Recent Trends in Bioinspired Metal Nanoparticles for Targeting Drug-Resistant Biofilms
Abstract
1. Introduction
2. Nanotechnology Approaches in Combatting MDR Biofilms
3. Overview of Metal Nanoparticles Synthesis
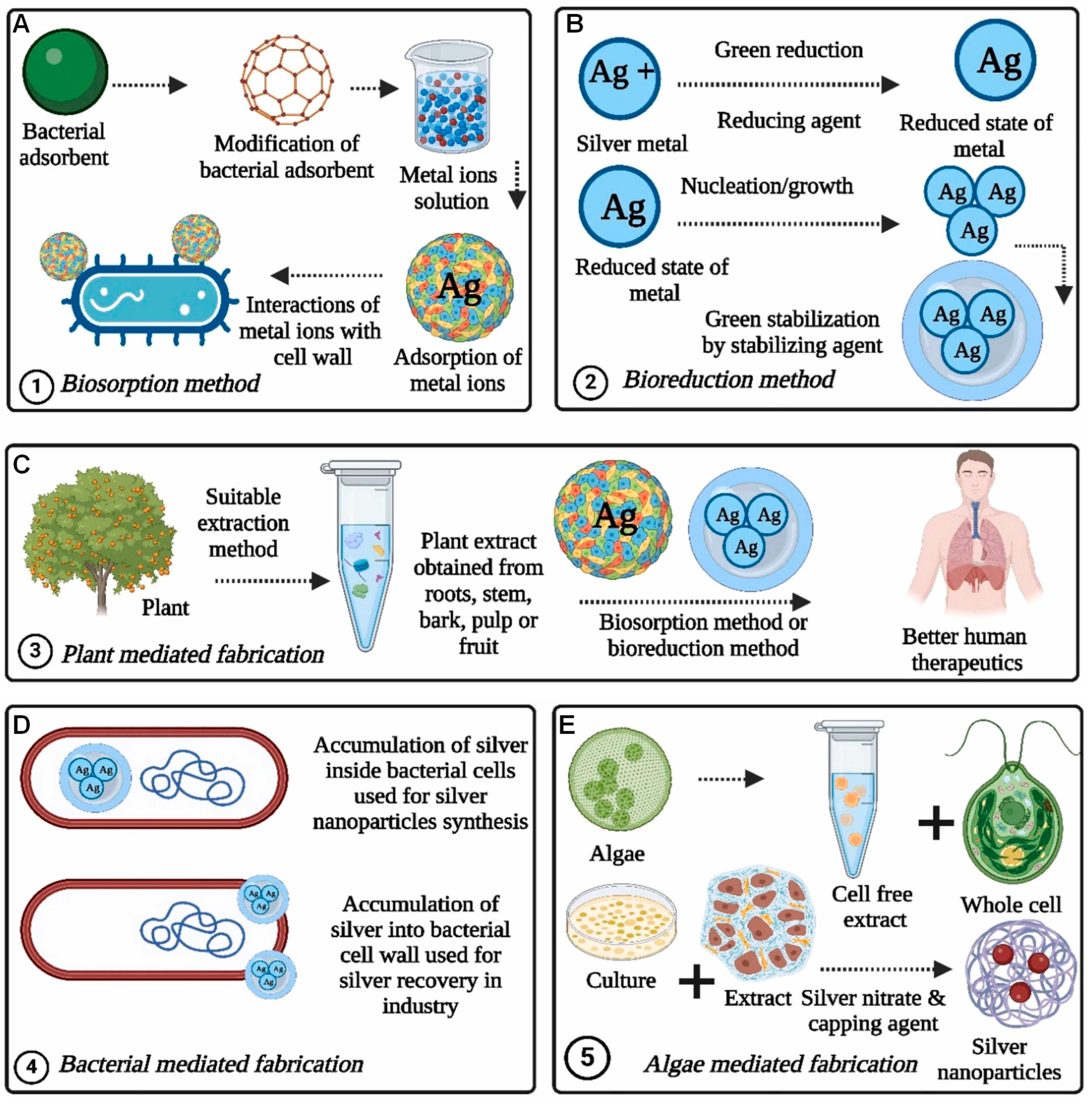
4. Bio-Inspired Synthesis of MNPs
5. Plant-Mediated Synthesis of MNPs
6. Microbial Synthesis of MNPs
6.1. Intracellular Synthesis of MNPs
6.2. Extracellular Synthesis of MNPs
| Microorganisms | Description | Extracellular/ Intracellular | Types of NPs | Shape | Size (nm) | References |
|---|---|---|---|---|---|---|
| Lysinibacillus sp. | Bacteria | Extracellular | Ag | Quasi-spherical | 7.5–14.7 | Pernas-Pleite et al. [114] |
| Cupriavidus sp. | Bacteria | Extracellular | Ag | Spherical | 10–50 | Ameen et al. [115] |
| Phormidesmis communis Strain AB_11_10 | Bacteria | Extracellular | Au | Quasi-spherical, triangular, and rectangular | 2–28 | Hamida et al. [116] |
| Streptomyces olivaceus (MSU3) | Bacteria | Extracellular | Ag | Spherical | 12.3 | Sanjivkumar et al. [117] |
| Alcaligenes sp. | Bacteria | Extracellular | Ag | Spherical | 30–50 | Divya et al. [118] |
| Penicillium chrysogenum MF318506 | Fungi | Extracellular | Ag | Spherical | 9.75–25.21 | Abd El Aty et al. [119] |
| Desmodesmus sp. | Algae | Intracellular | Ag | Spherical | 15–30 | Dağlıoğlu and Öztürk [120] |
| Portieria hornemannii | Algae | Extracellular | Ag | Spherical | 60–70 | Fatima et al. [121] |
| Micrococcus yunnanensis J2 | Bacteria | Extracellular | Au | Spherical | 53.8 | Jafari et al. [122] |
| Ganoderma applanatum | Fungi | Extracellular | Au | Cubic | 18.7 | Abdul-Hadi et al. [123] |
| Pseudoalteromonas sp. Bac178 | Bacteria | Intracellular | Au | Spherical | 26.12 | Patil et al. [100] |
| Gluconacetobacter liquefaciens | Bacteria | Intracellular | Au | Spherical | 11,232 | Liu et al. [99] |
| Paracoccus haeundaensis BC74171T | Bacteria | Extracellular | Au | Spherical | 20.93 ± 3.46 | Patil et al. [124] |
| Sargassum plagiophyllum | Algae | Extracellular | Au | Spherical | 65.87 | Dhas et al. [125] |
| Parmelia sulcata | Fungi | Extracellular | Au | Spherical | 54 | Gandhi et al. [126] |
| Aspergillus flavus | Fungi | Extracellular | Au | Spherical | 12 | Abu-Tahon et al. [127] |
| Streptomyces griseoruber | Bacteria | Extracellular | Se | Spherical | 100–250 | Ranjitha et al. [107] |
| Saccharomyces cerevisiae | Yeast | Intracellular | Se | Spherical | 50 | Faramarzi et al. [101] |
| Enterococcus faecalis | Bacteria | Intracellular | Se | Spherical | 29–195 | Shoeibi and Mashreghi [102] |
| Mariannaea sp. HJ. | Fungi | Intra and Extracellular | Se | Spherical | 45.19 and 212.65 | Zhang et al. [128] |
| Fusarium oxysporum | Fungi | Extracellular | Pt | Cubical, spherical, and triangular | 25 | Gupta et al. [129] |
| Pseudomonas kunmingensis ADR19 | Bacteria | Extracellular | Pt | Spherical | 3.95 | Eramabadi et al. [130] |
| Psychrobacter faecalis FZC6 | Bacteria | Extracellular | Pt | Spherical | 2.49 | |
| Vibrio fischeri NRRL B-11177 | Bacteria | Extracellular | Pt | Spherical | 3.84 | |
| Jeotgalicoccus coquinae ZC15 | Bacteria | Extracellular | Pt | Spherical | 5.74 |
7. Biomolecule-Mediated Synthesis of MNPs
8. MNP Characterization Methods
8.1. Visual Inspection and UV–Visible Spectrophotometry
8.2. FTIR Analysis
8.3. SEM, TEM, and EDX Analysis
8.4. XRD Analysis
8.5. Zeta Potential and Dynamic Light Scattering Analysis
9. Antibiofilm Properties of MNPs
9.1. Antibiofilm Efficacy of Biosynthesized AgNPs
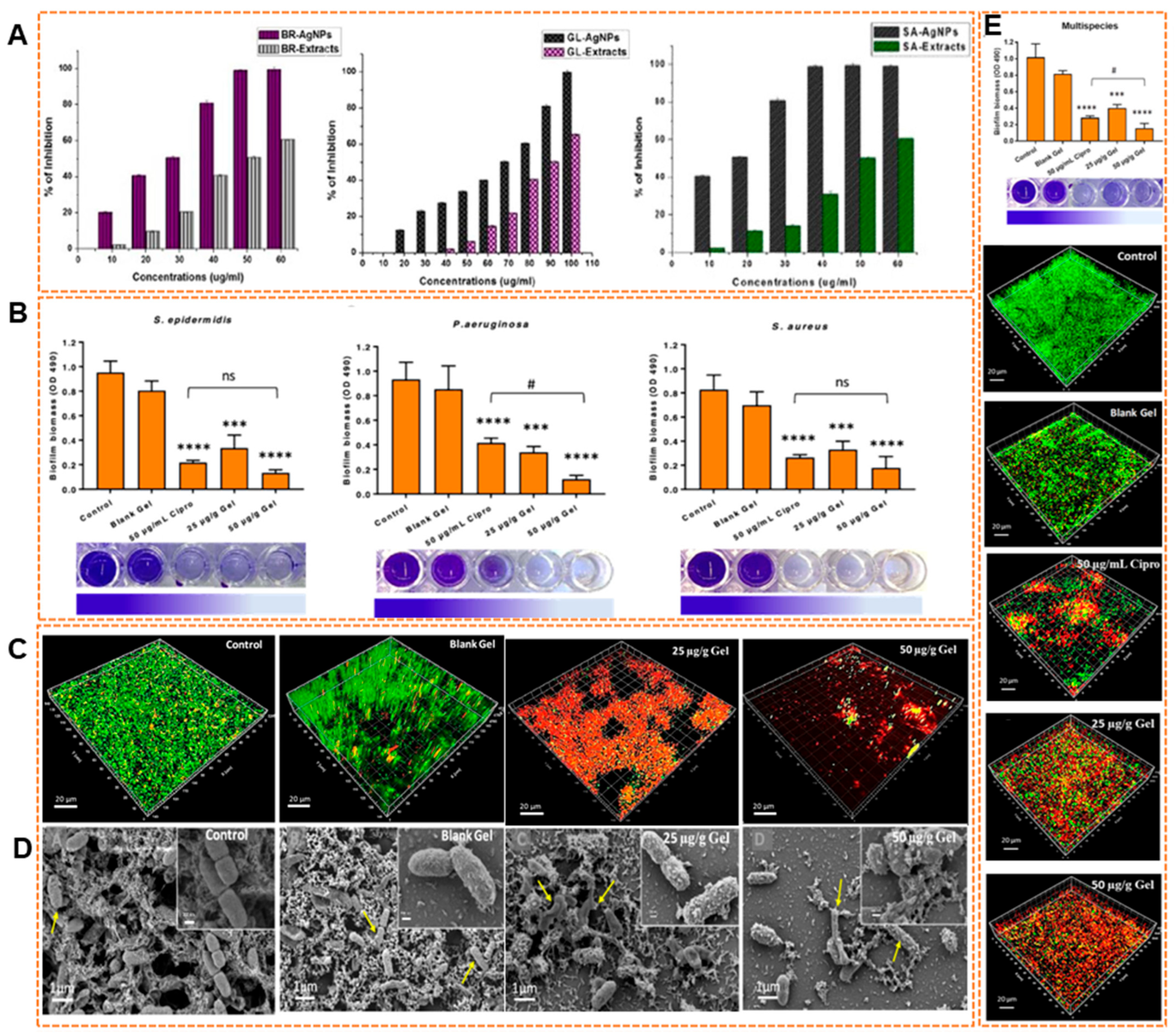
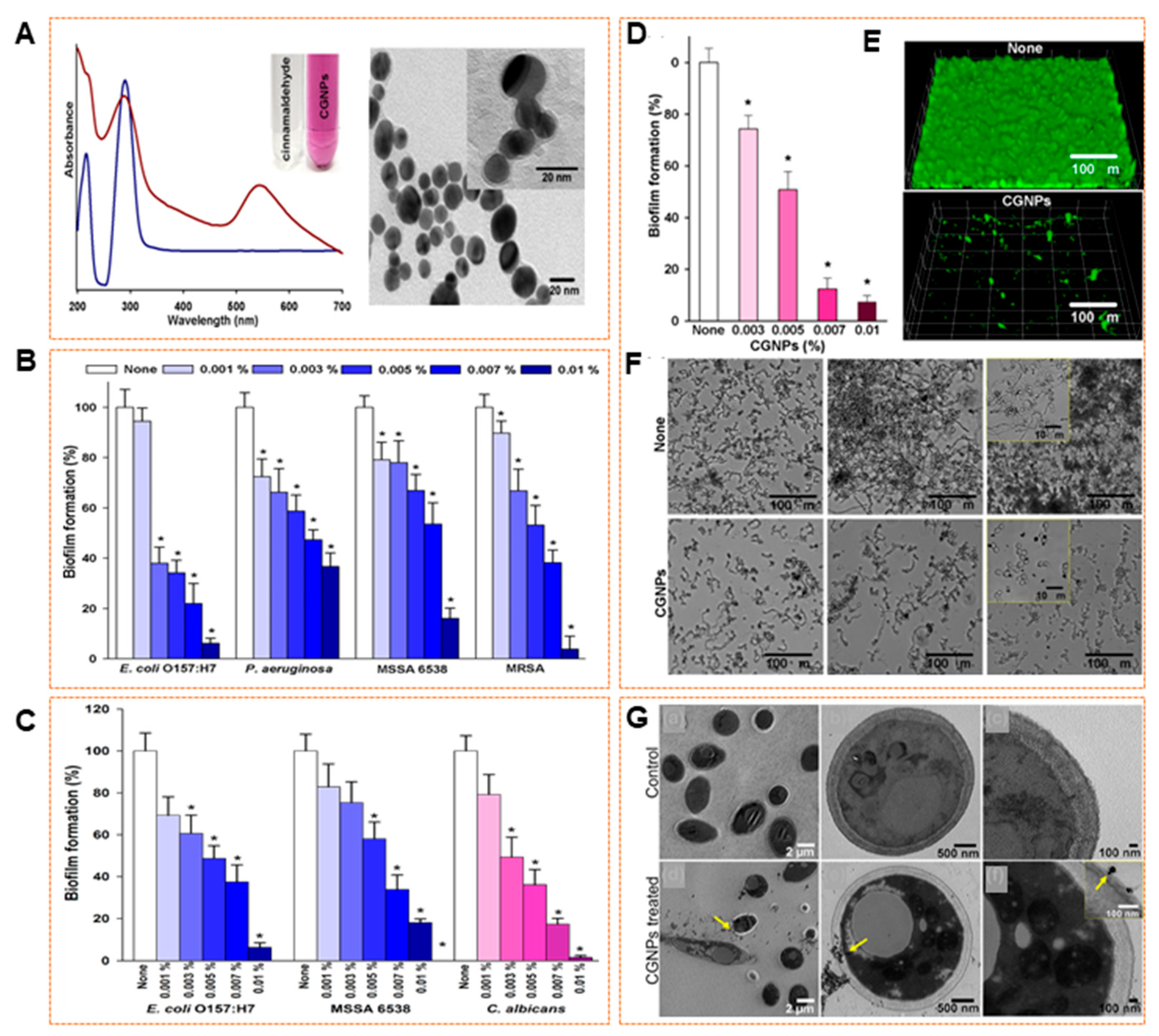
9.2. Antibiofilm Efficacy of Biosynthesized AuNPs
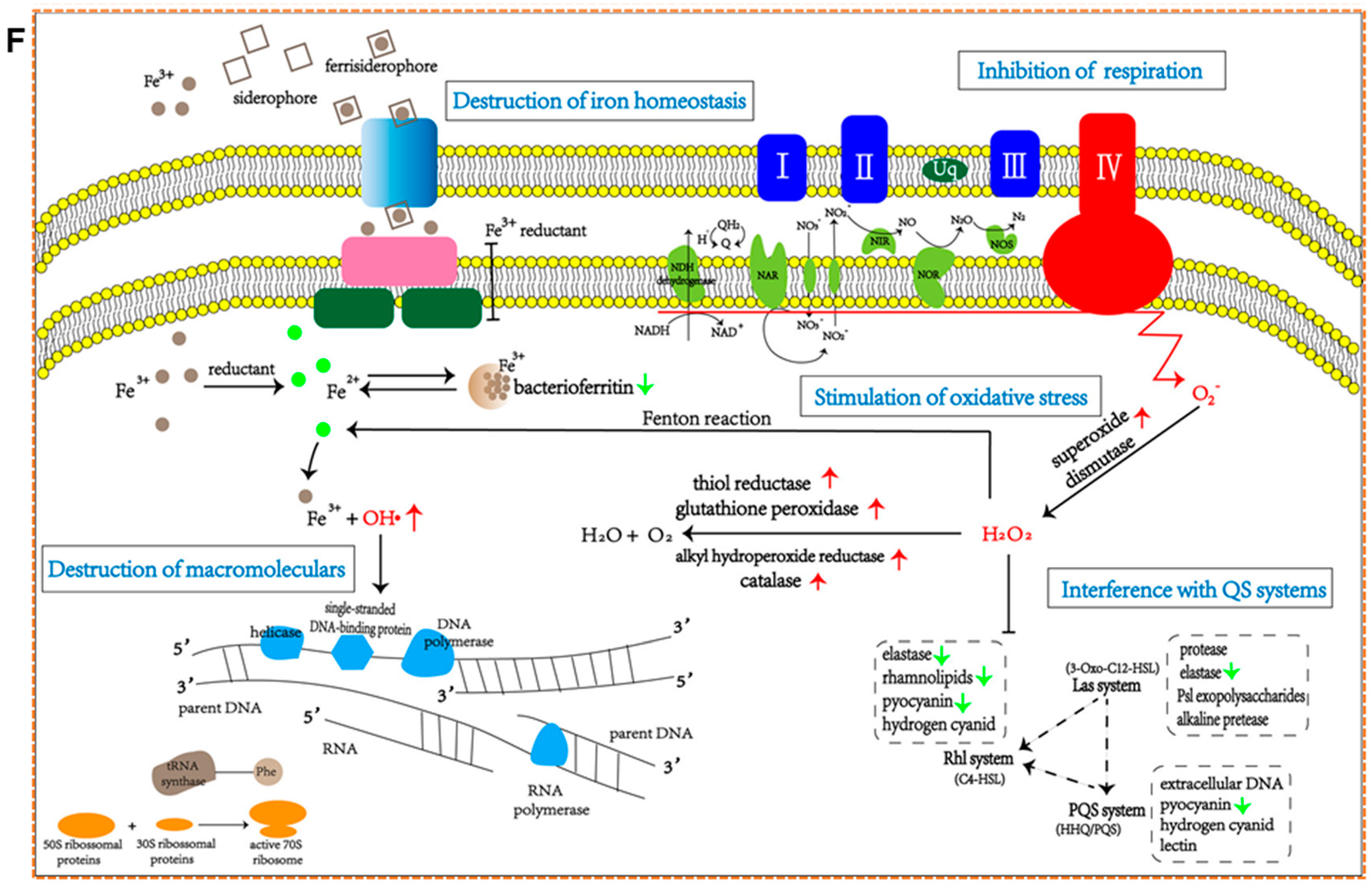
9.3. Antibiofilm Efficacy of Biosynthesized SeNPs
9.4. Antibiofilm Efficacy of Biosynthesized PtNPs
10. Conclusions
11. Future Perspectives
11.1. Toward Scalable and Consistent Green Synthesis
11.2. Understanding Mechanisms and Improving Target
11.3. Development of Multi-Functional Nanoplatforms
11.4. In Vivo Models and Safety Profiling
11.5. Navigating Regulatory and Commercialization Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habib, M.B.; Batool, G.; Shah, N.A.; Muhammad, T.; Akbar, N.S.; Shahid, A. Biofilm-Mediated Infections; Novel Therapeutic Approaches and Harnessing Artificial Intelligence for Early Detection and Treatment of Biofilm-Associated Infections. Microb. Pathog. 2025, 203, 107497. [Google Scholar] [CrossRef] [PubMed]
- Faleye, O.S.; Boya, B.R.; Lee, J.H.; Choi, I.; Lee, J. Halogenated Antimicrobial Agents to Combat Drug-Resistant Pathogens. Pharmacol. Rev. 2024, 76, 90–141. [Google Scholar] [CrossRef]
- Lan, J.; Zou, J.; Xin, H.; Sun, J.; Han, T.; Sun, M.; Niu, M. Nanomedicines as Disruptors or Inhibitors of Biofilms: Opportunities in Addressing Antimicrobial Resistance. J. Control. Release 2025, 381, 113589. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Lee, J.H.; Ma, J.Y.; Tan, Y.; Lee, J. Antivirulence Activities of Retinoic Acids against Staphylococcus aureus. Front. Microbiol. 2023, 14, 1224085. [Google Scholar] [CrossRef]
- Mulat, M.; Banicod, R.J.S.; Tabassum, N.; Javaid, A.; Karthikeyan, A. Multiple Strategies for the Application of Medicinal Plant-Derived Bioactive Compounds in Controlling Microbial Bioflim and Virulence Properties. Antibiotics 2025, 14, 555. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Wankhede, L.; Pulicharla, R.; Brar, S.K. Microplastic-Associated Biofilms in Wastewater Treatment Plants: Mechanisms and Impacts. J. Water Process Eng. 2025, 72, 107582. [Google Scholar] [CrossRef]
- Sabir, N.; Ikram, A.; Zaman, G.; Satti, L.; Gardezi, A.; Ahmed, A.; Ahmed, P. Bacterial Biofilm-Based Catheter-Associated Urinary Tract Infections: Causative Pathogens and Antibiotic Resistance. Am. J. Infect. Control 2017, 45, 1101–1105. [Google Scholar] [CrossRef]
- Faleye, O.O.; Lee, J.; Kim, Y.; Faleye, O.S.; Lee, J. Antibiofilm and Antivirulence Potentials of Iodinated Fmoc-Phenylalanine against Staphylococcus aureus. Microb. Pathog. 2024, 197, 107080. [Google Scholar] [CrossRef]
- Saharan, B.S.; Beniwal, N.; Duhan, J.S. From Formulation to Function: A Detailed Review of Microbial Biofilms and Their Polymer-Based Extracellular Substances. Microbe 2024, 5, 100194. [Google Scholar] [CrossRef]
- Ali, A.; Kang, E.T.; Xu, L. Nature-Inspired Anti-Fouling Strategies for Combating Marine Biofouling. Prog. Org. Coat. 2024, 189, 108349. [Google Scholar] [CrossRef]
- Sun, K.M.; Wang, J.; Ju, Q.; Zhao, Y.; Kong, X.; Chao, Y.; Tian, Y. The Mitigating Effects of Diatom-Bacteria Biofilm on Coastal Harmful Algal Blooms: A Lab-Based Study Concerning Species-Specific Competition and Biofilm Formation. J. Environ. Manag. 2023, 335, 117544. [Google Scholar] [CrossRef]
- Al-Madboly, L.A.; Aboulmagd, A.; El-Salam, M.A.; Kushkevych, I.; El-Morsi, R.M. Microbial Enzymes as Powerful Natural Anti-Biofilm Candidates. Microb. Cell Fact. 2024, 23, 343. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Jailani, A.; Lee, J.H.; Ahmed, B.; Lee, J. The Antibiofilm Effects of Antimony Tin Oxide Nanoparticles Against Polymicrobial Biofilms of Uropathogenic Escherichia coli and Staphylococcus aureus. Pharmaceutics 2023, 15, 1679. [Google Scholar] [CrossRef] [PubMed]
- Naga, N.G.; Magdy, H.M.; Negm, S.; El-kott, A.F.; AlShehri, M.A.; El-Metwally, M.M.; Abo-Neima, S.E.; Elsehly, E.M. Trends of Biomolecule-Conjugated Nanoparticles as Antibiofilm. Microb. Pathog. 2025, 202, 107396. [Google Scholar] [CrossRef]
- Mishra, A.; Pradhan, D.; Halder, J.; Biswasroy, P.; Rai, V.K.; Dubey, D.; Kar, B.; Ghosh, G.; Rath, G. Metal Nanoparticles against Multi-Drug-Resistance Bacteria. J. Inorg. Biochem. 2022, 237, 111938. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, S.; Blanco-Agudín, N.; Rodríguez, D.; Fernández-Vega, I.; Merayo-Lloves, J.; Quirós, L.M. Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases. Antibiotics 2025, 14, 289. [Google Scholar] [CrossRef]
- Algadi, H.; Alhoot, M.A.; Al-Maleki, A.R.; Purwitasari, N. Effects of Metal and Metal Oxide Nanoparticles against Biofilm-Forming Bacteria: A Systematic Review. J. Microbiol. Biotechnol. 2024, 34, 1748–1756. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Lazzarini Behrmann, I.C.; Daniel, M.A.; Sosa, G.L.; Owusu, E.; Parkin, I.P.; Candal, R.; Allan, E.; Vullo, D.L. Green Synthesis and Antibacterial-Antibiofilm Properties of Biogenic Silver Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100991. [Google Scholar] [CrossRef]
- Cruz, J.N.; Muzammil, S.; Ashraf, A.; Ijaz, M.U.; Siddique, M.H.; Abbas, R.; Sadia, M.; Saba; Hayat, S.; Lima, R.R. A Review on Mycogenic Metallic Nanoparticles and Their Potential Role as Antioxidant, Antibiofilm and Quorum Quenching Agents. Heliyon 2024, 10, e29500. [Google Scholar] [CrossRef]
- Kousar, R.; Khan, Z.U.H.; Sabahat, S.; Sun, J.; Muhammad, N.; Shah, N.S.; Iqbal, J.; Khasim, S.; Salam, M.A. Synergetic Comparative Study: Photocatalytic and Biological Investigations of Green-Synthesized Metal Oxide Nanoparticles. Nano-Struct. Nano-Objects 2024, 38, 101184. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Melnikov, M.Y. Hybrid Nanosystems of Antibiotics with Metal Nanoparticles—Novel Antibacterial Agents. Molecules 2023, 28, 1603. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Yang, Y.; Wu, J.; Cao, M.; Sun, L. One-Pot Synthesis of Functional Peptide-Modified Gold Nanoparticles for Gene Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128491. [Google Scholar] [CrossRef]
