Evaluating TnP as a Potential Therapeutic Agent for Retinopathy in Zebrafish Models
Abstract
1. Introduction
2. Results
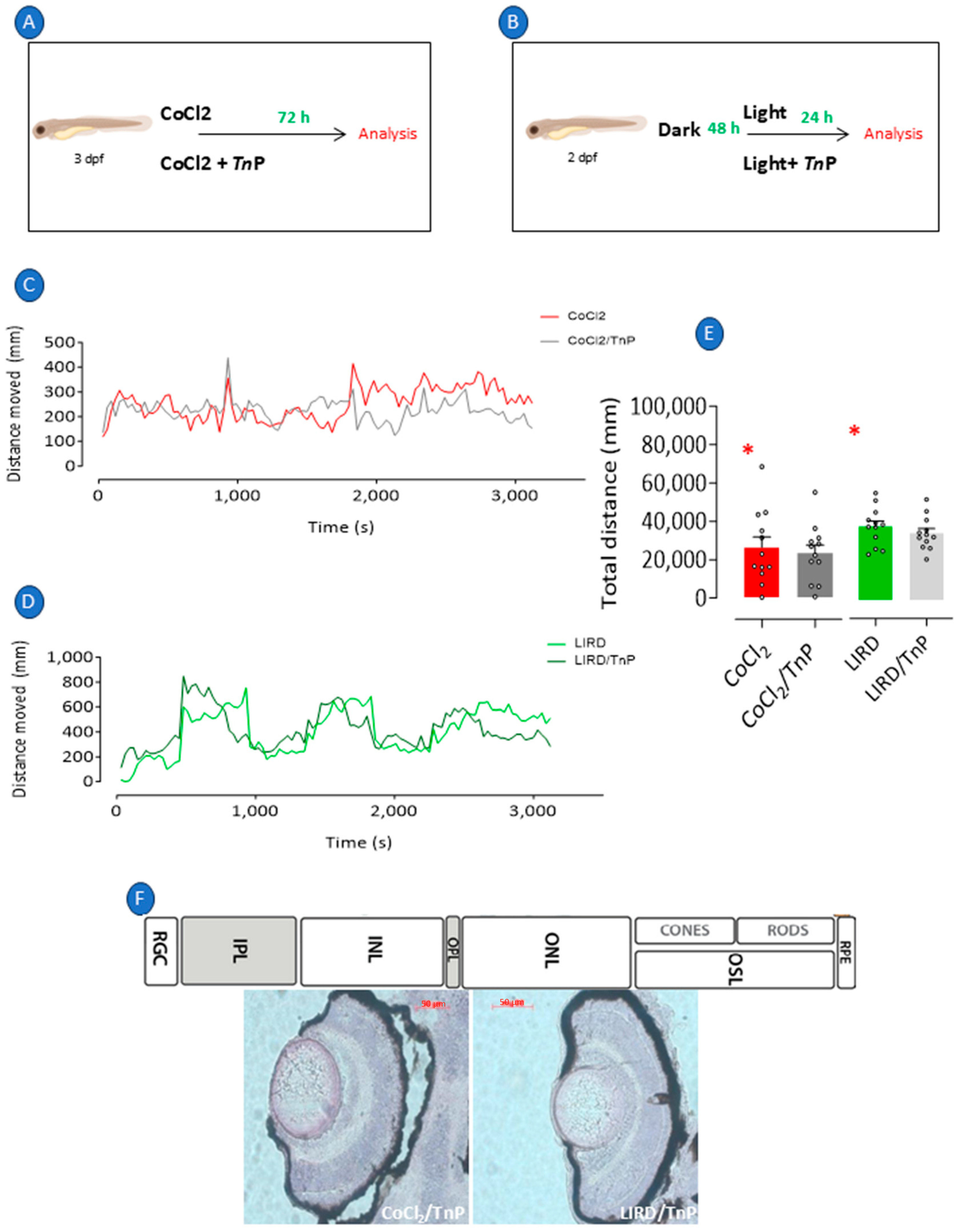
2.1. Recapitulation of Retinopathy in Zebrafish Larvae
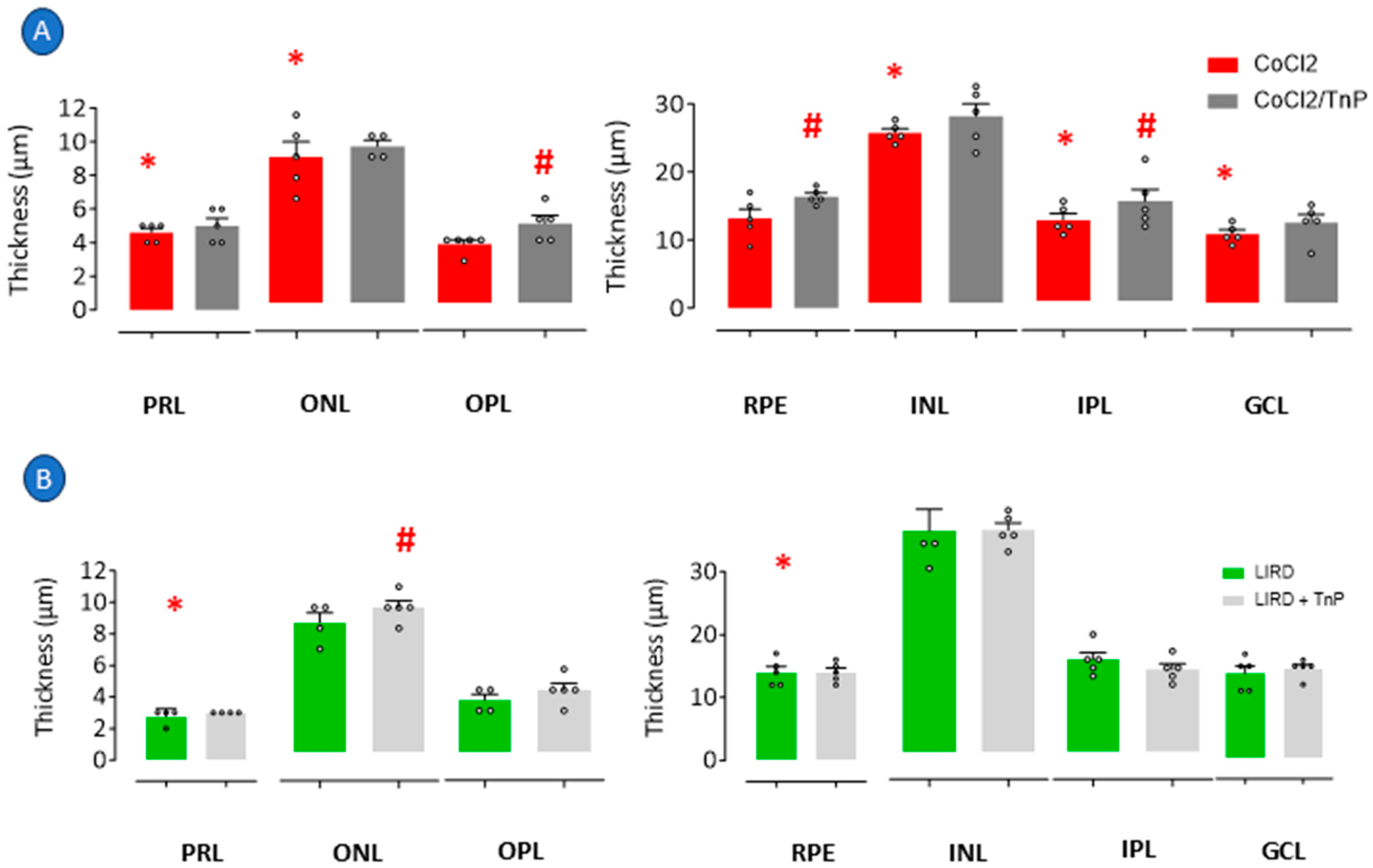
2.2. Prophylactic Effects of TnP in Retinopathy Models
2.3. Intrinsic Effects of TnP
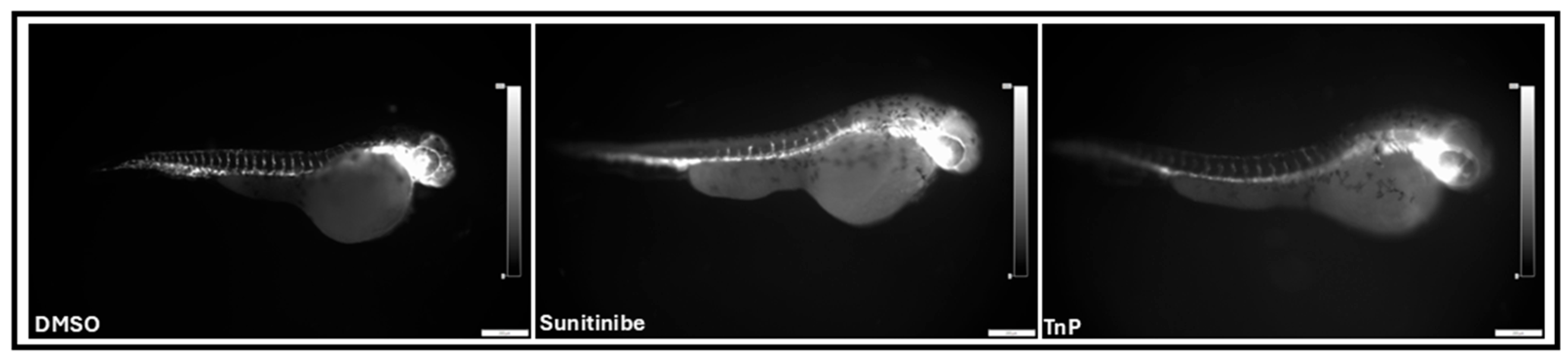
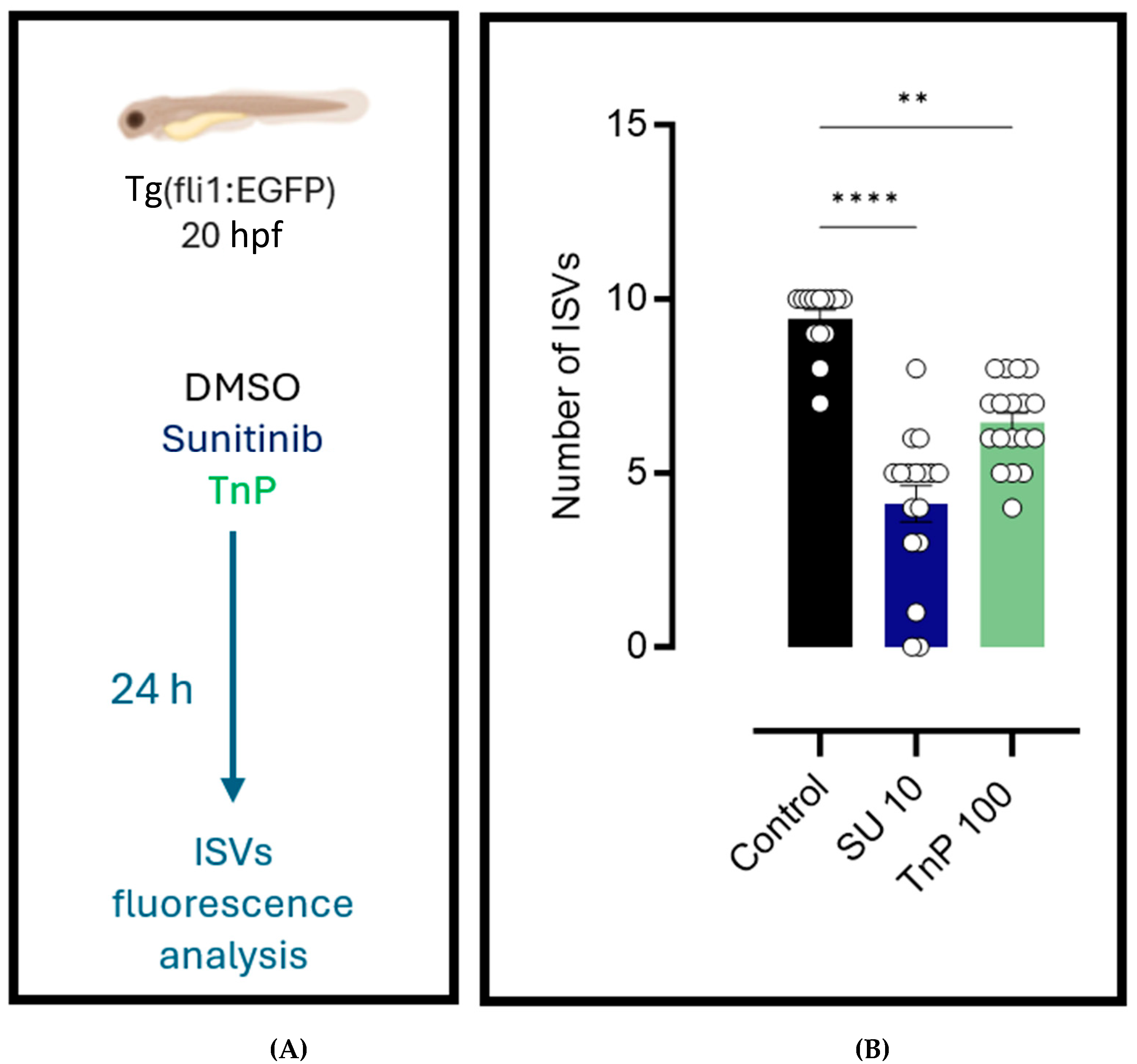
2.4. Antiangiogenic Effects of TnP
3. Discussion
3.1. Modulation of Retinal Degeneration and Regeneration: Effects of TnP in Hypoxia and LIRD Models
3.2. Study Limitations
- Molecular mechanisms: Gene expression or receptor interaction studies were not performed. Future work should incorporate a PCR, RNA-seq, or proteomics to elucidate TnP’s pathways.
- Functional assessment: The visual–motor response (VMR) was the sole behavioral metric. Complementary tests (e.g., optokinetic response tests, electroretinography) would strengthen the functional correlations.
- Comparator drugs: The absence of a standard anti-inflammatory control limited direct efficacy comparisons. Benchmarking against established therapies (e.g., anti-VEGF agents) is needed.
- Mechanistic insights: TnP’s proposed actions (e.g., integrin/VEGF modulation) remain hypothetical. Molecular docking, binding assays, and signaling studies are essential for their validation.
4. Materials and Methods
4.1. TnP Peptide
4.2. Zebrafish Husbandry
4.3. Zebrafish Anesthesia and Euthanasia
4.4. Cobalt Chloride-Induced Retinopathy and TnP Prophylactic Treatment
