Herbal Extract-Induced DNA Damage, Apoptosis, and Antioxidant Effects of C. elegans: A Comparative Study of Mentha longifolia, Scrophularia orientalis, and Echium biebersteinii
Abstract
1. Introduction
1.1. Scrophularia orientalis L.
1.2. Echium biebersteinii Laicata
1.3. Mentha longifolia (L.) L.
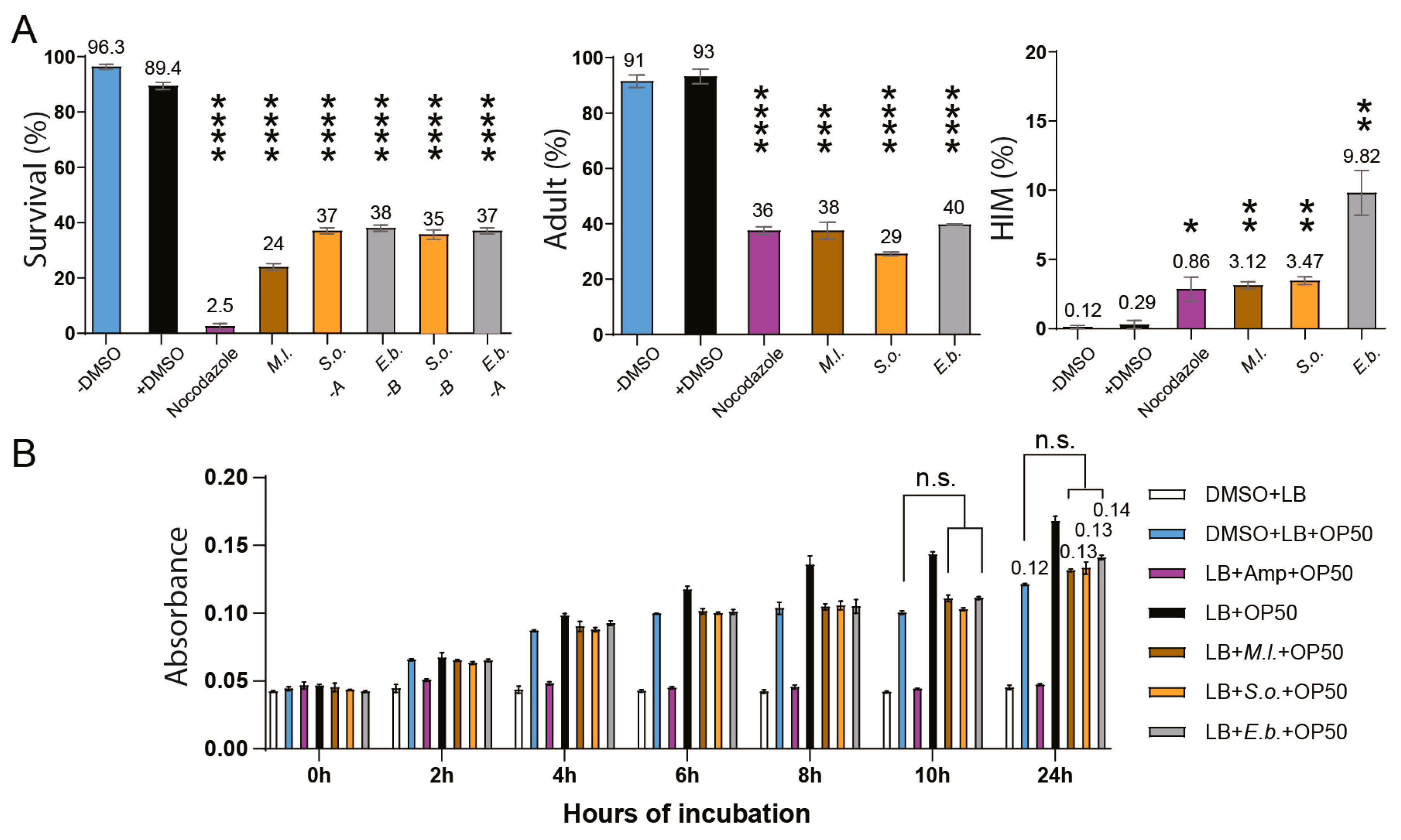
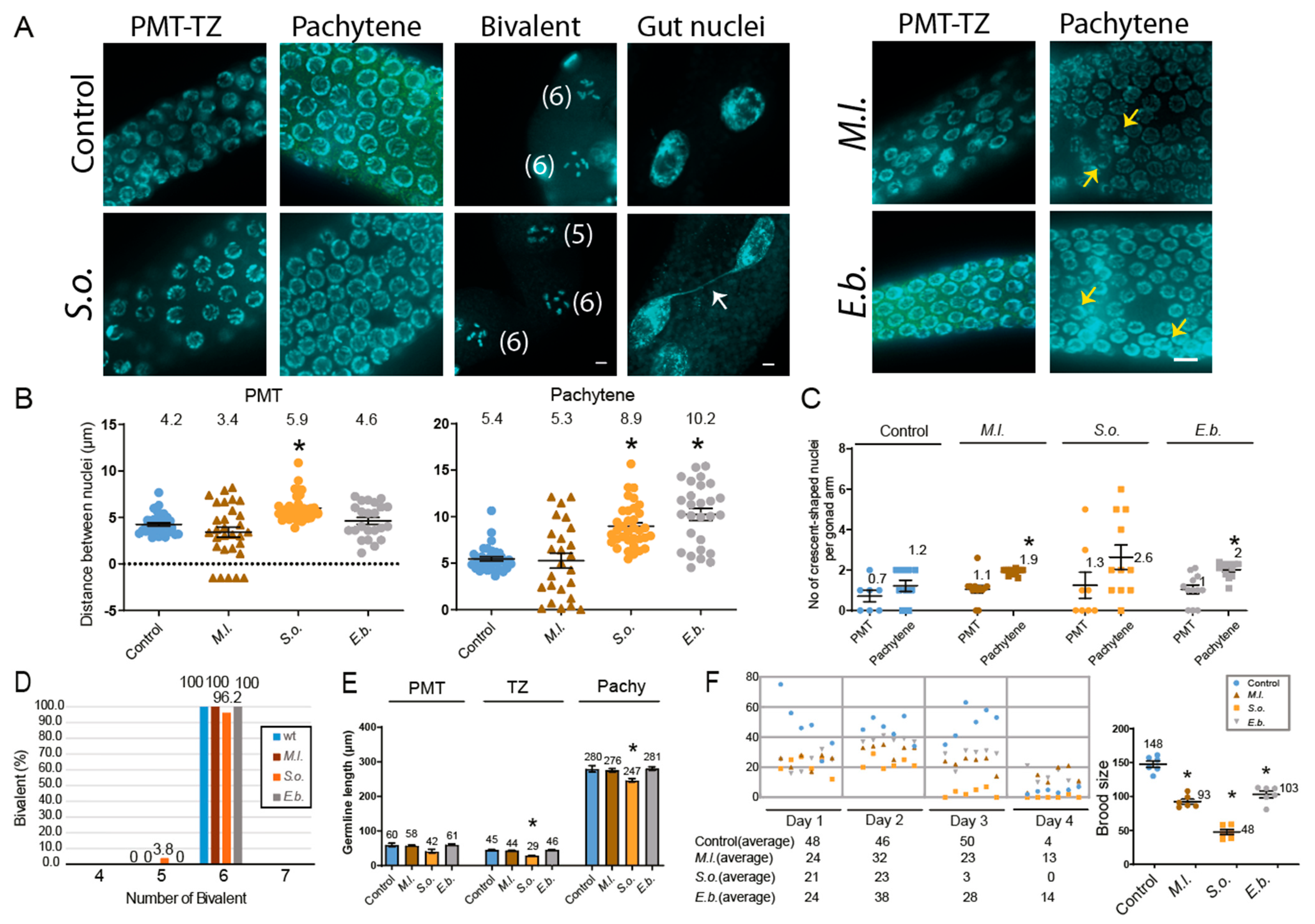
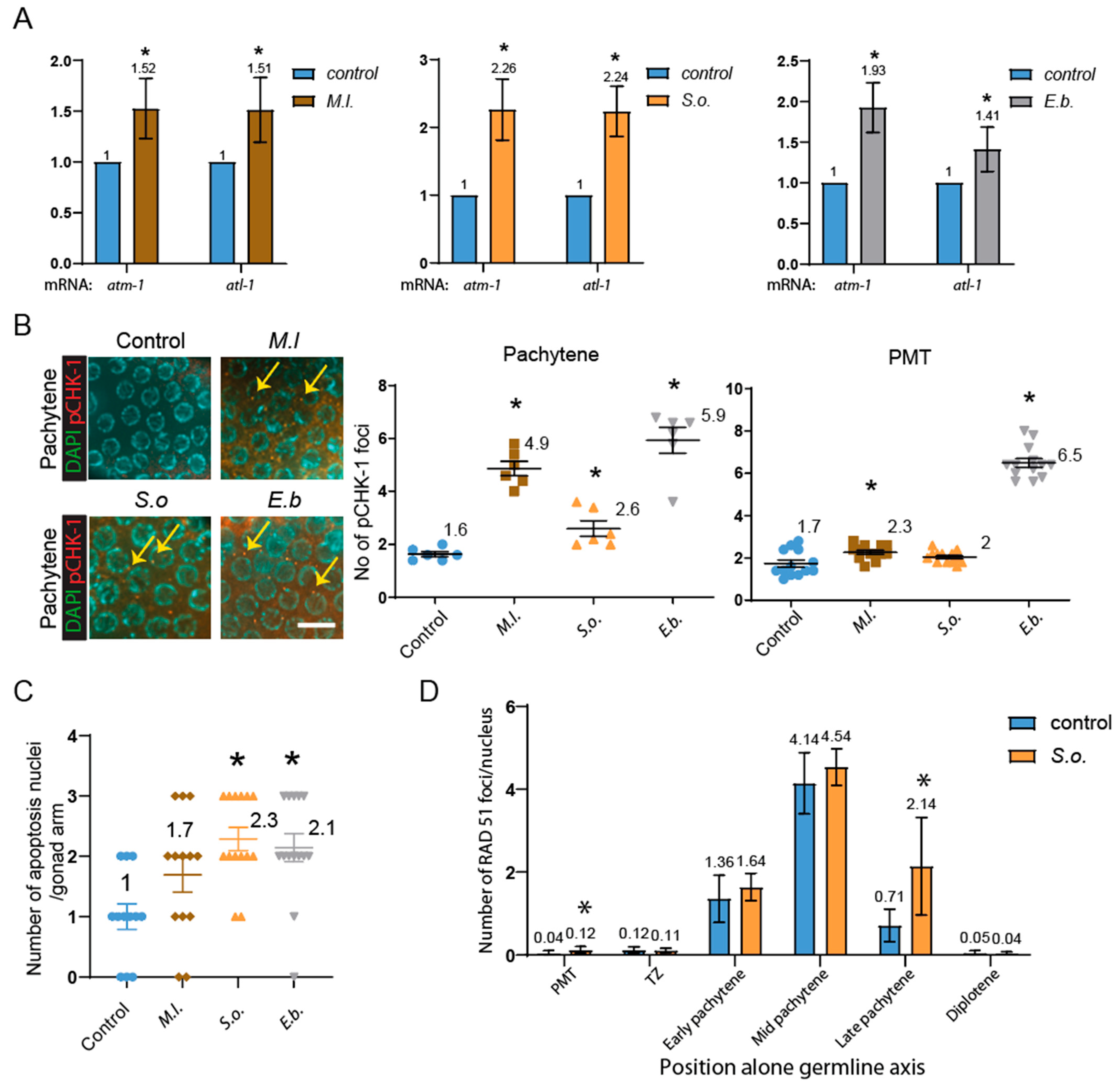
2. Results
3. Discussion
3.1. Herbal Extracts Induce Germline-Specific DNA Damage Checkpoint Activation and Meiotic Defects in C. elegans
3.2. Herbal Extracts Lead to Defective Mitotic and Meiotic Progression, Impaired DNA Repair, and DNA Damage Checkpoint Activation, Resulting in Germline Apoptosis
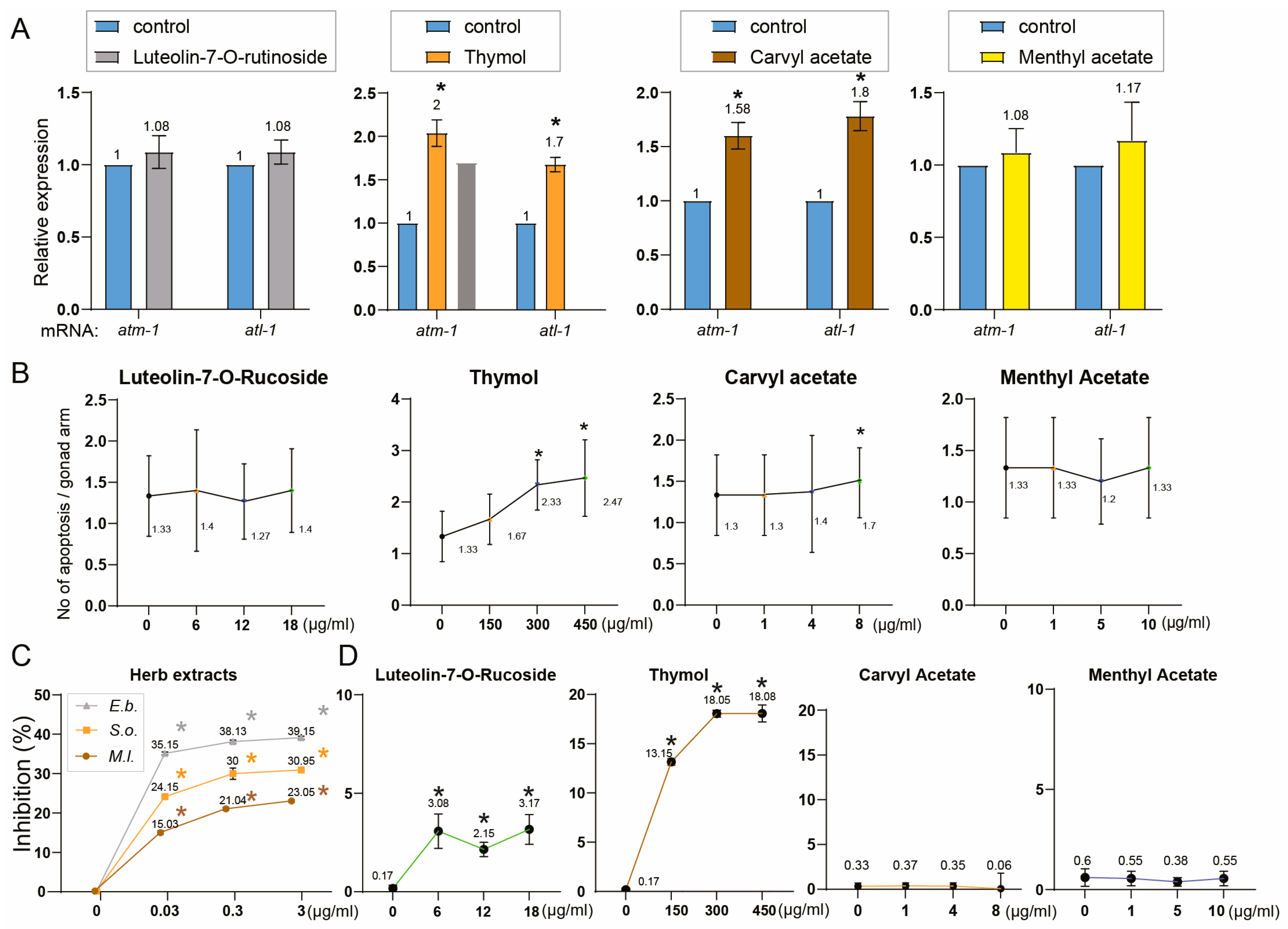
3.3. Phytochemical Composition Underlies the Biological Activities of Herbal Extracts: Four Common Compounds Identified—Thymol, Carvyl Acetate, Luteolin-7-O-Rutinoside, and Menthyl Acetate
3.4. Phytochemical Overlap Explains Parallel DNA Damage Responses Induced by S. orientalis and E. biebersteinii Extracts
3.5. Uncoupling Antioxidant Activity from Germline Toxicity in Herbal Extracts
4. Materials and Methods
4.1. Strains and Alleles
4.2. Herb Extraction
4.3. Survival, Larval Arrest/Lethality, and High Incidence of Males (HIM) Assay
4.4. LC–MS/MS Analysis
4.5. Immunofluorescence Assay
4.6. pCHK-1 Foci Quantification
4.7. Assessment of Germline Apoptosis
4.8. qRT-PCR
4.9. Chemical Reagents
4.10. Monitoring the Growth of E. coli
4.11. Quantitative Analysis of RAD-51 Foci
4.12. DPPH Free Radical Scavenging Assay
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahar, B.; Chongtham, N. Traditional uses and advances in recent research on wild aromatic plant Mentha longifolia and its pharmacological importance. Phytochem. Rev. 2024, 23, 529–550. [Google Scholar] [CrossRef]
- Jedrzejczyk, I.; Rewers, M. Genome size and ISSR markers for Mentha L. (Lamiaceae) genetic diversity assessment and species identification. Ind. Crops Prod. 2018, 120, 171–179. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of Genus Mentha: From Farm to Food Factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, S.; Esmaeili, F.; Lohrasebi, T. A Comprehensive Review of the Key Characteristics of the Genus Mentha, Natural Compounds and Biotechnological Approaches for the Production of Secondary Metabolites. Iran. J. Biotechnol. 2023, 21, e3605. [Google Scholar]
- Galíndez, J.d.S.; Lanza, A.D.; Matellano, L.F. Biologically Active Substances from the Genus Scrophularia. Pharm. Biol. 2002, 40, 45–59. [Google Scholar] [CrossRef]
- Scheunert, A.; Heubl, G. Against all odds: Reconstructing the evolutionary history of Scrophularia (Scrophulariaceae) despite high levels of incongruence and reticulate evolution. Org. Divers. Evol. 2017, 17, 323–349. [Google Scholar] [CrossRef]
- Ren, D.; Shen, Z.-Y.; Qin, L.-P.; Zhu, B. Pharmacology, phytochemistry, and traditional uses of Scrophularia ningpoensis Hemsl. J. Ethnopharmacol. 2021, 269, 113688. [Google Scholar] [CrossRef]
- Baltisberger, M.; Widmer, A. Chromosome numbers of plant species from the Canary Islands. Bot. Helv. 2006, 116, 9–30. [Google Scholar] [CrossRef]
- Jin, J.; Boersch, M.; Nagarajan, A.; Davey, A.K.; Zunk, M. Antioxidant Properties and Reported Ethnomedicinal Use of the Genus Echium (Boraginaceae). Antioxidants 2020, 9, 722. [Google Scholar] [CrossRef]
- Al-Dalahmeh, Y.; Almahmoud, S.A.J.; Al-Bataineh, N.; Alghzawi, T.A.; Alhamzani, A.G.; Al-Mutairi, A.A.; Al-Jaber, H.I.; Abu Orabi, S.T.; Bataineh, T.T.; Al-Sheraideh, M.S.; et al. Scrophularia peyronii Post. from Jordan: Chemical Composition of Essential Oil and Phytochemical Profiling of Crude Extracts and Their In Vitro Antioxidant Activity. Life 2023, 13, 1404. [Google Scholar] [CrossRef]
- Pasdaran, A.; Hamedi, A. The genus Scrophularia: A source of iridoids and terpenoids with a diverse biological activity. Pharm. Biol. 2017, 55, 2211–2233. [Google Scholar] [CrossRef]
- Lange, I.; Moschny, J.; Tamanyan, K.; Khutsishvili, M.; Atha, D.; Borris, R.P.; Koomoa, D.-L. Scrophularia orientalis extract induces calcium signaling and apoptosis in neuroblastoma cells. Int. J. Oncol. 2016, 48, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Lajimi, A.A.; Rezaie-Tavirani, M.; Mortazavi, S.A.; Barzegar, M.; Moghadamnia, S.H.; Rezaee, M.B. Study of Anti Cancer Property of Scrophularia striata Extract on the Human Astrocytoma Cell Line (1321). Iran. J. Pharm. Res. 2010, 9, 403–410. [Google Scholar][Green Version]
- Giessrigl, B.; Yazici, G.; Teichmann, M.; Kopf, S.; Ghassemi, S.; Atanasov, A.G.; Dirsch, V.M.; Grusch, M.; Jäger, W.; Ozmen, A.; et al. Effects of Scrophularia extracts on tumor cell proliferation, death and intravasation through lymphoendothelial cell barriers. Int. J. Oncol. 2012, 40, 2063–2074. [Google Scholar] [PubMed][Green Version]
- Kefi, S.; Essid, R.; Mkadmini, K.; Kefi, A.; Haddada, F.M.; Tabbene, O.; Limam, F. Phytochemical investigation and biological activities of Echium arenarium (Guss) extracts. Microb. Pathog. 2018, 118, 202–210. [Google Scholar] [CrossRef]
- Hosseini, N.; Abolhassani, M. Immunomodulatory properties of borage (Echium amoenum) on BALB/c mice infected with Leishmania major. J. Clin. Immunol. 2011, 31, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Bekhradnia, S.; Ebrahimzadeh, M.A. Antioxidant activity of Echium amoenum. Rev. Chim. J. 2016, 67, 223–226. [Google Scholar]
- Rabbani, M.; Sajjadi, S.; Vaseghi, G.; Jafarian, A. Anxiolytic effects of Echium amoenum on the elevated plus-maze model of anxiety in mice. Fitoterapia 2004, 75, 457–464. [Google Scholar] [CrossRef]
- Potdar, V.H.; Kibile, S.J. Evaluation of antidepressant-like effect of Citrus maxima leaves in animal models of depression. Iran. J. Basic Med. Sci. 2011, 14, 478. [Google Scholar]
- Shafaghi, B.; Naderi, N.; Tahmasb, L.; Kamalinejad, M. Anxiolytic effect of Echium amoenum L. in mice. J. Pharm. Res. 2002, 1, 37–41. [Google Scholar]
