Plant-Derived Exosomes: Nano-Inducers of Cross-Kingdom Regulations
Abstract
1. Introduction
2. Characterization of Exosomes
2.1. Physical Characterization of Exosomes
| Plants | Size | Shape | Zeta-Potential | Reference |
|---|---|---|---|---|
| Catharanthus roseus (L.) | 50 and 100 nm | Rounded hollow vesicle shape | −21.8 mV | [18] |
| Artemisia annua (L.) | 106.9 nm (Average) | Spherical | −22.5 mV | [33] |
| Asian ginseng (P. ginseng) | 241.1 ± 3.8 nm (Analyzed Further) 144.1 ± 2.8 nm 340.1 ± 15.9 nm | Cup-shaped | −27.4 ± 0.45 mV | [37] |
| 105.8 nm (Average) | Spherical | −20.7 mV | [51] | |
| 344.8 nm (Average) | Spherical | −25.4 mV | [52] | |
| 50–150nm | Spherical | −20.61 mV (Ultracentrifugation) −28.88mV (ExoQuick) −29.54 mV (Combination of Exo-Quick and Ultracentrifugation) | [53] | |
| 146.5 nm (Average) | Cup-shaped | −19.2 mV | [54] | |
| Arabidopsis thaliana | 50–150 nm | Spherical | −17.1 mV (Ultracentrifugation) −21.3 mV (ExoQuick) −25.9 mV (Combination of EXO-Quick and Ultracentrifugation) | [53] |
| Garlic (Allium sativum Linn) | 100 to 300 nm | Sphere-shaped | −7.8 mV | [55] |
| 100–300 nm | Sphere-shaped | −8 mV | [56] | |
| Curcumae Rhizoma (Curcuma longa L.) | 100–180 nm | Bowl-shaped | −20.90 mV | [57] |
| Cabbage (Brassica oleracea) | 100 nm (Average) | Spherical | −14.8 mV Cabbage −15.2 mV Red Cabbage | [58] |
| Tartary buckwheat (Fagopyrum tataricum) | 30–200 nm | Round- or Cup-shaped | −7.2 mV | [59] |
| Dandelion (Taraxacum officinale) | 142.5 nm (Average) | Disk-like or Spherical | −41.83 mV | [60] |
| Tomato (Solanum lycopersicum) | 140 to 170 nm | Spherical or oval-shaped | −24 mV (Approx) | [61] |
| Grapefruit (Citrus paradise) | 86 to 125 nm | Spherical or oval-shaped | −10 mV | [61] |
| Portulaca oleracea (L.) | 160 nm (Average) | Round | −31.4 mV | [62] |
| Turmeric (Curcuma longa) | 178 nm (Average) | Saucer-shaped | −21.7 mV | [63] |
2.2. Electrochemical Characterization of Exosomes
2.3. Biochemical Characterization of Exosomes
2.4. Characterization of Exosomes Based on Source
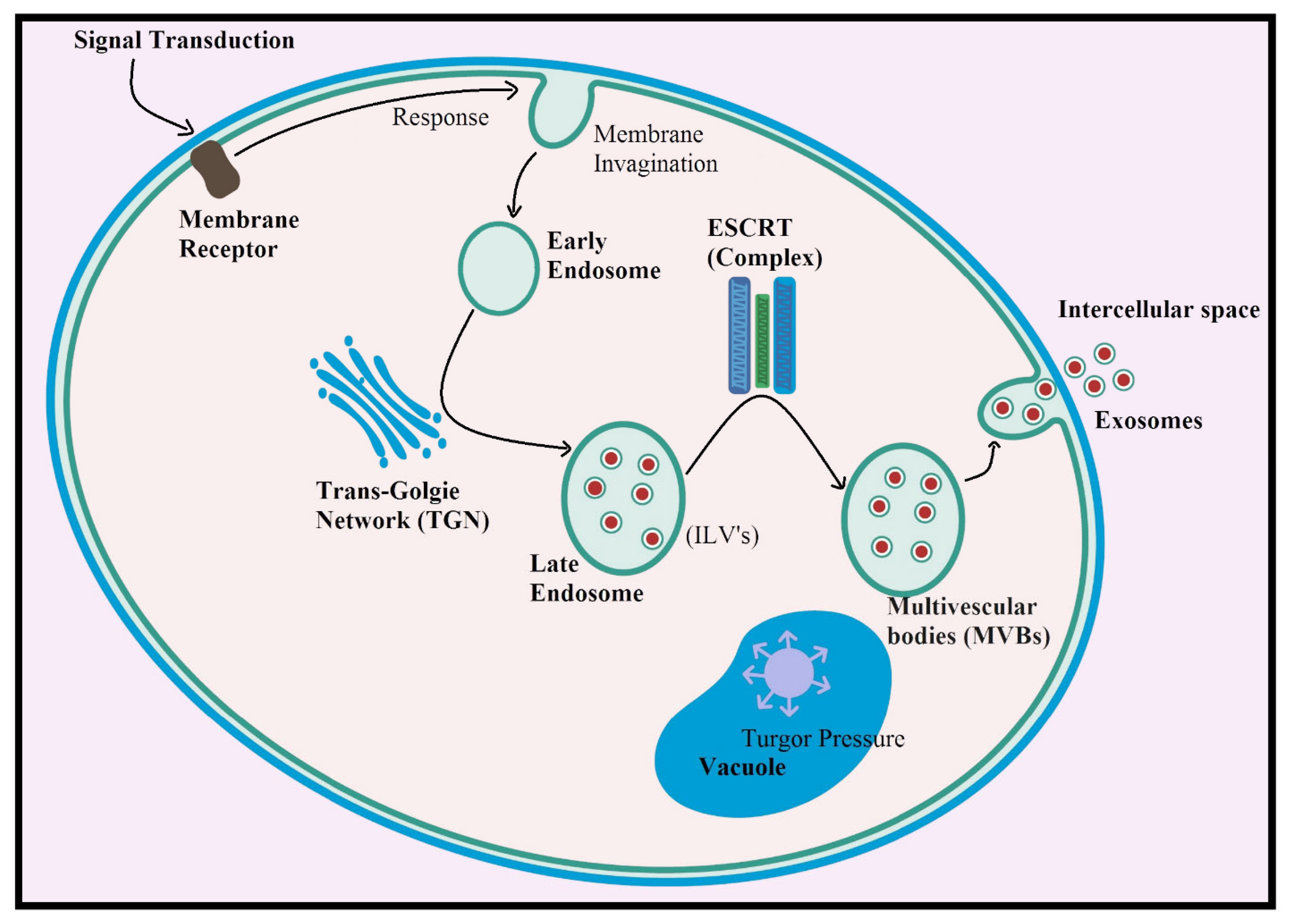
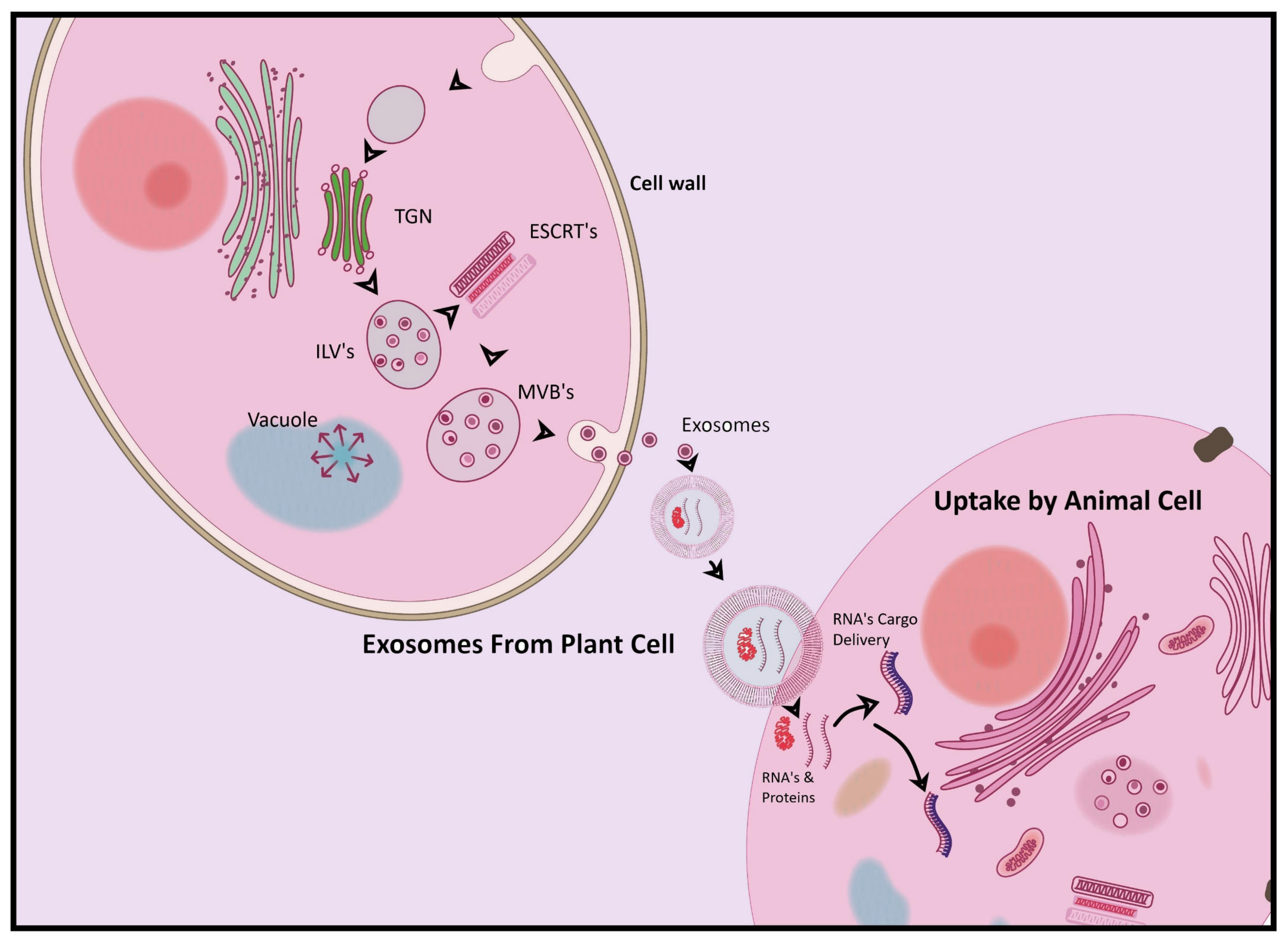
3. Biogenesis of Plant-Derived Exosomes
4. Methods for the Isolation of Exosomes
4.1. Ultracentrifugation Method
4.2. Immunoaffinity
4.3. Size-Exclusion Chromatography (SEC)
4.4. Ultrafiltration
4.5. Flow Field-Flow Fractionation
4.6. Precipitation
4.7. Other Methods of Isolation
5. Therapeutic Importance of Exosomes
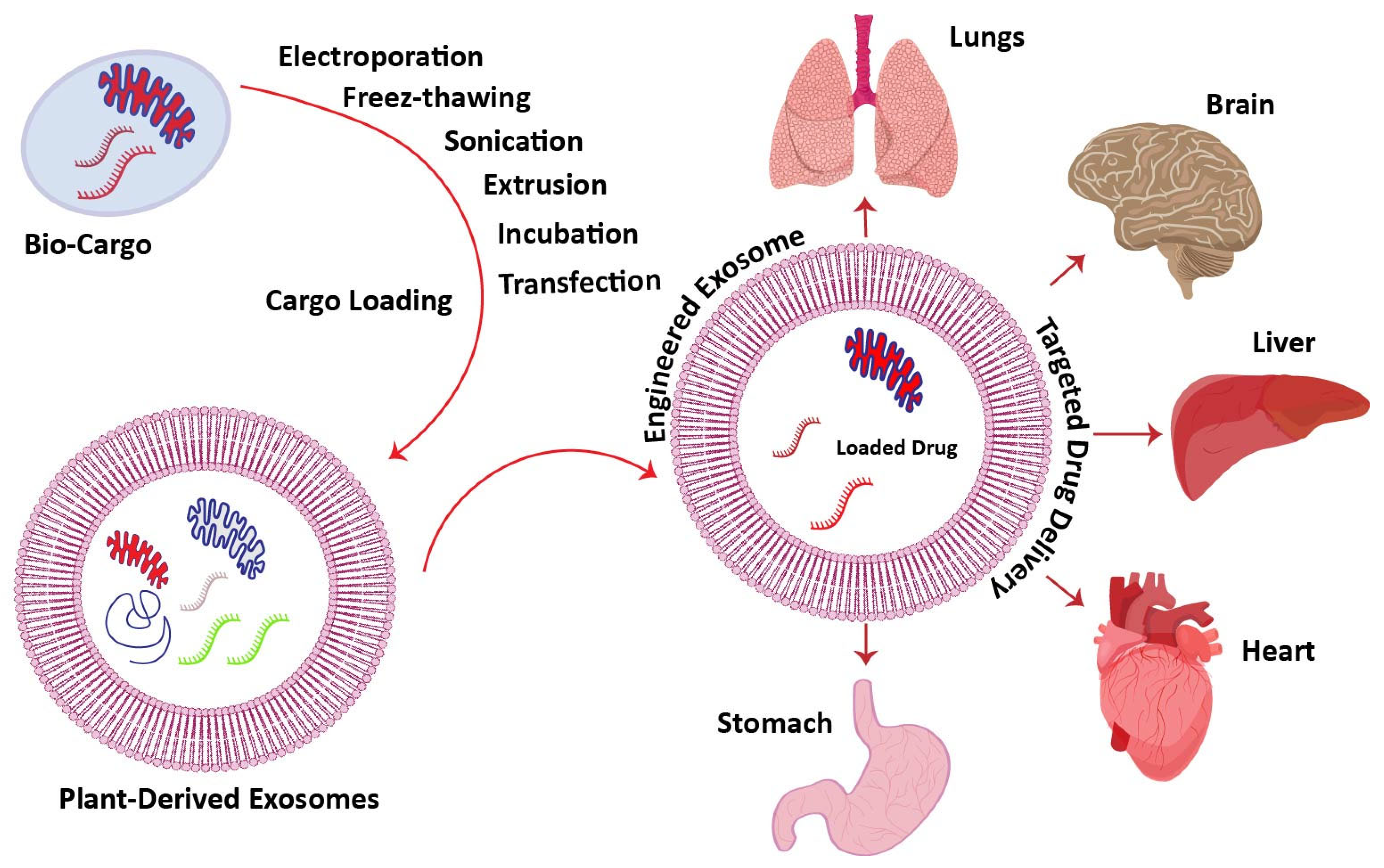
6. Plant-Derived Exosomes as a Targeted Drug Delivery Agent
6.1. Drug-Loading Methods for Targeted Treatments
6.1.1. Incubation
6.1.2. Extrusion
6.1.3. Sonication
6.1.4. Transfection
6.1.5. Electroporation
6.1.6. Freeze Thawing
7. Plant-Derived Exosomes as Cross-Kingdom Regulators
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christenhusz, M.; Byng, J. The number of known plant species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Ahmad, T. Shahabuddin. The uses of medicinal plants in the treatment of diseases. Eur. Acad. Res. 2013, 1, 1850–1853. [Google Scholar]
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants metabolites: Possibility of natural therapeutics against the COVID-19 pandemic. Front. Med. 2020, 7, 444. [Google Scholar] [CrossRef]
- Saminathan, A.; Zajac, M.; Anees, P.; Krishnan, Y. Organelle-level precision with next-generation targeting technologies. Nat. Rev. Mater. 2022, 7, 355–371. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Fox, A.S.; Yoon, S.B. DNA-induced transformation in Drosophila: Locus-specificity and the establishment of transformed stocks. Proc. Natl. Acad. Sci. USA 1970, 67, 1608–1615. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Johnstone, R.M. Exosomes biological significance: A concise review. Blood Cells Mol. Dis. 2006, 36, 315–321. [Google Scholar] [CrossRef]
- Ahn, S.H.; Ryu, S.W.; Choi, H.; You, S.; Park, J.; Choi, C. Manufacturing therapeutic exosomes: From bench to industry. Mol. Cells 2022, 45, 284–290. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Chernyshev, V.S.; Rachamadugu, R.; Tseng, Y.H.; Belnap, D.M.; Jia, Y.; Branch, K.J.; Butterfield, A.E.; Pease, L.F.; Bernard, P.S.; Skliar, M. Size and shape characterization of hydrated and desiccated exosomes. Anal. Bioanal. Chem. 2015, 407, 3285–3301. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Subha, D.; Harshnii, K.; Madhikiruba, K.G.; Nandhini, M.; Tamilselvi, K.S. Plant derived exosome-like nanovesicles: An updated overview. Plant Nano Biol. 2023, 3, 100022. [Google Scholar] [CrossRef]
- An, Q.; van Bel, A.J.E.; Hückelhoven, R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal. Behav. 2007, 2, 4–7. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Ou, X.; Wang, H.; Tie, H.; Liao, J.; Luo, Y.; Huang, W.; Yu, R.; Song, L.; Zhu, J. Novel plant-derived exosome-like nanovesicles from Catharanthus roseus: Preparation, characterization, and immunostimulatory effect via TNF-α/NF-κB/PU.1 axis. J. Nanobiotechnol. 2023, 21, 160. [Google Scholar] [CrossRef]
- Yamashita, T.; Takahashi, Y.; Takakura, Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 2018, 41, 835–842. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Oncel, S.S. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 2022, 347, 533–543. [Google Scholar] [CrossRef]
- Mahmoudi, M. The need for robust characterization of nanomaterials for nanomedicine applications. Nat. Commun. 2021, 12, 5246. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Théry, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Alzhrani, G.N.; Alanazi, S.T.; Alsharif, S.Y.; Albalawi, A.M.; Alsharif, A.A.; Abdel-Maksoud, M.S.; Elsherbiny, N. Exosomes: Isolation, characterization, and biomedical applications. Cell Biol. Int. 2021, 45, 1807–1831. [Google Scholar] [CrossRef]
- Lai, J.J.; Chau, Z.L.; Chen, S.Y.; Hill, J.J.; Korpany, K.V.; Liang, N.W.; Lin, L.H.; Lin, Y.H.; Liu, J.K.; Liu, Y.C.; et al. Exosome processing and characterization approaches for research and technology development. Adv. Sci. 2022, 9, e2103222. [Google Scholar] [CrossRef]
- Zebrowska, A.; Skowronek, A.; Wojakowska, A.; Widlak, P.; Pietrowska, M. Metabolome of exosomes: Focus on vesicles released by cancer cells and present in human body fluids. Int. J. Mol. Sci. 2019, 20, 3461. [Google Scholar] [CrossRef]
- Kugeratski, F.G.; Hodge, K.; Lilla, S.; McAndrews, K.M.; Zhou, X.; Hwang, R.F.; Zanivan, S.; Kalluri, R. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat. Cell Biol. 2021, 23, 631–641. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef]