4.5. Intense Light-Induced Retinal Damage and TnP Prophylactic Treatment
4.6. Visual–Motor Response
4.7. Hematoxylin/Eosin (H&E) Histological Analysis
4.8. Vasculature Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonade, D.; Kern, T.S. Photoreceptor Cells and RPE Contribute to the Development of Diabetic Retinopathy. Prog. Retin. Eye Res. 2021, 83, 100919. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hu, J.; Zhang, F.; Jin, Q.; Sun, X. High Glucose Promotes IL-17A-Induced Gene Expression through Histone Acetylation in Retinal Pigment Epithelium Cells. Int. Immunopharmacol. 2022, 110, 108893. [Google Scholar] [CrossRef]
- Middel, C.S.; Hammes, H.-P.; Kroll, J. Advancing Diabetic Retinopathy Research: Analysis of the Neurovascular Unit in Zebrafish. Cells 2021, 10, 1313. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, Y.; Chen, X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics. Front. Pharmacol. 2017, 8, 460. [Google Scholar] [CrossRef]
- Antman, E.M.; Bennett, J.S.; Daugherty, A.; Furberg, C.; Roberts, H.; Taubert, K.A. Use of Nonsteroidal Antiinflammatory Drugs. Circulation 2007, 115, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Moghadam-Kia, S.; Werth, V.P. Prevention and Treatment of Systemic Glucocorticoid Side Effects. Int. J. Dermatol. 2010, 49, 239–248. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Komegae, E.N.; Souza, T.A.M.; Grund, L.Z.; Lima, C.; Lopes-Ferreira, M. Multiple Functional Therapeutic Effects of TnP: A Small Stable Synthetic Peptide Derived from Fish Venom in a Mouse Model of Multiple Sclerosis. PLoS ONE 2017, 12, e0171796. [Google Scholar] [CrossRef]
