Parallel In Vitro and In Silico Studies of the Anti-Inflammatory Activity of Bioactive Compounds Found in Different Ethanolic Extracts of Bracts from B. x buttiana (var. Rose): A Comparative Analysis
Abstract
1. Introduction
2. Results
2.1. Comparison of the Amount of Ethanol and the Yield
2.2. Ethanol Concentration’s Impact on In Vitro Anti-Inflammatory Action
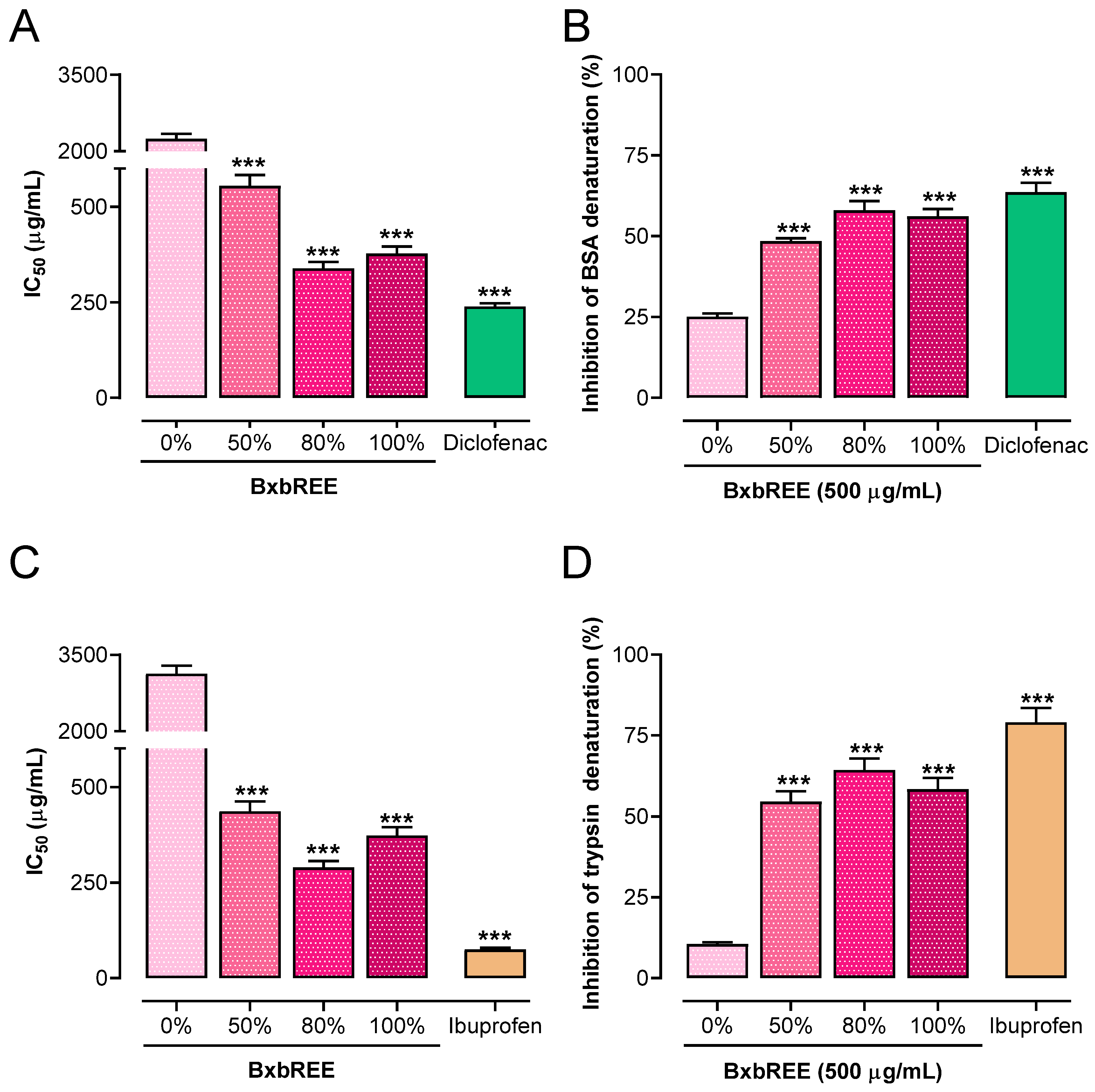
2.2.1. Impact of the BxbREE on Protein Denaturation
2.2.2. Effect of BxbREEs on Phospholipase Activity
2.2.3. BxbREEs’ Effects on Cyclooxygenase Activity
2.3. BxbREEs’ Effects on Erythrocyte Membrane Integrity Assay
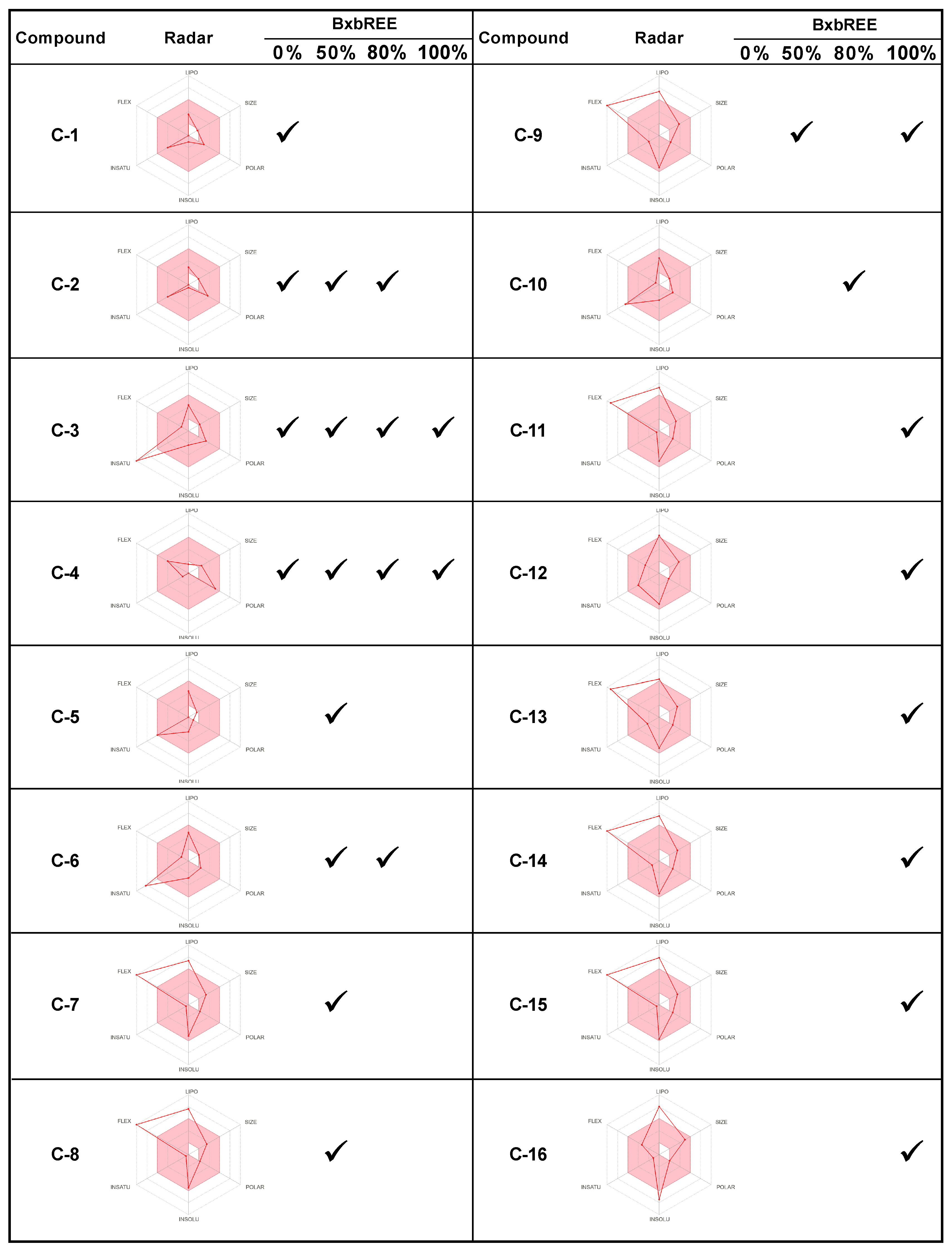
2.4. In Silico Analysis of the Compounds Found in BxbREE-0%, -50%, -80%, and -100%
3. Discussion
4. Materials and Methods
4.1. Chemicals Reagents
4.2. Plant Material
4.3. In Vitro Anti-Inflammation Activity Determination
4.3.1. Denaturation Assay Using Heat-Induced Bovine Serum Albumin
4.3.2. Proteinase Inhibitory Assay
4.4. Anti-Phospholipase Activity
4.5. Cyclooxygenase Inhibitory Activity of BxbREE
4.6. Erythrocyte Membrane Stabilization Assay
4.7. In Silico Evaluation of Pharmacokinetics Properties, Physicochemical Profile, and Drug-Likeness for Compounds in BxbREE
4.8. Presentation of Results and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| BxbREE | B. x buttiana (var. Rose) ethanolic extracts |
| C-1 | 2,5-Dimethyl-4-hydroxy-3-(2H)-Furanone |
| C-2 | 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl |
| C-3 | 2-Propenoic acid, 3-(2-hydroxyphenyl)-, (E)- |
| C-4 | 3-O-Methyl-d-glucose |
| C-5 | Benzofuran, 2,3-dihydro |
| C-6 | 2-Methoxy-4-vinylphenol |
| C-7 | 2-Methoxy-4-vinylphenol |
| C-8 | Hexadecanoic acid, ethyl ester |
| C-9 | 9,12-Octadecadienoic acid, ethyl ester |
| C-10 | Ethanone, 1-(2-hydroxy-5-methylphenyl)- |
| C-11 | n-Hexadecanoic acid |
| C-12 | Naphthalene, 3,4-dihydro-1,8-bis(trimethylsilyloxy)- |
| C-13 | 9,12-Octadecadienoic acid (Z,Z)- |
| C-14 | 9-Octadecenoic acid |
| C-15 | Octadecanoic acid |
| C-16 | Stigmasta-5,22-dien-3-ol |
| COX-1 | Cyclooxygenase-1 |
| COX-2 | Cyclooxygenase-2 |
| CYP450 | Cytochrome P450 |