- Elkommos-Zakhary, M.; Rajesh, N.; Beljanski, V. Exosome RNA sequencing as a tool in the search for cancer biomarkers. Non-Coding RNA 2022, 8, 75. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, S.; Li, M.; Gui, X.; Li, P. Isolation of exosome-like nanoparticles and analysis of microRNAs derived from coconut water based on small RNA high-throughput sequencing. J. Agric. Food Chem. 2018, 66, 2749–2757. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Y.; Li, Y.; Shi, T.; Luan, Y.; Yin, C. Exosome and virus infection. Front. Immunol. 2023, 14, 1154217. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, L.; Zhang, Y.; Lu, R. Plant-derived exosomes as a drug-delivery approach for the treatment of inflammatory bowel disease and colitis-associated cancer. Pharmaceutics 2022, 14, 822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, D.; Ma, X.; Wang, J.; Hou, W.; Zhang, W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed. Pharmacother. 2020, 128, 110237. [Google Scholar] [CrossRef]
- Liu, J.; Xiang, J.; Jin, C.; Ye, L.; Wang, L.; Gao, Y.; Lv, N.; Zhang, J.; You, F.; Qiao, H.; et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J. Nanobiotechnol. 2023, 21, 78. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Song, Y.; Kim, Y.; Ha, S.; Sheller-Miller, J.; Yoo, J.; Choi, C.; Park, C.H. The emerging role of exosomes as novel therapeutics: Biology, technologies, clinical applications, and the next. Am. J. Reprod. Immunol. 2021, 85, e13329. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.; Li, J.; Zeng, L. Plant-derived exosome-like nanovesicles: Current progress and prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef]
- Xu, X.-H.; Yuan, T.-J.; Dad, H.A.; Shi, M.-Y.; Huang, Y.-Y.; Jiang, Z.-H.; Peng, L.-H. Plant exosomes as novel nanoplatforms for microRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 2021, 21, 8151–8159. [Google Scholar] [CrossRef]
- Chuo, S.T.-Y.; Chien, J.C.-Y.; Lai, C.P.-K. Imaging extracellular vesicles: Current and emerging methods. J. Biomed. Sci. 2018, 25, 91. [Google Scholar] [CrossRef]
- Lim, J.; Choi, M.; Lee, H.; Kim, Y.-H.; Han, J.-Y.; Lee, E.S.; Cho, Y. Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires. J. Nanobiotechnol. 2019, 17, 1. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome theranostics: Biology and translational medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Cocozza, F.; Grisard, E.; Martin-Jaular, L.; Mathieu, M.; Théry, C. SnapShot: Extracellular vesicles. Cell 2020, 182, 262–262.e1. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, H.-T.; Wang, S.; Huang, S.-L.; Zheng, Y.; Wang, C.-Q.; Hu, B.-Y.; Qin, W.; Zou, T.-T.; Fu, Y.; et al. Isolation and characterization of exosomes for cancer research. J. Hematol. Oncol. 2020, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar] [PubMed]
- Li, M.; Li, S.; Du, C.; Zhang, Y.; Li, Y.; Chu, L.; Han, X.; Galons, H.; Zhang, Y.; Sun, H.; et al. Exosomes from different cells: Characteristics, modifications, and therapeutic applications. Eur. J. Med. Chem. 2020, 207, 112784. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, H.; Ruan, H.; Chen, Z.; Chen, S.; Wang, B.; Xie, Y. Isolation and identification of exosomes from feline plasma, urine and adipose-derived mesenchymal stem cells. BMC Vet. Res. 2021, 17, 272. [Google Scholar] [CrossRef]
- Mahdipour, E. Beta vulgaris juice contains biologically active exosome-like nanoparticles. Tissue Cell 2022, 76, 101800. [Google Scholar] [CrossRef]
- Cho, J.H.; Hong, Y.D.; Kim, D.; Park, S.J.; Kim, J.S.; Kim, H.-M.; Yoon, E.J.; Cho, J.-S. Confirmation of plant-derived exosomes as bioactive substances for skin application through comparative analysis of keratinocyte transcriptome. Appl. Biol. Chem. 2022, 65, 8. [Google Scholar] [CrossRef]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J. Pharm. Sci. 2022, 17, 53–69. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Prasad, D.; Kasiappan, R.; Chaudhari, S.R.; Sundaram, G.M. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci. Rep. 2020, 10, 4456. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.-H.; Wang, J.; Kim, Y.K.; Kwon, I.K. Isolation and characterization of ginseng-derived exosome-like nanoparticles with sucrose cushioning followed by ultracentrifugation. SN Appl. Sci. 2022, 4, 63. [Google Scholar] [CrossRef]
- Cao, M.; Yan, H.; Han, X.; Weng, L.; Wei, Q.; Sun, X.; Lu, W.; Wei, Q.; Ye, J.; Cai, X.; et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J. Immunother. Cancer 2019, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Jeong, H.; Jang, E.; Kim, E.; Yoon, Y.; Jang, S.; Jeong, H.-S.; Jang, G. Isolation of high-purity and high-stability exosomes from ginseng. Front. Plant Sci. 2023, 13, 1064412. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, S.; Zhu, Y.; Wang, R.; Wang, J. Amelioration of colitis progression by ginseng-derived exosome-like nanoparticles through suppression of inflammatory cytokines. J. Ginseng Res. 2023, 47, 627–637. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Bian, Y.; Jiang, X.; Zhu, F.; Yin, F.; Yin, L.; Song, X.; Guo, H.; Liu, J. Garlic-derived exosomes regulate PFKFB3 expression to relieve liver dysfunction in high-fat diet-fed mice via macrophage-hepatocyte crosstalk. Phytomedicine 2023, 112, 154679. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Li, W.; Jiang, X.; Yin, F.; Yin, L.; Zhang, Y.; Guo, H.; Liu, J. Garlic-derived exosomes carrying miR-396e shapes macrophage metabolic reprograming to mitigate the inflammatory response in obese adipose tissue. J. Nutr. Biochem. 2023, 113, 109249. [Google Scholar] [CrossRef]
- Yang, X.; Peng, Y.; Wang, Y.-e.; Zheng, Y.; He, Y.; Pan, J.; Liu, N.; Xu, Y.; Ma, R.; Zhai, J.; et al. Curcumae Rhizoma Exosomes-like nanoparticles loaded Astragalus components improve the absorption and enhance anti-tumor effect. J. Drug Deliv. Sci. Technol. 2023, 81, 104274. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef]
- Li, D.; Cao, G.; Yao, X.; Yang, Y.; Yang, D.; Liu, N.; Yuan, Y.; Nishinari, K.; Yang, X. Tartary buckwheat-derived exosome-like nanovesicles against starch digestion and their interaction mechanism. Food Hydrocoll. 2023, 141, 108739. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Z.; Wang, Y.; Liu, P.; Hu, K. Taraxacum officinale-derived exosome-like nanovesicles modulate gut metabolites to prevent intermittent hypoxia-induced hypertension. Biomed. Pharmacother. 2023, 161, 114572. [Google Scholar] [CrossRef]
- Kilasoniya, A.; Garaeva, L.; Shtam, T.; Spitsyna, A.; Putevich, E.; Moreno-Chamba, B.; Salazar-Bermeo, J.; Komarova, E.; Malek, A.; Valero, M.; et al. Potential of plant exosome vesicles from Grapefruit (Citrus × paradisi) and Tomato (Solanum lycopersicum) juices as functional ingredients and targeted drug delivery vehicles. Antioxidants 2023, 12, 943. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Z.; Xu, H.M.; Liang, Y.J.; Xu, J.; Yue, N.N.; Zhang, Y.; Tian, C.M.; Yao, J.; Wang, L.S.; Nie, Y.Q.; et al. Edible exosome-like nanoparticles from Portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4+CD8+T cells expansion. J. Nanobiotechnol. 2023, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Zhang, Y.; Yang, M.; Ma, Y.; Zhang, Y.; Xu, Q.; Tu, K.; Zhang, M. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J. Nanobiotechnol. 2022, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Huang, L.; Yang, Q.; Ao, Z.; Yang, R.; Krzesniak, J.; Lou, D.; Hu, L.; Dai, X.; Guo, F.; et al. Metabolomic analysis of exosomal-markers in esophageal squamous cell carcinoma. Nanoscale 2021, 13, 16457–16464. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Lu, J.; Ng, I.O.-L. Exosomes and cancer—Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022, 11, 54. [Google Scholar] [CrossRef]
