Synthesis and Combination Studies of Novel Dipeptide Nitriles with Curcumin for a Potent Synergistic Action Against Rhodesain, Cysteine Protease of Trypanosoma brucei rhodesiense
Abstract
1. Introduction
2. Results and Discussion
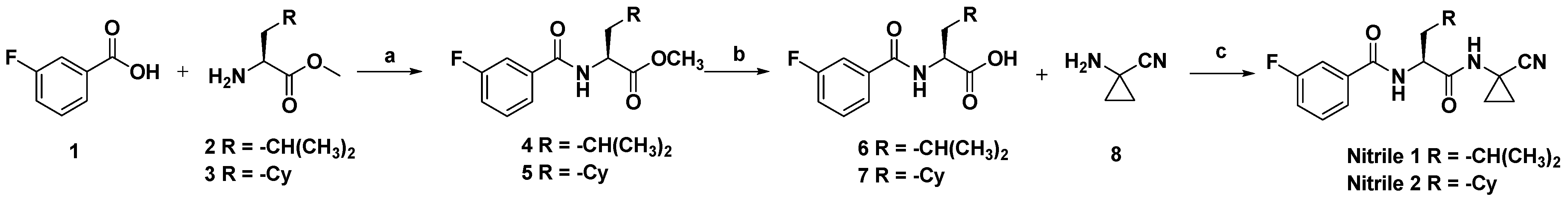
2.1. Chemistry
2.2. Biological Evaluation
3. Materials and Methods
3.1. Rhodesain Inhibition Assays
3.2. Calculation of the Combination Index
3.3. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NTDs | Neglected tropical diseases |
| WHO | World Health Organization |
| HAT | Human African trypanosomiasis |
| BBB | Blood–brain barrier |
| NECT | Nifurtimox-eflornithine combination |
| EMA | European Medicines Agency |
| Cbz | Benzyloxycarbonyl |
| AMC | Aminomethylcumarin |
References
- Neglected Tropical Diseases. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 4 April 2025).
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African Trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- MacLean, L.; Reiber, H.; Kennedy, P.G.; Sternberg, J.M. Stage Progression and Neurological Symptoms in Trypanosoma Brucei Rhodesiense Sleeping Sickness: Role of the CNS Inflammatory Response. PLoS Neglect. Trop. Dis. 2012, 6, e1857. [Google Scholar] [CrossRef]
- Kennedy, P.G.E.; Rodgers, J. Clinical and neuropathogenetic aspects of human african trypanosomiasis. Front. Immunol. 2019, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Papagni, R.; Novara, R.; Minardi, M.L.; Frallonardo, L.; Panico, G.G.; Pallara, E.; Cotugno, S.; Ascoli Bartoli, T.; Guido, G.; De Vita, E. Human african trypanosomiasis (sleeping sickness): Current knowledge and future challenges. Front. Trop. Dis. 2023, 4, 1087003. [Google Scholar] [CrossRef]
- Barrett, M.P. Transforming the chemotherapy of human African trypanosomiasis. Clin. Microbiol. Rev. 2025, 38, e0015323. [Google Scholar] [CrossRef]
- Lindner, A.K.; Lejon, V.; Barrett, M.P.; Blumberg, L.; Bukachi, S.A.; Chancey, R.J.; Edielu, A.; Matemba, L.; Mesha, T.; Mwanakasale, V.; et al. New WHO guidelines for treating rhodesiense human African trypanosomiasis: Expanded indications for fexinidazole and pentamidine. Lancet Infect. Dis. 2025, 25, e77–e85. [Google Scholar] [CrossRef] [PubMed]
- Kumeso, V.K.B.; Perdrieu, C.; Menétrey, C.; Kyhi, M.I.W.; Tete, D.N.; Camara, M.; Tampwo, J.; Kavunga, P.; Camara, M.L.; Kourouma, A.; et al. Effectiveness and safety of fexinidazole for gambiense human African trypanosomiasis and exploration of adherence in outpatients: A phase 3b, prospective, open-label, non-randomised, cohort study. Lancet Glob. Health 2025, 13, e900–e909. [Google Scholar] [CrossRef] [PubMed]
- Previti, S.; Di Chio, C.; Ettari, R.; Zappalà, M. Dual Inhibition of Parasitic Targets: A Valuable Strategy to Treat Malaria and Neglected Tropical Diseases. Curr. Med. Chem. 2022, 29, 2952–2978. [Google Scholar] [CrossRef]
- de Jesus Marinho, W.P.; de Oliveira Rios, É.; de Moura, R.O.; Nascimento, I.J.D.S. Target Selectivity of Cysteine Protease Inhibitors: A Strategy to Address Neglected Tropical Diseases. Curr. Med. Chem. 2025. [CrossRef]
- Sajid, M.; McKerrow, J.H. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 2002, 120, 1–21. [Google Scholar] [CrossRef]
- Nikolskaia, O.V.; de Lima, A.A.P.; Kim, Y.V.; Lonsdale-Eccles, J.D.; Fukuma, T.; Scharfstein, J.; Grab, D.J. Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease. J. Clin. Investig. 2006, 116, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.D.; McCulloch, R. Antigenic variation in trypanosomes: Enhanced phenotypic variation in a eukaryotic parasite. Adv. Parasitol. 2001, 49, 1–70. [Google Scholar]
