Evaluation of Statins Use in Hemodialysis Patients: A Retrospective Analysis of Clinical and Safety Outcomes
Abstract
1. Introduction
2. Results
2.1. Lipid Profile Changes Among the Study Participants
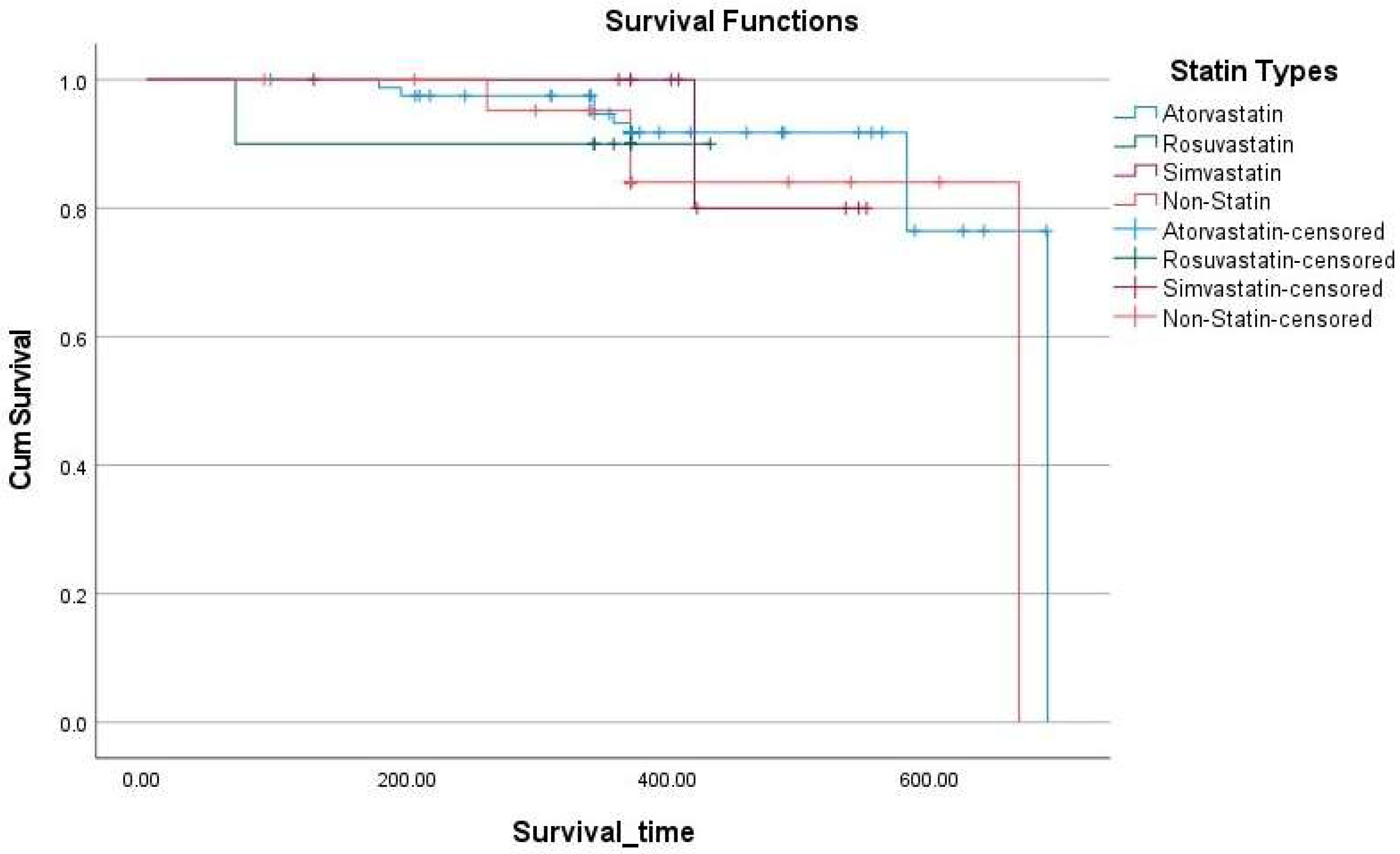
2.2. Association Between New Atherosclerotic Events and the Study Participants
2.3. Comparison of Liver Function and Creatine Kinase Levels Across Groups
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Endpoints
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESRD | End-Stage Renal Disease |
| CVDs | Cardiovascular Diseases |
| CAD | Coronary Artery Disease |
| ASCHD | Atherosclerotic Coronary Heart Disease |
| HD | Hemodialysis |
| CKD | Chronic Kidney Disease |
| CK | Creatine Kinase |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| HDL-C | High-Density Lipoprotein Cholesterol |
| TG | Triglycerides |
| TC | Total Cholesterol |
| AHA/ACC | American Heart Association/American College of Cardiology |
| CTT | The Cholesterol Treatment Trialists’ Collaboration |
| HF | Heart Failure |
| ACS | Acute Coronary Syndrome |
| PVD | Peripheral Vascular Disease |
| LFTs | Liver Function Tests |
| ALP | Alkaline Phosphatase |
| ALT | Alanine Transaminase |
| AST | Aspartate Aminotransferase |
References
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Anavekar, N.S.; McMurray, J.J.V.; Velazquez, E.J.; Solomon, S.D.; Kober, L.; Rouleau, J.-L.; White, H.D.; Nordlander, R.; Maggioni, A.; Dickstein, K.; et al. Relation Between Renal Dysfunction and Cardiovascular Outcomes After Myocardial Infarction. N. Engl. J. Med. 2004, 351, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; De Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of Estimated Glomerular Filtration Rate and Albuminuria with All-Cause and Cardiovascular Mortality in General Population Cohorts: A Collaborative Meta-Analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- United States Renal Data System. 2024 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; NIH: Bethesda, MD, USA, 2024.
- Calice-Silva, V.; Muenz, D.; Wong, M.M.Y.; McCullough, K.; Charytan, D.; Reichel, H.; Robinson, B.; Stengel, B.; Massy, Z.A.; Pecoits-Filho, R.; et al. International Practice Patterns of Dyslipidemia Management in Patients with Chronic Kidney Disease Under Nephrology Care: Is It Time to Review Guideline Recommendations? Lipids Health Dis. 2023, 22, 67. [Google Scholar] [CrossRef]
- Mathur, S.; Devaraj, S.; Jialal, I. Accelerated Atherosclerosis, Dyslipidemia, and Oxidative Stress in End-Stage Renal Disease. Curr. Opin. Nephrol. Hypertens. 2002, 11, 141–147. [Google Scholar] [CrossRef]
- Nakano, T. Atherosclerotic Diseases in Chronic Kidney Disease. J. Atheroscler. Thromb. 2025, 32, RV22030. [Google Scholar] [CrossRef]