- Patil, S.M.; Sawant, S.S.; Kunda, N.K. Exosomes as drug delivery systems: A brief overview and progress update. Eur. J. Pharm. Biopharm. 2020, 154, 259–269. [Google Scholar] [CrossRef]
- Öztürk, K.; Kaplan, M.; Çalış, S. Effects of nanoparticle size, shape, and zeta potential on drug delivery. Int. J. Pharm. 2024, 666, 124799. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Teng, Y.; He, J.; Zhong, Q.; Zhang, Y.; Lu, Z.; Guan, T.; Pan, Y.; Luo, X.; Feng, W.; Ou, C. Grape exosome-like nanoparticles: A potential therapeutic strategy for vascular calcification. Front. Pharmacol. 2022, 13, 1025768. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting zeta potential, the key feature of interfacial phenomena, with applications and recent advancements. ChemistrySelect 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Panigrahi, A.R.; Srinivas, L.; Panda, J. Exosomes: Insights and therapeutic applications in cancer. Transl. Oncol. 2022, 21, 101439. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Haraszti, R.A.; Didiot, M.C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef]
- Lam, S.M.; Zhang, C.; Wang, Z.; Ni, Z.; Zhang, S.; Yang, S.; Huang, X.; Mo, L.; Li, J.; Lee, B.; et al. A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat. Metab. 2021, 3, 909–922. [Google Scholar] [CrossRef]
- Peterka, O.; Jirásko, R.; Chocholoušková, M.; Kuchař, L.; Wolrab, D.; Hájek, R.; Vrána, D.; Strouhal, O.; Melichar, B.; Holčapek, M. Lipidomic characterization of exosomes isolated from human plasma using various mass spectrometry techniques. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158634. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Wang, Y.T.; Shi, T.; Srivastava, S.; Kagan, J.; Liu, T.; Rodland, K.D. Proteomic analysis of exosomes for discovery of protein biomarkers for prostate and bladder cancer. Cancers 2020, 12, 2335. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Liu, X.; Locasale, J.W. Metabolomics: A primer. Trends Biochem. Sci. 2017, 42, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Dong, J.-H.; Chen, L.; Liu, H.; Zheng, G.-E.; Chen, G.-Y.; Cheng, Y. Metabolomic identification of serum exosome-derived biomarkers for bipolar disorder. Oxidative Med. Cell. Longev. 2022, 2022, 5717445. [Google Scholar] [CrossRef]
- Ye, M.; Wang, J.; Pan, S.; Zheng, L.; Wang, Z.-W.; Zhu, X. Nucleic acids and proteins carried by exosomes of different origins as potential biomarkers for gynecologic cancers. Mol. Ther. Oncolytics 2022, 24, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cao, Y.; Köhler, J.; Lu, A.; Xu, S.; Wang, H. Unbiased RNA-Seq-driven identification and validation of reference genes for quantitative RT-PCR analyses of pooled cancer exosomes. BMC Genom. 2021, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef]
- Jiao, Y.; Xu, P.; Shi, H.; Chen, D.; Shi, H. Advances on liver cell-derived exosomes in liver diseases. J. Cell. Mol. Med. 2021, 25, 15–26. [Google Scholar] [CrossRef]
- Danesh, A.; Inglis, H.C.; Jackman, R.P.; Wu, S.; Deng, X.; Muench, M.O.; Heitman, J.W.; Norris, P.J. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood 2014, 123, 687–696. [Google Scholar] [CrossRef]
- Gao, M.; Gao, W.; Papadimitriou, J.M.; Zhang, C.; Gao, J.; Zheng, M. Exosomes—The enigmatic regulators of bone homeostasis. Bone Res. 2018, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Raimondo, S.; Alessandro, R. Extracellular vesicles from plants: Current knowledge and open questions. Int. J. Mol. Sci. 2021, 22, 5366. [Google Scholar] [CrossRef]
- Boccia, E.; Alfieri, M.; Belvedere, R.; Santoro, V.; Colella, M.; Del Gaudio, P.; Moros, M.; Dal Piaz, F.; Petrella, A.; Leone, A.; et al. Plant hairy roots for the production of extracellular vesicles with antitumor bioactivity. Commun. Biol. 2022, 5, 848. [Google Scholar] [CrossRef] [PubMed]
- Chukhchin, D.G.; Bolotova, K.; Sinelnikov, I.; Churilov, D.; Novozhilov, E. Exosomes in the phloem and xylem of woody plants. Planta 2019, 251, 12. [Google Scholar] [CrossRef] [PubMed]
- Di Raimo, R.; Mizzoni, D. Oral treatment with plant-derived exosomes restores redox balance in H2O2-treated mice. Antioxidants 2023, 12, 1169. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, G.; Liu, F.; Xie, M.; Zou, Y.; Wang, S.; Guo, Z.; Dong, J.; Ye, J.; Cao, Y.; et al. An enzyme-based system for extraction of small extracellular vesicles from plants. Sci. Rep. 2023, 13, 13931. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Fujita, D.; Arai, T.; Komori, H.; Shirasaki, Y.; Wakayama, T.; Nakanishi, T.; Tamai, I. Apple-derived nanoparticles modulate expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 cells. Mol. Pharm. 2018, 15, 5772–5780. [Google Scholar] [CrossRef]
- Trentini, M.; Zanolla, I.; Zanotti, F.; Tiengo, E.; Licastro, D.; Dal Monego, S.; Lovatti, L.; Zavan, B. Apple derived exosomes improve collagen type I production and decrease MMPs during aging of the skin through downregulation of the NF-κB pathway as mode of action. Cells 2022, 11, 3950. [Google Scholar] [CrossRef]
- Arai, M.; Komori, H.; Fujita, D.; Tamai, I. Uptake pathway of apple-derived nanoparticle by intestinal cells to deliver its cargo. Pharm. Res. 2021, 38, 523–530. [Google Scholar] [CrossRef]
- Komori, H.; Fujita, D.; Shirasaki, Y.; Zhu, Q.; Iwamoto, Y.; Nakanishi, T.; Nakajima, M.; Tamai, I. MicroRNAs in apple-derived nanoparticles modulate intestinal expression of Organic Anion-Transporting Peptide 2B1/SLCO2B1 in Caco-2 cells. Drug Metab. Dispos. 2021, 49, 803–809. [Google Scholar] [CrossRef]
- Teng, Y.; Xu, F.; Zhang, X.; Mu, J.; Sayed, M.; Hu, X.; Lei, C.; Sriwastva, M.S.; Kumar, A.; Sundaram, K.; et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol. Ther. 2021, 29, 2424–2440. [Google Scholar] [CrossRef] [PubMed]
- Ratnadewi, D.; Widjaja, C.H.; Barlian, A.; Amsar, R.M.; Ana, I.D.; Hidajah, A.C.; Notobroto, H.B.; Wungu, T.D.K. Isolation of native plant-derived exosome-like nanoparticles and their uptake by human cells. HAYATI J. Biosci. 2022, 30, 182–192. [Google Scholar] [CrossRef]
- Kalarikkal, S.P.; Sundaram, G.M. Edible plant-derived exosomal microRNAs: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol. Appl. Pharmacol. 2021, 414, 115425. [Google Scholar] [CrossRef]