- Yeşilada, E.; Honda, G.; Sezik, E.; Tabata, M.; Fujita, T.; Tanaka, T.; Takeda, Y.; Takaishi, Y. Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J. Ethnopharmacol. 1995, 46, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Kim, H.L.; Park, S.J.; Jung, H.J. Echium amoenum and Rosmarinic Acid Suppress the Growth and Metastasis of Gastric Cancer AGS Cells by Promoting Apoptosis and Inhibiting EMT. Int. J. Mol. Sci. 2024, 25, 12909. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.S. Herbs and Spices: New Processing Technologies; BoD–Books on Demand: Norderstedt, Germany, 2021. [Google Scholar]
- Brahmi, F.; Khodir, M.; Mohamed, C.; Pierre, D. Chemical Composition and Biological Activities of Mentha Species, in Aromatic and Medicinal Plants-Back to Nature; IntechOpen: London, UK, 2017. [Google Scholar]
- Al-Bayati, F.A. Isolation and identification of antimicrobial compound from Mentha longifolia L. leaves grown wild in Iraq. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Amzazi, S.; Ghoulami, S.; Bakri, Y.; Idrissi, A.I.; Fkih-Tétouani, S.; Benjouad, A. Human Immunodeficiency Virus Type 1 Inhibitory Activity of Mentha longifolia. Therapies 2003, 58, 531–534. [Google Scholar] [CrossRef]
- Gulluce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Patti, F.; Palmioli, A.; Vitalini, S.; Bertazza, L.; Redaelli, M.; Zorzan, M.; Rubin, B.; Mian, C.; Bertolini, C.; Iacobone, M.; et al. Anticancer Effects of Wild Mountain Mentha longifolia Extract in Adrenocortical Tumor Cell Models. Front. Pharmacol. 2020, 10, 1647. [Google Scholar] [CrossRef]
- Lui, D.Y.; Colaiacovo, M.P. Meiotic development in Caenorhabditis elegans. Adv. Exp. Med. Biol. 2013, 757, 133–170. [Google Scholar]
- Girard, C.; Roelens, B.; Zawadzki, K.A.; Villeneuve, A.M. Interdependent and separable functions of Caenorhabditis elegans MRN-C complex members couple formation and repair of meiotic DSBs. Proc. Natl. Acad. Sci. USA 2018, 115, E4443–E4452. [Google Scholar] [CrossRef]
- Kim, H.-M.; Colaiácovo, M.P.; Kim, S.K. ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the C. elegans Germline. PLoS Genet. 2014, 10, e1004723. [Google Scholar] [CrossRef]
- Kim, H.-M.; Colaiácovo, M.P. New Insights into the Post-Translational Regulation of DNA Damage Response and Double-Strand Break Repair in Caenorhabditis elegans. Genetics 2015, 200, 495–504. [Google Scholar] [CrossRef]
- Meng, Q.; Borris, R.P.; Kim, H.-M. Torenia sp. Extracts Contain Multiple Potent Antitumor Compounds with Nematocidal Activity, Triggering an Activated DNA Damage Checkpoint and Defective Meiotic Progression. Pharmaceuticals 2024, 17, 611. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Hu, A.; Xiao, W.; Borris, R.P.; Kim, H.-M. Therapeutic Potential of Lappula patula Extracts on Germline Development and DNA Damage Responses in C. elegans. Pharmaceuticals 2025, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Pathak, N.; Ren, X.; Borris, R.P.; Kim, H.-M. Exploring the Impact of Onobrychis cornuta and Veratrum lobelianum Extracts on C. elegans: Implications for MAPK Modulation, Germline Development, and Antitumor Properties. Nutrients 2023, 16, 1–22. [Google Scholar] [CrossRef]
- Zou, Y.; Luo, X.; Feng, Y.; Fang, S.; Tian, J.; Yu, B.; Li, J. Luteolin prevents THP-1 macrophage pyroptosis by suppressing ROS production via Nrf2 activation. Chem.-Biol. Interact. 2021, 345, 109573. [Google Scholar] [CrossRef]
- Shao, J.; Wang, C.; Li, L.; Liang, H.; Dai, J.; Ling, X.; Tang, H. Luteoloside Inhibits Proliferation and Promotes Intrinsic and Extrinsic Pathway-Mediated Apoptosis Involving MAPK and mTOR Signaling Pathways in Human Cervical Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1664. [Google Scholar] [CrossRef]
- Zhou, M.; Shen, S.; Zhao, X.; Gong, X. Luteoloside induces G0/G1 arrest and pro-death autophagy through the ROS-mediated AKT/mTOR/p70S6K signalling pathway in human non-small cell lung cancer cell lines. Biochem. Biophys. Res. Commun. 2017, 494, 263–269. [Google Scholar] [CrossRef]
- Lin, J.; Chen, J.; Zhang, Z.; Xu, T.; Shao, Z.; Wang, X.; Ding, Y.; Tian, N.; Jin, H.; Sheng, S.; et al. Luteoloside Inhibits IL-1β-Induced Apoptosis and Catabolism in Nucleus Pulposus Cells and Ameliorates Intervertebral Disk Degeneration. Front. Pharmacol. 2019, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; del Mar Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. PTR 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Slamenová, D.; Horváthová, E.; Sramková, M.; Marsálková, L. DNA-protective effects of two components of essential plant oils carvacrol and thymol on mammalian cells cultured in vitro. Neoplasma 2007, 54, 108–112. [Google Scholar]
- Li, Y.; Wen, J.-M.; Du, C.-J.; Hu, S.-M.; Chen, J.-X.; Zhang, S.-G.; Zhang, N.; Gao, F.; Li, S.-J.; Mao, X.-W.; et al. Thymol inhibits bladder cancer cell proliferation via inducing cell cycle arrest and apoptosis. Biochem. Biophys. Res. Commun. 2017, 491, 530–536. [Google Scholar] [CrossRef]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol Inhibits LPS-Stimulated Inflammatory Response via Down-Regulation of NF-κB and MAPK Signaling Pathways in Mouse Mammary Epithelial Cells. Inflammation 2014, 37, 214–222. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P.; Pizzo, S.V. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha piperita Grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Wu, X.; Zhao, Z.; Feng, X.; Bai, X.; Liu, X.; Zhao, J.; Takeda, S.; Qing, Y. Nonhomologous end joining and homologous recombination involved in luteolin-induced DNA damage in DT40 cells. Toxicol. Vitr. 2020, 65, 104825. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-C.; Chen, C.-Y.; Lin, C.-J.; Yang, T.-Y.; Chen, T.-H.; Wu, L.-C.; Wu, C.-C. Luteolin attenuates TGF-β1-induced epithelial–mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt–NF-κB–Snail pathway. Life Sci. 2013, 93, 924–933. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119 Pt A, 1–11. [Google Scholar] [CrossRef]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
