Combination Treatment with Free Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells
Abstract
1. Introduction
2. Results
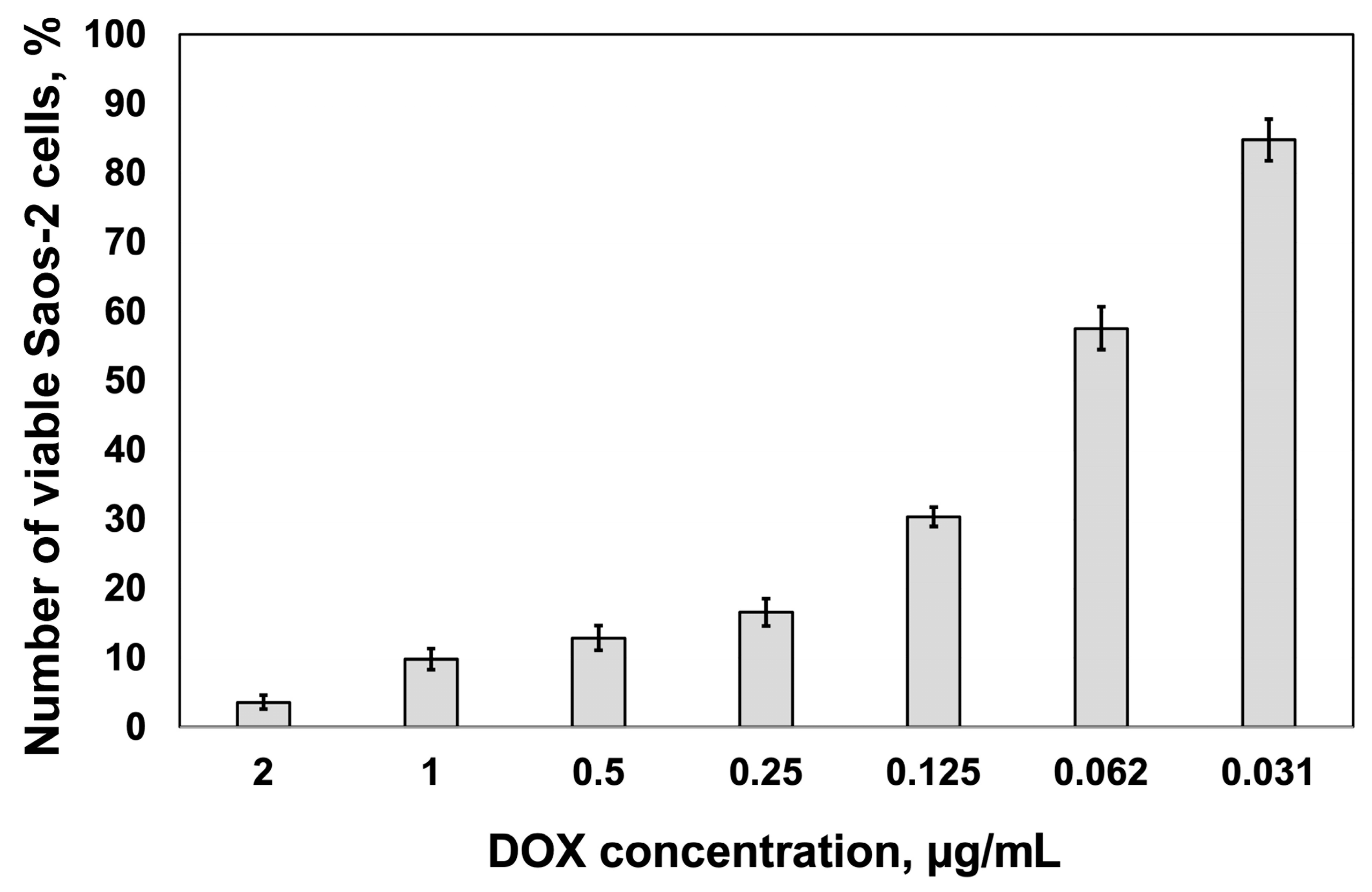
2.1. Cytotoxic Response
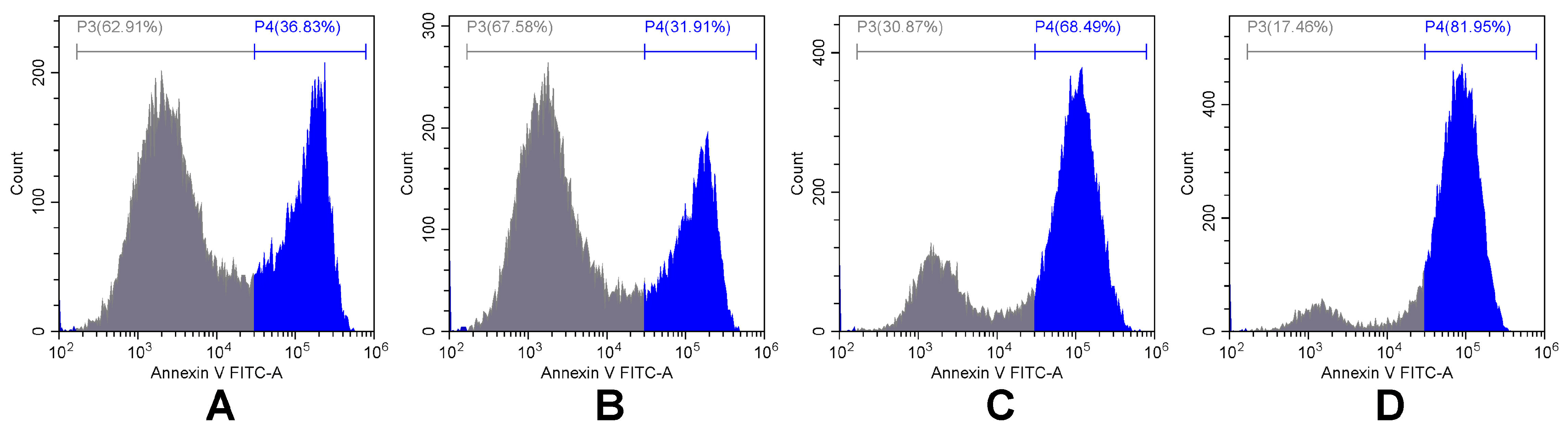
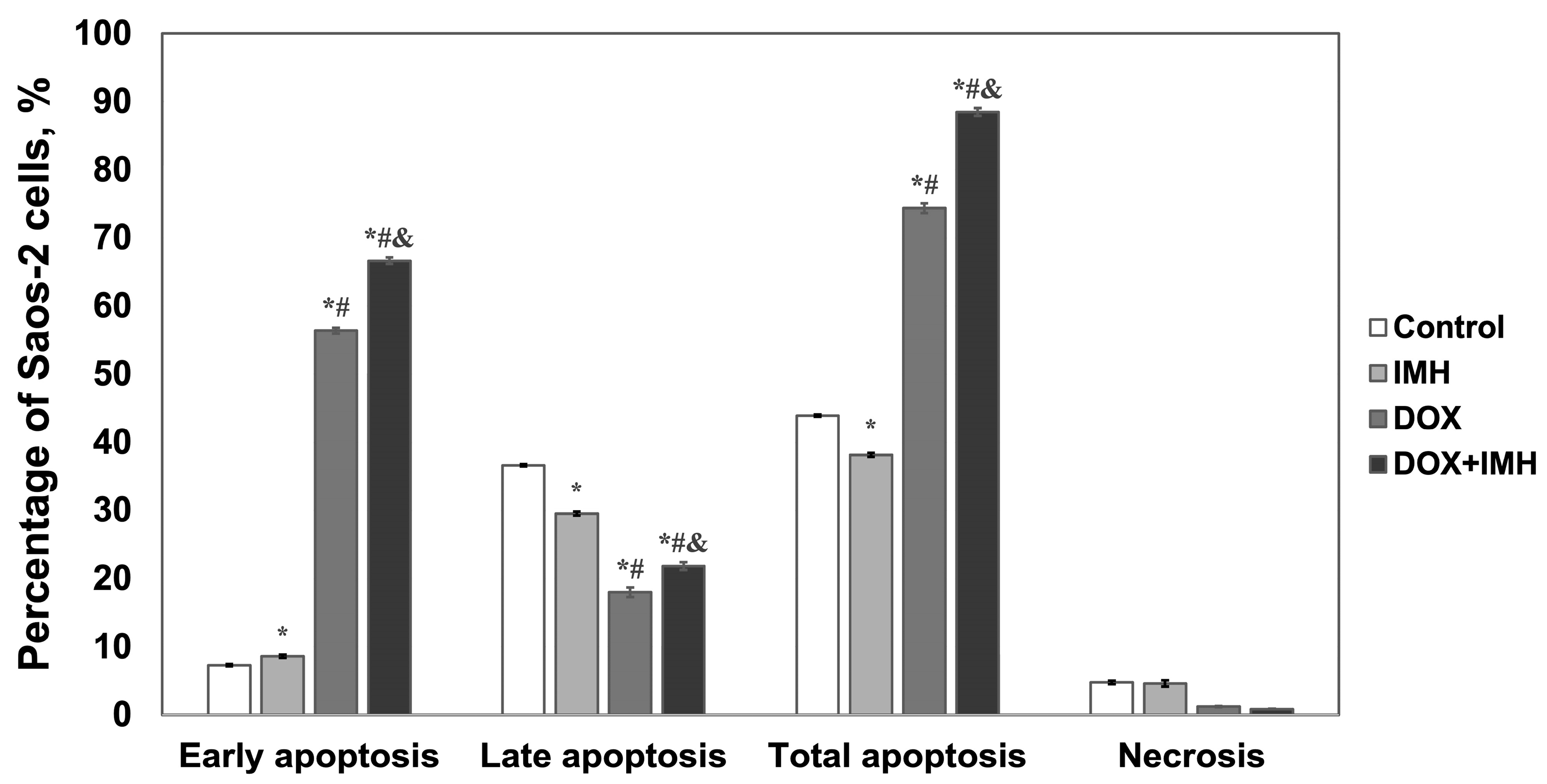
2.2. Apoptosis and Necrosis Detection
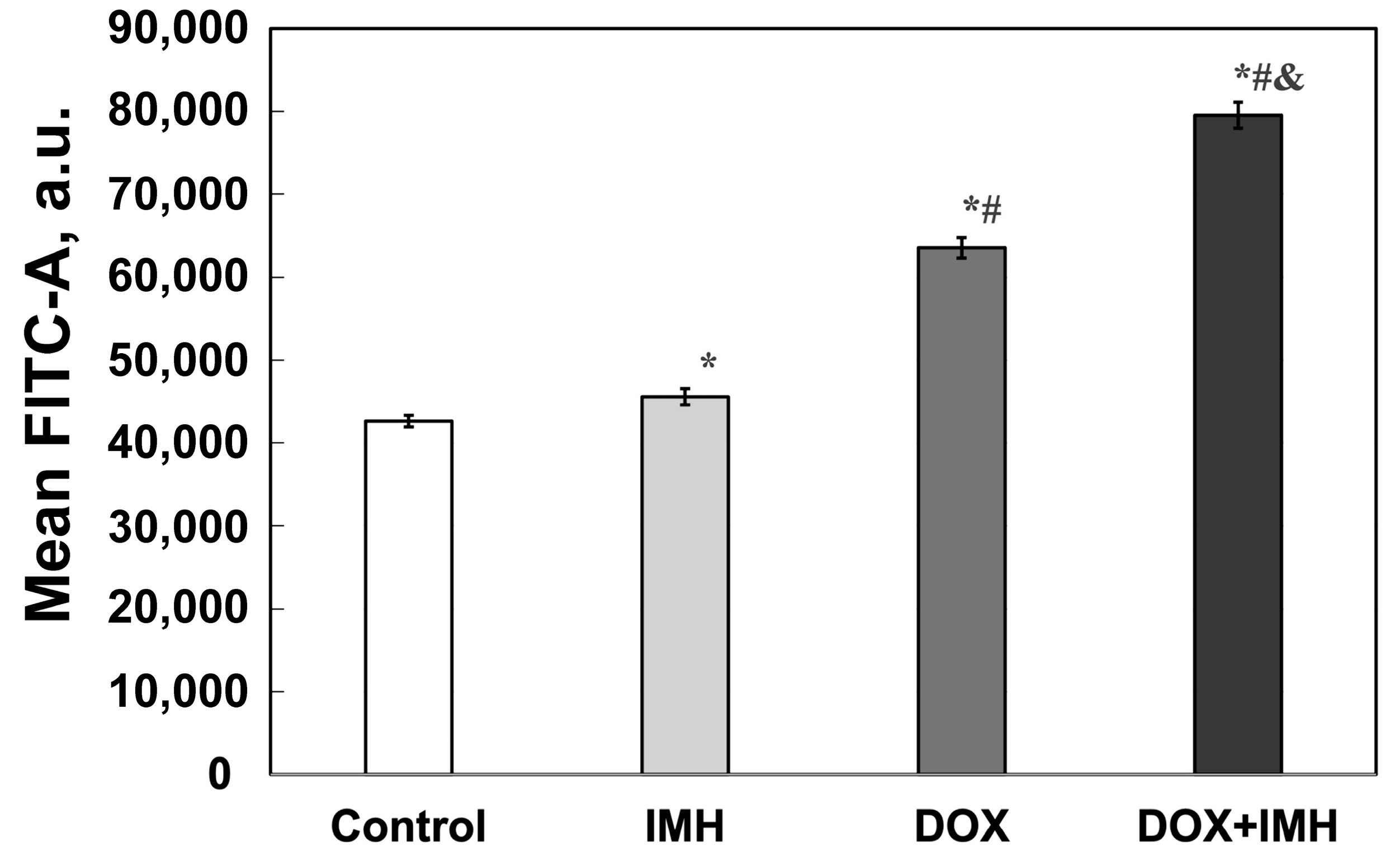
2.3. Reactive Oxygen Species Measurements
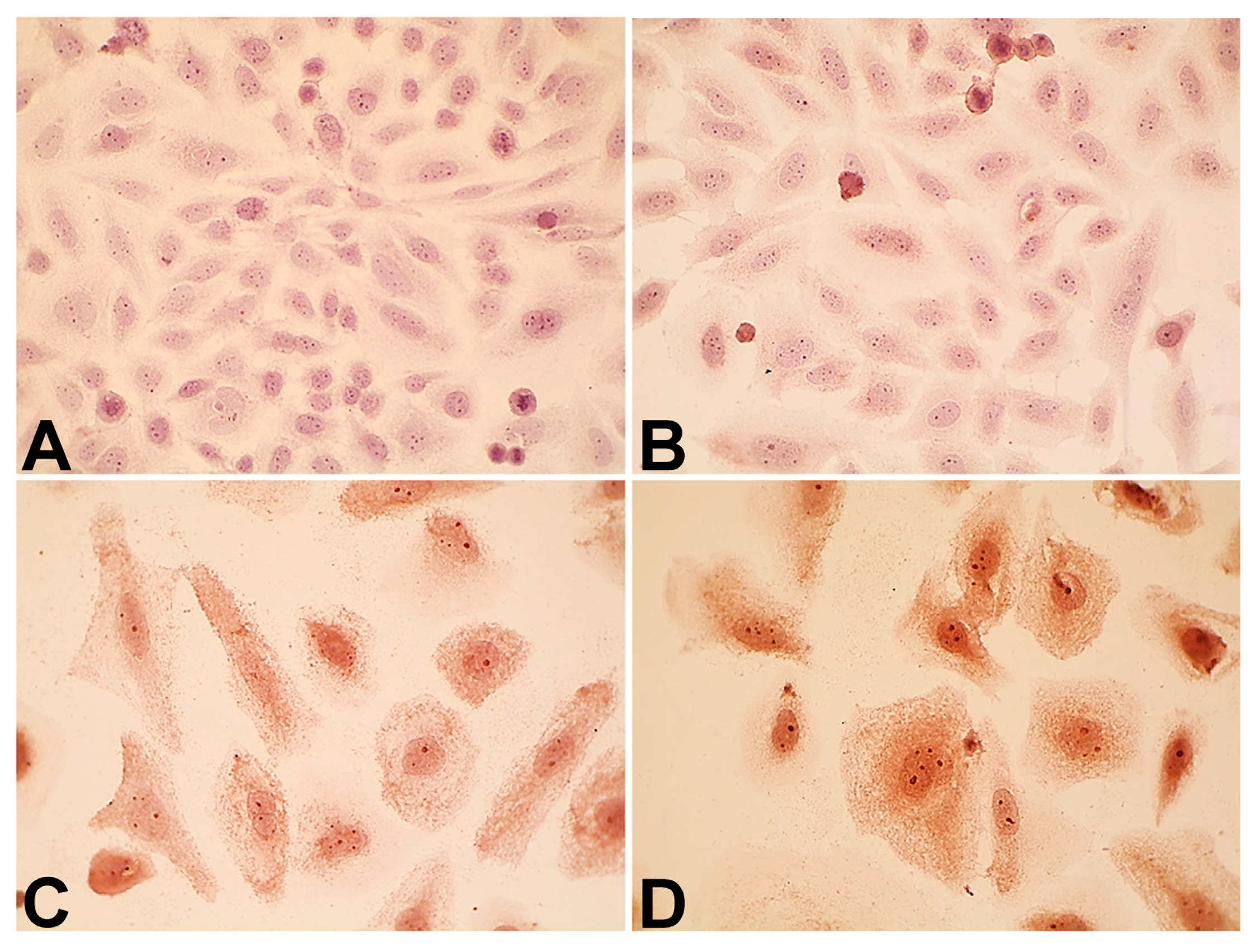
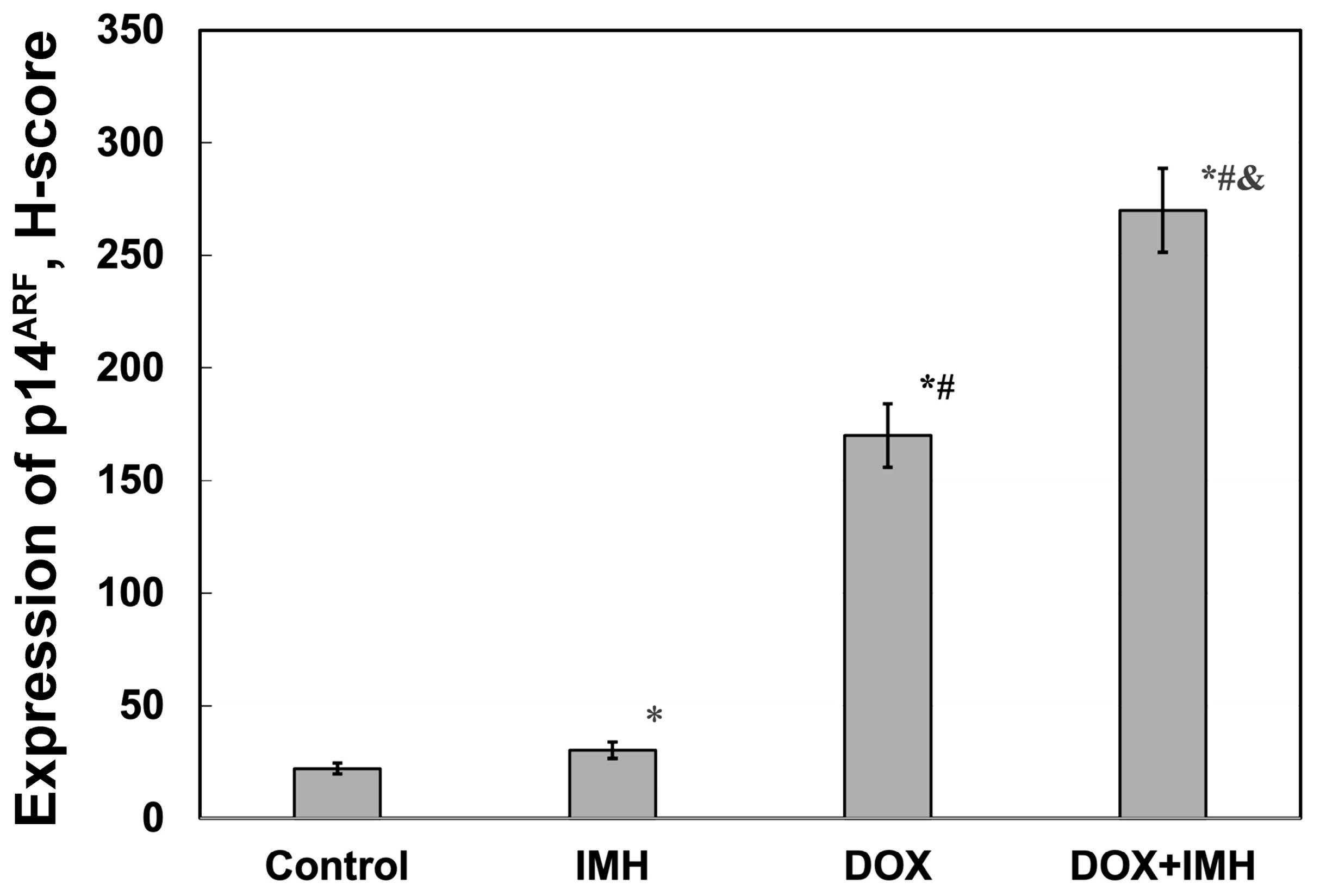
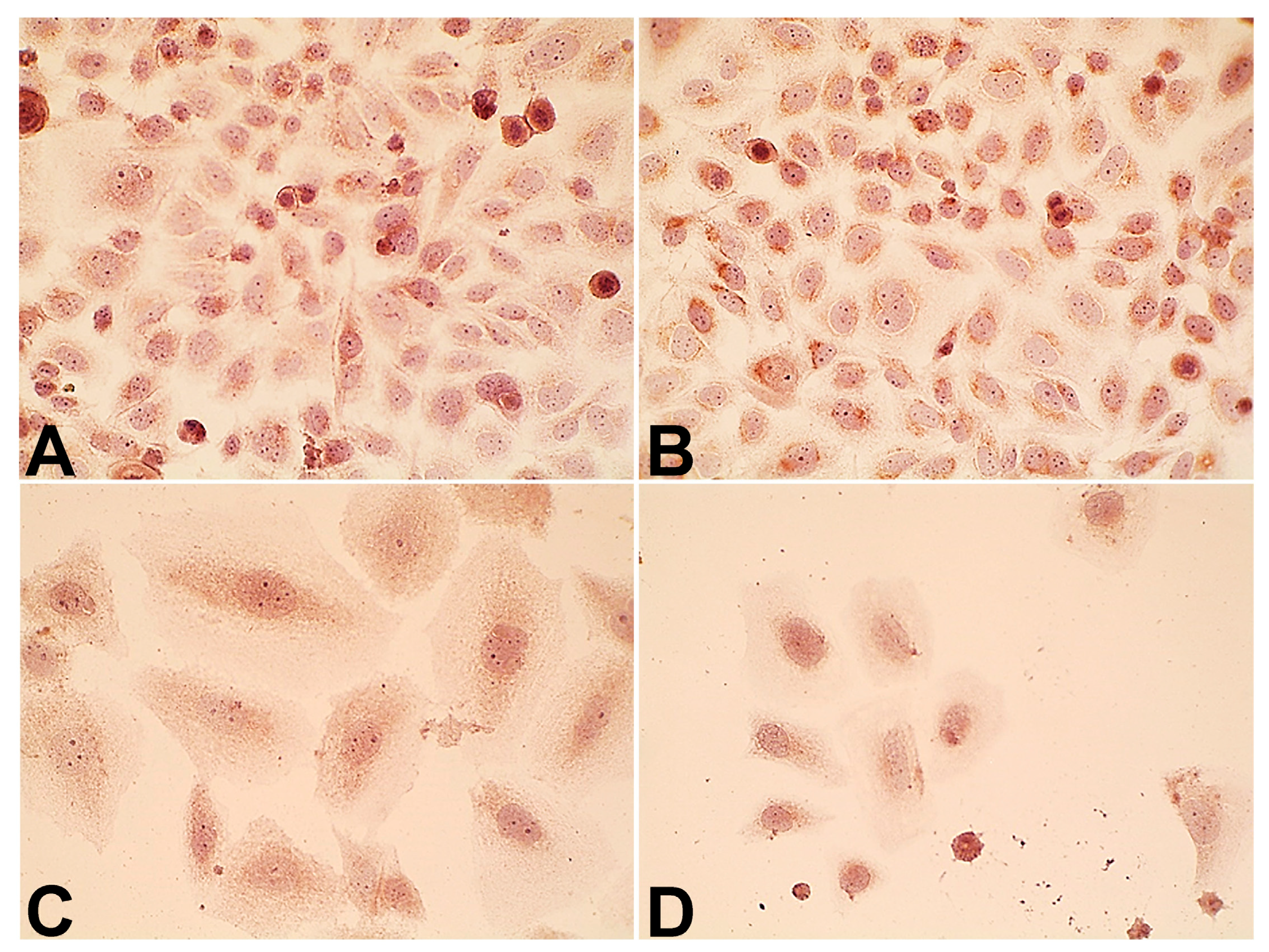
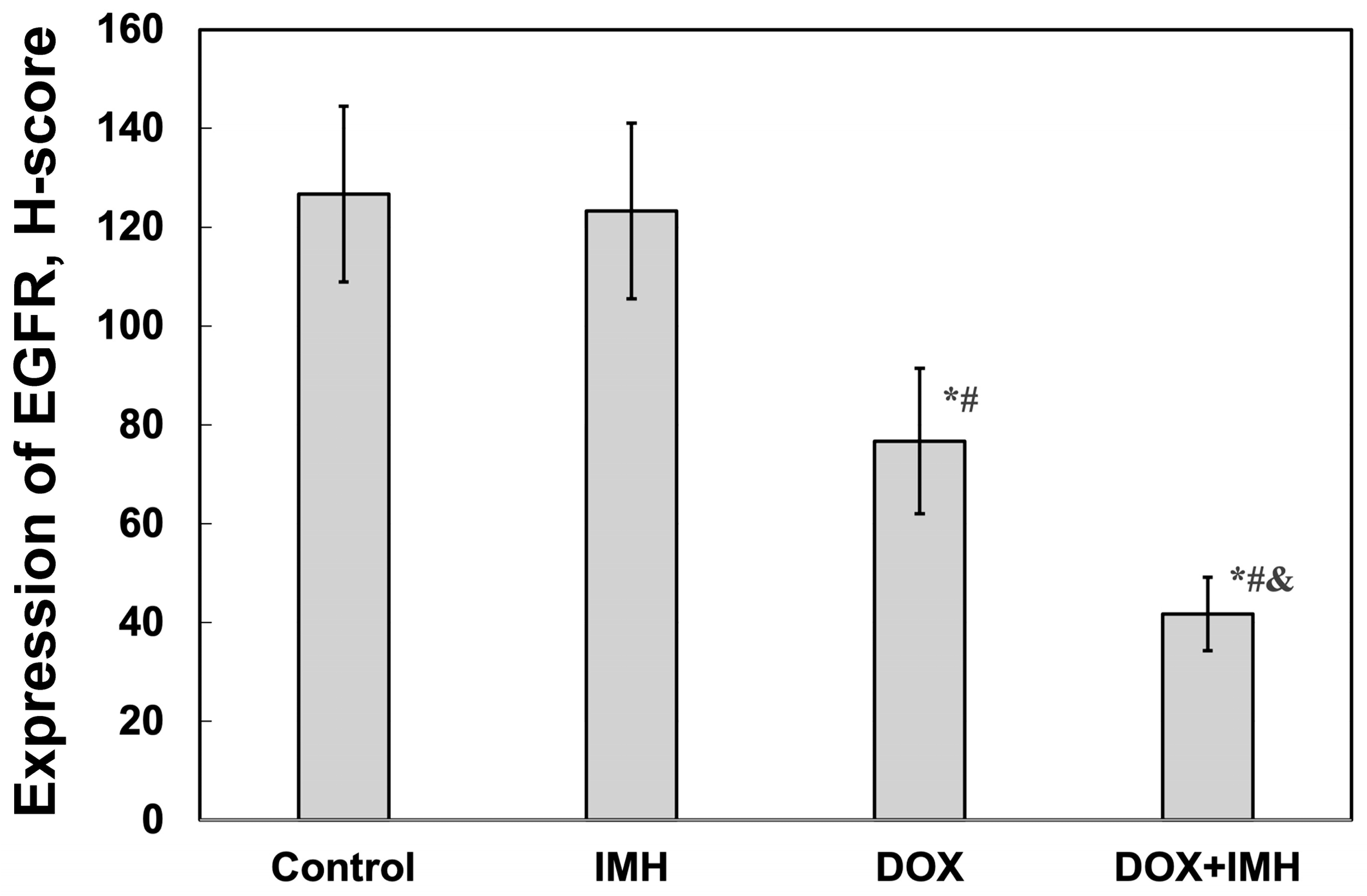
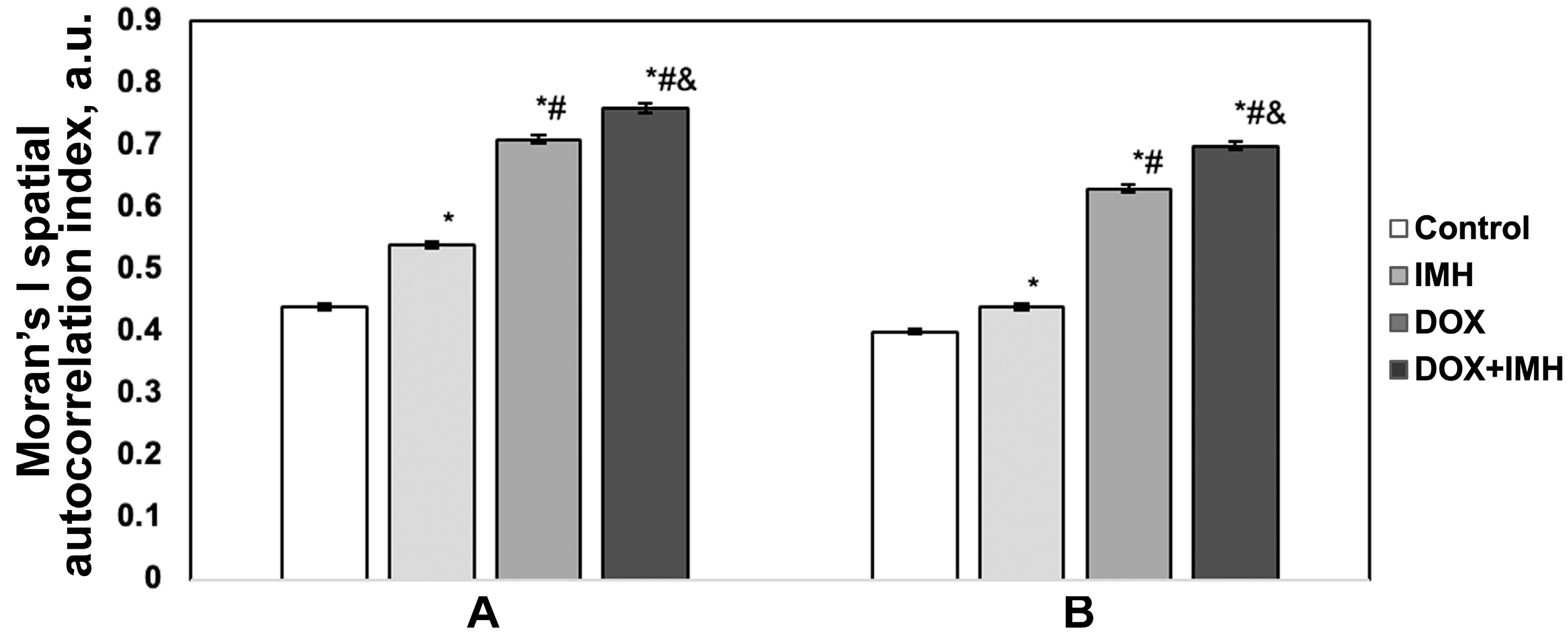
2.4. Expression of p14ARF and Epidermal Growth Factor Receptor
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Inductive Moderate Hyperthermia
4.3. Cell Viability
4.4. Flow Cytometry
4.5. Immunocytochemical Assay
4.6. Image Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Annexin V-FITC | Annexin V conjugated with fluorescein |
| DOX | Doxorubicin |
| EGFR | Epidermal growth factor receptor |
| FSC-A/SSC-A | Forward/side scatter area |
| IC50 | Half maximal inhibitory concentration |
| IMH | Inductive moderate hyperthermia |
| OS | Osteosarcoma |
| PI-ECD | Propidium iodide conjugated with energy-coupled dye |
| ROS | Reactive oxygen species |
References
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A Comprehensive Review. SICOT J. 2018, 4, 12. [Google Scholar] [CrossRef]
- Picci, P. Osteosarcoma (Osteogenic Sarcoma). Orphanet J. Rare Dis. 2007, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin Pathways: Pharmacodynamics and Adverse Effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kwok, J.C.; Richardson, D.R. Unexpected Anthracycline-Mediated Alterations in Iron-Regulatory Protein-RNA-Binding Activity: The Iron and Copper Complexes of Anthracyclines Decrease RNA-Binding Activity. Mol. Pharmacol. 2002, 62, 888–900. [Google Scholar] [CrossRef]
- Minotti, G.; Ronchi, R.; Salvatorelli, E.; Menna, P.; Cairo, G. Doxorubicin Irreversibly Inactivates Iron Regulatory Proteins 1 and 2 in Cardiomyocytes: Evidence for Distinct Metabolic Pathways and Implications for Iron-Mediated Cardiotoxicity of Antitumor Therapy. Cancer Res. 2001, 61, 8422–8428. [Google Scholar]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and Its Implications in Aging Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Wallrabenstein, T.; Daetwyler, E.; Oseledchyk, A.; Rochlitz, C.; Vetter, M. Pegylated Liposomal Doxorubicin (PLD) in Daily Practice-A Single Center Experience of Treatment with PLD in Patients with Comorbidities and Older Patients with Metastatic Breast Cancer. Cancer Med. 2023, 12, 13388–13396. [Google Scholar] [CrossRef]