- Lima, C.; Maleski, A.L.A.; Bernardo, J.T.G.; Zelli, V.C.; Komegae, E.N.; Lopes-Ferreira, M. TnP Peptide Suppresses Experimental Autoimmune Encephalomyelitis (EAE) in a Preclinical Mouse Model. Front. Immunol. 2022, 13, 857692. [Google Scholar] [CrossRef]
- Lima, C.; Falcão, M.A.P.; Pinto, F.J.; Bernardo, J.T.G.; Lopes-Ferreira, M. The Anti-Inflammatory Peptide TnP Is a Candidate Molecule for Asthma Treatment. Cells 2023, 12, 924. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Xu, G.-T.; Zhang, J.-F. Inflammation in Diabetic Retinopathy: Possible Roles in Pathogenesis and Potential Implications for Therapy. Neural Regen. Res. 2023, 18, 976. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Eto, S.F.; Lopes-Ferreira, M. Shedding Light on the Drug–Target Prediction of the Anti-Inflammatory Peptide TnP with Bioinformatics Tools. Pharmaceuticals 2022, 15, 994. [Google Scholar] [CrossRef] [PubMed]
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The Vascular Anatomy of the Developing Zebrafish: An Atlas of Embryonic and Early Larval Development. Dev. Biol. 2001, 230, 278–301. [Google Scholar] [CrossRef]
- Abrams, E.W.; Mullins, M.C. Early Zebrafish Development: It’s in the Maternal Genes. Curr. Opin. Genet. Dev. 2009, 19, 396–403. [Google Scholar] [CrossRef]
- Schmitt, E.A.; Dowling, J.E. Early Retinal Development in the Zebrafish, Danio Rerio: Light and Electron Microscopic Analyses. J. Comp. Neurol. 1999, 404, 515–536. [Google Scholar] [CrossRef]