- Chan, H.-W.; Lin, W.-C.; Kuo, D.-Y.; Chuang, H.-Y. Beta-Caryophyllene Augments Radiotherapy Efficacy in GBM by Modulating Cell Apoptosis and DNA Damage Repair via PPARγ and NF-κB Pathways. Phytother. Res. PTR 2025, 39, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Bakır, B.; Him, A.; Özbek, H.; Düz, E.; Tütüncü, M. Investigation of the anti-inflammatory and analgesic activities of -caryophyllene. Int. J. Essent. Oil Ther. 2008, 2, 41–44. [Google Scholar]
- Wójciak, M.; Feldo, M.; Borowski, G.; Kubrak, T.; Płachno, B.J.; Sowa, I. Antioxidant Potential of Diosmin and Diosmetin against Oxidative Stress in Endothelial Cells. Molecules 2022, 27, 8232. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Shao, J.; Dai, J.; Lin, Y.; Yang, X.; Ma, J.; He, Q.; Yang, B.; Yao, K.; Luo, P. Diosmetin protects against retinal injury via reduction of DNA damage and oxidative stress. Toxicol. Rep. 2016, 3, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, D.-H.; Park, S.-Y.; Seol, J.-W. Diosmetin inhibits tumor development and block tumor angiogenesis in skin cancer. Biomed. Pharmacother. 2019, 117, 109091. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Park, J.-K.; Choi, J.; Jang, H.; Seol, J.-W. Anti-inflammatory effects of natural flavonoid diosmetin in IL-4 and LPS-induced macrophage activation and atopic dermatitis model. Int. Immunopharmacol. 2020, 89, 107046. [Google Scholar] [CrossRef]
- Wu, H.-J.; Chan, W.-H. Genistein protects methylglyoxal-induced oxidative DNA damage and cell injury in human mononuclear cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2007, 21, 335–342. [Google Scholar] [CrossRef]
- Hu, X.; Wu, X.; Liu, H.; Cheng, Z.; Zhao, Z.; Xiang, C.; Feng, X.; Takeda, S.; Qing, Y. Genistein-induced DNA damage is repaired by nonhomologous end joining and homologous recombination in TK6 cells. J. Cell. Physiol. 2019, 234, 2683–2692. [Google Scholar] [CrossRef]
- Tominaga, Y.; Wang, A.; Wang, R.-H.; Wang, X.; Cao, L.; Deng, C.-X. Genistein inhibits Brca1 mutant tumor growth through activation of DNA damage checkpoints, cell cycle arrest, and mitotic catastrophe. Cell Death Differ. 2007, 14, 472–479. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-κB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit only NF-κB Activation along with Their Inhibitory Effect on iNOS Expression and NO Production in Activated Macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem.-Biol. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef]
- de Castilho da Silva, D.; Di Camillo Orfali, G.; Santana, M.G.; Palma, J.K.Y.; de Oliveira Assunção, I.R.; Marchesi, I.M.; Grizotto, A.Y.K.; Martinez, N.P.; Felliti, S.; Pereira, J.A.; et al. Antitumor effect of isoquercetin on tissue vasohibin expression and colon cancer vasculature. Oncotarget 2022, 13, 307–318. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, Y.; Zhang, X.; Chen, X.; Liu, Z.; Tian, X. Isoquercetin ameliorates myocardial infarction through anti-inflammation and anti-apoptosis factor and regulating TLR4-NF-κB signal pathway. Mol. Med. Rep. 2018, 17, 6675–6680. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef]
- Manna, K.; Das, U.; Das, D.; Kesh, S.B.; Khan, A.; Chakraborty, A.; Dey, S. Naringin inhibits gamma radiation-induced oxidative DNA damage and inflammation, by modulating p53 and NF-κB signaling pathways in murine splenocytes. Free Radic. Res. 2015, 49, 422–439. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhen, Y.; Chen, Y.; Zou, L.; Zhang, Y.; Hu, F.; Feng, J.; Shen, J.; Wei, B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NF-κB/COX-2-caspase-1 pathway in HeLa cervical cancer cells. Int. J. Oncol. 2014, 45, 1929–1936. [Google Scholar] [CrossRef]
- Do Nascimento, P.G.G.; Lemos, T.L.G.; Bizerra, A.M.C.; Arriaga, M.C.; Ferreira, D.A.; Santiago, G.M.P.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.A.; Pereira-Wilson, C.; Collins, A.R. Protective effects of Ursolic acid and Luteolin against oxidative DNA damage include enhancement of DNA repair in Caco-2 cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 692, 6–11. [Google Scholar] [CrossRef]
- Kassi, E.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Manoussakis, M.; Moutsatsou, P. Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. J. Cancer Res. Clin. Oncol. 2007, 133, 493–500. [Google Scholar] [CrossRef]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B.; Bobé, P. Potent Anti-Inflammatory Activity of Ursolic Acid, a Triterpenoid Antioxidant, Is Mediated through Suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef]
- Carrizzo, A.; Forte, M.; Damato, A.; Trimarco, V.; Salzano, F.; Bartolo, M.; Maciag, A.; Puca, A.A.; Vecchione, C. Antioxidant effects of resveratrol in cardiovascular, cerebral and metabolic diseases. Food Chem. Toxicol. 2013, 61, 215–226. [Google Scholar] [CrossRef]
- Sgambato, A.; Ardito, R.; Faraglia, B.; Boninsegna, A.; Wolf, F.I.; Cittadini, A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2001, 496, 171–180. [Google Scholar] [CrossRef]
- Hogg, S.J.; Chitcholtan, K.; Hassan, W.; Sykes, P.H.; Garrill, A. Resveratrol, Acetyl-Resveratrol, and Polydatin Exhibit Antigrowth Activity against 3D Cell Aggregates of the SKOV-3 and OVCAR-8 Ovarian Cancer Cell Lines. Obstet. Gynecol. Int. 2015, 2015, 279591. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, D.K. Anti-Inflammatory Responses of Resveratrol. Inflamm. Allergy Drug Targets 2007, 6, 168–173. [Google Scholar] [CrossRef]
- Wang, B.-W.; Jiang, Y.; Yao, Z.-L.; Chen, P.-S.; Yu, B.; Wang, S.-N. Aucubin Protects Chondrocytes Against IL-1β-Induced Apoptosis In Vitro And Inhibits Osteoarthritis In Mice Model. Drug Des. Dev. Ther. 2019, 13, 3529–3538. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Kuang, Z.; Wang, W.; Li, S.; Li, G.; Song, Y.; Li, H.; Cui, G.; Zhou, H.; Luo, H. Aucubin Exerts Anticancer Activity in Breast Cancer and Regulates Intestinal Microbiota. Evid.-Based Complement. Altern. Med. 2022, 2022, 4534411. [Google Scholar] [CrossRef]