- Lalmanach, G.; Boulange, A.; Serveau, C.; Lecaille, F.; Scharfstein, J.; Gauthier, F.; Authie, E. Congopain from Trypanosoma congolense: Drug target and vaccine candidate. Biol. Chem. 2002, 383, 739–749. [Google Scholar] [CrossRef]
- Caffrey, C.R.; Hansell, E.; Lucas, K.D.; Brinen, L.S.; Alvarez Hernandez, A.; Cheng, J.; Gwaltney, S.L., II; Roush, W.R.; Stierhof, Y.D.; Bogyo, M.; et al. Active site mapping, biochemical properties and subcellular localization of rhodesain, the major cysteine protease of Trypanosoma brucei rhodesiense. Mol. Biochem. Parasitol. 2001, 118, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Previti, S.; Ettari, R.; Di Chio, C.; Ravichandran, R.; Bogacz, M.; Hellmich, U.A.; Schirmeister, T.; Cosconati, S.; Zappalà, M. Development of Reduced Peptide Bond Pseudopeptide Michael Acceptors for the Treatment of Human African Trypanosomiasis. Molecules 2022, 27, 3765. [Google Scholar] [CrossRef]
- Di Chio, C.; Previti, S.; Amendola, G.; Ravichandran, R.; Wagner, A.; Cosconati, S.; Hellmich, U.A.; Schirmeister, T.; Zappalà, M.; Ettari, R. Development of novel dipeptide nitriles as inhibitors of rhodesain of Trypanosoma brucei rhodesiense. Eur. J. Med. Chem. 2022, 236, 114328. [Google Scholar] [CrossRef]
- Swenson, C.E.; Hunt, W.R.; Manfredi, C.; Beltran, D.J.; Hong, J.S.; Davis, B.R.; Suzuki, S.; Barillá, C.; Rab, A.; Chico, C.; et al. Evaluating elexacaftor/tezacaftor/ivacaftor (ETI Trikafta™) for treatment of patients with non-cystic fibrosis bronchiectasis (NCFBE): A clinical study protocol. PLoS ONE 2025, 20, e0316721. [Google Scholar] [CrossRef] [PubMed]
- Porcel-Pastrana, F.; Montero-Hidalgo, A.J.; G-García, M.E.; Gil-Duque, I.; Prats-Escribano, A.; Gahete, M.D.; Sarmento-Cabral, A.; Luque, R.M.; León-González, A.J. Cellular and Molecular Evidence of the Synergistic Antitumour Effects of Hydroxytyrosol and Metformin in Prostate Cancer. Int. J. Mol. Sci. 2025, 26, 1341. [Google Scholar] [CrossRef]
- Liu, R.; Li, L.; Zhang, P. Estimating the treatment effects of multiple drug combinations on multiple outcomes in hypertension. Cell Rep. Med. 2025, 6, 101947. [Google Scholar] [CrossRef]
- Veira, C.; Benítez, D.; Pérez-Díaz, L.; Álvarez, G.; Cerecetto, H.; Aguilera, E. Looking for ap-proved-medicines to be repositioned as anti-Trypanosoma cruzi agents. Identification of new chemo-types with good individual- or in combination-biological behaviours. Mem. Inst. Oswaldo Cruz. 2025, 120, e240183. [Google Scholar] [CrossRef]
- Trottier, B.; Yang, C.J.; Watanabe, D.; Marchetti, G.; Elbirt, D.; De Barra, E.; Gündüz, A.; Lee, S.H.; Vogelmann, R.; Robineau, O.; et al. Bictegravir/emtricitabine/tenofovir alafenamide in clinical practice for people with HIV: Final 24-month effectiveness and safety outcomes in key populations in the observational BICSTaR cohort. HIV Res. Clin. Pract. 2025, 26, 2456890. [Google Scholar] [CrossRef] [PubMed]
- Ettari, R.; Previti, S.; Di Chio, C.; Maiorana, S.; Allegra, A.; Schirmeister, T.; Zappalà, M. Drug synergism: Studies of combination of RK-52 and curcumin against rhodesain of Trypanosoma brucei rhodesiense. ACS Med. Chem. Lett. 2020, 11, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef]