- Rosa, J.G.S.; Lopes-Ferreira, M.; Lima, C. An Overview towards Zebrafish Larvae as a Model for Ocular Diseases. Int. J. Mol. Sci. 2023, 24, 5387. [Google Scholar] [CrossRef]
- Batista-Filho, J.; Falcão, M.A.P.; Maleski, A.L.A.; Soares, A.B.S.; Balan-Lima, L.; Disner, G.R.; Lima, C.; Lopes-Ferreira, M. Early Preclinical Screening Using Zebrafish (Danio Rerio) Reveals the Safety of the Candidate Anti-Inflammatory Therapeutic Agent TnP. Toxicol. Rep. 2021, 8, 13–22. [Google Scholar] [CrossRef]
- Pimentel Falcao, M.A.; Banderó Walker, C.I.; Rodrigo Disner, G.; Batista-Filho, J.; Silva Soares, A.B.; Balan-Lima, L.; Lima, C.; Lopes-Ferreira, M. Knockdown of MiR-26a in Zebrafish Leads to Impairment of the Anti-Inflammatory Function of TnP in the Control of Neutrophilia. Fish Shellfish. Immunol. 2021, 114, 301–310. [Google Scholar] [CrossRef]
- Rahimi, M.; Hossain, F.; Leahy, S.; Blair, N.P.; Jiang, X.; Shahidi, M. Inner Retinal Oxygen Delivery and Metabolism in Progressive Stages of Diabetic Retinopathy. Sci. Rep. 2024, 14, 4414. [Google Scholar] [CrossRef]
- Mendelsohn, B.A.; Kassebaum, B.L.; Gitlin, J.D. The Zebrafish Embryo as a Dynamic Model of Anoxia Tolerance. Dev. Dyn. 2008, 237, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, B.; Ceña, V.; Posadas, I. The Endoplasmic Reticulum Stress and the HIF-1 Signalling Pathways Are Involved in the Neuronal Damage Caused by Chemical Hypoxia. Br. J. Pharmacol. 2015, 172, 2838–2851. [Google Scholar] [CrossRef]
