PARP Inhibitors for Metastatic CRPC: More Answers than Questions, a Systematic Review and Meta-Analysis
Abstract
1. Introduction
- -
- What are the efficacy outcomes of PARPi-based therapies—monotherapy or in combination with ARSI—across distinct molecular subgroups in mCRPC?
- -
- What are the safety outcomes of PARPi therapies in monotherapy and combination strategies?
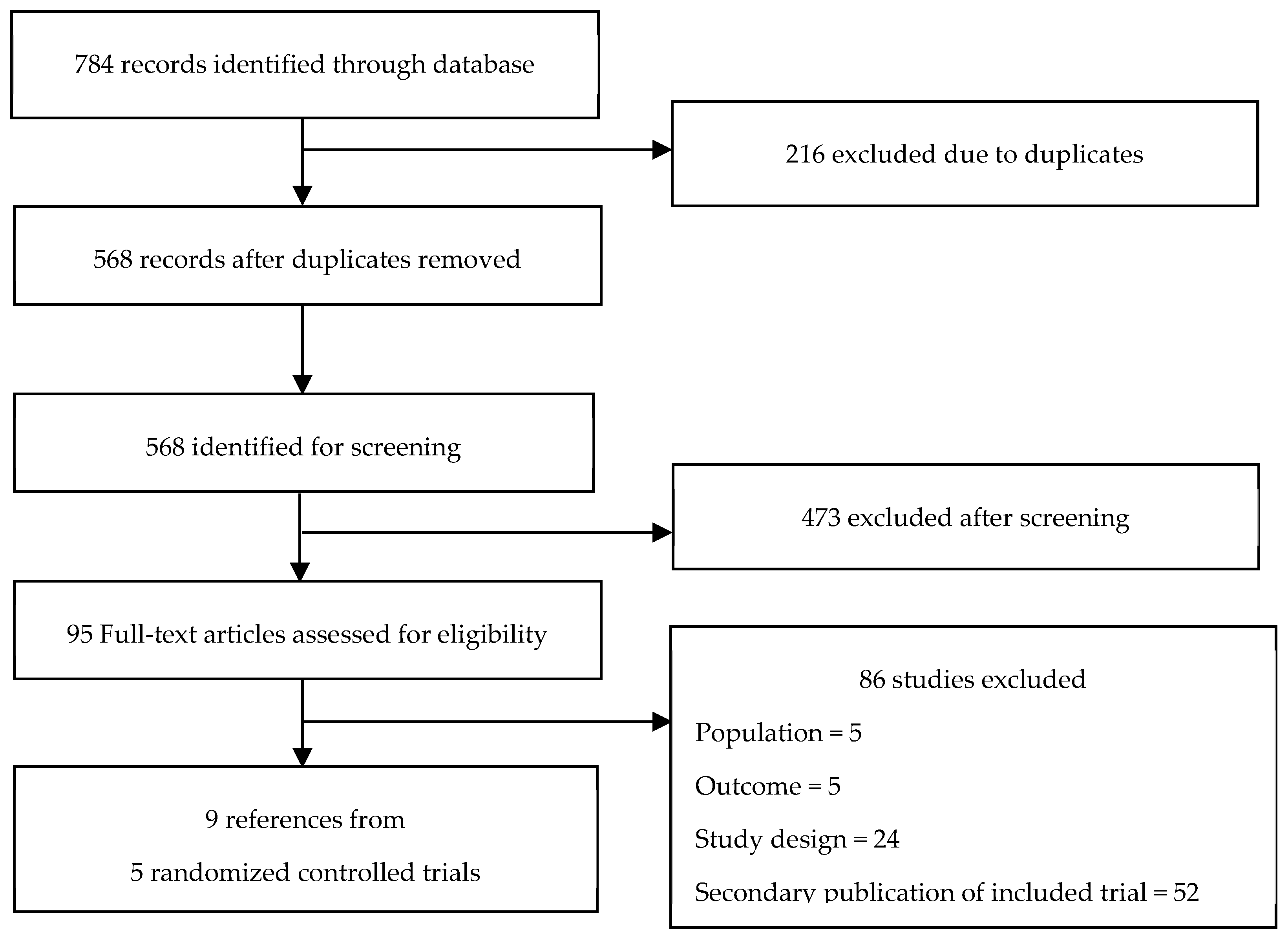
2. Methods
3. Results
3.1. Trials Characteristics
3.2. Quality Assessment
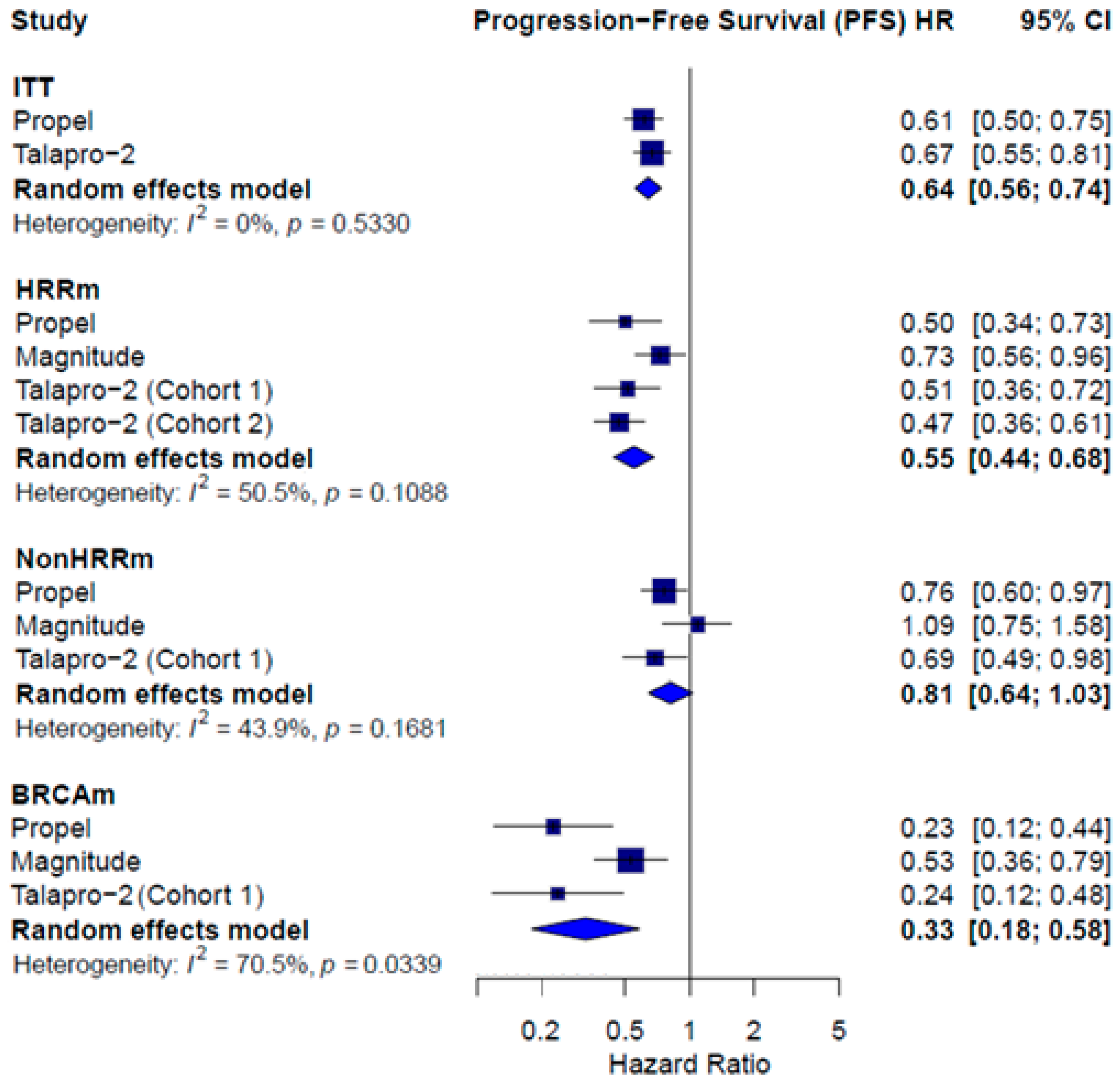
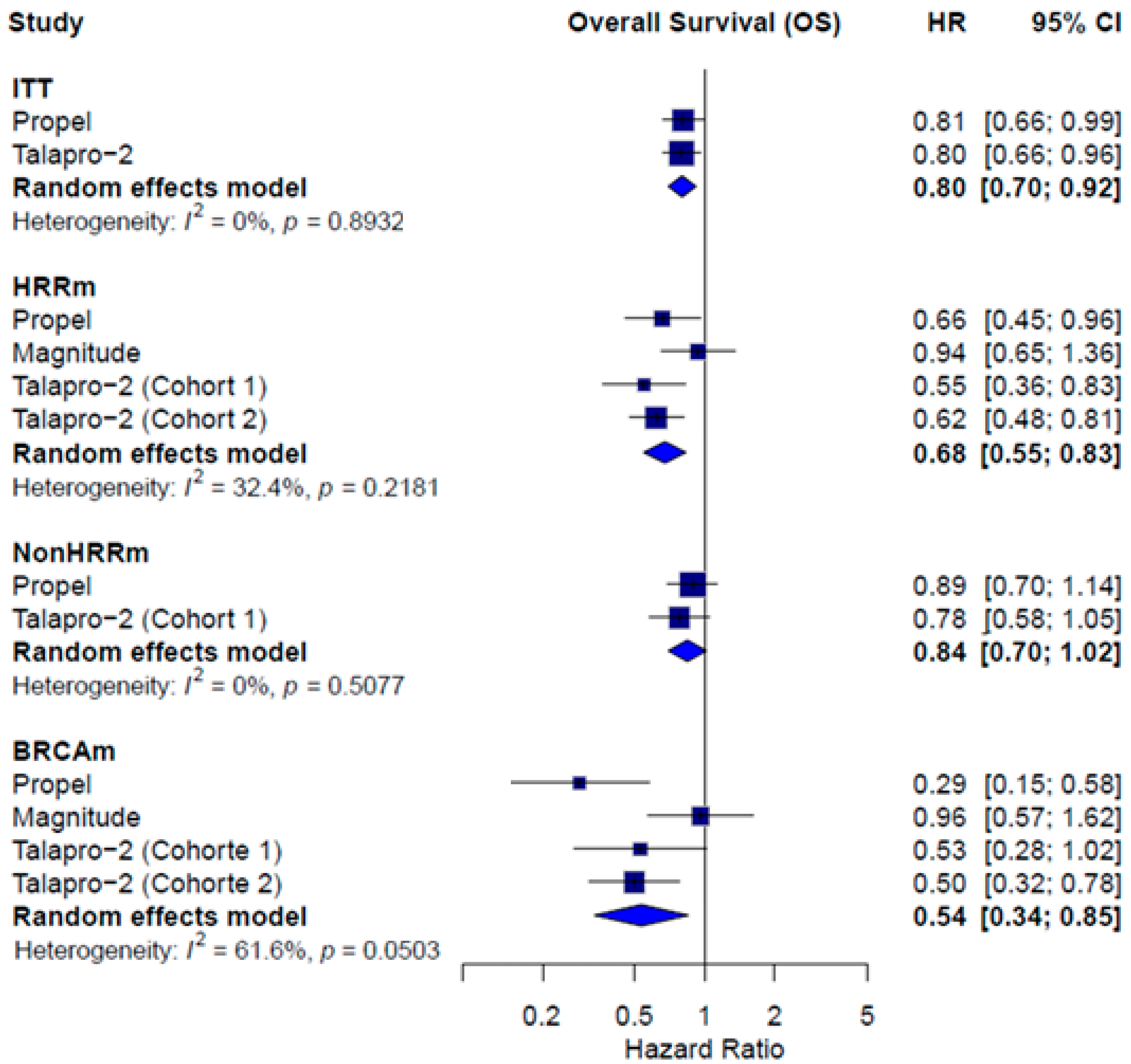