- Yin, C.; Zhu, H.; Lao, Y.; Jiang, Y.; Gong, L. MicroRNAs in the exosome-like nanoparticles from orange juice inhibit Citrus blue mold caused by Penicillium italicum. LWT—Food Sci. Technol. 2023, 182, 114781. [Google Scholar] [CrossRef]
- Stanly, C.; Alfieri, M.; Ambrosone, A. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the A375 human melanoma cell line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef] [PubMed]
- Setiadi, E.V.; Adlia, A.; Barlian, A.; Ayuningtyas, D.F.; Rachmawati, H. Development and characterization of a gel formulation containing golden cherry exosomes (Physalis minima) as a potential anti-photoaging. Pharm. Nanotechnol. 2024, 12, 56–67. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Park, Y.-S.; Kim, H.-S.; Kim, D.-H.; Lee, S.-H.; Lee, C.-H.; Lee, S.-H.; Kim, J.-E.; Lee, S.; Kim, H.M.; et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J. Control. Release 2023, 355, 184–198. [Google Scholar] [CrossRef]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Kocholata, M.; Prusova, M.; Malinska, H.A.; Maly, J.; Janouskova, O. Comparison of two isolation methods of tobacco-derived extracellular vesicles, their characterization and uptake by plant and rat cells. Sci. Rep. 2022, 12, 19896. [Google Scholar] [CrossRef]
- Kim, D.K.; Rhee, W.J. Antioxidative effects of carrot-derived nanovesicles in cardiomyoblast and neuroblastoma cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.-G.; Pham, C.V.; Chowdhury, R.; Patel, S.; Jaysawal, S.K.; Hou, Y.; Xu, H.; Jia, L.; Duan, A.; Tran, P.H.-L.; et al. Development of blueberry-derived extracellular nanovesicles for immunomodulatory therapy. Pharmaceutics 2023, 15, 2115. [Google Scholar] [CrossRef]
- De Robertis, M.; Sarra, A.; D’Oria, V.; Mura, F.; Bordi, F.; Postorino, P. Blueberry-Derived Exosome-Like Nanoparticles Counter the Response to TNF-α-Induced Change on Gene Expression in EA.hy926 Cells. Biomolecules 2020, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.L.; Nielsen, M.E. Plant exosomes: Using an unconventional exit to prevent pathogen entry? J. Exp. Bot. 2017, 69, 59–68. [Google Scholar] [CrossRef]
- Wu, B.; Liu, D.A.; Guan, L.; Myint, P.K.; Chin, L.; Dang, H.; Xu, Y.; Ren, J.; Li, T.; Yu, Z.; et al. Stiff matrix induces exosome secretion to promote tumour growth. Nat. Cell Biol. 2023, 25, 415–424. [Google Scholar] [CrossRef]
- Dawson, T.R.; Weaver, A.M. Niche tension controls exosome production. Nat. Cell Biol. 2023, 25, 377–378. [Google Scholar] [CrossRef]
- Liu, J.; Shapiro, J.I. Endocytosis and signal transduction: Basic science update. Biol. Res. Nurs. 2003, 5, 117–128. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, J.; Gao, C.; Zhuang, X.; Wang, J.; Jiang, L. Biogenesis of plant prevacuolar multivesicular bodies. Mol. Plant 2016, 9, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Li, X.; Bao, H.; Wang, Z.; Wang, M.; Fan, B.; Zhu, C.; Chen, Z. Biogenesis and function of multivesicular bodies in plant immunity. Front. Plant Sci. 2018, 9, 979. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The machinery of exosomes: Biogenesis, release, and uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Dmitrieff, S.; Nédélec, F. Membrane mechanics of endocytosis in cells with turgor. PLoS Comput. Biol. 2015, 11, e1004538. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Muallem, S.; Kim, S.H.; Kwon, K.B.; Kim, M.S. Exosomal release through TRPML1-mediated lysosomal exocytosis is required for adipogenesis. Biochem. Biophys. Res. Commun. 2019, 510, 409–415. [Google Scholar] [CrossRef]
- Adams, S.D.; Csere, J.; D’angelo, G.; Carter, E.P.; Romao, M.; Arnandis, T.; Dodel, M.; Kocher, H.M.; Grose, R.; Raposo, G.; et al. Centrosome amplification mediates small extracellular vesicle secretion via lysosome disruption. Curr. Biol. 2021, 31, 1403–1416.e1407. [Google Scholar] [CrossRef]
- Wu, X.; Showiheen, S.A.A.; Sun, A.R.; Crawford, R.; Xiao, Y.; Mao, X.; Prasadam, I. Exosomes Extraction and Identification. Methods Mol. Biol. 2019, 2054, 81–91. [Google Scholar] [PubMed]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-derived exosome-like nanoparticles: A concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef]
- Dash, M.; Palaniyandi, K.; Ramalingam, S.; Sahabudeen, S.; Raja, N.S. Exosomes isolated from two different cell lines using three different isolation techniques show variation in physical and molecular characteristics. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183490. [Google Scholar] [CrossRef]
- Xu, W.-M.; Li, A.; Chen, J.-J.; Sun, E.-J. Research development on exosome separation technology. J. Membr. Biol. 2023, 256, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Trentini, M.; Zanotti, F.; Tiengo, E.; Camponogara, F.; Degasperi, M.; Licastro, D.; Lovatti, L.; Zavan, B. An apple a day keeps the doctor away: Potential role of miRNA 146 on macrophages treated with exosomes derived from apples. Biomedicines 2022, 10, 415. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Mu, J.; Li, L.; Hu, J.; Lin, H.; Cao, J.; Gao, J. An antioxidative sophora exosome-encapsulated hydrogel promotes spinal cord repair by regulating oxidative stress microenvironment. Nanomedicine 2023, 47, 102625. [Google Scholar] [CrossRef]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on strategies and technologies for exosome isolation and purification. Front. Bioeng. Biotechnol. 2021, 9, 811971. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ludwig, S.; Muller, L.; Hong, C.S.; Kirkwood, J.M.; Ferrone, S.; Whiteside, T.L. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J. Extracell. Vesicles 2018, 7, 1435138. [Google Scholar] [CrossRef]
- Yousif, G.; Qadri, S.; Parray, A.; Akhthar, N.; Shuaib, A.; Haik, Y. Exosomes derived neuronal markers: Immunoaffinity isolation and characterization. Neuromol. Med. 2022, 24, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, M.; Yan, S.; Jiang, C.; Tofaris, G.K.; Davis, J.J. Alternating magnetic field-promoted nanoparticle mixing: The on-chip immunocapture of serum neuronal exosomes for Parkinson’s disease diagnostics. Anal. Chem. 2023, 95, 7906–7913. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.O.; Ahn, H.S.; Dao, T.N.T.; Hong, J.; Shin, W.; Lim, Y.M.; Chung, S.J.; Lee, J.H.; Liu, H.; Koo, B.; et al. Magnetic transferrin nanoparticles (MTNs) assay as a novel isolation approach for exosomal biomarkers in neurological diseases. Biomater. Res. 2023, 27, 12. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Lane, R.E.; Korbie, D.; Trau, M.; Hill, M.M. Optimizing size exclusion chromatography for extracellular vesicle enrichment and proteomic analysis from clinically relevant samples. Proteomics 2019, 19, e1800156. [Google Scholar] [CrossRef]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.; Beyer, K.; Borràs, F.E. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef]