- Wanner, C.; Tonelli, M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: Summary of Recommendation Statements and Clinical Approach to the Patient. Kidney Int. 2014, 85, 1303–1309. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Alhomoud, I.S.; Talasaz, A.; Mehta, A.; Kelly, M.S.; Sisson, E.M.; Bucheit, J.D.; Brown, R.; Dixon, D.L. Role of Lipoprotein(a) in Atherosclerotic Cardiovascular Disease: A Review of Current and Emerging Therapies. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2023, 43, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Guérin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Cardiovascular Disease in the Dialysis Population: Prognostic Significance of Arterial Disorders. Curr. Opin. Nephrol. Hypertens. 2006, 15, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Krane, V.; März, W.; Olschewski, M.; Mann, J.F.E.; Ruf, G.; Ritz, E. Atorvastatin in Patients with Type 2 Diabetes Mellitus Undergoing Hemodialysis. N. Engl. J. Med. 2005, 353, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Fellström, B.C.; Jardine, A.G.; Schmieder, R.E.; Holdaas, H.; Bannister, K.; Beutler, J.; Chae, D.-W.; Chevaile, A.; Cobbe, S.M.; Grönhagen-Riska, C.; et al. Rosuvastatin and Cardiovascular Events in Patients Undergoing Hemodialysis. N. Engl. J. Med. 2009, 360, 1395–1407. [Google Scholar] [CrossRef]
- Baigent, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The Effects of Lowering LDL Cholesterol with Simvastatin plus Ezetimibe in Patients with Chronic Kidney Disease (Study of Heart and Renal Protection): A Randomised Placebo-Controlled Trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef]
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J.; et al. Author Correction: Lipid Management in Patients with Chronic Kidney Disease. Nat. Rev. Nephrol. 2019, 15, 121. [Google Scholar] [CrossRef]
- Hohenstein, B. Lipoprotein(a) in Nephrological Patients. Clin. Res. Cardiol. Suppl. 2017, 12 (Suppl. 1), 27–30. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Herrington, W.; Emberson, J.; Mihaylova, B.; Blackwell, L.; Reith, C.; Solbu, M.; Mark, P.; Fellström, B.; Jardine, A.; et al. Impact of Renal Function on the Effects of LDL Cholesterol Lowering with Statin-Based Regimens: A Meta-Analysis of Individual Participant Data from 28 Randomised Trials. Lancet Diabetes Endocrinol. 2016, 4, 829–839. [Google Scholar] [CrossRef]
- Echefu, G.; Stowe, I.; Burka, S.; Basu-Ray, I.; Kumbala, D. Pathophysiological Concepts and Screening of Cardiovascular Disease in Dialysis Patients. Front. Nephrol. 2023, 3, 1198560. [Google Scholar] [CrossRef]