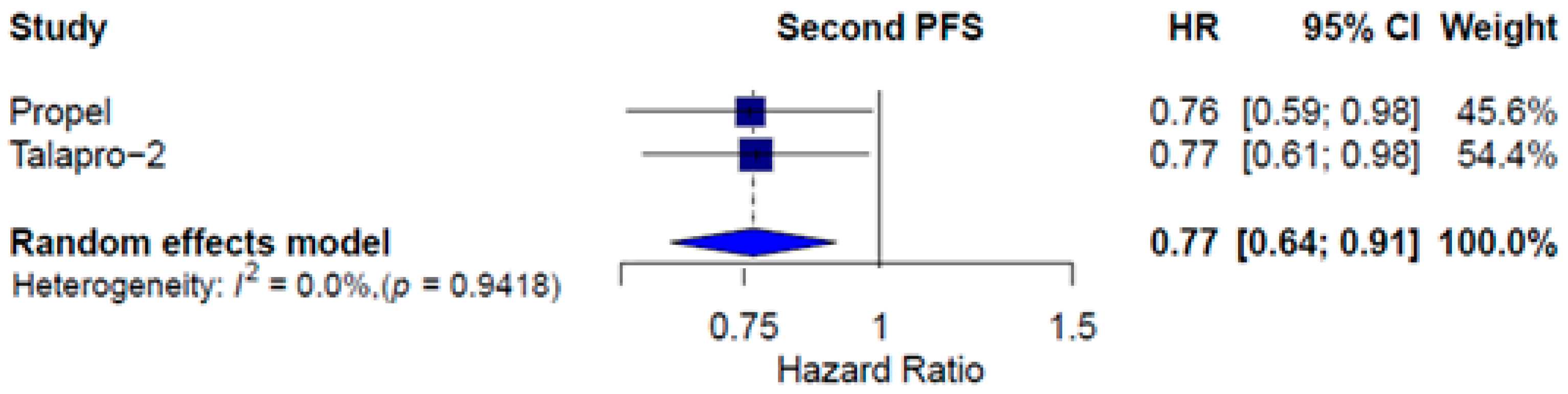
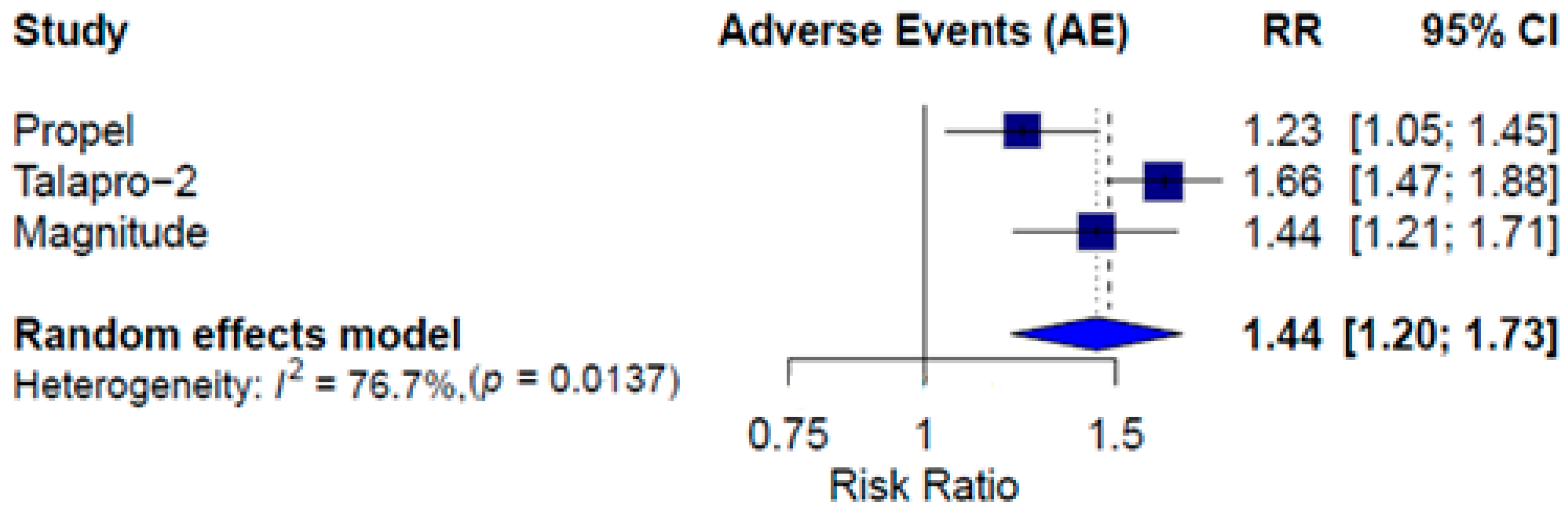
3.3. Combined Therapy Efficacy
3.4. Combined Therapy Safety
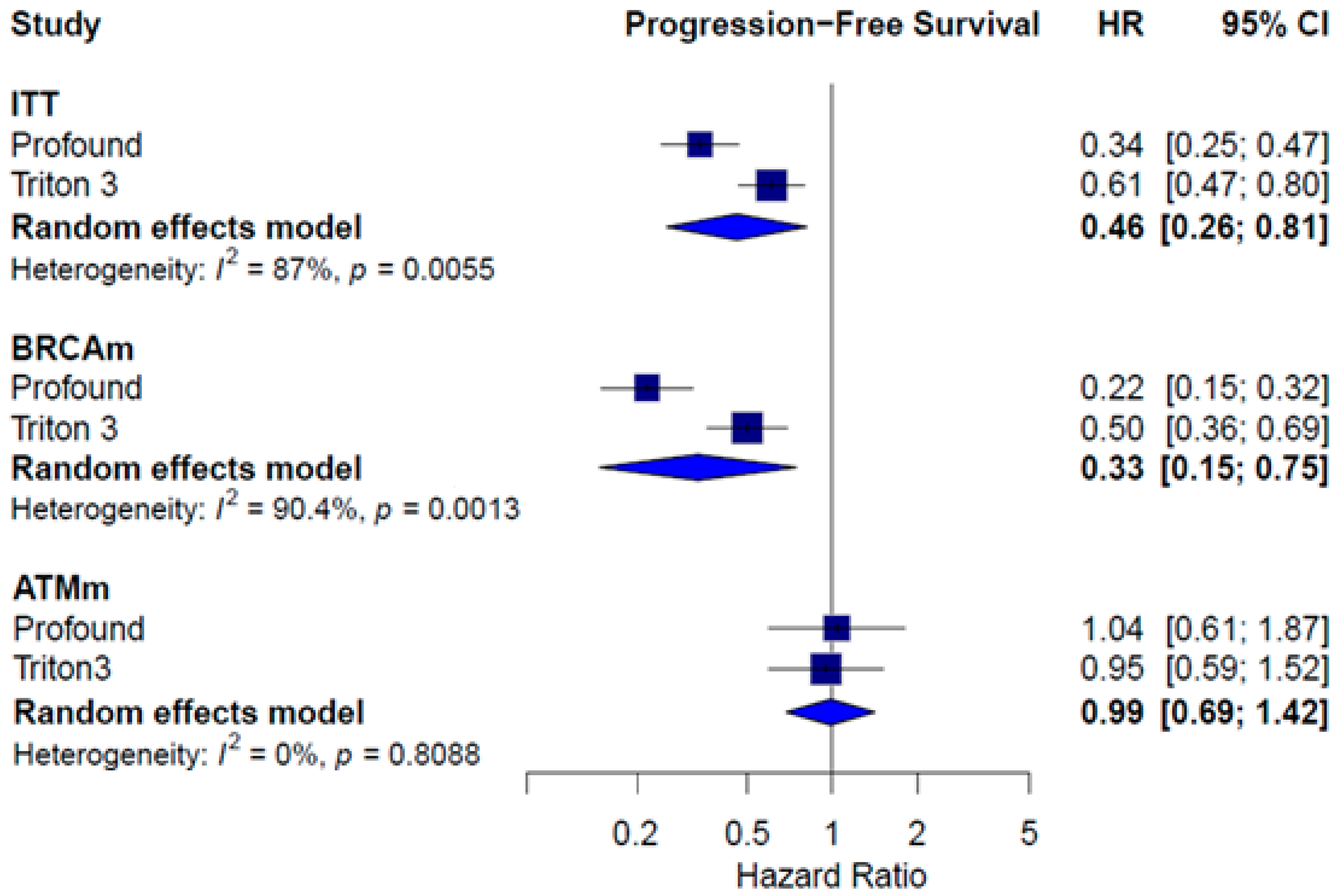
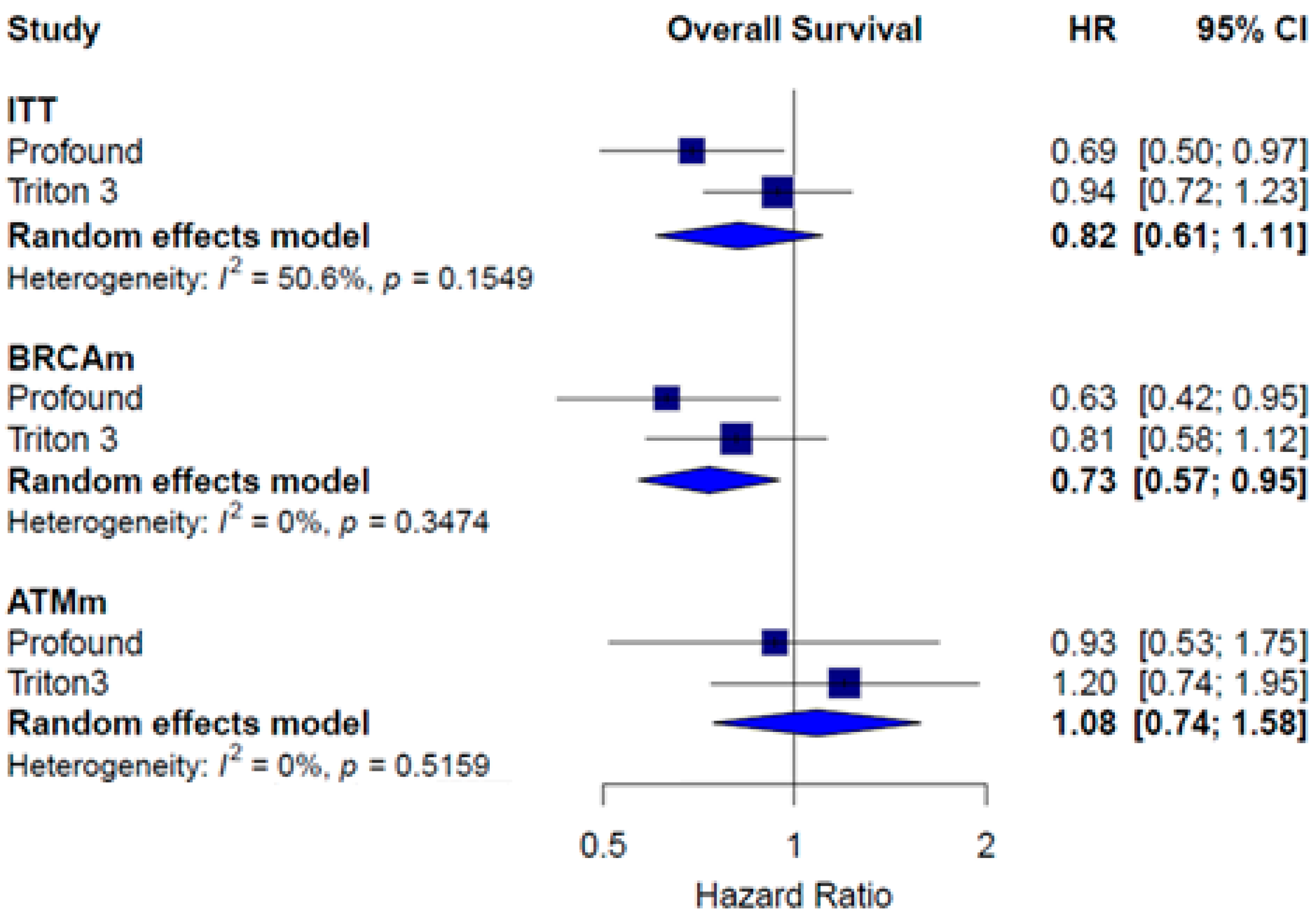
3.5. Monotherapy Efficacy
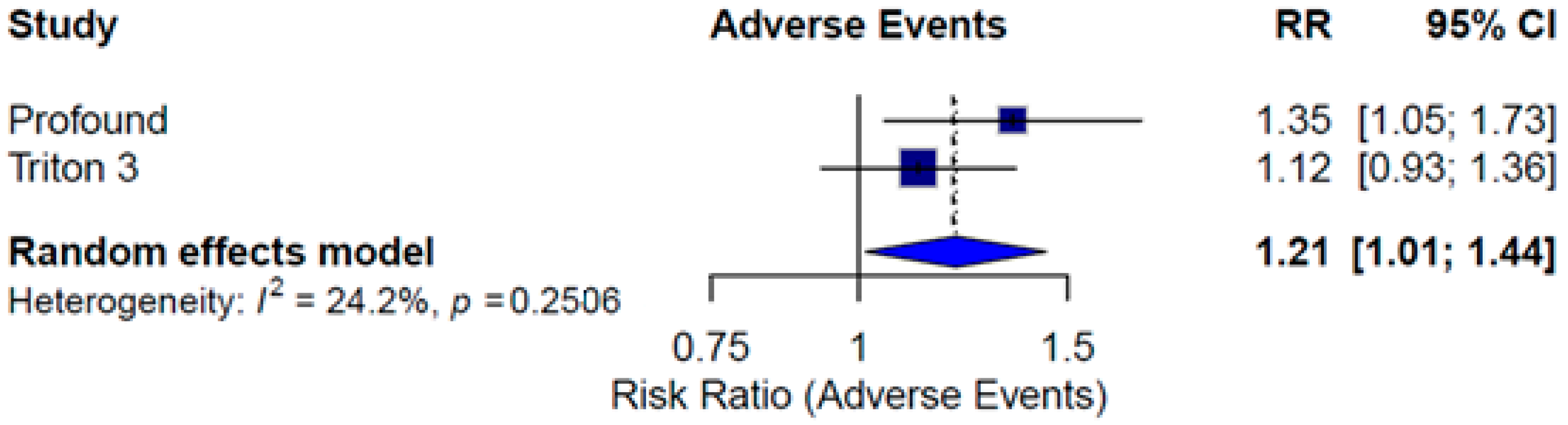
3.6. Monotherapy Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAP | Abiraterone acetate with prednisone |
| AEs | Adverse Events |
| ARPI | Androgen Receptor Pathway Inhibitor |
| ARSi | Androgen Receptor Signaling Inhibitors |
| ASCO | American Society of Clinical Oncology |
| BRCAm | BRCA mutation |
| CI | Confidence intervals |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DDR | DNA damage response |
| ESMO | European Society of Medical Oncology |
| HR | Hazard ratio |
| HRR | Homologous Recombination Repair |
| HRRm | Homologous Recombination Repair Gene Mutation |
| ITT | Intention-to-treat |
| mCRPC | Metastatic castration-resistant prostate cancer |
| mCSPC | Metastatic castration-sensitive prostate cancer |
| NGS | Next-generation sequencing |
| nmCRPC | Non-metastatic castration-resistant prostate cancer |
| non-HRRm | Without HRR mutations |
| OS | Overall survival |
| PARPi | PARP inhibitors |
| PC | Prostate cancer |
| PFS | Progression-free survival. |
| PFS2 | Second progression-free survival |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCTs | Randomized trials |
| rPFS | Radiographic progression-free survival |
| RR | Risk Ratio |
| RoB | Cochrane’s Risk of Bias |
References
- GLOBOCAN—World Health Organization. Cancer Today. Available online: https://gco.iarc.fr/today/ (accessed on 22 August 2023).
