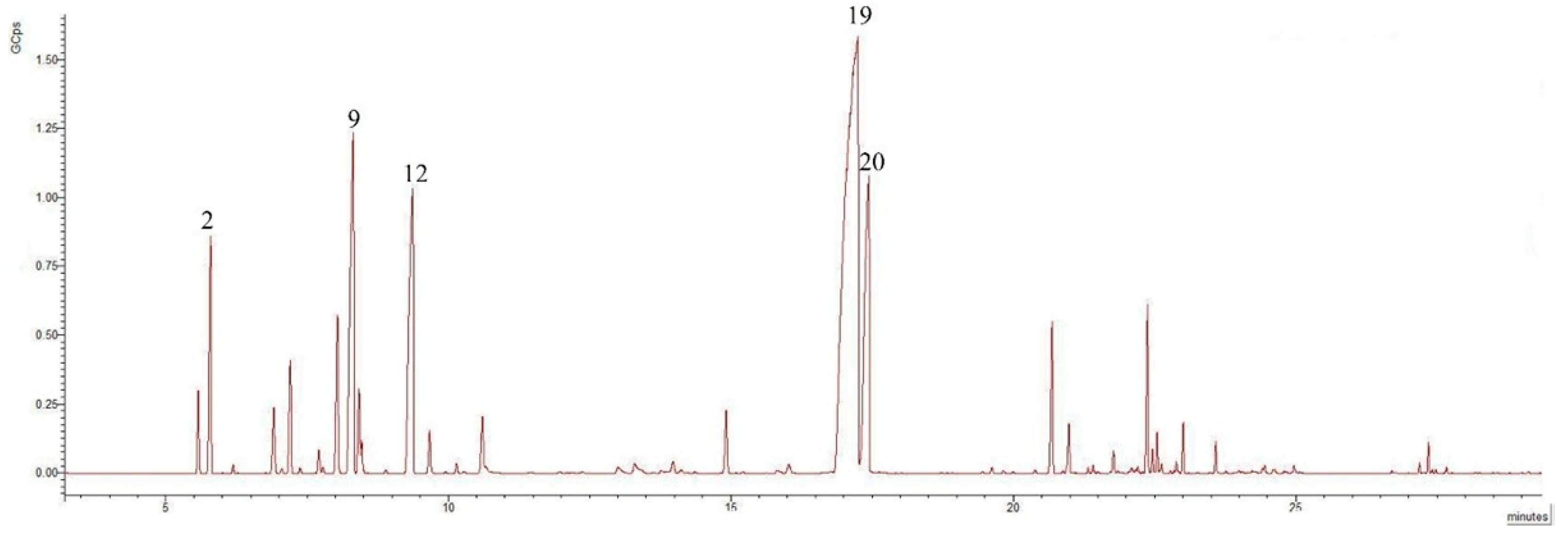
| No. | Compounds | Formula | Class | RIc | RIl | % of Total-Leaves | % of Total-Flowers | p-Value |
|---|---|---|---|---|---|---|---|---|
| 1 | α-Thujene | C10H16 | MH | 929 | 924 | 0.86 | 1.04 | 0.078 |
| 2 | α-Pinene | C10H16 | MH | 933 | 936 | 3.72 | 4.09 | 0.188 |
| 3 | Camphene | C10H16 | MH | 950 | 950 | tr | tr | 1.000 |
| 4 | 1-Octen-3-ol | C8H16O | O | 977 | 980 | 1.05 | 1.13 | 0.001 *** |
| 5 | (−)-β-Pinene | C10H16 | MH | 982 | 984 | 1.45 | 1.78 | 0.030 * |
| 6 | 3-Octanol | C8H18O | O | 987 | 988 | tr | ND | 0.005 ** |
| 7 | α-Phellandrene | C8H16 | MH | 1009 | 1008 | 0.29 | 0.34 | 0.046 * |
| 8 | α-Terpinene | C10H16 | MH | 1017 | 1015 | 2.41 | 2.83 | 0.036 * |
| 9 | p-Cymene | C10H14 | MH | 1019 | 1020 | 9.12 | 10.10 | 0.067 |
| 10 | D-Limonene | C10H16 | MH | 1020 | 1024 | 1.03 | 1.83 | 0.030 * |
| 11 | β-Phellandrene | C10H16 | MH | 1022 | 1025 | 0.37 | 0.42 | 0.046 * |
| 12 | γ-Terpinene | C10H16 | MH | 1050 | 1057 | 8.43 | 9.70 | 0.015 * |
| 13 | trans-β-Terpineol | C10H18O | MO | 1061 | 1159 | 0.67 | 0.70 | 0.194 |
| 14 | Linalool | C10H18O | MO | 1090 | 1099 | 1.06 | 0.96 | 0.009 ** |
| 15 | Endoborneol | C10H18O | MO | 1162 | 1165 | tr | tr | 1.000 |
| 16 | L-terpinen-4-ol | C10H18O | MO | 1176 | 1179 | 0.62 | tr | 0.122 |
| 17 | L-α-Terpineol | C10H18O | MO | 1190 | 1186 | 0.30 | 0.31 | 0.614 |
| 18 | Thymol methyl ether | C11H16O | MO | 1229 | 1237 | 1.00 | 1.05 | 0.023 * |
| 19 | Thymol | C10H14O | MO | 1298 | 1301 | 47.13 | 45.37 | 0.069 |
| 20 | Carvacrol | C10H14O | MO | 1300 | 1298 | 12.75 | 10.86 | 0.004 ** |
| 21 | Caryophyllene | C15H24 | SH | 1414 | 1417 | 2.41 | 2.46 | 0.434 |
| 22 | γ-Muurolene | C15H24 | SH | 1471 | 1476 | 0.26 | 0.37 | 0.100 |
| 23 | β-Bisabolene | C15H24 | SH | 1486 | 1481 | 2.46 | 2.35 | 0.244 |
| 24 | γ-Cadinene | C15H24 | SH | 1510 | 1513 | 0.54 | 0.29 | 0.904 |