- Bhilkar, P.R.; Bodhne, A.S.; Yerpude, S.T.; Madankar, R.S.; Somkuwar, S.R.; Daddemal-Chaudhary, A.R.; Lambat, A.P.; Desimone, M.; Sharma, R.; Chaudhary, R.G. Phyto-Derived Metal Nanoparticles: Prominent Tool for Biomedical Applications. OpenNano 2023, 14, 100192. [Google Scholar] [CrossRef]
- Vadakkan, K.; Rumjit, N.P.; Ngangbam, A.K.; Vijayanand, S.; Nedumpillil, N.K. Novel Advancements in the Sustainable Green Synthesis Approach of Silver Nanoparticles (AgNPs) for Antibacterial Therapeutic Applications. Coord. Chem. Rev. 2024, 499, 215528. [Google Scholar] [CrossRef]
- Saeed, Z.; Pervaiz, M.; Ejaz, A.; Hussain, S.; Shaheen, S.; Shehzad, B.; Younas, U. Garlic and Ginger Extracts Mediated Green Synthesis of Silver and Gold Nanoparticles: A Review on Recent Advancements and Prospective Applications. Biocatal. Agric. Biotechnol. 2023, 53, 102868. [Google Scholar] [CrossRef]
- Akhter, M.S.; Rahman, M.A.; Ripon, R.K.; Mubarak, M.; Akter, M.; Mahbub, S.; Al Mamun, F.; Sikder, M.T. A Systematic Review on Green Synthesis of Silver Nanoparticles Using Plants Extract and Their Bio-Medical Applications. Heliyon 2024, 10, e29766. [Google Scholar] [CrossRef]
- Sarkar, S.; Roy, A.; Mitra, R.; Kundu, S.; Banerjee, P.; Acharya Chowdhury, A.; Ghosh, S. Escaping the ESKAPE Pathogens: A Review on Antibiofilm Potential of Nanoparticles. Microb. Pathog. 2024, 194, 106842. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 47. [Google Scholar] [CrossRef]
- Kulkarni, D.; Sherkar, R.; Shirsathe, C.; Sonwane, R.; Varpe, N.; Shelke, S.; More, M.P.; Pardeshi, S.R.; Dhaneshwar, G.; Junnuthula, V.; et al. Biofabrication of Nanoparticles: Sources, Synthesis, and Biomedical Applications. Front. Bioeng. Biotechnol. 2023, 11, 1159193. [Google Scholar] [CrossRef]
- Metz, K.M.; Sanders, S.E.; Pender, J.P.; Dix, M.R.; Hinds, D.T.; Quinn, S.J.; Ward, A.D.; Duffy, P.; Cullen, R.J.; Colavita, P.E. Green Synthesis of Metal Nanoparticles via Natural Extracts: The Biogenic Nanoparticle Corona and Its Effects on Reactivity. ACS Sustain. Chem. Eng. 2015, 3, 1610–1617. [Google Scholar] [CrossRef]
- Gopireddy, R.R.; Maruthapillai, A.; Arockia Selvi, J.; Mahapatra, S. Determination of Potential Genotoxic Impurity Hydrazine Hydrate in Ibrutinib by RP-Liquid Chromatography. Mater. Today Proc. 2018, 34, 430–436. [Google Scholar] [CrossRef]
- Vanga, S.; Rani, S. A Review on Green Synthesis, Characterization and Applications of Plant Mediated Metal Nanoparticles. Next Res. 2025, 2, 100356. [Google Scholar] [CrossRef]
- Balaji, S.; Pandian, M.S.; Ganesamoorthy, R.; Karchiyappan, T. Green Synthesis of Metal Oxide Nanoparticles Using Plant Extracts: A Sustainable Approach to Combat Antimicrobial Resistance. Environ. Nanotechnol. Monit. Manag. 2025, 23, 101066. [Google Scholar] [CrossRef]
- Banu, K.S.; Chakraborty, P. An Overview of Bio-Assisted Nanoparticles: Synthesis, Application and Challenges in Nature’s Toolbox. Nano-Struct. Nano-Objects 2024, 39, 101317. [Google Scholar] [CrossRef]
- Yan, Q.; Yang, Z.; Chen, Z. Recent Advances in Microbial Nanomaterials/Nanoparticles Synthesis and Rare Earth Elements Recovery from Rare Earth Mine Wastewater: A Review. Chem. Eng. J. 2025, 510, 161647. [Google Scholar] [CrossRef]
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B.A. Microbial Synthesis of Gold Nanoparticles: Current Status and Future Prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Al-sahlany, S.T.G.; Niamah, A.K.; Verma, D.K.; Prabhakar, P.; Patel, A.R.; Thakur, M.; Singh, S. Applications of Green Synthesis of Nanoparticles Using Microorganisms in Food and Dairy Products: Review. Processes 2025, 13, 1560. [Google Scholar] [CrossRef]
- Michalec, S.; Nieckarz, W.; Klimek, W.; Lange, A.; Matuszewski, A.; Piotrowska, K.; Hotowy, A.; Kunowska-Slósarz, M.; Sosnowska, M. Green Synthesis of Silver Nanoparticles from Chlorella vulgaris Aqueous Extract and Their Effect on Salmonella enterica and Chicken Embryo Growth. Molecules 2025, 30, 1521. [Google Scholar] [CrossRef]
- Singh, N.A.; Narang, J.; Garg, D.; Jain, V.; Payasi, D.; Suleman, S.; Swami, R.K. Nanoparticles Synthesis via Microorganisms and Their Prospective Applications in Agriculture. Plant Nano Biol. 2023, 5, 100047. [Google Scholar] [CrossRef]
- Ghasemi, S.; Dabirian, S.; Kariminejad, F.; Koohi, D.E.; Nemattalab, M.; Majidimoghadam, S.; Zamani, E.; Yousefbeyk, F. Process Optimization for Green Synthesis of Silver Nanoparticles Using Rubus discolor Leaves Extract and Its Biological Activities against Multi-Drug Resistant Bacteria and Cancer Cells. Sci. Rep. 2024, 14, 4130. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, Y.; Perez-Davalos, L.; LeDuc, R.; Zahedi, R.P.; Labouta, H.I. Omics Approaches for the Assessment of Biological Responses to Nanoparticles. Adv. Drug Deliv. Rev. 2023, 200, 114992. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morales, O.; Gueye, M.; Lacombe, L.; Nowak, C.; Schmachtenberg, R.; Hörner, M.; Jerez-Longres, C.; Mohsenin, H.; Wagner, H.J.; Weber, W. Synthetic Biology as Driver for the Biologization of Materials Sciences. Mater. Today Bio 2021, 11, 100115. [Google Scholar] [CrossRef]
- Barrett-Catton, E.; Ross, M.L.; Asuri, P. Multifunctional Hydrogel Nanocomposites for Biomedical Applications. Polymers 2021, 13, 856. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, S.; Gholap, A.D.; Kumari, R.; Tanwar, R.; Kumar, V.; Khalid, M.; Faiyazuddin, M. A Comprehensive Review on the Biomass-Mediated Synthesis of Silver Nanoparticles: Opportunities and Challenges. J. Environ. Chem. Eng. 2025, 13, 115133. [Google Scholar] [CrossRef]
- Rana, G.; Dhiman, V.K.; Ali, S.K.; Chauhan, A.; Jabir, M.S.; Ghotekar, S. Emerging Developments in Plant-Based Metal Nanomaterials for Diverse Versatile Applications—A Review. Results Chem. 2025, 15, 102231. [Google Scholar] [CrossRef]
- Thatyana, M.; Dube, N.P.; Kemboi, D.; Manicum, A.L.E.; Mokgalaka-Fleischmann, N.S.; Tembu, J.V. Advances in Phytonanotechnology: A Plant-Mediated Green Synthesis of Metal Nanoparticles Using Phyllanthus Plant Extracts and Their Antimicrobial and Anticancer Applications. Nanomaterials 2023, 13, 2616. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Fawzy, M.; Hosny, M.; Abd El-Monaem, E.M.; Tamer, T.M.; Omer, A.M. Green Synthesis of Platinum Nanoparticles Using Atriplex halimus Leaves for Potential Antimicrobial, Antioxidant, and Catalytic Applications. Arab. J. Chem. 2022, 15, 103517. [Google Scholar] [CrossRef]
- El-Gebaly, A.S.; Sofy, A.R.; Hmed, A.A.; Youssef, A.M. Green Synthesis, Characterization and Medicinal Uses of Silver Nanoparticles (Ag-NPs), Copper Nanoparticles (Cu-NPs) and Zinc Oxide Nanoparticles (ZnO-NPs) and Their Mechanism of Action: A Review. Biocatal. Agric. Biotechnol. 2024, 55, 103006. [Google Scholar] [CrossRef]
- Bharathi, D.; Josebin, M.D.; Vasantharaj, S.; Bhuvaneshwari, V. Biosynthesis of Silver Nanoparticles Using Stem Bark Extracts of Diospyros montana and Their Antioxidant and Antibacterial Activities. J. Nanostruct. Chem. 2018, 8, 83–92. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Oladzadabbasabadi, N.; Dheyab, M.A.; Lalabadi, M.A.; Sheibani, S.; Ghasemlou, M.; Esmaeili, Y.; Barrow, C.J.; Naebe, M.; Timms, W. Emerging Trends in Smart and Sustainable Nano-Biosensing: The Role of Green Nanomaterials. Ind. Crops Prod. 2025, 223, 120108. [Google Scholar] [CrossRef]
- Habeeb, H.; Thoppil, J.E. Green Synthesis of Silver Nanoparticles from Wrightia arborea: Physiochemical Profiling and Genotoxic Potency. Inorg. Chem. Commun. 2023, 156, 111179. [Google Scholar] [CrossRef]
- Moosavy, M.H.; de la Guardia, M.; Mokhtarzadeh, A.; Khatibi, S.A.; Hosseinzadeh, N.; Hajipour, N. Green Synthesis, Characterization, and Biological Evaluation of Gold and Silver Nanoparticles Using Mentha spicata Essential Oil. Sci. Rep. 2023, 13, 7230. [Google Scholar] [CrossRef]
- Karan, T.; Gonulalan, Z.; Erenler, R.; Kolemen, U.; Eminagaoglu, O. Green Synthesis of Silver Nanoparticles Using Sambucus ebulus Leaves Extract: Characterization, Quantitative Analysis of Bioactive Molecules, Antioxidant and Antibacterial Activities. J. Mol. Struct. 2024, 1296, 136836. [Google Scholar] [CrossRef]
- Susanna, D.; Balakrishnan, R.M.; Ponnan Ettiyappan, J. Ultrasonication-Assisted Green Synthesis and Characterization of Gold Nanoparticles from Nothapodytes foetida: An Assessment of Their Antioxidant, Antibacterial, Anticancer and Wound Healing Potential. J. Drug Deliv. Sci. Technol. 2023, 87, 104740. [Google Scholar] [CrossRef]
- Keshtmand, Z.; Khademian, E.; Poorjafari Jafroodi, P.; Abtahi, M.S.; Tavakkoli Yaraki, M. Green Synthesis of Selenium Nanoparticles Using Artemisia chamaemelifolia: Toxicity Effects through Regulation of Gene Expression for Cancer Cells and Bacteria. Nano-Struct. Nano-Objects 2023, 36, 101049. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Aramesh, N.; Hasnain, M.S.; Nayak, A.K.; Varma, R.S. Greener Fabrication of Metal Nanoparticles Using Plant Materials: A Review. Chem. Phys. Impact 2023, 7, 100255. [Google Scholar] [CrossRef]