- Waterhouse, D.N.; Tardi, P.G.; Mayer, L.D.; Bally, M.B. A Comparison of Liposomal Formulations of Doxorubicin with Drug Administered in Free Form: Changing Toxicity Profiles. Drug Saf. 2001, 24, 903–920. [Google Scholar] [CrossRef]
- Barnes, F.; Greenebaum, B. Role of Radical Pairs and Feedback in Weak Radio Frequency Field Effects on Biological Systems. Environ. Res. 2018, 163, 165–170. [Google Scholar] [CrossRef]
- Loboda, A.; Smolanka Sr, I.; Orel, V.E.; Syvak, L.; Golovko, T.; Dosenko, I.; Lyashenko, A.; Smolanka, I.; Dasyukevich, O.; Tarasenko, T.; et al. Efficacy of Combination Neoadjuvant Chemotherapy and Regional Inductive Moderate Hyperthermia in the Treatment of Patients With Locally Advanced Breast Cancer. Technol. Cancer Res. Treat. 2020, 19, 1533033820963599. [Google Scholar] [CrossRef] [PubMed]
- Consales, C.; Merla, C.; Marino, C.; Benassi, B. Electromagnetic Fields, Oxidative Stress, and Neurodegeneration. Int. J. Cell Biol. 2012, 2012, 683897. [Google Scholar] [CrossRef] [PubMed]
- Orel, V.; Shevchenko, A.; Romanov, A.; Tselepi, M.; Mitrelias, T.; Barnes, C.H.W.; Burlaka, A.; Lukin, S.; Shchepotin, I. Magnetic Properties and Antitumor Effect of Nanocomplexes of Iron Oxide and Doxorubicin. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 47–55. [Google Scholar] [CrossRef]
- Wong, S.Y.; Benjamin, P.; Hore, P.J. Magnetic Field Effects on Radical Pair Reactions: Estimation of B1/2 for Flavin-Tryptophan Radical Pairs in Cryptochromes. Phys. Chem. Chem. Phys. 2023, 25, 975–982. [Google Scholar] [CrossRef]
- Zadeh-Haghighi, H.; Simon, C. Magnetic Field Effects in Biology from the Perspective of the Radical Pair Mechanism. J. R. Soc. Interface 2022, 19, 20220325. [Google Scholar] [CrossRef]
- Buchachenko, A.L.; Kuznetsov, D.A. Magnetic Field Affects Enzymatic ATP Synthesis. J. Am. Chem. Soc. 2008, 130, 12868–12869. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Wójcik-Piotrowicz, K.; Guzdek, P.; Gil, K.; Kaszuba-Zwoińska, J. Protein Expression Changes during Phagocytosis Influenced by Low-Frequency Electromagnetic Field Exposure. Int. J. Biol. Macromol. 2022, 217, 481–491. [Google Scholar] [CrossRef]
- Ferdous, M.S.; Koupaie, E.H.; Eskicioglu, C.; Johnson, T. An Experimental 13.56 MHz Radio Frequency Heating System for Efficient Thermal Pretreatment of Wastewater Sludge. Prog. Electromagn. Res. B 2017, 79, 83–101. [Google Scholar] [CrossRef]