| hG-IIA | Human group IIA secreted phospholipase A2 |
| pG-IB | Secretory phospholipase A2 group IB |
| PLA2 | Phospholipase A2 |
References
- Janeway, C.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Zotova, N.; Zhuravleva, Y.; Chereshnev, V.; Gusev, E. Acute and Chronic Systemic Inflammation: Features and Differences in the Pathogenesis, and Integral Criteria for Verification and Differentiation. Int. J. Mol. Sci. 2023, 24, 1144. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, T. The molecular mechanisms of chronic inflammation development. Front. Immunol. 2012, 3, 323. [Google Scholar] [CrossRef]
- Eggleton, P.; Haigh, R.; Winyard, P.J.R. Consequence of neo-antigenicity of the ‘altered self’. Rheumatology 2008, 47, 567–571. [Google Scholar] [CrossRef]
- Williams, L.A.D.; O’Connar, A.; Latore, L.; Dennis, O.; Ringer, S.; Whittaker, J.A.; Conrad, J.; Vogler, B.; Rosner, H.; Kraus, W. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Med. J. 2008, 57, 327–331. [Google Scholar]
- Al-Shidhani, A.; Al-Rawahi, N.; Al-Rawahi, A.J.O.M.J. Non-steroidal anti-inflammatory drugs (NSAIDs) use in primary health care centers in A’Seeb, Muscat: A clinical audit. Oman Med. J. 2015, 30, 366. [Google Scholar] [CrossRef]
- Ong, C.; Lirk, P.; Tan, C.; Seymour, R. An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin. Med. Res. 2007, 5, 19–34. [Google Scholar] [CrossRef]
- Chandra, S.; Dey, P.; Bhattacharya, S.J.J.A.P.E.R. Preliminary in vitro assessment of anti-inflammatory property of Mikania scandens flower extract. J. Adv. Pharm. Edu. Res. 2012, 2, 25–31. [Google Scholar]
- Mitra, R.; Ghosh, S.; Mukherjee, G.; Chowdhury, A.A. Secondary Metabolites: Treasure Trove for Future Medicine. In Plant Specialized Metabolites: Phytochemistry, Ecology and Biotechnology; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 1–45. [Google Scholar]
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar]
- Abarca-Vargas, R.; Pena Malacara, C.F.; Petricevich, V.L.J.A. Characterization of chemical compounds with antioxidant and cytotoxic activities in bougainvillea x buttiana holttum and standl, (Var. rose) extracts. Antioxidants 2016, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Lee, S.W.; Ahn, J.H. Phenolic C-glycoside synthesis using microbial systems. Curr. Opin. Biotechnol. 2022, 78, 102827. [Google Scholar] [CrossRef] [PubMed]