- Lithi, I.J.; Ahmed Nakib, K.I.; Chowdhury, A.M.S.; Sahadat Hossain, M. A Review on the Green Synthesis of Metal (Ag, Cu, and Au) and Metal Oxide (ZnO, MgO, Co3O4, and TiO2) Nanoparticles Using Plant Extracts for Developing Antimicrobial Properties. Nanoscale Adv. 2025, 7, 2446–2473. [Google Scholar] [CrossRef]
- Elmitwalli, O.S.M.M.S.; Kassim, D.A.K.; Algahiny, A.T.; Henari, F.Z. Green Synthesis of Metal Nanoparticles Using Cinnamomum-Based Extracts and Their Applications. Nanotechnol. Sci. Appl. 2025, 18, 93–114. [Google Scholar] [CrossRef]
- Monica Ahmad, N.; Husaini Mohamed, A.; Hasan, N.A.; Zainal- Abidin, N.; Zaini Nawahwi, M.; Mohamad Azzeme, A. Effect of Optimisation Variable and the Role of Plant Extract in the Synthesis of Nanoparticles Using Plant-Mediated Synthesis Approaches. Inorg. Chem. Commun. 2024, 161, 111839. [Google Scholar] [CrossRef]
- Kumari, K.A.; Reddy, G.B.; Vishnu, T.; Mittapelli, V. Sustainable Green Synthesis of Silver Nanoparticles Using Chloris barbata Leaf Extract with Its Bioactivity against Multidrug Resistant Bacterial Strains and as an Antioxidant Agent. Mater. Today Proc. 2023, 92, 586–593. [Google Scholar] [CrossRef]
- Wei, Z.; Zhuo, S.; Zhang, Y.; Wu, L.; Gao, X.; He, S.; Bo, X.; Zhou, W. Machine Learning Reshapes the Paradigm of Nanomedicine Research. Acta Pharm. Sin. B, 2025; in press. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorpeh, F. Toxicity Prediction of Nanoparticles Using Machine Learning Approaches. Toxicology 2024, 501, 153697. [Google Scholar] [CrossRef] [PubMed]
- Gastelum-cabrera, M.; Mendez-pfeiffer, P.; Ballesteros-monrreal, M.G.; Velasco-rodríguez, B.; Martínez-flores, P.D.; Silva-bea, S.; Domínguez-arca, V.; Prieto, G.; Barbosa, S.; Otero, A.; et al. Phytosynthesis and Characterization of Silver Nanoparticles from Antigonon leptopus: Assessment of Antibacterial and Cytotoxic Properties. Pharmaceutics 2025, 17, 672. [Google Scholar] [CrossRef]
- Mwakalesi, A.J.; Lema, E.S. Green Synthesis and Characterization of Silver Nanoparticles from Aqueous Extract of Harrisonia abyssinica Fruits. Eng. Proc. 2025, 87, 24. [Google Scholar]
- Lekkala, V.D.V.V.; Muktinutalapati, A.V.; Lebaka, V.R.; Lomada, D.; Korivi, M.; Li, W.; Reddy, M.C. Green Synthesis and Characterization of Silver Nanoparticles from Tinospora cordifolia Leaf Extract: Evaluation of Their Antioxidant, Anti-Inflammatory, Antibacterial, and Antibiofilm Efficacies. Nanomaterials 2025, 15, 381. [Google Scholar] [CrossRef]
- Muthu, K.; Rajeswari, S.; Akilandaeaswari, B.; Nagasundari, S.M.; Rangasamy, R. Synthesis, Characterisation and Photocatalytic Activity of Silver Nanoparticles Stabilised by Punica granatum Seeds Extract. Mater. Technol. 2020, 36, 684–693. [Google Scholar] [CrossRef]
- Jebril, S.; Khanfir Ben Jenana, R.; Dridi, C. Green Synthesis of Silver Nanoparticles Using Melia azedarach Leaf Extract and Their Antifungal Activities: In Vitro and in Vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Heikal, Y.M.; Şuţan, N.A.; Rizwan, M.; Elsayed, A. Green Synthesized Silver Nanoparticles Induced Cytogenotoxic and Genotoxic Changes in Allium cepa L. Varies with Nanoparticles Doses and Duration of Exposure. Chemosphere 2020, 243, 125430. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M.; Esmail, G.A.; Al-Dhabi, N.A.; Arasu, M.V. Green Synthesis and Characterization of Silver Nanoparticles from Moringa oleifera Flower and Assessment of Antimicrobial and Sensing Properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111836. [Google Scholar] [CrossRef]
- Singh, S.R.; Kittur, B.; Bhavi, S.M.; Thokchom, B.; Padti, A.C.; Bhat, S.S.; Bajire, S.K.; Shastry, R.P.; Srinath, B.S.; Sillanpää, M.; et al. The Effect of Clitoria ternatea L. Flowers-Derived Silver Nanoparticles on A549 and L-132 Human Cell Lines and Their Antibacterial Efficacy in Caenorhabditis elegans In Vivo. Hybrid Adv. 2025, 8, 100359. [Google Scholar] [CrossRef]
- Sagadevan, S.; Vennila, S.; Muthukrishnan, L.; Gurunathan, K.; Oh, W.C.; Paiman, S.; Mohammad, F.; Al-Lohedan, H.A.; Jasni, A.H.; Fatimah, I.; et al. Exploring the Therapeutic Potentials of Phyto-Mediated Silver Nanoparticles Formed via Calotropis procera (Ait.) R. Br. Root Extract. J. Exp. Nanosci. 2020, 15, 217–232. [Google Scholar] [CrossRef]
- Al-Nuairi, A.G.; Mosa, K.A.; Mohammad, M.G.; El-Keblawy, A.; Soliman, S.; Alawadhi, H. Biosynthesis, Characterization, and Evaluation of the Cytotoxic Effects of Biologically Synthesized Silver Nanoparticles from Cyperus conglomeratus Root Extracts on Breast Cancer Cell Line MCF-7. Biol. Trace Elem. Res. 2020, 194, 560–569. [Google Scholar] [CrossRef]
- Gomathi, A.C.; Xavier Rajarathinam, S.R.; Mohammed Sadiq, A.; Rajeshkumar, S. Anticancer Activity of Silver Nanoparticles Synthesized Using Aqueous Fruit Shell Extract of Tamarindus indica on MCF-7 Human Breast Cancer Cell Line. J. Drug Deliv. Sci. Technol. 2020, 55, 101376. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Kim, D.S.; Kim, D.Y.; Shin, H.S. Exploiting Fruit Waste Grape Pomace for Silver Nanoparticles Synthesis, Assessing Their Antioxidant, Antidiabetic Potential and Antibacterial Activity against Human Pathogens: A Novel Approach. Nanomaterials 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green Synthesis of Silver Nanoparticles Using Cauliflower Waste and Their Multifaceted Applications in Photocatalytic Degradation of Methylene Blue Dye and Hg2+ Biosensing. SN Appl. Sci. 2020, 2, 738. [Google Scholar] [CrossRef]
- Panchamoorthy, R.; Mohan, U.; Muniyan, A. Apium graveolens Reduced Phytofabricated Gold Nanoparticles and Their Impacts on the Glucose Utilization Pattern of the Isolated Rat Hemidiaphragm. Heliyon 2022, 8, e08805. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Kala, S.M.J.; Pushparaj, T.L. Biogenic Synthesis of Gold Nanoparticles Using Jasminum auriculatum Leaf Extract and Their Catalytic, Antimicrobial and Anticancer Activities. J. Drug Deliv. Sci. Technol. 2020, 57, 101620. [Google Scholar] [CrossRef]
- Kaval, U.; Hoşgören, H. Biosynthesis, Characterization, and Biomedical Applications of Gold Nanoparticles with Cucurbita moschata Duchesne Ex Poiret Peel Aqueous Extracts. Molecules 2024, 29, 923. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Zangeneh, A. Novel Green Synthesis of Hibiscus Sabdariffa Flower Extract Conjugated Gold Nanoparticles with Excellent Anti-Acute Myeloid Leukemia Effect in Comparison to Daunorubicin in a Leukemic Rodent Model. Appl. Organomet. Chem. 2020, 34, e5271. [Google Scholar] [CrossRef]
- Patil, M.P.; Bayaraa, E.; Subedi, P.; Piad, L.L.A.; Tarte, N.H.; Kim, G. Do Biogenic Synthesis, Characterization of Gold Nanoparticles Using Lonicera japonica and Their Anticancer Activity on HeLa Cells. J. Drug Deliv. Sci. Technol. 2019, 51, 83–90. [Google Scholar] [CrossRef]
- Chokkalingam, M.; Singh, P.; Huo, Y.; Soshnikova, V.; Ahn, S.; Kang, J.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.C. Facile Synthesis of Au and Ag Nanoparticles Using Fruit Extract of Lycium chinense and Their Anticancer Activity. J. Drug Deliv. Sci. Technol. 2019, 49, 308–315. [Google Scholar] [CrossRef]
- Zhang, T.; Dang, M.; Zhang, W.; Lin, X. Gold Nanoparticles Synthesized from Euphorbia fischeriana Root by Green Route Method Alleviates the Isoprenaline Hydrochloride Induced Myocardial Infarction in Rats. J. Photochem. Photobiol. B Biol. 2020, 202, 111705. [Google Scholar] [CrossRef] [PubMed]
- Doan, V.D.; Huynh, B.A.; Nguyen, T.D.; Cao, X.T.; Nguyen, V.C.; Nguyen, T.L.H.; Nguyen, H.T.; Le, V.T. Biosynthesis of Silver and Gold Nanoparticles Using Aqueous Extract of Codonopsis pilosula Roots for Antibacterial and Catalytic Applications. J. Nanomater. 2020, 2020, 8492016. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; AlYahya, S.; Jalal, M.; Khan, H.M.; Asiri, S.M.M.; Ahmad, W.; Mahdi, A.A.; et al. Biogenic Gold Nanoparticles as Potent Antibacterial and Antibiofilm Nano-Antibiotics against Pseudomonas aeruginosa. Antibiotics 2020, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Mulla, N.A.; Otari, S.V.; Bohara, R.A.; Yadav, H.M.; Pawar, S.H. Rapid and Size-Controlled Biosynthesis of Cytocompatible Selenium Nanoparticles by Azadirachta indica Leaves Extract for Antibacterial Activity. Mater. Lett. 2020, 264, 127353. [Google Scholar] [CrossRef]
- Krishnan, M.; Ranganathan, K.; Maadhu, P.; Thangavelu, P.; Kundan, S.; Arjunan, N. Leaf Extract of Dillenia indica as a Source of Selenium Nanoparticles with Larvicidal and Antimicrobial Potential toward Vector Mosquitoes and Pathogenic Microbes. Coatings 2020, 10, 626. [Google Scholar] [CrossRef]
- Al-Radadi, N.S.; Adam, S.I.Y. Green Biosynthesis of Pt-Nanoparticles from Anbara Fruits: Toxic and Protective Effects on CCl4 Induced Hepatotoxicity in Wister Rats. Arab. J. Chem. 2020, 13, 4386–4403. [Google Scholar] [CrossRef]
- Kumar, P.V.; Jelastin Kala, S.M.; Prakash, K.S. Green Synthesis Derived Pt-Nanoparticles Using Xanthium strumarium Leaf Extract and Their Biological Studies. J. Environ. Chem. Eng. 2019, 7, 103146. [Google Scholar] [CrossRef]