- Li, Z.; Deng, J.; Sun, J.; Ma, Y. Hyperthermia Targeting the Tumor Microenvironment Facilitates Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 595207. [Google Scholar] [CrossRef]
- Brummelhuis, I.S.G.; Crezee, J.; Witjes, J.A. Evaluation of Thermal Dose Effect in Radiofrequency-Induced Hyperthermia with Intravesical Chemotherapy for Nonmuscle Invasive Bladder Cancer. Int. J. Hyperth. 2023, 40, 2157498. [Google Scholar] [CrossRef]
- Wust, P.; Veltsista, P.D.; Oberacker, E.; Yavvari, P.; Walther, W.; Bengtsson, O.; Sterner-Kock, A.; Weinhart, M.; Heyd, F.; Grabowski, P.; et al. Radiofrequency Electromagnetic Fields Cause Non-Temperature-Induced Physical and Biological Effects in Cancer Cells. Cancers 2022, 14, 5349. [Google Scholar] [CrossRef] [PubMed]
- Orel, V.B.; Papazoglou, A.S.; Tsagkaris, C.; Moysidis, D.V.; Papadakos, S.; Galkin, O.Y.; Orel, V.E.; Syvak, L.A. Nanotherapy Based on Magneto-Mechanochemical Modulation of Tumor Redox State. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1868. [Google Scholar] [CrossRef]
- Nizhelska, O.I.; Marynchenko, L.V.; Piasetskyi, V.I. Biological risks of using non-thermal non-ionizing electromagnetic fields. Innov. Biosyst. Bioeng. 2020, 4, 95–109. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-Term Outcomes Among Patients With Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Roussakow, S. Neo-Adjuvant Chemotherapy Alone or with Regional Hyperthermia for Soft-Tissue Sarcoma. Lancet Oncol. 2017, 18, e629. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, J.; Jin, Y.; Zhang, F.; Yang, X. Intratumoral Radiofrequency Hyperthermia-Enhanced Chemotherapy of Liposomal Doxorubicin on Hepatocellular Carcinoma. Am. J. Transl. Res. 2018, 10, 3619–3627. [Google Scholar]
- Salvador, D.; Bastos, V.; Oliveira, H. Hyperthermia Enhances Doxorubicin Therapeutic Efficacy against A375 and MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 35. [Google Scholar] [CrossRef]
- Orel, V.E.; Diedkov, A.G.; Ostafiichuk, V.V.; Lykhova, O.O.; Kolesnyk, D.L.; Orel, V.B.; Dasyukevich, O.Y.; Rykhalskyi, O.Y.; Diedkov, S.A.; Prosvietova, A.B. Combination Treatment with Liposomal Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells. Pharmaceuticals 2024, 17, 133. [Google Scholar] [CrossRef]
- Kullenberg, F.; Degerstedt, O.; Calitz, C.; Pavlović, N.; Balgoma, D.; Gråsjö, J.; Sjögren, E.; Hedeland, M.; Heindryckx, F.; Lennernäs, H. In Vitro Cell Toxicity and Intracellular Uptake of Doxorubicin Exposed as a Solution or Liposomes: Implications for Treatment of Hepatocellular Carcinoma. Cells 2021, 10, 1717. [Google Scholar] [CrossRef]