- Davis, C.N.; Phillips, H.; Tomes, J.J.; Swain, M.T.; Wilkinson, T.J.; Brophy, P.M.; Morphew, R.M. The importance of extracellular vesicle purification for downstream analysis: A comparison of differential centrifugation and size exclusion chromatography for helminth pathogens. PLoS Neglected Trop. Dis. 2019, 13, e0007191. [Google Scholar] [CrossRef]
- Mol, E.A.; Goumans, M.J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine 2017, 13, 2061–2065. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 2343. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A review of exosomal isolation methods: Is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Bellotti, C.; Lang, K.; Kuplennik, N.; Sosnik, A.; Steinfeld, R. High-grade extracellular vesicles preparation by combined size-exclusion and affinity chromatography. Sci. Rep. 2021, 11, 10550. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lian, J.Q.; Wang, P.Z.; Pan, L.; Ji, X.Y.; Bai, X.F.; Jia, Z.S. Isolation of exosomes derived from dendritic cells by ultrafiltration centrifugalization and their morphologic characteristics. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2007, 23, 1119–1121. [Google Scholar]
- He, L.; Zhu, D.; Wang, J.; Wu, X. A highly efficient method for isolating urinary exosomes. Int. J. Mol. Med. 2019, 43, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Allen, C.L.; Benjamin-Davalos, S.; Koroleva, M.; MacFarland, D.; Minderman, H.; Ernstoff, M.S. A rapid exosome isolation using ultrafiltration and size exclusion chromatography (REIUS) method for exosome isolation from melanoma cell lines. Methods Mol. Biol. 2021, 2265, 289–304. [Google Scholar]
- Guerreiro, E.M.; Vestad, B.; Steffensen, L.A.; Aass, H.C.D.; Saeed, M.; Øvstebø, R.; Costea, D.E.; Galtung, H.K.; Søland, T.M. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS ONE 2018, 13, e0204276. [Google Scholar] [CrossRef]
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.A.; Grauls, G.; Mariman, E.C.; Wouters, E.F.M.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R.; et al. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci. Rep. 2017, 7, 15297. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Wagner, M.; Holzschuh, S.; Traeger, A.; Fahr, A.; Schubert, U.S. Asymmetric flow field-flow fractionation in the field of nanomedicine. Anal. Chem. 2014, 86, 5201–5210. [Google Scholar] [CrossRef]
- Bian, J.; Gobalasingham, N.; Purchel, A.; Lin, J. The power of field-flow fractionation in characterization of nanoparticles in drug delivery. Molecules 2023, 28, 4169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053. [Google Scholar] [CrossRef]
- Wahlund, K.G. Flow field-flow fractionation: Critical overview. J. Chromatogr. A 2013, 1287, 97–112. [Google Scholar] [CrossRef]
- Gao, J.; Li, A.; Hu, J.; Feng, L.; Liu, L.; Shen, Z. Recent developments in isolating methods for exosomes. Front. Bioeng. Biotechnol. 2022, 10, 1100892. [Google Scholar] [CrossRef]
- Coughlan, C.; Bruce, K.D.; Burgy, O.; Boyd, T.D.; Michel, C.R.; Garcia-Perez, J.E.; Adame, V.; Anton, P.; Bettcher, B.M.; Chial, H.J.; et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr. Protoc. Cell Biol. 2020, 88, e110. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.H.; Kim, J.Y.; Lim, J.E.; Im, Y.H. Cytokine profiling in serum-derived exosomes isolated by different methods. Sci. Rep. 2020, 10, 14069. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Palau, X.; Serra, A.; Wong, A.S.; Sandin, S.; Lai, M.K.; Chen, C.P.; Kon, O.L.; Sze, S.K. Extracellular vesicles are rapidly purified from human plasma by PRotein Organic Solvent PRecipitation (PROSPR). Sci. Rep. 2015, 5, 14664. [Google Scholar] [CrossRef]
- Kırbaş, O.K.; Bozkurt, B.T.; Asutay, A.B.; Mat, B.; Ozdemir, B.; Öztürkoğlu, D.; Ölmez, H.; İşlek, Z.; Şahin, F.; Taşlı, P.N. Optimized isolation of extracellular vesicles from various organic sources using aqueous two-phase system. Sci. Rep. 2019, 9, 19159. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Y.; Qian, X. Target-specific exosome isolation through aptamer-based microfluidics. Biosensors 2022, 12, 2572022. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, Q.; Cheng, L.; Wang, Y.; Li, M.; Yang, Q.; Hu, L.; Lou, D.; Li, J.; Dong, X.; et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat. Methods 2021, 18, 212–218. [Google Scholar] [CrossRef]
- Jackson, K.K.; Mata, C.; Marcus, R.K. A rapid capillary-channeled polymer (C-CP) fiber spin-down tip approach for the isolation of plant-derived extracellular vesicles (PDEVs) from 20 common fruit and vegetable sources. Talanta 2023, 252, 123779. [Google Scholar] [CrossRef]
- Dyball, L.E.; Smales, C.M. Exosomes: Biogenesis, targeting, characterization and their potential as “Plug & Play” vaccine platforms. Biotechnol. J. 2022, 17, e2100646. [Google Scholar]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef] [PubMed]
- Shivji, G.G.; Dhar, R.; Devi, A. Role of exosomes and its emerging therapeutic applications in the pathophysiology of non-infectious diseases. Biomarkers 2022, 27, 534–548. [Google Scholar] [CrossRef]
- de Freitas, R.C.C.; Hirata, R.D.C.; Hirata, M.H.; Aikawa, E. Circulating extracellular vesicles as biomarkers and drug delivery vehicles in cardiovascular diseases. Biomolecules 2021, 11, 388. [Google Scholar] [CrossRef]
- Rahbarghazi, R.; Jabbari, N.; Sani, N.A.; Asghari, R.; Salimi, L.; Kalashani, S.A.; Feghhi, M.; Etemadi, T.; Akbariazar, E.; Mahmoudi, M.; et al. Tumor-derived extracellular vesicles: Reliable tools for Cancer diagnosis and clinical applications. Cell Commun. Signal. 2019, 17, 73. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Cerione, R.A.; Antonyak, M.A. Extracellular vesicles and their roles in cancer progression. Methods Mol. Biol. 2021, 2174, 143–170. [Google Scholar] [PubMed]
- Martellucci, S.; Orefice, N.S.; Angelucci, A.; Luce, A.; Caraglia, M.; Zappavigna, S. Extracellular Vesicles: New Endogenous Shuttles for miRNAs in Cancer Diagnosis and Therapy? Int. J. Mol. Sci. 2020, 21, 6486. [Google Scholar] [CrossRef]
- Salehi, M.; Sharifi, M. Exosomal miRNAs as novel cancer biomarkers: Challenges and opportunities. J. Cell. Physiol. 2018, 233, 6370–6380. [Google Scholar] [CrossRef]
- Dutta, S.; Hornung, S.; Taha, H.B.; Bitan, G. Biomarkers for parkinsonian disorders in CNS-originating EVs: Promise and challenges. Acta Neuropathol. 2023, 145, 515–540. [Google Scholar] [CrossRef]
- Hill, A.F. Extracellular vesicles and neurodegenerative diseases. J. Neurosci. 2019, 39, 9269–9273. [Google Scholar] [CrossRef] [PubMed]
- Hornung, S.; Dutta, S.; Bitan, G. CNS-derived blood exosomes as a promising source of biomarkers: Opportunities and challenges. Front. Mol. Neurosci. 2020, 13, 38. [Google Scholar] [CrossRef]
- Kavya, A.N.V.L.; Subramanian, S.; Ramakrishna, S. Therapeutic applications of exosomes in various diseases: A review. Biomater. Adv. 2022, 134, 112579. [Google Scholar]