- Yakubu, J.; Pandey, A.V. Innovative Delivery Systems for Curcumin: Exploring Nanosized and Conventional Formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef]
- Chou, T.C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Schirmeister, T.; Kesselring, J.; Jung, S.; Schneider, T.H.; Weickert, A.; Becker, J.; Lee, W.; Bamberger, D.; Wich, P.R.; Distler, U.; et al. Quantum chemical-based protocol for the rational design of covalent inhibitors. J. Am. Chem. Soc. 2016, 138, 8332–8335. [Google Scholar] [CrossRef]








| 1/64 × IC50 | 1/4 × IC50 | 1/2 × IC50 | IC50 | 2 × IC50 | 4 × IC50 | |
|---|---|---|---|---|---|---|
| Nitrile 1 | 0.004 μM | 0.066 μM | 0.13 μM | 0.26 μM | 0.53 μM | 1.06 μM |
| Curcumin | 0.26 μM | 4.09 μM | 8.18 μM | 16.36 μM | 32.71 μM | 65.42 μM |
| Nitrile 1 + curcumin | 0.004 + 0.26 μM | 0.066 + 4.09 μM | 0.13 + 8.18 μM | 0.26 +16.36 μM | 0.53 + 32.71 μM | 1.06 +65.42 μM |
| 1/64 × IC50 | 1/4 × IC50 | 1/2 × IC50 | IC50 | 2 × IC50 | 4 × IC50 | |
|---|---|---|---|---|---|---|
| Nitrile 2 | 0.0022 μM | 0.035 μM | 0.07 μM | 0.14 μM | 0.28 μM | 0.56 μM |
| Curcumin | 0.26 μM | 4.09 μM | 8.18 μM | 16.36 μM | 32.71 μM | 65.42 μM |
| Nitrile 2 + curcumin | 0.0022+ 0.26 μM | 0.035+ 4.09 μM | 0.07+ 8.18 μM | 0.14+ 16.36 μM | 0.28+ 32.71 μM | 0.56+ 5.42 μM |
| Affected Fraction (fa) | % Rhodesain Inhibition | CI | Diagnosis CI |
|---|---|---|---|
| 0.5 | 50% | 0.9744 | Slight synergism |
| 0.6 | 60% | 0.8723 | Slight synergism |
| 0.7 | 70% | 0.7927 | Moderate synergism |
| 0.8 | 80% | 0.7289 | Moderate synergism |
| 0.9 | 90% | 0.6796 | Synergism |
| 0.99 | 99% | 0.6622 | Synergism |
| Affected Fraction (fa) | % Rhodesain Inhibition | CI | Diagnosis CI |
|---|---|---|---|
| 0.5 | 50% | 1.1414 | Additive |
| 0.6 | 60% | 0.9654 | Slight synergism |
| 0.7 | 70% | 0.8216 | Moderate synergism |
| 0.8 | 80% | 0.6942 | Synergism |
| 0.9 | 90% | 0.5662 | Synergism |
| 0.99 | 99% | 0.3843 | Synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Chio, C.; Starvaggi, J.; Previti, S.; De Luca, F.; Natale, B.; Cosconati, S.; Schirmeister, T.; Zappalà, M.; Ettari, R. Synthesis and Combination Studies of Novel Dipeptide Nitriles with Curcumin for a Potent Synergistic Action Against Rhodesain, Cysteine Protease of Trypanosoma brucei rhodesiense. Pharmaceuticals 2025, 18, 847. https://doi.org/10.3390/ph18060847
Di Chio C, Starvaggi J, Previti S, De Luca F, Natale B, Cosconati S, Schirmeister T, Zappalà M, Ettari R. Synthesis and Combination Studies of Novel Dipeptide Nitriles with Curcumin for a Potent Synergistic Action Against Rhodesain, Cysteine Protease of Trypanosoma brucei rhodesiense. Pharmaceuticals. 2025; 18(6):847. https://doi.org/10.3390/ph18060847
Chicago/Turabian StyleDi Chio, Carla, Josè Starvaggi, Santo Previti, Fabiola De Luca, Benito Natale, Sandro Cosconati, Tanja Schirmeister, Maria Zappalà, and Roberta Ettari. 2025. "Synthesis and Combination Studies of Novel Dipeptide Nitriles with Curcumin for a Potent Synergistic Action Against Rhodesain, Cysteine Protease of Trypanosoma brucei rhodesiense" Pharmaceuticals 18, no. 6: 847. https://doi.org/10.3390/ph18060847
APA StyleDi Chio, C., Starvaggi, J., Previti, S., De Luca, F., Natale, B., Cosconati, S., Schirmeister, T., Zappalà, M., & Ettari, R. (2025). Synthesis and Combination Studies of Novel Dipeptide Nitriles with Curcumin for a Potent Synergistic Action Against Rhodesain, Cysteine Protease of Trypanosoma brucei rhodesiense. Pharmaceuticals, 18(6), 847. https://doi.org/10.3390/ph18060847