- Fanoro, O.T.; Parani, S.; Maluleke, R.; Lebepe, T.C.; Varghese, R.J.; Mgedle, N.; Mavumengwana, V.; Oluwafemi, O.S. Biosynthesis of Smaller-Sized Platinum Nanoparticles Using the Leaf Extract of Combretum erythrophyllum and Its Antibacterial Activities. Antibiotics 2021, 10, 1275. [Google Scholar] [CrossRef]
- Aygun, A.; Gülbagca, F.; Ozer, L.Y.; Ustaoglu, B.; Altunoglu, Y.C.; Baloglu, M.C.; Atalar, M.N.; Alma, M.H.; Sen, F. Biogenic Platinum Nanoparticles Using Black Cumin Seed and Their Potential Usage as Antimicrobial and Anticancer Agent. J. Pharm. Biomed. Anal. 2020, 179, 112961. [Google Scholar] [CrossRef] [PubMed]
- Alfryyan, N.; Kordy, M.G.M.; Abdel-Gabbar, M.; Soliman, H.A.; Shaban, M. Characterization of the Biosynthesized Intracellular and Extracellular Plasmonic Silver Nanoparticles Using Bacillus cereus and Their Catalytic Reduction of Methylene Blue. Sci. Rep. 2022, 12, 12495. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Turner, R.J. Editorial: Nanomicrobiology: Emerging Trends in Microbial Synthesis of Nanomaterials and Their Applications. Front. Microbiol. 2021, 12, 751693. [Google Scholar] [CrossRef]
- Kazemi, S.; Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent Advances in Green Synthesized Nanoparticles: From Production to Application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Otari, S.V.; Patil, R.M.; Ghosh, S.J.; Thorat, N.D.; Pawar, S.H. Intracellular Synthesis of Silver Nanoparticle by Actinobacteria and Its Antimicrobial Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Markus, J.; Mathiyalagan, R.; Kim, Y.J.; Abbai, R.; Singh, P.; Ahn, S.; Perez, Z.E.J.; Hurh, J.; Yang, D.C. Intracellular Synthesis of Gold Nanoparticles with Antioxidant Activity by Probiotic lactobacillus Kimchicus DCY51T Isolated from Korean Kimchi. Enzyme Microb. Technol. 2016, 95, 85–93. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biosynthesis of Gold and Selenium Nanoparticles by Purified Protein from Acinetobacter sp. SW 30. Enzyme Microb. Technol. 2018, 111, 81–86. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 626834. [Google Scholar] [CrossRef]
- Liu, Y.; Perumalsamy, H.; Kang, C.H.; Kim, S.H.; Hwang, J.S.; Koh, S.C.; Yi, T.H.; Kim, Y.J. Intracellular Synthesis of Gold Nanoparticles by Gluconacetobacter liquefaciens for Delivery of Peptide CopA3 and Ginsenoside and Anti-Inflammatory Effect on Lipopolysaccharide-Activated Macrophages. Artif. Cells Nanomed. Biotechnol. 2020, 48, 777–788. [Google Scholar] [CrossRef]
- Patil, Y.M.; Rajpathak, S.N.; Deobagkar, D.D. Characterization and DNA Methylation Modulatory Activity of Gold Nanoparticles Synthesized by Pseudoalteromonas Strain. J. Biosci. 2019, 44, 15. [Google Scholar] [CrossRef]
- Faramarzi, S.; Anzabi, Y.; Jafarizadeh-Malmiri, H. Nanobiotechnology Approach in Intracellular Selenium Nanoparticle Synthesis Using Saccharomyces cerevisiae—Fabrication and Characterization. Arch. Microbiol. 2020, 202, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Shoeibi, S.; Mashreghi, M. Biosynthesis of Selenium Nanoparticles Using Enterococcus faecalis and Evaluation of Their Antibacterial Activities. J. Trace Elem. Med. Biol. 2017, 39, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Gaidhani, S.V.; Yeshvekar, R.K.; Shedbalkar, U.U.; Bellare, J.H.; Chopade, B.A. Bio-Reduction of Hexachloroplatinic Acid to Platinum Nanoparticles Employing Acinetobacter calcoaceticus. Process Biochem. 2014, 49, 2313–2319. [Google Scholar] [CrossRef]
- Dagci, I.; Acar, M.; Turhan, F.; Mavi, A.; Unver, Y. Extracellular Production of Azurin by Reusable Magnetic Fe3O4 Nanoparticle-Immobilized Pseudomonas aeruginosa. J. Biotechnol. 2024, 394, 48–56. [Google Scholar] [CrossRef]
- Uthra, C.; Muralitharan, G.; Nagaraj, K.; Thajjudin, N.; Kaliyaperumal, R.; Badgujar, N.P.; Shah, F.; Abhijith, S.M. Exploring Biogenic Microbial Synthesis and Biomedical Applications of Silver Nanoparticles: A Comprehensive Review. Results Chem. 2024, 8, 101555. [Google Scholar] [CrossRef]
- Campaña, A.L.; Saragliadis, A.; Mikheenko, P.; Linke, D. Insights into the Bacterial Synthesis of Metal Nanoparticles. Front. Nanotechnol. 2023, 5, 1216921. [Google Scholar] [CrossRef]
- Ranjitha, V.R.; Ravishankar, V.R. Extracellular Synthesis of Selenium Nanoparticles from an Actinomycetes Streptomyces griseoruber and Evaluation of Its Cytotoxicity on HT-29 Cell Line. Pharm. Nanotechnol. 2018, 6, 61–68. [Google Scholar] [CrossRef]
- Prema, P.; Subha Ranjani, S.; Ramesh Kumar, K.; Veeramanikandan, V.; Mathiyazhagan, N.; Nguyen, V.H.; Balaji, P. Microbial Synthesis of Silver Nanoparticles Using Lactobacillus plantarum for Antioxidant, Antibacterial Activities. Inorg. Chem. Commun. 2022, 136, 109139. [Google Scholar] [CrossRef]
- John Wilson, J.; Ponseetha Lakshmi, M.; Sivakumar, T.; Ponmanickam, P.; Sevarkodiyone, S.P. Green Synthesis of Silver Nanoparticles Using Bacillus subtilis (P3) and Its Larvicidal, Histopathological and Biotoxicity Efficacy. S. Afr. J. Bot. 2022, 151, 309–318. [Google Scholar] [CrossRef]
- Hu, D.; Yu, S.; Yu, D.; Liu, N.; Tang, Y.; Fan, Y.; Wang, C.; Wu, A. Biogenic Trichoderma Harzianum-Derived Selenium Nanoparticles with Control Functionalities Originating from Diverse Recognition Metabolites against Phytopathogens and Mycotoxins. Food Control 2019, 106, 106748. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Olowe, O.M.; Ayilara, M.S.; Fasusi, O.A.; Omotayo, O.P.; Fadiji, A.E.; Onwudiwe, D.C.; Babalola, O.O. Biosynthesis of Nanoparticles Using Microorganisms: A Focus on Endophytic Fungi. Heliyon 2024, 10, e39636. [Google Scholar] [CrossRef]
- Romano, I.; Vitiello, G.; Gallucci, N.; Di Girolamo, R.; Cattaneo, A.; Poli, A.; Di Donato, P. Extremophilic Microorganisms for the Green Synthesis of Antibacterial Nanoparticles. Microorganisms 2022, 10, 1885. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Biofactories: Engineered Nanoparticles: Via Genetically Engineered Organisms. Green Chem. 2019, 21, 4583–4603. [Google Scholar] [CrossRef]
- Pernas-pleite, C.; Conejo-martínez, A.M.; Marín, I.; Abad, J.P. Silver Nanoparticles (AgNPs) from Lysinibacillus sp. Culture Broths: Antibacterial Activity, Mechanism Insights, and Synergy with Classical Antibiotics. Biomolecules 2025, 15, 731. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; AlYahya, S.; Govarthanan, M.; ALjahdali, N.; Al-Enazi, N.; Alsamhary, K.; Alshehri, W.A.; Alwakeel, S.S.; Alharbi, S.A. Soil Bacteria Cupriavidus sp. Mediates the Extracellular Synthesis of Antibacterial Silver Nanoparticles. J. Mol. Struct. 2020, 1202, 127233. [Google Scholar] [CrossRef]
- Hamida, R.S.; Almotwaa, S.M.; Al-Otaibi, W.A.; Alqhtani, H.A.; Ali, M.A.; Bin-Meferij, M.M. Apoptotic Induction by Biosynthesized Gold Nanoparticles Using Phormidesmis communis Strain AB_11_10 against Osteosarcoma Cancer. Biomedicines 2024, 12, 1570. [Google Scholar] [CrossRef]
- Sanjivkumar, M.; Vaishnavi, R.; Neelakannan, M.; Kannan, D.; Silambarasan, T.; Immanuel, G. Investigation on Characterization and Biomedical Properties of Silver Nanoparticles Synthesized by an Actinobacterium Streptomyces olivaceus (MSU3). Biocatal. Agric. Biotechnol. 2019, 17, 151–159. [Google Scholar] [CrossRef]
- Divya, M.; Kiran, G.S.; Hassan, S.; Selvin, J. Biogenic Synthesis and Effect of Silver Nanoparticles (AgNPs) to Combat Catheter-Related Urinary Tract Infections. Biocatal. Agric. Biotechnol. 2019, 18, 101037. [Google Scholar] [CrossRef]
- Abd El Aty, A.A.; Mohamed, A.A.; Zohair, M.M.; Soliman, A.A.F. Statistically Controlled Biogenesis of Silver Nano-Size by Penicillium chrysogenum MF318506 for Biomedical Application. Biocatal. Agric. Biotechnol. 2020, 25, 101592. [Google Scholar] [CrossRef]
- Dağlıoğlu, Y.; Yılmaz Öztürk, B. A Novel Intracellular Synthesis of Silver Nanoparticles Using Desmodesmus sp. (Scenedesmaceae): Different Methods of Pigment Change. Rend. Lincei 2019, 30, 611–621. [Google Scholar] [CrossRef]
- Fatima, R.; Priya, M.; Indurthi, L.; Radhakrishnan, V.; Sudhakaran, R. Biosynthesis of Silver Nanoparticles Using Red Algae Portieria hornemannii and Its Antibacterial Activity against Fish Pathogens. Microb. Pathog. 2020, 138, 103780. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Rokhbakhsh-Zamin, F.; Shakibaie, M.; Moshafi, M.H.; Ameri, A.; Rahimi, H.R.; Forootanfar, H. Cytotoxic and Antibacterial Activities of Biologically Synthesized Gold Nanoparticles Assisted by Micrococcus yunnanensis Strain J2. Biocatal. Agric. Biotechnol. 2018, 15, 245–253. [Google Scholar] [CrossRef]
- Abdul-Hadi, S.Y.; Owaid, M.N.; Rabeea, M.A.; Abdul Aziz, A.; Jameel, M.S. Rapid Mycosynthesis and Characterization of Phenols-Capped Crystal Gold Nanoparticles from Ganoderma applanatum, Ganodermataceae. Biocatal. Agric. Biotechnol. 2020, 27, 101683. [Google Scholar] [CrossRef]
- Patil, M.P.; Kang, M.J.; Niyonizigiye, I.; Singh, A.; Kim, J.O.; Seo, Y.B.; Kim, G. Do Extracellular Synthesis of Gold Nanoparticles Using the Marine Bacterium Paracoccus haeundaensis BC74171T and Evaluation of Their Antioxidant Activity and Antiproliferative Effect on Normal and Cancer Cell Lines. Colloids Surf. B Biointerfaces 2019, 183, 110455. [Google Scholar] [CrossRef]