- Rangel-Ramírez, V.V.; González-Sánchez, H.M.; Lucio-García, C. Exosomes: From biology to immunotherapy in infectious diseases. Infect. Dis. 2023, 55, 79–107. [Google Scholar] [CrossRef]
- Wang, C.; Xu, M.; Fan, Q.; Li, C.; Zhou, X. Therapeutic potential of exosome-based personalized delivery platform in chronic inflammatory diseases. Asian J. Pharm. Sci. 2023, 18, 100772. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Hall, D.C.; Rapaport, J.A.; Paradise, C.R. Exosomes and hair restoration. Adv. Cosmet. Surg. 2023, 6, 31–41. [Google Scholar] [CrossRef]
- Zhang, L.; He, F.; Gao, L.; Cong, M.; Sun, J.; Xu, J.; Wang, Y.; Hu, Y.; Asghar, S.; Hu, L.; et al. Engineering exosome-like nanovesicles derived from Asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int. J. Nanomed. 2021, 16, 1575–1586. [Google Scholar] [CrossRef]
- Barzin, M.; Bagheri, A.M.; Ohadi, M.; Abhaji, A.M.; Salarpour, S.; Dehghannoudeh, G. Application of plant-derived exosome-like nanoparticles in drug delivery. Pharm. Dev. Technol. 2023, 28, 383–402. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant exosome-like nanovesicles: Emerging therapeutics and drug delivery nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Yi, Q.; Xu, Z.; Thakur, A.; Zhang, K.; Liang, Q.; Liu, Y.; Yan, Y. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol. Res. 2023, 190, 106733. [Google Scholar] [CrossRef]
- Li, A.; Li, D.; Gu, Y.; Liu, R.; Tang, X.; Zhao, Y.; Qi, F.; Wei, J.; Liu, J. Plant-derived nanovesicles: Further exploration of biomedical function and application potential. Acta Pharm. Sin. B 2023, 13, 3300–3320. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Miller, D.P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.K.; Zhang, L.; Yan, J.; et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of Porphyromonas gingivalis. iScience 2019, 21, 308–327. [Google Scholar] [CrossRef]
- Yang, C.; Merlin, D. Nanoparticle-mediated drug delivery systems for the treatment of IBD: Current perspectives. Int. J. Nanomed. 2019, 14, 8875–8889. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Nanda, H.S.; Peng, X.; Zhou, Y. Exosomes, a new star for targeted delivery. Front. Cell Dev. Biol. 2021, 9, 751079. [Google Scholar] [CrossRef] [PubMed]
- Hamed, K.M.; Dighriri, I.M.; Baomar, A.F.; Alharthy, B.T.; Alenazi, F.E.; Alali, G.H.; Alenazy, R.H.; Alhumaidi, N.T.; Alhulayfi, D.H.; Alotaibi, Y.B.; et al. Overview of methotrexate toxicity: A comprehensive literature review. Cureus 2022, 14, e29518. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine 2017, 12, 1927–1943. [Google Scholar] [CrossRef]
- Chen, X.; Ji, S.; Yan, Y.; Lin, S.; He, L.; Huang, X.; Chang, L.; Zheng, D. Engineered plant-derived nanovesicles facilitate tumor therapy: Natural bioactivity plus drug controlled release platform. Int. J. Nanomed. 2023, 18, 4779–4804. [Google Scholar] [CrossRef]
- Kim, K.; Yoo, H.J.; Jung, J.H.; Lee, R.; Hyun, J.K.; Park, J.H.; Na, D.; Yeon, J.H. Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J. Funct. Biomater. 2020, 11, 22. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Dhar, R.; Mukerjee, N.; Mukherjee, D.; Devi, A.; Jha, S.K.; Gorai, S. Plant-derived exosomes: A new dimension in cancer therapy. Phytother. Res. 2024, 38, 1721–1723. [Google Scholar] [CrossRef]
- Yin, L.; Yan, L.; Yu, Q.; Wang, J.; Liu, C. Characterization of the MicroRNA profile of ginger exosome-like nanoparticles and their anti-Inflammatory effects in intestinal Caco-2 cells. J. Agric. Food Chem. 2022, 70, 4725–4734. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, C.; Xiao, K. Engineered extracellular vesicles-like biomimetic nanoparticles as an emerging platform for targeted cancer therapy. J. Nanobiotechnol. 2023, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, S.; Liu, L.; Dang, P.; Liu, Y.; Sun, Z.; Qiao, B.; Wang, C. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Zheng, M.; Zhu, C.; Wang, G.; Xia, Y.; Blumenthal, E.J.; Mao, W.; Wan, Y. Engineered extracellular vesicles for concurrent Anti-PDL1 immunotherapy and chemotherapy. Bioact. Mater. 2022, 9, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, H.; Shi, W.; Chen, L.; Chen, T.; Chen, G.; Wang, W.; Lan, J.; Huang, Z.; Zhang, J.; et al. Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J. Nanobiotechnol. 2021, 19, 439. [Google Scholar] [CrossRef]
- Cheng, Q.; Shi, X.; Han, M.; Smbatyan, G.; Lenz, H.J.; Zhang, Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J. Am. Chem. Soc. 2018, 140, 16413–16417. [Google Scholar] [CrossRef]
- Zeng, L.; Shi, W.; Wang, H.; Cheng, X.; Chen, T.; Wang, L.L.; Lan, J.; Sun, W. Codelivery of π-π Stacked Dual Anticancer Drugs Based on Aloe-Derived Nanovesicles for Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2022, 14, 27686–27702. [Google Scholar] [CrossRef]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef]
- Martínez-Delgado, A.A.; Khandual, S.; Villanueva–Rodríguez, S.J. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Song, Q.; Yin, X.; Song, R.; Chen, G. Preparation, characterization, and in vitro anticancer activity evaluation of broccoli-derived extracellular vesicle-coated astaxanthin nanoparticles. Molecules 2022, 27, 3955. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Mu, J.; Hu, X.; Samykutty, A.; Zhuang, X.; Deng, Z.; Zhang, L.; Cao, P.; Yan, J.; Miller, D.; et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget 2016, 7, 25683–25697. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.G.; Guo, P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Huang, H.; Yi, X.; Wei, Q.; Li, M.; Cai, X.; Lv, Y.; Weng, L.; Mao, Y.; Fan, W.; Zhao, M.; et al. Edible and cation-free kiwi fruit derived vesicles mediated EGFR-targeted siRNA delivery to inhibit multidrug resistant lung cancer. J. Nanobiotechnol. 2023, 21, 41. [Google Scholar] [CrossRef]
- del Pozo-Acebo, L.; López de las Hazas, M.-C.; Tomé-Carneiro, J.; del Saz-Lara, A.; Gil-Zamorano, J.; Balaguer, L.; Chapado, L.A.; Busto, R.; Visioli, F.; Dávalos, A. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol. Res. 2022, 185, 106472. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Raimondo, S.; Giavaresi, G.; Lorico, A.; Alessandro, R. Extracellular vesicles as biological shuttles for targeted therapies. Int. J. Mol. Sci. 2019, 20, 1848. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Khan, M.I. Plant-derived extracellular vesicles and their exciting potential as the future of next-generation drug delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
- Jeong, K.; Yu, Y.J.; You, J.Y.; Rhee, W.J.; Kim, J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab A Chip 2020, 20, 548–557. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Kenari, A.N.; Cheng, L.; Hill, A.F. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods 2020, 177, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, L.; Zhu, C.; Zheng, Q.; Wang, G.; Tong, J.; Fang, Y.; Xia, Y.; Cheng, G.; He, X.; et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Cancer Res. 2018, 78, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.W.; Kang, M.; Kim, H.Y.; Han, J.; Kang, S.; Lee, J.-R.; Jeong, G.-J.; Kwon, S.P.; Song, S.Y.; Go, S.; et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano 2018, 12, 8977–8993. [Google Scholar] [CrossRef]