- Gandaglia, G.; Karakiewicz, P.I.; Briganti, A.; Passoni, N.M.; Schiffmann, J.; Trudeau, V.; Graefen, M.; Montorsi, F.; Sun, M. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur. Urol. 2015, 68, 325–334. [Google Scholar] [CrossRef]
- Gillessen, S.; Attard, G.; Beer, T.M.; Beltran, H.; Bjartell, A.; Bossi, A.; Briganti, A.; Bristow, R.G.; Chi, K.N.; Clarke, N. Management of patients with advanced prostate cancer: Report of the advanced prostate cancer consensus conference 2019. Eur. Urol. 2020, 77, 508–547. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Abida, W.; Armenia, J.; Gopalan, A.; Brennan, R.; Walsh, M.; Barron, D.; Danila, D.; Rathkopf, D.; Morris, M.; Slovin, S. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Olmos, D.; Lorente, D.; Alameda, D.; Cattrini, C.; Romero-Laorden, N.; Lozano, R.; Lopez-Casas, P.P.; Capone, C.; Vanden Broecke, A.M.; Trevisan, M. Presence of somatic/germline homologous recombination repair (HRR) mutations and outcomes in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) receiving first-line (1L) treatment stratified by BRCA status. J. Clin. Oncol. 2023, 41, 5003. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP inhibitors: Clinical relevance, mechanisms of action and tumor resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.; Sartor, O.; Agarwal, N.; Olmos, D. 610O Final overall survival (OS) analysis of PROfound: Olaparib vs. physician’s choice of enzalutamide or abiraterone in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations. Ann. Oncol. 2020, 31, S508. [Google Scholar] [CrossRef]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U.; et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef]
- Chi, K.N.; Rathkopf, D.; Smith, M.R.; Efstathiou, E.; Attard, G.; Olmos, D.; Lee, J.Y.; Small, E.J.; Pereira de Santana Gomes, A.J.; Roubaud, G.; et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 3339–3351. [Google Scholar] [CrossRef]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.; Loredo, E.; Procopio, G.; de Menezes, J.; Girotto, G.; Arslan, C.; et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid. 2022, 1, EVIDoa2200043. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; De Giorgi, U.; Young Joung, J.; Fong, P.C.C.; et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomised, placebo-controlled, phase 3 trial. Lancet 2023, 402, 291–303. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.; Carles, J.; Fay, A.P.; Matsubara, N.; Szczylik, C.; De Giorgi, U.; Joung, J.Y.; Fong, P.C.C.; Voog, E.; et al. Final overall survival (OS) with talazoparib (TALA) + enzalutamide (ENZA) as first-line treatment in unselected patients with metastatic castration-resistant prostate cancer (mCRPC) in the phase 3 TALAPRO-2 trial. J. Clin. Oncol. 2025, 43, LBA18. [Google Scholar] [CrossRef]
- Fizazi, K.; Azad, A.; Matsubara, N.; Carles, J.; Fay, A.P.; De Giorgi, U.; Joung, J.Y.; Fong, P.C.C.; Voog, E.; Jones, R.J.; et al. Final overall survival (OS) with talazoparib (TALA) + enzalutamide (ENZA) as first-line (1L) treatment in patients (pts) with homologous recombination repair (HRR)-deficient metastatic castration-resistant prostate cancer (mCRPC) in the phase 3 TALAPRO-2 trial. J. Clin. Oncol. 2025, 43, LBA141. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, version 6.0 (Updated July 2019). 2019. Available online: www.training.cochrane.org/handbook (accessed on 4 April 2020).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Wiercioch, W.; Brozek, J.; Etxeandia-Ikobaltzeta, I.; Mustafa, R.A.; Manja, V.; Brignardello-Petersen, R.; Neumann, I.; Falavigna, M.; Alhazzani, W. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J. Clin. Epidemiol. 2017, 81, 101–110. [Google Scholar] [CrossRef]
- Fu, R.; Gartlehner, G.; Grant, M.; Shamliyan, T.; Sedrakyan, A.; Wilt, T.J.; Griffith, L.; Oremus, M.; Raina, P.; Ismaila, A.; et al. Conducting Quantitative Synthesis When Comparing Medical Interventions: AHRQ and the Effective Health Care Program. In Methods Guide for Effectiveness and Comparative Effectiveness Reviews; AHRQ Methods for Effective Health Care; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008; Available online: http://www.ncbi.nlm.nih.gov/books/NBK49407/ (accessed on 4 April 2020).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Chi, K.N.; Sandhu, S.; Smith, M.R.; Attard, G.; Saad, M.; Olmos, D.; Castro, E.; Roubaud, G.; Pereira de Santana Gomes, A.J.; Small, E.J.; et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: Second interim analysis of the randomized phase III MAGNITUDE trial. Ann. Oncol. 2023, 34, 772–782. [Google Scholar] [CrossRef]
- Khoshkar, Y.; Westerberg, M.; Adolfsson, J.; Bill-Axelson, A.; Olsson, H.; Eklund, M.; Akre, O.; Garmo, H.; Aly, M. Mortality in men with castration-resistant prostate cancer—A long-term follow-up of a population-based real-world cohort. BJUI Compass 2022, 3, 173–183. [Google Scholar] [CrossRef]
- Wang, B.-R.; Chen, Y.-A.; Kao, W.-H.; Lai, C.-H.; Lin, H.; Hsieh, J.-T. Developing New Treatment Options for Castration-Resistant Prostate Cancer and Recurrent Disease. Biomedicines 2022, 10, 1872. [Google Scholar] [CrossRef]
- FDA Center for Drug Evaluation and Research. FDA Approves Olaparib for HRR Gene-Mutated Metastatic Castration-Resistant Prostate Cancer. FDA. 2021. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer (accessed on 22 August 2023).