- Patel, P.N.; Giugliano, R.P. Low-Density Lipoprotein Cholesterol Lowering Therapy for the Secondary Prevention of Atherosclerotic Cardiovascular Disease. Glob. Cardiol. Sci. Pract. 2020, 2020, 39. [Google Scholar] [CrossRef]
- Wanner, C.; Krane, V.; März, W.; Olschewski, M.; Asmus, H.-G.; Krämer, W.; Kühn, K.-W.; Kütemeyer, H.; Mann, J.F.E.; Ruf, G.; et al. Randomized Controlled Trial on the Efficacy and Safety of Atorvastatin in Patients with Type 2 Diabetes on Hemodialysis (4D Study): Demographic and Baseline Characteristics. Kidney Blood Press. Res. 2004, 27, 259–266. [Google Scholar] [CrossRef]
| Socio-Economic and Clinical Variables | No. of Patients (n) | Percentage (%) |
|---|---|---|
| Male | 64 | 35.8 |
| Female | 115 | 64.2 |
| Age (Mean ± SD) | 66.6 ± 14.7 years | |
| Body Mass Index (Mean ± SD) | 28.6 ± 7.7 kg/m2 | |
| Comorbidities | ||
| Diabetes Mellitus | 134 | 74.9 |
| Hypertension | 168 | 93.9 |
| CV events | ||
| None | 84 | 46.9 |
| ACS | 19 | 10.6 |
| HF | 25 | 14.0 |
| ACS and HF | 49 | 27.4 |
| RHD with MS | 2 | 1.1 |
| Atherosclerotic Events History | ||
| None | 108 | 60.3 |
| Stroke | 64 | 35.8 |
| PAD | 3 | 1.7 |
| Stroke and PAD | 4 | 2.2 |
| Statin Therapy | ||
| Atorvastatin | 124 | 69.3 |
| Rosuvastatin | 10 | 5.6 |
| Simvastatin | 14 | 7.8 |
| Not on Statin Therapy | 31 | 17.3 |
| Distribution of Atorvastatin Doses | ||
| 10 mg | 15 | 12.1 |
| 20 mg | 45 | 36.3 |
| 40 mg | 56 | 45.2 |
| 60 mg | 2 | 1.6 |
| 80 mg | 6 | 4.8 |
| Distribution of Rosuvastatin Doses | ||
| 10 mg | 5 | 50 |
| 20 mg | 3 | 30 |
| 40 mg | 2 | 20 |
| Distribution of Simvastatin Doses | ||
| 10 mg | 3 | 21.4 |
| 20 mg | 8 | 57.2 |
| 40 mg | 3 | 21.4 |
| Socio-Economic and Clinical Variables | Statin Users n (%) | Non-Statin Users n (%) |
|---|---|---|
| Male | 49 (33.1%) | 15 (48.4%) |
| Female | 99 (66.9%) | 16 (51.6%) |
| Age (Mean ± SD) | 67.62 (13.23) | 61.90 (19.91) |
| Body Mass Index (Mean ± SD) | 29.40 (7.79) | 25.06 (6.17) |
| Comorbidities | ||
| Diabetes Mellitus | 122 (82.4%) | 12 (38.7%) |
| Hypertension | 140 (94.6%) | 28 (90.3%) |
| CV events | ||
| None | 60 (40.5%) | 24 (77.4%) |
| ACS | 18 (12.2%) | 1 (3.2%) |
| HF | 22 (14.9%) | 3 (9.7%) |
| ACS and HF | 46 (31.1%) | 3 (9.7%) |
| RHD with MS | 2 (1.4%) | 0 (0.0%) |
| Atherosclerotic Events History | ||
| None | 86 (58.1%) | 22 (71.0%) |
| Stroke | 55 (37.2%) | 9 (29.0%) |
| PAD | 3 (2.0%) | 0 (0.0%) |
| Stroke and PAD | 4 (2.7%) | 0 (0.0%) |
| Participant Groups | Lipid Profile (mmol/L) | Duration | Mean | SD | t-Value | p-Value |
|---|---|---|---|---|---|---|
| Atorvastatin | LDL-C | Baseline | 2.058 | 0.818 | 0.609 | 0.54 |
| Follow-up | 2.003 | 0.769 | ||||
| TG | Baseline | 1.730 | 1.113 | 0.642 | 0.52 | |
| Follow-up | 1.656 | 1.019 | ||||