- Wang, S.; Konorev, E.A.; Kotamraju, S.; Joseph, J.; Kalivendi, S.; Kalyanaraman, B. Doxorubicin Induces Apoptosis in Normal and Tumor Cells via Distinctly Different Mechanisms. Intermediacy of H(2)O(2)- and P53-Dependent Pathways. J. Biol. Chem. 2004, 279, 25535–25543. [Google Scholar] [CrossRef]
- Saito, K.; Iioka, H.; Kojima, C.; Ogawa, M.; Kondo, E. Peptide-based Tumor Inhibitor Encoding Mitochondrial P14ARF Is Highly Efficacious to Diverse Tumors. Cancer Sci. 2016, 107, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.-S.; Chang, J.-H.; Hung, W.-Y.; Yang, Y.-C.; Chien, M.-H. The Interplay of Reactive Oxygen Species and the Epidermal Growth Factor Receptor in Tumor Progression and Drug Resistance. J. Exp. Clin. Cancer Res. 2018, 37, 61. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.L.; Loughran, Ö.; La Thangue, N.B. p14ARF Regulates E2F Activity. Oncogene 2002, 21, 4220–4230. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.P.M.; Thomas, D.G.; Giordano, T.J.; Baker, L.H.; McDonagh, K.T. Cell Surface Expression of Epidermal Growth Factor Receptor and Her-2 with Nuclear Expression of Her-4 in Primary Osteosarcoma. Cancer Res. 2004, 64, 2047–2053. [Google Scholar] [CrossRef]
- Sevelda, F.; Mayr, L.; Kubista, B.; Lötsch, D.; van Schoonhoven, S.; Windhager, R.; Pirker, C.; Micksche, M.; Berger, W. EGFR Is Not a Major Driver for Osteosarcoma Cell Growth in Vitro but Contributes to Starvation and Chemotherapy Resistance. J. Exp. Clin. Cancer Res. 2015, 34, 134. [Google Scholar] [CrossRef] [PubMed]
- Damerell, V.; Pepper, M.S.; Prince, S. Molecular Mechanisms Underpinning Sarcomas and Implications for Current and Future Therapy. Sig. Transduct. Target. Ther. 2021, 6, 246. [Google Scholar] [CrossRef]
- Lafage-Proust, M.-H.; Roche, B.; Langer, M.; Cleret, D.; Vanden Bossche, A.; Olivier, T.; Vico, L. Assessment of Bone Vascularization and Its Role in Bone Remodeling. Bonekey Rep. 2015, 4, 662. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Rankin, E.B.; Castellini, L.; Fernandez-Alcudia, J.; Lagory, E.L.; Andersen, R.; Rhodes, S.D.; Wilson, T.L.S.; Mohammad, K.S.; Castillo, A.B.; et al. Oxygen-Sensing PHDs Regulate Bone Homeostasis through the Modulation of Osteoprotegerin. Genes. Dev. 2015, 29, 817–831. [Google Scholar] [CrossRef]