- Jo, W.; Jeong, D.; Kim, J.; Cho, S.; Jang, S.C.; Han, C.; Kang, J.Y.; Gho, Y.S.; Park, J. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab A Chip 2014, 14, 1261–1269. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Xi, X.M.; Xia, S.J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie 2021, 76, 61–67. [Google Scholar]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Danilushkina, A.A.; Emene, C.C.; Barlev, N.A.; Gomzikova, M.O. Strategies for engineering of extracellular vesicles. Int. J. Mol. Sci. 2023, 24, 13247. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, X.; Huang, H.; Tang, S.; Chai, Y.; Xu, Z.; Li, M.; Chen, X.; Liu, J.; et al. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J. Nanobiotechnol. 2022, 20, 279. [Google Scholar] [CrossRef]
- Chong, Z.X.; Yeap, S.K. Transfection types, methods and strategies: A technical review. PeerJ 2021, 9, e11165. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Kandimalla, R.; Wallen, M.; Tyagi, N.; Wilcher, S.; Yan, J.; Schultz, D.J.; Spencer, W.; et al. Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett. 2021, 505, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Shirley, S.A.; Heller, R.; Heller, L.C. Chapter 7—Electroporation Gene Therapy. In Gene Therapy of Cancer, 3rd ed.; Lattime, E.C., Gerson, S.L., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 93–106. [Google Scholar]
- Nikyar, A.; Bolhassani, A. Electroporation: An effective method for in vivo gene delivery. Drug Deliv. Lett. 2022, 12, 35–45. [Google Scholar] [CrossRef]
- Lennaárd, A.J.; Mamand, D.R.; Wiklander, R.J.; El Andaloussi, S.; Wiklander, O.P.B. Optimised electroporation for loading of extracellular vesicles with doxorubicin. Pharmaceutics 2021, 14, 38. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.-A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Nguyen, T.N.G.; Lee, B.J.; Duan, W. Aspirin-loaded nanoexosomes as cancer therapeutics. Int. J. Pharm. 2019, 572, 118786. [Google Scholar] [CrossRef]
- Ferreira, D.; Moreira, J.N.; Rodrigues, L.R. New advances in exosome-based targeted drug delivery systems. Crit. Rev. Oncol. Hematol. 2022, 172, 103628. [Google Scholar] [CrossRef]
- Liu, G.; Kang, G.; Wang, S.; Huang, Y.; Cai, Q. Extracellular vesicles: Emerging players in plant defense against pathogens. Front. Plant Sci. 2021, 12, 757925. [Google Scholar] [CrossRef]
- Urzì, O.; Gasparro, R.; Ganji, N.R.; Alessandro, R. Plant-RNA in extracellular vesicles: The secret of cross-kingdom communication. Membranes 2022, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Munhoz da Rocha, I.F.; Amatuzzi, R.F.; Lucena, A.C.R.; Faoro, H.; Alves, L.R. Cross-kingdom extracellular vesicles EV-RNA communication as a mechanism for host-pathogen interaction. Front. Cell. Infect. Microbiol. 2020, 10, 593160. [Google Scholar] [CrossRef] [PubMed]
- Stanton, B.A. Extracellular vesicles and host–pathogen interactions: A review of inter-Kingdom signaling by small noncoding RNA. Genes 2021, 12, 1010. [Google Scholar] [CrossRef] [PubMed]
- Sriwastva, M.K.; Deng, Z.B.; Wang, B.; Teng, Y.; Kumar, A.; Sundaram, K.; Mu, J.; Lei, C.; Dryden, G.W.; Xu, F.; et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022, 23, e53365. [Google Scholar] [CrossRef]
- Olmi, L.; Pepe, G.; Helmer-Citterich, M.; Canini, A.; Gismondi, A. Looking for plant microRNAs in human blood samples: Bioinformatics evidence and perspectives. Plant Foods Hum. Nutr. 2023, 78, 399–406. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Y.; Zhang, K.; Liu, Y.; Liang, Q.; Thakur, A.; Liu, W.; Yan, Y. Plant-derived extracellular vesicles (PDEVs) in nanomedicine for human disease and therapeutic modalities. J. Nanobiotechnol. 2023, 21, 114. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef]
- Stanly, C.; Moubarak, M.; Fiume, I.; Turiák, L. Membrane transporters in Citrus clementina fruit juice-derived nanovesicles. Int. J. Mol. Sci. 2019, 20, 6205. [Google Scholar] [CrossRef]
- Urzì, O.; Cafora, M.; Ganji, N.R.; Tinnirello, V.; Gasparro, R.; Raccosta, S.; Manno, M.; Corsale, A.M.; Conigliaro, A.; Pistocchi, A.; et al. Lemon-derived nanovesicles achieve antioxidant and anti-inflammatory effects activating the AhR/Nrf2 signaling pathway. iScience 2023, 26, 107041. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, M.; Shao, C.; Ji, N.; Zhang, H.; Li, C. Momordica charantia-derived extracellular vesicles provide antioxidant protection in ulcerative colitis. Molecules 2023, 28, 6182. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, X.-J.; Cai, H.; Zhu, Y.-H.; Huang, L.-Y.; Wang, W.; Luo, L.; Qi, S.-H. Momordica charantia-derived extracellular vesicles-like nanovesicles inhibited glioma proliferation, migration, and invasion by regulating the PI3K/AKT signaling pathway. J. Funct. Foods 2022, 90, 104968. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, H.W.; Hwang, J.H.; Eom, J.Y.; Kim, D.H.; Park, J.; Tae, H.J. Plum-derived exosome-like nanovesicles induce differentiation of osteoblasts and reduction of osteoclast activation. Nutrients 2023, 15, 2107. [Google Scholar] [CrossRef] [PubMed]


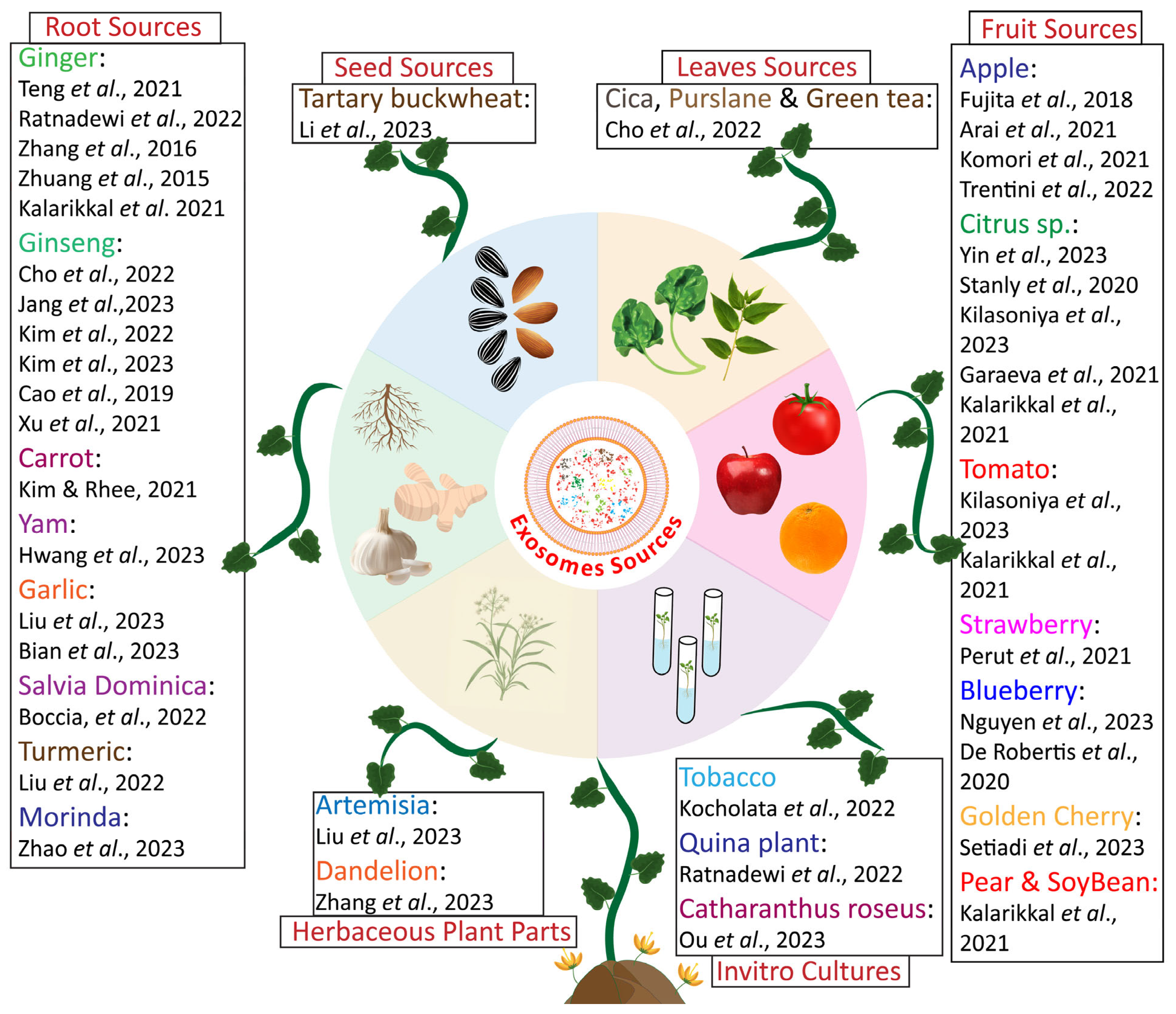
| Plant Source | Exosome Source | Isolation Method (IM) | Therapeutic Potential | Targeted Disease | Cargo Loaded | Bioactivity Validated in | Reference |
|---|---|---|---|---|---|---|---|
| Artemisia annua L. | Herbaceous plant parts | Sucrose gradient separation | Inter-kingdom communication, tumor regression | Cancer | None | Mice/Cells | [33] |
| Cica (Cantella asiatica) | Leaves | Ultracentrifugation aqueous two-phase system (ATPS) | Cosmeceutical product | Skin health/aging | None | Cells | [48] |
| Purslane (Portulaca oleracea) | Leaves | Ultracentrifugation | Cosmeceutical product | Skin health/aging | None | Cells | [48] |
| Green tea (Camellia sinensis) | Leaves | Ultracentrifugation aqueous two-phase system (ATPS) | Cosmeceutical product | Skin health/aging | None | Cells | [48] |
| Ginseng (P. ginseng) | Roots | Ultracentrifugation Aqueous two-phase systems (ATPS) | Cosmeceutical product | Skin health/aging | None | Cells | [48] |
| Ginger (Zingiber officinalis) | Rhizome Roots | Sucrose gradient separation | Prospective protective agent against alcohol induced live injury | Alcohol-induced liver damage | None | Mice/Cells | [71] |
| Rhizome | Sucrose gradient separation | Effective for the treatment and prevention of colitis-associated cancer and inflammatory bowel disease | Inflammatory bowel disease and colitis-associated cancer | None | Mice/Cells | [72] | |