- Park, K.S.; Chang, I.-M. Anti-Inflammatory Activity of Aucubin by Inhibition of Tumor Necrosis Factor-α Production in RAW 264.7 Cells. Planta Medica 2004, 70, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, V.; Lauridsen, S.T.; Daneshvar, B.; Jakobsen, J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000, 154, 201–210. [Google Scholar] [CrossRef]
- Jang, S.H.; Lim, J.W.; Morio, T.; Kim, H. Lycopene inhibits Helicobacter pylori-induced ATM/ATR-dependent DNA damage response in gastric epithelial AGS cells. Free Radic. Biol. Med. 2012, 52, 607–615. [Google Scholar] [CrossRef]
- Jeong, Y.; Lim, J.W.; Kim, H. Lycopene Inhibits Reactive Oxygen Species-Mediated NF-κB Signaling and Induces Apoptosis in Pancreatic Cancer Cells. Nutrients 2019, 11, 762. [Google Scholar] [CrossRef]
- Hadad, N.; Levy, R. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signaling. Free Radic. Biol. Med. 2012, 53, 1381–1391. [Google Scholar] [CrossRef]
- Beeharry, N.; E Lowe, J.; Hernandez, A.R.; A Chambers, J.; Fucassi, F.; Cragg, P.J.; Green, M.H.; Green, I.C. Linoleic acid and antioxidants protect against DNA damage and apoptosis induced by palmitic acid. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2003, 530, 27–33. [Google Scholar] [CrossRef]
- Lauson, C.B.N.; Tiberti, S.; Corsetto, P.A.; Conte, F.; Tyagi, P.; Machwirth, M.; Ebert, S.; Loffreda, A.; Scheller, L.; Sheta, D.; et al. Linoleic acid potentiates CD8+ T cell metabolic fitness and antitumor immunity. Cell Metab. 2023, 35, 633–650.e9. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Gil, J.; Sierra-Magro, A.; Morales-Garcia, J.A.; Sanz-SanCristobal, M.; Alonso-Gil, S.; Cortes-Canteli, M.; Niso-Santano, M.; Martínez-Chacón, G.; Fuentes, J.M.; Santos, A.; et al. Neuroprotective and Anti-Inflammatory Effects of Linoleic Acid in Models of Parkinson’s Disease: The Implication of Lipid Droplets and Lipophagy. Cells 2022, 11, 2297. [Google Scholar] [CrossRef]
- Bilgic, S.; Ozgocmen, M. The protective effect of misoprostol against doxorubicin induced liver injury. Biotech. Histochem. 2019, 94, 583–591. [Google Scholar] [CrossRef]
- Li, B.; Hu, Y.; Chen, Y.; Liu, K.; Rong, K.; Hua, Q.; Fu, S.; Yang, X.; Zhou, T.; Cheng, X.; et al. Homoplantaginin alleviates intervertebral disc degeneration by blocking the NF-κB/MAPK pathways via binding to TAK1. Biochem. Pharmacol. 2024, 226, 116389. [Google Scholar] [CrossRef]
- Ying, X.-X.; Li, H.-B.; Chu, Z.-Y.; Zhai, Y.-J.; Leng, A.-J.; Liu, X.; Xin, C.; Zhang, W.-J.; Kang, T.-G. HPLC determination of malondialdehyde in ECV304 cell culture medium for measuring the antioxidant effect of vitexin-4″-O-glucoside. Arch. Pharmacal Res. 2008, 31, 878–885. [Google Scholar] [CrossRef]
- Wei, W.; Ying, X.; Zhang, W.; Chen, Y.; Leng, A.; Jiang, C.; Liu, J. Effects of vitexin-2″-O-rhamnoside and vitexin-4″-O-glucoside on growth and oxidative stress-induced cell apoptosis of human adipose-derived stem cells. J. Pharm. Pharmacol. 2014, 66, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Van Buul, P.P.; Van Duyn-Goedhart, A.; De Rooij, D.G.; Sankaranarayanan, K. Differential radioprotective effects of misoprostol in DNA repair-proficient and -deficient or radiosensitive cell systems. Int. J. Radiat. Biol. 1997, 71, 259–264. [Google Scholar]
- Gálvez, M.; Martín-Cordero, C.; Ayuso, M.J. Iridoids as DNA topoisomerase I poisons. J. Enzyme Inhib. Med. Chem. 2005, 20, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Basso, E.; Regazzo, G.; Fiore, M.; Palma, V.; Traversi, G.; Testa, A.; Degrassi, F.; Cozzi, R. Resveratrol affects DNA damage induced by ionizing radiation in human lymphocytes in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 806, 40–46. [Google Scholar] [CrossRef]
- Kao, C.-L.; Huang, P.-I.; Tsai, P.-H.; Tsai, M.-L.; Lo, J.-F.; Lee, Y.-Y.; Chen, Y.-J.; Chen, Y.-W.; Chiou, S.-H. Resveratrol-induced apoptosis and increased radiosensitivity in CD133-positive cells derived from atypical teratoid/rhabdoid tumor. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 219–228. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Colaiacovo, M.P. DNA Damage Sensitivity Assays in Caenorhabditis elegans. Bio-Protocol 2015, 5, e1487. [Google Scholar] [CrossRef]
- Colaiácovo, M.P.; MacQueen, A.J.; Martinez-Perez, E.; McDonald, K.; Adamo, A.; La Volpe, A.; Villeneuve, A.M. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell 2003, 5, 463–474. [Google Scholar] [CrossRef]
- Kelly, K.; Dernburg, A.F.; Stanfield, G.M.; Villeneuve, A.M. Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 2000, 156, 617–630. [Google Scholar] [CrossRef]
- Ren, X.; Tian, S.; Meng, Q.; Kim, H.-M. Histone Demethylase AMX-1 Regulates Fertility in a p53/CEP-1 Dependent Manner. Front. Genet. 2022, 13, 929716. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, S.; Beese-Sims, S.E.; Chen, J.; Shin, N.; Colaiácovo, M.P.; Kim, H.-M.; Copenhaver, G.P. Histone demethylase AMX-1 is necessary for proper sensitivity to interstrand crosslink DNA damage. PLoS Genet. 2021, 17, e1009715. [Google Scholar] [CrossRef]
- Sayed, S.M.A.; Siems, K.; Schmitz-Linneweber, C.; Luyten, W.; Saul, N. Enhanced Healthspan in Caenorhabditis elegans Treated with Extracts From the Traditional Chinese Medicine Plants Cuscuta chinensis Lam. and Eucommia ulmoides Oliv. Front. Pharmacol. 2021, 12, 604435. [Google Scholar] [CrossRef]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Tania, U.H.; Hassan, M.R.; Eshita, N.J.; Akhter, R.; Shahriar, M. Evaluation of In vitro Antioxidant and In vivo Pharmacological Activity of Leaf Extracts of Hoya parasitica (Wall.). J. Appl. Pharm. Sci. 2016, 6, 163–170. [Google Scholar] [CrossRef][Green Version]
- Kajimoto, T.; Hidaka, M.; Shoyama, K.; Nohara, T. Iridoids from Scrophularia ningpoensis. Phytochemistry 1989, 28, 2701–2704. [Google Scholar] [CrossRef]