- Waszkowiak, K.; Gliszczyńska-Świgło, A.; Barthet, V.; Skręty, J. Effect of extraction method on the phenolic and cyanogenic glucoside profile of flaxseed extracts and their antioxidant capacity. J. Am. Oil Chem. Soc. 2015, 92, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Vargas, R.; Petricevich, V.L. Bougainvillea genus: A review on phytochemistry, pharmacology, and toxicology. Evid. Based Complement. Altern. Med. 2018, 2018, 9070927. [Google Scholar] [CrossRef]
- Abarca-Vargas, R.; Zamilpa, A.; Petricevich, V.L. Development and validation of conditions for extracting flavonoids content and evaluation of antioxidant and cytoprotective activities from Bougainvillea x buttiana Bracteas (var. rose). Antioxidants 2019, 8, 264. [Google Scholar] [CrossRef]
- Castañeda-Corral, G.; Cedillo-Cortezano, M.; Aviles-Flores, M.; López-Castillo, M.; Acevedo-Fernández, J.J.; Petricevich, V.L.J.P. Antinociceptive and Anti-Inflammatory Activities of Acetonic Extract from Bougainvillea x buttiana (var. Rose). Pharmaceuticals 2024, 17, 1037. [Google Scholar] [CrossRef]
- Figueroa, L.A.; Navarro, L.B.; Vera, M.P.; Petricevich, V.L. Antioxidant activity, total phenolic and flavonoid contents, and cytotoxicity evaluation of Bougainvillea xbuttiana. Int. J. Pharm. Pharm. Sci. 2014, 6, 497–502. [Google Scholar]
- Gil AA, P.; Navarro, L.B.; Vera, M.P.; Petricevich, V.L. Anti-inflammatory and antinociceptive activities of the ethanolic extract of Bougainvillea xbuttiana. J. Ethnopharmacol. 2012, 144, 712–719. [Google Scholar]
- Schuh, M.G.; Boldini, D.; Sieber, S.A. Synergizing Chemical Structures and Bioassay Descriptions for Enhanced Molecular Property Prediction in Drug Discovery. J. Chem. Inf. Model. 2024, 64, 4640–4650. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Loo, T.W.; Clarke, D.M. Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J. Membr. Biol. 2005, 206, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Hakkola, J.; Hukkanen, J.; Turpeinen, M.; Pelkonen, O. Inhibition and induction of CYP enzymes in humans: An update. Arch. Toxicol. 