- Dhas, T.S.; Sowmiya, P.; Kumar, V.G.; Ravi, M.; Suthindhiran, K.; Borgio, J.F.; Narendrakumar, G.; Kumar, V.R.; Karthick, V.; Kumar, C.M.V. Antimicrobial Effect of Sargassum plagiophyllum Mediated Gold Nanoparticles on Escherichia coli and Salmonella typhi. Biocatal. Agric. Biotechnol. 2020, 26, 101627. [Google Scholar] [CrossRef]
- Gandhi, A.D.; Murugan, K.; Umamahesh, K.; Babujanarthanam, R.; Kavitha, P.; Selvi, A. Lichen Parmelia sulcata Mediated Synthesis of Gold Nanoparticles: An Eco-Friendly Tool against Anopheles stephensi and Aedes aegypti. Environ. Sci. Pollut. Res. 2019, 26, 23886–23898. [Google Scholar] [CrossRef]
- Abu-Tahon, M.A.; Ghareib, M.; Abdallah, W.E. Environmentally Benign Rapid Biosynthesis of Extracellular Gold Nanoparticles Using Aspergillus flavus and Their Cytotoxic and Catalytic Activities. Process Biochem. 2020, 95, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, H.; Bai, J.; Li, Y.; Yang, J.; Ma, Q.; Qu, Y. Biosynthesis of Selenium Nanoparticles Mediated by Fungus mariannaea sp. HJ and Their Characterization. Colloids Surf. A Physicochem. Eng. Asp. 2019, 571, 9–16. [Google Scholar] [CrossRef]
- Gupta, K.; Chundawat, T.S. Bio-Inspired Synthesis of Platinum Nanoparticles from Fungus fusarium Oxysporum: Its Characteristics, Potential Antimicrobial, Antioxidant and Photocatalytic Activities. Mater. Res. Express 2019, 6, 1050d6. [Google Scholar] [CrossRef]
- Eramabadi, P.; Masoudi, M.; Makhdoumi, A.; Mashreghi, M. Microbial Cell Lysate Supernatant (CLS) Alteration Impact on Platinum Nanoparticles Fabrication, Characterization, Antioxidant and Antibacterial Activity. Mater. Sci. Eng. C 2020, 117, 111292. [Google Scholar] [CrossRef]
- Mosallam, F.M.; El-Sayyad, G.S.; Fathy, R.M.; El-Batal, A.I. Biomolecules-Mediated Synthesis of Selenium Nanoparticles Using Aspergillus oryzae Fermented Lupin Extract and Gamma Radiation for Hindering the Growth of Some Multidrug-Resistant Bacteria and Pathogenic Fungi. Microb. Pathog. 2018, 122, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, L.; Wang, K.; Li, X.; Li, J.; Zhang, S.; Zhao, H.; Jiang, X.; Guan, W.; Yang, L. Bio-mediated Synthesis—A Sustainable Strategy for Nanomaterials Preparation: A Comprehensive Bibliometric Review. Nano Sel. 2021, 2, 2275–2290. [Google Scholar] [CrossRef]
- Dhaka, A.; Chand Mali, S.; Sharma, S.; Trivedi, R. A Review on Biological Synthesis of Silver Nanoparticles and Their Potential Applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Theodosiou, M.; Chalmpes, N.; Gournis, D.; Sakellis, E.; Boukos, N.; Kostakis, M.; Thomaidis, N.S.; Efthimiadou, E.K. Amino Acid Driven Synthesis of Gold Nanoparticles: A Comparative Study on Their Biocompatibility. Mater. Chem. Phys. 2024, 319, 129260. [Google Scholar] [CrossRef]
- Ponjavic, M.; Stevanovic, S.; Nikodinovic-Runic, J.; Jeremic, S.; Cosovic, V.R.; Maksimovic, V. Bacterial Nanocellulose as Green Support of Platinum Nanoparticles for Effective Methanol Oxidation. Int. J. Biol. Macromol. 2022, 223, 1474–1484. [Google Scholar] [CrossRef]
- Lafta, M.Z.; Hameed Al-Samarrai, R.R.; Bouaziz, M. Green Synthesis of Silver and Gold Nanoparticles Using Quercetin Extracted from Arctium lappa by HPLC, Characterization and Estimation of Antioxidant Activity. Results Chem. 2025, 13, 102028. [Google Scholar] [CrossRef]
- Vijayaraj, R.; Altaff, K.; Jayaprakashvel, M.; Muthezhilan, R.; Saran, B.; Kurinjinathan, P.; Jeyaperumal, S.; Perumal, V.; Kumar, R.M.S.; Govindan, L. Chitin-Derived Silver Nanoparticles for Enhanced Food Preservation: Synthesis, Characterization, and Antimicrobial Potential. Micro 2023, 3, 912–929. [Google Scholar] [CrossRef]
- Zainol Abidin, N.A.; Kormin, F.; Zainol Abidin, N.A.; Bakar, M.F.A.; Moujdin, I.A. Synthesis and Characterization of Curcumin-Chitosan Loaded Gold Nanoparticles by Oryctes rhinoceros’ Chitin for Cosmeceutical Application. Molecules 2023, 28, 1799. [Google Scholar] [CrossRef]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Lenggoro, W. Development and Characterization of Carrageenan/Nanocellulose/Silver Nanoparticles Bionanocomposite Film from Kappaphycus alvarezii Seaweed for Food Packaging. Int. J. Biol. Macromol. 2025, 311, 143922. [Google Scholar] [CrossRef]
- Chang, L.; Ahmad, B.; Tong, A.; Wu, Z.; Xu, P.; Gao, Z.; Yin, C.; Luan, M.; Tong, C.; Liu, B. Green Synthesis of Silver Nanoparticles Using Fructus Aurantii-Loaded Citrus Pectin Hydrogel for Accelerated Healing of MRSA-Infected Diabetic Wounds. Int. J. Biol. Macromol. 2025, 315, 144222. [Google Scholar] [CrossRef]
- Alsadooni, J.F.K.; Haghi, M.; Barzegar, A.; Feizi, M.A.H. The Effect of Chitosan Hydrogel Containing Gold Nanoparticle Complex with Paclitaxel on Colon Cancer Cell Line. Int. J. Biol. Macromol. 2023, 247, 125612. [Google Scholar] [CrossRef] [PubMed]
- Borker, S.; Pokharkar, V. Engineering of Pectin-Capped Gold Nanoparticles for Delivery of Doxorubicin to Hepatocarcinoma Cells: An Insight into Mechanism of Cellular Uptake. Artif. Cells Nanomed. Biotechnol. 2018, 46, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Nandana, C.N.; Christeena, M.; Bharathi, D. Synthesis and Characterization of Chitosan/Silver Nanocomposite Using Rutin for Antibacterial, Antioxidant and Photocatalytic Applications. J. Clust. Sci. 2021, 33, 269–279. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, H.; Li, Z.; Huang, D.; Nong, L.; Ning, Z.; Hu, Z.; Xu, C.; Yan, J.K. Pectin-Decorated Selenium Nanoparticles as a Nanocarrier of Curcumin to Achieve Enhanced Physicochemical and Biological Properties. IET Nanobiotechnol. 2019, 13, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J. Tree Gum Stabilised Selenium Nanoparticles: Characterisation and Antioxidant Activity. IET Nanobiotechnol. 2018, 12, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, S.; Sudarsanakumar, C. Elucidating the Interaction of L-Cysteine-Capped Selenium Nanoparticles and Human Serum Albumin: Spectroscopic and Thermodynamic Analysis. New J. Chem. 2017, 41, 9521–9530. [Google Scholar] [CrossRef]
- Velmurugan, P.; Shim, J.; Kim, K.; Oh, B.T. Prunus × yedoensis Tree Gum Mediated Synthesis of Platinum Nanoparticles with Antifungal Activity against Phytopathogens. Mater. Lett. 2016, 174, 61–65. [Google Scholar] [CrossRef]
- Elamin, M.B.; Ali, S.M.A.; Chrouda, A.; Alhaidari, L.M.; Alrouqi, R.M.; Al-Shammari, A.T.; Alsuqiani, A.A.; Jaffrezic-Renault, N. Green Synthesis of Gum Arabic Platinum Nanoparticle: Electrochemical Sensor Application for Glyphosate Detection. Polym. Adv. Technol. 2023, 34, 3407–3414. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, Y.; Yue, P.; Li, H.; Wu, Y.; Hao, X.; Peng, F. Structure, Stability, Antioxidant Activity, and Controlled-Release of Selenium Nanoparticles Decorated with Lichenan from Usnea longissima. Carbohydr. Polym. 2023, 299, 120219. [Google Scholar] [CrossRef]
- Kavin, T.; Murugaiyah, V.; Tan, J.K.; Kassim, M.N.I.; Ramakrishna, S.; Vigneswari, S. Eco-Friendly Synthesis of Silver Nanoparticles Using Coffea Arabica Husk for Enhanced Antibacterial and Anti-Cancer Applications. Biomass Bioenergy 2025, 194, 107625. [Google Scholar] [CrossRef]
- Joseph, J.; Chandrasekaran, R.; Palani, S. Anti-Proliferative Activities of Carthamidin Mediated Gold Nanoparticles against Breast Cancer: An in-Vitro Approach. Nano-Struct. Nano-Objects 2024, 39, 101324. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Jia, H.; Zhou, J.; Xi, J.; Wang, J.; Chen, X.; Wu, L. Green Synthesis of Platinum Nanoparticles by Nymphaea tetragona Flower Extract and Their Skin Lightening, Antiaging Effects. Arab. J. Chem. 2023, 16, 104391. [Google Scholar] [CrossRef]
- Olaoye, A.B.; Owoeye, S.S.; Nwobegu, J.S. Facile Green Synthesis of Plant-Mediated Selenium Nanoparticles (SeNPs) Using Moringa Oleifera Leaf and Bark Extract for Targeting α-Amylase and α-Glucosidase Enzymes in Diabetes Management. Hybrid Adv. 2024, 7, 100281. [Google Scholar] [CrossRef]
- Jalal, M.; Azam Ansari, M.; Alshamrani, M.; Ghazanfar Ali, S.; Jamous, Y.F.; Alyahya, S.A.; Alhumaidi, M.S.; Altammar, K.A.; Alsalhi, A.; Khan, H.M.; et al. Crinum latifolium Mediated Biosynthesis of Gold Nanoparticles and Their Anticandidal, Antibiofilm and Antivirulence Activity. J. Saudi Chem. Soc. 2023, 27, 101644. [Google Scholar] [CrossRef]
- Ponsanti, K.; Tangnorawich, B.; Natphopsuk, S.; Toommee, S.; Pechyen, C. Physicochemical Aspects of Platinum Nanoparticles (PtNPs) from Biological Synthesis: Influence of Plant Leaf Based Extracts as the Reducing Agent. Int. J. Precis. Eng. Manuf.-Green Technol. 2024, 11, 1097–1113. [Google Scholar] [CrossRef]
- Ridha, D.M.; Al-Awdy, M.J.; Abd Al-Zwaid, A.J.; Balakit, A.A.; Al-Dahmoshi, H.O.M.; Alotaibi, M.H.; El-Hiti, G.A. Antibacterial and Antibiofilm Activities of Selenium Nanoparticles-Antibiotic Conjugates against Anti-Multidrug-Resistant Bacteria. Int. J. Pharm. 2024, 658, 124214. [Google Scholar] [CrossRef]
- He, H.; Liu, C.; Shao, C.; Wu, Y.; Huang, Q. Green Synthesis of Ultrasmall Selenium Nanoparticles (SeNPs) Using Hericium erinaceus Polysaccharide (HEP) as Nanozymes for Efficient Intracellular Antioxidation. Mater. Lett. 2022, 317, 132079. [Google Scholar] [CrossRef]