- Miyazawa, M.; Okuno, Y.; Nakamura, S.-I.; Kameoka, H. Suppression of SOS-inducing activity of chemical mutagens by cinnamic acid derivatives from Scrophulia ningpoensis in the Salmonella typhimurium TA1535/pSK1002 umu test. J. Agric. Food Chem. 1998, 46, 904–910. [Google Scholar] [CrossRef]
- Qian, J.; Hunkler, D.; Rimpler, H. Iridoid-related aglycone and its glycosides from Scrophularia ningpoensis. Phytochemistry 1992, 31, 905–911. [Google Scholar] [CrossRef]
- Fujita, T.; Sezik, E.; Tabata, M.; Yesilada, E.; Honda, G.; Takeda, Y.; Tanaka, T.; Takaishi, Y. Traditional medicine in Turkey VII. Folk medicine in middle and west Black Sea regions. Econ. Bot. 1995, 49, 406–422. [Google Scholar] [CrossRef]
- Amabeoku, G.J.; Erasmus, S.J.; Ojewole, J.A.O.; Mukinda, J.T. Antipyretic and antinociceptive properties of Mentha longifolia Huds. (Lamiaceae) leaf aqueous extract in rats and mice. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 645. [Google Scholar] [CrossRef]
- Karimian, P.; Gholamreza, K.; Amirghofran, Z. Anti-inflammatory effect of Mentha longifolia in lipopolysaccharide-stimulated macrophages: Reduction of nitric oxide production through inhibition of inducible nitric oxide synthase. J. Immunotoxicol. 2013, 10, 393–400. [Google Scholar] [CrossRef]
- Afkar, S.; Somaghian, S.A. Determining of chemical composition, anti-pathogenic and anticancer activity of Mentha longifolia essential oil collected from Iran. Nat. Prod. Res. 2024, 31, 1–9. [Google Scholar] [CrossRef]
- Beheshtian, N.; Karimi, E.; Asili, J.; Beheshtin, N.; Le, H.H.; Shakeri, M. Mentha longifolia L. Inhibits Colorectal Cancer Cell Proliferation and Induces Apoptosis via Caspase Regulation. Int. J. Transl. Med. 2023, 3, 416–425. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.; Al-Askar, A.A. Anticandidal and anti-carcinogenic activities of Mentha longifolia (Wild Mint) extracts in vitro. J. King Saud Univ. Sci. 2020, 32, 2046–2052. [Google Scholar] [CrossRef]
- Asemani, Y.; BayaT, M.; Malek-Hosseini, S.; Amirghofran, Z. Modulation of in vitro proliferation and cytokine secretion of human lymphocytes by Mentha longifolia extracts. Avicenna J. Phytomed. 2019, 9, 34–43. [Google Scholar]
- Bai, X.; Aimila, A.; Aidarhan, N.; Duan, X.; Maiwulanjiang, M. Chemical constituents and biological activities of essential oil from Mentha longifolia: Effects of different extraction methods. Int. J. Food Prop. 2020, 23, 1951–1960. [Google Scholar] [CrossRef]
- Dadkhah, A.; Fatemi, F.; Rasooli, A.; Malayeri, M.R.M.; Torabi, F. Assessing the effect of Mentha longifolia essential oils on COX-2 expression in animal model of sepsis induced by caecal ligation and puncture. Pharm. Biol. 2018, 56, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Haikal, A.; El-Neketi, M.; Helal, M.G.; Abou-Zeid, L.A.; Hassan, M.A.; Gohar, A.A. Anti-asthmatic and antioxidant activity of flavonoids isolated from Mentha longifolia subspecies typhoides (Briq.) Harley. and Mentha longifolia subspecies schimperi (Briq.) Briq. on ovalbumin-induced allergic asthma in mice: In-vivo and in-silico study. J. Ethnopharmacol. 2025, 339, 119133. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.; Gogoi, A.; Tamang, R.; Dutta, P.; Perveen, K.; Alshaikh, A.N.; Begum, T. Exploring the bioactive potential of Mentha longifolia from Northeast India: An inclusive study on phytochemical composition and biological activities. J. Essent. Oil Bear. Plants 2024, 27, 1102–1120. [Google Scholar] [CrossRef]
- Pham, D.V.; Dam, N.A.L.; Dinh, N.A.; Nguyen, T.D. Anti-inflammatory activities of some Mentha essential oils in lipopolysaccharide-activated macrophages. Tạp Chí Nghiên Cứu Dược Và Thông Tin Thuốc 2025, 22, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, H.; Azimirad, M.; Abdemohamadi, E.; Pezzani, R.; Zali, M.R.; Yadegar, A. Pleiotropic effects of Mentha longifolia L. extract on the regulation of genes involved in inflammation and apoptosis induced by Clostridioides difficile ribotype 001. Front. Microbiol. 2023, 14, 1273094. [Google Scholar] [CrossRef]
- Ibrahim, A. anti-acetylcholinesterase, anti-inflammatory and antioxidant activities of Mentha longifolia for treating Alzheimer disease. Pharm. Lett. 2016, 8, 34–39. [Google Scholar]
- Janifer, R.X.; Bajpjpai, P.; Phani, K.G.; Pal, M.M.; Jitendra, K.; Chaurasia, O.; Shashi, B.S. Determination of Total Phenols, Free Radical Scavenging and Antibacterial Activities of Mentha longifolia Linn. Hudson from the Cold Desert, Ladakh, India. Pharmacogn. J. 2010, 2, 470–475. [Google Scholar] [CrossRef]
- Stanisavljevic, D.M.; Stojicevic, S.S.; Djordjevic, S.M.; Zlatkovic, B.P.; Velickovic, D.T.; Karabegovic, I.T.; Lazic, M.L. Antioxidant activity, the content of total phenols and flavonoids in the ethanol extracts of Mentha longifolia (L.) Hudson dried by the use of different techniques. Chem. Ind. Chem. Eng. Q. 2012, 18, 411–420. [Google Scholar] [CrossRef]
- Al-janabi, A.A.; Sahib, A.A.; Ali, F.J. Studying The Effect of Mentha longifolia Plant Extract In Inhibition Growth of Some Bacteria and Inhibiting the Emergence Fourth Stage Larvae of Mosquitoes Aedes Aegypti. Indian J. Forensic Med. Toxicol. 2020, 14, 1629. [Google Scholar]
- Al-Mijalli, S.H.; Mrabti, N.N.; Ouassou, H.; Sheikh, R.A.; Abdallah, E.M.; Assaggaf, H.; Bakrim, S.; Alshahrani, M.M.; Al Awadh, A.A.; Qasem, A.; et al. Phytochemical Variability, In Vitro and In Vivo Biological Investigations, and In Silico Antibacterial Mechanisms of Mentha piperita Essential Oils Collected from Two Different Regions in Morocco. Foods 2022, 11, 3466. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Klimek-Szczykutowicz, M.; El-Ansary, D.O.; Mahmoud, E.A. Polyphenol Profile and Antimicrobial and Cytotoxic Activities of Natural Mentha × piperita and Mentha longifolia Populations in Northern Saudi Arabia. Processes 2020, 8, 479. [Google Scholar] [CrossRef]
- Ghazyzadeh, M.; Abolfazl, D.; Kazemi, S.; Harandi, A.; Ghasempour, M. Evaluation of the Antibacterial Activity of Mentha longifolia Essential Oil against Enterococcus faecalis and its Chemical Composition. J. Dent. 2025. [Google Scholar] [CrossRef]