- FDA Center for Drug Evaluation and Research. FDA Grants Accelerated Approval to Rucaparib for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer. FDA. 2021. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate (accessed on 22 August 2023).
- FDA Center for Drug Evaluation and Research. FDA Approves Talazoparib with Enzalutamide for HRR Gene-Mutated Metastatic Castration-Resistant Prostate Cancer. FDA. 2023. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate (accessed on 22 August 2023).
- FDA Center for Drug Evaluation and Research. FDA Approves Olaparib with Abiraterone and Prednisone (or Prednisolone) for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer. FDA. 2023. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-abiraterone-and-prednisone-or-prednisolone-brca-mutated-metastatic-castration (accessed on 22 August 2023).
- FDA Center for Drug Evaluation and Research. FDA Approves Niraparib and Abiraterone Acetate Plus Prednisone for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer. FDA. 2023. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-and-abiraterone-acetate-plus-prednisone-brca-mutated-metastatic-castration (accessed on 22 August 2023).
- Piulats, J.M.; Azad, A.A.; Laird, A.D.; Matsubara, N.; Fizazi, K.; Shore, N.D.; Karsh, L.; Liu, G.; Fay, A.P.; Carles, J.; et al. Abstract CT018: TMPRSS2-ERG and RB1 as candidate predictive biomarkers for efficacy in TALAPRO-2: Phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) vs. placebo (PBO) + ENZA as first-line (1L) treatment in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). Cancer Res. 2024, 84, CT018. [Google Scholar] [CrossRef]
- Messina, C.; Giunta, E.F.; Signori, A.; Rebuzzi, S.E.; Banna, G.L.; Maniam, A.; Buti, S.; Cattrini, C.; Fornarini, G.; Bauckneht, M.; et al. Combining PARP Inhibitors and Androgen Receptor Signalling Inhibitors in Metastatic Prostate Cancer: A Quantitative Synthesis and Meta-analysis. Eur. Urol. Oncol. 2024, 7, 179–188. [Google Scholar] [CrossRef]
- Roberto, M.; Di Civita, M.A.; Marinelli, D.; Torchia, A.; Cara, N.; Maltese, G.; Speranza, I.; Santini, D. PARP inhibitor-based treatment in metastatic, castration-resistant prostate cancer (mCRPC): A systematic review and meta-analysis. BJUI Compass 2025, 6, e455. [Google Scholar] [CrossRef]
- Sayyid, R.K.; Klaassen, Z.; Berlin, A.; Roy, S.; Brandão, L.R.; Bernardino, R.; Chavarriaga, J.; Jiang, D.M.; Spratt, D.E.; Fleshner, N.E.; et al. Poly(adenosine diphosphate-ribose) polymerase inhibitor combinations in first-line metastatic castrate-resistant prostate cancer setting: A systematic review and meta-analysis. BJU Int. 2023, 132, 619–630. [Google Scholar] [CrossRef]

| Author (Year)/ Study | Clark N, 2022 PROpel [14] | Chi K, 2023 MAGNITUDE [13,25] | Agarwal N, 2023/2025; Fizazi K, 2025 TALAPRO-2 [15,16,17] |
|---|---|---|---|
| Population | Patients with mCRPC without prior systemic treatment for mCRPC | Patients with mCRPC without prior systemic treatment for mCRPC | Patients with mCRPC without prior systemic treatment for mCRPC |
| Previous therapies | Prior docetaxel treatment Olaparib: 97 (24.3%) Placebo: 98 (24.7%) Prior treatment with NHA Olaparib: 1 (0.3%) Placebo: 0 | Androgen deprivation therapy Niraparib+AAP: 204 (96.2%) Placebo+AAP: 201 (95.3%) AR-targeted therapy for nmCRPC or mCSPC Niraparib+AAP: 8 (3.8%) Placebo+AAP: 5 (2.4%) Taxane chemotherapy for mCSPC Niraparib+AAP: 41 (19.3%) Placebo+AAP: 44 (20.9%) AAP (≤4 months) for mCRPC Niraparib+AAP: 50 (23.6%) Placebo+AAP: 48 (22.7%) Others # Niraparib+AAP: 52 (24.5%) Placebo+AAP: 58 (27.5%) | Previous docetaxel chemotherapy Talazoparib plus enzalutamide: 86 (21%) Placebo plus enzalutamide: 93 (23%) Previous treatment with novel hormonal therapy Talazoparib plus enzalutamide: 23 (6%) Placebo plus enzalutamide: 27 (7%) Abiraterone Talazoparib plus enzalutamide: 21 (5%) Placebo plus enzalutamide: 25 (6%) Orteronel Talazoparib plus enzalutamide: 2 (<1%) Placebo plus enzalutamide: 2 (<1%) |
| Intervention, n | Olaparib (300 mg BID) and AAP (1000 mg/day) N = 399 HRRm = 111 (27.8%) Non-HRRm = 279 (69.9%) BRCA1 = 9 (2.3%) BRCA2 = 38 (9.5%) | Niraparib (200 mg/day) and AAP (1000 mg/day) N = 335 HRRm = 212 (63.2%) Non-HRRm = 123 (36.7%) * * Futility analysis during the study’s development | Talazoparib (0,5 mg) and enzalutamide (160 mg/day) (N = 402) HRRm = 85 (21%) BRCA1/2 = 27 (7%) Non-HRRm = 317 (79%) |