2020, 94, 3671–3722. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Kosala, K.; Widodo, M.A.; Santoso, S.; Karyono, S. In vitro and in vivo anti-inflammatory activities of Coptosapelta flavescens Korth Root’s methanol extract. J. Appl. Pharm. Sci. 2018, 8, 042–048. [Google Scholar]
- Kaymaz, K.; Hensel, A.; Beikler, T. Polyphenols in the prevention and treatment of periodontal disease: A systematic review of in vivo, ex vivo and in vitro studies. Fitoterapia 2019, 132, 30–39. [Google Scholar] [CrossRef]
- Jahangir, S.; John, P.; Bhatti, A.; Aslam, M.M.; Malik, J.M.; Anderson, J.R.; Peffers, M.J. LC-MS/MS-Based Serum Protein Profiling for Identification of Candidate Biomarkers in Pakistani Rheumatoid Arthritis Patients. Life 2022, 12, 464. [Google Scholar] [CrossRef]
- Otunola, G.A.; Afolayan, A.J.J.B.; Equipment, B. In vitro antibacterial, antioxidant and toxicity profile of silver nanoparticles green-synthesized and characterized from aqueous extract of a spice blend formulation. Biotechnol. Biotechnol. Equip. 2018, 32, 724–733. [Google Scholar] [CrossRef]
- Russo, A.; Acquaviva, R.; Campisi, A.; Sorrenti, V.; Di Giacomo, C.; Virgata, G.; Barcellona, M.; Vanella, A. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol. Toxicol. 2000, 16, 91–98. [Google Scholar] [CrossRef]
- Vargas, R.A.; Guerrero, R.V.; Petricevich, V.L. Evaluation of anti-arthritic potential of partitioned extracts of Bougainvillea x buttiana (var. Rose) holttum and standl. Int. J. Pharm. Pharm. Sci. 2018, 10, 117. [Google Scholar] [CrossRef]
- Yesmin, S.; Paul, A.; Naz, T.; Rahman, A.B.M.A.; Akhter, S.F.; Wahed, M.I.I.; Bin Emran, T.; Siddiqui, S.A. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clin. Phytosci. 2020, 6, 59. [Google Scholar] [CrossRef]
- Kardile, M.V.; Mahajan, U.B.; Shaikh, H.M.; Goyal, S.N.; Patil, C.R. Membrane stabilization assay for anti-inflammatory activity yields false positive results for samples containing traces of ethanol and methanol. World J. Pharm. Pharm. Sci. 2016, 5, 493–497. [Google Scholar]