- Taylor, S.; Chen, J.; Luo, J.; Hitchcock, P. Light-Induced Photoreceptor Degeneration in the Retina of the Zebrafish. In Retinal Development. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 247–254. [Google Scholar]
- Baksheeva, V.E.; Tiulina, V.V.; Tikhomirova, N.K.; Gancharova, O.S.; Komarov, S.V.; Philippov, P.P.; Zamyatnin, A.A.; Senin, I.I.; Zernii, E.Y. Suppression of Light-Induced Oxidative Stress in the Retina by Mitochondria-Targeted Antioxidant. Antioxidants 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, S.; Hurst, J.; Rensinghoff, F.; Tsai, T.; Grauthoff, S.; Satgunarajah, Y.; Dick, H.B.; Schnichels, S.; Joachim, S.C. Degenerative Effects of Cobalt-Chloride Treatment on Neurons and Microglia in a Porcine Retina Organ Culture Model. Exp. Eye Res. 2017, 155, 107–120. [Google Scholar] [CrossRef]
- Mirshahi, A.; Hoehn, R.; Lorenz, K.; Kramann, C.; Baatz, H. Anti-Tumor Necrosis Factor Alpha for Retinal Diseases: Current Knowledge and Future Concepts. J. Ophthalmic Vis. Res. 2012, 7, 39–44. [Google Scholar] [PubMed]
- Kim, G.H.; Paik, S.-S.; Park, Y.S.; Kim, H.G.; Kim, I.-B. Amelioration of Mouse Retinal Degeneration After Blue LED Exposure by Glycyrrhizic Acid-Mediated Inhibition of Inflammation. Front. Cell. Neurosci. 2019, 13, 319. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Dick, A.D.; Brézin, A.P.; Nguyen, Q.D.; Thorne, J.E.; Kestelyn, P.; Barisani-Asenbauer, T.; Franco, P.; Heiligenhaus, A.; Scales, D.; et al. Adalimumab in Patients with Active Noninfectious Uveitis. N. Engl. J. Med. 2016, 375, 932–943. [Google Scholar] [CrossRef]