- Tourabi, M.; Metouekel, A.; Ghouizi, A.E.L.; Jeddi, M.; Nouioura, G.; Laaroussi, H.; Hosen, E.; Benbrahim, K.F.; Bourhia, M.; Salamatullah, A.M.; et al. Efficacy of various extracting solvents on phytochemical composition, and biological properties of Mentha longifolia L. leaf extracts. Sci. Rep. 2023, 13, 18028. [Google Scholar] [CrossRef]
- Shen, X.; Eichhorn, T.; Greten, H.J.; Efferth, T. Effects of Scrophularia ningpoensis Hemsl. on Inhibition of Proliferation, Apoptosis Induction and NF-?B Signaling of Immortalized and Cancer Cell Lines. Pharmaceuticals 2012, 5, 189–208. [Google Scholar] [CrossRef]
- Azadmehr, A.; Goudarzvand, M.; Saadat, P.; Ebrahimi, H.; Hajiaghaee, R.; Miri, N.S.; Fallahnezhad, S.; Norian, R.; Rahmani, A.; Baee, M. Immunomodulatory and anti-inflammatory effects of Scrophularia megalantha ethanol extract on an experimental model of multiple sclerosis. Res. J. Pharmacogn. 2019, 6, 43–50. [Google Scholar]
- Li, Y.M.; Han, Z.H.; Jiang, S.H.; Jiang, Y.; Yao, S.D.; Zhu, D.Y. Fast repairing of oxidized OH radical adducts of dAMP and dGMP by phenylpropanoid glycosides from Scrophularia ningpoensis Hemsl. Acta Pharmacol. Sin. 2000, 21, 1125–1128. [Google Scholar]
- Bahmani, M.; Saatloo, N.V.; Maghsoudi, R.; Momtaz, H.; Saki, K.; Kazemi-Ghoshchi, B.; Asadzadeh, J.; Sotoudeh, A.; Emami, F. A comparative study on the effect of ethanol extract of wild Scrophularia deserti and streptomycin on Brucellla melitensis. J. Herbmed Pharmacol. 2013, 2, 17–20. [Google Scholar]
- Fernández, M.A.; García, M.D.; Sáenz, M.T. Antibacterial activity of the phenolic acids fractions of Scrophularia frutescens and Scrophularia sambucifolia. J. Ethnopharmacol. 1996, 53, 11–14. [Google Scholar] [CrossRef]
- Ayobi, H.; Jamalifar, H.; Pour Mohammadi, F.; Goodarzi, S.; Fazeli, M.R.; Attar, F.; Hadjiakhoondi, A.; Yassa, N. Antibacterial Effects of Scrophularia striata Extract on Pseudomonas aeruginosa. J. Med. Plants 2014, 13, 73–80. [Google Scholar]
- Lewenhofer, V.; Schweighofer, L.; Ledermüller, T.; Eichsteininger, J.; Kählig, H.; Zehl, M.; Nguyen, C.H.; Krupitza, G.; Özmen, A.; Krenn, L. Chemical Composition of Scrophularia lucida and the Effects on Tumor Invasiveness in Vitro. Front. Pharmacol. 2018, 9, 304. [Google Scholar] [CrossRef]
- Musa, A.; Ahmed, S.R.; Hussein, S.; Youssif, K.A.; El-Ghorab, A.H.; Al Haidari, R.A.; Mostafa, M.A.; Almaghrabi, M.; Aldakhil, T.Y.; Mohamed, M.A.; et al. Prominent antidiabetic and anticancer investigation of Scrophularia deserti extract: Integration of experimental and computational approaches. J. Mol. Struct. 2024, 1315, 138769. [Google Scholar] [CrossRef]
- Namvaran, A.; Fazeli, M.; Farajnia, S.; Hamidian, G.; Rezazadeh, H. Effects of Scrophularia oxysepala Methanolic Extract on Early Stages of Dimethylhydrazine-Induced Colon Carcinoma in Rats: Apoptosis Pathway Approach. Adv. Pharm. Bull. 2022, 12, 835–841. [Google Scholar] [CrossRef]
- Azadmehr, A.; Oghyanous, K.A.; Hajiaghaee, R.; Amirghofran, Z.; Azadbakht, M. Antioxidant and Neuroprotective Effects of Scrophularia striata Extract Against Oxidative Stress-Induced Neurotoxicity. Cell. Mol. Neurobiol. 2013, 33, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.-H.; Yang, C.-H.; Kim, S.-C. Inhibitory effect of Scrophulariae Radix extract on TNF-alpha, IL-1beta, IL-6 and Nitric Oxide production in lipopolysaccharide—activated Raw 264.7 cells. Korea J. Herbol. 2005, 20, 7–16. [Google Scholar]
- Díaz, A.M.; Abad, M.J.; Fernández, L.; Silván, A.M.; De Santos, J.; Bermejo, P. Phenylpropanoid glycosides from Scrophularia scorodonia: In vitro anti-inflammatory activity. Life Sci. 2004, 74, 2515–2526. [Google Scholar] [CrossRef]
- Fernández, M.A.; Sáenz, M.T.; García, M.D. Anti-inflammatory Activity in Rats and Mice of Phenolic Acids Isolated from Scrophularia frutescens. J. Pharm. Pharmacol. 1998, 50, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Giner, R.-M.; Villalba, M.-L.; Recio, M.-C.; Máñez, S.; Cerdá-Nicolás, M.; Rı́os, J.-L. Anti-inflammatory glycoterpenoids from Scrophularia auriculata. Eur. J. Pharmacol. 2000, 389, 243–252. [Google Scholar] [CrossRef]
- Mao, G.; Sun, L.; Xu, J.; Li, Y.; Dunzhu, C.; Zhang, L.; Qian, F. Scrodentoids H and I, a Pair of Natural Epimerides from Scrophularia dentata, Inhibit Inflammation through JNK-STAT3 Axis in THP-1 Cells. Evid.-Based Complement. Altern. Med. 2020, 2020, 1842347. [Google Scholar] [CrossRef]
- Pham, T.N.A.; Kim, H.L.; Lee, D.-R.; Choi, B.-K.; Yang, S.H. Anti-inflammatory Effects of Scrophularia buergeriana Extract Mixture Fermented with Lactic Acid Bacteria. Biotechnol. Bioprocess Eng. 2022, 27, 370–378. [Google Scholar] [CrossRef]
- Shin, N.-R.; Lee, A.Y.; Song, J.-H.; Yang, S.; Park, I.; Lim, J.-O.; Jung, T.-Y.; Ko, J.-W.; Kim, J.-C.; Lim, K.S.; et al. Scrophularia buergeriana attenuates allergic inflammation by reducing NF-κB activation. Phytomedicine 2020, 67, 153159. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Ren, Q.; Wang, Y.; Zhou, L.; Fu, Y.; Sai, C.; Pella, S.S.; Guo, Y.; Gao, L.-N. Polysaccharides of Scrophularia ningpoensis Hemsl.: Extraction, Antioxidant, and Anti-Inflammatory Evaluation. Evid.-Based Complement. Altern. Med. 2020, 2020, 8899762. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur. J. Pharmacol. 2008, 588, 78–84. [Google Scholar] [CrossRef]
- Jeong, E.J.; Ma, C.J.; Lee, K.Y.; Kim, S.H.; Sung, S.H.; Kim, Y.C. KD-501, a standardized extract of Scrophularia buergeriana has both cognitive-enhancing and antioxidant activities in mice given scopolamine. J. Ethnopharmacol. 2009, 121, 98–105. [Google Scholar] [CrossRef]
- Lee, H.J.; Spandidos, D.A.; Tsatsakis, A.; Margina, D.; Izotov, B.N.; Yang, S.H. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int. J. Mol. Med. 2019, 43, 2144–2152. [Google Scholar] [CrossRef]
- Shiri, H.; Karimpour, A.; Sattari, M.; Hemmati, S.; Seyyedebrahimi, S.; Panahi, G. Evaluation of Antioxidant Potential and Free Radical Scavenging Activity of Methanol Extract from Scrophularia striata. Acta Biochim. Iran. 2023, 1, 71–77. [Google Scholar] [CrossRef]