- Amujoyegbe, O.O.; Agbedahunsi, J.M.; Akinpelu, B.A.; Oyedapo, O.O. In vitro evaluation of membrane stabilizing activities of leaf and root extracts of Calliandra portoricensis (JACQ) benth on sickle and normal human erythrocytes. Int. Res. J. Pharm. Pharmacol. 2012, 2, 198–203. [Google Scholar]
- Wu, Y.; Bao, J.; Liu, Y.; Wang, X.; Lu, X.; Wang, K. In vitro and in silico analysis of the bindings between legacy and novel per-and polyfluoroalkyl substances and human serum albumin. Toxics 2024, 12, 46. [Google Scholar] [CrossRef]
- Trujillo-Correa, A.I.; Quintero-Gil, D.C.; Diaz-Castillo, F.; Quiñones, W.; Robledo, S.M.; Martinez-Gutierrez, M. In vitro and in silico anti-dengue activity of compounds obtained from Psidium guajava through bioprospecting. BMC Complement. Altern. Med. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Oyedapo, O.O.; Akinpelu, B.A.; Akinwunmi, K.F.; Adeyinka, M.O.; Sipeolu, F.O. Red blood cell membrane stabilizing potentials of extracts of Lantana camara and its fractions. Int. J. Plant Physiol. Biochem. 2010, 2, 46–51. [Google Scholar]
- Shinde, U.; Phadke, A.; Nair, A.; Mungantiwar, A.; Dikshit, V.; Saraf, M. Membrane stabilizing activity—A possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 1999, 70, 251–257. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V.J.C. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]





| Ethanol Concentration in the Extract | Recovery (%) |
|---|---|
| 0% | 30.31 ± 0.69 * |
| 50% | 29.28 ± 0.72 * |
| 80% | 19.49 ± 0.51 * |
| 100% | 7.82 ± 0.18 |
| Clave | C-3 | C-5 | C-6 | C-10 | C-12 |
|---|---|---|---|---|---|
| Chemical name | 2-Propenoic acid, 3-(2-hydroxyphenyl)-, (E)- | Benzofuran, 2,3-dihydro- | 2-Methoxy-4-vinylphenol | Ethanone, 1-(2-hydroxy-5-methylphenyl)- | Naphthalene, 3,4-dihydro-1,8-bis(trimethylsilyloxy)- |
| Identified in: | BxbREE-0%, -50%, -80%, -100% | BxbREE-50% | BxbREE-50%, -80% | BxbREE-80% | BxbREE-100% |
| CYP isoform | |||||
| CYP450 2C9 Substrate/ Inhibitor | Non-substrate/ non-inhibitor | Non-substrate/ non-inhibitor | Non-substrate/ non-inhibitor | Non-substrate/ non-inhibitor | Non-substrate/ non-inhibitor |