| Comparator, n | AAP (1000 mg/day) and placebo (N = 397) HRRm = 115 (29%) Non-HRRm = 273 (68.8%) BRCA1 = 3 (0.8%); BRCA2 = 35 (8.8%) | AAP (1000 mg/day) and placebo (N = 335) HRRm = 211 (62.9%) Non-HRRm = 124 (37.1%) * * Futility analysis during the study’s development | Placebo plus enzalutamide (160 mg/day) (N = 404) HRRm = 84 (21%) BRCA1/2 = 32 (8%) Non-HRRm = 319 (79%) |
| Median PFS | ITT HR = 0.61; 95% CI 0.50 to 0.75 Subgroups HRRm HR = 0.50; 95% CI 0.34 to 0.73 Non-HRRm HR = 0.76; 95% CI 0.60 to 0.97 BRCAm HR = 0.23; 95% CI 0.12 to 0.44 | Subgroups HRRm HR = 0.73; 95% CI 0.56 to 0.96 Non-HRRm HR = 1.09; 95% CI 0.75 to 1.58 BRCAm HR = 0.53; 95% CI 0.36 to 0.79 | ITT HR = 0.67; 95% CI 0.55 to 0.81 Subgroups HRRm Talapro-2 (cohort 1) HR = 0.51; 95% CI 0.36 to 0.72 Talapro-2 (cohort 2) HR = 0.47; 95% CI 0.36 to 0.61 Non-HRRm Talapro (cohort 1) HR = 0.69; 95% CI 0.49 to 0.98 BRCAm Talapro (cohort 1) HR = 0.24; 95% CI 0.12 to 0.48 |
| Median OS | ITT HR = 0.81; 95% CI 0.66 to 0.99 Subgroup HRRm HR = 0.66; 95% CI, 0.45 to 0.96 Non-HRRm HR = 0.89; 95% CI 0.70 to 1.14 BRCAm HR = 0.29; 95% CI 0.15 to 0.58 | Subgroup HRRm HR = 0.94; 95% CI 0.65 to 1.36 BRCAm HR = 0.96; 95% CI 0.57 to 1.62 | ITT HR = 0.80; 95% CI 0.66 to 0.96 Subgroups HRRm Talapro-2 (cohort 1) HR = 0.55; 95% 0.36 to 0.83 Talapro-2 (cohort 2) HR = 0.62; 95% CI 0.48 to 0.81 Non-HRRm Talapro-2 (cohort 1) HR = 0.78; 95% CI 0.58 to 1.05 BCRAm Talapro-2 (cohort 1) HR = 0.53; 95% CI 0.28 to 1.02 Talapro-2 (cohort 2) HR = 0.50; 95% CI 0.32 to 0.78 |
| Author (Year)/ Study | De Bono, 2020 PROfound [10,11] | Fizazi, 2023 TRITON 3 [12] |
|---|---|---|
| Population | Patients with mCRPC with previous treatment with enzalutamide, abiraterone, or chemotherapy allowed Cohort A (n = 245) BRCA1, BRCA2, or ATM alteration Cohort B (n = 142) alteration in any of 12 prespecified genes (BRIP1, BARD1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, and RAD54L) | Patients with mCRPC with a BRCA1, BRCA2, or ATM alteration with previous treatment with enzalutamide, abiraterone, not chemotherapy |
| Previous therapies | Previous new hormonal agent Cohort A and B Enzalutamide only Olaparib: 105 (41%); control: 54 (41%) Abiraterone only Olaparib: 100 (39%); control: 54 (41%) Enzalutamide and abiraterone Olaparib: 51 (20%); control: 23 (18%) Cohort A Enzalutamide only Olaparib: 68 (42%); control: 40 (48%) Abiraterone only Olaparib: 62 (38%); control: 29 (35%) Enzalutamide and abiraterone Olaparib: 32 (20%); control: 14 (17%) Previous taxane use Cohort A and B Olaparib: 170 (66%); control: 84 (64%) Docetaxel only Olaparib: 115 (45%); control: 58 (44%) Cabazitaxel only Olaparib: 3 (1%); control: 0 Docetaxel and cabazitaxel Olaparib: 51 (20%); control: 26 (20%) Paclitaxel only Olaparib: 1 (<1%); control: 0 Cohort A Previous taxane use Olaparib: 106 (65%); control: 52 (63%) Docetaxel only Olaparib: 74 (46%); control: 32 (39%) Cabazitaxel only Olaparib: 2 (1%); control: 0 Docetaxel and cabazitaxel Olaparib: 29 (18%); control: 20 (24%) Paclitaxel only Olaparib: 1 (<1%); control: 0 | Second-generation ARPI Abiraterone acetate Rucaparib: 150 (56%); control: 80 (59%) Apalutamide Rucaparib: 8 (3%); control: 1 (1%) Enzalutamide Rucaparib: 119 (44%); control: 61 (45%) Docetaxel for hormone-sensitive prostate cancer Rucaparib: 63 (23%); control: 28 (21%) Therapy for castration-resistant prostate cancer 0 Rucaparib: 48 (18%); control: 26 (19%) ≥1 Rucaparib: 222 (82%); control: 109 (81%) Assigned control medication Docetaxel Rucaparib: not applicable; control: 75 (56%) Abiraterone acetate Rucaparib: not applicable; control: 28 (21%) Enzalutamide Rucaparib: not applicable; control: 32 (24%) |
| Intervention, n | Olaparib (300 mg BID) Cohort A = 162 Cohort B = 94 | Rucaparib (600 mg BID) N = 270 |
| Comparator, n | Physician’s choice of enzalutamide (160 mg/day) or AAP (1000 mg/day) Cohort A = 8 Cohort B = 48 | Physician’s choice of enzalutamide (160 mg/day) N = 32 (24%), or AAP (1000 mg/day) N = 28 (21%), or Docetaxel (75 mg/m2 every 3 weeks, up to a maximum of 10 cycles) |
| Median PFS and OS | Results for Cohort A and B: PFS (independent review) Olaparib: 5.8 months; control: 3.5 months HR = 0.49; 95% CI, 0.38–0.63; p < 0.001) OS Olaparib: 17.5 months; control: 14.3 months HR = 0.67; 95% CI 0.49–0.93; p = 0.006 Cohort A PFS (independent review) Olaparib: 7.4 months; control: 3.6 months HR = 0.34; 95% CI, 0.25 to 0.47; p < 0.001 OS Olaparib: 19.1 months; control: 14.7 months HR = 0.69; 95% CI 0.50–0.97; p = 0.006 Cohort B PFS (independent review) Olaparib: 4.8 months; control: 3.3 months HR 0.88 | Results for ITT population: PFS (independent review) Rucaparib: 10.2 (8.3–11.2) months; control: 6.4 (5.6–8.2) months HR = 0.61; 95% CI, 0.47–0.80; p < 0.001 OS Rucaparib: 23.6 (19.7–25.0) months; control: 20.9 (17.5–24.4) months HR = 0.94; 95% CI, 0.72 to 1.23 |