- Zengin, G.; Stefanucci, A.; Rodrigues, M.J.; Mollica, A.; Custodio, L.; Aumeeruddy, M.Z.; Mahomoodally, M.F. Scrophularia lucida L. Scrophularia lucida L. as a valuable source of bioactive compounds for pharmaceutical applications: In vitro antioxidant, anti-inflammatory, enzyme inhibitory properties, in silico studies, and HPLC profiles. J. Pharm. Biomed. Anal. 2019, 162, 225–233. [Google Scholar] [CrossRef]
- Akşit, Z. Chemical composition and antimicrobial activity of Scrophularia catariifolia Boiss. & Heldr essential oil. J. Essent. Oil Bear. Plants 2025, 28, 37–43. [Google Scholar]
- Jafari, A.A.; Shohrati, M.; Mahmoudi, R.; Hoseini, R.H.; Nosratpour, S.; Pajohi-Alamoti, M.; Latifi, A.M. Chemical composition and biological activities of Scrophularia striata extracts. Minerva Biotecnol. 2014, 26, 183–189. [Google Scholar]
- Lin, T.; Huang, L.; Cheng, N.; Wang, Y.; Ning, Z.; Huang, S.; Wu, Y.; Chen, T.; Su, S.; Lin, Y. The in vitro and in vivo antibacterial activities of uniflorous honey from a medicinal plant, Scrophularia ningpoensis Hemsl., and characterization of its chemical profile with UPLC-MS/MS. J. Ethnopharmacol. 2022, 296, 115499. [Google Scholar] [CrossRef] [PubMed]
- Renda, G.; Kalaycı, Y.; Korkmaz, B.; Karaoglu, S.A.; Yaylı, N. Chemical Composition and Antimicrobial Activity of the Essential Oils of Five Scrophularia L. Species from Turkey. Rec. Nat. Prod. 2017, 11, 521–531. [Google Scholar] [CrossRef]
- Sharafati-chaleshtori, R.; Rafieian-kopaei, M. Screening of antibacterial effect of the Scrophularia Striata against E. coli in vitro. J. Herbmed Pharmacol. 2014, 3, 31–34. [Google Scholar]
- Stavri, M.; Mathew, K.T.; Gibbons, S. Antimicrobial constituents of Scrophularia deserti. Phytochemistry 2006, 67, 1530–1533. [Google Scholar] [CrossRef]
- Tavarideh, F.; Pourahmad, F.; Nemati, M. Diversity and antibacterial activity of endophytic bacteria associated with medicinal plant, Scrophularia striata. Vet. Res. Forum 2022, 13, 409–415. [Google Scholar]
- Yook, K.-D. Antimicrobial activity and cytotoxicity test of Scrophularia ningpoensis hemsl extracts against Klebsiella pneumoniae. J. Korea Soc. Comput. Inf. 2016, 21, 135–139. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Najafi, F.; Tahvilian, R.; Zangeneh, A.; Moradi, R. Assessment of In Vitro Antibacterial Properties of the Hydroalcoholic Extract of Scrophularia striata Against Staphylococcus aureus (ATCC No. 25923). Int. J. Pharmacogn. Phytochem. Res. 2017, 9. [Google Scholar] [CrossRef]
- Amirghofran, Z.; Naseri, N.; Kalantar, K. Anti-inflammatory activity of Echium amoenum extract on macrophages mediated by inhibition of inflammatory mediators and cytokines expression. Res. Pharm. Sci. 2018, 13, 73. [Google Scholar] [CrossRef]
- Asghari, B.; Mafakheri, S.; Zarrabi, M.; Erdem, S.; Orhan, I.; Bahadori, M. Therapeutic target enzymes inhibitory potential, antioxidant activity, and rosmarinic acid content of Echium amoenum. S. Afr. J. Bot. 2019, 120, 191–197. [Google Scholar] [CrossRef]
- Farahani, M.; Branch, Q.; Azad, I. Antiviral effect assay of aqueous extract of Echium amoenum-L against HSV-1. Zahedan J. Res. Med. Sci. 2013, 15, 46–48. [Google Scholar]
- Abolhassani, M. Antiviral activity of borage (Echium amoenum). Arch. Med. Sci. 2010, 6, 366–369. [Google Scholar] [CrossRef]
- Alamholo, M. Antiradical and antibacterial activity of Echium altissimum extracts on human infective bacteria and chemical composition analysis. Microbiol. Metab. Biotechnol. 2020, 3, 19–27. [Google Scholar]
- El-Tantawy, H.M.; Hassan, A.R.; Taha, H.E. Antioxidant potential and LC/MS metabolic profile of anticancer fractions from Echium angustifolium Mill. aerial parts. J. Appl. Pharm. Sci. 2021, 11, 200–208. [Google Scholar] [CrossRef]
- El-Tantawy, H.M.; Hassan, A.R.; Taha, H.E. Anticancer mechanism of the non-polar extract from Echium angustifolium Mill. aerial parts in relation to its chemical content. Egypt. J. Chem. 2022, 65, 17–26. [Google Scholar] [CrossRef]
- Fazeli, M. The effect of Echium amoenum hydro-methanolic extract on MCF-7 and MDA-MB468 breast cancer cell lines and increasing the cytotoxicity of doxorubicin. Iran. J. Physiol. Pharmacol. 2023, 7, 84–94. [Google Scholar]
- Abed, A.; Minaiyan, M.; Ghannadi, A.; Mahzouni, P.; Babavalian, M.R. Effect of Echium amoenum Fisch. et Mey a Traditional Iranian Herbal Remedy in an Experimental Model of Acute Pancreatitis. Int. Sch. Res. Not. 2012, 2012, 141548. [Google Scholar] [CrossRef] [PubMed]
- Kitessa, S.M.; Nichols, P.D.; Abeywardena, M. Purple Viper's Bugloss (Echium plantagineum) Seed Oil in Human Health, in Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 951–958. [Google Scholar]
- Mir, M. Echium oil: A valuable source of n-3 and n-6 fatty acids. Oléagineux Corps Gras Lipides 2008, 15, 252–256. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentão, P.; Domínguez-Perles, R.; Baenas, N.; Andrade, P.B.; Miyamoto, S. Integrated Analysis of COX-2 and iNOS Derived Inflammatory Mediators in LPS-Stimulated RAW Macrophages Pre-Exposed to Echium plantagineum L. Bee Pollen Extract. PLoS ONE 2013, 8, e59131. [Google Scholar] [CrossRef]
- Moreira, R.; Fernandes, F.; Valentão, P.; Pereira, D.M.; Andrade, P.B. Echium plantagineum L. honey: Search of pyrrolizidine alkaloids and polyphenols, anti-inflammatory potential and cytotoxicity. Food Chem. 2020, 328, 127169. [Google Scholar] [CrossRef]
- Larki, R.A.; Zayerzadeh, E.; Harzandi, N.; Anissian, A. Protective Effects of Echium amoenum on Oxidative Stress and Gene Expression Induced by Permethrin in Wistar Rats. Hepat. Mon. 2020, 20, e103774. [Google Scholar]
- Abbaszadeh, S.; Rajabian, T.; Taghizadeh, M. Antioxidant Activity, Phenolic and Flavonoid Contents of Echium Species from Different Geographical Locations of Iran. J. Med. Plants By-Prod. 2013, 2, 23–31. [Google Scholar]