| CYP450 2D6 Substrate/Inhibitor | Non-substrate/non-inhibitor | Non-substrate/ non-inhibitor | Non-substrate/non-inhibitor | Non-substrate/ non-inhibitor | Non-substrate/ non-inhibitor |
| CYP450 3A4 Substrate/Inhibitor | Non-substrate/ non-inhibitor | Non-substrate/ non-inhibitor | Non-substrate/non-inhibitor | Non-substrate/ non-inhibitor | Substrate/ non-inhibitor |
| CYP450 1A2 Inhibitor | Non-inhibitor | Inhibitor | Non-inhibitor | Inhibitor | Inhibitor |
| CYP450 2C19 Inhibitor | Non-inhibitor | Inhibitor | Non-inhibitor | Non-inhibitor | Inhibitor |
| CYP Inhibitory Promiscuity | Low CYP inhibitory promiscuity | Low CYP inhibitory promiscuity | Low CYP inhibitory promiscuity | Low CYP inhibitory promiscuity | High CYP inhibitory promiscuity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castañeda-Corral, G.; Cedillo-Cortezano, M.; Petricevich, V.L. Parallel In Vitro and In Silico Studies of the Anti-Inflammatory Activity of Bioactive Compounds Found in Different Ethanolic Extracts of Bracts from B. x buttiana (var. Rose): A Comparative Analysis. Pharmaceuticals 2025, 18, 821. https://doi.org/10.3390/ph18060821
Castañeda-Corral G, Cedillo-Cortezano M, Petricevich VL. Parallel In Vitro and In Silico Studies of the Anti-Inflammatory Activity of Bioactive Compounds Found in Different Ethanolic Extracts of Bracts from B. x buttiana (var. Rose): A Comparative Analysis. Pharmaceuticals. 2025; 18(6):821. https://doi.org/10.3390/ph18060821
Chicago/Turabian StyleCastañeda-Corral, Gabriela, Mayra Cedillo-Cortezano, and Vera L. Petricevich. 2025. "Parallel In Vitro and In Silico Studies of the Anti-Inflammatory Activity of Bioactive Compounds Found in Different Ethanolic Extracts of Bracts from B. x buttiana (var. Rose): A Comparative Analysis" Pharmaceuticals 18, no. 6: 821. https://doi.org/10.3390/ph18060821
APA StyleCastañeda-Corral, G., Cedillo-Cortezano, M., & Petricevich, V. L. (2025). Parallel In Vitro and In Silico Studies of the Anti-Inflammatory Activity of Bioactive Compounds Found in Different Ethanolic Extracts of Bracts from B. x buttiana (var. Rose): A Comparative Analysis. Pharmaceuticals, 18(6), 821. https://doi.org/10.3390/ph18060821