| Peeled Hawaiian ginger roots | Sucrose gradient separation | Treatment of viral infections like COVID-19 | Lung inflammation | None | Mice/Cells | [102] | |
| Rhizome var. Gajah | Ultracentrifugation and precipitation (Polyethylene glycol 6000) (PEG-6000) | Potential drug delivery agent and potential nano-nutrient carrier | Not specified | None | Cells | [103] | |
| Fresh Rhizome | PEG precipitation | miRNA capacity for targeting transcriptome of SARS-CoV-2 | SARS-CoV-2 | mi-RNA | None | [104] | |
| Garlic (Allium sativum L.) | Bulbs | PEG precipitation Ultracentrifugation | Regulation of 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3) expression for inhibition of inflammatory response in mice | Nonalcoholic fatty liver disease | None | Mice/Cells | [55] |
| Bulbs | Microfiltration followed by PEG precipitation, then ultracentrifugation, followed by microfiltration | Regulation of (PFKFB3) expression for mediation of glucose metabolic reprogramming leading to attenuation of inflammatory responses | Chronic Inflammation | None | Mice/Cells | [56] | |
| Curcumae Rhizoma (Curcuma longa L.) | Rhizome | Sucrose gradient separation | Potential nano carrier for Astragalus components to enhance anti-tumor activity | Cancer | Astragalus components (AC) | Mice/Cells | [57] |
| Tartary buckwheat (Fagopyrum tataricum) | Seeds | Sucrose gradient separation | Prospective natural ingredients for the regulation of postprandial glucose | Not specified | None | None | [59] |
| Dandelion (Taraxacum officinale) | Herbaceous Part | Ultracentrifugation | Effective for the reduction in intermittent hypoxia-induced hypertension | Hypoxia-induced hypertension | None | Mice | [60] |
| Tomato (Solanum lycopersicum) | Fruit | Ultracentrifugation | Potential drug delivery agent | Not specified | None | Cells | [61] |
| Grapefruit (Citrus paradise) | Fruit | Ultracentrifugation | Potential drug delivery agent | Not specified | None | Cells | [61] |
| Fruit | Ultracentrifugation | Potential carrier of proteins to human cells | Not specified | Proteins | Mice/Cells | [109] | |
| Edible portion of fruit | PEG Precipitation | miRNA capacity for targeting the transcriptome of SARS-CoV-2 | SARS-CoV-2 | mi-RNA | None | [104] | |
| Fruit Juice | Ultracentrifugation | Inhibition of tumor proliferation | Cancer | None | Cells | [106] | |
| Turmeric (Curcuma longa) | Rhizome | Sucrose gradient separation | Colitis treatment | Ulcerative colitis | None | Mice/Cells | [63] |
| Salvia dominica | Hairy roots | Ultracentrifugation | Prospective antitumor agent | Not specified | None | Cells | [93] |
| Morinda officinalis | Roots | Ultracentrifugation | Drug carriers and therapeutic agents | Not specified | None | Mice/Cells | [96] |
| Strawberry (Fragaria x ananassa) | Fruits | Ultracentrifugation | Potential drug carrier | Not specified | None | Cells | [97] |
| Apple | Fruit (Fuji apples) | Ultracentrifugation | mRNA expression modulation of intestinal transporters | Human epithelial colorectal adenocarcinoma | None | Cells | [98] |
| Fruit (Golden Delicious) (Malus domestica sp.) | Ultracentrifugation | Induce an anti-inflammatory effect in primary dermal fibroblasts | Skin aging | None | Cells | [99] | |
| Fruit (Sun Fuji) (Mallus pumila) | Ultracentrifugation | Regulation of mRNA expression of intestinal transport materials | Not specified | None | Cells | [100] | |
| Fruit (Sun Fuji) (Malus pumila) | Ultracentrifugation | mRNA expression regulation of intestinal transporters | Not specified | None | Cells | [101] | |
| Fruit (Golden delicious) (Malus domestica sp.) | Ultracentrifugation | Anti-inflammatory effect | Inflammation | None | Cells | [129] | |
| Quina plant (Cinchona ledgeriana) | Friable Callus | Ultracentrifugation and Precipitation (Polyethylene glycol 6000) (PEG-6000) | Potential drug delivery agent and potential nano-nutrient carrier | Not specified | None | Cells | [103] |
| Citrus (Citrus reticulate) | Fruit Juice | Ultracentrifugation, followed by sucrose gradient centrifugation | Inhibition of citrus blue mold on citrus fruit | Citrus blue mold caused by Penicillium italicum (plant disease) | None | Fungus in vitro | [105] |
| Sweet orange (C. sinensis) | Fruit Juice | Ultracentrifugation | Inhibition of tumor proliferation | Cancer | None | Cells | [106] |
| Lemon (C. limon) | Fruit Juice | Ultracentrifugation | Inhibition of tumor proliferation | Cancer | None | Cells | [106] |
| Bitter orange (C. aurantium) | Fruit Juice | Ultracentrifugation | Inhibition of tumor proliferation | Cancer | None | Cells | [106] |
| Golden Cherry (Physalis minima) | Fruits | PEG precipitation | Treatment of photoaging | Anti-photoaging | None | None | [107] |
| Yam (Dioscorea japonica) | Tuber (Fresh Juice) | Sucrose gradient separation | Stimulation of osteoblasts formation in mice leading to prevention of osteoporosis | Osteoporosis | None | Mice/Cells | [108] |
| Flos Sophorae Immaturus (Sophora japonica L.) | Flowers | Ultracentrifugation | Promotion of spinal cord repair by regulation of oxidative stress in microenvironment, prospectively use for CNS diseases treatment | Spinal cord injury | None | Mice/Cells | [130] |
| Tobacco (Nicotiana tabacum) | Callus culture and BY-2 suspension culture | Ultracentrifugation and Precipitation | Potential carrier for cellular uptake | Not specified | None | Cells | [110] |
| Carrot (Daucus carota subsp. Sativus) | Fresh Juice of Edible Taproot | Ultrafiltration followed by size exclusion chromatography | Possible curative for Parkinson’s disease and myocardial infarction | Parkinson’s disease and myocardial infarction | None | Cells | [111] |
| Blueberry | Fruits (Apoplastic Fluid) | Ultracentrifugation | Immunomodulatory therapies | Not specified | None | Cells | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, T.U.; Li, H.; Martuscelli, M.; Aiello, F.; Esposito, L.; Ashraf, K.; Guo, M.; Mohsin, A. Plant-Derived Exosomes: Nano-Inducers of Cross-Kingdom Regulations. Pharmaceuticals 2025, 18, 1005. https://doi.org/10.3390/ph18071005
Rehman TU, Li H, Martuscelli M, Aiello F, Esposito L, Ashraf K, Guo M, Mohsin A. Plant-Derived Exosomes: Nano-Inducers of Cross-Kingdom Regulations. Pharmaceuticals. 2025; 18(7):1005. https://doi.org/10.3390/ph18071005
Chicago/Turabian StyleRehman, Touseef Ur, Huiliang Li, Maria Martuscelli, Francesca Aiello, Luigi Esposito, Kamran Ashraf, Meijin Guo, and Ali Mohsin. 2025. "Plant-Derived Exosomes: Nano-Inducers of Cross-Kingdom Regulations" Pharmaceuticals 18, no. 7: 1005. https://doi.org/10.3390/ph18071005
APA StyleRehman, T. U., Li, H., Martuscelli, M., Aiello, F., Esposito, L., Ashraf, K., Guo, M., & Mohsin, A. (2025). Plant-Derived Exosomes: Nano-Inducers of Cross-Kingdom Regulations. Pharmaceuticals, 18(7), 1005. https://doi.org/10.3390/ph18071005