- Magdy, G.; Aboelkassim, E.; Abd Elhaleem, S.M.; Belal, F. A Comprehensive Review on Silver Nanoparticles: Synthesis Approaches, Characterization Techniques, and Recent Pharmaceutical, Environmental, and Antimicrobial Applications. Microchem. J. 2024, 196, 109615. [Google Scholar] [CrossRef]
- Khan, M.R.; Urmi, M.A.; Kamaraj, C.; Malafaia, G.; Ragavendran, C.; Rahman, M.M. Green Synthesis of Silver Nanoparticles with Its Bioactivity, Toxicity and Environmental Applications: A Comprehensive Literature Review. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100872. [Google Scholar] [CrossRef]
- Seerangaraj, V.; Sathiyavimal, S.; Shankar, S.N.; Nandagopal, J.G.T.; Balashanmugam, P.; Al-Misned, F.A.; Shanmugavel, M.; Senthilkumar, P.; Pugazhendhi, A. Cytotoxic Effects of Silver Nanoparticles on Ruellia tuberosa: Photocatalytic Degradation Properties against Crystal Violet and Coomassie Brilliant Blue. J. Environ. Chem. Eng. 2021, 9, 105088. [Google Scholar] [CrossRef]
- Tharani, S.; Bharathi, D.; Ranjithkumar, R. Extracellular Green Synthesis of Chitosan-Silver Nanoparticles Using Lactobacillus reuteri for Antibacterial Applications. Biocatal. Agric. Biotechnol. 2020, 30, 101838. [Google Scholar] [CrossRef]
- Zarei, Z.; Razmjoue, D.; Moazeni, M.; Azarnivand, H.; Oroojalian, F. Salvia Sclarea L. (Clary Sage) Flower Extract-Mediated Green Synthesis of Silver Nanoparticles and Their Characterization, Antibacterial, Antifungal, and Scolicidal Activities against Echinococcus granulosus. Microbe 2024, 5, 100216. [Google Scholar] [CrossRef]
- Akhtar, S.; Zuhair, F.; Nawaz, M.; Khan, F.A. Green Synthesis, Characterization, Morphological Diversity, and Colorectal Cancer Cytotoxicity of Gold Nanoparticles. RSC Adv. 2024, 14, 36576–36592. [Google Scholar] [CrossRef]
- Sripriya, N.; Vasantharaj, S.; Mani, U.; Shanmugavel, M.; Jayasree, R.; Gnanamani, A. Encapsulated Enhanced Silver Nanoparticles Biosynthesis by Modified New Route for Nano-Biocatalytic Activity. Biocatal. Agric. Biotechnol. 2019, 18, 101045. [Google Scholar] [CrossRef]
- Ansari, N.; Ali, A.; Akhtar, M.S.; Hasan, S.; Khatoon, T.; Khan, A.R.; Wabaidur, S.M.; Rahman, Q.I. Green Synthesis of Zinc Oxide Marigold Shaped Clusters Using Eucalyptus Globulus Leaf Extract as Robust Photocatalyst for Dyes Degradation under Sunlight. Mater. Sci. Semicond. Process. 2024, 173, 108087. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Gandhi, A.D.; Vadakkan, K.; Mahadeva Rao, U.S.; Babujanarthanam, R. Plumeria pudica Jacq. Flower Extract—Mediated Silver Nanoparticles: Characterization and Evaluation of Biomedical Applications. Inorg. Chem. Commun. 2021, 126, 108470. [Google Scholar] [CrossRef]
- Cherian, T.; Merlin, T.; Rajendran, K.; Thomas, J. Biogenic Production and Characterization of SeNPs (Selenium Nanoparticles) Utilizing Aqueous Fruit Extract of Morus alba and Assessment of Their Biological Potentialities. Results Surf. Interfaces 2025, 19, 100562. [Google Scholar] [CrossRef]
- Bharathi, D.; Bhuvaneshwari, V. Evaluation of the Cytotoxic and Antioxidant Activity of Phyto-Synthesized Silver Nanoparticles Using Cassia Angustifolia Flowers. Bionanoscience 2019, 9, 155–163. [Google Scholar] [CrossRef]
- Sizochenko, N.; Mikolajczyk, A.; Syzochenko, M.; Puzyn, T.; Leszczynski, J. Zeta Potentials (ζ) of Metal Oxide Nanoparticles: A Meta-Analysis of Experimental Data and a Predictive Neural Networks Modeling. NanoImpact 2021, 22, 100317. [Google Scholar] [CrossRef]
- Souza, I.D.L.; Saez, V.; Mansur, C.R.E. Lipid Nanoparticles Containing Coenzyme Q10 for Topical Applications: An Overview of Their Characterization. Colloids Surf. B Biointerfaces 2023, 230, 113491. [Google Scholar] [CrossRef]
- Manimaran, M.; Norizan, M.N.; Kassim, M.H.M.; Adam, M.R.; Abdullah, N.; Norrrahim, M.N.F. Critical Review on the Stability and Thermal Conductivity of Water-Based Hybrid Nanofluids for Heat Transfer Applications. RSC Adv. 2025, 15, 14088–14125. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, H.M.; Kong, P.; Zhu, Y.M.; Dai, S.G.; Zheng, G. Concentration Measurement of Particles by Number Fluctuation in Dynamic Light Backscattering. Powder Technol. 2013, 246, 499–503. [Google Scholar] [CrossRef]
- Larichev, Y.V. Application of DLS for Metal Nanoparticle Size Determination in Supported Catalysts. Chem. Pap. 2021, 75, 2059–2066. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-Selenium and Its Nanomedicine Applications: A Critical Review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef]
- Belloso Daza, M.V.; Scarsi, A.; Gatto, F.; Rocchetti, G.; Pompa, P.P.; Cocconcelli, P.S. Role of Platinum Nanozymes in the Oxidative Stress Response of Salmonella Typhimurium. Antioxidants 2023, 12, 1029. [Google Scholar] [CrossRef]
- Wahab, S.; Salman, A.; Khan, Z.; Khan, S.; Krishnaraj, C.; Yun, S. Il Metallic Nanoparticles: A Promising Arsenal against Antimicrobial Resistance—Unraveling Mechanisms and Enhancing Medication Efficacy. Int. J. Mol. Sci. 2023, 24, 14897. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.M.; Surdu, V.A.; Ficai, A.; Ficai, D.; Grumezescu, A.M.; Andronescu, E. Green Synthesis of Metal and Metal Oxide Nanoparticles: A Review of the Principles and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 15397. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-Biofilm and Antibacterial Activities of Silver Nanoparticles Synthesized by the Reducing Activity of Phytoconstituents Present in the Indian Medicinal Plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef]
- Al-Wrafy, F.A.; Al-Gheethi, A.A.; Ponnusamy, S.K.; Noman, E.A.; Fattah, S.A. Nanoparticles Approach to Eradicate Bacterial Biofilm-Related Infections: A Critical Review. Chemosphere 2022, 288, 132603. [Google Scholar] [CrossRef]
- Irshad, A.; Jawad, R.; Sharif, S.; Joly, N.; Ishtiaq, U.; Martin, P.; Mushtaq, Q. Bioengineering of Glucan Coated Silver Nanoparticles as Dynamic Biomedical Compound; in Vitro and in Vivo Studies. Microb. Pathog. 2024, 197, 107005. [Google Scholar] [CrossRef]
- El Deeb, B.A.; Faheem, G.G.; Bakhit, M.S. Biosynthesis of Silver Nanoparticles by Talaromyces funiculosus for Therapeutic Applications and Safety Evaluation. Sci. Rep. 2025, 15, 13750. [Google Scholar] [CrossRef]
- Abeleda, H.E.P.; Javier, A.P.; Murillo, A.Q.M.; Baculi, R.Q. Alpha-Amylase Conjugated Biogenic Silver Nanoparticles as Innovative Strategy against Biofilm-Forming Multidrug Resistant Bacteria. Biocatal. Agric. Biotechnol. 2020, 29, 101784. [Google Scholar] [CrossRef]
- Haidari, H.; Kopecki, Z.; Bright, R.; Cowin, A.J.; Garg, S.; Goswami, N.; Vasilev, K. Ultrasmall AgNP-Impregnated Biocompatible Hydrogel with Highly Effective Biofilm Elimination Properties. ACS Appl. Mater. Interfaces 2020, 12, 41011–41025. [Google Scholar] [CrossRef] [PubMed]
- Onodera, A.; Nishiumi, F.; Kakiguchi, K.; Tanaka, A.; Tanabe, N.; Honma, A.; Yayama, K.; Yoshioka, Y.; Nakahira, K.; Yonemura, S.; et al. Short-Term Changes in Intracellular ROS Localisation after the Silver Nanoparticles Exposure Depending on Particle Size. Toxicol. Rep. 2015, 2, 574–579. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic Potential of Silver Nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, D.; Wadaan, M.A.; Mythili, R.; Lee, J. Synthesis of Chitosan and Gum Arabic Functionalized Silver Nanocomposite for Efficient Removal of Methylene Blue and Antibacterial Activity. Polym. Adv. Technol. 2024, 35, e6356. [Google Scholar] [CrossRef]
- Velmathi, G.; Sekar, V.; Santhanam, A. Biogenic Synthesis of Silver Nanoparticles from Deinococcus radiodurans Encapsulated with Curcumin for Enhanced Antibiofilm Activity. Microb. Pathog. 2025, 203, 107462. [Google Scholar] [CrossRef]
- Aboelmaati, M.G.; Abdel Gaber, S.A.; Soliman, W.E.; Elkhatib, W.F.; Abdelhameed, A.M.; Sahyon, H.A.; El-Kemary, M. Biogenic and Biocompatible Silver Nanoparticles for an Apoptotic Anti-Ovarian Activity and as Polydopamine-Functionalized Antibiotic Carrier for an Augmented Antibiofilm Activity. Colloids Surf. B Biointerfaces 2021, 206, 111935. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Liao, S.; Jiang, C.; Wang, L.; Tang, Y.; Wu, G.; Dai, G.; Chen, L. Quantitative Proteomics Reveals the Mechanism of Silver Nanoparticles against Multidrug-Resistant Pseudomonas aeruginosa Biofilms. J. Proteome Res. 2020, 19, 3109–3122. [Google Scholar] [CrossRef]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef]
- Eker, F.; Akdaşçi, E.; Duman, H.; Bechelany, M.; Karav, S. Gold Nanoparticles in Nanomedicine: Unique Properties and Therapeutic Potential. Nanomaterials 2024, 14, 1854. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.; Lee, J.H.; Lee, J. Direct One-Pot Synthesis of Cinnamaldehyde Immobilized on Gold Nanoparticles and Their Antibiofilm Properties. Colloids Surf. B Biointerfaces 2017, 160, 639–648. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Chen, J.; Kalaiselvi, V.; Divya, M.; González-Sánchez, Z.I.; Durán-Lara, E.F.; Vaseeharan, B. Antibacterial and Antibiofilm Activities of Marine Polysaccharide Laminarin Formulated Gold Nanoparticles: An Ecotoxicity and Cytotoxicity Assessment. J. Environ. Chem. Eng. 2021, 9, 105514. [Google Scholar] [CrossRef]