- Zahm, A.M.; Bucaro, M.A.; Ayyaswamy, P.S.; Srinivas, V.; Shapiro, I.M.; Adams, C.S.; Mukundakrishnan, K. Numerical Modeling of Oxygen Distributions in Cortical and Cancellous Bone: Oxygen Availability Governs Osteonal and Trabecular Dimensions. Am. J. Physiol.-Cell Physiol. 2010, 299, C922–C929. [Google Scholar] [CrossRef]
- Hannah, S.S.; McFadden, S.; McNeilly, A.; McClean, C. “Take My Bone Away?” Hypoxia and Bone: A Narrative Review. J. Cell. Physiol. 2021, 236, 721–740. [Google Scholar] [CrossRef]
- Zelmer, A.R.; Starczak, Y.; Solomon, L.B.; Richter, K.; Yang, D.; Atkins, G.J. Saos-2 Cells Cultured under Hypoxia Rapidly Differentiate to an Osteocyte-like Stage and Support Intracellular Infection by Staphylococcus Aureus. Physiol. Rep. 2023, 11, e15851. [Google Scholar] [CrossRef] [PubMed]
- Ramazi, S.; Salimian, M.; Allahverdi, A.; Kianamiri, S.; Abdolmaleki, P. Synergistic Cytotoxic Effects of an Extremely Low-Frequency Electromagnetic Field with Doxorubicin on MCF-7 Cell Line. Sci. Rep. 2023, 13, 8844. [Google Scholar] [CrossRef]
- Hou, C.-H.; Lin, F.-L.; Hou, S.-M.; Liu, J.-F. Hyperthermia Induces Apoptosis through Endoplasmic Reticulum and Reactive Oxygen Species in Human Osteosarcoma Cells. Int. J. Mol. Sci. 2014, 15, 17380–17395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Ren, Q.Q.; Ye, T.H.; Liu, Y.M.; Zheng, C.S.; Zhou, G.F.; Xia, X.W. Sublethal hyperthermia enhances anticancer activity of doxorubicin in chronically hypoxic HepG2 cells through ROS-dependent mechanism. Biosci. Rep. 2021, 41, BSR20210442. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia Treatment as a Promising Anti-Cancer Strategy: Therapeutic Targets, Perspective Mechanisms and Synergistic Combinations in Experimental Approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef]
- Santini, S.J.; Cordone, V.; Falone, S.; Mijit, M.; Tatone, C.; Amicarelli, F.; Di Emidio, G. Role of Mitochondria in the Oxidative Stress Induced by Electromagnetic Fields: Focus on Reproductive Systems. Oxidative Med. Cell. Longev. 2018, 2018, 5076271. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of Doxorubicin Is Mediated through Mitochondrial Iron Accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef]
- Stanley, S.A.; Friedman, J.M. Electromagnetic Regulation of Cell Activity. Cold Spring Harb. Perspect. Med. 2019, 9, a034322. [Google Scholar] [CrossRef]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-Induced Oxidative Stress in Cancer Treatments: Angel or Devil? Redox Biol. 2023, 63, 102754. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and Polymersomes: A Comparative Review towards Cell Mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Zhen, C.; Zhang, G.; Wang, S.; Wang, J.; Fang, Y.; Shang, P. Electromagnetic Fields Regulate Iron Metabolism in Living Organisms: A Review of Effects and Mechanism. Progress. Biophys. Mol. Biol. 2024, 188, 43–54. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Martinovich, G.G.; Martinovich, I.V.; Voinarouski, V.V.; Grigorieva, D.V.; Gorudko, I.V.; Panasenko, O.M. Free Radicals and Signal Transduction in Cells. Biophysics 2023, 68, 537–551. [Google Scholar] [CrossRef]
- Wydra, R.J.; Oliver, C.E.; Anderson, K.W.; Dziubla, T.D.; Hilt, J.Z. Accelerated Generation of Free Radicals by Iron Oxide Nanoparticles in the Presence of an Alternating Magnetic Field. RSC Adv. 2015, 5, 18888–18893. [Google Scholar] [CrossRef]
- Gallagher, S.; Kefford, R.F.; Rizos, H. Enforced Expression of p14ARF Induces P53-Dependent Cell Cycle Arrest but Not Apoptosis. Cell Cycle 2005, 4, 465–472. [Google Scholar] [CrossRef]
- Damalas, A.; Velimezi, G.; Kalaitzakis, A.; Liontos, M.; Papavassiliou, A.G.; Gorgoulis, V.; Angelidis, C. Loss of p14ARF Confers Resistance to Heat Shock- and Oxidative Stress-Mediated Cell Death by Upregulating β-Catenin. Int. J. Cancer 2011, 128, 1989–1995. [Google Scholar] [CrossRef]
- Lee, J.A.; Ko, Y.; Kim, D.H.; Lim, J.S.; Kong, C.-B.; Cho, W.H.; Jeon, D.-G.; Lee, S.-Y.; Koh, J.-S. Epidermal Growth Factor Receptor: Is It a Feasible Target for the Treatment of Osteosarcoma? Cancer Res. Treat. 2012, 44, 202–209. [Google Scholar] [CrossRef]
- Ulasov, I.V.; Foster, H.; Butters, M.; Yoon, J.-G.; Ozawa, T.; Nicolaides, T.; Figueroa, X.; Hothi, P.; Prados, M.; Butters, J.; et al. Precision Knockdown of EGFR Gene Expression Using Radio Frequency Electromagnetic Energy. J. Neurooncol. 2017, 133, 257–264. [Google Scholar] [CrossRef]
- Lindner, A.U.; Salvucci, M.; McDonough, E.; Cho, S.; Stachtea, X.; O’Connell, E.P.; Corwin, A.D.; Santamaria-Pang, A.; Carberry, S.; Fichtner, M.; et al. An Atlas of Inter- and Intra-Tumor Heterogeneity of Apoptosis Competency in Colorectal Cancer Tissue at Single-Cell Resolution. Cell Death Differ. 2022, 29, 806–817. [Google Scholar] [CrossRef]
- Xu, M.; Kumar, A.; LeBeau, J.M. Correlating Local Chemical and Structural Order Using Geographic Information Systems-Based Spatial Statistics. Ultramicroscopy 2023, 243, 113642. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gorman, B.P.; Hering, A.S. Objective Identification of Local Spatial Structure for Material Characterization. Stat. Anal. Data Min. ASA Data Sci. J. 2020, 13, 377–393. [Google Scholar] [CrossRef]