| TC | Baseline | 3.769 | 1.059 | 1.062 | 0.29 | |
| Follow-up | 3.654 | 0.974 | ||||
| HDL-C | Baseline | 0.900 | 0.245 | −0.969 | 0.33 | |
| Follow-up | 0.925 | 0.262 | ||||
| Rosuvastatin | LDL-C | Baseline | 2.607 | 0.942 | 1.160 | 0.28 |
| Follow-up | 2.113 | 1.136 | ||||
| TG | Baseline | 1.482 | 0.855 | −0.723 | 0.49 | |
| Follow-up | 1.766 | 1.003 | ||||
| TC | Baseline | 4.398 | 1.165 | 1.143 | 0.29 | |
| Follow-up | 3.785 | 1.390 | ||||
| HDL-C | Baseline | 1.011 | 0.304 | 0.869 | 0.41 | |
| Follow-up | 0.930 | 0.344 | ||||
| Simvastatin | LDL-C | Baseline | 1.550 | 0.520 | −1.608 | 0.15 |
| Follow-up | 1.901 | 0.651 | ||||
| TG | Baseline | 1.480 | 0.865 | −1.555 | 0.16 | |
| Follow-up | 1.845 | 0.963 | ||||
| TC | Baseline | 3.268 | 0.818 | −1.188 | 0.27 | |
| Follow-up | 3.632 | 0.863 | ||||
| HDL-C | Baseline | 0.997 | 0.333 | 0.570 | 0.58 | |
| Follow-up | 0.952 | 0.228 | ||||
| Not on Statin Therapy | LDL-C | Baseline | 2.678 | 0.630 | −0.796 | 0.44 |
| Follow-up | 2.820 | 0.821 | ||||
| TG | Baseline | 1.351 | 0.527 | 0.553 | 0.59 | |
| Follow-up | 1.282 | 0.458 | ||||
| TC | Baseline | 4.391 | 0.670 | −0.586 | 0.56 | |
| Follow-up | 4.515 | 0.915 | ||||
| HDL-C | Baseline | 1.089 | 0.269 | −0.495 | 0.63 | |
| Follow-up | 1.107 | 0.235 |
| Participant Groups | Atherosclerotic Events | Total (%) | Chi-Square Test χ2 | p-Value | ||
|---|---|---|---|---|---|---|
| No (%) | Yes (%) | |||||
| Atorvastatin | No | 51 (28.5) | 4 (2.2) | 55 (30.7) | 0.127 | 0.72 |
| Yes | 113 (63.1) | 11 (6.1) | 124 (69.3) | |||
| Rosuvastatin | No | 157 (87.7) | 12 (6.7) | 169 (94.4%) | 6.48 | 0.01 |
| Yes | 7 (3.9) | 3 (1.7) | 10 (5.6) | |||
| Simvastatin | No | 150 (83.8) | 15 (8.4) | 165 (92.2) | 1.389 | 0.24 |
| Yes | 14 (7.8) | 0 (0.0) | 14 (7.8) | |||
| Not on Statin Therapy | No | 134 (74.9) | 14 (7.8) | 148 (82.7) | 1.29 | 0.25 |
| Yes | 30 (16.8) | 1 (0.6) | 31 (17.3) | |||
| B | SE | Wald | df | Sig. | Exp(B) | 95.0% CI for Exp(B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Statin Types | 1.186 | 3 | 0.756 | |||||
| Rosuvastatin | 0.436 | 1.077 | 0.164 | 1 | 0.685 | 1.547 | 0.187 | 12.778 |
| Simvastatin | 0.059 | 1.084 | 0.003 | 1 | 0.956 | 1.061 | 0.127 | 8.882 |
| Non-Statin | 0.665 | 0.630 | 1.114 | 1 | 0.291 | 1.944 | 0.566 | 6.676 |
| Participant Groups | LFTs (U/L) | Duration | Mean | SD | t-Value | p-Value |
|---|---|---|---|---|---|---|
| Atorvastatin | AST | Baseline | 17.57 | 9.56 | −0.627 | 0.53 |
| Follow-up | 24.08 | 97.09 | ||||
| ALT | Baseline | 14.27 | 8.42 | −0.945 | 0.35 | |
| Follow-up | 24.08 | 97.09 | ||||
| ALP | Baseline | 147.61 | 97.83 | −1.766 | 0.08 | |
| Follow-up | 160.57 | 100.41 | ||||
| CK Level | Baseline | 56.41 | 46.04 | 0.454 | 0.65 | |