| 25 | δ-Cadinene | C15H24 | SH | 1519 | 1522 | 0.57 | 0.45 | 0.380 |
| 26 | Caryophyllene oxide | C15H24O | SO | 1576 | 1578 | 0.50 | 0.36 | 0.005 ** |
| Monoterpene hydrocarbons | 27.68 | 32.13 | 0.049 * | |||||
| Oxygenated monoterpene | 63.53 | 59.25 | 0.021 * | |||||
| Sesquiterpene hydrocarbons | 6.24 | 5.92 | 0.417 | |||||
| Oxygenated sesquiterpene | 0.50 | 0.36 | 0.005 ** | |||||
| Others | 1.05 | 1.13 | 0.028 * | |||||
| Total | 99.00 | 98.79 | 0.677 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaouchi, O.; Todorova, V.; Ivanova, S.; Dzhambazova, E.; Fernane, F.; Zerrouki, N.D.; Peychev, L.; Saracheva, K.; Shishmanova-Doseva, M.; Peychev, Z. In Vivo Evaluation of the Analgesic and Anti-Inflammatory Activity of Thymus numidicus Essential Oil. Pharmaceuticals 2025, 18, 1031. https://doi.org/10.3390/ph18071031
Chaouchi O, Todorova V, Ivanova S, Dzhambazova E, Fernane F, Zerrouki ND, Peychev L, Saracheva K, Shishmanova-Doseva M, Peychev Z. In Vivo Evaluation of the Analgesic and Anti-Inflammatory Activity of Thymus numidicus Essential Oil. Pharmaceuticals. 2025; 18(7):1031. https://doi.org/10.3390/ph18071031
Chicago/Turabian StyleChaouchi, Ouardia, Velislava Todorova, Stanislava Ivanova, Elizabet Dzhambazova, Farida Fernane, Nacira Daoudi Zerrouki, Lyudmil Peychev, Kremena Saracheva, Michaela Shishmanova-Doseva, and Zhivko Peychev. 2025. "In Vivo Evaluation of the Analgesic and Anti-Inflammatory Activity of Thymus numidicus Essential Oil" Pharmaceuticals 18, no. 7: 1031. https://doi.org/10.3390/ph18071031
APA StyleChaouchi, O., Todorova, V., Ivanova, S., Dzhambazova, E., Fernane, F., Zerrouki, N. D., Peychev, L., Saracheva, K., Shishmanova-Doseva, M., & Peychev, Z. (2025). In Vivo Evaluation of the Analgesic and Anti-Inflammatory Activity of Thymus numidicus Essential Oil. Pharmaceuticals, 18(7), 1031. https://doi.org/10.3390/ph18071031