- Fugit, K.D.; Xiang, T.X.; Choi du, H.; Kangarlou, S.; Csuhai, E.; Bummer, P.M.; Anderson, B.D. Mechanistic model and analysis of doxorubicin release from liposomal formulations. J. Control. Release 2015, 217, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Macleod, K.; Abra, R.M.; Huang, A.H.; Hahn, G.M. Hyperthermia induces doxorubicin release from long-circulating liposomes and enhances their anti-tumor efficacy. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; More, N.; Schein, P.S. Doxorubicin-induced chronic cardiotoxicity and its protection by liposomal administration. Cancer Res. 1982, 42, 1817–1825. [Google Scholar]
- Gallego, B.; Murillo, D.; Rey, V.; Huergo, C.; Estupiñán, Ó.; Rodríguez, A.; Tornín, J.; Rodríguez, R. Addressing Doxorubicin Resistance in Bone Sarcomas Using Novel Drug-Resistant Models. Int. J. Mol. Sci. 2022, 23, 6425. [Google Scholar] [CrossRef]
- Post, S.M. Mouse Models of Sarcomas: Critical Tools in Our Understanding of the Pathobiology. Clin. Sarcoma Res. 2012, 2, 20. [Google Scholar] [CrossRef]
- Fogh, J.; Trempe, G. New Human Tumor Cell Lines. In Human Tumor Cells In Vitro; Fogh, J., Ed.; Springer: Boston, MA, USA, 1975; ISBN 978-1-4757-1649-8. [Google Scholar]
- Capes-Davis, A.; Freshney, R.I. Freshney’s Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 1–832. [Google Scholar]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Logue, S.E.; Elgendy, M.; Martin, S.J. Expression, Purification and Use of Recombinant Annexin V for the Detection of Apoptotic Cells. Nat. Protoc. 2009, 4, 1383–1395. [Google Scholar] [CrossRef]
- Vincenzi, F.; Rotondo, J.C.; Pasquini, S.; Di Virgilio, F.; Varani, K.; Tognon, M. A3 Adenosine and P2X7 Purinergic Receptors as New Targets for an Innovative Pharmacological Therapy of Malignant Pleural Mesothelioma. Front. Oncol. 2021, 11, 679285. [Google Scholar] [CrossRef] [PubMed]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS Using Oxidized DCFDA and Flow-Cytometry. In Advanced Protocols in Oxidative Stress II; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 57–72. ISBN 978-1-60761-411-1. [Google Scholar]