- Qais, F.A.; Ahmad, I.; Altaf, M.; Alotaibi, S.H. Biofabrication of Gold Nanoparticles Using Capsicum annuum Extract and Its Antiquorum Sensing and Antibiofilm Activity against Bacterial Pathogens. ACS Omega 2021, 6, 16670–16682. [Google Scholar] [CrossRef]
- Velasco, A.T.; Fernando, S.I.D.; Judan Cruz, K.G. LasR/RhlR Expression Linked to Quorum Sensing-Mediated Biofilm Formation in Pseudomonas aeruginosa Using Gold Nanoparticles Synthesized with Ethnobotanical Extracts. Bionanoscience 2020, 10, 876–884. [Google Scholar] [CrossRef]
- Penman, R.; Kariuki, R.; Shaw, Z.L.; Dekiwadia, C.; Christofferson, A.J.; Bryant, G.; Vongsvivut, J.; Bryant, S.J.; Elbourne, A. Gold Nanoparticle Adsorption Alters the Cell Stiffness and Cell Wall Bio-Chemical Landscape of Candida albicans Fungal Cells. J. Colloid Interface Sci. 2024, 654, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of Gold Nanoparticles (AuNPs) on Pathogenic Biofilm Formation and Invasion to Host Cells. Sci. Rep. 2016, 6, 26667. [Google Scholar] [CrossRef]
- Wu, J.; Huo, X.; Liu, J.; Bu, F.; Zhang, P. Multifunctional NIR-II Nanoplatform for Disrupting Biofilm and Promoting Infected Wound Healing. Colloids Surf. B Biointerfaces 2025, 245, 114330. [Google Scholar] [CrossRef]
- Jawad, K.H.; Jamagh, F.K.; Sulaiman, G.M.; Hasoon, B.A.; Albukhaty, S.; Mohammed, H.A.; Abomughaid, M.M. Antibacterial and Antibiofilm Activities of Amikacin-Conjugated Gold Nanoparticles: A Promising Formulation for Contact Lens Preservation. Inorg. Chem. Commun. 2024, 162, 112286. [Google Scholar] [CrossRef]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Junaid, S.; Vallini, G. Biogenic Selenium and Tellurium Nanoparticles Synthesized by Environmental Microbial Isolates Efficaciously Inhibit Bacterial Planktonic Cultures and Biofilms. Front. Microbiol. 2015, 6, 584. [Google Scholar] [CrossRef]
- Rajkhowa, S.; Hussain, S.Z.; Agarwal, M.; Zaheen, A.; Al-Hussain, S.A.; Zaki, M.E.A. Advancing Antibiotic-Resistant Microbe Combat: Nanocarrier-Based Systems in Combination Therapy Targeting Quorum Sensing. Pharmaceutics 2024, 16, 1160. [Google Scholar] [CrossRef]
- Hamman, N.; Ramburrun, P.; Dube, A. Selenium Nanoparticle Activity against S. mutans Biofilms as a Potential Treatment Alternative for Periodontitis. Pharmaceutics 2024, 16, 450. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Zonaro, E.; Lemire, J.A.; Demeter, M.; Vallini, G.; Turner, R.J.; Lampis, S. Antimicrobial Activity of Biogenically Produced Spherical Se-Nanomaterials Embedded in Organic Material against Pseudomonas aeruginosa and Staphylococcus aureus Strains on Hydroxyapatite-Coated Surfaces. Microb. Biotechnol. 2017, 10, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Kanak, K.R.; Dass, R.S.; Pan, A. Anti-Quorum Sensing Potential of Selenium Nanoparticles against LasI/R, RhlI/R, and PQS/MvfR in Pseudomonas aeruginosa: A Molecular Docking Approach. Front. Mol. Biosci. 2023, 10, 1203672. [Google Scholar] [CrossRef] [PubMed]
- Asadpour, L.; Mehrbakhsh Bandari, M.A.; Sojoudi Masouleh, R. Amikacin-Loaded Selenium Nanoparticles Improved Antibacterial and Antibiofilm Activity of Amikacin against Bovine Mastitis-Causing Staphylococcus aureus. Heliyon 2025, 11, e41103. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, K.K.; Selvaraj, B.; Subramanian, K.; Binsuwaidan, R.; Saeed, M. Antibiofilm and Antivirulence Activity of Selenium Nanoparticles Synthesized from Cell-Free Extract of Moderately Halophilic Bacteria. Microb. Pathog. 2024, 193, 106740. [Google Scholar] [CrossRef]
- Hassan, M.G.; Hawwa, M.T.; Baraka, D.M.; El-Shora, H.M.; Hamed, A.A. Biogenic Selenium Nanoparticles and Selenium/Chitosan-Nanoconjugate Biosynthesized by Streptomyces parvulus MAR4 with Antimicrobial and Anticancer Potential. BMC Microbiol. 2024, 24, 21. [Google Scholar] [CrossRef]
- Haddadian, A.; Robattorki, F.F.; Dibah, H.; Soheili, A.; Ghanbarzadeh, E.; Sartipnia, N.; Hajrasouliha, S.; Pasban, K.; Andalibi, R.; Ch, M.H.; et al. Niosomes-Loaded Selenium Nanoparticles as a New Approach for Enhanced Antibacterial, Anti-Biofilm, and Anticancer Activities. Sci. Rep. 2022, 12, 21938. [Google Scholar] [CrossRef]
- Garg, D.; Kumar, V.; Mercy Merlin, S.S.; Sachdev, A.; Matai, I. Antimicrobial Hydrogels Incorporating Nanoselenium@reduced Graphene Oxide Nanocomposites for Biofilm Inhibition. New J. Chem. 2023, 48, 2421–2438. [Google Scholar] [CrossRef]
- Prateeksha; Singh, B.R.; Shoeb, M.; Sharma, S.; Naqvi, A.H.; Gupta, V.K.; Singh, B.N. Scaffold of Selenium Nanovectors and Honey Phytochemicals for Inhibition of Pseudomonas aeruginosa Quorum Sensing and Biofilm Formation. Front. Cell. Infect. Microbiol. 2017, 7, 93. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s Frontier in Combatting Infectious and Inflammatory Diseases: Prevention and Treatment. Signal Transduct. Target. Ther. 2024, 9, 34. [Google Scholar] [PubMed]
- Sonkusre, P.; Singh Cameotra, S. Biogenic Selenium Nanoparticles Inhibit Staphylococcus Aureus Adherence on Different Surfaces. Colloids Surf. B Biointerfaces 2015, 136, 1051–1057. [Google Scholar] [CrossRef]
- Rajendran, S.; Prabha, S.S.; Rathish, R.J.; Singh, G.; Al-Hashem, A. Antibacterial Activity of Platinum Nanoparticles. In Nanotoxicity Prevention and Antibacterial Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 275–281. [Google Scholar] [CrossRef]
- Vukoja, D.; Vlainić, J.; Ljolić Bilić, V.; Martinaga, L.; Rezić, I.; Brlek Gorski, D.; Kosalec, I. Innovative Insights into In Vitro Activity of Colloidal Platinum Nanoparticles against ESBL-Producing Strains of Escherichia coli and Klebsiella pneumoniae. Pharmaceutics 2022, 14, 1714. [Google Scholar] [CrossRef]
- Krishnasamy, N.; Ramadoss, R.; Vemuri, S.; Sujai, G.N.S. Optimizing Desmostachya bipinnata-Derived Platinum Nanoparticles for Enhanced Antibacterial and Biofilm Reduction. Microb. Pathog. 2024, 196, 107004. [Google Scholar] [CrossRef] [PubMed]
- Hasoon, B.A.; Hasan, D.M.A.; Jawad, K.H.; Shakaer, S.S.; Sulaiman, G.M.; Hussein, N.N.; Mohammed, H.A.; Abomughaid, M.M.; Ramesh, T. Promising Antibiofilm Formation: Liquid Phase Pulsed Laser Ablation Synthesis of Graphene Oxide@Platinum Core-Shell Nanoparticles. PLoS ONE 2024, 19, e0310997. [Google Scholar] [CrossRef]
- Ranpariya, B.; Salunke, G.; Karmakar, S.; Babiya, K.; Sutar, S.; Kadoo, N.; Kumbhakar, P.; Ghosh, S. Antimicrobial Synergy of Silver-Platinum Nanohybrids with Antibiotics. Front. Microbiol. 2021, 11, 610968. [Google Scholar] [CrossRef]
- Rajkumari, J.; Busi, S.; Vasu, A.C.; Reddy, P. Facile Green Synthesis of Baicalein Fabricated Gold Nanoparticles and Their Antibiofilm Activity against Pseudomonas aeruginosa PAO1. Microb. Pathog. 2017, 107, 261–269. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Salaam, A.; Ajibade, A. Green Synthesis of Silver Nanoparticle Using Oscillatoria sp. Extract, Its Antibacterial, Antibiofilm Potential and Cytotoxicity Activity. Heliyon 2019, 5, e02502. [Google Scholar] [CrossRef]
- Shanthi, S.; David Jayaseelan, B.; Velusamy, P.; Vijayakumar, S.; Chih, C.T.; Vaseeharan, B. Biosynthesis of Silver Nanoparticles Using a Probiotic Bacillus licheniformis Dahb1 and Their Antibiofilm Activity and Toxicity Effects in Ceriodaphnia cornuta. Microb. Pathog. 2016, 93, 70–77. [Google Scholar] [CrossRef]
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M.; et al. Green Synthesis of Silver, Gold and Silver/Gold Bimetallic Nanoparticles Using the Gloriosa superba Leaf Extract and Their Antibacterial and Antibiofilm Activities. Microb. Pathog. 2016, 101, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Majumdar, A.G.; Gullani, H.; Roy, A.L.; Dash, A.K.; Behera, P.K.; Parwez, Z.; Giri, S.; Mohanty, P.S.; Ray, L. Green Synthesis of Silver-Chitosan Nanocomposite Exhibits Promising Antibiofilm Properties against Pathogenic Bacteria Escherichia coli and Staphylococcus aureus. Microbe 2025, 6, 100264. [Google Scholar] [CrossRef]
- Sattari-Maraji, A.; Nikchi, M.; Shahmiri, M.; Ahadi, E.M.; Firoozpour, L.; Moazeni, E.; Jabalameli, F.; Pourmand, M.R.; Kharrazi, S. Jellein-I-Conjugated Gold Nanoparticles: Insights into the Antibacterial, Antibiofilm Activities against MRSA, and Anticancer Properties. J. Drug Deliv. Sci. Technol. 2024, 101, 106218. [Google Scholar] [CrossRef]
- Hudaya, I.R.; Situmorang, V.C.; Hardianto, F.A.; Putra, E.D.L.; Satria, D.; Hertriani, T.; Hartati, R.; Septama, A.W. Biofabrication of Silver Nanoparticles Using Artocarpus heterophyllus Leaves Extract: Characterization and Evaluation of Its Antibacterial, Antibiofilm, and Antioxidant Activities. J. Cryst. Growth 2024, 644, 127827. [Google Scholar] [CrossRef]
- Mostafa, H.Y.; El-Sayyad, G.S.; Nada, H.G.; Ellethy, R.A.; Zaki, E.G. Promising Antimicrobial and Antibiofilm Activities of Orobanche aegyptiaca Extract-Mediated Bimetallic Silver-Selenium Nanoparticles Synthesis: Effect of UV-Exposure, Bacterial Membrane Leakage Reaction Mechanism, and Kinetic Study. Arch. Biochem. Biophys. 2023, 736, 109539. [Google Scholar] [CrossRef]
- El-Deeb, B.; Al-Talhi, A.; Mostafa, N.; Abou-assy, R. Biological Synthesis and Structural Characterization of Selenium Nanoparticles and Assessment of Their Antimicrobial Properties. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 45, 135–170. [Google Scholar]