| Follow-up | 53.48 | 53.37 | ||||
| Rosuvastatin | AST | Baseline | 14.22 | 5.24 | −0.388 | 0.71 |
| Follow-up | 15.44 | 8.05 | ||||
| ALT | Baseline | 12.78 | 5.45 | −0.944 | 0.37 | |
| Follow-up | 15.44 | 8.05 | ||||
| ALP | Baseline | 133.11 | 36.87 | −0.282 | 0.78 | |
| Follow-up | 145.00 | 131.26 | ||||
| CK Level | Baseline | 97.22 | 74.04 | 1.870 | 0.10 | |
| Follow-up | 46.44 | 23.51 | ||||
| Simvastatin | AST | Baseline | 23.56 | 24.22 | 1.445 | 0.19 |
| Follow-up | 11.22 | 3.87 | ||||
| ALT | Baseline | 18.44 | 21.89 | 0.943 | 0.37 | |
| Follow-up | 11.22 | 3.87 | ||||
| ALP | Baseline | 122.89 | 58.88 | −0.167 | 0.87 | |
| Follow-up | 126.56 | 20.09 | ||||
| CK Level | Baseline | 56.50 | 35.61 | 2.353 | 0.05 | |
| Follow-up | 37.63 | 16.50 | ||||
| Not on Statin Therapy | AST | Baseline | 16.68 | 8.13 | 0.620 | 0.54 |
| Follow-up | 14.89 | 15.82 | ||||
| ALT | Baseline | 13.11 | 9.83 | −0.987 | 0.34 | |
| Follow-up | 14.89 | 15.82 | ||||
| ALP | Baseline | 180.37 | 295.29 | 0.412 | 0.69 | |
| Follow-up | 168.11 | 186.16 | ||||
| CK Level | Baseline | 46.63 | 28.92 | −0.760 | 0.46 | |
| Follow-up | 57.95 | 67.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, A.S.; Albekery, M.A.; Alanazi, A.A.; Alhomoud, I.S.; Alamer, K.A.; Shawaqfeh, M.; Alshammari, R.H.; Alhejaili, F.; Al Sahlawi, M.; Aldossary, I.; et al. Evaluation of Statins Use in Hemodialysis Patients: A Retrospective Analysis of Clinical and Safety Outcomes. Pharmaceuticals 2025, 18, 911. https://doi.org/10.3390/ph18060911
Alotaibi AS, Albekery MA, Alanazi AA, Alhomoud IS, Alamer KA, Shawaqfeh M, Alshammari RH, Alhejaili F, Al Sahlawi M, Aldossary I, et al. Evaluation of Statins Use in Hemodialysis Patients: A Retrospective Analysis of Clinical and Safety Outcomes. Pharmaceuticals. 2025; 18(6):911. https://doi.org/10.3390/ph18060911
Chicago/Turabian StyleAlotaibi, Abdulmalik S., Mohamed A. Albekery, Ahmed A. Alanazi, Ibrahim S. Alhomoud, Khalid A. Alamer, Mohammad Shawaqfeh, Reem H. Alshammari, Fayez Alhejaili, Muthana Al Sahlawi, Ibrahim Aldossary, and et al. 2025. "Evaluation of Statins Use in Hemodialysis Patients: A Retrospective Analysis of Clinical and Safety Outcomes" Pharmaceuticals 18, no. 6: 911. https://doi.org/10.3390/ph18060911
APA StyleAlotaibi, A. S., Albekery, M. A., Alanazi, A. A., Alhomoud, I. S., Alamer, K. A., Shawaqfeh, M., Alshammari, R. H., Alhejaili, F., Al Sahlawi, M., Aldossary, I., Aljuayl, H. A., Alkathiri, M., Alharbi, S., Albekairy, A., & Alkatheri, A. (2025). Evaluation of Statins Use in Hemodialysis Patients: A Retrospective Analysis of Clinical and Safety Outcomes. Pharmaceuticals, 18(6), 911. https://doi.org/10.3390/ph18060911