- Adel Pilerood, S.; Prakash, J. Evaluation of nutritional composition and antioxidant activity of Borage (Echium amoenum) and Valerian (Valerian officinalis). J. Food Sci. Technol. 2014, 51, 845–854. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Jaradat, N.; Qneibi, M.; Hawash, M.; Emwas, N. Free radicals and enzymes inhibitory potentials of the traditional medicinal plant Echium angustifolium. Eur. J. Integr. Med. 2020, 38, 101196. [Google Scholar] [CrossRef]
- Aouadi, K.; Hajlaoui, H.; Arraouadi, S.; Ghannay, S.; Snoussi, M.; Kadri, A. HPLC/MS Phytochemical Profiling with Antioxidant Activities of Echium humile Desf. Extracts: ADMET Prediction and Computational Study Targeting Human Peroxiredoxin 5 Receptor. Agronomy 2021, 11, 2165. [Google Scholar] [CrossRef]
- Safaeian, L.; Javanmard, S.H.; Ghanadian, M.; Seifabadi, S. Cytoprotective and antioxidant effects of Echium amoenum anthocyanin-rich extract in human endothelial cells (HUVECs). Avicenna J. Phytomed. 2015, 5, 157–166. [Google Scholar] [PubMed]
- Aouadi, K.; Hajlaoui, H.; Arraouadi, S.; Ghannay, S.; Snoussi, M.; Kadri, A. Phytochemical Profiling, Antimicrobial and α-Glucosidase Inhibitory Potential of Phenolic-Enriched Extracts of the Aerial Parts from Echium humile Desf.: In Vitro Combined with In Silico Approach. Plants 2022, 11, 1131. [Google Scholar] [CrossRef]
- Kavehei, M.; Sefidroo, S.H. Investigating the Antimicrobial Activity of Different Extracts of Echium on Selected Gram-Positive and Gram-Negative Bacteria. Tabari Biomed. Stud. Res. J. 2023, 5, 18–24. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M.; Akbarzadeh, M. Chemical Composition and Antimicrobial Activity of Essential Oil of Echium italicum L. J. Essent. Oil Bear. Plants 2009, 12, 557–561. [Google Scholar] [CrossRef]
- Fatemeh, N.; Fazilati, M.; Dousti, B.; Mir Derikvand, R. Evaluation of the Antifungal effects of various Extracts of the Aerial part and Root of Echium italicum on Candida albicans compared with two common antibiotics. Yafteh 2019, 21, 122–134. [Google Scholar]
- Sabour, M.; Hakemi vala, M.; Malayeri, H.O.A. Evaluation of the antibacterial effect of Echium amoenum Fisch. et Mey. against multidrug resistant Acinetobacter baumannii strains isolated from burn wound infection. Nov. Biomed. 2015, 3, 38–42. [Google Scholar]
- Shariatifar, N.; Fathabad, A.E.; Madihi, S. Antibacterial activity of aqueous and ethanolic extracts of Echium amoenum on food-borne pathogens. J. Food Saf. Hyg. 2016, 2, 63–66. [Google Scholar]
- Tabata, M.; Tsukada, M.; Fukui, H. Antimicrobial Activity of Quinone Derivatives from Echium lycopsis Callus Cultures. Planta Medica 1982, 44, 234–236. [Google Scholar] [CrossRef]
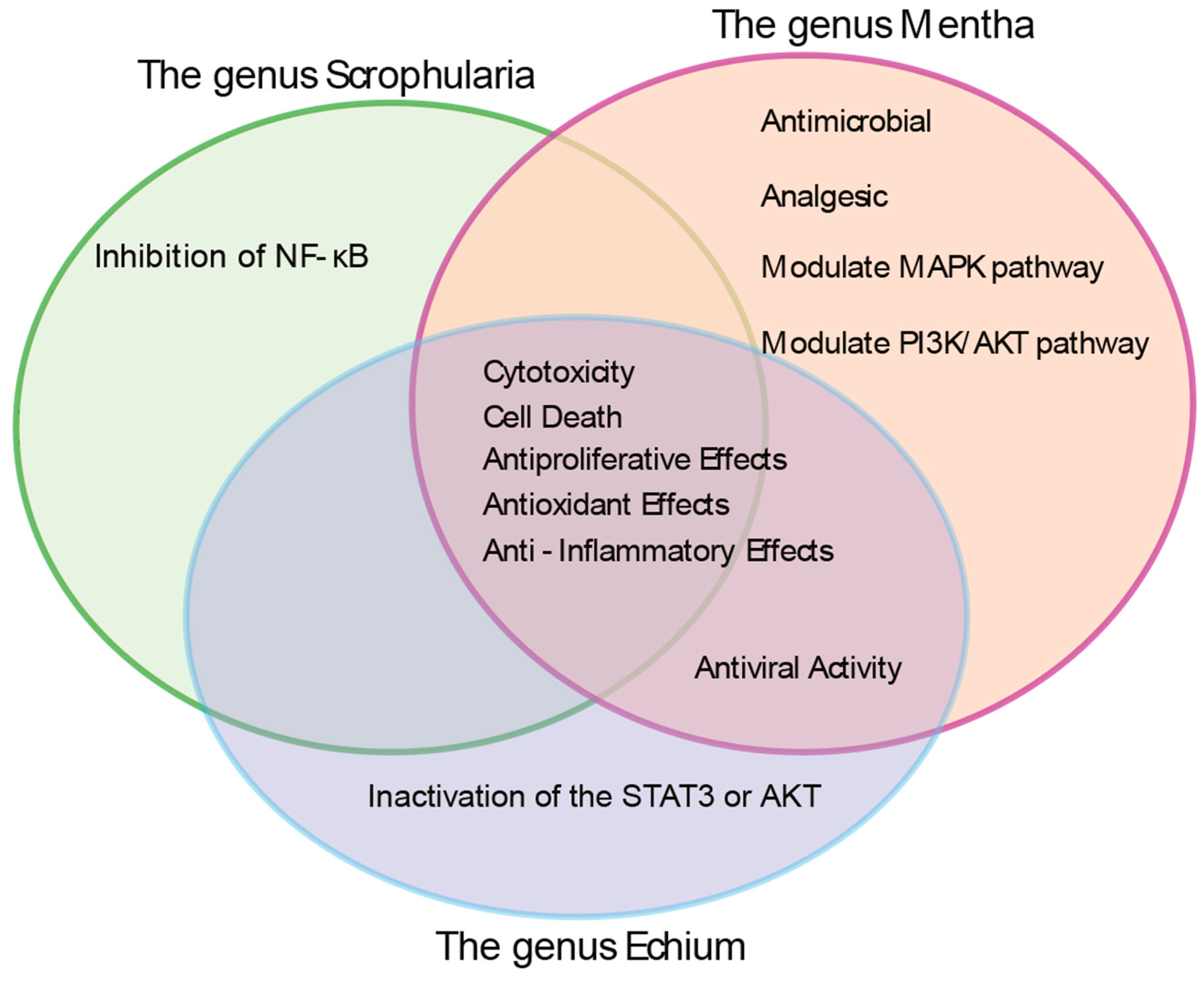
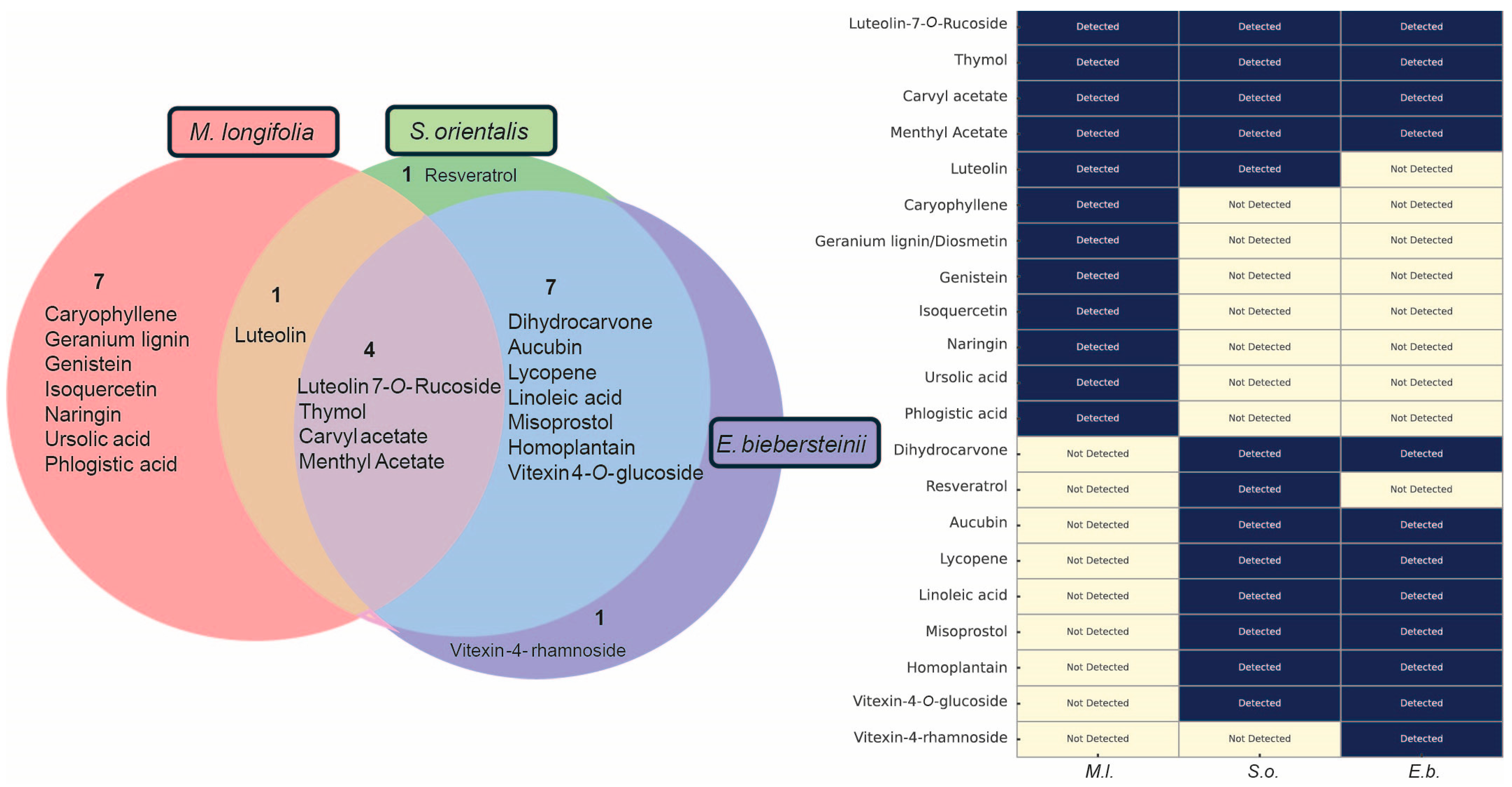
| Genus | Taxonomy | Characteristics | Distribution | Sample Used in This Study |
|---|---|---|---|---|
| Mentha | Defined as 18–30 species across five sections: Mentha, Preslia, Audibertia, Eriodontes, Pulegium. Includes M. spicata, M. aquatica, M. arvensis, M. longifolia [1,2,3,4] | Aromatic, herbaceous perennials with extensive stolons [4] | Widely distributed: Northern Pakistan, Europe, Nepal, India, Western China, Germany, UK, Egypt, Nigeria, Turkey [1] | Mentha longifolia |
| Scrophularia | Genus Scrophularia (Scrophulariaceae); ~300 species [5] | Mostly herbaceous perennials; also subshrubs, biennials, or annuals [6] | Temperate Asia, Mediterranean Europe, North America [7] | Scrophularia orientalis |
| Echium | Genus Echium (Boraginaceae); ~60 species, 30 in Canary Islands, 24 endemic [8] | Annual, biennial, or perennial flowering plants [8,9] | Native to North Africa, Europe, Macaronesia (Azores, Madeira, Canaries, Cape Verde) [8,9] | Echium biebersteinii |
| No | Compounds | Antioxidant | DNA Damage Response/Repair | Antitumor | Anti-Inflammatory |
|---|---|---|---|---|---|
| 1 | Luteolin-7-O-Rucoside | [36] | [37] | [38] | [39] |
| 2 | Thymol | [40] | [41] | [42] | [43] |
| 3 | Carvyl acetate | ||||
| 4 | Menthyl Acetate | [44] | |||
| 5 | Luteolin | [45] | [46] | [47] | [48] |
| 6 | Caryophyllene | [49] | [50] | [51] | [52] |
| 7 | Geranium lignin/Diosmetin | [53] | [54] | [55] | [56] |
| 8 | Genistein | [57] | [58] | [59] | [60] |
| 9 | Isoquercetin | [61] | [62] | [63] | |
| 10 | Naringin | [64] | [65] | [66] | |
| 11 | Ursolic acid | [67] | [68] | [69] | [70] |
| 12 | Phlogistic acid | ||||
| 13 | Dihydrocarvone | ||||
| 14 | Resveratrol | [71] | [72] | [73] | [74] |
| 15 | Aucubin | [75] | [76] | [77] | |
| 16 | Lycopene | [78] | [79] | [80] | [81] |
| 17 | Linoleic acid | [82] | [83] | [84] | |
| 18 | Misoprostol | [85] | |||
| 19 | Homoplantain | [86] | |||
| 20 | Vitexin-4-O-glucoside | [87] | |||
| 21 | Vitexin-4-rhamnoside | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, A.; Meng, Q.; Borris, R.P.; Kim, H.-M. Herbal Extract-Induced DNA Damage, Apoptosis, and Antioxidant Effects of C. elegans: A Comparative Study of Mentha longifolia, Scrophularia orientalis, and Echium biebersteinii. Pharmaceuticals 2025, 18, 1030. https://doi.org/10.3390/ph18071030
Hu A, Meng Q, Borris RP, Kim H-M. Herbal Extract-Induced DNA Damage, Apoptosis, and Antioxidant Effects of C. elegans: A Comparative Study of Mentha longifolia, Scrophularia orientalis, and Echium biebersteinii. Pharmaceuticals. 2025; 18(7):1030. https://doi.org/10.3390/ph18071030
Chicago/Turabian StyleHu, Anna, Qinghao Meng, Robert P. Borris, and Hyun-Min Kim. 2025. "Herbal Extract-Induced DNA Damage, Apoptosis, and Antioxidant Effects of C. elegans: A Comparative Study of Mentha longifolia, Scrophularia orientalis, and Echium biebersteinii" Pharmaceuticals 18, no. 7: 1030. https://doi.org/10.3390/ph18071030
APA StyleHu, A., Meng, Q., Borris, R. P., & Kim, H.-M. (2025). Herbal Extract-Induced DNA Damage, Apoptosis, and Antioxidant Effects of C. elegans: A Comparative Study of Mentha longifolia, Scrophularia orientalis, and Echium biebersteinii. Pharmaceuticals, 18(7), 1030. https://doi.org/10.3390/ph18071030