- Ullah, A.; Mirani, Z.A.; Binbin, S.; Wang, F.; Chan, M.W.H.; Aslam, S.; Yonghong, L.; Hasan, N.; Naveed, M.; Hussain, S.; et al. An Elucidative Study of the Anti-Biofilm Effect of Selenium Nanoparticles (SeNPs) on Selected Biofilm Producing Pathogenic Bacteria: A Disintegrating Effect of SeNPs on Bacteria. Process Biochem. 2023, 126, 98–107. [Google Scholar] [CrossRef]
- Gurkok, S.; Ozdal, M.; Cakici, T.; Kurbanoglu, E.B. Antimicrobial, Antibiofilm, and Antiurease Activities of Green-Synthesized Zn, Se, and ZnSe Nanoparticles against Streptococcus salivarius and Proteus mirabilis. Bioprocess Biosyst. Eng. 2025, 48, 589–603. [Google Scholar] [CrossRef]
- Dlugaszewska, J.; Dobrucka, R. Effectiveness of Biosynthesized Trimetallic Au/Pt/Ag Nanoparticles on Planktonic and Biofilm Enterococcus faecalis and Enterococcus faecium Forms. J. Clust. Sci. 2019, 30, 1091–1101. [Google Scholar] [CrossRef]


| Plant Name | Plant Part Used | Type of NPs | Shape | Size (nm) | References |
|---|---|---|---|---|---|
| Antigonon leptopus | Leaf | Ag | Spherical | 4–20 | Gastelum-Cabrera et al. [64] |
| Harrisonia abyssinica | Fruit | Ag | Spherical | 2–24 | Mwakalesi et al. [65] |
| Tinospora cordifolia | Leaf | Ag | Spherical | 125 | Lekkala et al. [66] |
| Punica granatum | Seeds | Ag | Spherical | 10–35 | Muthu et al. [67] |
| Melia azedarach | Leaf | Ag | Spherical | 18–30 | Jebril et al. [68] |
| Eichhornia crassipes | Leaf | Ag | Spherical, cubic, rod and hexagonal | 57.93, 56.44 and 58.25 | Heikal et al. [69] |
| Moringa oleifera | Flower | Ag | Spherical | 8 | Bindhu et al. [70] |
| Clitoria ternatea | Flower | Ag | Quasi-spherical | 3.21–43.7 | Singh et al. [71] |
| Calotropis procera | Root | Ag | Spherical and square | 38–44 | Sagadevan et al. [72] |
| Cyperus conglomeratus | Root | Ag | Spherical | 70–100 | Al-Nuairi et al. [73] |
| Tamarindus indica | Fruit | Ag | Spherical | 20–52 | Gomathi et al. [74] |
| Vitis vinifera | Fruit waste | Ag | Spherical | 22–35 | Saratale et al. [75] |
| Brassica oleracea var. botrytis | Flower waste | Ag | Spherical | 5–50 | Kadam et al. [76] |
| Apium graveolens | Leaf and stem | Au | Spherical | 4–15 | Panchamoorthy et al. [77] |
| Jasminum auriculatum | Leaf | Au | Spherical | 8–37 | Balasubramanian et al. [78] |
| Cucurbita moschata | Fruit peel | Au | Spherical | 18.10 | Kaval and Hosgoren [79] |
| Hibiscus sabdariffa | Flower | Au | Spherical | 15–45 | Zangeneh et al. [80] |
| Lonicera japonica | Flower | Au | Spherical, triangular and hexagonal | 10–40 | Patil et al. [81] |
| Lycium chinense | Fruit | Ag and Au | Spherical | 50–200 and 20–100 | Chokkalingam et al. [82] |
| Euphorbia fischeriana | Root | Au | Core–shell | 20–60 | Zhang et al. [83] |
| Codonopsis pilosula | Root | Au | Spherical | 20 ± 3.2 | Doan et al. [84] |
| Tinospora cordifolia | Stem | Au | Spherical | 16.1 | Ali et al. [85] |
| Azadirachta indica | Leaf | Se | Spherical | 142–168 | Mulla et al. [86] |
| Dillenia indica | Leaf | Se | Oval | 50–900 | Krishnan et al. [87] |
| Phoenix dactylifera | Fruit | Pt | Quasi- spherical | 2.3–3 | Al-Radadi et al. [88] |
| Xanthium strumarium | Leaf | Pt | Cubic | 22 | Kumar et al. [89] |
| Combretum erythrophyllum | Leaf | Pt | Spherical | 1.04 ± 0.26 | Fanoro et al. [90] |
| Nigella sativa | Leaf | Pt | Spherical | 3.47 ± 1.31 | Aygun et al. [91] |
| Biomolecules | Type of NP | Shape | Size (nm) | References |
|---|---|---|---|---|
| Chitin | Ag | Rod | 15–70 length and 5–10 breadth | Vijayaraj et al. [137] |
| Curcumin-chitosan | Au | Spherical | 128.27 | Zainol Abidin et al. [138] |
| Carrageenan/nanocellulose | Ag | Spherical | 20–200 | Jaffar et al. [139] |
| Fructus aurantii-loaded Citrus pectin | Ag | Spherical | 5–32 | Chang et al. [140] |
| Chitosan | Au | Spherical | 45–60 | Alsadooni et al. [141] |
| Pectin | Au | Spherical | 14 | Borker and Pokharkar [142] |
| Rutin/chitosan | Ag | Spherical | 23–78 | Bharathi et al. [143] |
| Pectin | Se | Spherical | ~61 | Wu et al. [144] |
| Gum kondagogu | Se | spheroids | 44.4–200 | Kora [145] |
| L-cystine | Se | Spherical | 60 | Prasanth and Sudarsanakumar [146] |
| Prunus × yedoensis—gum | Pt | Circular | 10–20 | Velmurugan et al. [147] |
| Gum arabic | Pt | Spherical | 6–10 | Elamin et al. [148] |
| Lichenan from Usnea longissima | Se | Spherical | 76 | Yang et al. [149] |
| Nanoparticles | Synthesis Source | Antibiofilm Activity | Proposed Mechanism | References |
|---|---|---|---|---|
| Ag | Deinococcus radiodurans | E. coli and S. aureus | Generation of ROS, leading to oxidative stress that damages bacterial DNA and proteins, resulting in protein denaturation and disruption of metabolic processes | Velmathi et al. [188] |
| Au | Baicalein | P. aeruginosa PAO1 | Inhibits EPS synthesis, reducing the structural integrity of biofilms and impairing bacterial adhesion and communication. | Rajkumari et al. [219] |
| Ag | Oscillatoria sp. | E. coli 35218, S. aureus, P. aeruginosa, Citrobacter, S. typhi, E. coli 11775, and Bacillus sp. | Interferes with bacterial replication by deactivating DNA-binding enzymes, thus preventing DNA synthesis and cell division. | Adebayo-Tayo et al. [220] |
| Ag | Bacillus licheniformis Dahb1 | V. parahaemolyticus DAV1 | Triggers premature detachment of bacterial cells by disrupting biofilm matrix stability, likely through oxidative stress and signaling interference. | Shanthi et al. [221] |
| Ag/Au bimetallic NPs | Gloriosa superba | S. aureus, S. pneumoniae, K. pneumoniae, and E. coli | Disrupts bacterial cell membrane integrity, causing leakage of intracellular contents and loss of membrane potential essential for cell viability. | Gopinath et al. [222] |
| Ag-CS nanocomposite | Streptomyces strain RB7AG and chitosan | E. coli and S. aureus | Induces ROS production and disrupts membrane permeability, enhancing chitosan’s cationic interaction with bacterial surfaces for effective biofilm penetration. | Behera et al. [223] |
| Au | Jellein-I peptide conjugated | MRSA | Interferes with protein function by binding to key enzymes and structural proteins in MRSA, leading to impaired biofilm architecture and cellular metabolism. | Sattari-Maraji et al. [224] |
| Au | Cinnamaldehyde | C. albicans | Disrupts biofilm formation by inhibiting hyphal growth and morphogenesis in C. albicans. | Ramasamy et al. [193] |
| Au | Artocarpus heterophyllus | A. baumannii and MRSA | Inhibits initial biofilm establishment by blocking bacterial adhesion and proliferation, likely by targeting surface adhesins and cell division proteins. | Hudaya et al. [225] |
| Ag-Se | Orobanche aegyptiaca | S. aureus | Suppresses the production of exopolysaccharides by interfering with bacterial signaling pathways that regulate EPS biosynthesis. | Mostafa et al. [226] |
| Au | Capsicum annum | P. aeruginosa PAO1 | Inhibits quorum-sensing networks responsible for the regulation of virulence genes and biofilm maturation in P. aeruginosa. | Qais et al. [195] |
| Se | Providencia vermicola BGRW | S. aureus, B. cereus, E. coli, Proteus sp. P. aeruginosa, and S. enteritidis | Disrupts the structural matrix of mature biofilms by degrading the glycocalyx and inhibiting biofilm matrix cohesion. | El-Deeb et al. [227] |
| Se | B. subtilis BSN313 | P. aeruginosa ATCC 9027, S. typhi ATCC 14028, and S. aureus ATCC 25923 | Alters bacterial surface properties such as hydrophobicity and electrostatic interactions, hindering cell aggregation and biofilm integrity. | Ullah et al. [228] |
| Se | Pseudomonas aeruginosa OG1 | S. salivarius and P. mirabilis | Reduces biofilm formation by inhibiting oxidative stress response, impairing bacterial adhesion and biofilm stabilization. | Gurkok et al. [229] |
| Au/Pt/Ag | Lamii albi flos | Enterococcal strain | Targets sessile bacterial populations by penetrating biofilms and disrupting intracellular processes, leading to the eradication of planktonic escape cells. | Dlugaszewska et al. [230] |
| Pt | Desmostachya bipinnata | S. aureus, Enterococcus faecalis, and S. mutants | Promotes intracellular ROS accumulation, resulting in oxidative damage to essential biomolecules and impairment of metabolic pathways in multidrug-resistant pathogens. | Krishnasamy et al. [216] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharathi, D.; Lee, J. Recent Trends in Bioinspired Metal Nanoparticles for Targeting Drug-Resistant Biofilms. Pharmaceuticals 2025, 18, 1006. https://doi.org/10.3390/ph18071006
Bharathi D, Lee J. Recent Trends in Bioinspired Metal Nanoparticles for Targeting Drug-Resistant Biofilms. Pharmaceuticals. 2025; 18(7):1006. https://doi.org/10.3390/ph18071006
Chicago/Turabian StyleBharathi, Devaraj, and Jintae Lee. 2025. "Recent Trends in Bioinspired Metal Nanoparticles for Targeting Drug-Resistant Biofilms" Pharmaceuticals 18, no. 7: 1006. https://doi.org/10.3390/ph18071006
APA StyleBharathi, D., & Lee, J. (2025). Recent Trends in Bioinspired Metal Nanoparticles for Targeting Drug-Resistant Biofilms. Pharmaceuticals, 18(7), 1006. https://doi.org/10.3390/ph18071006