- Freeman, S.S.; Allen, S.W.; Ganti, R.; Wu, J.; Ma, J.; Su, X.; Neale, G.; Dome, J.S.; Daw, N.C.; Khoury, J.D. Copy Number Gains in EGFR and Copy Number Losses in PTEN Are Common Events in Osteosarcoma Tumors. Cancer 2008, 113, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Müer, A.; Overkamp, T.; Gillissen, B.; Richter, A.; Pretzsch, T.; Milojkovic, A.; Dörken, B.; Daniel, P.T.; Hemmati, P. p14ARF-Induced Apoptosis in P53 Protein-Deficient Cells Is Mediated by BH3-Only Protein-Independent Derepression of Bak Protein through Down-Regulation of Mcl-1 and Bcl-xL Proteins. J. Biol. Chem. 2012, 287, 17343–17352. [Google Scholar] [CrossRef] [PubMed]
- Detre, S.; Jotti, G.S.; Dowsett, M. A “Quickscore” Method for Immunohistochemical Semiquantitation: Validation for Oestrogen Receptor in Breast Carcinomas. J. Clin. Pathol. 1995, 48, 876–878. [Google Scholar] [CrossRef]
- González-García, I.; Solé, R.V.; Costa, J. Metapopulation Dynamics and Spatial Heterogeneity in Cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 13085–13089. [Google Scholar] [CrossRef]
- Orel, V.E.; Ashykhmin, A.; Golovko, T.; Rykhalskyi, O.; Orel, V.B. Texture Analysis of Tumor and Peritumoral Tissues Based on 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Hybrid Imaging in Patients with Rectal Cancer. J. Comput. Assist. Tomogr. 2021, 45, 820–828. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orel, V.E.; Diedkov, A.G.; Ostafiichuk, V.V.; Lyalkin, S.A.; Tkachenko, I.O.; Kolesnyk, D.L.; Orel, V.B.; Dasyukevich, O.Y.; Rykhalskyi, O.Y.; Movchan, O.V.; et al. Combination Treatment with Free Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells. Pharmaceuticals 2025, 18, 852. https://doi.org/10.3390/ph18060852
Orel VE, Diedkov AG, Ostafiichuk VV, Lyalkin SA, Tkachenko IO, Kolesnyk DL, Orel VB, Dasyukevich OY, Rykhalskyi OY, Movchan OV, et al. Combination Treatment with Free Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells. Pharmaceuticals. 2025; 18(6):852. https://doi.org/10.3390/ph18060852
Chicago/Turabian StyleOrel, Valerii E., Anatolii G. Diedkov, Vasyl V. Ostafiichuk, Sergii A. Lyalkin, Igor O. Tkachenko, Denys L. Kolesnyk, Valerii B. Orel, Olga Yo. Dasyukevich, Oleksandr Yu. Rykhalskyi, Oleksii V. Movchan, and et al. 2025. "Combination Treatment with Free Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells" Pharmaceuticals 18, no. 6: 852. https://doi.org/10.3390/ph18060852
APA StyleOrel, V. E., Diedkov, A. G., Ostafiichuk, V. V., Lyalkin, S. A., Tkachenko, I. O., Kolesnyk, D. L., Orel, V. B., Dasyukevich, O. Y., Rykhalskyi, O. Y., Movchan, O. V., Galkin, A. Y., & Prosvietova, A. B. (2025). Combination Treatment with Free Doxorubicin and Inductive Moderate Hyperthermia for Sarcoma Saos-2 Cells. Pharmaceuticals, 18(6), 852. https://doi.org/10.3390/ph18060852