| Event | PROpel [14] | MAGNITUDE [13] | TALAPRO-2 [15] | |||
|---|---|---|---|---|---|---|
| Olaparib and AAP (N = 398) | Placebo and AAP (N = 396) | Niraparib and AAP (N = 212) | Placebo and AAP (N = 211) | Talazoparib and Enzalutamide (N = 398) | Placebo and Enzalutamide (N = 401) | |
| Any | 188 (47.2) | 152 (38.4) | 142 (67.0) | 98 (46.4) | 299 (75.1) | 181 (45.1) |
| Anemia | 60 (15.1) ǂ | 13 (3.3) ǂ | 63 (29.7) | 16 (7.6) | 185 (46.5) | 17 (4.2) |
| Neutropenia | 14 (6.6) | 3 (1.4) | 73 (18.3) | 6 (1.5) | ||
| Fatigue | 9 (2.3) | 6 (1.5) | 7 (3.3) | 9 (4.3) | 16 (4.0) | 8 (2.0) |
| Thrombocytopenia | 14 (6.6) | 5 (2.4) | 29 (7.3) | 4 (1.0) | ||
| Back pain | 3 (0.8) | 4 (1.0) | 51 (24.1) | 2 (1.0) | 10 (2.5) | 4 (1.0) |
| Leukopenia | 5 (2.4) | 1 (0.5) | 25 (6.3) | 0 | ||
| Decreased appetite | 4 (1.0) | 0 | 6 (2.8) | 1 (0.5) | 5 (1.3) | 4 (1.0) |
| Fall | 2 (0.9) | 6 (2.8) | 9 (2.3) | 8 (2.0) | ||
| Asthenia | 2 (0.9) | 1 (0.5) | 11 (2.8) | 3 (0.7) | ||
| Hypertension | 14 (3.5) | 13 (3.3) | 31 (14.6) | 26 (12.3) | 21 (5.3) | 30 (7.5) |
| Lymphopenia | 20 (5.0) | 4 (1.0) | ||||
| Hypokalemia | 6 (2.8) | 6 (2.8) | ||||
| Urinary tract infection | 8 (2.0) | 4 (1.0) | ||||
| Event | PROfound [10,11] | TRITON3 [12] | ||
|---|---|---|---|---|
| Olaparib (N = 256) | Control (N = 130) | Rucaparib (N = 270) | Control (N = 130) | |
| Any, n (%) | 130 (50.8) | 49 (37.7) | 161 (59.6) | 69 (53.1) |
| Anemia, n (%) | 55 (21.5) | 7 (5.4) | 64 (23.7) | 1 (0.8) |
| Neuropathy ‡, n (%) | 25 (9.3) | 4 (3.1) | ||
| Neutropenia, n (%) | 10 (3.9) | 0 | 20 (7.4) | 10 (7.7) |
| Thrombocytopenia, n (%) | 9 (3.5) | 0 | 16 (5.9) | 0 |
| Fatigue/asthenia, n (%) | 7 (2.7) | 7 (5.4) | 19 (7.0) | 12 (9.2) |
| Pneumonia, n (%) | 6 (2.3) | 1 (0.8) | ||
| Dyspnea, n (%) | 6 (2.3) | 0 | 1 (0.4) | 2 (1.5) |
| Vomiting, n (%) | 6 (2.3) | 1 (0.8) | 2 (0.7) | 1 (0.8) |
| Pulmonary embolism, n (%) | 6 (2.3) | 1 (0.8) | ||
| Urinary tract infection, n (%) | 4 (1.6) | 5 (3.8) | ||
| Hypertension, n (%) | 3 (1.2) | 3 (2.3) | 16 (5.9) | 7 (5.4) |
| Sepsis, n (%) | 3 (1.2) | 3 (2.3) | ||
| Nausea, n (%) | 3 (1.2) | 0 | 7 (2.6) | 1 (0.8) |
| Back pain, n (%) | 2 (0.8) | 2 (1.5) | 9 (3.3) | 5 (3.8) |
| Febrile neutropenia, n (%) | 2 (0.7) | 8 (6.2) | ||
| Subgroup | Treatment | PFS HR [CI] | OS HR [CI] | PFS2 HR [CI] | Grade ≥3 AEs RR [CI] |
|---|---|---|---|---|---|
| Monotherapy | ITT | 0.46 [0.26; 0.81] | 0.82 [0.61; 1.11] | 1.21 [1.01; 1.44] | |
| BRCAm | 0.33 [0.15; 0.75] | 0.73 [0.57; 0.95] | |||
| ATMm | 0.99 [0.69; 1.42] | 1.08 [0.74; 1.58] | |||
| Combined therapy | ITT | 0.64 [0.56; 0.74] | 0.80 [0.70; 0.92] | 0.77 [0.64; 0.91] | 1.44 [1.20; 1.73] |
| HRRm | 0.55 [0.44; 0.68] | 0.68 [0.55; 0.83] | |||
| Non-HRRm | 0.81 [0.64; 1.03] | 0.84 [0.70; 1.02] | |||
| BRCAm | 0.33 [0.18; 0.58] | 0.54 [0.34; 0.85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manneh, R.; Molina-Cerrillo, J.; de Velasco, G.; Ibatá, L.; Martínez, S.; Ruiz-Granados, Á.; Alonso-Gordoa, T. PARP Inhibitors for Metastatic CRPC: More Answers than Questions, a Systematic Review and Meta-Analysis. Pharmaceuticals 2025, 18, 1015. https://doi.org/10.3390/ph18071015
Manneh R, Molina-Cerrillo J, de Velasco G, Ibatá L, Martínez S, Ruiz-Granados Á, Alonso-Gordoa T. PARP Inhibitors for Metastatic CRPC: More Answers than Questions, a Systematic Review and Meta-Analysis. Pharmaceuticals. 2025; 18(7):1015. https://doi.org/10.3390/ph18071015
Chicago/Turabian StyleManneh, Ray, Javier Molina-Cerrillo, Guillermo de Velasco, Linda Ibatá, Susan Martínez, Álvaro Ruiz-Granados, and Teresa Alonso-Gordoa. 2025. "PARP Inhibitors for Metastatic CRPC: More Answers than Questions, a Systematic Review and Meta-Analysis" Pharmaceuticals 18, no. 7: 1015. https://doi.org/10.3390/ph18071015
APA StyleManneh, R., Molina-Cerrillo, J., de Velasco, G., Ibatá, L., Martínez, S., Ruiz-Granados, Á., & Alonso-Gordoa, T. (2025). PARP Inhibitors for Metastatic CRPC: More Answers than Questions, a Systematic Review and Meta-Analysis. Pharmaceuticals, 18(7), 1015. https://doi.org/10.3390/ph18071015