- Novikova, Y.P.; Gancharova, O.S.; Eichler, O.V.; Philippov, P.P.; Grigoryan, E.N. Preventive and Therapeutic Effects of SkQ1-Containing Visomitin Eye Drops against Light-Induced Retinal Degeneration. Biochemistry 2014, 79, 1101–1110. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten Years of Anti-Vascular Endothelial Growth Factor Therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Lahne, M.; Nagashima, M.; Hyde, D.R.; Hitchcock, P.F. Reprogramming Müller Glia to Regenerate Retinal Neurons. Annu. Rev. Vis. Sci. 2020, 6, 171–193. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, J.G.S.; Bernardo, J.T.G.; Álvarez, Y.; Kennedy, B.; Lima, C.; Lopes-Ferreira, M. Evaluating TnP as a Potential Therapeutic Agent for Retinopathy in Zebrafish Models. Pharmaceuticals 2025, 18, 840. https://doi.org/10.3390/ph18060840
Rosa JGS, Bernardo JTG, Álvarez Y, Kennedy B, Lima C, Lopes-Ferreira M. Evaluating TnP as a Potential Therapeutic Agent for Retinopathy in Zebrafish Models. Pharmaceuticals. 2025; 18(6):840. https://doi.org/10.3390/ph18060840
Chicago/Turabian StyleRosa, João Gabriel Santos, Jefferson Thiago Gonçalves Bernardo, Yolanda Álvarez, Breandán Kennedy, Carla Lima, and Monica Lopes-Ferreira. 2025. "Evaluating TnP as a Potential Therapeutic Agent for Retinopathy in Zebrafish Models" Pharmaceuticals 18, no. 6: 840. https://doi.org/10.3390/ph18060840
APA StyleRosa, J. G. S., Bernardo, J. T. G., Álvarez, Y., Kennedy, B., Lima, C., & Lopes-Ferreira, M. (2025). Evaluating TnP as a Potential Therapeutic Agent for Retinopathy in Zebrafish Models. Pharmaceuticals, 18(6), 840. https://doi.org/10.3390/ph18060840








